Abstract
The study of immune system aging is of relevance, considering its myriad of interactions and role in protecting and maintaining body homeostasis. While mouse models have been extensively used to study immune system aging, little is known on how the main immune populations progress over time and what is the impact of sex. To contribute to filling this gap, male and female BALB/cByJ mice were longitudinally evaluated, from 3 to 18 months old, for the main blood populations, assessed by flow cytometry. Using linear mixed-effect models, we observed that the percentages of neutrophils, monocytes, eosinophils, and total natural killer (NK) cells increase with aging, while those of B cells, T cells (including CD4+ and CD8+ subsets), and Ly6C+ NK cells decrease. Males present higher percentages of neutrophils and classical monocytes Ly6Chigh over time, while females present higher percentages of total T cells, both CD4+ and CD8+, eosinophils, and NK cells. Males and females display similar percentages of B cells, even though with opposite accelerated progressions over time. This study revealed that mouse models recapitulate what is observed in humans during aging: an overall proportional decrease in the adaptive and an increase in the innate immune cells. Additionally, it uncovers an age-related sexual dimorphism in the proportion of immune cells in circulation, further strengthening the need to explore the impact of sex when addressing immune system aging using mouse models.
Keywords: Adaptive immune, system, Aging, Animal model, Blood, Gender differences, Immunosenescence, Innate immune system, Linear mixed models, Sex
The increasingly aged population, and its inherent association with health decline, challenges the scientific community to better understand the physiology of healthy aging. The immune system is one of the central body systems, given its own functions and the myriad of interactions it establishes with other organ systems. Therefore, it is relevant to understand how the immune system ages, influences, and is influenced by aging.
The immune system senescence, also known as immunosenescence, is characterized by progressive alterations in the proportion and function of immune cells throughout aging. In the innate immune system aging, while changes in cell function (eg, reduction in antigen presentation and in phagocytic capacity) have been consistently reported, studies exploring the numbers and frequency of cells mainly in humans often present inconsistent findings (1–4). With respect to the adaptive immune system, studies mostly in humans showed that a characteristic feature of aging is the reduction in the number of T and B cells, which is accompanied by a shrinking of their repertoire diversity, an increase of memory and activated cells, and a reduction in naïve cells (5–7). However, a comprehensive characterization of the age-related cellular characterization is poorly studied in rodents.
The cumulative alterations of the immune system over time ultimately lead to increased susceptibility to infection and reduced responsiveness to vaccination (8,9). Of interest, men and women experience those alterations differently, being the immune response typically stronger in women than in men (9,10). In other species (eg, other mammals, insects, and reptiles), a similar trend is observed regarding the immune response sexual dimorphism (9). In addition, human studies have further denoted a sexual dimorphism in age-related changes of blood cellular composition; however, information on animal models, especially rodents, is still scarce (11–14).
The mouse model has been unique to understanding human physiology in health and disease. Most published reports studying immunosenescence compare young versus aged mice, with a cross-sectional experimental design (15–17), missing therefore the opportunity to study inter- and intravariability in the aging processes. To contribute in filling this gap, in the present study, we investigated the individual progressions of the main blood adaptive and innate immune cell populations and whether those were sex-dependent in mice.
Method
Animals
Male and female BALB/cByJ mice, 6 weeks old, were purchased from Charles River and maintained until 18 months of age at the Life and Health Sciences Research Institute animal facility. Mice were initially housed in groups of 5 per cage under standard laboratory conditions (light/dark cycle of 12/12 hours; 22°C; 55% humidity), food and water ad libitum. Males were regrouped whenever aggressive behaviors were observed. Health monitoring was performed according to FELASA guidelines, confirming the Specified Pathogen-Free health status of sentinel animals maintained in the same animal room. Mice well-being was routinely evaluated: Animals were euthanized whenever open wounds from aggressions, visible tumors, or over 20% weight loss (in relation to the highest weight achieved) were observed. Blood samples were collected from the same animal at approximately 3, 6, 10, 12, 15, and 18 months of age.
Three independent experiments (referred as set 1, set 2, and set 3) were performed, and the number of mice used is listed in Supplementary Table 1. A total sample size of 218 mice (approximately 35 mice per sex/per experiment/set) was used. Sample size was previously determined using G*Power 3.1.2 (18) assuming an analysis of variance for repeated measures, a medium effect size [(f(V) = 0.25)], an alpha of 0.05, a statistical power of 0.8, and 2 groups with 6 measurements.
At the end of the study, mice were anesthetized with a mixture of ketamine (75 mg/kg, intraperitoneally [i.p.]) and medetomidine (1 mg/kg, i.p.) and were transcardially perfused with 0.9% saline. All procedures were carried out following European Regulations (European Union Directive 2010/63/EU). The animal facility and people directly involved in animal experimentation were certified by the Portuguese regulatory entity—Direção Geral de Alimentação e Veterinária (DGAV). All experiments were approved by the Ethics Committee of the University of Minho and by the national competent entity DGAV (#009458).
Flow Cytometry
Peripheral blood was collected through a small incision on the tail to heparin-coated capillaries. Fifty microliters of blood were incubated for 20 minutes in the dark, at room temperature (RT), with the following combination of previously titrated antibodies (all from BioLegend, San Diego, CA, USA): anti-mouse CD49b FITC (clone: DX5); anti-mouse CD62L PE (clone: MEL-14); anti-mouse CD19 PercpCy5.5 (clone: 6D5); anti-mouse CD3 PECy7 or APC (clone: 145-2C11); anti-mouse CD44 APC or BV605 (clone: IM7); anti-mouse CD4 BV421 (clone: RM4-5); anti-mouse CD8 BV510 (clone: 53-6.7); anti-mouse Ly6G BV650 (clone: 1A8); anti-mouse Ly6C BV711 (clone: HK1.4); and anti-mouse CD45.2 BV785 or PECy7 (clone: 104). Erythrocytes were lysed with ammonium–chloride–potassium buffer (0. 15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2 EDTA) for 10 minutes in the dark, RT. Samples were washed twice with fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline with 0.5% bovine serum albumin and 0.01% sodium azide) and centrifuged at 300 ×g for 5 minutes, RT. Samples were acquired (minimum of 100 000 CD45.2+ events/sample) on a BD LSRII flow cytometer using the FACS DIVA software (Becton and Dickinson, Franklin Lakes, NJ, USA). Samples from each set timepoint were acquired in the same run. Data were analyzed using FlowJo software (Becton and Dickinson), and the different immune cell populations were defined as follows: eosinophils (CD45.2+ Ly6C− SSC-Ahigh), NK cells (CD45.2+ CD49b+; negative for CD4, CD8, CD19, and Ly6G; subcategorized as Ly6C− and Ly6C+), neutrophils (CD45.2+ Ly6G+; negative for CD4, CD8, and CD19), classical monocytes (CD45.2+ Ly6Chigh; negative for CD4, CD8, CD19), B cells (CD45.2+ CD19+; negative for CD3 and LY6G), total T cells (CD45.2+ CD3+; negative for CD19 and LY6G), among which CD4+ and CD8+ T cells were defined (CD4+ CD8- and CD4- CD8+, respectively); CD4+ and CD8+ T cells were also divided in distinct activation subsets based on the expression of CD62L (CD62L−; CD62Lint; CD62Lhigh). The gating strategy used is represented in Supplementary Figures 1 and 2.
Statistical Analysis
Mixed-design analysis of variance (ANOVA), the most used statistical approach in biological sciences to explore between-subject differences in repeated measures data sets, does not allow missing values. In a longitudinal experimental design, losing subjects over time (in this case due to mice death) results in extra loss of information. To overcome this gap, we took advantage of linear mixed models for repeated measures that allow exploring the progression/trajectory of the main cell populations of the peripheral immune system over time/during aging and to dissect the impact of sex on that progression. This approach prevented listwise deletion from missing data (considering that some mice died during the 18 months evaluation, as given in Supplementary Table 1). The survival rate in all sets combined was 89% for females and 44% for males, comprising an overall survival rate of 67%. All the analyses were performed with combined results from the 3 sets. Due to an atypical leukocyte profile, 4 females were excluded from the analysis (1 from set 1, 1 from set 2, and 2 from set 3) throughout the whole evaluation.
The relation between time and the percentage of cells can either be linear or quadratic. Time, represented by each month of evaluation, was introduced in the models either as a linear variable (Time) or as a quadratic variable (by calculating the time square; Time2). To avoid collinearity, time was centered to the mean (cTime = each timepoint – mean of time points) and only then computed cTime2. The cTime, sex, cTime × sex (interaction), cTime2, and cTime2 × sex (interaction) were used as fixed factors. A multivariate analysis was performed with all independent variables analyzed simultaneously. Whenever a significant interaction between Time and Sex is observed, the slope of the progression over time for females is B[cTime], and for males is B[cTime] + B[cTime × Sex]. Whenever B[cTime] presents negative values, a negative B[cTime2] indicates an acceleration and a positive B[cTime2] a deceleration over time. On the other hand, whenever B[cTime] presents positive values, a positive B[cTime2] indicates an acceleration and a negative B[cTime2] a deceleration over time. A random intercept per subject was introduced in the model to analyze the changes in the percentage of immune cells over time considering the within-subject correlations between the percentage of immune cells. Models were tested for various covariance structures (Level 1: repeated covariance type and Level 2: random covariance type), and the pair with the lowest Akaike’s Information Criteria was used. The estimation method used was the restricted maximum likelihood.
The variable “sets” were also included as covariates in the models (results given in Supplementary Material) to account for interexperiments variability. Additionally, the same models were tested with standardized measures, z-scores (computed considering the mean of each population for all the time points at each set independently). To assess the possible bias caused by missing data (mice that died before the last time point), the same analyses were performed only with survivors.
Normal distribution of the residuals from each model was checked based on skewness (≤3) and kurtosis (≤8), and all the populations present a normal distribution (excepting CD62L− populations and total NK cells).
The variance of each immune cell population at the various time points was calculated on normalized values (z-scores).
A one-way repeated measures ANOVA and multiple comparisons with Bonferroni correction were used to identify the differences in the expression of CD44 among the different CD4+ and CD8+ T-cell subsets (CD62L−; CD62Lint; CD62Lhigh).
A significant result was considered for p value ≤ .05. The statistical procedures were performed in IBM SPSS Version 25 (IBM Corp., Armonk, NY, USA), and the graphs were designed using Prism7 (GraphPad Software, San Diego, CA, USA).
Results
Age and Sex-Related Alterations in the Percentage of Blood Innate Immune Cells
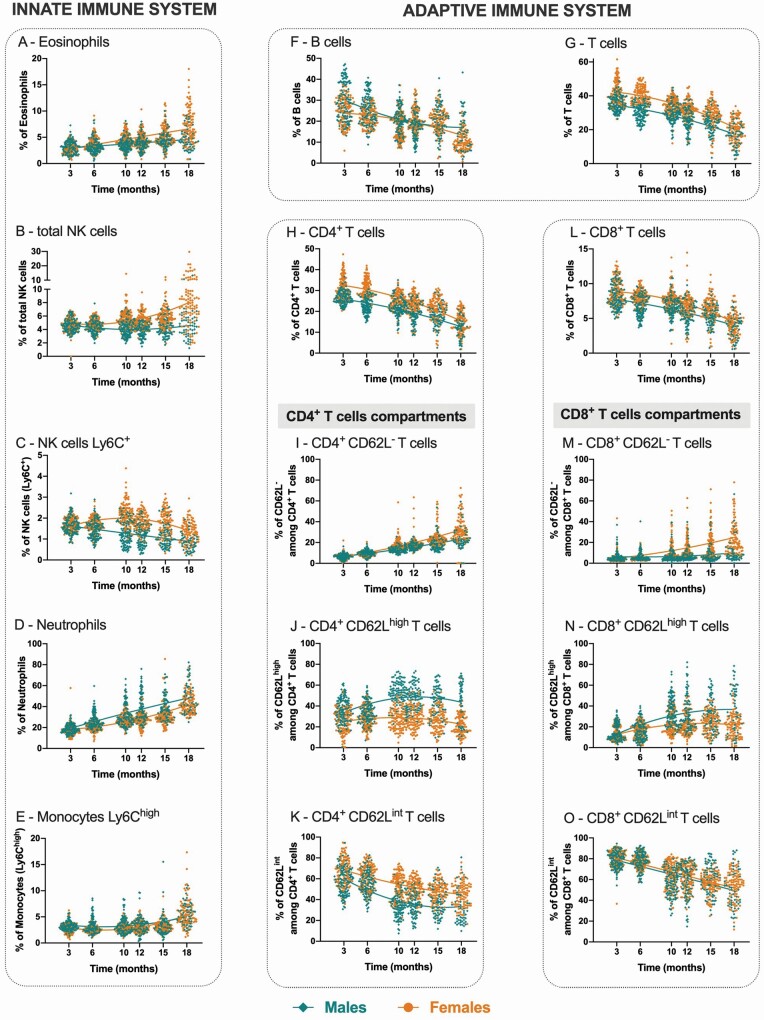
The results are presented as a combination of 3 independent experiments (referred as set 1, set 2, and set 3). The percentage of blood innate immune cells varies with age (eosinophils, total NK cells, neutrophils, and classical monocytes increase, while Ly6C+ NK cells decrease). Sex dimorphism was observed in the progression of most cell populations throughout aging. Specifically:
(1) Eosinophils (Figure 1A and Table 1): males present lower cell percentages than females (B[sex] = −0.889, the reference category is females) and a slower linear increase over time (+0.225% per month for females vs +0.129% per month for males; B[cTime] = 0.225 and B[cTime × Sex] = −0.096).
(2) Total NK cells (Figure 1B and Table 1): males present lower cell percentages (B[sex] = −1.410) than females. Male percentages remained constant throughout time, while in females an increase is observed over time (+0.138% per month for females vs −0.008% for males; B[cTime] = 0.138 and B[cTime × Sex] = −0.146) with a mild acceleration (B[cTime2] = 0.007; meaning that for each month there is an increment [acceleration] of 0.007% added to B[cTime] for the first month, 0.028% for the second, 0.063% for the third, and so forth—same rationale for the following models). A similar profile is observed for Ly6C− NK cells (Supplementary Figure 3 and Supplementary Table 2).
(3) Ly6C+ NK cells (Figure 1C and Table 1): males present lower cell percentages than females (B[sex] = −0.681) and a faster decrease over time (−0.017% per month for females vs −0.049% for males; B[cTime] = −0.017 and B[cTime × Sex] = −0.032). Females display an accelerated decrease over time, while males present only a linear decrease (−0.007% per month for females vs 0.000% for males; B[cTime2] = −0.007 and B[cTime2 × Sex] = 0.007).
(4) Neutrophils (Figure 1D and Table 1): males present higher cell percentages than females (B[sex] = 8.308) and a faster increase over time (+1.639% per month for females vs +1.950% for males; B[Time] = 1.639 and B[cTime × Sex] = 0.311). A slight accelerated increase in the percentage of neutrophils during aging is observed, mainly for females (+0.055% per month for females vs −0.025% for males; B[cTime2] = 0.055 and B[cTime2 × Sex] = −0.080).
(5) Monocytes Ly6Chigh (Figure 1F and Table 1): males present higher cell percentages (B[sex] = 0.617), and similar increase over time is observed for both sexes (+0.176% per month; B[Time] = 0.176 and [cTime × Sex] not significant). The percentual increase of monocytes during aging gradually accelerates over time, more pronouncedly in females (+0.024% per month for females vs +0.015% for males; B[cTime2] = 0.024 and B[cTime2 × Sex] = −0.009).
Figure 1.
Longitudinal evaluation of blood immune cells. Percentages of the main innate (eosinophils [A], total NK cells [B], Ly6C+ NK cells [C], neutrophils [D], and monocytes Ly6Chigh [E]) and adaptive immune cell populations over time (total B cells [F], total T cells [G], CD4+ T cells [H], CD8+ T cells [L], and activation compartments within CD4+ and CD8+ T cells: CD62L− [I and M, respectively], CD62Lhigh [J and N, respectively] and CD62Lint [K and O, respectively]). The representation combines the results of 3 independent experimental sets. Each dot represents one animal, where males are depicted as diamonds (teal in online version) and females as circles (orange in online version). Lines represent the best-fit equation representative of the data (either linear regression or second-order polynomial [quadratic] functions).
Table 1.
Linear Mixed Models Testing the Effect of Time (aging) and Sex on the Percentages of the Blood Main Innate Immune System Cells—a Multivariate Analysis
| Dependent Variable | Parameters | Estimate (B) | SE | p † | 95% CI | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Eosinophils | Intercept | 4.582 | 0.101 | <.001 | 4.383 | 4.780 |
| cTime | 0.225 | 0.017 | <.001 | 0.191 | 0.259 | |
| Sex* | −0.889 | 0.146 | <.001 | −1.177 | −0.601 | |
| cTime × Sex* | −0.096 | 0.028 | .001 | −0.150 | −0.041 | |
| cTime2 | 0.002 | 0.003 | .425 | −0.003 | 0.008 | |
| cTime2 × Sex* | 0.004 | 0.005 | .331 | −0.005 | 0.013 | |
| Total NK cells | Intercept | 5.410 | 0.086 | <.001 | 5.241 | 5.579 |
| cTime | 0.138 | 0.016 | <.001 | 0.106 | 0.169 | |
| Sex* | −1.410 | 0.125 | <.001 | −1.655 | −1.164 | |
| cTime × Sex* | −0.146 | 0.025 | <.001 | −0.195 | −0.097 | |
| cTime2 | 0.007 | 0.003 | .012 | 0.002 | 0.012 | |
| cTime2 × Sex* | 0.003 | 0.004 | .462 | −0.005 | 0.011 | |
| Ly6C+ NK cells | Intercept | 1.913 | 0.037 | <.001 | 1.839 | 1.986 |
| cTime | −0.017 | 0.004 | <.001 | −0.024 | −0.010 | |
| Sex* | −0.681 | 0.054 | <.001 | −0.787 | −0.575 | |
| cTime × Sex* | −0.032 | 0.006 | <.001 | −0.043 | −0.020 | |
| cTime2 | −0.007 | 0.001 | <.001 | −0.008 | −0.006 | |
| cTime2 × Sex* | 0.007 | 0.001 | <.001 | 0.005 | 0.009 | |
| Neutrophils | Intercept | 27.114 | 0.660 | <.001 | 25.815 | 28.412 |
| cTime | 1.639 | 0.075 | <.001 | 1.490 | 1.787 | |
| Sex* | 8.308 | 0.951 | <.001 | 6.436 | 10.181 | |
| cTime × Sex* | 0.311 | 0.123 | .012 | 0.068 | 0.554 | |
| cTime2 | 0.055 | 0.013 | <.001 | 0.030 | 0.081 | |
| cTime2 × Sex* | −0.080 | 0.020 | <.001 | −0.120 | −0.041 | |
| Monocytes Ly6Chigh | Intercept | 2.751 | 0.085 | <.001 | 2.584 | 2.917 |
| cTime | 0.176 | 0.066 | .008 | 0.047 | 0.306 | |
| Sex* | 0.617 | 0.123 | <.001 | 0.375 | 0.858 | |
| cTime × Sex* | −0.060 | 0.093 | .518 | −0.243 | 0.123 | |
| cTime2 | 0.024 | 0.001 | <.001 | 0.021 | 0.027 | |
| cTime2 × Sex* | −0.009 | 0.002 | <.001 | −0.014 | −0.005 |
Note: CI = confidence interval; SE = standard error.
*Reference category is female.
†Statistically significant results (p < .05) are highlighted in bold.
Age and Sex-Related Alterations in the Percentage of Blood Adaptive Immune Cells
Alike the innate immune cells, the percentage of most cell populations varies during aging in a process commonly moderated by sex.Specifically:
(1) B cells (Figure 1F and Table 2): males and females display similar cell percentages (B[sex] not significant), decreasing with time, more rapidly in males (−0.697% per month for females vs −0.908% for males; B[Time] = −0.697 and B[cTime × Sex] = −0.211). Interestingly, females present an accelerated decrease and males present a decelerated decrease over time (−0.035% per month for females vs +0.061% for males; B[cTime2] = −0.035 and B[cTime2 × Sex] = 0.096).
(2) T cells (Figure 1G and Table 2): males present lower cell percentages than females (B[sex] = −6.995) and a slower decrease over time (−1.474% per month for females vs −1.158% for males; B[Time] = −1.474 and B[cTime × Sex] = 0.316), with an overall acceleration independent of sex (B[cTime2] = −0.058 and B[cTime2 × Sex] not significant).
(3) CD4+ and CD8+ T-cell subsets (Figure 1H and L, respectively; Table 2): for both cell subsets males present lower cell percentages than females (BCD4+[Sex] = −5.403 and BCD8+[Sex] = −1.390). Moreover, while for CD8+ T cells males and females present a similar decrease in the percentage of cells over time (−0.296% per month; B[Time] = −0.296 and B[cTime × Sex] not significant), for CD4+ T cells females present a faster decrease cells than males (−1.174% per month for females vs −0.895% for males; B[Time] = −1.174 and B[cTime × Sex] = 0.279). The acceleration profile is similar in both sexes for CD4+ T cells (B[cTime2] = −0.037), though for CD8+ T cells a more pronounced acceleration is observed in females than in males (−0.018% per month for females vs −0.009% for males; B[cTime2] = −0.018 and B[cTime2 × Sex] = 0.009).
Table 2.
Linear Mixed Models Testing the Effect of Time (aging) and Sex on the Percentages of the Blood Main Adaptive Immune System Cells—a Multivariate Analysis
| Dependent Variable | Parameters | Estimate (B) | SE | p † | 95% CI | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| B cells | Intercept | 20.193 | 0.437 | <.001 | 19.333 | 21.053 |
| cTime | −0.697 | 0.056 | <.001 | −0.806 | −0.587 | |
| Sex* | −0.230 | 0.635 | .718 | −1.477 | 1.018 | |
| cTime × Sex* | −0.211 | 0.086 | .015 | −0.381 | −0.041 | |
| cTime2 | −0.035 | 0.011 | .002 | −0.058 | −0.013 | |
| cTime2 × Sex* | 0.096 | 0.017 | <.001 | 0.062 | 0.130 | |
| T cells | Intercept | 34.433 | 0.417 | <.001 | 33.612 | 35.254 |
| cTime | −1.474 | 0.052 | <.001 | −1.577 | −1.372 | |
| Sex* | −6.995 | 0.604 | <.001 | −8.184 | −5.806 | |
| cTime × Sex* | 0.316 | 0.082 | <.001 | 0.155 | 0.477 | |
| cTime2 | −0.058 | 0.009 | <.001 | −0.075 | −0.040 | |
| cTime2 × Sex* | 0.017 | 0.014 | .221 | −0.010 | 0.045 | |
| CD4+ T cells | Intercept | 25.802 | 0.323 | <.001 | 25.165 | 26.438 |
| cTime | −1.174 | 0.038 | <.001 | −1.250 | −1.098 | |
| Sex* | −5.403 | 0.469 | <.001 | −6.326 | −4.480 | |
| cTime × Sex* | 0.279 | 0.061 | <.001 | 0.159 | 0.399 | |
| cTime2 | −0.037 | 0.007 | <.001 | −0.050 | −0.023 | |
| cTime2 × Sex* | 0.010 | 0.011 | .359 | −0.011 | 0.031 | |
| CD62L− among CD4+ T cells | Intercept | 17.174 | 0.402 | <.001 | 16.381 | 17.966 |
| cTime | 1.789 | 0.072 | <.001 | 1.647 | 1.932 | |
| Sex* | −2.682 | 0.581 | <.001 | −3.826 | −1.538 | |
| cTime × Sex* | −0.749 | 0.113 | <.001 | −0.971 | −0.527 | |
| cTime2 | 0.051 | 0.009 | <.001 | 0.034 | 0.069 | |
| cTime2 × Sex* | −0.051 | 0.014 | <.001 | −0.078 | −0.023 | |
| CD62Lint among CD4+ T cells | Intercept | 53.645 | 0.756 | <.001 | 52.158 | 55.133 |
| cTime | −1.547 | 0.089 | <.001 | −1.723 | −1.371 | |
| Sex* | −16.141 | 1.115 | <.001 | −18.333 | −13.949 | |
| cTime × Sex* | −0.073 | 0.139 | .601 | −0.347 | 0.201 | |
| cTime2 | 0.048 | 0.018 | .006 | 0.014 | 0.083 | |
| cTime2 × Sex* | 0.115 | 0.027 | <.001 | 0.062 | 0.168 | |
| CD62Lhigh among CD4+ T cells | Intercept | 28.844 | 0.800 | <.001 | 27.270 | 30.418 |
| cTime | −0.198 | 0.101 | .051 | −0.396 | 0.001 | |
| Sex* | 19.334 | 1.171 | <.001 | 17.030 | 21.639 | |
| cTime × Sex* | 0.748 | 0.158 | <.001 | 0.436 | 1.059 | |
| cTime2 | −0.091 | 0.019 | <.001 | −0.128 | −0.055 | |
| cTime2 × Sex* | −0.090 | 0.029 | .002 | −0.146 | −0.033 | |
| CD8+ T cells | Intercept | 7.480 | 0.149 | <.001 | 7.186 | 7.774 |
| cTime | −0.296 | 0.067 | <.001 | −0.428 | −0.164 | |
| Sex* | −1.390 | 0.213 | <.001 | −1.811 | −0.970 | |
| cTime × Sex* | 0.022 | 0.095 | .816 | −0.164 | 0.209 | |
| cTime2 | −0.018 | 0.002 | <.001 | −0.021 | −0.015 | |
| cTime2 × Sex* | 0.009 | 0.003 | <.001 | 0.004 | 0.015 | |
| CD62L− among CD8+ T cells | Intercept | 11.359 | 0.646 | <.001 | 10.084 | 12.633 |
| cTime | 1.372 | 0.092 | <.001 | 1.189 | 1.554 | |
| Sex* | −4.240 | 0.928 | <.001 | −6.071 | −2.409 | |
| cTime × Sex* | −1.061 | 0.146 | <.001 | −1.349 | −0.774 | |
| cTime2 | 0.062 | 0.014 | <.001 | 0.034 | 0.089 | |
| cTime2 × Sex* | −0.033 | 0.021 | .119 | −0.075 | 0.009 | |
| CD62Lint among CD8+ T cells | Intercept | 65.384 | 0.898 | <.001 | 63.618 | 67.150 |
| cTime | −1.950 | 0.103 | <.001 | −2.153 | −1.746 | |
| Sex* | −1.263 | 1.309 | .335 | −3.837 | 1.311 | |
| cTime × Sex* | 0.121 | 0.165 | .464 | −0.204 | 0.446 | |
| cTime2 | 0.032 | 0.019 | .092 | −0.005 | 0.070 | |
| cTime2 × Sex* | 0.002 | 0.029 | .936 | −0.055 | 0.060 | |
| CD62Lhigh among CD8+ T cells | Intercept | 22.334 | 0.932 | <.001 | 20.501 | 24.167 |
| cTime | 0.588 | 0.095 | <.001 | 0.401 | 0.775 | |
| Sex* | 6.978 | 1.352 | <.001 | 4.318 | 9.638 | |
| cTime × Sex* | 0.975 | 0.152 | <.001 | 0.674 | 1.276 | |
| cTime2 | −0.087 | 0.018 | <.001 | −0.121 | −0.052 | |
| cTime2 × Sex* | 0.024 | 0.027 | <.001 | −0.030 | 0.077 |
Note: CI = confidence interval; SE = standard error.
*Reference category is female.
†Statistically significant results (p < .05) are highlighted in bold.
CD4+ and CD8+ T cells can be divided into subsets/compartments based on their activation state. Mouse studies, based mostly on C57BL/6 mice, define T cells as: naïve, CD44low/−CD62Lhigh; central memory, CD44high CD62Lhigh; and effector memory, CD44high CD62Llow/− (19,20). However, the expression profile of CD44 and CD62L is distinct in Balb/c mice. Considering that in Balb/c mice 3 clear populations can be identified based on CD62L in CD4+ and CD8+ T cells (Supplementary Figure 2G and H), this study only used this marker to define the T-cell compartments. Within CD4+ T cells, CD62L− cells present the highest expression of CD44, and CD62Lint the lowest; within CD8+ T cells, CD62L− and CD62Lhigh present the highest expression of CD44 and CD62Lint the lowest (Supplementary Figure 4). The percentage of CD62L− T cells increases over time (in both sexes among CD4+ T cells, and exclusively for females among CD8+ T cells); CD62Lint T cells decrease with aging both in CD4+ and CD8+ T cells. While females have a higher percentage of CD62Lint cells than males among CD4+ T cells; CD62Lhigh T cells increase almost exclusively on males. More precisely:
(1) CD62L− T cells (Figure 1I and M; Table 2): males present lower percentages of CD62L− cells among CD4+ and CD8+ T cells (BCD4+CD62L-[Sex] = −2.682 and BCD8+CD62L−[Sex] = −4.240), and these percentages increase with aging for both sexes. Interestingly, the increase of CD4+CD62L− T cells is slower in males than in females (+1.789% per month for females vs +1.040% for males; B[Time] = 1.789 and B[cTime × Sex] = −0.749), while the increase in the CD8+CD62L− T cells percentages is more pronouncedly observed for females (1.372% per month for females vs 0.311% for males; B[Time] = 1.372 and B[cTime × Sex] = −1.061).
(2) CD62Lhigh T cells (Figure 1J and N; Table 2): the percentage of this CD4+ T-cell subset is higher in males than in females (B[sex] = 19.334). While cell percentages decrease with aging in females, males display an increase (−0.198% per month for females vs +0.550% for males; B[cTime] = −0.198, p = .051 and B[cTime × Sex] = 0.748). Additionally, females present an acceleration over time, while males present a deceleration (−0.091% per month for females vs −0.181% for males; B[cTime2] = −0.091 and B[cTime2 × Sex] = −0.090). Regarding CD8+ T cells (Figure 1N and Table 2), males present higher percentages of CD62Lhigh cells than females (B[Sex] = 6.978), with a faster increase over time (0.588% per month for females vs 1.563% for males; B[cTime] = 0.588; B[cTime × Sex] = 0.975) and overall deceleration (−0.087% per month for females vs −0.063% for males; B[cTime2] = −0.087 and B[cTime2 × Sex] = +0.024).
(3) CD62Lint T cells (Figure 1K and O; Table 2): cell percentages decrease over time in both CD4+ T cells and CD8+ T cells (−1.547% per month [BCD4+[Time] = −1.547] and −1.959% per month [BCD8+[Time] = −1.959], respectively), with males and females presenting a similar decrease of CD62Lint T cells within the CD8+ T-cell subset (B[cTime × Sex] and B[cTime2 × Sex] not significant), and males presenting a more pronounced deceleration of CD62Lint T cells within the CD4+ T-cell subsets overtime than females (0.046% per month for females vs 0.161% for males; B[cTime2] = +0.046, B[cTime2 × Sex] = +0.115, B[cTime × Sex] not significant). Males present lower percentages of CD62Lint cells within the CD4+ T-cell subsets than females (B[Sex] = −16 141) and similar profiles regarding the percentages of CD62Lint CD8+ T cells (B[Sex] not significant).
Control of the Interexperimental Set Variability and Exclusion of Mice That Died During the Course of the Experiment Have No Impact on the Overall Observations
Because we compiled the data from 3 independent experiments, and to control for the heterogeneity, the percentages of cells from each of the 3 sets were standardized by calculating the z-scores. As shown in Supplementary Figure 5, the progression for standardized measures is similar to that presented in Figure 1 (unstandardized measures). The results from the linear mixed models are identical in the standardized and unstandardized measures except for (a) B cells: sex becomes a significant parameter and the significance in the interaction between time and sex is lost when considering standardized values; and (b) CD4+CD62Lint T cells: the quadratic effect of time is lost when considered standardized values (data not shown).
To control for a potential effect of individual experimental sets in the linear mixed models with unstandardized values, the variable “set” was also included (Supplementary Tables 3 and 4). When controlling for the “set” effect, the significant parameters remain the same with estimates within the 95% confidence interval of the first model.
Moreover, the total variability within immune cell populations increases with aging, as demonstrated by the increased variance in most populations at 18 months of age (Figure 2). Total NK cells present the highest variability observed at 18 months of age, more evidently denoted in female mice. Exploring the covariates associated with this age-related increased variability will contribute to the understanding of intervariability in the aging process.
Figure 2.
Variance of each immune cell population at various time points (overall—top panel; only on females—middle panel; only on males—bottom panel). Variance calculated on normalized values (z-scores). Dark grey corresponds to the lowest and highest variances (in online version blue corresponds to the lowest variances and red to the highest).
As the survival rate, especially in males, is relatively low (44%), it is conceivable that mice that died during the experiments might be different and not representative of the overall population. To explore the possible impact of a listwise exclusion of all mice that died throughout the experiment, linear mixed models were also built using only the survivors; no impact was observed on the main conclusions (Supplementary Figure 6).
Discussion
This longitudinal study shows that the percentages of immune cells in mice vary with aging and that males and females show distinct progression profiles. More specifically, the percentage of neutrophils, monocytes, eosinophils, and total NK cells increases with age, while that of Ly6C+ NK cells, B and T cells (both CD4+ and CD8+) decreases. These observations reveal an overall decrease of adaptive and an increase of innate immune cells, a signature that is in line with some of the known age-related changes of the immune system that occur in humans (2,12,17,21–24). Of relevance, a skew in hematopoiesis during aging, favoring the myeloid lineage, has been shown both in humans and rodents (25,26), which can contribute to the observed increased proportion of circulating innate cells. In the same line of evidence, both aged humans and mice present increased levels of total monocytes in comparison to young subjects (2,17). However, with respect to classical monocytes, the population explored in this study, humans and inbred mice seem to differ: in humans, CD14high decrease or remain constant with aging (11,12), whereas in mice, Ly6Chigh are increased in old mice, as demonstrated here and by others (17). This discrepancy might be related to genetic variability since CD-1 mice, which present higher genetic variability than inbred strains, also display decreased numbers of classical monocytes in aged mice (27). Moreover, a reduction in monocytes’ chemotaxis has been described (3), which might explain the observed accumulation of these cells in circulation. The percentage of human neutrophils increases with age (21), similarly to what we observed here for mice. Neutrophils’ capacity to migrate to inflamed tissues is also impaired in aged animals (28,29), potentially contributing to their accumulation in the blood. Concerning NK cells, we observed an overall decrease of Ly6C+ NK cells and an increase of total NK cells. In humans, an increase in total NK cells during aging has been extensively reported (12,30–32) as we show for female mice. This is in contrast with reports from experiments using C57BL/6 mice in which NK cells are selected as NK1.1+ cells (33), a molecule not expressed in Balb/c mice (34). We, and others using Balb/c mice (35), define NK cells as CD49b+ (and negative for CD4, CD8, CD19, and Ly6G). Recently, the expression of CD49b was seen to occur also in atypical innate lymphoid cells (ILC1) (36), which might indicate that we are selecting a broader population of cells rendering the comparison between studies difficult. An NK cell subset based on the expression of Ly6C was identified, even though the expression of this marker on NK cells is still poorly characterized. It was reported that mature NK cells (NK1.1+ CD11b+) expressing Ly6C present a resting/inert phenotype with a poor proliferative potential (37), and that memory NK cells express high levels of Ly6C (and low levels of CD27) upon viral infection (38,39). Curiously, CD27− CD11b+ NK cells, considered the most mature cells, were shown to decrease in the blood of aged mice (15), which is in line with our findings. However, the mouse CD27− NK cells present some similarities with human CD56dim NK cells (40), which have been described to be increased in older individuals (32,41). Considering the absence of CD56 expression in mice (40), additional markers should be used to better characterize the dynamics of the various NK cells subpopulations during aging to allow a more accurate comparison with humans.
As for the adaptive immune system, it has been extensively described that human B and T (both CD4+ and CD8+ subsets) percentages and absolute numbers decrease with aging (11,12,22,23), as observed here in mice. Moreover, studies in rats and mice also evidenced a decrease in splenic T cells (or CD4+ T cells) in old animals (16,42).
Considering the importance to explore sex as a biological variable that might influence preclinical research (43) and the lack of reports tackling this issue in mouse healthy aging, we explored sex-related differences in the percentage of immune cells over time.
We show that sex is a significant and confounding factor in the progression of the main blood populations of the immune system evaluated. Males present overall higher percentages of neutrophils and classical monocytes compared to females. By contrast, females present higher percentages of total T cells, and both CD4+ and CD8+ T cells, eosinophils, and NK cells. Even though males and females present similar percentages of B cells, their progression over time reveals opposing acceleration profiles. Altogether, this clearly demonstrates an age-related sexual dimorphism in the proportion of immune cells in circulation. Sex differences in basal conditions, as shown here, are still poorly characterized, though extensively explored during immune responses both in humans and in animal models. In studies comparing the immune response in males versus females, females usually present a stronger immune response resulting in a more efficient pathogen clearance but increased susceptibility to autoimmunity (9,10). Additionally, in aged individuals, women have higher genomic accessibility for genes (ATAC-seq) related to adaptive immunity and lower for monocytes and inflammation-related genes than men (11). As described here, the percentages of total T cells, both CD4+ and CD8+ subsets, are lower in males and decrease during aging in both sexes. Accordingly, human cross-sectional studies demonstrated an identical profile, even though the impact of sex was only observed for total T cells and CD4+ T cells (11,12). These findings might be related to the fact that thymic function decreases with aging differently in men and women. As no direct method is available to quantify thymic function, this has been estimated using various surrogates, of which the quantification of T-cell receptor excision circles (TRECs) in peripheral blood (44) is the most used. The decrease of TRECs with age, from 20 to 70 years of age, is greater in men than in women (31), thus suggesting an accelerated decrease of thymic function in men, which might explain the greater percentages of T cells in women.
The most well-described age-related alteration in the adaptive immune system, mainly in humans, is the shift in the T-cell compartments from a naïve into memory and effector phenotypes (5,6,45). For strains comparisons, a note is necessary on the classification of T-cell compartments in mice. The classical classification of T-cell compartments in mice, retrieved essentially from the C57BL/6 strain, is based on the expression of CD44 and CD62L (naïve T cells as CD44low/−CD62Lhigh, central memory T cells as CD44high CD62Lhigh, and effector memory T cells as CD44high CD62Llow/−) (19,20). However, the expression profile of CD44 and CD62L is distinct in Balb/c mice, as shown by others (46) and also here. Balb/c mice present higher expression of CD62L than C57BL/6 mice (46), possibly explaining some of the strain differences. In Balb/c mice, 3 distinct populations based on the expression of CD62L are defined: CD62L−, CD62Lint, and CD62Lhigh. Considering the median fluorescence intensity of CD44 on each population, we propose that CD62L− T cells are effector memory cells due to their highest expression of CD44; CD62Lhigh T cells are central memory cells due to their intermediate expression of CD44; and CD62Lint T cells are naïve cells due to their lowest expression of CD44 (19,20). Using this classification, we observe that CD62L− T cells (effector memory cells) increase over time (in both sexes among CD4+ T cells, and exclusively for females on CD8+ T cells); CD62Lint T cells (naïve cells) decrease with aging both in CD4+ and CD8+ T cells, where females have clearly more naïve CD4+ T cells; and CD62Lhigh T cells (central memory cells) increase almost exclusively on males. Concordantly, in humans, the decrease in naïve T cells is more pronounced in CD8+ T cells, with women displaying higher relative amounts of naïve T cells than men (11,12,47). On the other hand, men present a higher proportion of effector T cells than women (47), mostly at young ages (11). To note, here we present the proportion of cells among either CD4+ or CD8+ T cells, while human studies present mostly absolute counts or percentages among total peripheral blood mononuclear cells (11,47), which can explain some of the inconsistencies found. Moreover, the dynamics of T-cell compartments seem to display considerable species differences, which is of relevance when age is considered. For instance, the proportion of T-cell compartments in the adult mouse is more similar to that of neonate humans than adults (48). Overall, irrespectively from the T-cell compartments analyzed, the alterations in the proportion of cells are strongly influenced by sex, reinforcing the need to investigate both males and females when addressing the impact of aging on T cells.
Potential mechanisms responsible for sex-related differences are likely related to the effects of sex hormones on the immune system and to genetic factors, as described elsewhere (8,9,49). The increased variability in the proportion of immune cells with aging, which has also been reported at the transcriptional level (50), reinforces the need to consider individual progressions when analyzing biological parameters in age-related research. Longitudinal studies and mixed-effects models are 2 strategies that should be implemented more often when studying immune system aging.
The concordance across human and mouse studies suggests that some of the aging and sex-specific signatures described are conserved across species, which gives the confidence to use animal models when seeking further understanding of the sex-related discrepancies in immune system aging.
Taken together, this longitudinal study comprehensively explores the evolution of the main immune cell populations during aging, revealing an overall decrease in the adaptive immune cells and an increase in innate cells. It also reveals that sex plays an essential role in the mouse immune system aging, as described in humans, and must be taken into account when designing and planning experiments with rodents.
Supplementary Material
Acknowledgments
We acknowledge Gisela Armada, João Canto-Gomes, Ângela Mesquita, Rita Melo-Miranda, and Rogério Castro for all the help in blood samples’ collection and processing.
Contributor Information
Cláudia Serre-Miranda, Life and Health Sciences Research Institute, School of Medicine, University of Minho, Braga, Portugal; ICVS/3B’s—PT Government Associate Laboratory, Braga/Guimarães, Portugal.
Susana Roque, Life and Health Sciences Research Institute, School of Medicine, University of Minho, Braga, Portugal; ICVS/3B’s—PT Government Associate Laboratory, Braga/Guimarães, Portugal.
Palmira Barreira-Silva, Life and Health Sciences Research Institute, School of Medicine, University of Minho, Braga, Portugal; ICVS/3B’s—PT Government Associate Laboratory, Braga/Guimarães, Portugal.
Claudia Nobrega, Life and Health Sciences Research Institute, School of Medicine, University of Minho, Braga, Portugal; ICVS/3B’s—PT Government Associate Laboratory, Braga/Guimarães, Portugal.
Neide Vieira, Life and Health Sciences Research Institute, School of Medicine, University of Minho, Braga, Portugal; ICVS/3B’s—PT Government Associate Laboratory, Braga/Guimarães, Portugal.
Patrício Costa, Life and Health Sciences Research Institute, School of Medicine, University of Minho, Braga, Portugal; ICVS/3B’s—PT Government Associate Laboratory, Braga/Guimarães, Portugal.
Joana Almeida Palha, Life and Health Sciences Research Institute, School of Medicine, University of Minho, Braga, Portugal; ICVS/3B’s—PT Government Associate Laboratory, Braga/Guimarães, Portugal.
Margarida Correia-Neves, Life and Health Sciences Research Institute, School of Medicine, University of Minho, Braga, Portugal; ICVS/3B’s—PT Government Associate Laboratory, Braga/Guimarães, Portugal.
Funding
This work was supported by National funds, through the Foundation for Science and Technology (FCT)—project UIDB/50026/2020 and UIDP/50026/2020; and by the projects NORTE-01-0145-FEDER-000013 and NORTE-01-0145-FEDER-000023, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). C.S.-M. was supported by an FCT PhD grant (SFRH/BD/112494/2015). C.N., S.R., and N.V. are junior researchers under the scope of the FCT Transitional Rule DL57/2016.
Conflict of Interest
None declared.
Author Contributions
C.S.-M., J.A.P., and M.C.-N. conceptualized, designed, and supervised the study; C.S.-M. performed the experiment and processed the samples, analyzed the data, prepared the figures, performed the statistical analysis, and drafted the first version of the manuscript; S.R., P.B.-S., C.N., and N.V. collected and processed samples; P.C. supervised the statistical analysis; and all authors discussed the results and contributed to the final version of the manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–887. doi: 10.1038/nri3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Della Bella S, Bierti L, Presicce P, et al. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122(2):220–228. doi: 10.1016/j.clim.2006.09.012 [DOI] [PubMed] [Google Scholar]
- 3. Panda A, Arjona A, Sapey E, et al. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30(7):325–333. doi: 10.1016/j.it.2009.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mocchegiani E, Giacconi R, Cipriano C, Malavolta M. NK and NKT cells in aging and longevity: role of zinc and metallothioneins. J Clin Immunol. 2009;29(4):416–425. doi: 10.1007/s10875-009-9298-4 [DOI] [PubMed] [Google Scholar]
- 5. Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127(3):274–281. doi: 10.1016/j.mad.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 6. Nikolich-Žugich J. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J Immunol. 2014;193(6):2622–2629. doi: 10.4049/jimmunol.1401174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Britanova OV, Putintseva EV, Shugay M, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol. 2014;192(6):2689–2698. doi: 10.4049/jimmunol.1302064 [DOI] [PubMed] [Google Scholar]
- 8. Gubbels Bupp MR, Potluri T, Fink AL, Klein SL. The confluence of sex hormones and aging on immunity. Front Immunol. 2018;9:1269. doi: 10.3389/fimmu.2018.01269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 10. Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309–321. doi: 10.1111/acel.12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Márquez EJ, Chung CH, Marches R, et al. Sexual-dimorphism in human immune system aging. Nat Commun. 2020;11(1):751. doi: 10.1038/s41467-020-14396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patin E, Hasan M, Bergstedt J, et al. ; Milieu Intérieur Consortium . Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat Immunol. 2018;19(3):302–314. doi: 10.1038/s41590-018-0049-7 [DOI] [PubMed] [Google Scholar]
- 13. Strindhall J, Skog M, Ernerudh J, et al. The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: the Swedish HEXA immune study. Age (Dordr). 2013;35(3):985–991. doi: 10.1007/s11357-012-9400-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdullah M, Chai PS, Chong MY, et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. 2012;272(2):214–219. doi: 10.1016/j.cellimm.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 15. Nair S, Fang M, Sigal LJ. The natural killer cell dysfunction of aged mice is due to the bone marrow stroma and is not restored by IL-15/IL-15Rα treatment. Aging Cell. 2015;14(2):180–190. doi: 10.1111/acel.12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Naseery NI, Mousa HSE, Noreldin AE, El-Far AH, Elewa YHA. Aging-associated immunosenescence via alterations in splenic immune cell populations in rat. Life Sci. 2020;241:117168. doi: 10.1016/j.lfs.2019.117168 [DOI] [PubMed] [Google Scholar]
- 17. Puchta A, Naidoo A, Verschoor CP, et al. TNF drives monocyte dysfunction with age and results in impaired anti-pneumococcal immunity. PLoS Pathog. 2016;12(1):e1005368. doi: 10.1371/journal.ppat.1005368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
- 19. Rosenblum MD, Way SS, Abbas AK. Regulatory T cell memory. Nat Rev Immunol. 2016;16(2):90–101. doi: 10.1038/nri.2015.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Del Zotto G, Principi E, Antonini F, et al. Comprehensive phenotyping of peripheral blood T lymphocytes in healthy mice. Cytometry A. 2021;99(3): 243–250. doi: 10.1002/cyto.a.24246 [DOI] [PubMed] [Google Scholar]
- 21. Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol. 2016;83(4):255–266. doi: 10.1111/sji.12413 [DOI] [PubMed] [Google Scholar]
- 22. Paganelli R, Quinti I, Fagiolo U, et al. Changes in circulating B cells and immunoglobulin classes and subclasses in a healthy aged population. Clin Exp Immunol. 1992;90(2):351–354. doi: 10.1111/j.1365-2249.1992.tb07954.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muggen AF, de Jong M, Wolvers-Tettero ILM, et al. The presence of CLL-associated stereotypic B cell receptors in the normal BCR repertoire from healthy individuals increases with age. Immun Ageing. 2019;16:22. doi: 10.1186/s12979-019-0163-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chong Y, Ikematsu H, Yamaji K, et al. CD27(+) (memory) B cell decrease and apoptosis-resistant CD27(−) (naive) B cell increase in aged humans: implications for age-related peripheral B cell developmental disturbances. Int Immunol. 2005;17(4):383–390. doi: 10.1093/intimm/dxh218 [DOI] [PubMed] [Google Scholar]
- 25. Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111(12):5553–5561. doi: 10.1182/blood-2007-11-123547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rundberg Nilsson A, Soneji S, Adolfsson S, Bryder D, Pronk CJ. Human and murine hematopoietic stem cell aging is associated with functional impairments and intrinsic megakaryocytic/erythroid bias. PLoS One. 2016;11(7):e0158369. doi: 10.1371/journal.pone.0158369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strohacker K, Breslin WL, Carpenter KC, McFarlin BK. Aged mice have increased inflammatory monocyte concentration and altered expression of cell-surface functional receptors. J Biosci. 2012;37(1):55–62. doi: 10.1007/s12038-011-9169-z [DOI] [PubMed] [Google Scholar]
- 28. Wenisch C, Patruta S, Daxböck F, Krause R, Hörl W. Effect of age on human neutrophil function. J Leukoc Biol. 2000;67(1):40–45. doi: 10.1002/jlb.67.1.40 [DOI] [PubMed] [Google Scholar]
- 29. Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J Immunol. 2013;190(4):1746–1757. doi: 10.4049/jimmunol.1201213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schindowski K, Fröhlich L, Maurer K, Müller WE, Eckert A. Age-related impairment of human T lymphocytes’ activation: specific differences between CD4(+) and CD8(+) subsets. Mech Ageing Dev. 2002;123(4):375–390. doi: 10.1016/s0047-6374(01)00396-7 [DOI] [PubMed] [Google Scholar]
- 31. Clave E, Araujo IL, Alanio C, et al. Human thymopoiesis is influenced by a common genetic variant within the TCRA-TCRD locus. Sci Transl Med. 2018;10(457):eaao2966. doi: 10.1126/scitranslmed.aao2966 [DOI] [PubMed] [Google Scholar]
- 32. Almeida-Oliveira A, Smith-Carvalho M, Porto LC, et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol. 2011;72(4):319–329. doi: 10.1016/j.humimm.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 33. Beli E, Duriancik DM, Clinthorne JF, Lee T, Kim S, Gardner EM. Natural killer cell development and maturation in aged mice. Mech Ageing Dev. 2014;135:33–40. doi: 10.1016/j.mad.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carlyle JR, Mesci A, Ljutic B, et al. Molecular and genetic basis for strain-dependent NK1.1 alloreactivity of mouse NK cells. J Immunol. 2006;176(12):7511–7524. doi: 10.4049/jimmunol.176.12.7511 [DOI] [PubMed] [Google Scholar]
- 35. Arase H, Saito T, Phillips JH, Lanier LL. Cutting edge: the mouse NK cell-associated antigen recognized by DX5 monoclonal antibody is CD49b (alpha 2 integrin, very late antigen-2). J Immunol. 2001;167(3):1141–1144. doi: 10.4049/jimmunol.167.3.1141 [DOI] [PubMed] [Google Scholar]
- 36. Wong E, Xu RH, Rubio D, et al. Migratory dendritic cells, group 1 innate lymphoid cells, and inflammatory monocytes collaborate to recruit NK cells to the virus-infected lymph node. Cell Rep. 2018;24(1):142–154. doi: 10.1016/j.celrep.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Omi A, Enomoto Y, Kiniwa T, Miyata N, Miyajima A. Mature resting Ly6C(high) natural killer cells can be reactivated by IL-15. Eur J Immunol. 2014;44(9):2638–2647. doi: 10.1002/eji.201444570 [DOI] [PubMed] [Google Scholar]
- 38. Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16(2):112–123. doi: 10.1038/nri.2015.9 [DOI] [PubMed] [Google Scholar]
- 39. Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x [DOI] [PubMed] [Google Scholar]
- 41. Hazeldine J, Hampson P, Lord JM. Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell. 2012;11(5):751–759. doi: 10.1111/j.1474-9726.2012.00839.x [DOI] [PubMed] [Google Scholar]
- 42. Xie J, Zhang J, Wu H, et al. The influences of age on T lymphocyte subsets in C57BL/6 mice. Saudi J Biol Sci. 2017;24(1):108–113. doi: 10.1016/j.sjbs.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller LR, Marks C, Becker JB, et al. Considering sex as a biological variable in preclinical research. FASEB J. 2017;31(1):29–34. doi: 10.1096/fj.201600781R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dion ML, Sékaly RP, Cheynier R. Estimating thymic function through quantification of T-cell receptor excision circles. Methods Mol Biol. 2007;380:197–213. doi: 10.1007/978-1-59745-395-0_12 [DOI] [PubMed] [Google Scholar]
- 45. Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17(5):468–475. doi: 10.1016/j.coi.2005.07.020 [DOI] [PubMed] [Google Scholar]
- 46. Tu L, Poe JC, Kadono T, et al. A functional role for circulating mouse L-selectin in regulating leukocyte/endothelial cell interactions in vivo. J Immunol. 2002;169(4):2034–2043. doi: 10.4049/jimmunol.169.4.2034 [DOI] [PubMed] [Google Scholar]
- 47. Yan J, Greer JM, Hull R, et al. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing. 2010;7:4. doi: 10.1186/1742-4933-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beura LK, Hamilton SE, Bi K, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532(7600):512–516. doi: 10.1038/nature17655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gubbels Bupp MR. Sex, the aging immune system, and chronic disease. Cell Immunol. 2015;294(2):102–110. doi: 10.1016/j.cellimm.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 50. Martinez-Jimenez CP, Eling N, Chen HC, et al. Aging increases cell-to-cell transcriptional variability upon immune stimulation. Science. 2017;355(6332):1433–1436. doi: 10.1126/science.aah4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.