Abstract
Age-dependent differences in methylation at specific cytosine–guanine (CpG) sites have been used in “epigenetic clock” formulas to predict age. Deviations of epigenetic age from chronological age are informative of health status and are associated with adverse health outcomes, including mortality. In most cases, epigenetic clocks are performed on methylation from DNA extracted from circulating blood cells. However, the effect of neoplastic cells in the circulation on estimation and interpretation of epigenetic clocks is not well understood. Here, we explored this using Fischer 344 (F344) rats, a strain that often develops large granular lymphocyte leukemia (LGLL). We found clear histological markers of LGLL pathology in the spleens and livers of 27 out of 61 rats aged 17–27 months. We assessed DNA methylation by reduced representation bisulfite sequencing with coverage of 3 million cytosine residues. Although LGLL broadly increased DNA methylation variability, it did not change epigenetic aging. Despite this, the inclusion of rats with LGLL in clock training sets significantly altered predictor selection probability at 83 of 121 commonly utilized CpG sites. Furthermore, models trained on rat samples that included individuals with LGLL had greater absolute age error than those trained exclusively rats free of LGLL (39% increase; p < .0001). We conclude that the epigenetic signals for aging and LGLL are distinct, such that LGLL assessment is not necessary for valid measures of epigenetic age in F344 rats. The precision and architecture of constructed epigenetic clock formulas, however, can be influenced by the presence of neoplastic hematopoietic cells in training set populations.
Keywords: Aging, Cancer, Mononuclear cell leukemia
Alterations in epigenetic modifications of DNA are a hallmark of aging. The age-dependent divergence in methylation profiles of monozygotic twins suggests that external lifestyle factors and/or stochastic internal processes contribute to lifelong drift in DNA methylation (1). Large-scale interrogation of methylation at individual cytosine residues has identified sites where percent methylation is strongly correlated with age (2,3). These findings led to the development of “epigenetic clocks” that accurately estimate age across multiple tissues using weighted averages of methylation at a selected group of cytosine–guanine (CpG) sites (4,5). It has been argued that individuals with a methylation signature “older” than chronological age are experiencing accelerated aging. Accelerated methylation age has been observed in Werner’s syndrome (6) and HIV infection (7) and has been associated with mortality risk in the general population (8). Another line of epigenetic clock research has assessed genetic and genomic properties of the specific CpG sites composing clock formulas to gain insights into the molecular mechanisms of aging (5,9–11).
Most epigenetic clocks were developed using whole-blood DNA. However, because white blood cell composition changes systematically with age and may be affected by acute and chronic diseases (12,13), this raises the possibility that changes attributed to intrinsic cellular aging may be due to different cell composition in the source material. Blood-related diseases such as leukemias, lymphomas, or myelomas, which are characterized by dramatic changes in composition and epigenetic status of white blood cells and the presence of abnormal circulating cells (14), may be particularly disruptive to epigenetic clock modeling. The inclusion of individuals with hematopoietic malignancies within training data could affect subsequently generated epigenetic clock formulas. The few studies applying previously generated epigenetic clock formulas to test populations with hematopoietic malignancies have yielded discordant results: Age acceleration was observed with B-cell lymphoma (15) but not with acute myeloid leukemia (16).
Rodents provide an attractive model system for aging studies, as their shorter life spans allow for aging studies to be conducted on rapid time scales. Accordingly, epigenetic clocks have been developed in mice and rats and used to track interventions that shorten or prolong life span (17–19). Furthermore, the investigation into genetic and genomic architectures of these clocks has provided insight into mechanisms underlying the aging process (9,18,20). Initial rodent epigenetic clocks were developed in mice, which are extensively used for studies involving genetic manipulation. However, rats confer several advantages over mice for longitudinal aging studies. Their larger body size allows for sufficient volumes of blood to be drawn repeatedly, as well as better spatial resolution of the brain and heart tissue during in vivo imaging (21). Additionally, rats offer a well-established model for cognitive aging that mimics individual differences seen in humans (22).
A potential bias in rodent studies using blood-based epigenetic clock assessment is that laboratory rodents are frequently affected by hematopoietic malignancies. Indeed, hematological cancers are the most frequent cause of death in aged mice and rats (23). This problem is especially pronounced with Fischer 344 (F344) rats, a strain widely used in aging research in general and first used to calculate a rat epigenetic clock (9). F344 rats often spontaneously develop large granular lymphocyte leukemia (LGLL), which is characterized by atypical mononuclear cells in spleen tissue and circulating blood; in more advanced cases, lesions are seen in the liver and then in the bone marrow (24). Rats with LGLL display marked hemolytic anemia and leukocytosis (25). LGLL typically emerges in F344 populations at 18 months of age and is the leading cause of death after 20 months (24,26). The goal of the present study was to assess the impact of hematopoietic disease on epigenetic clock function using spontaneous LGLL in aged F344 rats. In particular, we sought to address (a) whether the presence of LGLL would accelerate epigenetic aging, and (b) how it would affect the precision and CpG composition of newly generated epigenetic clock formulas.
Method
Animals
All animals used herein were part of a previously published study (9) whose procedures were approved by the Institutional Animal Care and Use Committee of the National Institute on Aging Intramural Research Program (NIA-IRP) in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. In brief, male F344 CDF rats were obtained from the NIA Aged Rodent Colony. Rats were singly housed after arrival at the NIA-IRP animal facility, where they had ad libitum access to standard house chow and water and were maintained on a 12 hours on–12 hours off light cycle. A total of 162 animals were used in the study (6 for each month of age between 1 and 27 months). Three animals died before a blood sample could be collected and another 2 died after the blood was collected but before the scheduled necropsy for liver and spleen collection. One juvenile rat and one middle-aged rat died prematurely and were therefore excluded from subsequent analysis. Rats assessed for LGLL were euthanized within 4 weeks of blood draw. At the time of euthanasia, internal organs were dissected and stored in neutral buffered 10% formalin for subsequent pathological analysis.
LGLL Staging
Previous research indicates that the presence of LGLL is extremely rare before the age of 18 months (24). Therefore, we assessed LGLLpathology in animals 17 months of age and older (n = 61). As a negative control, we also evaluated spleens and livers of six 6-month-old rats and six 12-month-old rats. Following established diagnostic procedures (26), organs were cut into 5–6 µm sections and stained with hematoxylin and eosin (H&E; Histoserv, Inc., Germantown, MD). Each slide was evaluated on a light microscope at progressively higher magnifications from 4× to 40× by a member of the study team (G.E.F.) and reviewed by a certified veterinary pathologist (L.R.B.). Disagreements were resolved by joint review and discussion. Splenic tissues were assessed for infiltration of the red pulp by neoplastic cells and effacement of the white pulp; liver tissues were assessed for infiltration of hepatic sinusoids by neoplastic cells and degeneration of hepatocytes. Each animal was assigned a score of 0 (absence of LGLL) to 3 on the basis of established criteria (27–29), summarized in Table 1. Rats were not evaluated for other age-related pathologies aside from LGLL.
Table 1.
Descriptions of Splenic and Liver Pathology by Large Granular Lymphocyte Leukemia Stage
| Stage | Description |
|---|---|
| 0 | Lesion free. |
| 1 | Leukemic cells in the splenic red pulp. |
| 2 | Infiltration of splenic red pulp with leukemic cells and evident effacement of the marginal zone. Leukemic cells evident in the hepatic sinusoids. |
| 3 | Severe infiltration of the splenic red pulp with effacement of the marginal zone and peri-arteriolar lymphatic sheath, resulting in complete loss of splenic architecture. Centrilobular hepatocellular degeneration and necrosis are evident. |
Adopted From Ref. (29).
DNA Methylation Scores
The raw data and procedures used to generate methylation scores for these samples have already been described (9). In brief, blood was treated with proteinase K and RNAse A, followed by DNA extraction. For each rat, a single sample of 100 ng DNA was digested with Msp I and ligated to TruSeq barcoded adapters. DNA fragments between 200 and 300 bp were selected with magnetic beads, bisulfite-treated, PCR-amplified, and sequenced on an Illumina HiSeq2500 sequencer (8 libraries per lane) with 100 bp single-end reads. Sequences were trimmed of adapter DNA using CutAdapt, aligned with BS-Seeker2, and methylation beta values calculated at all cytosine residues. Aligned data were filtered to keep only sites with greater than 10× coverage in at least 80% of the samples. Samples that failed bisulfite sequencing quality control or had more than 20% missing methylation scores were excluded (Supplementary Table 1), leaving a total of 84 young rats, 27 aged rats free of LGLL (“LGLL−”), and 24 aged rats with LGLL at any stage (“LGLL+”). Subsequent methylation analysis was restricted to the 3 002 770 sites with complete coverage in all 135 analyzed rats. Genomic annotations were derived of database “rn6” from the Rat Genome Sequencing Consortium.
Statistical Analysis
Analysis was performed using GraphPad Prism or R on an NIA Computational Biology Core high-performance workstation. Threshold for statistical significance in epigenome-wide linear modeling was adjusted using Bonferroni correction to account for a number of tests (methylation at 3 × 106 cytosine residues). Predictor selection was performed using the elastic net algorithm implemented in the R package “glmnet” (30), with alpha fixed at 0.5 to weight penalization midway between ridge and LASSO. The regularization parameter lambda was adjusted to minimize mean-squared error using either 5-fold or 10-fold cross-validation, depending on whether the modeling was performed only on the 51 aged rats or on the all-ages 135 rat cohort, respectively. A probability threshold of 0.5 was used to interpret predictions from logistic regression models. Identification of de novo DNA motifs was performed using the HOMER (31) function “findMotifsGenome” with a window of 200 bp surrounding target sites.
Monte Carlo analyses (Figures 3 and 4) contained 200 simulations, each with 84 rats, a number representing 75% of the total LGLL− population. Half of the simulations contained 84 randomly selected LGLL− rats. The other half (“mixed”) contained 18 randomly selected LGLL+ (all 3 stages) rats and 66 randomly selected LGLL− rats, thereby maintaining a consistent ratio of LGLL− and LGLL+ rats in each simulation. To test LGLL classification models (Supplementary Figures 4 and 5), a series of Monte Carlo analyses were run, each with 100 simulations. In each simulation, 75% of LGLL+ and 75% of aged LGLL− rats were partitioned into a training set to build a model, which was then applied to the remaining test set animals and evaluated for sensitivity and specificity. LGLL presence was treated as a binary condition in all Monte Carlo analyses.
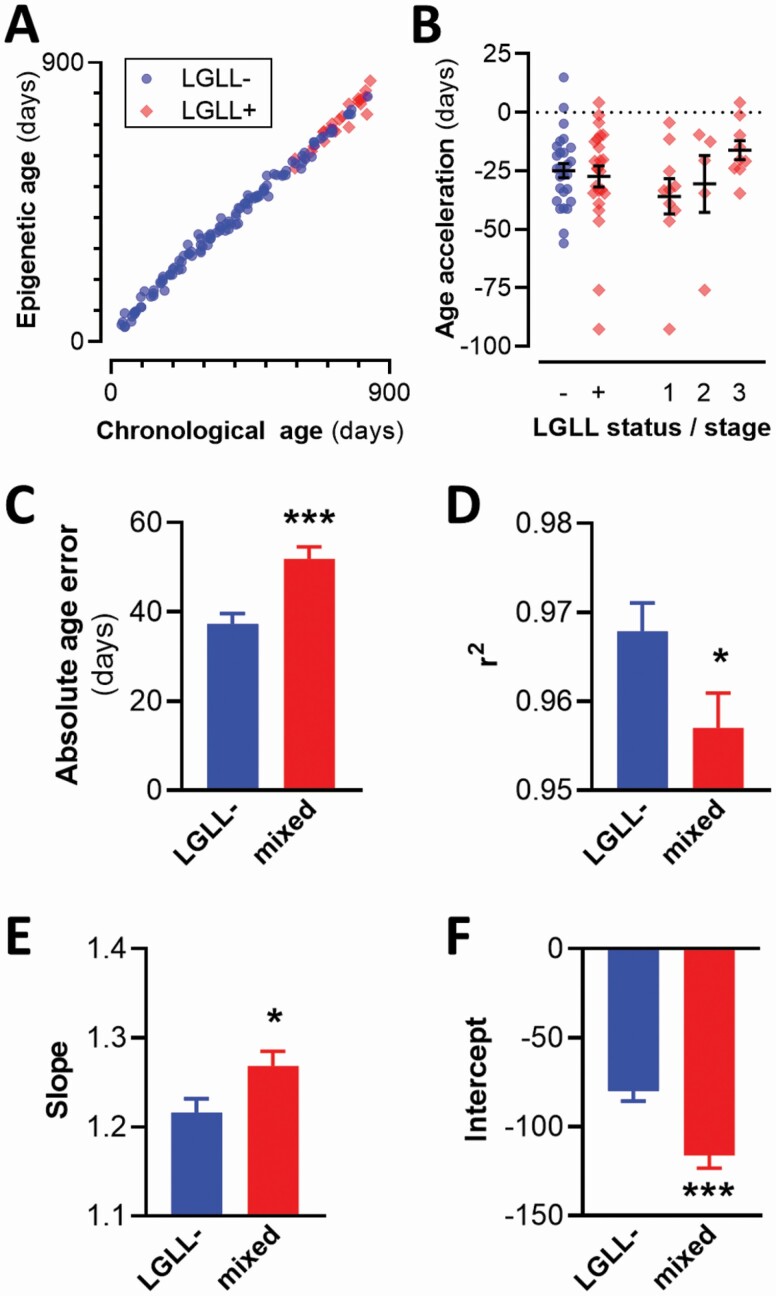
Figure 3.
LGLL reduces epigenetic clock precision without accelerating epigenetic aging. (A) Sample epigenetic clock trained on all rats, with chronological and epigenetic ages plotted. r2 = 0.99. (B) Age acceleration of rats presented in panel A, grouped by LGLL status and score. (C–F) Results from 2 sets of 100 simulations generating epigenetic clock models trained exclusively on LGLL− rats or on mixed populations that included LGLL− and LGLL+ rats from all 3 stages of pathology. Each training set contained 84 randomly selected rats; proportions of LGLL+ and LGLL− were constant in the mixed population simulations. (C) Comparison of absolute age error. (D–F) Evaluation of models fitting actual age versus epigenetic age: fit (D), slope (E), and intercept (F). All plots are shown as mean ± SEM. LGLL = large granular lymphocyte leukemia. *p <.05; ***p <.001.
Figure 4.
LGLL affects elastic net predictor selection during epigenetic clock generation. (A) Scatter plot of individual cytosine residues selected in epigenetic clock models. X-axis, rate of selection in 100 models trained exclusively on LGLL− rats. Y-axis, rate of selection in 100 models trained with mixed populations containing both LGLL+ (all 3 stages) and LGLL− rats. Diagonal line indicates equivalent selection rate (slope = 1); sites below it were biased toward usage in LGLL− simulations; sites above the line were biased toward usage in mixed simulations. Lightly-colored squares/diamonds close to the diagonal line represent sites where bias was not statistically significant. Gray circles represent selection rates from an additional 100 simulations with randomly permuted LGLL scores. (B) Scatter plot of regions of DNA, defined as stretches of DNA with no gaps between residues greater than 250 bp. X-axis, the total number of sites within region used as predictors across 100 simulations training epigenetic clock models on populations of LGLL− rats. Y-axis, number of sites used as predictors in 100 simulations with mixed populations of LGLL− and LGLL+ rats. (C) Net selection bias measures of regions plotted in panel B. Net selection bias is calculated as the absolute value of difference between the total number of sites selected in mixed simulations versus LGLL− simulations. The top 50 regions from real data (diamonds and squares) and random permutations (gray circles) are listed in rank order. (D) Selection rates of individual sites present within the region of DNA immediately upstream of the Trnak-cuu gene. Chromosomal annotations are drawn to scale; reduced representation bisulfite sequencing (RRBS) coverage DNA fragment is 344 bp long. LGLL = large granular lymphocyte leukemia.
Influence of LGLL on elastic net predictor selection was assessed by calculating selection bias, defined as the number of times a particular site or region was selected in 100 simulations with LGLL-training data, subtracted by the number of times it was selected in corresponding mixed population simulations. A large number of CpG sites were used in very few models; to prevent our analysis from overemphasizing small biases at sites rarely used (lower-left corner in Figure 4A), we restricted the analysis to sites selected in at least 10% of simulations. To assess whether a bias was statistically significant, we used a random permutation-based false discovery rate (FDR) approach. We ran 100 additional simulations in which LGLL scores were randomly permuted, and used the observed standard deviation of bias scores in this data set to normalize scores from real and permuted data into number of standard deviations beyond the null hypothesis (bias = 0). Treating these as z-scores, we calculated p values based on a normal distribution and took the ratio of indexed real:permuted p values to compute FDR-adjusted q values. Cutoff for significance was set at q < .05.
Results
LGLL Pathology
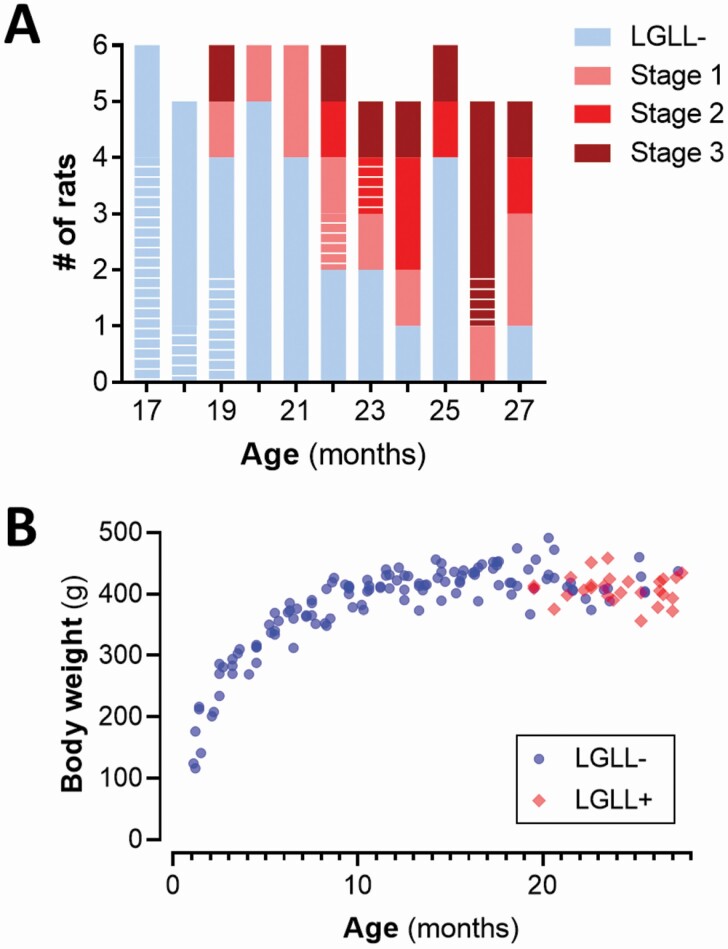
Among the 61 rats older than 17 months evaluated for the LGLL staging, 27 had morphological changes consistent with LGLL (Supplementary Figure 1): 11 rats at Stage 1, 6 rats at Stage 2, and 10 rats at Stage 3 (Figure 1A). Within this cohort, LGLL+ rats skewed older, p = .0007. LGLL was not associated with a significant difference in body weight (Figure 1B; age-adjusted effect of LGLL p = .33).
Figure 1.
LGLL prevalence by age. (A) Prevalence of LGLL stage by age of rats. White-stippled bar segments indicate rats that were assessed for LGLL but excluded from final DNA methylation analysis. (B) Individual body weights of rats. LGLL = large granular lymphocyte leukemia.
LGLL-Associated Changes in DNA Methylation
Global methylation, calculated by averaging methylation scores across all 3 million sites, displayed greater variability among LGLL+ rats than aged LGLL− controls (0.0596 ± 0.0025 [SD] vs 0.0690 ± 0.0006; F23,26 = 17.69, p < .0001). Methylation at individual cytosines was more variable in LGLL+ rats (Figure 2; Supplementary Table 2); the standard deviation of methylation scores was increased by at least 0.1 at 6 689 sites but similarly decreased at only 51 sites (exact binomial test p <2e−16). The increase in variability was not likely a byproduct of the older age of LGLL+ rats, because methylation variability was nearly identical in non-LGLL rats above or below the group median age (Supplementary Figure 2A). Additionally, the influence of LGLL on DNA methylation variability was stronger, not weaker, when the analysis was restricted to rats 22 months of age or older (Supplementary Figure 2B), a subgroup in which there was not an age difference between LGLL+ and LGLL− rats (p = .3). None of the 3 million CpG sites examined displayed average methylation that differed significantly between LGLL+ and LGLL− rats (Figure 2C). However, restricting the analysis to rats with the most advanced stage of LGLL did reveal a limited number of differentially methylated sites (Supplementary Figure 3 and Supplementary Table 3).
Figure 2.
Increased variability in CpG methylation in rats with LGLL. (A) Scatter plot of 3 million individual cytosine residues. X-axis, difference in the standard deviation of methylation fraction between LGLL+ and LGLL− rats. Points to the right of zero (vertical line) indicate sites with greater variability in LGLL+ rats. Y-axis, average methylation fraction in all animals. Inset, individual methylation scores at representative CpG site demonstrating increased variability in LGLL+ rats. (B) Frequency distribution of sites based on the standard deviations of methylation scores. (C) Manhattan plot depicting the age-adjusted influence of LGLL on average methylation at individual CpG sites across rat genome. Horizontal line at top of graph indicates the Bonferroni-adjusted threshold for statistical significance. LGLL = large granular lymphocyte leukemia; CpG = cytosine–guanine.
We performed HOMER analysis to identify transcription factor binding motifs enriched close to CpG sites affected by LGLL. To obtain statistically significant and robust motifs, large numbers of sites are required. Therefore, we used 2 approaches to broaden the definition of differentially methylated CpG sites: grouping all LGLL stages together or splitting LGLL Stage 3, and relaxing statistical thresholds for positive identification (Supplementary Table 4). The most significant hit in all cases contained a CCGG consensus sequence that appeared in close proximity to both hyper- and hypo-methylated CpG sites and was predicted to bind the transcription factors Elk4 or Elf1.
To test whether there might be a signal across a constellation of sites that is not evident at individual CpG residues, we attempted multivariate modeling using linear and nonlinear approaches. Linear and logistic elastic net regression models (Supplementary Figure 4), as well as support vector machine and decision tree modeling (Supplementary Figure 5), all failed to reliably identify rats with LGLL. Most models had poor sensitivity, usually only identifying leukemic status in rats with Stage 3 LGLL. Collectively these data suggest that LGLL introduces random variability in DNA methylation, with a systematic signal only becoming evident at the most advanced stage of disease pathology.
Impact of LGLL on the Epigenetic Clock
We next explored the influence of LGLL on epigenetic clock modeling. We first generated a new epigenetic clock model using elastic net regularization trained on all rats (Figure 3A). LGLL was not associated with a significant change in age acceleration/deceleration (Figure 3B). Even the subset of rats with Stage 3 LGLL did not display significant age acceleration/deceleration (Figure 3B). To further explore the influence of LGLL on epigenetic clock modeling, we ran 100 pairs of analyses on randomly selected subsets of rats. In each analysis, we constructed 2 epigenetic clock models: one trained exclusively on LGLL− rats, the other on a mix of LGLL+ (all stages) and LGLL− rats, using identical proportions in each simulation. All models were trained on 84 rats, a number representing 75% of the total LGLL− population. When each rat’s average epigenetic age was taken across simulations using mixed populations, there was again no significant effect of LGLL on age acceleration or absolute age error (Supplementary Figure 6). Predictions from each epigenetic clock model were fitted to their respective training data for evaluation. Models trained on populations containing LGLL+ rats had worse precision, most evident in a 39% increase in absolute age error (Figure 3C). Furthermore, the formulas fitting actual age as a function of epigenetic age from simulations containing LGLL+ rats had significantly lower Pearson correlation coefficients (Figure 3D), slopes further from 1 (Figure 3E), and intercepts further from 0 (Figure 3F). Training a final epigenetic clock model on all 111 LGLL− rats resulted in a model containing 129 CpG sites (Supplementary Table 5), 117 of which were common to models generated in the Monte Carlo simulations. This model was very well fit to its training set (r2 = 0.999; mean absolute age error 7.19 days).
The 200 regression models generated in the Monte Carlo simulations used anywhere from 31 to 127 individual cytosine residues’ percent methylation as predictors. A total of 4 612 unique sites were selected at least once as predictors for epigenetic clock models. Only 12 of these sites were significantly affected by Stage 3 LGLL using FDR criteria; none were among the 18 sites significant at the more stringent Bonferroni level. These results suggest a minimal overlap between epigenetic responses to aging and LGLL.
Many sites used in the Monte Carlo simulations showed selection bias in which the rate of inclusion in models differed in simulations trained on LGLL-rats versus mixed populations containing both LGLL− and LGLL+ rats (Figure 4A). To identify sites with statistically significant bias, we used a permutation-based FDR adjustment, comparing observed results to 100 simulations in which rats’ LGLL scores were randomly permuted. We found that 83 of the 123 CpG sites appearing in at least 10% of simulations showed significant selection bias at the q < .05 level. The most dramatic bias in selection was for the cytosine at position 1:216661895 in exon 2 of the Cdkn1c gene. This CpG was selected as a predictor in 98/100 LGLL- simulations, but only 6/100 simulations trained on mixed populations. Most sites with significant bias were selected less frequently when LGLL+ rats were introduced into training data (Figure 4A; points below diagonal line). There were fewer sites biased toward selection in mixed population simulations (above diagonal); interestingly, 2 of the sites showing the greatest bias in this direction were in close proximity to one another, immediately upstream of the Trnak-cuu transcription start site.
The observation of clustered sites with similar selection bias propelled us to look at more broadly defined regions of DNA in which predictor selection by elastic net was affected by LGLL. We operationally defined a “region” of DNA to continue until the first gap of more than 250 bp between sites, a threshold chosen based on the distribution of observed site–site gaps (Supplementary Figure 7). We then added the total number of sites selected within each region across all 100 simulations for each population type (Figure 4B). We found that 50 of 82 analyzed regions had a bias that was statistically significant at the FDR-adjusted q < .05 level. A majority of the most biased regions were selected more in the LGLL− only simulations (Figure 4C), meaning that the inclusion of LGLL+ rats in a training set reduces the likelihood that they are selected as predictors in model generation. The single most biased region was a 344 bp fragment of DNA immediately upstream of the Trnak-cuu transcription start site containing 5 individual cytosine residues that were used as predictors in epigenetic clock models. All 5 of these residues showed bias toward mixed populations (Figure 4D). Age-adjusted methylation scores in this region were not significantly affected by LGLL status, whether analyzed individually (p > .39) or averaged into a single regional methylation score calculated for each rat (p = .28).
Discussion
Here we show that the presence of LGLL in F344 rats is associated with broadly increased interindividual variability in DNA methylation, assessed by reduced representation bisulfite sequencing at 3 million cytosine residues. A limited number of sites were differentially methylated in the presence of LGLL, but only in rats with an advanced stage of disease pathology. Epigenetic aging was not accelerated in rats with any stage of LGLL. However, the inclusion of leukemic rats in training data reduced the precision of subsequently generated clock models and profoundly affected their CpG composition.
The F344 is one of the 3 rat strains that have been provided by the National Institute on Aging’s Aged Research Colony since its inception in 1974 (32), which may account in part for its widespread use in aging research. F344 rats frequently develop LGLL after 17 months of age, often resulting in death (24,26). It is unclear whether the development of this condition introduces a systematic bias in the epigenetic signal that accompanies biological aging. To address this question, we tested the influence of LGLL on white blood cell DNA methylation in male F344 rats ranging from 1 to 27 months old. We found that LGLL did not affect epigenetic aging, regardless of whether all LGLL+ rats were analyzed together as a group or separated by disease stage. The lack of an effect was surprising, considering that the average survival of an F344 rat from the first identification of leukemic cells in peripheral blood is 5 weeks (33); therefore, in these rats, the epigenetic clock is not a good predictor of survival. The majority of our rats with Stage 2 LGLL had neoplastic cells visible in the liver. As LGLL has been shown to originate in the spleen (24), liver involvement indicates that neoplastic cells were circulating in the peripheral blood. Indeed, there was minimal overlap between the sites with significantly different methylation in the presence of Stage 3 LGLL and the sites selected in 200 simulations of epigenetic clock formula generation. These results indicate that epigenetic age is a valid measure in F344 rats even without assessment for LGLL pathology, although its association with mortality is questionable.
In contrast to the minimal impact on epigenetic age calculations, the inclusion of LGLL+ rats in training sets profoundly affected the creation of epigenetic clock formulas. These models had reduced precision in accurately calculating chronological age across all rats. Interestingly, the lack of precision in our models trained on mixed populations of rats appeared to be driven at least in part by increased variability in the methylation scores of LGLL+ rats at certain residues, where methylation was otherwise strongly associated with chronological age. In addition to reduced precision, these models used different CpG sites as predictors. Because hematopoietic neoplasia is common in aged mice and rats (23), these results suggest that caution should be employed when analyzing CpG composition of rodent epigenetic clocks.
Much has been discussed on the use of epigenetic clocks in humans to estimate mortality. While it has been clearly demonstrated that epigenetic age acceleration—the discrepancy between epigenetic clock prediction and actual chronological age—can predict health outcomes including mortality, the effect size of this information is relatively small. For example, a human study found that the epigenetic clock was modestly sensitive to risk factors for coronary heart disease, but did not predict outcomes (34). Furthermore, a mouse study using different pools of CpGs and selection algorithms found an explicit trade-off between model accuracy in predicting chronological age and sensitivity to antiaging dwarfism mutations (35). Newer generation epigenetic clocks that are tuned on health characteristics instead of age perform better, especially in predicting mortality (36), but their reliability is still too small for translational purposes.
This is the first study to document epigenetic changes associated with spontaneous LGLL in F344 rats. We found an increase in variability of methylation at individual sites, reminiscent of observations in human acute myeloid leukemia and several leukemic cell lines (37). We could not identify any individual cytosine bases where there were reliable changes in methylation that occurred broadly across all stages of LGLL. This may be because of low signal to noise due to healthy cells with presumably normal patterns of DNA methylation; abnormal leukocytes account for anywhere from 20% to 90% of circulating white blood cells in rats with LGLL at varying stages of progression (38). When we restricted the analysis to the most advanced stage of LGLL, we found a limited number of differentially methylated sites. The most significantly associated site was hypermethylated in rats with LGLL and located 70 bp upstream of the transcription start site for the inositol transporter Slc2a13. Interestingly, a human study found that expression of SLC2A13 in bone marrow was a prognostic indicator of survival time in patients with acute myeloid leukemia (39). Under the traditional assumption that hypermethylation at the promoter of a gene suppresses transcription, our findings are congruent with the observation in humans. Several other sites that we found to be most significantly altered in LGLL have been implicated in various human cancers, though not necessarily related to hematopoietic tissues. TUBB4A expression is a prognostic indicator in clear cell renal cell carcinoma (40). MEIS2 is hypermethylated and transcriptionally downregulated in prostate cancer, and its methylation status is a prognostic biomarker for recurrence (41). NFIX, which regulates hematopoietic lineage specification (42), is proposed as a “master regulator” for metastasis in lung cancer (43). Finally, our data identified DNA-binding motifs for Elk4 protein proximal to sites with altered methylation. This transcription factor is associated with a number of human cancers (44,45). Collectively these results suggest that epigenetic events in rat LGLL bear similarities to a variety of human cancers involving both blood and solid tumors.
There were several important limitations to this study. First, we used only male rats due to availability from the NIA Aged Rodent Colony at the time this work was conducted. LGLL has been documented in female rats (24,26), and it would be important for future work to explore potential sex differences in the phenomena addressed here. Second, we did not assess rats for other age-related pathologies; rats with advanced stages of LGLL might be expected to develop complications due to impaired immune function. Thus, while the LGLL associated changes that we documented may be direct consequences of transformation within white blood cells, we cannot exclude the possibility that they are mediated indirectly through other health complications. Finally, sample sizes in certain analyses were limited. There were only 9 rats with Stage 3 LGLL in our sample, which may have reduced the generalizability of some of our findings. In most analyses, we combined all LGLL stages together into a single group (n = 24); however, to identify differentially methylated CpG sites, we had to isolate rats with Stage 3 LGLL (n = 9). Nevertheless, the key findings of our study—the impact of LGLL on the architecture and function of epigenetic clock models—remain.
In summary, we show that LGLL, a disease that is common in aged F344 rats, has a broad impact on methylation variability in white blood cell DNA. Despite this, the presence of LGLL did not accelerate epigenetic aging. Given that rats with LGLL survive only for a few weeks and that as many as 50% of F344 rats develop LGLL, our findings also suggest a strong disconnect between epigenetic signals associated with chronological aging and risk of death. However, the inclusion of LGLL+ rats adversely affected the precision and composition of newly generated epigenetic clock formulas. Thus, diverse potential effects of circulating neoplastic cells on epigenetic clock modeling should be considered.
Supplementary Material
Acknowledgments
We thank Toshiko Tanaka for advising on statistical analyses and assistance with the Manhattan plots, Supriyo De for access to NIA Computational Biology Core high-performance workstations, Osorio Meirelles for advising on randomized permutation, Ravi Tharakan for advising on DNA methylation, Brian Clopper for logistical support with rat spleen and liver tissue, and Heather DeMali for digital microscopy imaging.
Contributor Information
Giovanni E Finesso, Comparative Medicine Section, National Institute on Aging—Intramural Research Program, National Institutes of Health, Baltimore, Maryland, USA.
Ross A McDevitt, Comparative Medicine Section, National Institute on Aging—Intramural Research Program, National Institutes of Health, Baltimore, Maryland, USA.
Roshni Roy, Laboratory of Molecular Biology and Immunology, National Institute on Aging—Intramural Research Program, National Institutes of Health, Baltimore, Maryland, USA.
Lauren R Brinster, Office of Research Services, Division of Veterinary Resources, National Institutes of Health, Bethesda, Maryland, USA.
Andrea Di Francesco, Translational Gerontology Branch, National Institute on Aging—Intramural Research Program, National Institutes of Health, Baltimore, Maryland, USA; Calico Life Sciences, South San Francisco, California, USA.
Theresa Meade, Comparative Medicine Section, National Institute on Aging—Intramural Research Program, National Institutes of Health, Baltimore, Maryland, USA.
Rafael de Cabo, Translational Gerontology Branch, National Institute on Aging—Intramural Research Program, National Institutes of Health, Baltimore, Maryland, USA.
Luigi Ferrucci, Translational Gerontology Branch, National Institute on Aging—Intramural Research Program, National Institutes of Health, Baltimore, Maryland, USA.
Kathy A Perdue, Comparative Medicine Section, National Institute on Aging—Intramural Research Program, National Institutes of Health, Baltimore, Maryland, USA.
Funding
This work was supported entirely by the National Institute on Aging Intramural Research Program.
Conflict of Interest
None declared.
Author Contributions
G.E.F., K.A.P., R.D., and L.F. conceived the project. K.A.P. and T.M. performed necropsies and collected rat tissue; L.R.B. and G.E.F. assessed pathology. Statistical analyses were designed and performed by L.F. and R.A.M. HOMER motif identification was performed by R.R. A.D. purified nucleic acids and assisted with interpretation of results. The manuscript was written by R.A.M., L.F., K.A.P., and G.E.F. with assistance from all coauthors.
References
- 1. Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102(30):10604–10609. doi: 10.1073/pnas.0500398102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bocklandt S, Lin W, Sehl ME, et al. Epigenetic predictor of age. PLoS One. 2011;6(6):e14821. doi: 10.1371/journal.pone.0014821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maegawa S, Hinkal G, Kim HS, et al. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 2010;20(3):332–340. doi: 10.1101/gr.096826.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–367. doi: 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maierhofer A, Flunkert J, Oshima J, Martin GM, Haaf T, Horvath S. Accelerated epigenetic aging in Werner syndrome. Aging (Albany NY). 2017;9(4):1143–1152. doi: 10.18632/aging.101217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Horvath S, Levine AJ. HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis. 2015;212(10):1563–1573. doi: 10.1093/infdis/jiv277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen BH, Marioni RE, Colicino E, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY). 2016;8(9):1844–1865. doi: 10.18632/aging.101020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levine M, McDevitt RA, Meer M, et al. A rat epigenetic clock recapitulates phenotypic aging and co-localizes with heterochromatin. Elife. 2020;9: e59201. doi: 10.7554/eLife.59201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Z, Leung D, Thrush K, et al. Underlying features of epigenetic aging clocks in vivo and in vitro. Aging Cell. 2020;19(10):e13229. doi: 10.1111/acel.13229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malousi A, Andreou AZ, Georgiou E, Tzimagiorgis G, Kovatsi L, Kouidou S. Age-dependent methylation in epigenetic clock CpGs is associated with G-quadruplex, co-transcriptionally formed RNA structures and tentative splice sites. Epigenetics. 2018;13(8):808–821. doi: 10.1080/15592294.2018.1514232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–139. doi: 10.1038/ni1033 [DOI] [PubMed] [Google Scholar]
- 13. Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol. 2016;83(4):255–266. doi: 10.1111/sji.12413 [DOI] [PubMed] [Google Scholar]
- 14. Hu D, Shilatifard A. Epigenetics of hematopoiesis and hematological malignancies. Genes Dev. 2016;30(18):2021–2041. doi: 10.1101/gad.284109.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dugué PA, Bassett JK, Joo JE, et al. DNA methylation-based biological aging and cancer risk and survival: pooled analysis of seven prospective studies. Int J Cancer. 2018;142(8):1611–1619. doi: 10.1002/ijc.31189 [DOI] [PubMed] [Google Scholar]
- 16. Søraas A, Matsuyama M, de Lima M, et al. Epigenetic age is a cell-intrinsic property in transplanted human hematopoietic cells. Aging Cell. 2019;18(2):e12897. doi: 10.1111/acel.12897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petkovich DA, Podolskiy DI, Lobanov AV, Lee SG, Miller RA, Gladyshev VN. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 2017;25(4):954–960.e6. doi: 10.1016/j.cmet.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stubbs TM, Bonder MJ, Stark AK, et al. ; BI Ageing Clock Team . Multi-tissue DNA methylation age predictor in mouse. Genome Biol. 2017;18(1):68. doi: 10.1186/s13059-017-1203-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang T, Tsui B, Kreisberg JF, et al. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. 2017;18(1):57. doi: 10.1186/s13059-017-1186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coninx E, Chew YC, Yang X, et al. Hippocampal and cortical tissue-specific epigenetic clocks indicate an increased epigenetic age in a mouse model for Alzheimer’s disease. Aging (Albany NY). 2020;12(20):20817–20834. doi: 10.18632/aging.104056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellenbroek B, Youn J. Rodent models in neuroscience research: is it a rat race? Dis Model Mech. 2016;9(10):1079–1087. doi: 10.1242/dmm.026120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gallagher M, Stocker AM, Koh MT. Mindspan: lessons from rat models of neurocognitive aging. ILAR J. 2011;52(1):32–40. doi: 10.1093/ilar.52.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Snyder JM, Ward JM, Treuting PM. Cause-of-death analysis in rodent aging studies. Vet Pathol. 2016;53(2):233–243. doi: 10.1177/0300985815610391 [DOI] [PubMed] [Google Scholar]
- 24. Stromberg PC, Vogtsberger LM. Pathology of the mononuclear cell leukemia of Fischer rats. I. Morphologic studies. Vet Pathol. 1983;20(6):698–708. doi: 10.1177/030098588302000605 [DOI] [PubMed] [Google Scholar]
- 25. Stromberg PC, Vogtsberger LM, Marsh LR. Pathology of the mononuclear cell leukemia of Fischer rats. III. Clinical chemistry. Vet Pathol. 1983;20(6):718–726. doi: 10.1177/030098588302000607 [DOI] [PubMed] [Google Scholar]
- 26. Goodman DG, Ward JM, Squire RA, Chu KC, Linhart MS. Neoplastic and nonneoplastic lesions in aging F344 rats. Toxicol Appl Pharmacol. 1979;48(2):237–248. doi: 10.1016/0041-008x(79)90029-2 [DOI] [PubMed] [Google Scholar]
- 27. Losco PE, Ward JM. The early stage of large granular lymphocyte leukemia in the F344 rat. Vet Pathol. 1984;21(3):286–291. doi: 10.1177/030098588402100304 [DOI] [PubMed] [Google Scholar]
- 28. Stefanski SA, Greenwell A, Merrick BA, Brown TT, Reynolds SH. Proliferating cell nuclear antigen staining of Fischer-344/N rat spleens affected by large granular lymphocyte leukemia. Toxicol Pathol. 1995;23(1):1–6. doi: 10.1177/019262339502300101 [DOI] [PubMed] [Google Scholar]
- 29. Maronpot RR, Nyska A, Foreman JE, Ramot Y. The legacy of the F344 rat as a cancer bioassay model (a retrospective summary of three common F344 rat neoplasms). Crit Rev Toxicol. 2016;46(8):641–675. doi: 10.1080/10408444.2016.1174669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zou H, Hastie T. Regularization and variable selection via the elastic net (vol B 67, pg 301, 2005). J R Stat Soc B. 2005;67:768–768. doi: 10.1111/j.1467-9868.2005.00527.x [DOI] [Google Scholar]
- 31. Heinz S, Benner C, Spann N, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–589. doi: 10.1016/j.molcel.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sprott RL. Development of animal models of aging at the National Institute of Aging. Neurobiol Aging. 1991;12(6):635–638. doi: 10.1016/0197-4580(91)90113-x [DOI] [PubMed] [Google Scholar]
- 33. Moloney WC, Boschetti AE, King VP. Spontaneous leukemia in Fischer rats. Cancer Res. 1970;30(1):41–43. https://cancerres.aacrjournals.org/content/30/1/41 [PubMed] [Google Scholar]
- 34. Horvath S, Gurven M, Levine ME, et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17(1):171. doi: 10.1186/s13059-016-1030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thompson MJ, Chwiałkowska K, Rubbi L, et al. A multi-tissue full lifespan epigenetic clock for mice. Aging (Albany, NY). 2018;10(10):2832–2854. doi: 10.18632/aging.101590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany, NY). 2018;10(4):573–591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jelinek J, Liang S, Lu Y, et al. Conserved DNA methylation patterns in healthy blood cells and extensive changes in leukemia measured by a new quantitative technique. Epigenetics. 2012;7(12):1368–1378. doi: 10.4161/epi.22552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stromberg PC, Vogtsberger LM, Marsh LR, Wilson FD. Pathology of the mononuclear cell leukemia of Fischer rats. II. Hematology. Vet Pathol. 1983;20(6):709–717. doi: 10.1177/030098588302000606 [DOI] [PubMed] [Google Scholar]
- 39. Lai B, Lai Y, Zhang Y, Zhou M, Sheng L, OuYang G. The solute carrier family 2 genes are potential prognostic biomarkers in acute myeloid leukemia. Technol Cancer Res Treat. 2020;19:1533033819894308. doi: 10.1177/1533033819894308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu WH, Xu Y, Wang J, et al. Prognostic value and immune infiltration of novel signatures in clear cell renal cell carcinoma microenvironment. Aging (Albany, NY). 2019;11(17):6999–7020. doi: 10.18632/aging.102233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nørgaard M, Haldrup C, Bjerre MT, et al. Epigenetic silencing of MEIS2 in prostate cancer recurrence. Clin Epigenetics. 2019;11(1):147. doi: 10.1186/s13148-019-0742-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O’Connor C, Campos J, Osinski JM, Gronostajski RM, Michie AM, Keeshan K. Nfix expression critically modulates early B lymphopoiesis and myelopoiesis. PLoS One. 2015;10(3):e0120102. doi: 10.1371/journal.pone.0120102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rahman NIA, Abdul Murad NA, Mollah MM, Jamal R, Harun R. NFIX as a master regulator for lung cancer progression. Front Pharmacol. 2017;8:540. doi: 10.3389/fphar.2017.00540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peng C, Zeng W, Su J, et al. Cyclin-dependent kinase 2 (CDK2) is a key mediator for EGF-induced cell transformation mediated through the ELK4/c-Fos signaling pathway. Oncogene. 2016;35(9):1170–1179. doi: 10.1038/onc.2015.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sizemore GM, Pitarresi JR, Balakrishnan S, Ostrowski MC. The ETS family of oncogenic transcription factors in solid tumours. Nat Rev Cancer. 2017;17(6):337–351. doi: 10.1038/nrc.2017.20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.