Abstract
In this study, we investigated how the concentrations, pairwise correlations and ratios of 202 free circulating blood metabolites and lipids vary with age in a panel of n = 1 882 participants with an age range from 48 to 94 years. We report a statistically significant sex-dependent association with age of a panel of metabolites and lipids involving, in women, linoleic acid, α-linoleic acid, and carnitine, and, in men, monoacylglycerols and lysophosphatidylcholines. Evaluating the association of correlations among metabolites and/or lipids with age, we found that phosphatidylcholines correlations tend to have a positive trend associated with age in women, and monoacylglycerols and lysophosphatidylcholines correlations tend to have a negative trend associated with age in men. The association of ratio between molecular features with age reveals that decanoyl-l-carnitine/lysophosphatidylcholine ratio in women “decrease” with age, while l-carnitine/phosphatidylcholine and l-acetylcarnitine/phosphatidylcholine ratios in men “increase” with age. These results suggest an age-dependent remodeling of lipid metabolism that induces changes in cell membrane bilayer composition and cell cycle mechanisms. Furthermore, we conclude that lipidome is directly involved in this age-dependent differentiation. Our results demonstrate that, using a comprehensive approach focused on the changes of concentrations and relationships of blood metabolites and lipids, as expressed by their correlations and ratios, it is possible to obtain relevant information about metabolic dynamics associated with age.
Keywords: Correlation analysis, Gender differences, Human aging, Lipids, Metabolomics
Aging is a very complex process, influenced by genetic, environmental, and lifestyle factors (1,2), and involves progressive systemic dysregulation, affecting all levels of an organism, from molecules to organs (3,4). Metabolomics, that is the comprehensive analysis of small molecule profiles measured in a biological sample like blood or urine (5,6), is an excellent approach to obtain a global representation of the metabolic status of an organism with respect to a healthy status or a particular pathophysiological condition (7–9). The analysis of metabolomic profiles obtained from participants of different ages, performed using an integrative systems biology approach (10), allows the comprehensive description of the metabolic dynamics and can help to quantify and decipher the relationships between molecular features and aging process (4,11). Studies have been conducted in humans, highlighting how the metabolome is sex and age-dependent, indicating sex-specific association of certain genetic loci with several metabolites and lipid species: the levels of many metabolites (among them fatty acids, including 10 long-chain fatty acids, polyunsaturated fatty acids, glutamine, tyrosine, and histidine) and variation thereof are highly dependent on sex and age, and that sex differentially influences the levels and variation over time of many metabolites (12,13).
Correlations and ratios among molecules, and not only their levels, bear relevant biological information: Because molecules behave in an orchestrated way through metabolic pathways, changes in their association patterns, as represented by correlations and ratios (14,15), can provide information on the remodulation of biochemical reaction networks and metabolic pathways associated with age or sexual dimorphism, thus suggesting mechanisms through which molecules may modify cell membranes and affect hormonal activities, mitochondrial metabolism, and cell responses to oxidative stress (11,16).
In this study, making use of publicly available data, we took a comprehensive system biology approach, focusing on the association of the blood circulating unconjugated metabolites and lipids with age and sex in a large population cohort with an age range between 48 and 98 years (17). We investigated how metabolite and lipid abundances correlate with age groups, but also how the correlation and the ratios between metabolites and lipids change in groups of participants of different (increasing) ages.
Material and Methods
Experimental Data
We used data from the TwinGene project (17) that includes a longitudinal cohort from the Swedish Twin Register and a matched subcontrol cohort stratified on age and sex. The cohort was selected by Ganna et al. (17). This data set is a valid representation of a population consisting of not related participants and with a wide age range. It contains 202 quantified blood metabolites and lipids measured on n = 2 139 participants (nW = 921 women [43%] and nM = 1 218 men [57%]) with an overall age range of 47.6–93.9 years (women age range = 48.4–93.9 years and men age range = 47.6–93.3 years) and with an overall average age of 68.8 (women average age = 68.8 years and men average age = 68.7 years). This data set was used to identify potential molecular features and metabolic pathways associated with the sex-related aging process. Data were downloaded from the MetaboLights database (https://www.ebi.ac.uk/metabolights/) with accession number MTBLS93. Briefly, metabolomic profiling was performed on ultra-performance liquid chromatography to quadrupole time-of-flight mass spectrometry with an atmospheric electrospray interface operating in positive ion mode. The first step was the detection, alignment, grouping, and assignment of metabolites, performed by Ganna et al. (17), using the XCSM software. For the metabolic annotation, 4 approaches were performed by the authors: (a) based on matching accurate mass, fragmentation pattern, and retention time with their in-house spectral library of authentic standards collected; (b) based on spectrum and/or m/z similarities, but not retention time, and the annotation relies on the information of public databases; (c) based on the combination of spectral data, accurate mass, and retention time to assign the metabolite to a specific chemical class; (d) the other approaches failed in the annotation of the metabolite and the metabolite was annotated as “unknown.” Combining these approaches, m = 202 molecular features, divided into m1 = 36 metabolites and m2 = 166 lipids and lipid precursors, were assigned in the original publication (Supplementary Table 1).
For further details, we refer the reader to the original publication (17).
Data Preprocessing
Removal of outliers
To obtain a uniform study population, we removed those participants showing outlying blood metabolites and lipid profiles under the assumption of the presence of possibly undiagnosed pathophysiological conditions. Outliers were removed using a Principal Components Analysis (PCA) based approach. Hotelling’s T2 values were calculated from PCA scores; samples whose T2 values exceeded the 95% confidence ellipsis were considered outliers and were removed from subsequent analysis. The optimal number of significant principal components to be retained (at the α = 0.05 level) was determined using a statistical test based on the Tracy–Widom distribution (18). A total of 117 women (18%) and 140 men (11%) were removed from the analysis. This left n = 1 882 (nW = 804 women, 43%, nM = 1 078 men, 57%) samples/participants available for further analysis.
Subject stratification
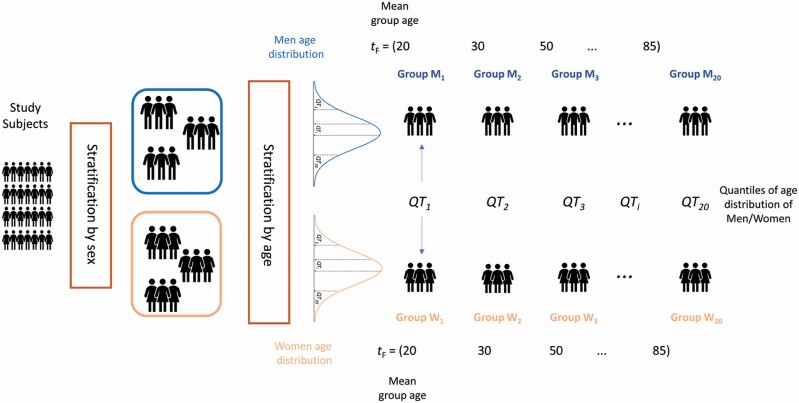
The nW = 804 women and the nM = 1 078 men were separately stratified by age in 20 groups, Wt (for women) and Mt (for men) with t = 1,2, …, 20 of size wi and mi by taking the 20 quantiles QT1, QT2, …, QT20 of the women and men age distributions, reflecting the 5th, 10th, …, 95th, and 100th percentiles of the sex-specific age distribution. Consequently, each Wt group and Mt group had approximately 5% of the sex-specific sample (≅40 for women and ≅54 for men). The age characteristics for each women and men group are given in Supplementary Table 2. A graphical illustration is shown in Figure 1. For each Wt and Mt group, we defined the corresponding data matrices Wt and Mt of size wi × p and mi× p containing the concentrations of the p = 202 metabolites and lipids measured on the wi and mi participants in the corresponding group. Each set of data matrices is associated with a 1 × 20 vector tM (respectively tF) containing the average age of the M1, M2, …, M20 group (respectively W1, W2, …, W20).
Figure 1.
Overview of stratification of the study participants. Participants are first stratified by sex and then by age. Women and men are divided into 20 groups according to the 20 quantiles obtained from the age distribution of the 2 sex-specific groups.
Statistical Analysis
Estimation of the average concentration of molecular features specific to age groups
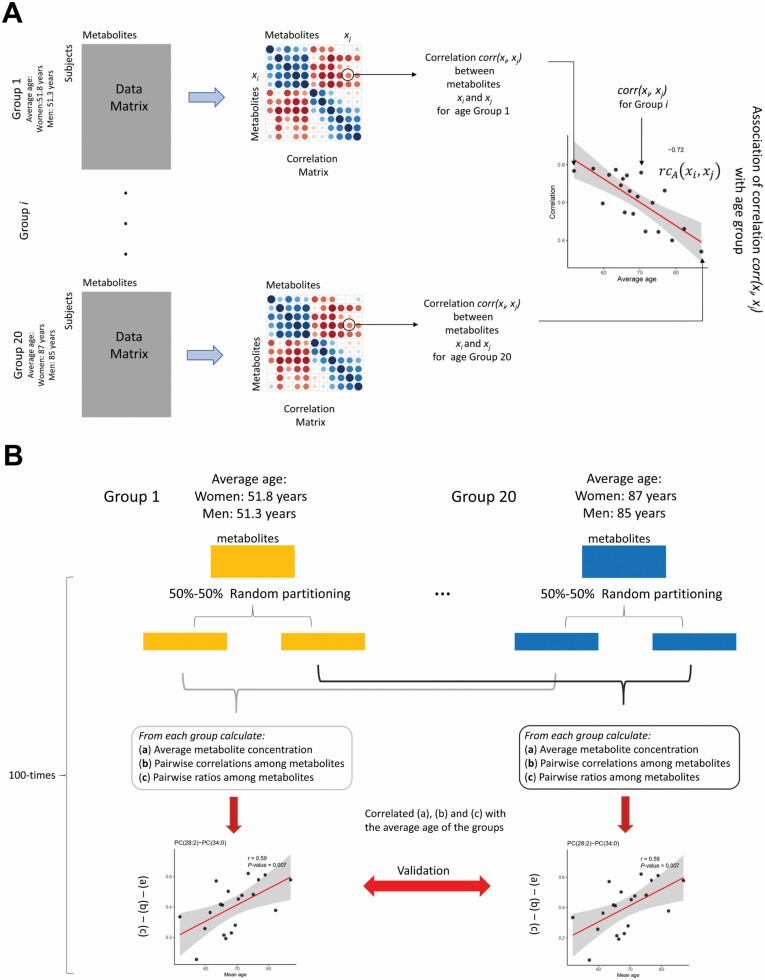
For each data set Wt and Mt we calculated the mean abundance mi between each molecular feature xi. As for the correlation case, we obtained thus 20 values for each metabolite–lipid, representing the changes of the average abundance of molecular feature xi associated with the age groups (a graphical representation is shown in Figure 2A):
Figure 2.
(A) Overview of the statistical procedure used to establish the association (eqn (6)) between the metabolite and lipids pairwise correlations (eqn (3)), with the group age (Figure 1). In the case of ratios (eqn (7)), the correlation matrix is replaced with the matrix of pairwise ratios, for average abundances is replaced by the vectors of means (eqn (8)). (B). Overview of the data-splitting procedure used to validate the results. Each subject group is randomly split into 2 halves, obtaining 2 sets of 20 groups. The analysis is performed on the first set, while the second set is used for validation. The procedure is repeated 100 times: Only results validated more than 50% of the times are considered significant.
We considered the standard mean estimation:
| (1) |
For each feature, we thus obtained 20 mean values:
| (2) |
Estimation of correlations between molecular features specific to age groups
For each data set Wt and Mt of size wi × p and mi × p, we calculated the correlation rij between each pair of molecular features xi, xj. For each pair, we obtained thus 20 correlation values, representing the evolution of the strength of the relationship between molecular features xi, xj associated with different age groups (Figure 2A):
| (3) |
We used Winsorized correlation coefficients that are robust toward the shape, sample size, and outliers in the metabolite concentration distribution (19) to estimate the correlation rij within molecular features pairs. The Winsorized correlation coefficient is obtained by replacing the k smallest observations with the (k + 1)st smallest observation, and the k largest observations with the (k + 1)st largest observation. In this way, the observations are Winsorized at each end of both xi, and xj. The Pearson’s correlation coefficient is then calculated on the Winsorized variables (20). A 10% Winsorization was used. Among the ½p(p−1) possible correlations we retained for further analysis only those pairs of molecular features for which the correlation rijwas found to be significant at the α = 0.01 level in at least 10 of the 20 data sets Wt and Mt.
Estimation of ratios between molecular features specific to age groups
For each data set Wt and Mt, we calculated the ratio qij between each pair of molecular features xi, xj. As for the correlation case, we thus obtained 20 values for each pair, representing the evolution of the ratio magnitude of molecular features xi and xj (Figure 2A). We considered the unbiased ratio estimator proposed by van Kempen and van Vliet (21) which is defined as:
| (4) |
where is the mean of xi, is the mean of xj, var(xi) is the variance of xi, cov(xi,xj) is the covariance between xi and xj, and n is the sample size. For each ratio, we thus obtained 20 ratio values:
| (5) |
Because we were looking for ratio values varying over the 20 age groups, we retained for further analysis only those ratios qij for which the relative variation between qij(t = 1) and qij(t = 20) was larger than 10%.
Estimation of the association with average group age of the correlation and ratios among molecular features
The association rcA(xi, xj) of the correlation of each pair of molecular features xi, xj with the average group age tM was estimated by taking the Winsorized correlation between the vectors of correlations defined in eqn (3) and the average group age vector tM (respectively, tF):
| (6) |
The association rqA(xi, xj) of the ratio of each pair of molecular features xi, xj with the average group age tM was estimated in a similar fashion:
| (7) |
The association raA(xi, xj) of the mean abundance of each molecular features xi with the average group age tM was estimated as:
| (8) |
We considered to be associated with age only the correlations, ratios, or mean abundances of those molecular features for which |rcA(xi, xj)|≥ 0.65, |rqA(xi, xj)|≥ 0.65 and |raA(xij)|≥ 0.65 and p < .01 after correction (fdr) for multiple testing with the Benjamini–Hochberg method. Correction for multiple testing (Benjamini–Hochberg) was applied at all analysis stages. This choice is based on both statistical and biological considerations. There are 20 age groups, which means that the sample size available to estimate the correlation between metabolite concentrations and associations (correlations and ratios) is 20: With 20 observations, it is possible to assess significance at α = 0.01 with 80% power only of correlations |r| ≥ 0.65. In addition, there are ~20 participants per age group, thus metabolite–metabolite correlation |r| ≥ 0.65 can be estimated. Biologically the 0.65 threshold is justified by considering that the majority of correlations observed in metabolomics studies are below 0.6 (22,23): Setting a higher threshold allows to focus on correlations that really stand out of the background correlation.
Validation of the results
To validate the results of the analysis described in the previous sections, that is, the existence of an association between average age group and metabolite and lipid concentration (eqn (8)), correlations (eqn (6)), and ratios (eqn (7)), we implemented a data splitting approach (24,25). Basically, we randomly split each of the 20 age groups into 2 halves and performed the analysis independently on the 2 data splits to ascertain if the results could be reproduced. To consider the variability due to the random splitting, the overall procedure was repeated generating k = 100 different pairs of data splits: Analysis was repeated on the 100 pairs of data. We considered to be valid only those results that were confirmed in at least 50% of the splits (Figure 2B). In this way, we could obtain an estimation of the reproducibility and robustness of the results by mimicking validation in an external cohort: A portion of the data is used to suggest a hypothesis, and a second, independent portion is used to test it. Note that this approach can be rephrased in an inferential setting and implies that Type I error (ie, the risk of false positives) is controlled (conservatively) at the 0.01 level (26) after correction for multiple testing. The downside of such an approach is a potential loss of power, due to the reduction of the sample size used to estimate correlation. However, this approach is effective in giving valid inference after the selection of a hypothesis, estimating nuisance parameters, and avoiding overfitting (26).
Software
All calculations and plots were performed in R (version 3.3.2). The function “win.cor,” implemented in WRS2 package, was used to calculate the Winsorized correlations.
Results
Association of Metabolite and Lipid Abundances With Age
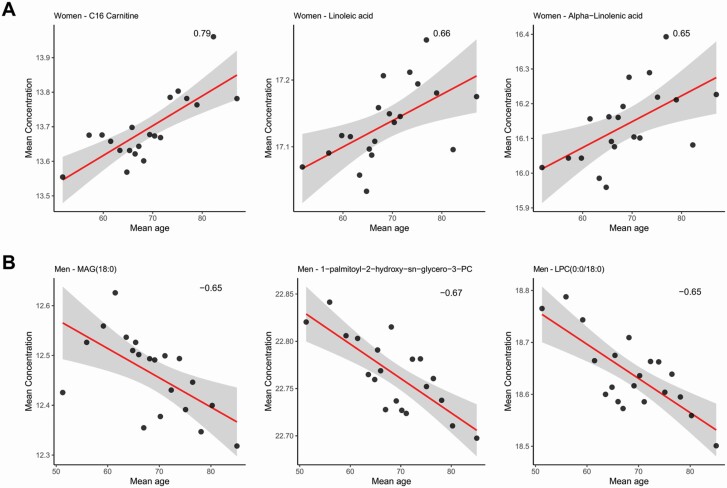
Starting from a total of n = 202 metabolites and lipids, a total of pW = 3 (women) and pM = 3 (men) compounds were found statistically significant (adjusted p ≤ .01 and absolute value of ≥ 0.65, see eqn (8)) in more than 50% of splits obtained performing the validation method.
In particular, in the women cohort, we observed a positive correlation of the concentrations of carnitine with = 0.79 and an adjusted p = .0009 in the 79% of validation splits, linoleic acid with = 0.66 and an adjusted p = .001 in the 59% of validation splits, and α-linoleic acid with = 0.65 and an adjusted p = .01 in the 66% of validation splits with the average age of women group (Figure 3A).
Figure 3.
Correlations between average metabolites and lipids concentrations and the average age of the 20 subject groups: women (A) and men (B). LPC = lysophosphatidylcholine; PC = phosphatidylcholine; PE = phosphatidylethanolamine; MAG = monoacylglycerol. See Figure 2 for an overview of the statistical procedure.
The age groups that we used here are data-driven and are not physiologically informed. In particular, the first group of women (W1) corresponds to a 6-year age bin that likely represents perimenopausal women, given that the average age of menopause in women in the Western world is 51 years (27). Although this does not affect statistical analysis, we shall consider that menopausal transition aligns with age.
In the men cohort, we observed negative correlation with age of monoacylglycerol (MAG), especially MAG (18:0) with = −0.65 and an adjusted p = .005 in the 53% of validation splits, and lysophosphatidylcholines (LPCs), especially 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC [16:0]) with = −0.67 and an adjusted p = .005 in the 58% of validation splits, and LPC (0:0/18:0) with = −0.65 and an adjusted p = .008 in the 62% of validation splits (Figure 3B).
For a complete overview of the results for all metabolites see Supplementary Table 3.
Association of the Correlation Among Molecular Features With Age
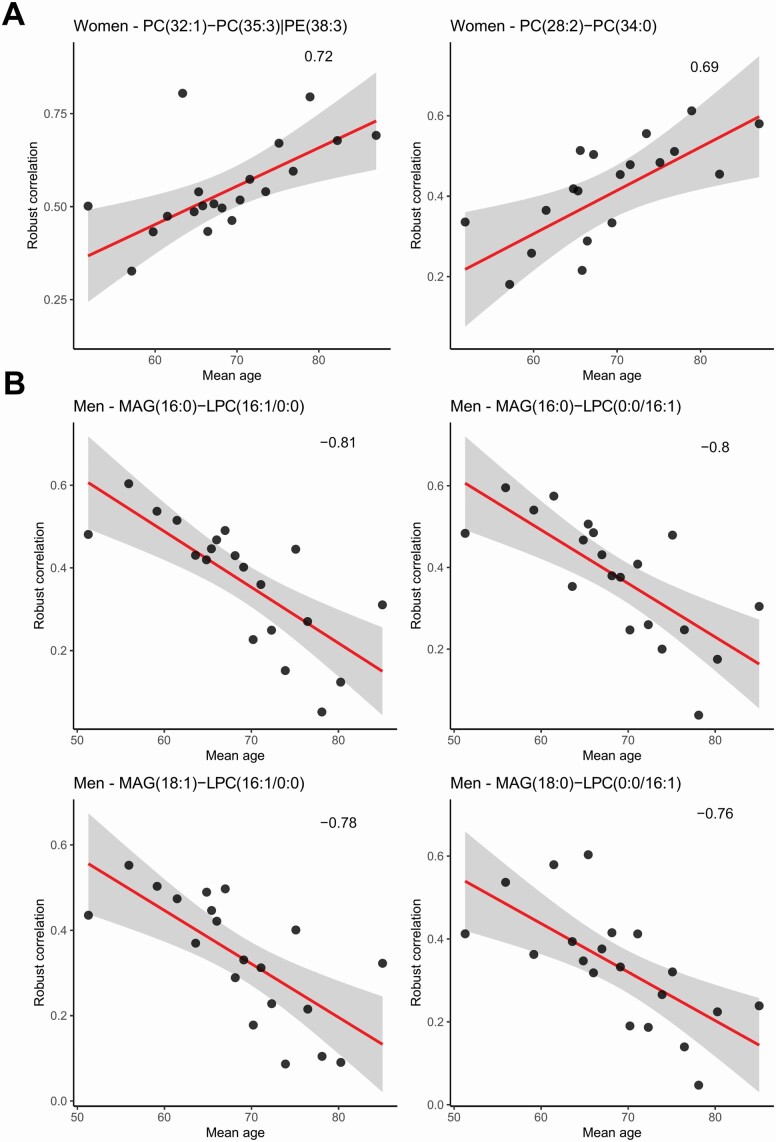
Starting from a total of n = 20 301 metabolites and lipids correlations, a total of cW = 2 (women) and cM = 4 (men) correlations among molecules result to be statistically significant (adjusted p ≤ .01 and absolute value of ≥ 0.65, see eqn (6)) in more than 50% of splits after the validation method.
In the women cohort, the correlations between phosphocholines (PCs), especially between (a) PC (28:2)–PC (32:1) with = 0.69 and an adjusted p = .008 in the 57% of validation splits and (b) PC (32:1)–PC (35:3) | PE (38:3) with = 0.72 and an adjusted p = .008 in the 54% of validation splits, tend to increase with age (Figure 4A).
Figure 4.
Correlations between metabolites and lipids correlations and the average age of the 20 subject groups: women (A) and men (B). LPC = lysophosphatidylcholine; PC = phosphatidylcholine; PE = phosphatidylethanolamine; MAG = monoacylglycerol. See Figure 2 for an overview of the statistical procedure.
In Figure 4B, correlations between MAG and LPC, especially (a) MAG (16:0)–LPC (16:1/0:0) with = −0.81 and an adjusted p = .002 in the 53% of validation splits, (b) MAG (16:0)–LPC (0:0/16:1) with = −0.80 and an adjusted p = .002 in the 59% of validation splits, (c) MAG (18:1)–LPC (16:1/0:0) with = −0.78 and an adjusted p = .003 in the 60% of validation splits, and (d) MAG (18:0)–LPC (0:0/16:1) with = −0.76 and an adjusted p = .004 in the 53% of validation splits, decrease with the average age of men-specific groups. The levels of these lipids vary in a similar fashion, decreasing with the age.
For a complete overview of the results for all metabolites see Supplementary Table 3.
Association of the Ratios Among Molecular Features With Age
Alterations in the ratios between 2 single lipids and/or metabolites may point at perturbations in pathways relevant for a certain specific phenotype and they could influence the physiological course of aging. In this light, pairwise ratios may serve as potential biomarkers of the aging process (28,29).
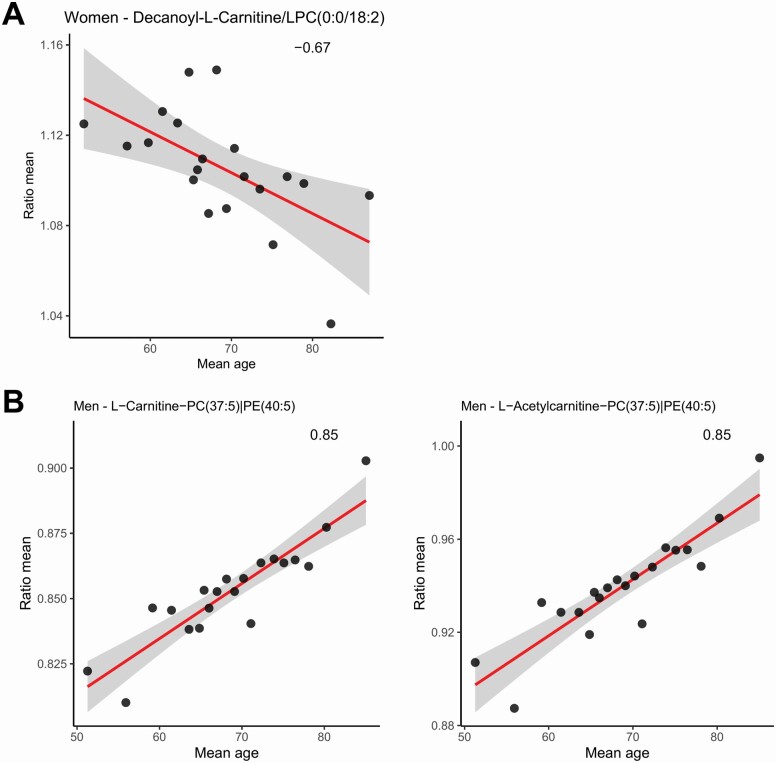
Starting from a total of n = 20 301 metabolites and lipids ratios, after the validation method, we found only qW =1 (women) and qM = 2 (men) ratios between molecules whose variation is significantly associated with the average age (adjusted p ≤ 0.01 and absolute value of ≥ 0.65, see eqn (7)). In particular, the ratio between decanoyl-l-carnitine/LPC (0:0/18:2) with = −0.67 and an adjusted p = .002 in the 56% of validation splits shows a negative association with the average age of the women cohort (Figure 5A).
Figure 5.
Correlations between average metabolites and lipids ratios and the average age of the 20 subject groups: women (A) and men (B). GCA = glycocholic acid; PC = phosphatidylcholine; PE = phosphatidylethanolamine; MAG = monoacylglycerol. See Figure 2 for an overview of the statistical procedure.
In Figure 5B, the ratios between l-carnitine/PC (37:5) with = 0.85 and an adjusted p = 1 × 10−4 in the 55% of validation splits and l-acetylcarnitine/PC (37:5) with = 0.85 and an adjusted p = 2 × 10−4 in the 51% of validation splits tend to be positively correlated with the average age of men-specific groups.
For a complete overview of the results for all metabolites see Supplementary Table 3.
Discussion
To shed light on the molecular mechanisms possibly associated with age, we studied how the concentration, correlations, and ratios of and among circulating blood metabolites and lipids vary with subject age groups, considering men and women separately to highlight possible dependencies on sex. Using different approaches, in the original paper, Ganna et al. (17) demonstrated that LPC (18:1) and LPC (18:2) are not directly associated with coronary heart disease (CHD), but they found an age-dependent negative trend of these 2 lipids in association with CHD risk. Moreover, MAG (18:2) and sphingomyelin (28:1) have a positive correlation with the CHD risk. Our results support the usefulness of the metabolomic analysis conjugated with a system biology approach for the identification of age-related metabolites and their association patterns, providing additional information compared to what is already known from the literature.
In women, the levels of carnitine, linoleic acid, and α-linoleic acid show a positive correlation with (group) age. These significant correlations are of particular interest because previous studies showed that the age-dependent carnitine serum levels increase more with age in adult women than men (30,31), and the endogenous biosynthesis of carnitine depends on the production, by lysosomal protein degradation, of trimethyl-lysine (32) whose homeostasis is regulated by dietary intake, intestinal absorption, and renal reabsorption. Carnitine also plays an important role in carnitine-shuttle biochemical reactions and in the energy pool metabolism, inducing an expression of intramitochondrial alterations (30,33), fundamental in linoleic acid metabolism. Previous studies report that the reduction of estrogens activity and the increase of testosterone levels induce modification of the rate of conversion of linoleic acid and α-linolenic acid into n − 3 long-chain polyunsaturated fatty acids, inducing changes in cell membrane composition and in cell cycle mechanisms (34,35). Endogenous biosynthesis of carnitine depends on the production, by lysosomal protein degradation, of trimethyl-lysine (32). The homeostasis of this molecule is regulated by dietary intake, intestinal absorption, and renal reabsorption. Carnitine also plays an important role in carnitine-shuttle biochemical reactions and in the energy pool metabolism, inducing an expression of intramitochondrial alterations (30,33), fundamental in linoleic acid metabolisms, whose activity shows age-dependent dysregulation (36). In addition to the role of polyunsaturated fatty acids as energy sources, they have several functions, as cellular signaling pathways (37) and as structural components of cell membranes (38), inducing age-dependent changes (39).
The negative correlation of LPCs concentrations with age, molecularly associated with the reduction of MAGs levels by the MAG lipase enzyme activity (36,40), induces a skeletal muscle mitochondrial dysfunction (41); the decreasing of LPCs is, generally, also associated with the increase of body mass index (BMI) but, in an older population, this effect is associated, firstly, with the increasing of age-dependent inflammation, depending on an overall remodulation of cell membrane and mitochondrial dysfunction.
Because the pairwise correlations among molecules can be used as a proxy to describe the underlying metabolic network (10), here we consider the correlations observed as the result of the combination of all reactions and regulatory processes occurring in the metabolic network (18,42) at a given age.
In women, the correlations between PC (28:2)–PC (32:1) and PC (32:1)–PC(35:3) | PE(38:3) tend to increase with age. During the menopause period, a global dysregulation on liver enzymes is induced, causing the synthesis of PCs from choline (43,44). The interactions of PCs are associated with the remodulation of membranes integrity, promoting their conservation and directly affecting the membrane permeability, increasing the fluidity of the bilayer and protecting it from peroxidative damage (38,45), a frequent phenomenon in advanced age (46). Correlations between MAG (16:0)–LPC (16:1/0:0), MAG (16:0)–LPC (0:0/16:1), MAG (18:1)–LPC (16:1/0:0), and MAG (18:0)–LPC (0:0/16:1) decrease with age in men, and this has been related to the increase of the MAG lipase enzyme activity that determines the hydrolysis of MAG into glycerol and fatty acid alkyl ester (36,40) and to the impaired mitochondrial oxidative capacity associated with low levels of LPCs in advanced age (36,41).
The alterations in the ratios between 2 single lipids and/or metabolites may point at perturbations in pathways relevant for a certain specific phenotype. We considered the pairwise ratios as potential biomarkers (28,29) of the aging process. We found that only the ratio between decanoyl-l-carnitine/LPC(0:0/18:2) shows a negative association with the average age in women, and, at best of our knowledge, this association has never been reported. We can speculate that decreasing levels of LPCs and the increasing levels of decanoyl-l-carnitine induce, synergistically, a mitochondrial dysfunction (36,41), contributing to age-dependent metabolic changes and being an indirect result of aging (47). In contrast, the ratios between l-carnitine/PC(37:5) and l-acetylcarnitine/PC(37:5) tend to be positively correlated with the average age of men-specific groups.
Little is known about these molecular ratios. As said before, carnitine plays a role in carnitine-shuttle biochemical reactions: carnitine palmitoyltransferase 1 enzyme is involved in the reversible acylation of l-carnitine, producing l-acetylcarnitine, and this event is fundamental in fatty acid beta-oxidation, maintenance of acyl-coenzyme A pools, and energy metabolism (30). The carnitine-shuttle activity could generate a specific remodeling of mitochondrial fatty acids oxidation, promoting a modification in the mitochondrial membrane lipidome (48), increasing PCs fraction (49,50). Although, actually, the overall aging molecular mechanisms are unclear, our results show that lipids (ie, LPC, MAG, PC, PE, linoleic acid) and carnitine are fundamental in the age-related metabolic pathways.
Strengths and Limitations
One of the strengths of this study is a large number of patients with a very wide age range (47.6–93.9 years) whose metabolome was analyzed. We implemented a stringent validation of the results using a repeated data resampling to account for varaibility and to obtain robust estimate of metabolite concentrations, correlations, and ratios calculated at the age group level to eliminate subject-to-subject variability.
One limitation of this study is the lack of availability of the clinical data (ie, BMI, waist circumference, systolic and diastolic blood pressures) associated with the participants’ metabolite data, publicly available on the MetaboLights public database, resulting in an incomplete representation of the pathophysiological conditions of the cohort, indicating that we could not correct at the individual level for such factors in the analysis.
Conclusions
In this study, we presented a comprehensive biology approach to highlight potential molecular features concentrations, associations, and ratios directly associated with the increase of the age of a sex- and age-matched population. We showed that linoleic acid, α-linoleic acid, and carnitine have, in the women cohort, a positive correlation trend with age, while MAGs and LPCs have, in the men cohort, a negative correlation trend with age. These results highlight, in women, the effect of the reduction of estrogens activity and the increase of testosterone levels on the linoleic acid metabolism and on the energy pool metabolism that induces the overall changes in cell membrane composition and cell cycle mechanisms. In men, low levels of LPCs concentrations are directly connected with the reduction of MAGs levels by the MAG lipase enzymatic activity that induces mitochondrial dysfunction.
Analyzing the pairwise correlations among molecules, we observed that PCs/PCs correlations tend to have a positive trend associated with the average ages of women, while MAGs/LPCs correlations tend to have a negative trend associated with men average ages. These results, in both cases, suggest an age-dependent remodeling of fatty acid metabolism that induces, overall, remodeling of cell and mitochondrial membranes and modification in terms of fluidity of membranes bilayers.
We studied the pairwise ratios as potential biomarkers of aging. In women, the decanoyl-l-carnitine/LPC ratio has a negative association with the increasing of the average ages, while in men the ratios between l-carnitine/PC and l-acetylcarnitine/PC have a positive association with the increase of age, suggesting, in both cases, a radical remodeling of the dynamic membrane fluidity and carnitine-shuttle activity.
This study brings forward the concept that correlation and ratios among molecular features, and not only abundances along, could be used to investigate the dynamic of molecular mechanisms and their association with age.
Supplementary Material
Acknowledgments
The authors acknowledge the support and the use of resources of Instruct-ERIC, a Landmark ESFRI project, and specifically the CERM/CIRMMP Italy Centre.
Contributor Information
Francesca Di Cesare, Magnetic Resonance Center (CERM), University of Florence, Firenze, Italy.
Claudio Luchinat, Magnetic Resonance Center (CERM), University of Florence, Firenze, Italy; Department of Chemistry “Ugo Schiff,” University of Florence, Sesto Fiorentino, Italy.
Leonardo Tenori, Magnetic Resonance Center (CERM), University of Florence, Firenze, Italy; Department of Chemistry “Ugo Schiff,” University of Florence, Sesto Fiorentino, Italy.
Edoardo Saccenti, Laboratory of Systems and Synthetic Biology, Wageningen University & Research, Wageningen, The Netherlands.
Funding
This research received no external funding.
Conflict of Interest
None declared.
References
- 1. Karasik D, Demissie S, Cupples LA, Kiel DP. Disentangling the genetic determinants of human aging: biological age as an alternative to the use of survival measures. J Gerontol A Biol Sci Med Sci. 2005;60(5):574–587. doi: 10.1093/gerona/60.5.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kerber RA, O’Brien E, Cawthon RM. Gene expression profiles associated with aging and mortality in humans. Aging Cell. 2009;8(3):239–250. doi: 10.1111/j.1474-9726.2009.00467.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoffman JM, Lyu Y, Pletcher SD, Promislow DEL. Proteomics and metabolomics in ageing research: from biomarkers to systems biology. Essays Biochem. 2017;61(3):379–388. doi: 10.1042/EBC20160083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jové M, Maté I, Naudí A, et al. Human aging is a metabolome-related matter of gender. J Gerontol A Biol Sci Med Sci. 2016;71(5):578–585. doi: 10.1093/gerona/glv074 [DOI] [PubMed] [Google Scholar]
- 5. Vignoli A, Ghini V, Meoni G, et al. High-throughput metabolomics by 1D NMR. Angew Chem Int Ed Engl. 2019;58(4):968–994. doi: 10.1002/anie.201804736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckhart AD, Beebe K, Milburn M. Metabolomics as a key integrator for “omic” advancement of personalized medicine and future therapies. Clin Transl Sci. 2012;5(3):285–288. doi: 10.1111/j.1752-8062.2011.00388.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vignoli A, Tenori L, Luchinat C, Saccenti E. Age and sex effects on plasma metabolite association networks in healthy subjects. J Proteome Res. 2018;17(1):97–107. doi: 10.1021/acs.jproteome.7b00404 [DOI] [PubMed] [Google Scholar]
- 8. Vignoli A, Tenori L, Giusti B, et al. NMR-based metabolomics identifies patients at high risk of death within two years after acute myocardial infarction in the AMI-Florence II cohort. BMC Med. 2019;17(1):3. doi: 10.1186/s12916-018-1240-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vignoli A, Paciotti S, Tenori L, et al. Fingerprinting Alzheimer’s disease by 1H nuclear magnetic resonance spectroscopy of cerebrospinal fluid. J Proteome Res. 2020;19(4):1696–1705. doi: 10.1021/acs.jproteome.9b00850 [DOI] [PubMed] [Google Scholar]
- 10. Rosato A, Tenori L, Cascante M, De Atauri Carulla PR, Martins Dos Santos VAP, Saccenti E. From correlation to causation: analysis of metabolomics data using systems biology approaches. Metabolomics. 2018;14(4):37. doi: 10.1007/s11306-018-1335-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu Z, Zhai G, Singmann P, et al. Human serum metabolic profiles are age dependent. Aging Cell. 2012;11(6):960–967. doi: 10.1111/j.1474-9726.2012.00865.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Darst BF, Koscik RL, Hogan KJ, Johnson SC, Engelman CD. Longitudinal plasma metabolomics of aging and sex. Aging (Albany NY). 2019;11(4):1262–1282. doi: 10.18632/aging.101837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mittelstrass K, Ried JS, Yu Z, et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011;7(8):e1002215. doi: 10.1371/journal.pgen.1002215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petersen AK, Krumsiek J, Wägele B, et al. On the hypothesis-free testing of metabolite ratios in genome-wide and metabolome-wide association studies. BMC Bioinformatics. 2012;13(1):120. doi: 10.1186/1471-2105-13-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Altmaier E, Ramsay SL, Graber A, Mewes HW, Weinberger KM, Suhre K. Bioinformatics analysis of targeted metabolomics—uncovering old and new tales of diabetic mice under medication. Endocrinology. 2008;149(7):3478–3489. doi: 10.1210/en.2007-1747 [DOI] [PubMed] [Google Scholar]
- 16. Barbieri M, Boccardi V, Papa M, Paolisso G. Metabolic journey to healthy longevity. Horm Res. 2009;71(suppl 1):24–27. doi: 10.1159/000178032 [DOI] [PubMed] [Google Scholar]
- 17. Ganna A, Salihovic S, Sundström J, et al. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet. 2014;10(12):e1004801. doi: 10.1371/journal.pgen.1004801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saccenti E. Correlation patterns in experimental data are affected by normalization procedures: consequences for data analysis and network inference. J Proteome Res. 2017;16(2):619–634. doi: 10.1021/acs.jproteome.6b00704 [DOI] [PubMed] [Google Scholar]
- 19. Tuğran E, Kocak M, Mirtagioğlu H, Yiğit S, Mendes M. A simulation based comparison of correlation coefficients with regard to type I error rate and power. J Data Anal Inf Process. 2015;3(3):87–101. doi: 10.4236/jdaip.2015.33010 [DOI] [Google Scholar]
- 20. Wilcox RR. Some results on a Winsorized correlation coefficient. Br J Math Stat Psychol. 1993;46(2):339–349. doi: 10.1111/j.2044-8317.1993.tb01020.x [DOI] [Google Scholar]
- 21. van Kempen GM, van Vliet LJ. Mean and variance of ratio estimators used in fluorescence ratio imaging. Cytometry. 2000;39(4):300–305. doi: [DOI] [PubMed] [Google Scholar]
- 22. Camacho D, Fuente ADL, Mendes P. The origins of correlations in metabolomics data. Metabolomics. 2005;1(1):53–63. doi: 10.1007/s11306-005-1107-3 [DOI] [Google Scholar]
- 23. Jahagirdar S, Saccenti E. On the use of correlation and MI as a measure of metabolite–metabolite association for network differential connectivity analysis. Metabolites. 2020;10(4): 171. doi: 10.3390/metabo10040171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cox DR. A note on data-splitting for the evaluation of significance levels. Biometrika. 1975;62(2):441–444. doi: 10.1093/biomet/62.2.441 [DOI] [Google Scholar]
- 25. Rubin D, Dudoit S, van der Laan M. A method to increase the power of multiple testing procedures through sample splitting. Stat Appl Genet Mol Biol. 2006;5:Article19. doi: 10.2202/1544-6115.1148 [DOI] [PubMed] [Google Scholar]
- 26. DiCiccio CJ, DiCiccio TJ, Romano JP. Exact tests via multiple data splitting. Stat Probab Lett. 2020;166:108865. doi: 10.1016/j.spl.2020.108865 [DOI] [Google Scholar]
- 27. Lindh-Åstrand L, Hoffmann M, Järvstråt L, Fredriksson M, Hammar M, Spetz Holm AC. Hormone therapy might be underutilized in women with early menopause. Hum Reprod. 2015;30(4):848–852. doi: 10.1093/humrep/dev017 [DOI] [PubMed] [Google Scholar]
- 28. Zelezniak A, Sheridan S, Patil KR. Contribution of network connectivity in determining the relationship between gene expression and metabolite concentration changes. PLoS Comput Biol. 2014;10(4):e1003572. doi: 10.1371/journal.pcbi.1003572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krumsiek J, Stückler F, Suhre K, et al. Network-based metabolite ratios for an improved functional characterization of genome-wide association study results. bioRxiv, doi: 10.1101/048512, 13 April 2016, preprint: not peer reviewed. [DOI] [Google Scholar]
- 30. Mitchell SL, Uppal K, Williamson SM, et al. The carnitine shuttle pathway is altered in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018;59(12):4978–4985. doi: 10.1167/iovs.18-25137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malaguarnera G, Catania VE, Bonfiglio C, Bertino G, Vicari E, Malaguarnera M. Carnitine serum levels in frail older subjects. Nutrients. 2020;12(12):3887. doi: 10.3390/nu12123887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. doi: 10.1016/j.cmet.2007.10.013 [DOI] [PubMed] [Google Scholar]
- 33. Judit B, Andras S, Katalin K, Bela M. Mass spectrometric analysis of L-carnitine and its esters: potential biomarkers of disturbances in carnitine homeostasis. Curr Mol Med. 2020;20(5):336–354. doi: 10.2174/1566524019666191113120828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Janssen CI, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res. 2014;53:1–17. doi: 10.1016/j.plipres.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 35. Cybulska AM, Skonieczna-Żydecka K, Drozd A, et al. Fatty acid profile of postmenopausal women receiving, and not receiving, hormone replacement therapy. Int J Environ Res Public Health. 2019;16(21):4273. doi: 10.3390/ijerph16214273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson AA, Stolzing A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell. 2019;18(6):e13048. doi: 10.1111/acel.13048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sokoła-Wysoczańska E, Wysoczański T, Wagner J, et al. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders—a review. Nutrients. 2018;10(10):1561. doi: 10.3390/nu10101561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Z, Agellon LB, Allen TM, et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3(5):321–331. doi: 10.1016/j.cmet.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 39. Chung KW. Advances in understanding of the role of lipid metabolism in aging. Cells. 2021;10(4):880. doi: 10.3390/cells10040880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grabner GF, Zimmermann R, Schicho R, Taschler U. Monoglyceride lipase as a drug target: at the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol Ther. 2017;175:35–46. doi: 10.1016/j.pharmthera.2017.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Semba RD, Zhang P, Adelnia F, et al. Low plasma lysophosphatidylcholines are associated with impaired mitochondrial oxidative capacity in adults in the Baltimore Longitudinal Study of Aging. Aging Cell. 2019;18(2):e12915. doi: 10.1111/acel.12915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Steuer R, Kurths J, Fiehn O, Weckwerth W. Observing and interpreting correlations in metabolomic networks. Bioinformatics. 2003;19(8):1019–1026. doi: 10.1093/bioinformatics/btg120 [DOI] [PubMed] [Google Scholar]
- 43. Auro K, Joensuu A, Fischer K, et al. A metabolic view on menopause and ageing. Nat Commun. 2014;5:4708. doi: 10.1038/ncomms5708 [DOI] [PubMed] [Google Scholar]
- 44. Cui X, Yu X, Sun G, et al. Differential metabolomics networks analysis of menopausal status. PLoS One. 2019;14(9):e0222353. doi: 10.1371/journal.pone.0222353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rabini RA, Moretti N, Staffolani R, et al. Reduced susceptibility to peroxidation of erythrocyte plasma membranes from centenarians. Exp Gerontol. 2002;37(5):657–663. doi: 10.1016/s0531-5565(02)00006-2 [DOI] [PubMed] [Google Scholar]
- 46. Akila VP, Harishchandra H, D’souza V, D’souza B. Age related changes in lipid peroxidation and antioxidants in elderly people. Indian J Clin Biochem. 2007;22(1):131–134. doi: 10.1007/BF02912896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haas RH. Mitochondrial dysfunction in aging and diseases of aging. Biology. 2019;8(2):48. doi: 10.3390/biology8020048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lum H, Sloane R, Huffman KM, et al. Plasma acylcarnitines are associated with physical performance in elderly men. J Gerontol A Biol Sci Med Sci. 2011;66(5):548–553. doi: 10.1093/gerona/glr006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Burstein MT, Titorenko VI. A mitochondrially targeted compound delays aging in yeast through a mechanism linking mitochondrial membrane lipid metabolism to mitochondrial redox biology. Redox Biol. 2014;2:305–307. doi: 10.1016/j.redox.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Janikiewicz J, Szymański J, Malinska D, et al. Mitochondria-associated membranes in aging and senescence: structure, function, and dynamics. Cell Death Dis. 2018;9(3):332. doi: 10.1038/s41419-017-0105-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.