Abstract
Background
Previous findings regarding the association between benzodiazepine exposure and dementia have conflicted, though many have not accounted for anticholinergic exposure. The goal of this study was to evaluate the association of benzodiazepine exposure with the risk of developing dementia, accounting for the anticholinergic burden.
Methods
Using a retrospective cohort design, we identified veterans 65 or older without dementia during a 10-year baseline period and then followed participants for 5 years to evaluate the risk of dementia diagnosis. The primary exposure was cumulative benzodiazepine exposure. Cox proportional hazards survival model was used to examine the association between benzodiazepine exposure and dementia, adjusting for anticholinergic burden and other demographic and clinical characteristics associated with increased dementia risk.
Results
Of the 528 006 veterans in the study cohort, 28.5% had at least one fill for a benzodiazepine. Overall, 7.9% developed a diagnosis of dementia during the observation period. Compared to veterans with no exposure to benzodiazepines, the adjusted hazard ratios for dementia risk were 1.06 (95% confidence interval [CI] 1.02–1.10) for low benzodiazepine exposure, 1.05 (95% CI 1.01–1.09) for medium benzodiazepine exposure, and 1.05 (95% CI 1.02–1.09) for high benzodiazepine exposure.
Conclusions
Cumulative benzodiazepine exposure was minimally associated with increased dementia risk when compared with nonuse but did not increase in a dose-dependent fashion with higher exposure. Veterans with low benzodiazepine exposure had essentially the equivalent risk of developing dementia as veterans with high exposure. While benzodiazepines are associated with many side effects for older adults, higher cumulative use does not appear to increase dementia risk.
Keywords: Cognitive impairment, Sedative hypnotic, Veterans affairs
Benzodiazepines are widely prescribed to older adults (1) despite the association of their use with a variety of adverse effects, including falls (2), fractures (3), and motor vehicle accidents (4). In addition, following opioids, benzodiazepines are the second most common medication class implicated in prescription drug overdose (5). Particularly concerning for older adults, benzodiazepines adversely affect cognition: In a review of 68 randomized, placebo-controlled trials, benzodiazepines consistently induced both amnestic and non-amnestic cognitive impairment, with larger effects among participants aged 65 years or older (6). Given these concerns, multiple professional societies have cautioned against long-term use of benzodiazepines among older adults given the high potential for side effects (7,8).
Benzodiazepine use is associated with sedation and inattention as well as declines across several cognitive domains including reduced visuospatial ability, speed of processing, and visual learning (9). Additionally, conditions for which benzodiazepines may be prescribed (eg, depression, anxiety, posttraumatic stress disorder, and anxiety) have also been independently associated with an increased risk of dementia (10). While benzodiazepines’ adverse impact on cognition is clear, the link between benzodiazepine exposure and development of dementia is less so. Several studies have supported an association between benzodiazepine use and increased risk for dementia, including 2 recent analyses published by Billioti de Gage et al. (11,12). However, 2 other recent cohort studies (13,14) and a case–control analysis (15) did not find increased risk related to benzodiazepine exposure. An additional recent study specifically limited to patients with mood disorders also did not find an association between benzodiazepine exposure and dementia (16).
One potential reason for the conflicting results is that these prior analyses, while accounting for a variety of potential confounders, did not account for anticholinergic exposure, which, if chronic, can cause Alzheimer-type pathology (17) and does appear to be associated with incident dementia (18). Adults who are prescribed benzodiazepines may also be more likely to take anticholinergic medications, exposure to which then confounds the apparent relationship of benzodiazepines with dementia. We sought to evaluate the association of cumulative benzodiazepine exposure and incident dementia utilizing veterans administration (VA) data over a 15-year period from FY2000 to FY2015, accounting for the important confounder of anticholinergic exposure. We hypothesized that high cumulative exposure to benzodiazepines would be associated with an increased risk of dementia.
Method
We linked prescription data from the VA Pharmacy Benefits Management service with the Corporate Data Warehouse patient data to evaluate the association between cumulative benzodiazepine exposure and incident dementia. The VA Ann Arbor Healthcare System institutional review board approved this study.
Study Cohort
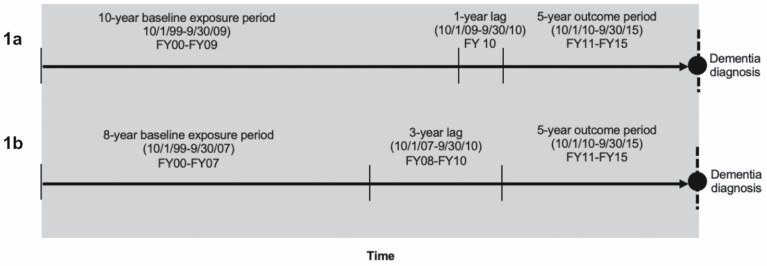
Data were drawn from a 100% sample of VHA data from FY2000 to FY2015. This time span was split into 3 periods: a 10-year exposure period (FY2000–FY2009), a 1-year lag period (FY2010), and a 5-year outcome period (FY2011–FY2015). Veterans were eligible for the study cohort if they were older than 54 years at the start of the baseline (ie, October 1, 1999) and, during each year of the baseline exposure period, they met the following criteria: (a) had at least one outpatient or inpatient service encounter, (b) had at least one prescription claim, and (c) did not have a diagnosis of dementia. We required that the cohort meet the above criteria for each year of the baseline exposure period in order to ascertain cumulative prescription benzodiazepine exposure. By requiring patients to be older than 54 years at the start of baseline, all cohort veterans were aged 65 years or older at the start of the outcome period for dementia ascertainment.
Incident Dementia Diagnosis
Incident dementia was determined during the 5-year study outcome period from FY2011 to FY2015 (Figure 1). We identified a diagnosis of Alzheimer’s disease or related dementia during the observation period from outpatient and inpatient service encounters each year (see Supplementary Table 1 for International Classification of Disease, Ninth Revision codes).
Figure 1.
Study timeline for primary (1a) and sensitivity (1b) analyses.
Cumulative Benzodiazepine Exposure
Using prescription data from VA Pharmacy Benefits Management, we identified benzodiazepine exposure during the 10-year baseline exposure period. All benzodiazepine prescriptions were converted to lorazepam equivalents (Supplementary Table 2) (19,20). A daily dose equivalent of lorazepam 1 mg per day was considered the standardized daily dose (SDD). We then calculated the total medication dose for each benzodiazepine prescription fill in lorazepam equivalents by multiplying the tablet strength by the number of tablets dispensed. Using methods similar to Gray et al. (14,18), we calculated the total standardized daily dose (TSDD) by summing the SDD for all benzodiazepine prescriptions during the 10-year exposure window. We categorized cumulative use as no use, low use (1–30 TSDD), medium use (31–365 TSDD), and high use (366+ TSDD) based on the distribution and clinically meaningful cut-points. For example, a TSDD of 365 meant that a patient received 365 days of daily dose-equivalent of lorazepam 1 mg during the 10-year baseline exposure period. (That exposure could have occurred during 365 continuous days or been intermittent throughout the 10-year exposure period.) To limit protopathic bias, we excluded benzodiazepine exposure in the year preceding the observation period (ie, FY2010) because benzodiazepine prescriptions that close to an incident diagnosis could have been prescribed for prodromal symptoms of dementia (21). Figure 1 demonstrates the study baseline exposure and outcome periods.
Covariates
We selected covariates for inclusion that are potentially associated with incident dementia risk based on a review of the literature (10). Sociodemographic factors included age, sex, education, and income; education and median income were determined from Census data based on the veteran’s ZIP Code (22). Clinical conditions included indicators of cardiovascular risk (eg, stroke, diabetes, hypertension, myocardial infarction, congestive heart failure, angina, arrhythmia, hyperlipidemia, and peripheral artery disease) as well as depression, anxiety, posttraumatic stress disorder, insomnia, traumatic brain injury, tobacco use, and alcohol use. Clinical conditions were assessed from ICD-9-CM codes during the 10-year baseline and 1-year lag period.
Lastly, studies have consistently demonstrated an association between exposure to medications with strong anticholinergic properties and an increased risk of dementia (18,23,24). To account for such exposure, we calculated cumulative strong anticholinergic medication burden during the same 10-year baseline exposure period from which benzodiazepine exposure was ascertained (ie, FY2000–FY2009). We calculated the total medication dose for each anticholinergic medication fill by multiplying the tablet strength by the number of tablets dispensed. We then calculated the SDD by dividing by the minimum effective dose per day recommended for use in older adults (18,24). For each veteran, the TSDD was calculated by summing the SDD for all anticholinergic medication fills during the baseline exposure period. As with benzodiazepines, prescriptions during the FY2010-lag period were excluded. TSDD anticholinergic medication exposures were categorized as no use, 1–30, 31–365, 366–1 095, and >1 095 based on previous studies (18,24).
Statistical Analysis
For descriptive purposes, we identified the most commonly prescribed benzodiazepines among veterans during the baseline period, characterized by half-life (20). Next, we summarized the sociodemographic and clinical characteristics of the cohort by benzodiazepine exposure groups.
We used a Cox proportional hazards survival model to evaluate the association between cumulative benzodiazepine exposure and incident dementia. Participants were censored for (a) death, (b) gap in care for greater than 12 months from a last inpatient or outpatient service encounter (ie, on Day 366 following their last encounter), or (c) end of the study period. We fit the following models sequentially to meet our study objectives: Model 1 was unadjusted, Model 2 added patient sociodemographic characteristics (eg, age, income, education) and clinical conditions associated with incident dementia risk, and Model 3 then added the 10-year cumulative anticholinergic medication exposure during the baseline exposure period. The assumption of proportional hazards was assessed by examining plots of Schoenfeld residuals by transformed time for each variable. Plots did not suggest nonproportional hazards.
We conducted several sensitivity analyses. First, given the potential that benzodiazepine use preceding a dementia diagnosis may be in response to prodromal symptoms of dementia, we extended the lag time to 3 years to help minimize potential protopathic bias. Second, we assessed whether results remained consistent for the risk of developing Alzheimer’s disease specifically (ICD-9-CM codes 290.0, 290.1x, 290.2x, 290.3, 294.10, 294.11, 331.0). Third, among veterans with high benzodiazepine exposure (ie, those with a TSDD 366+), we evaluated if the risk of incident dementia differed by the recency of benzodiazepine exposure (ie, recent users, past users, or continuous users) (14). Lastly, to differentially account for the competing risk of death, we reran the analysis with death as a competing risk using Fine and Gray subdistribution hazard models.
All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC).
Results
At the start of the outcome period in FY2010, the analytic cohort included 528 066 veterans. The mean age was 77.0 years (SD 7.4) and 97.7% were male. About 28.5% of veterans in the study cohort filled at least one prescription for a benzodiazepine during the exposure period; 7.9% of veterans in the study cohort received a diagnosis of dementia during the outcome period, of which 34.0% received benzodiazepines during the baseline exposure period.
Baseline sociodemographic, clinical characteristics, and anticholinergic exposure by the level of benzodiazepine exposure are given in Table 1. Generally, those with higher benzodiazepine exposure had a higher prevalence of mental health disorders and insomnia, though they did not have a higher burden of other medical conditions. Those with the highest benzodiazepine exposure also had the highest burden of high-level anticholinergic exposure (ie, TSDD >1 095) and the smallest proportion with no anticholinergic exposure: Among those in the top tertile of benzodiazepine exposure, 43.9% had the highest category of anticholinergic exposure, while 19.8% had no exposure. Among the study cohort, lorazepam, temazepam, diazepam, clonazepam, and alprazolam were the most commonly prescribed benzodiazepines during the baseline exposure period (Table 2).
Table 1.
Baseline Sociodemographic and Clinical Characteristics of Veterans at the Start of Observation Period, FY2010
| Benzodiazepine Exposure* | |||||
|---|---|---|---|---|---|
| Characteristics, N (%) | Overall (N = 528 066) | None (N = 377 784), N (%) | Low (N = 33 613), N (%) | Medium (N = 46 551), N (%) | High (N = 70 118), N (%) |
| Age, mean (SD) | 77.0 (7.4) | 77.4 (7.2) | 75.8 (7.3) | 76.2 (7.5) | 75.4 (7.6) |
| Male | 515 979 (97.7) | 370 295 (98.0) | 32 419 (96.4) | 45 125 (96.9) | 68 140 (97.2) |
| % with less than high school education, mean (SD) | 15.7 (9.0) | 15.6 (9.0) | 15.8 (9.1) | 15.9 (9.1) | 15.9 (8.9) |
| Median household income ($), mean (SD) | 49 620 (19 106.9) | 49 825 (19 248.7) | 49 787 (19 124.0) | 48 989 (18 815.6) | 48 860 (18 485.8) |
| Depression | 67 643 (12.8) | 30 316 (8.0) | 5 230 (15.6) | 9 955 (21.4) | 22 142 (31.6) |
| Anxiety | 33 556 (6.4) | 8 960 (2.4) | 2 008 (6.0) | 5 120 (11.0) | 17 468 (24.9) |
| Posttraumatic stress disorder | 33 567 (6.4) | 13 182 (3.5) | 2 252 (6.7) | 4 743 (10.2) | 13 390 (19.1) |
| Alcohol disorder | 15 560 (2.9) | 9 164 (2.4) | 1 551 (4.6) | 1 838 (3.9) | 3 007 (4.3) |
| Diabetes | 201 714 (38.2) | 145 371 (38.5) | 13 151 (39.1) | 18 053 (38.8) | 25 139 (35.9) |
| Hypertension | 388 628 (73.6) | 279 973 (74.1) | 25 023 (74.4) | 34 123 (73.3) | 49 509 (70.6) |
| Insomnia | 19 351 (3.7) | 7 299 (1.9) | 1 529 (4.5) | 3 442 (7.4) | 7 081 (10.1) |
| Stroke | 22 290 (4.2) | 14 921 (3.9) | 1 806 (5.4) | 2 298 (4.9) | 3 265 (4.7) |
| Traumatic brain injury | 2 531 (0.5) | 1 443 (0.4) | 223 (0.7) | 350 (0.8) | 515 (0.7) |
| Tobacco use disorder | 47 481 (9.0) | 30 472 (8.1) | 3 573 (10.6) | 4 877 (10.5) | 8 559 (12.2) |
| Myocardial infarction | 23 416 (4.4) | 15 279 (4.0) | 1 863 (5.5) | 2 624 (5.6) | 3 650 (5.2) |
| Coronary heart failure | 48 723 (9.2) | 32 416 (8.6) | 3 872 (11.5) | 5 342 (11.5) | 7 093 (10.1) |
| Angina | 12 628 (2.4) | 7 807 (2.1) | 1 138 (3.4) | 1 549 (3.3) | 2 134 (3.0) |
| Arrhythmia | 98 457 (18.6) | 69 106 (18.3) | 7 001 (20.8) | 9 580 (20.6) | 12 770 (18.2) |
| Hyperlipidemia | 358 767 (67.9) | 259 062 (68.6) | 22 372 (66.6) | 31 132 (66.9) | 46 201 (65.9) |
| Peripheral artery disease | 44 881 (8.5) | 30 919 (8.2) | 3 376 (10.0) | 4 394 (9.4) | 6 192 (8.8) |
| Cumulative anticholinergic medication use† | |||||
| None | 218 301 (41.3) | 184 238 (48.8) | 8 926 (26.6) | 11 238 (24.1) | 13 899 (19.8) |
| TSDD 1–30 | 42 132 (8.0) | 32 118 (8.5) | 3 297 (9.8) | 3 322 (7.1) | 3 395 (4.8) |
| TSDD 31–365 | 107 502 (20.4) | 74 047 (19.6) | 9 218 (27.4) | 11 645 (25.0) | 12 592 (18.0) |
| TSDD 366–1 095 | 53 454 (10.1) | 32 349 (8.6) | 4 634 (13.8) | 7 025 (15.1) | 9 446 (13.5) |
| TSDD >1 095 | 106 677 (20.2) | 55 032 (14.6) | 7 538 (22.4) | 13 321 (28.6) | 30 786 (43.9) |
*Cumulative benzodiazepine exposure: total standardized daily dose (TSDD) based on lorazepam equivalents summed over the 10-year exposure period, categorized as none (0), low (1–30), medium (31–365), and high (366+).
†Cumulative anticholinergic exposure: TSDD based on the minimum effective daily dose and summed over the 10-year exposure period.
Table 2.
Cumulative Benzodiazepine Exposure During the Study Baseline Exposure Period by Medication Type*
| Medication | Patients With Benzodiazepine Use (N = 150 282) N (%) |
Total Benzodiazepine Exposure Person-Days (N = 271 625 248) N (%) |
|---|---|---|
| Short-acting † | ||
| Lorazepam | 53 464 (35.6) | 58 543 918 (21.6) |
| Temazepam | 50 910 (33.9) | 30 421 821 (11.2) |
| Clonazepam | 31 094 (20.7) | 81 344 862 (29.9) |
| Alprazolam | 30 315 (20.2) | 62 472 212 (23.0) |
| Oxazepam | 8 917 (5.9) | 7 276 693 (2.7) |
| Triazolam | 734 (0.5) | 262 178 (0.1) |
| Estazolam | 4 (0.0) | 539 (0.0) |
| Long-acting | ||
| Diazepam | 32 360 (21.5) | 25 861 540 (9.5) |
| Chlordiazepoxide | 4 138 (2.8) | 3 753 102 (1.4) |
| Clorazepate | 895 (0.6) | 1 219 814 (0.4) |
| Flurazepam | 662 (0.4) | 689 124 (0.3) |
| Halazepam | 1 (0.0) | 420 (0.0) |
| Prazepam | 4 (0.0) | 106 (0.0) |
*Column percentages do not sum to 100% because patients could receive >1 benzodiazepine during the exposure period.
†Benzodiazepines are classified as short- or long-acting by half-life (ie, time taken for blood concentration to fall to half its peak value) (20).
Benzodiazepine Exposure and Dementia Risk
We fit 3 separate Cox proportional hazards survival models to characterize the relationship between cumulative benzodiazepine exposure and dementia risk. In unadjusted analysis (Model 1), cumulative benzodiazepine exposure was associated with increased dementia risk in a dose-dependent fashion when compared with nonuse (high benzodiazepine exposure [TSDD 366+]: hazard ratio [HR] 1.39, 95% confidence interval [CI] 1.35–1.43; medium benzodiazepine exposure [TSDD 31–365]: HR 1.31, 95% CI 1.27–1.35; low benzodiazepine exposure [1–30]: HR 1.20, 95% CI 1.15–1.24; Table 3). Model 2 included patient sociodemographic and clinical characteristics associated with incident dementia risk; inclusion of these variables reduced the strength of the association of benzodiazepine exposure and dementia risk. Compared to nonuse, high exposure (HR 1.12, 95% CI 1.08–1.15), medium exposure (HR 1.09, 95% CI 1.05–1.13), and low benzodiazepine exposure (HR 1.09, 95% CI 1.05–1.14) were only slightly associated with dementia risk. In Model 3, which included 10-year cumulative anticholinergic exposure, benzodiazepine exposure remained minimally associated with increased dementia risk when compared with nonuse, but did not increase in a dose-dependent fashion with higher exposure. Veterans with low benzodiazepine exposure (HR 1.06, 95% CI 1.02–1.10) had essentially the equivalent, slightly elevated risk of developing dementia as veterans with high benzodiazepine exposure (HR 1.05, 95% CI 1.02–1.09).
Table 3.
Association of Incident Dementia During Follow-up Years With 10-Year Benzodiazepine Exposure*
| Hazard Ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| Benzodiazepine Exposure† | Follow-up Time‡, Person-Years | Incident Dementia (N = 41 630), N (%) | Model 1: Unadjusted | Model 2: Demographics + Clinical Characteristics§ | Model 3: Model 2 + Anticholinergic Exposure |
| None | 1 509 015 | 27 529 (66.1) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Low | 133 754 | 2 923 (7.0) | 1.20 (1.15–1.24) | 1.09 (1.05–1.14) | 1.06 (1.02–1.10) |
| Medium | 180 894 | 4 314 (10.4) | 1.31 (1.27–1.35) | 1.09 (1.05–1.13) | 1.05 (1.01–1.09) |
| High | 271 486 | 6 864 (16.5) | 1.39 (1.35–1.43) | 1.12 (1.08–1.15) | 1.05 (1.02–1.09) |
*7.9% (N = 34 766) of veterans in the study cohort received a diagnosis of dementia during the follow-up period, of which 34.0% received benzodiazepines during the baseline period.
†Benzodiazepine exposure: total standardized daily dose summed over the 10-year exposure window, categorized as none (0), low (1–30), medium (31–365), and high (366+).
‡Follow-up time starts from October 1, 2010 until September 30, 2015, incident dementia, gap in VA care more than 365 days or death, whichever is earliest.
§Model 2: Adjusted for patient sociodemographic (age, income, education) and clinical conditions (stroke, diabetes, hypertension, myocardial infarction, congestive heart failure, angina, arrhythmia, hyperlipidemia, peripheral artery disease, depression, anxiety, posttraumatic stress disorder, insomnia, traumatic brain injury, tobacco, and alcohol use).
Sensitivity Analyses
We conducted several sensitivity analyses. First, to address potential protopathic bias (21), we increased the lag period to 3 years (Figure 1). In adjusted models controlling for patient sociodemographic, clinical characteristics, and anticholinergic medication exposure (ie, Model 3), only the highest level of benzodiazepine exposure was slightly associated with increased risk of dementia (HR 1.03, 95% CI 1.00–1.07). Low and medium levels of benzodiazepine exposure were not significantly associated with incident dementia (Supplementary Table 2). Next, we evaluated the association between benzodiazepine exposure and incident risk specifically of Alzheimer’s disease. In adjusted models, compared to nonuse, none of the benzodiazepine exposure levels were associated with the risk of Alzheimer’s disease (Supplementary Table 3). Among veterans with the highest use of benzodiazepines (TSDD 366+), we found that the timing of benzodiazepine exposure (ie, recent, past, or continuous use) was not associated with dementia risk: Veterans with continuous use of benzodiazepines during the 10-year baseline exposure period were just as likely as veterans with predominately recent or past use of benzodiazepines to develop dementia (Supplementary Table 4). Lastly, when we fit the Fine and Gray models with death as the competing risk, we found that while low levels of benzodiazepine exposure were associated with a higher risk of dementia (subdistribution HR 1.06, 95% CI 1.02–1.10), medium and high levels were not (subdistribution HR 1.03, 95% CI 0.99–1.06 and subdistribution HR 1.01, 95% CI 0.98–1.05, respectively; Supplementary Table 5).
Discussion
In this analysis of a large national sample of older veterans, we did not find a strong association between benzodiazepine use and dementia risk. When controlling for important covariates including cumulative anticholinergic exposure, Veterans with low benzodiazepine exposure had a nearly identical—or slightly higher—risk of developing dementia when compared with veterans with high benzodiazepine exposure, which argues against a causal relationship (25). In sensitivity analyses, no level of benzodiazepine exposure was associated with increased risk of developing Alzheimer’s disease specifically nor did the timing of benzodiazepine exposure (ie, recent, past, or continuous use) influence dementia risk.
The results from previous studies evaluating the association between cumulative benzodiazepine exposure and dementia risk have been inconsistent. Some studies have found an increased risk of dementia with benzodiazepine use (11,12), while others consistent with our findings, found no association (13–16). Our results differ from 2 recent analyses published by Billioti de Gage et al. (11,12), demonstrating an association between benzodiazepine exposure and dementia. In their first analysis, a prospective analysis of 1 063 adults who were dementia-free at baseline, new use of a benzodiazepine was associated with increased risk of dementia, with an adjusted HR of 1.60 (11). Their finding was consistent across multiple sensitivity analyses. However, in that analysis, the benzodiazepine prescribing could have been for prodromal or early symptoms of dementia such as depression or impaired sleep, which may occur before dementia is diagnosed—in other words, the prescribed benzodiazepine may have been for a symptom related to a neurodegenerative process not yet diagnosed as frank dementia. In a subsequent analysis designed to address this potential protopathic bias (21), the authors again found that benzodiazepine use was associated with an increased risk of Alzheimer’s disease (adjusted odds ratio 1.51) (12). While these studies controlled for a variety of covariates linked to dementia risk, neither study controlled for cumulative anticholinergic medication exposure, which can potentially confound the relationship between benzodiazepine exposure and dementia risk.
A more recent analysis of a prospective, population-based cohort of 3 434 older adults in the United States by Gray et al. (14) did not find that benzodiazepine exposure was associated with incident dementia. Furthermore, in a large nationwide cohort and nested case–control study in Denmark, the authors found that among 235 465 patients with affective disorders, benzodiazepine exposure was not associated with subsequent dementia (16). In a cohort study of 10 263 patients in Canada, Nafti et al. (13) found that while benzodiazepine exposure was associated with an increased risk of cognitive impairment (adjusted HR 1.32), there was no significant association between benzodiazepine use and the risk of dementia or Alzheimer’s disease. Furthermore, in a case–control study among 26 459 patients in the United Kingdom, Imfeld et al. (15) found that when controlling for protopathic bias by increasing the lag period to 3 years, there was no association between earlier benzodiazepine exposure and subsequent risk of Alzheimer’s disease or vascular dementia.
Relatively few studies examining the association between benzodiazepine exposure and dementia have accounted for anticholinergic use. Anticholinergic medications impair short-term cognition by blocking acetylcholine in the central nervous system, where it plays an essential role in cognitive function including memory and attention (26). Beyond these short-term effects, Alzheimer’s disease is associated with loss of cholinergic neurons in the basal forebrain (27), while cholinesterase inhibitors (eg, donepezil), which decrease the breakdown of acetylcholine, are the current mainstays of treatment for Alzheimer’s disease. In a cohort study in the United Kingdom, Grossi et al. (28) found no association between benzodiazepine exposure and dementia risk when controlling for anticholinergic exposure as a dichotomous variable. However, the study only included a small number of participants who had any use of benzodiazepines (N = 227) over a 10-year follow-up period. In a more recent cohort study among 3 526 community-dwelling patients within primary care in the Netherlands that controlled for anticholinergic exposure as a dichotomous variable, the authors again found no association between benzodiazepine use and dementia risk (29). Neither study evaluated the impact of cumulative dose and anticholinergic exposure.
This analysis represents one of the largest prospective cohort studies to evaluate the association between benzodiazepine exposure and dementia. We included a variety of covariates for inclusion that are associated with incident dementia risk (10) and ours is the first to also account for the cumulative dose of anticholinergic exposure as a potential confounder. Our analysis has several limitations. First, prescribing is a proxy for benzodiazepine exposure but does not capture actual medication use. Second, detection of baseline comorbidities and a dementia diagnosis are based on administrative claims data, which may contribute to misclassification bias and residual confounding. Third, our sample includes a high proportion of men, and our study results may not generalize to other clinical populations. Fourth, our study observation period ends after 5 years, which leaves dementia diagnoses past that period unobserved, which may also underestimate dementia risk. Lastly, our estimates of benzodiazepine and anticholinergic exposures may be underestimated because they do not account for medications received outside of the VA, such as through Medicare Part D. However, Part D did not begin until 2006 and did not cover benzodiazepines until 2013, limiting the potential for unobserved exposures.
While our study does not show a link between benzodiazepine exposure and dementia risk, benzodiazepines clearly impair cognition, including changes in memory, concentration, and attention (6,13). In a review of 68 randomized, placebo-controlled trials where participants underwent neuropsychological testing before and after benzodiazepine administration, Tannenbaum et al. (6) demonstrated that benzodiazepines consistently induced both amnestic and non-amnestic cognitive impairment. Such impairments can limit functional ability and threaten independence for older adults. Furthermore, the use of benzodiazepines among older adults has been associated with a variety of other noncognitive significant adverse effects including falls and sedation, which should limit their routine use among older adults to avoid medication-related harms.
Conclusions
This study represents one of the largest prospective studies evaluating the association between benzodiazepine use and dementia. Cumulative benzodiazepine exposure was not meaningfully associated with an increased risk of incident dementia. Differences from previous studies may be due to failure to account for other important confounding factors such as cumulative anticholinergic medication use. While benzodiazepines are associated with many serious side effects for older adults, higher cumulative use does not appear to increase dementia risk.
Funding
This work was supported by the National Institute of Drug Abuse (R01 DA045705-S1). L.B.G. was also supported, in part, by grant K23AG066864 from the National Institute on Aging. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit.
Conflict of Interest
None declared.
Author Contributions
L.B.G. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: L.B.G. and D.T.M; acquisition, analysis, or interpretation of data: all authors; drafting of the manuscript: L.B.G., R.V.I., and D.T.M.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: R.V.I. and H.M.K.; obtaining funding: D.T.M.; administrative, technical, or material support: D.T.M.; supervision: D.T.M.
Supplementary Material
No related articles have been published or submitted from this study.
Contributor Information
Lauren B Gerlach, Department of Psychiatry, University of Michigan, Ann Arbor, USA; Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, USA.
Hyungjin Myra Kim, Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, USA; Center for Clinical Management Research, VA Ann Arbor Healthcare System, Michigan, USA.
Rosalinda V Ignacio, Center for Clinical Management Research, VA Ann Arbor Healthcare System, Michigan, USA; Department of Biostatistics, School of Public Health, University of Michigan, Ann Arbor, USA.
Julie Strominger, Center for Clinical Management Research, VA Ann Arbor Healthcare System, Michigan, USA.
Donovan T Maust, Department of Psychiatry, University of Michigan, Ann Arbor, USA; Center for Clinical Management Research, VA Ann Arbor Healthcare System, Michigan, USA.
References
- 1. Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70(2):97–106. doi: 10.1176/appi.ps.201800321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–1960. doi: 10.1001/archinternmed.2009.357 [DOI] [PubMed] [Google Scholar]
- 3. Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Hazardous benzodiazepine regimens in the elderly: effects of half-life, dosage, and duration on risk of hip fracture. Am J Psychiatry. 2001;158(6):892–898. doi: 10.1176/appi.ajp.158.6.892 [DOI] [PubMed] [Google Scholar]
- 4. Dassanayake T, Michie P, Carter G, Jones A. Effects of benzodiazepines, antidepressants and opioids on driving: a systematic review and meta-analysis of epidemiological and experimental evidence. Drug Saf. 2011;34(2):125–156. doi: 10.2165/11539050-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 5. Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am J Public Health. 2016;106(4):686–688. doi: 10.2105/AJPH.2016.303061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29(8):639–658. doi: 10.1007/BF03262280 [DOI] [PubMed] [Google Scholar]
- 7. American Geriatrics Society. Ten things physicians and patients should question.http://www.choosingwisely.org/societies/american-geriatrics-society/. Published 2015. Accessed August 1, 2017.
- 8. Salzman C. The APA task force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151–152. doi: 10.1176/ajp.148.2.151 [DOI] [PubMed] [Google Scholar]
- 9. Stewart SA. The effects of benzodiazepines on cognition. J Clin Psychiatry. 2005;66(suppl 2):9–13. [PubMed] [Google Scholar]
- 10. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 11. Billioti de Gage S, Begaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case–control study. BMJ. 2014;349(sep09 2):g5205–g5205. doi: 10.1136/bmj.g5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nafti M, Sirois C, Kröger E, Carmichael PH, Laurin D. Is benzodiazepine use associated with the risk of dementia and cognitive impairment—not dementia in older persons? The Canadian Study of Health and Aging. Ann Pharmacother. 2020;54(3):219–225. doi: 10.1177/1060028019882037 [DOI] [PubMed] [Google Scholar]
- 14. Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016;352:i90. doi: 10.1136/bmj.i90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imfeld P, Bodmer M, Jick SS, Meier CR. Benzodiazepine use and risk of developing Alzheimer’s disease or vascular dementia: a case–control analysis. Drug Saf. 2015;38(10):909–919. doi: 10.1007/s40264-015-0319-3 [DOI] [PubMed] [Google Scholar]
- 16. Osler M, Jørgensen MB. Associations of benzodiazepines, Z-drugs, and other anxiolytics with subsequent dementia in patients with affective disorders: a nationwide cohort and nested case–control study. Am J Psychiatry. 2020;177(6):497–505. doi: 10.1176/appi.ajp.2019.19030315 [DOI] [PubMed] [Google Scholar]
- 17. Perry EK, Kilford L, Lees AJ, Burn DJ, Perry RH. Increased Alzheimer pathology in Parkinson’s disease related to antimuscarinic drugs. Ann Neurol. 2003;54(2):235–238. doi: 10.1002/ana.10639 [DOI] [PubMed] [Google Scholar]
- 18. Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401–407. doi: 10.1001/jamainternmed.2014.7663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galanter M, Kleber H. The American Psychiatric Publishing Textbook of Substance Abuse Treatment. Arlington, VA: American Psychiatric Publishing Inc.; 2008. [Google Scholar]
- 20. Ashton CH. The Ashton Manual. Benzodiazepines: How They Work and How to Withdraw. Newcastle upon Tyne, UK: New Castle University; 2002. [Google Scholar]
- 21. Tamim H, Monfared AA, LeLorier J. Application of lag-time into exposure definitions to control for protopathic bias. Pharmacoepidemiol Drug Saf. 2007;16(3):250–258. doi: 10.1002/pds.1360 [DOI] [PubMed] [Google Scholar]
- 22. ZCTA. U.S. Census Bureau. Zip Code™ Tabulation Areas (ZCTAs™). https://www.census.gov/geo/reference/zctas.html. Accessed August 1, 2018.
- 23. Chatterjee S, Talwar A, Aparasu RR. Anticholinergic medications and risk of dementia in older adults: where are we now? Expert Opin Drug Saf. 2020;19(10):1251–1267. doi: 10.1080/14740338.2020.1811227 [DOI] [PubMed] [Google Scholar]
- 24. Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case–control study. JAMA Intern Med. 2019;179(8):1084–1093. doi: 10.1001/jamainternmed.2019.0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shakir SA, Layton D. Causal association in pharmacovigilance and pharmacoepidemiology: thoughts on the application of the Austin Bradford-Hill criteria. Drug Saf. 2002;25(6):467–471. doi: 10.2165/00002018-200225060-00012 [DOI] [PubMed] [Google Scholar]
- 26. Newman EL, Gupta K, Climer JR, Monaghan CK, Hasselmo ME. Cholinergic modulation of cognitive processing: insights drawn from computational models. Front Behav Neurosci. 2012;6:24. doi: 10.3389/fnbeh.2012.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev Neurother. 2008;8(11):1703–1718. doi: 10.1586/14737175.8.11.1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grossi CM, Richardson K, Fox C, et al. Anticholinergic and benzodiazepine medication use and risk of incident dementia: a UK cohort study. BMC Geriatr. 2019;19(1):276. doi: 10.1186/s12877-019-1280-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hafdi M, Hoevenaar-Blom MP, Beishuizen CRL, Moll van Charante EP, Richard E, van Gool WA. Association of benzodiazepine and anticholinergic drug usage with incident dementia: a prospective cohort study of community-dwelling older adults. J Am Med Dir Assoc. 2020;21(2):188–193.e3. doi: 10.1016/j.jamda.2019.05.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.