Abstract
Objectives
Little is known with certainty about the natural history of spinal disease progression in ankylosing spondylitis (AS). Our objective was to discover if there were distinct patterns of change in vertebral involvement over time and to study associated clinical factors.
Methods
Data were analysed from the Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS) observational cohort. All patients met modified New York Criteria for AS and had ≥2 sets of radiographs scored by modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) by two independent readers between 2002 and 2017. Group-based trajectory modelling (GBTM) was used to classify patients into distinct groups of longitudinal mSASSS considering sociodemographic and clinical covariables. The optimal trajectory model and number of trajectories was selected using Nagin’s Bayesian information criterion (BIC).
Results
A total of 561 patients with 1618 radiographs were analysed. The optimum number of trajectory groups identified was four (BIC −4062). These groups were subsequently categorized as: non-progressors (204 patients), late-progressors (147 patients), early-progressors (107 patients) and rapid-progressors (103 patients). Baseline predictors associated with higher spinal disease burden groups included: baseline mSASSS, male gender, longer disease duration, elevated CRP and smoking history. In addition, time-varying anti-TNF use per year was associated with decreased mSASSS progression only in the rapid-progressor group.
Conclusions
GBTM identified four distinct patterns of spinal disease progression in the PSOAS cohort. Male gender, longer disease duration, elevated CRP and smoking were associated with higher spinal disease groups. Independent confirmation in other AS cohorts is needed to confirm these radiographic patterns.
Keywords: longitudinal modeling, ankylosing spondylitis
Rheumatology key messages.
Four groups of ankylosing spondylitis spinal progression were identified using group-based trajectory modeling.
Tumour necrosis factor inhibitors were negatively associated only with the rapid-progressor group.
Introduction
Structural damage, encompassing both bony destruction and aberrant bone formation of the spine, is one of the most notable and recognizable, yet heterogeneous clinical manifestations of radiographic axial spondyloarthritis/ankylosing spondylitis (AS) [1]. It is a major determinant of physical function in AS patients [2]. Structural damage is a key domain in axial spondyloarthritis [including non-radiographic axial spondyloarthritis (nr-axSpA) and AS] recommended by the Assessment of Spondyloarthritis International Society (ASAS) and OMERACT as a core set for studying if medications are disease modifying [3].
Despite the above importance for understanding structural damage in the context of patient care, little is known for certain about the natural history of spinal disease change in AS. Past and current spinal fusion/radiographic progression studies to date have used an analytical approach that assumed linearity. In a 12-year longitudinal follow-up of the OASIS cohort, Ramiro et al. suggested that a linear model was the best fit at a group levelling in their longitudinal analyses [4]. However, the same authors also acknowledged the highly variable course at a specific patient level with simple visualization of individual progression curves revealing oscillating patterns of steep progression and relative quiescence. Understanding this variability in response to interventions is necessary to move therapeutic efforts forward in randomized clinical trials and help explain potential conflicting results regarding the outcome of pharmacotherapy on AS spinal disease.
Trajectory modelling has been employed in medical research to study patterns of behaviour, medication adherence, and the natural history of chronic diseases such as cardiovascular disease and chronic kidney disease, thus allowing for discussion regarding prognosis and treatment initiation discussion [5–9]. Trajectory modelling in rheumatology has been mainly limited to studies of pain and disease activity [10, 11]. In axSpA/AS, Molto et al. had previously shown that almost one-third of patients with early disease were on a trajectory of persistent high disease activity [12]. In osteoarthritis, recent studies have found that these techniques can reveal distinct patterns of knee joint space narrowing over time [13, 14].
Therefore, the overall goal of this study was to identify common patterns of change of spinal disease over time in well-characterized patients with AS enrolled in a longitudinal observational cohort. We applied group-based trajectory modelling, an analytical method that allowed us to incorporate all data collected on individuals over time of variable follow-up to identify distinctive radiographic spinal disease groups [15]. We hypothesized that trajectory modelling may inform risk stratification and decision making by patients and their providers by yielding distinct patterns of radiographic spinal disease over time. In this study we aim to: characterize the patients’ radiographic disease trajectory and examine the association of each radiographic trajectory with clinical factors.
Methods
Patients
Patients were participants in the Prospective Study of Outcomes in AS (PSOAS), an observational study begun in 2002 [16, 17]. Entry criteria for this cohort include ≥18 years of age and meeting modified New York Criteria for AS [18]. Patients were recruited from the investigators’ clinics, patient support groups (such as the Spondylitis Association of America), and community rheumatologists. Patients were included from five study sites: Cedars-Sinai Medical Center in Los Angeles, California, the University of Texas Health Science Center at Houston (UTH), the NIH Clinical Center, the University of California at San Francisco (UCSF), and the Princess Alexandra Hospital in Brisbane, Australia (PAH). The research carried out followed the Helsinki Declaration, each institution had the study approved by their respective institutional review boards (IRB), and each participating patient reviewed and signed an informed consent form. All patients with ≥2 sets of spinal radiographs since the time enrolment in the cohort began in 2002 through to July 2017 were included in this study.
Imaging and clinical assessments
Lateral radiographs of the lumbar spine and cervical spine were taken at the baseline visit and every 2 years to assess structural severity/progression assessed by the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) [19]. Outcomes were ascertained from time of cohort entry until the last set of radiographs per patient up to 2017. The mSASSS for each radiographic set was based on readings by a central, expert musculoskeletal radiologist (T.J.L.) and a second, PSOAS study-site rheumatologist experienced in AS-research. T.J.L. was blinded to all clinical aspects of the patient’s record including treatment. All mSASSS values further underwent quality assurance by the PSOAS Data Management and Statistical Core (DMSC) [20]. Discrepancies between the two readers and/or serial readings were adjudicated by a third investigator (J.D.R.).
Clinical variables were extracted from the PSOAS Research Electronic Data Capture (REDCAP) database after Case Report Form quality checks by the DMSC [20] We queried baseline sociodemographic variables as well as longitudinal CRP values, disease-specific patient-reported outcomes and medications associated with each radiographic set. Medications, including biologics, were at the discretion of each treating rheumatologist and patient. CRP was dichotomized as elevated vs non-elevated of the lab’s reference range at the time of the radiographic set analysed. TNF inhibitor (TNFi) utilization was reported as a yes/no categorical variable over the time interval between radiographic sets per patient. Non-steroidal anti-inflammatory medications (NSAIDs) intake was calculated by an NSAID equivalence score or ‘ASAS-NSAID score’ as well between radiographic sets per patient [21].
Statistical analysis
In order to construct mSASSS trajectories, we used group-based trajectory modelling (GBTM). GBTM is a nonlinear regression model approach that allows measurement points to be separated by different intervals and permits individual patient-level variability [15]. To characterize mSASSS trajectories over a period of 15 years, observation points were set up every 2 years per the observational cohort protocol with a zero-inflated Poisson model used, given the non-negative, integer distribution of mSASSS data. Time of the mSASSS assessment was considered as a time variable in modelling. Comparisons were made between models allowing for varying number of trajectory groups, as well as polynomial modelling for time. Latent class models with different numbers of trajectories were built individually to obtain the Nagin Bayesian information criterion (BIC) to assess model fit [15].
All individuals had posterior probability of group membership (based on their individual mSASSS profile) of each trajectory group calculated; with patients assigned to their highest probability trajectory group. Mean group assignment possibilities, which indicate the average possibility of being assigned to the trajectory for all individual patients in that trajectory, were then computed. A higher mean group assignment possibility indicates better fit with a predefined minimum cutoff of >0.7 considered acceptable [17].
The number of trajectories was determined by exploring the models with the lowest BIC, average posterior group membership probabilities and the number of patients in each group. Trajectory groups with >10% (>50 patients) of the cohort were used for model cutoff. We then computed the percentage of mSASSS change for each year in each of the trajectories. Analyses were performed using Statistical Analysis Software (SAS) version 9.4 (SAS Institute Inc). SAS PROC TRAJ, a custom SAS module available for free download, was used for GBTM (http://www.andrew.cmu.edu/user/bjones/download.htm).
Our modelling adjusted for risk factors of structural spinal damage: gender, baseline smoking, elevated CRP, disease duration as well as disease activity [as measured by the Bath Ankylosing Disease Activity Index (BASDAI)] [22]. Due to the collinearity of age and disease duration, only disease duration was included in our adjusted models. Additionally, we also adjusted our GBTM models to assess the associations between trajectory shape with longitudinal CRP and longitudinal medication utilization using the time-varying covariates (TCOV) statement considering their previously reported association with radiographic disease [23–25]. We further conducted comparisons of baseline characteristics among trajectory groups using analysis of variance or its non-parametric counterpart Kruskal–Wallis test for continuous variables, and χ2 (χ2) test for categorical variables. All analyses were performed at 5% level of significance.
Results
Our final modelling included 561 PSOAS patients with ≥2 radiographic sets available for mSASSS scoring (Fig. 1). A total of 1618 radiographs were analysed. Patients were mostly male (75%), white (80%) and HLA-B27 positive (84%) (Table 1). Less than half of patients (42%) were exposed to TNFi medications before study entry.
Fig. 1.
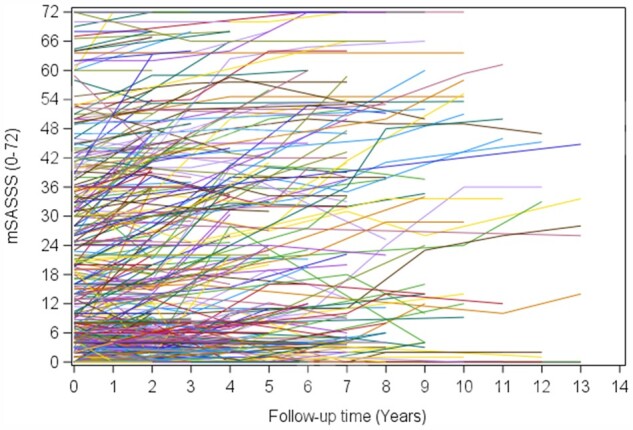
Individual patient mSASSS scores over time
Time in years is along the x-axis and total Modified Stokes Ankylosing Spinal Score is along the y-axis. Each individual line (n=561) represents a patient in the PSOAS cohort from time in cohort entry with all complete mSASSS scores included with at least two sets of radiographs.
Table 1.
Sociodemographic and clinical characteristics of the PSOAS group-based trajectory modelling cohort
| Characteristics | n = 561 |
|---|---|
| Male, n (%) | 424 (75.58) |
| White, n (%) | 453 (80.75) |
| Education >high school, n (%) | 467 (81.08) |
| Smoking history, n (%) | 226 (40.29) |
| HLA-B27 positivity | 472 (84.74) |
| Number of X-ray sets, median (IQR) | 3 (2, 4) |
| Follow up years, median (IQR) | 4.17 (2.25, 6.67) |
| Age at baseline, mean (s.d.) | 41.79 (13.09) |
| Disease duration at baseline, median (IQR) | 15.00 (7.00, 25.00) |
| Number of comorbidities, median (IQR) | 2.00 (1.00, 3.00) |
| BASFI Score, median (IQR) | 22.70 (9.20, 44.00) |
| BASDAI Score, median (IQR) | 3.20 (1.60, 5.39) |
| First observed CRP (mg/dL), median (IQR) | 0.40 (0.20, 1.00) |
| First observed ESR (mm/h), median (IQR) | 11.00 (5.00, 21.50) |
| Baseline mSASSS, median (IQR) | 5.00 (0.00, 26.00) |
| Baseline TNFi use, n (%) | 236 (42.07) |
| Baseline NSAID use, n (%) | 402 (71.66) |
IQR: interquartile range.
The optimum number of trajectory groups identified was four groups (Table 2). The posterior probability of these groups was very high, with the lowest average posterior probability being >0.94, far greater than the recommended value of 0.7 suggesting good fit of the model (group 1: 0.96; group 2: 0.95; group 3: 0.94; group 4: 0.97). The potential misclassification error of each group (difference between the theoretical proportion in each group and actual group assignment) was small, <2% (Supplementary Table S1, available at Rheumatology online).
Table 2.
Model fit for group-based trajectory modelling of Longitudinal mSASSS according to number of groups
| Number of groups | BIC (n = 561)a | BIC (n = 1618)b |
|---|---|---|
| 2 | −8130.15 | −8142.34 |
| 3 | −6038.86 | −6051.05 |
| 4 | −5091.02 | −5112.73 |
| 5c | −5449.14 | −5470.85 |
Note: The true BIC lies within these two BIC values: aBIC = Bayesian information criterion (for the total number of patients). bBIC = Bayesian information criterion [for the total number of observations across time (mSASSS radiographs)]. cOne or more of the groups have <10% of the observations.
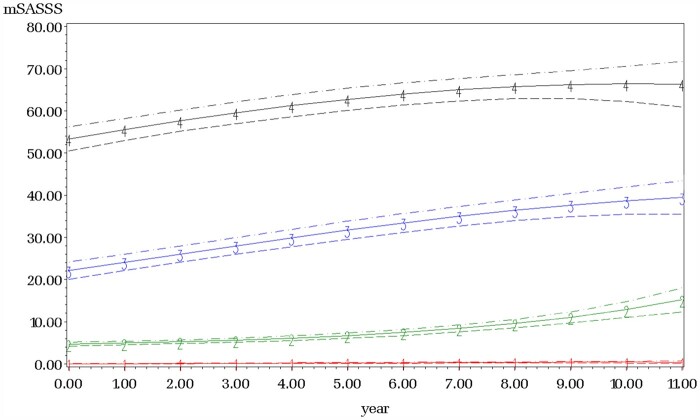
All four groups had distinct shapes, with curvilinear/quadratic functions identified as significant in all trajectory groups with adequate number of patients in each group adjusting for our baseline risk factors of: CRP, disease duration, mSASSS, gender and smoking (Tables 3 and 4; Fig. 2). Group 1 had 204 (37%) patients with an average of mSASSS (s.e.) of 0.04 (1.24) at cohort entry and were named ‘non-progressors’. Group 2 had 147 (26%) patients and, while they had an average mSASSS of 4.68 (1.05) at the time of entry in the cohort, they had increased rates of mSASSS progression in a curvilinear fashion over time (‘late progressors’). Group 3 had 107 (19%) patients and had a higher average mSASSS of 22.09 (1.04) at cohort entry and had slowing in their rate of mSASSS change in a curvilinear fashion and was named ‘early progressors’. Lastly, group 4 (n = 103, 18%) had a similar curve as in group 3 but had an average baseline mSASSS of 53.35 (1.02) and was named ‘rapid progressors’.
Table 3.
mSASSS trajectory model results for each group
| Variable | Coefficient estimate | P-value |
|---|---|---|
| Non-progressors (n = 204, 36.4%) | ||
| Intercept* | −3.23 | <0.001 |
| Linear* | 0.48 | <0.001 |
| Quadratic* | −0.02 | 0.02 |
| TNFi use (longitudinal) | 0.52 | 0.06 |
| Elevated CRP (longitudinal) | 0.40 | 0.13 |
| Late progressors (n = 147, 26.2%) | ||
| Intercept* | 1.54 | <0.001 |
| Linear* | 0.04 | 0.07 |
| Quadratic* | 0.01 | 0.01 |
| TNFi use (longitudinal) | 0.09 | 0.08 |
| Elevated CRP (longitudinal) | −0.06 | 0.26 |
| Early progressors (n = 107, 19.1%) | ||
| Intercept* | 3.10 | <0.001 |
| Linear* | 0.09 | <0.001 |
| Quadratic* | −0.01 | 0.01 |
| TNFi use (longitudinal) | −0.04 | 0.23 |
| Elevated CRP (longitudinal) | −0.02 | 0.52 |
| Rapid progressors (n = 103, 18.4%) | ||
| Intercept* | 3.98 | <0.001 |
| Linear* | 0.04 | <0.001 |
| Quadratic* | −0.01 | 0.03 |
| TNFi use (longitudinal)* | −0.09 | <0.001 |
| Elevated CRP(longitudinal) | −0.02 | 0.43 |
P <0.05.
Table 4.
Baseline Predictors of Trajectory group membership in the 4-group mSASSS model (non-progressor group is the reference group)
| Variable | Coefficient estimate | P-value |
|---|---|---|
| Late progressors | ||
| Baseline mSASSS* | −2.09 | <0.001 |
| Gender (Male vs Female)* | 0.58 | 0.02 |
| Disease duration (≥20 years vs <20 years)* | 0.01 | <0.001 |
| Smoking history (Yes vs No) | 0.19 | 0.63 |
| Hx of TNFi (Yes vs No) | 0.09 | 0.72 |
| CRP (Elevated vs Non-elevated) | 0.04 | 0.70 |
| BASDAI (≥4 vs <4) | 0.003 | 0.95 |
| Early Progressors | ||
| Baseline mSASSS* | −4.24 | <0.001 |
| Gender (Male vs Female)* | 1.40 | <0.001 |
| Disease duration (≥20 years vs <20 years)* | 0.13 | <0.001 |
| Smoking history (Yes vs No) | 0.65 | 0.14 |
| Hx of TNFi (Yes vs No) | 0.51 | 0.11 |
| CRP (elevated vs non-elevated) | 0.28 | 0.01 |
| BASDAI (≥4 vs <4) | −0.02 | 0.66 |
| Rapid Progressors | ||
| Baseline mSASSS* | −5.87 | <0.001 |
| Gender (Male vs Female)* | 2.46 | <0.001 |
| Disease duration (≥20 years vs <20 years)* | 0.15 | <0.001 |
| Smoking history (Yes vs No)* | 0.91 | 0.04 |
| Hx of TNFi (Yes vs No) | 0.11 | 0.74 |
| CRP (Elevated vs Non-elevated)* | 0.30 | 0.01 |
| BASDAI (≥4 vs <4) | 0.04 | 0.52 |
P <0.05.
Fig. 2.
Longitudinal mSASSS trajectory groups
Time in years is along the x-axis and total Modified Stokes Ankylosing Spinal Score is along the y-axis. The solid lines represent the estimated mean with dotted lines representing the 95% confidence interval. Trajectory groups from this patient cohort (n =561) are: Group 1 (red line) Non-Progressors, Group 2 (green line) Late Progressors, Group 3 (blue line) Early Progressors and Group 4 (black line) Rapid Progressors. Adjustments included time-variant (TNF inhibitor use and abnormal CRP) and time-invariant risk-factors (e.g. gender, smoking and disease duration).
The trajectory modelling results for each group are shown in Table 3. Adding our predetermined model TCOV of longitudinal CRP (elevated vs no elevated), TNFi use and NSAID use, only CRP and TNFi use were found to be significant and improved the overall model fit [BIC −4044 (w/TCOV) vs −5091 (w/o TCOV)].
Baseline predictors of group assignment when compared with the non-progressors included baseline mSASSS values, male gender and disease duration for late, early and rapid progressor trajectory groups. Elevated CRP was found to be a predictor of early and rapid progressor trajectory groups. Smoking was only found to be associated with the rapid progressor group in comparison with non-progressors; however, history of TNFi use or disease activity, as measured by BASDAI, were not (Table 4). Educational level was not found to be a predictor of group assignment (Supplementary Table 2, available at Rheumatology online).
When we modelled for our longitudinal covariables of CRP, TNFi and NSAID medication utilization, data for 16 patients could not be analysed due to incomplete data, hence reducing the total number of patients to 546 overall. Longitudinal TNFi usage was significantly and inversely associated with the shape of our ‘rapid progressors’ group, with an associated decrease of 8% mSASSS/year compared with non-TNFi use (Supplementary Table 3, available at Rheumatology online). This within-trajectory group effect was not observed in the other trajectory groups. Longitudinal elevated CRP or NSAID utilization (ASAS-NSAID score ≥50% vs <50%) did not seem to have a significant effect associated with the shape of the trajectory groups.
Lastly, we included relevant baseline characteristics among our trajectory groups (Table 5). Significant differences were noted among age, disease duration, CRP, white race and baseline mSASSS (Table 5). The differences in age, disease duration and baseline mSASSS were only noted for our non-progressor group compared with the other three trajectory groups in pair-wise comparisons (Supplementary Table S4, available at Rheumatology online). No significant differences were found with disease activity, educational level, physical functioning, TNFi medication utilization, extra musculoskeletal manifestations of disease or HLA-B27 status.
Table 5.
Select baseline characteristic comparisons between trajectory groups of AS patients
| Variable | Non-progressors n = 204 (36.4%) | Late progressors n = 147 (26.2%) | Early progressors n = 107 (19.1%) | Rapid progressors n = 103 (18.4%) |
|---|---|---|---|---|
| White race* | 155 (75.98) | 122 (82.99) | 81 (75.70) | 95 (92.23) |
| HLA B-27 positivity | 173 (85.22) | 129 (88.97) | 87 (82.08) | 83 (80.58) |
| Married* | 97 (47.55) | 79 (53.74) | 54 (50.47) | 67 (65.05) |
| Education (high school graduate) | 173 (85.22) | 122 (83.56) | 83 (77.57) | 89 (87.25) |
| Ever smoke | 79 (38.73) | 68 (46.26) | 41 (38.32) | 38 (36.89) |
| Age at baseline* | 38.27 (11.18) | 44.14 (13.43) | 42.36 (14.46) | 44.80 (13.21) |
| Age of disease onset | 24.76 (10.39) | 25.00 (8.61) | 23.66 (9.48) | 24.86 (10.17) |
| Disease duration at baseline* | 12.00 (6.00, 18.00) | 17.00 (9.00, 27.00) | 18.00 (6.00, 28.00) | 20.00 (8.00, 29.00) |
| Number of comorbidities* | 1.00 (1.00, 3.00) | 2.00 (1.00, 3.00) | 1.50 (1.00, 3.00) | 2.00 (1.00, 4.00) |
| First observed CRP* | 0.43 (0.17, 1.23) | 0.44 (0.22, 1.10) | 0.32 (0.15, 0.60) | 0.40 (0.17, 0.98) |
| First observed ESR | 12.00 (5.00, 25.50) | 11.00 (6.00, 22.00) | 9.00 (5.00, 16.00) | 10.00 (5.00, 20.00) |
| TNFi ever use | 144 (70.59) | 94 (63.95) | 75 (70.09) | 79 (76.70) |
| NSAID use | 159 (77.94) | 99 (67.35) | 77 (71.96) | 67 (65.05) |
| Baseline BASDAI score | 3.40 (1.61, 5.33) | 3.16 (1.53, 5.09) | 3.29 (1.49, 5.44) | 3.57 (1.91, 5.35) |
| Baseline BASFI score | 22.85 (9.00, 42.60) | 22.00 (9.50, 43.70) | 24.20 (10.80, 44.30) | 31.30 (14.60, 56.70) |
| Baseline mSASSS total* | 3 (0,13) | 6 (0, 34) | 6 (0, 30) | 11(0, 36) |
P <0.05.
Discussion
The goal of this study was to understand the natural history of spinal structural change over time in AS. GBTM was chosen over more traditional longitudinal modelling of mSASSS change over time because we assumed that AS spinal disease consists of clusters of distinct trajectories. In our study, we found that there were four major groups/patterns of radiographic disease in the PSOAS cohort that we designated non-progressors, late progressors, early progressors and rapid progressors based on their trajectory shape over time. We found that clinical factors that were more associated with greater mSASSS disease/trajectory group included male gender, white race, elevated CRP and smoking history. In our groups, we did not find significant difference in HLA-B27 positivity. The non-progressor group was younger and has less disease duration compared with other groups. When modelling for potential disease modifying factors, we found in the rapid progressor group that TNFi utilization was associated with decreased progression as measured by mSASSS.
This study adds to our understanding of changes in AS spinal involvement over a clinically significant period in the lifetime of an AS patient. Previous work in this area has focussed on finding the mean, linear change of AS patients over time. We found that by using GBTM, radiographic progression by subgroup was best explained by curvilinear/quadratic functions. We also showed these rates of spinal damage (e.g. increasing or decreasing change in mSASSS) depended on trajectory group membership. To the best of our knowledge, this is the first study that uses GBTM and shows these patterns of spinal disease. We also confirmed known associations of race, gender, smoking and CRP elevation with increasing mSASSS over time.
Understanding the radiographic course of AS has important implications for research and clinical practice. Current studies of disease-modifying agents on spinal structural disease have been mixed, with only some studies showing an effect of TNFi and NSAIDs on radiographic disease. In fact, a recent meta-analysis of 24 clinical trials assessing radiographic progression over time has shown mixed results regarding TNFi effects of slowing radiographic progression, with sensitivity analyses showing a potentially important or clinical impactful slowing effect of TNFi medications only over a 4-year interval [26]. Our results may help interpret these heterogeneous findings because we show a group-dependent effect; namely, only patients of our rapid-progressor group demonstrated a decrease in their change in mSASSS on TNFi medications. Moreover, the benefit of TNFi treatment in our rapid-progressor group was time-dependent as earlier intervention would lead to greater decreases in mSASSS given the curvilinear trajectory. This has important clinical implications as this suggests that while all patients may not benefit from pharmacotherapy, our rapid-progressor patient subgroup, which has the highest disease burden of spinal disease, is a group that benefits from use of TNFi medication potentially independent of CRP elevation. This finding could be paradigm-shifting – if confirmed in subsequent clinical trials – as current treatment recommendations are based on disease activity.
There are limitations and strengths in our study. For example, our cohort was mostly white, well-educated, HLA-B27 positive and from North America and Australia who met modified New York criteria for AS. This may limit the generalizability of our study to other races/patient populations; nevertheless, our cohort allowed us to have a more homogeneous population to examine this disease-specific outcome. We also did not have socioeconomic status data available in our cohort which may impact patients’ access to healthcare. More importantly, because we studied radiographic patient data available from cohort entry regardless of the time of disease onset/AS diagnosis, we were unable to capture how radiographic disease starts from inception of disease and/or when patients previously with nr-axSpA met modified New York Criteria. Thus, we were unable to study patients at disease onset, although our cohort yielded distinct groups over a 15-year period. Considering the timeframe of the mSASSS observations (2002–2017) we also did not examine IL-17A inhibitors, which became available more recently. Lastly, self-reported disease duration was a predictor of group membership of all groups compared with non-progressors with average difference 5–8 years. This difference could lead to bias among our trajectory groups; however, our length of follow-up mitigates some of this concern. Strengths of our study include a large number of patients and complete sets of spinal imaging with centralized assessment that allowed us to implement GBTM of AS spinal disease. We were also able to study longitudinal associations with sociodemographic, disease specific and pharmacotherapy with AS spinal disease given our observational cohort study design.
In conclusion, our finding that identified four groups of AS spinal progression may help elucidate the heterogeneity of radiographic AS spinal disease. Future studies in different AS populations are needed to validate our findings of distinct subgroups and their differing curvilinear functions of mSASSS spinal progression. We also hope that by identifying these subgroups we will allow for more mechanistic studies that discover the underlying pathophysiology of AS spinal damage and hyperostotic changes. Understanding the patterns of changes in spinal involvement in AS along with the identification of clinical factors that are associated with them may offer new insights for patients, clinicians and researchers to seek answers about those at greatest risk, the underlying mechanisms, and the potential therapeutic targets for different subgroups of AS spinal disease.
Supplementary Material
Acknowledgements
M.C.H. is supported by the UTHealth Center for Clinical and Translational Sciences KL2 program [5KL2-TR003168-02]. The authors would like to thank the staff and participants of the PSOAS cohort. Special thanks is given to Dr Michael Ward, a key member of the PSOAS investigators.
Funding: This work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) [P01-052915–06], Spondylitis Association of America (SAA) and Eli Lilly and Company.
Disclosure statement: The authors reports the following disclosures: M.C.H.: personal fees for consulting from Novartis and UCB; M.L.: none; L.S.G.: AbbVie, personal fees from Eli Lilly, personal fees from Gilead, personal fees from GSK, personal fees from Janssen, grants and personal fees from Novartis, grants and personal fees from Pfizer, grants and personal fees from UCB; M.A.B.: none; A.T.: none; M.H.R. none; M.L.I.: none; J.D.R.: personal fees from Novartis; M.H.W.: personal fees for consulting and advisory board participation for the following entities; Novartis, Pfizer, GSK and UCB; T.J.L.: none. T.H. and M.S. are employees and shareholders of Eli Lilly.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, SAA, CATS or Eli Lilly.
Data availability statement
Data is available upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Taurog JD, Chhabra A, Colbert RA.. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016;374:2563–74. [DOI] [PubMed] [Google Scholar]
- 2. Poddubnyy D, Listing J, Haibel H. et al. Functional relevance of radiographic spinal progression in axial spondyloarthritis: results from the GErman SPondyloarthritis Inception Cohort. Rheumatology 2018;57:703–11. [DOI] [PubMed] [Google Scholar]
- 3. van der Heijde D, Calin A, Dougados M. et al. Selection of instruments in the core set for DC-ART, SMARD, physical therapy, and clinical record keeping in ankylosing spondylitis. Progress report of the ASAS Working Group. Assessments in Ankylosing Spondylitis. J Rheumatol 1999;26:951–4. [PubMed] [Google Scholar]
- 4. Ramiro S, Stolwijk C, van Tubergen A. et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis 2015;74:52–9. [DOI] [PubMed] [Google Scholar]
- 5. Schubert KO, Air T, Clark SR. et al. Trajectories of anxiety and health related quality of life during pregnancy. PLoS One 2017;12:e0181149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elmer J, Fogliato R, Setia N. et al. Trajectories of prescription opioids filled over time. PLoS One 2019;14:e0222677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burckhardt P, Nagin DS, Padman R.. Multi-trajectory models of chronic kidney disease progression. AMIA Annu Symp Proc 2016;2016:1737–46. [PMC free article] [PubMed] [Google Scholar]
- 8. Theodore RF, Broadbent J, Nagin D. et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension 2015;66:1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elmer J, Jones BL, Nagin DS.. Comparison of parametric and nonparametric methods for outcome prediction using longitudinal data after cardiac arrest. Resuscitation 2020;148:152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Courvoisier DS, Alpizar-Rodriguez D, Gottenberg JE. et al. Rheumatoid arthritis patients after initiation of a new biologic agent: trajectories of disease activity in a large multinational cohort study. EBioMedicine 2016;11:302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. James RJE, Walsh DA, Ferguson E.. General and disease-specific pain trajectories as predictors of social and political outcomes in arthritis and cancer. BMC Med 2018;16:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molto A, Tezenas du Montcel S, Wendling D. et al. Disease activity trajectories in early axial spondyloarthritis: results from the DESIR cohort. Ann Rheum Dis 2017;76:1036–41. [DOI] [PubMed] [Google Scholar]
- 13. Bartlett SJ, Ling SM, Mayo NE, Scott SC, Bingham C. 3rd. Identifying common trajectories of joint space narrowing over two years in knee osteoarthritis. Arthritis Care Res 2011;63:1722–8. [DOI] [PubMed] [Google Scholar]
- 14. Collins JE, Neogi T, Losina E.. Trajectories of structural disease progression in knee osteoarthritis. Arthritis Care Res 2020;73:1354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagin D. Group-Based modeling of development. Cambridge: Harvard University Press, 2005. [Google Scholar]
- 16. Rahbar MH, Lee M, Hessabi M, Tahanan A. et al. Harmonization, data management, and statistical issues related to prospective multicenter studies in ankylosing spondylitis (AS): experience from the Prospective Study Of Ankylosing Spondylitis (PSOAS) cohort. Contemp Clin Trials Commun 2018;11:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reveille JD, Lee M, Gensler LS. et al. The changing profile of ankylosing spondylitis in the biologic era. Clin Rheumatol 2020;39:2641–51. [DOI] [PubMed] [Google Scholar]
- 18. van der Linden S, Valkenburg HA, Cats A.. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 19. Creemers MC, Franssen MJ, van't Hof MA. et al. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005;64:127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rahbar MH, Lee M, Hessabi M, Tahanan A. et al. Harmonization, data management, and statistical issues related to prospective multicenter studies in ankylosing spondylitis (AS): experience from the Prospective Study Of Ankylosing Spondylitis (PSOAS) cohort. Contemp Clin Trials Commun 2018;11:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dougados M, Simon P, Braun J. et al. ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 2011;70:249–51. [DOI] [PubMed] [Google Scholar]
- 22. Garrett S, Jenkinson T, Kennedy LG. et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 23. Poddubnyy D, Rudwaleit M, Haibel H. et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis 2012;71:1616–22. [DOI] [PubMed] [Google Scholar]
- 24. Haroon N, Inman RD, Learch TJ. et al. The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum 2013;65:2645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Tubergen A, Ramiro S, van der Heijde D. et al. Development of new syndesmophytes and bridges in ankylosing spondylitis and their predictors: a longitudinal study. Ann Rheum Dis 2012;71:518–23. [DOI] [PubMed] [Google Scholar]
- 26. Karmacharya P, Duarte-Garcia A, Dubreuil M. et al. Effect of therapy on radiographic progression in axial spondyloarthritis: a systematic review and meta-analysis. Arthritis Rheumatol 2020;72:733–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon reasonable request.