Abstract
Objectives
ANCA-associated vasculitis (AAV) is a rare autoimmune disorder that commonly involves the kidney. Early identification of kidney involvement, assessing treatment-response and predicting outcome are important clinical challenges. Here, we assessed the potential utility of interval kidney biopsy in AAV.
Methods
In a tertiary referral centre with a dedicated vasculitis service, we identified patients with AAV who had undergone interval kidney biopsy, defined as a repeat kidney biopsy (following an initial biopsy showing active AAV) undertaken to determine the histological response in the kidney following induction immunosuppression. We analysed biochemical, histological and outcome data, including times to kidney failure and death for all patients.
Results
We identified 57 patients with AAV who underwent at least one interval kidney biopsy (59 interval biopsies in total; median time to interval biopsy ∼130 days). Of the 59 interval biopsies performed, 24 (41%) patients had clinically suspected active disease at time of biopsy which was confirmed histologically in only 42% of cases; 35 (59%) patients were in clinical disease-remission, and this was correct in 97% of cases. The clinician’s impression was incorrect in one in four patients. Hematuria at interval biopsy did not correlate with histological activity. Interval biopsy showed fewer acute lesions and more chronic damage compared with initial biopsy and led to immunosuppressive treatment-change in 75% (44/59) of patients. Clinical risk prediction tools tended to operate better using interval biopsy data.
Conclusion
Interval kidney biopsy is useful for determining treatment-response and subsequent disease management in AAV. It may provide better prognostic information than initial kidney biopsy and should be considered for inclusion into future clinical trials and treatment protocols for patients with AAV.
Keywords: ANCA vasculitis, interval kidney biopsy, hematuria
Rheumatology key messages.
Interval kidney biopsy provides important information that guides clinical management of patients with anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV).
Hematuria correlates poorly with disease activity within the kidney.
Current renal risk prediction tools might operate more effectively using interval biopsy parameters.
Introduction
Systemic vasculitis associated with anti-neutrophil cytoplasm antibodies (ANCA), ANCA-associated vasculitis (AAV), is a rare autoimmune disorder characterized by leucocyte infiltration and necrosis of predominantly the small vessels [1]. Kidney involvement is seen in two-thirds of patients, classically as a pauci-immune necrotizing glomerulonephritis with crescents [2]. Induction immunosuppressive treatment for AAV with renal involvement typically involves glucocorticoids alongside cyclophosphamide or rituximab [3]. Once a patient attains disease remission, there follows a course of maintenance immunosuppression.
Identifying AAV early, assessing its response to treatment, and risk-stratifying patients at greatest long-term risk remain important clinical challenges. In those with renal involvement, the measurement of kidney function using serum creatinine is often inadequate because substantial renal damage can occur before function is impaired to a detectable extent [4, 5]. Furthermore, changes in serum creatinine concentration during follow-up may be due to changes in vasculitis disease activity or due to glomerular scarring or tubular injury from infections or medications, scenarios that require different clinical management. Other commonly used measures such as hematuria, circulating CRP and serum ANCA titre lack specificity and sensitivity and do not adequately reflect disease activity [6, 7]. Recent data suggest that glucocorticoid-sparing [8, 9], or indeed glucocorticoid-free [10], regimens are able to treat AAV effectively while reducing treatment-related complications.
As clinicians move towards using lower doses of induction immunosuppression in patients with AAV, confirming treatment-induced disease remission becomes even more important as unchecked smouldering inflammation can lead to the accrual of organ damage and increase long-term mortality. Disease activity scores such as the BVAS [11] have limitations as they are often unable to distinguish active disease from established damage. Interval kidney biopsy is an established component of immunological monitoring in kidney transplantation [12], and its utility in assessing treatment response in lupus nephritis is increasingly recognized [13]. There are, however, few data on the use of interval kidney biopsy in AAV [14].
Here, we assessed the utility of initial and interval kidney biopsy in a large cohort of patients with ANCA-associated glomerulonephritis with long-term follow-up. We defined interval kidney biopsy as a repeat kidney biopsy (following an initial biopsy showing active AAV) undertaken to determine the histological response in the kidney following induction immunosuppression. Specifically, we sought to determine: (i) the correlation between clinically determined and histologically verified disease activity during follow-up; and (ii) the extent to which an interval biopsy leads to changes in clinical management.
Patients and methods
Study design and study participants
We conducted a retrospective cohort study of patients with AAV presenting to the Edinburgh Vasculitis Service and undergoing kidney biopsy. This met the criteria for a service evaluation study and hence did not require approval from a research ethics committee. All patients provided informed consent for treatment and received standard care according to our unit protocol. Patients were included if they had undergone an interval kidney biopsy. An interval biopsy was defined as a repeat kidney biopsy (following an initial biopsy showing active AAV) undertaken to determine the histological response in the kidney following induction immunosuppression.
Standard of care
Within our specialist vasculitis service, induction immunosuppressive treatment for AAV comprises oral glucocorticoids with either intravenous cyclophosphamide or intravenous rituximab, or a combination of cyclophosphamide and rituximab. Plasma exchange is used in some patients, mainly those with pulmonary haemorrhage or those with severe kidney involvement. Choice of immunosuppressive regimen is based on patient and disease factors. In general, patients are transitioned from induction to maintenance immunosuppression ∼3 months following diagnosis. The decision to perform an interval biopsy is made on an individual patient basis; these are not protocol biopsies.
Definitions
The diagnosis of AAV was made in accordance with the 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides Criteria [15]. Disease remission was defined as a BVAS of 0 for at least two months while taking prednisone at a daily dose of ≤7.5 mg in conjunction with the treating clinician’s assessment of clinically silent disease. A major biopsy complication was defined as an adverse event that required hospital admission, blood transfusion, endovascular intervention, surgery, or resulted in death. For biopsy data analysis, a crescent was defined as two or more layers of proliferating cells in Bowman’s space. A normal glomerulus was defined by the absence of necrosis, crescent formation, endocapillary proliferation and segmental or global glomerulosclerosis. Glomeruli with minor mesangial or ischaemic changes only were included in this category. Interstitial fibrosis and tubular atrophy (IFTA) and interstitial nephritis were defined as mild (<25%), moderate (25–50%) and severe (>50%). Active AAV kidney disease on interval biopsy was defined by the presence of necrotizing lesions and/or cellular crescents. End-stage kidney disease was defined according to the Kidney Disease: Improving Global Outcomes definition [16] as an estimated glomerular filtration rate (eGFR) <15 mL/min/1.73m2 for 3 months, dialysis for 3 months or receipt of a kidney transplant.
Collection of clinical and histological data
Baseline demographic data were collected at date of initial kidney biopsy. Biochemical data were collected at presentation and at 12-monthly intervals to a total of 36 months follow-up. We accepted a range of 3 months on either side of the intended date. eGFR was calculated using the CKD Epidemiology Collaboration (CKD-EPI) formula. Biochemical data were also collected at time of interval biopsy. Urinalysis data were collected from prior to the initial and interval biopsy.
All kidney biopsies were reviewed by an experienced nephropathologist. Biopsy reports were reviewed, and information related to glomeruli (total number and number of necrotizing lesions, cellular crescents, fibrocellular crescents and global glomerulosclerosis), IFTA and interstitial nephritis were collected. Clinical impression at time of biopsy was determined by review of clinical correspondence.
Renal risk classifications were determined for all patients in accordance with the criteria outlined by Berden et al. and Brix et al. for both initial and interval kidney biopsies [17, 18]. In brief, the Berden classification categorized biopsies according to whether 50% or more glomeruli on biopsy were normal (focal), contained cellular crescents (crescentic) or were globally sclerosed (sclerotic). If there was no predominant feature, then biopsies were classified as mixed. Biopsies containing <10 glomeruli were excluded from Berden classification. The Brix Risk Score was based upon the percentage of normal glomeruli, the percentage of IFTA and the eGFR at time of biopsy and categorized patients into low, medium or high risk.
Statistical analysis
Data were tested for normality with log transformation as appropriate. Data are presented as median (interquartile range) or mean (s.e.m.) where indicated. Comparison of continuous variables was performed by Student’s t test, Mann–Whitney test or Wilcoxon rank sum test as appropriate or mixed effects model if ≥3 groups with post-hoc Šídák multiple comparisons test. Kaplan–Meier curves analysed by log-rank (Mantel–Cox) test. Comparison between categorical variables was by a χ2 test or Fisher’s exact test where appropriate. Results were considered significant if two-sided P < 0.05. Statistical analysis was performed using Prism (version 9, GraphPad Software Inc, San Diego, CA, USA) and R (version 3.4, R Foundation, Vienna, Austria).
Results
Patient characteristics
We identified 218 patients with AAV who underwent a kidney biopsy. Of these, 57 patients underwent interval kidney biopsy. Of those, 55 patients had one interval kidney biopsy and two patients had two interval kidney biopsies. For the purposes of our analysis, the patients who had two interval biopsies were counted twice. Their index biopsy (showing active AAV) was unique to each interval biopsy.
Baseline patient demographics are shown in Table 1 and Supplementary Table S1, available at Rheumatology online. The median time to interval biopsy was 130 days. All patients had completed induction immunosuppression by the time of interval biopsy. Patients undergoing interval kidney biopsy were younger and were more likely to have been given a cyclophosphamide-based induction immunosuppression regimen and were less likely to have received rituximab. Compared with time of initial kidney biopsy, patients had improved biochemical, hematological and immunological parameters at the time of interval kidney biopsy (Supplementary Table S2 and Supplementary Fig. S1, available at Rheumatology online). Of the nine patients requiring renal replacement therapy at disease presentation, only one patient was receiving this at the time of interval biopsy. There were no major complications from kidney biopsy in our cohort.
Table 1.
Baseline demographics
| Baseline characteristics | Interval biopsy (n = 59) |
|---|---|
| Age (years) | 60 (48–65) |
| Male | 30 (51%) |
| Autoantibody status | |
| MPO positive | 27 (46%) |
| PR3 positive | 24 (41%) |
| ANCA negative | 3 (5%) |
| Dual positive | 5 (8%) |
| Organ involvement | 3 (1–3) |
| Kidney | 59 (100%) |
| Lung | 27 (46%) |
| ENT | 19 (32%) |
| Nerve | 14 (24%) |
| Joint | 10 (17%) |
| Eye | 12 (20%) |
| Other | 11 (19%) |
| Blood results | |
| Creatinine (mg/dL) | 2.53 (1.66–3.56) |
| eGFR (mL/min/1.73m2) | 26 (14–39) |
| Haemoglobin (g/dL) | 9.8 (8.2–10.7) |
| Platelets (x109/L) | 356 (287–455) |
| CRP (mg/L) | 86 (26–151) |
| Urine PCR (mg/mmol) | 141 (88–312) |
| Renal replacement therapy | 9 (15%) |
| Immunosuppression | |
| Glucocorticoid | 59 (100%) |
| Cyclophosphamide | 48 (81%) |
| Rituximab | 20 (34%) |
| PEX | 28 (47%) |
| Time to interval biopsy (days) | 130 (110–170) |
Data are presented as median (interquartile range) or number of patients (%). MPO positive and PR3 positive refer to patients who are solely positive for that specific autoantibody. Dual positive refers to patients who are positive for two out of three of MPO, PR3 and GBM autoantibodies.
eGFR: estimated glomerular filtration rate; PCR: protein creatinine ratio; PR3: proteinase 3.
Histopathological findings
Biopsy data were available for all patients (Table 2 and Fig. 1). In general, there were significantly more normal and globally sclerosed glomeruli on interval biopsy compared with initial biopsy, and fewer necrotizing lesions and crescents (both cellular and fibrocellular). All initial kidney biopsies had some degree of interstitial inflammation whereas ∼20% of interval biopsies had no interstitial inflammation. There was a tendency for interval biopsies to show more extensive IFTA.
Table 2.
Histopathological findings on biopsy
| Biopsy findings | Initial biopsy | Interval biopsy |
|---|---|---|
| (n = 59) | (n = 59) | |
| Total glomeruli (n) | 19 (13–27) | 19 (13–24) |
| Glomeruli (%) | ||
| Normal | 26 (17–48) | 39 (21–60)* |
| Necrotizing lesions | 25 (12–52) | 0 (0–0)* |
| Cellular crescents | 16 (0–33) | 0 (0–0)* |
| Fibrocellular crescents | 0 (0–14) | 0 (0–0)* |
| Globally sclerosed | 7 (0–18) | 22 (9–40)* |
| Interstitial inflammation | ||
| Nil | 0 (0%) | 11 (19%)* |
| Mild | 43 (73%) | 40 (68%) |
| Moderate | 8 (14%) | 7 (12%) |
| Severe | 8 (14%) | 1 (2%)* |
| IFTA | ||
| Mild | 32 (54%) | 21 (36%)* |
| Moderate | 17 (29%) | 19 (32%) |
| Severe | 10 (17%) | 19 (32%) |
Data are presented as median (interquartile range) or number of patients (%). Total glomeruli data are presented as number of glomeruli in sample. All other glomeruli data are presented as percentage of normal glomeruli. Analysis by Wilcoxon matched-pairs signed rank test or χ2 test as appropriate, *P <0.05. IFTA: interstitial fibrosis and tubular atrophy.
Fig. 1.
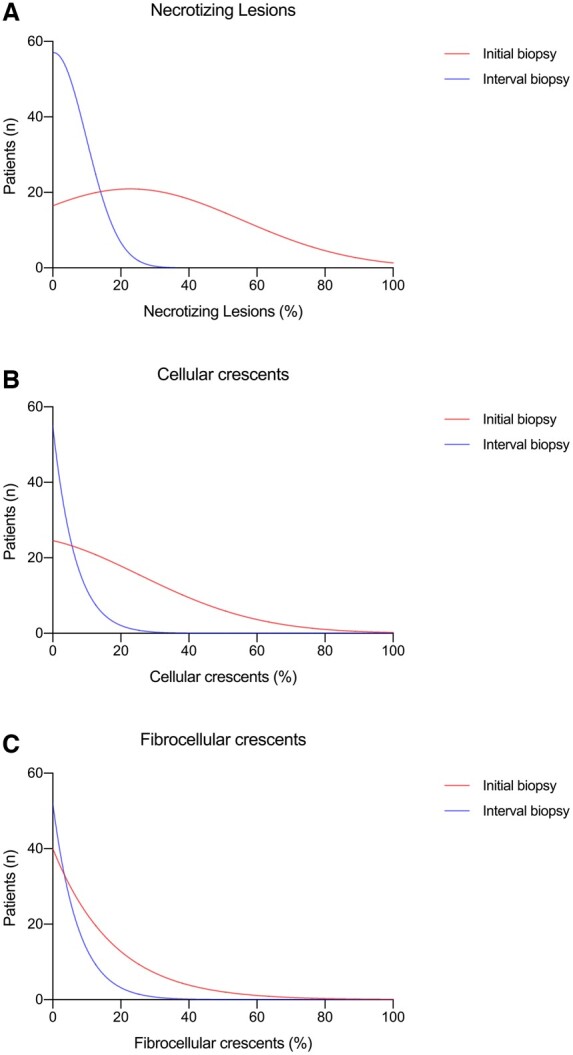
Histopathology of initial and interval kidney biopsy
Distribution of initial (red) and interval (blue) biopsy necrotizing lesions, cellular and fibrocellular crescents.
Interval kidney biopsies showing active AAV (n = 10) had significantly more necrotizing lesions and cellular crescents compared with those interval biopsies showing inactive disease (n = 46) (Supplementary Table S3, available at Rheumatology online). Three biopsies showed a new pathology in the kidney; two showed membranous nephropathy; and one showed a drug-related injury. Although there was a tendency for interval biopsies with active disease to have more extensive interstitial inflammation than those with inactive disease, IFTA was comparable between the two groups.
Clinical impression, interval biopsy findings and changes to immunosuppressive treatment
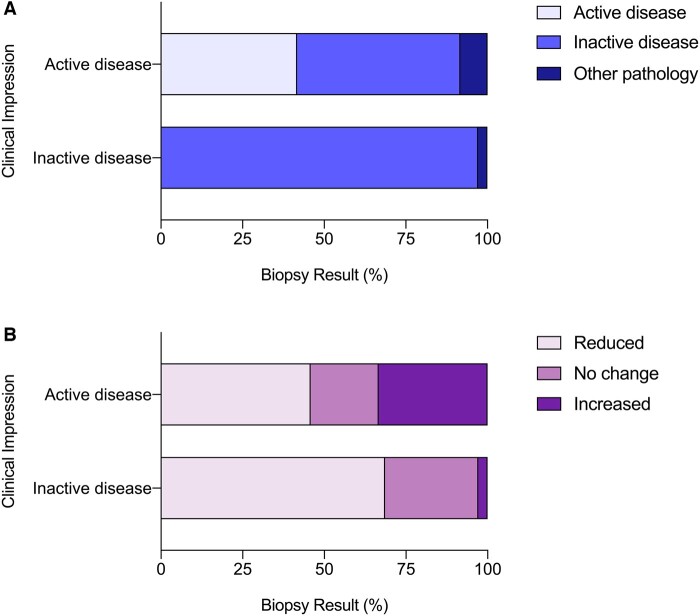
At the time of interval biopsy, 24 patients (41%) were clinically suspected to have ongoing disease activity; 35 patients (59%) were thought to be in clinical disease-remission. Overall, the clinician’s impression was incorrect in one in four biopsies (15/59) (Fig. 2A). For patients with suspected active AAV within the kidney, this was confirmed on biopsy in only 42% (10/24) of cases whereas 50% (12/24) showed inactive disease and 8% (2/24) showed an alternative and new pathology. For patients suspected of having inactive disease, this was confirmed on biopsy in 97% (34/35) of cases while 3% (1/35) showed a new pathology.
Fig. 2.
Biopsy findings and treatment change
The biopsy findings (A) and change in immunosuppression following biopsy (B) for patients who were clinically suspected of active disease (n = 24) or inactive disease (n = 35) are shown.
Following interval biopsy, immunosuppressive treatment was changed in 75% (44/59) of patients (Fig. 2B). Importantly, 46% (11/24) of patients with previously suspected active disease had a reduction in immunosuppression following interval biopsy; similarly, 3% (1/35) of patients with previously suspected inactive disease had an escalation in immunosuppression. Nine patients received augmented immunosuppression. These patients were treated with glucocorticoid (89%), rituximab (78%), cyclophosphamide (44%) and/or plasma exchange (22%). All patients were treated with either rituximab or cyclophosphamide, and two patients were treated with both.
Change in clinical biomarkers and urinalysis at time of interval biopsy
We went on to compare the change in biomarkers from the time of initial to interval kidney biopsy between patients with no evidence of disease activity (n = 49) and those with active disease (n = 10) on interval biopsy. These are biomarkers used routinely by the clinician to aid in the assessment of AAV disease activity (Supplementary Fig. S2, available at Rheumatology online). Patients with inactive disease on interval biopsy had had a significant fall in serum creatinine (–26%) and increase in eGFR (+45%) compared with a 9% increase in serum creatinine and 9% fall in eGFR for those with ongoing disease activity (P <0.05 for between group comparisons for both). Haemoglobin had increased to a greater extent in those with inactive disease compared with those with active disease (+2.4 g/dL vs +0.5 g/dL, P <0.05), and proteinuria had fallen to a greater extent (urine protein: creatinine: –72 mg/mmol vs +54 mg/mmol, P <0.05). There were no differences observed for high-sensitivity C-reactive protein (–88 mg/l vs –44 mg/l, P = 0.35), platelet count (–98 109/l vs –34 109/l, P = 0.07), MPO ANCA titre (–32 IU/ml vs –28 IU/ml, P = 0.91) or PR3 ANCA titre (–125 IU/ml vs –31 IU/ml, P = 0.31). Interestingly, hematuria, often used as a correlate of active kidney disease in AAV [19], did not discriminate between those with inactive or active disease on interval kidney biopsy (Fig. 3); 60% of patients with active disease on interval biopsy had no hematuria on urinalysis whereas 59% of patients with inactive disease on interval biopsy had some degree of hematuria.
Fig. 3.
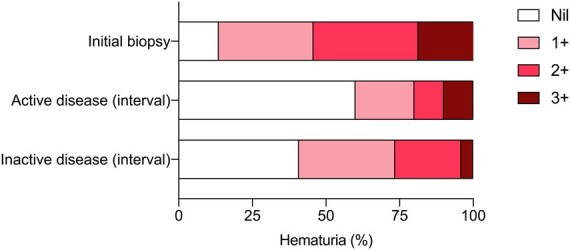
Presence of hematuria on urinalysis
Hematuria data for patients who had an interval biopsy shown at initial biopsy (n=59) and then for those with active disease (n = 10) and inactive disease (n = 49) on interval biopsy. Analysis comparing active disease and inactive disease groups by Chi-square test or Fisher’s exact test as appropriate. No significant differences between groups.
Interval kidney biopsy and long-term kidney function
Of our study cohort, 55 patients had follow-up data to 12 months and 49 patients to 36 months. At 12 months, 7% (4/55) had reached end-stage kidney disease and 4% (2/55) had died. At 36 months, these figures were 6% (3/49) and 8% (4/49), respectively.
Renal risk scoring
We categorized all kidney biopsies using the Berden and Brix classification tools (Fig. 4), respectively [17, 18]. These were developed to enable prediction of long-term kidney outcome based on initial histopathological features, with the Brix score incorporating clinical parameters. When considering all initial and interval biopsies together, and using the Berden Classification, there were no significant changes in the proportion classified as focal (initial vs interval biopsy: 21% vs 36%, P = 0.08), sclerotic (4% vs 15%, P = 0.09) or mixed (62% vs 49%, P = 0.17), whereas significantly fewer interval biopsies were classified as crescentic (13% vs 0%, P <0.05). Using the Brix score, there were no changes in biopsies classified as low (initial vs interval biopsy: 31% vs 36%, P = 0.56), medium (51% vs 53%, P = 0.85) or high risk (19% vs 12%, P = 0.31).
Fig. 4.
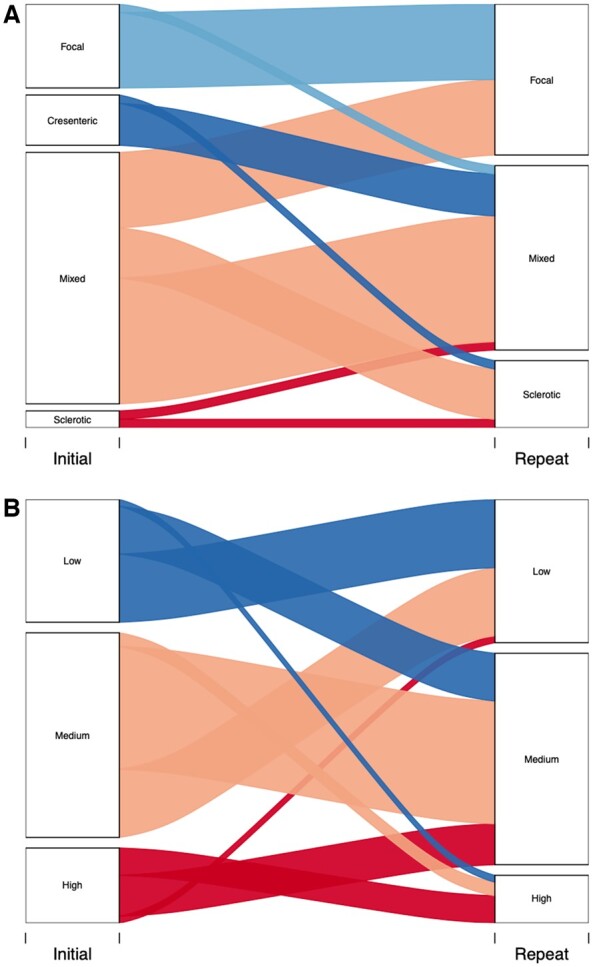
Renal risk scoring
Berden risk classification (A) and Brix risk score (B) were determined for patients based on initial and interval biopsy. Six initial biopsies and six interval biopsies were excluded from Berden Classification as they had <10 glomeruli leaving a total of 53 biopsies for each group. All initial and interval biopsies were suitable for Brix risk score giving a total of 59 biopsies for each group. Analysis by Chi-square test or Fisher’s exact test as appropriate.
Next, for both classification tools we evaluated how the risk classification changed between initial and interval biopsy for individual patients. For the Berden classification, 90% of biopsies (9/10) initially classified as focal remained focal on interval biopsy, with one biopsy re-classified as mixed. Those initial biopsies classified as crescentic became either mixed (83%, 5/6) or sclerotic (17%, 1/6). Fifty percent of initial biopsies (15/30) classified as mixed remained mixed on interval assessment with 30% (9/30) becoming focal and 20% (6/30) becoming sclerotic. Finally, biopsies originally classified as sclerotic either remained sclerotic (1/2) or became mixed (1/2) on interval classification. Using the Brix score, the majority of biopsies classified as low remained low (10/18) on interval assessment, while 39% (7/18) changed to medium and 6% (1/18) entered the high class. Sixty percent (18/30) of biopsies classified as medium remained medium on interval biopsy, while 33% (10/30) became low and 7% (2/30) became high. Of biopsies initially classified as high, the majority improved to medium (6/11) on interval assessment with 36% (4/11) remaining high and 9% (1/11) becoming low.
Finally, we evaluated whether the Berden and Brix risk classification tools operated differently with respect to kidney outcome if calculated using interval biopsy parameters compared with initial biopsy parameters. For the Berden classification, and using initial biopsy data, each of the four groups showed an improvement in eGFR over the 36-month follow-up period (baseline vs 36-month eGFR: focal, 47 ± 7 ml/min/1.73m2vs 67 ± 11 mL/min/1.73m2; crescentic, 19 ± 5 ml/min/1.73m2vs 37 ± 10 mL/min/1.73m2; mixed, 28 ± 4 ml/min/1.73m2vs 44 ± 4 ml/min/1.73m2; sclerotic, 23 ± 20 mL/min/1.73m2vs 35 ± 13 mL/min/1.73m2), with no differences between groups (Supplementary Fig. S3A, available at Rheumatology online). Although these figures were not significantly different using interval kidney biopsy data, there was a trend for the sclerotic group to show less of an increase in eGFR than those patients in the focal and mixed groups (Supplementary Fig. S3B, available at Rheumatology online). For the Brix classification, the low, medium and high-risk groups all demonstrated increases in eGFR from baseline to 36 months (low, 42 ± 7 ml/min/1.73m2vs 54 ± 7 ml/min/1.73m2; medium, 30 ± 4 ml/min/1.73m2vs 50 ± 5 ml/min/1.73m2; high, 12 ± 2 ml/min/1.73m2vs 30 ± 5 ml/min/1.73m2). Using the interval biopsy, the low and medium risk groups showed similar increases in eGFR over the 36-month period (low, 45 ± 6 ml/min/1.73m2vs 66 ± 6 ml/min/1.73m2; medium, 24 ± 3 ml/min/1.73m2vs 39 ± 3 ml/min/1.73m2); however, the high-risk group demonstrated an increase of 2 ml/min/1.73m2 (17 ± 5 ml/min/1.73m2vs 19 ± 6 ml/min/1.73m2), significantly less than that seen using initial biopsy data (P <0.01) (Supplementary Fig. S3C and D, available at Rheumatology online).
Discussion
This is the largest study to date examining the utility of interval kidney biopsy in patients with AAV. We have shown that interval biopsy provides important information that guides clinical management of these patients. Of note, in 25% of cases the clinician’s impression of anticipated interval biopsy findings was incorrect. In particular, in patients where ongoing AAV disease activity was suspected this was confirmed in less than half of all cases. Interval biopsy findings may therefore prevent inappropriate escalation of immunosuppression in many patients. Our data also show that routinely used biomarkers of AAV disease activity, particularly hematuria, correlate poorly with disease activity within the kidney. We suggest that current renal risk prediction tools might operate more effectively using interval biopsy parameters. Finally, our data demonstrate the natural history of renal histopathology in AAV from presentation with active disease through to treatment-induced disease remission.
The clinical utility of interval kidney biopsy is recognized in lupus nephritis. Marinaki and colleagues showed that interval biopsy findings modified treatment in >75% of patients [20], findings supported by Malvar et al., who also showed that interval biopsy was safe in a study of 75 patients with lupus nephritis [13]. To our knowledge there is only one previous study that has explored the role of interval kidney biopsy in patients with AAV. Hruskova et al. reported on 17 patients who underwent protocolized interval biopsy [14]. Similar to our own findings, they showed a decrease in acute lesions on interval biopsy with an increase in chronic lesions. Their data also suggested that the proportion of normal glomeruli remains constant throughout the disease process. In contrast, we found a higher proportion of normal glomeruli on interval biopsy. There may be a number of explanations for these differences. First, the study by Hruskova included younger patients (average age 49 years) with more aggressive disease on initial biopsy (>50% of patients had >50% cellular crescents; ∼25% of patients were receiving dialysis) and more chronic lesions on interval biopsy [14]. Second, their small study included patients diagnosed between 1991 and 1995, a time when AAV treatment was different to current standard of care. Third, interval biopsy in their study was carried out later in the disease (at ∼13 months compared with ∼4 months in the current study).
Our study is the first to compare the change in Brix risk score and Berden Classification from initial kidney biopsy to interval biopsy. Brix risk score changed in ∼50% of patients between initial and interval biopsy, ∼30% of patients showed an improvement in risk score whereas the remaining ∼20% progressed. With respect to the Berden Classification, a similar ∼50% of patients’ biopsies changed class on interval biopsy with ∼30% demonstrating progression (mostly from crescentic to mixed, or mixed to sclerotic), and ∼20% improving, mostly from mixed to focal. Our data also suggest that when using these tools to predict longer-term kidney function at the individual patient level, data from an interval biopsy might be more useful than those from initial biopsy. This should be explored further in future studies. The study by Hruskova also examined risk categorization at both initial and interval biopsy using the Berden classification [14, 17]. In their cohort, the majority of patients’ biopsies showed progression in histopathological classification, particularly from the crescentic to mixed categories.
Our study examined routinely used clinical biomarkers of systemic and renal AAV disease activity. As expected, at the time of interval kidney biopsy (∼4 months following initial biopsy) a number of these had improved compared with timing of initial biopsy. Those patients with inactive kidney disease demonstrated a greater reduction in serum creatinine and proteinuria, and rise in eGFR and haemoglobin, compared with patients with active disease on interval biopsy. It is interesting that CRP and PR3 (but not MPO) ANCA titre fell to a lesser extent in those with ongoing AAV disease within the kidney, although these did not reach significance. Interestingly, most (85%) patients had some degree of hematuria – often considered a marker of active glomerular disease – at time of interval biopsy; this includes the ∼60% of patients subsequently shown to have inactive kidney disease. This is particularly important as currently defining remission is based on structured clinical assessment using validated disease activity scores such as the BVAS [21]. This requires the treating clinician to determine whether markers such as hematuria are due to active kidney disease or not. Thus, our findings suggest that these clinical biomarkers lack sensitivity and cannot be reliably used to discriminate active from inactive kidney disease in patients with AAV.
Importantly, kidney biopsy was a safe procedure in this study. This is in keeping with a recent meta-analysis of over 115 000 biopsies, which showed that clinically significant bleeding (requiring a blood transfusion) occurs in ∼1.6% of patients [22]. Performing a kidney biopsy in the elderly, a population with a rising incidence of AAV [23], has also been shown to be safe and to provide important prognostic information for these patients [24].
We recognize the limitations of our study. Its retrospective nature and inclusion of patients from a single tertiary AAV referral centre. However, our study population is comparable in terms of demographics, including baseline level of kidney function, to several large randomized controlled trial populations, and we show similar patient outcomes [9, 25, 26]. In addition, European Guidelines for the management of AAV now recommend patients are managed in specialist centres [21]. Our centre does not protocolize interval kidney biopsies and decisions are based on a balance of benefits and risks for the individual patient. Our study was not large enough to answer important patient-centred questions: does the additional information gained by interval biopsy lead to meaningful reductions in death, end-stage kidney disease and complications of immunosuppression such as diabetes, fractures and infections. We accept these limitations; however, it is unlikely that there will ever be a randomized trial of sufficient size to directly test this. Our findings contribute to the body of evidence that should be weighed to decide whether an interval biopsy is likely to confer more benefit or harm in any individual.
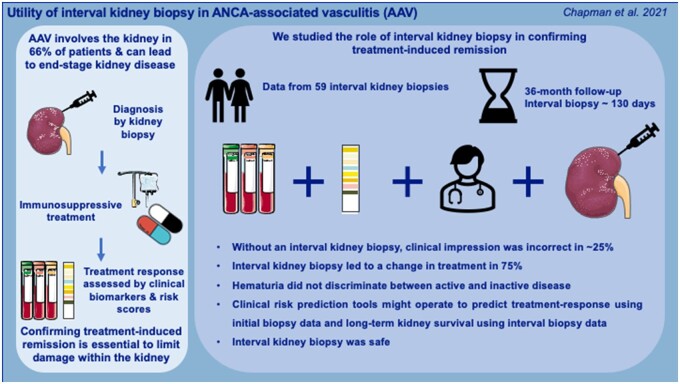
In summary, we have examined the utility of interval kidney biopsy in patients with AAV and have demonstrated this to be clinically useful in determining treatment-response within the kidney and subsequent disease management. Interval kidney biopsy may also provide additional prognostic information. Our data add to the literature on the safe practice of interval kidney biopsy within the contexts of kidney transplantation and lupus nephritis [12, 13, 20, 27]. Given the vogue towards glucocorticoid-limiting, and altogether avoiding, treatment regimens, and with a shift towards more personalized medicine, the need for better strategies to confirm disease remission at the histological level is increasingly important [8–10]. Our data support the role of interval kidney biopsy in AAV on an individual patient basis to assess disease activity (Fig. 5).
Fig. 5.
Utility of interval kidney biopsy in ANCA-associated vasculitis (AAV)
Supplementary Material
Acknowledgements
Research idea and study design: ND; data acquisition: GBC, COCB, RL and ND; data analysis/interpretation: GBC, TEF, FAC, DP, EMH and RWH; statistical analysis: GBC, RWH and ND; DCK, COCB and ND provided supervision. Each author contributed manuscript drafting and revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The data underlying this article are available in the article and in its online supplementary material.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Pearce FA, Lanyon PC, Grainge MJ. et al. Incidence of ANCA-associated vasculitis in a UK mixed ethnicity population. Rheumatology 2016;55:1656–63. [DOI] [PubMed] [Google Scholar]
- 2. Hunter RW, Welsh N, Farrah TE, Gallacher PJ, Dhaun N.. ANCA associated vasculitis. BMJ 2020;369:m1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wallace ZS, Miloslavsky EM.. Management of ANCA associated vasculitis. BMJ 2020;368:m421. [DOI] [PubMed] [Google Scholar]
- 4. Hewitt SM, Dear J, Star RA.. Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol 2004;15:1677–89. [DOI] [PubMed] [Google Scholar]
- 5. McAdoo SP, Tanna A, Randone O. et al. Necrotizing and crescentic glomerulonephritis presenting with preserved renal function in patients with underlying multisystem autoimmune disease: a retrospective case series. Rheumatology 2015;54:1025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luqmani RA. Disease assessment in systemic vasculitis. Nephrol Dial Transplant 2015;30(Suppl 1):i76–82. [DOI] [PubMed] [Google Scholar]
- 7. Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA.. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis–a meta-analysis. Rheumatology 2012;51:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pepper RJ, McAdoo SP, Moran SM. et al. A novel glucocorticoid-free maintenance regimen for anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology 2019;58:373. [DOI] [PubMed] [Google Scholar]
- 9. Walsh M, Merkel PA, Peh CA. et al. Jayne DRW and investigators P. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med 2020;382:622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farrah TE, Prendecki M, Hunter RW. et al. Glucocorticoid-free treatment of severe anti-neutrophil cytoplasm antibody-associated vasculitis. Nephrol Dial Transplant 2021;36:739–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hellmich B, Flossmann O, Gross WL. et al. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis 2007;66:605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilkinson A. Protocol transplant biopsies: are they really needed? Clin J Am Soc Nephrol 2006;1:130–7. [DOI] [PubMed] [Google Scholar]
- 13. Malvar A, Alberton V, Lococo B. et al. Kidney biopsy-based management of maintenance immunosuppression is safe and may ameliorate flare rate in lupus nephritis. Kidney Int 2020;97:156–62. [DOI] [PubMed] [Google Scholar]
- 14. Hruskova Z, Honsova E, Berden AE. et al. Repeat protocol renal biopsy in ANCA-associated renal vasculitis. Nephrol Dial Transplant 2014;29:1728–32. [DOI] [PubMed] [Google Scholar]
- 15. Jennette JC, Falk RJ, Bacon PA. et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 16. Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–30. [DOI] [PubMed] [Google Scholar]
- 17. Berden AE, Ferrario F, Hagen EC. et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 2010;21:1628–36. [DOI] [PubMed] [Google Scholar]
- 18. Brix SR, Noriega M, Tennstedt P. et al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int 2018;94:1177–88. [DOI] [PubMed] [Google Scholar]
- 19. Lv L, Chang DY, Li ZY. et al. Persistent hematuria in patients with antineutrophil cytoplasmic antibody-associated vasculitis during clinical remission: chronic glomerular lesion or low-grade active renal vasculitis? BMC Nephrol 2017;18:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marinaki S, Kapsia E, Liapis G. et al. Clinical impact of repeat renal biopsies in patients with lupus nephritis: renal biopsy is essential especially later in the course of the disease. Eur J Rheumatol 2020;7:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yates M, Watts RA, Bajema IM, Cid MC. et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 2016;75:1583–94. [DOI] [PubMed] [Google Scholar]
- 22. Poggio ED, McClelland RL, Blank KN. et al. Systematic review and meta-analysis of native kidney biopsy complications. Clin J Am Soc Nephrol 2020;15:1595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGoon MD, Frost AE, Oudiz RJ. et al. Ambrisentan therapy in patients with pulmonary arterial hypertension who discontinued bosentan or sitaxsentan due to liver function test abnormalities. Chest 2009;135:122–9. [DOI] [PubMed] [Google Scholar]
- 24. Navaratnarajah A, Sambasivan K, Cook TH. et al. Predicting long-term renal and patient survival by clinicopathological features in elderly patients undergoing a renal biopsy in a UK cohort. Clin Kidney J 2019;12:512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stone JH, Merkel PA, Spiera R. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flossmann O, Berden A, de Groot K. et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011;70:488–94. [DOI] [PubMed] [Google Scholar]
- 27. Pascual M, Vallhonrat H, Cosimi AB. et al. The clinical usefulness of the renal allograft biopsy in the cyclosporine era: a prospective study. Transplantation 1999;67:737–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.