Abstract
Objectives
To investigate the dynamics of response of synovitis to IL-17A inhibition with secukinumab in patients with active PsA using Power Doppler ultrasound.
Methods
The randomized, placebo-controlled, Phase III ULTIMATE study enrolled PsA patients with active ultrasound synovitis and clinical synovitis and enthesitis having an inadequate response to conventional DMARDs and naïve to biologic DMARDs. Patients were randomly assigned to receive either weekly subcutaneous secukinumab (300 or 150 mg according to the severity of psoriasis) or placebo followed by 4-weekly dosing thereafter. The primary outcome was the mean change in the ultrasound Global EULAR and OMERACT Synovitis Score (GLOESS) from baseline to week 12. Key secondary endpoints included ACR 20 and 50 responses.
Results
Of the 166 patients enrolled, 97% completed 12 weeks of treatment (secukinumab, 99%; placebo, 95%). The primary end point was met, and the adjusted mean change in GLOESS was higher with secukinumab than placebo [−9 (0.9) vs −6 (0.9), difference (95% CI): −3 (−6, −1); one-sided P=0.004] at week 12. The difference in GLOESS between secukinumab and placebo was significant as early as one week after initiation of treatment. All key secondary endpoints were met. No new or unexpected safety findings were reported.
Conclusion
This unique ultrasound study shows that apart from improving the signs and symptoms of PsA, IL-17A inhibition with secukinumab leads to a rapid and significant reduction of synovitis in PsA patients.
Trial registration
ClinicalTrials.gov; NCT02662985.
Keywords: PsA, Power Doppler ultrasound, OMERACT, GLOESS, clinical outcome, responsiveness, synovitis, joints, secukinumab, biological DMARDs
Rheumatology key messages.
Importance of GLOESS using Power Doppler ultrasound (PDUS) for detecting synovitis in RA has been established.
ULTIMATE is the first randomized controlled trial to show the applicability of GLOESS using PDUS in PsA.
The GLOESS results confirm rapid and early response to secukinumab on synovitis in PsA.
Introduction
PsA is characterized by inflammation of synovial membranes and entheseal sites leading to pain, structural damage, impairment of physical function and quality of life [1–5]. Abrogation of inflammation in the joints is a central goal for the treatment of PsA, like in any other form of inflammatory arthritis. However, to date the effects of drug therapy on disease are usually measured indirectly, through assessing the impact on signs and symptoms of disease, rather than directly assessing inflammation at joint level. Hence, little is known about the dynamic effect of DMARDs on synovitis.
Ultrasound in B-mode combined with Power Doppler (PD; the association named PDUS), permits visualization of both morphological and functional changes of synovium [6, 7]. The EULAR and the OMERACT have recently standardized the use of PDUS for detecting synovitis and developed a composite scoring system at joint and patient level: the Global EULAR-OMERACT Synovitis Score (GLOESS), which has shown high responsiveness to treatment and excellent reliability in RA patients [8–11], suggesting the possibility to be used to monitor treatment response in inflammatory arthritis.
Secukinumab, a human monoclonal antibody that directly inhibits IL-17A, has demonstrated sustained efficacy on signs and symptoms, inhibition of structural damage progression, and a favourable long-term safety profile in patients with PsA over 5 years [12–14]. However, little is known of its direct effect on synovitis (and enthesitis) and the dynamics of such response. To investigate this, we initiated the ULTIMATE study, which is the first PDUS-based randomized placebo-controlled trial in PsA that primarily focused on synovial responses rather than on signs and symptoms of disease. Hence, the primary aim of the ULTIMATE study was to evaluate whether treatment with secukinumab inhibits synovitis, as measured by PDUS, in patients with active PsA who failed conventional synthetic DMARDs (csDMARDs) therapy and were naïve to biological DMARDs (bDMARDs). Herein, we present the primary efficacy data of secukinumab on synovitis in patients with active PsA.
Methods
Patients and study design
Biologic-naïve patients (aged ≥18 years) with a diagnosis of PsA for at least 6 months, fulfilling the CASPAR criteria, and having an inadequate response to csDMARDs and an active disease based on tender joint count (TJC) ≥3 of 78 joints and swollen joint count (SJC) ≥3 of 76 joints were considered eligible for this study. In addition, patients had to present active PDUS synovitis according to a pre-defined cut-off (Supplementary Fig. S1 and Table S1, available at Rheumatology online) at screening and baseline and at least one clinical enthesitis at screening and baseline. Patients could continue to receive MTX, glucocorticoids and NSAIDs at a stable standard dose from 1 month prior to screening to 24 weeks (Supplementary Fig. S2, available at Rheumatology online).
Key exclusion criteria included (i) evidence of an ongoing infection or malignant process; (ii) prior treatment with bDMARDs, including tumor necrosis factor inhibitors; (iii) active ongoing inflammatory conditions other than PsA; (iv) active systemic infection within 2 weeks before randomization; (v) history of ongoing, chronic or recurrent infectious disease or evidence of tuberculosis infection; (vi) known infection with human immunodeficiency virus or hepatitis B or C at screening or randomization; and (vii) history of lymphoproliferative disease, any known malignancy, or malignancy of any organ system within the past 5 years. Detailed inclusion and exclusion criteria are listed in the Supplementary Table S1, available at Rheumatology online.
ULTIMATE (NCT02662985) was a multicentre, randomized, double-blind, placebo-controlled, 52-week Phase III study (Supplementary Fig. S2, available at Rheumatology online). The study was initiated on 22 August 2016 (first patient, first visit), and conducted across 37 active sites in 17 countries. This study consisted of a 1- to 4-week screening phase, followed by a 12-week, double-blind, placebo-controlled treatment period (TP 1; baseline to week 12); a 12-week open-label period (TP 2; week 12 to week 24); a 6-month, open-label extension period (TP 3; week 24 to week 52); and a 12-week safety follow-up period (week 52 to week 64; Supplementary Fig. S2, available at Rheumatology online).
Enrolled patients were randomized (1:1) using Interactive Response Technology (IRT) to receive either subcutaneous secukinumab (300 mg or 150 mg) or placebo weekly followed by 4-weekly dosing at Weeks 4 and 8 in a double-blind manner (Supplementary Fig. S2, available at Rheumatology online). Patients received secukinumab 300 mg or 150 mg according to the severity of skin disease. The open-label phase started at week 12 (TP 2), and all patients (including the placebo group) received secukinumab 300 mg or 150 mg depending on the skin severity through IRT every 4 weeks until week 52 in an open-label manner. Patients, study centre personnel (including ultrasound and clinical investigators) and data analysts were fully blinded to the treatment assigned to patients at randomization for the first 12 weeks of the study (TP 1). The ultrasound and clinical investigators remained blinded from each other until the final database lock.
The study protocol and its amendments were reviewed and approved by the independent ethics committee or institutional review board for each participating centre. The study was conducted according to the International Council for Harmonization (ICH) E6 Guideline for Good Clinical Practice (GCP) that has its origin in the Declaration of Helsinki [15]. Written informed consent was obtained from all enrolled patients. Data were collected in accordance with the GCP guidelines by the study investigators and analysed by the sponsor.
Assessment of joints by ultrasound
PDUS evaluation was performed at screening; baseline; and Weeks 1, 2, 4, 6, 8 and 12. The following 24 joints were evaluated bilaterally: metacarpophalangeal (MCP) joints 1–5, proximal interphalangeal (PIP) joints 1–5, metatarsophalangeal (MTP) joints 1–5, distal interphalangeal (DIP) joints 2–5, wrists, elbows, shoulders (glenohumeral), knees and ankles (tibiotalar). The joints were scanned at each visit from the dorsal aspect, with the joint in a neutral position, except for the knee, which was examined in a flexed position (30°). All recesses of each joint were scanned, and the detection of maximal grading of PDUS synovitis in one of these recesses determined the final grade of the joint.
All PDUS evaluations were performed at each site by an independent examiner, expert in musculoskeletal ultrasound, with >5 years of experience, and blinded to the clinical evaluation. To ensure homogeneity of PDUS synovitis scoring, all ultrasound investigators completed an extensive 2-day training session, including examination of patients with PsA. In addition, ultrasound settings were not changed during the study, standardized joint and probe positions were used, and software was not upgraded. Centres were advised to create a fixed study setting to be used at each evaluation.
Medium- to high-level ultrasound machines [ESAOTE, Italy, Acuson, USA, Logic Series 9, 7 and enext GE, USA, Siemens, USA, or other, such as Toshiba Xario 200, Toshiba Aplio (300, 400), Japan, Aloka Arietta V70, and Samsung HS60] were used, which employed high frequency (12–18 MHz) transducers. Doppler parameters were adjusted according to the device used (range of pulse repetition frequency 400–800 Hz; Doppler frequency 7–14.1 MHz).
PDUS synovitis was defined according to the EULAR-OMERACT definition as a hypoechoic synovial hypertrophy (SH) detected in B-mode, which may show PD signal. At each visit, PDUS synovitis was graded semi-quantitatively (0–3) according to the EULAR-OMERACT PDUS composite score (Table 1) [8, 11]. In addition, single components of this composite score (i.e. hypoechoic SH and PD synovial signal) were scored separately at each visit.
Table 1.
Ultrasound scoring system for B-mode and PD signal at joint level
| B-mode: inflammatory or active synovial hypertrophy | |
| Grade 0 | No hypoechoic synovial thickening |
| Grade 1 | Minimal hypoechoic synovial thickening filling the angle between the periarticular bones, without bulging over the line linking tops of the bones |
| Grade 2 | Hypoechoic synovial thickening bulging over the line linking tops of the periarticular bones but without extension along the bone diaphysis |
| Grade 3 | Hypoechoic synovial thickening bulging over the line linking tops of the periarticular bones and with extension to at least one of the bone diaphysis |
| PD signal | |
| Grade 0 | No flow (PD signal) in the synovium |
| Grade 1 | Up to three single spots signals or up to two confluent spots or one confluent spot plus up to two single spots |
| Grade 2 | Vessel signals in less than half of the area of the synovium (<50%) |
| Grade 3 | Vessel signals in more than half of the area of the synovium (>50%) |
Grades: 0, normal joint; 1, minimal synovitis; 2, moderate synovitis; 3, severe synovitis.
PD: Power Doppler; PDUS: Power Doppler ultrasonography.
The GLOESS for the 24 paired joints was calculated as the sum of each PDUS composite score for all joints examined, giving a potential score ranging from 0 to 144. As previously reported, GLOESS incorporates both B-mode and PD measures of synovitis and allows for the evaluation of changes in the activity and morphology of synovitis. To help in grading severity, an atlas with examples of B-mode and PD grading for all joints examined was available in each centre.
All images were recorded, anonymized and sent for central reading for the first patient enrolled at each centre to allow a verification of the consistent scoring across sites. Training sessions and central reading of the images collected from the first included patient enrolled in each site were considered adequate to ensure a homogeneous rating across sites.
Clinical and safety assessments
Joints were assessed clinically for tenderness and swelling to calculate the TJC and SJC. In addition, ACR 20, 50 and 70 responses and their core components and the mean change from baseline in HAQ Disability Index (HAQ-DI) were evaluated. Safety assessments, including adverse events (AEs), serious AEs and AEs of special interest occurring during the first 12 weeks were performed in all patients receiving at least one dose of study drug.
Statistical analysis
This study was designed to test the superiority of secukinumab compared with placebo at a 5% significance level with a two-sided test. No data applying the EULAR-OMERACT composite PDUS score at the joint or patient level (GLOESS) in PsA were previously reported; however, the mean change from baseline to week 12 was assumed based on the abatacept treatment effect from a previous PDUS study in RA [16]. Assuming a difference in the mean change from baseline to week 12 in GLOESS (primary objective) of −6 with a pooled s.d. of 13.2, a total of 218 patients (109 patients per arm) were estimated to achieve a power of 90%.
Blinded sample size re-estimation (SSR) was performed after the completion of week 12 for the first 60 patients and substantiated by data collection from the first 72 enrolled patients to reassess variability of the disease and adjust sample size calculation accordingly. A protocol amendment was introduced to reduce the study sample size from 218 patients to 164 patients (82 patients per arm) with the power relaxed to 80% and a one-sided (α = 5%) superiority test vs placebo for the primary objective. The detailed SSR has been provided in Supplementary Table S2, available at Rheumatology online.
The efficacy analyses were performed on the full analysis set, which comprised all patients who were randomized and had study treatment assigned. The primary and key secondary endpoints were analysed according to a pre-defined statistical hierarchy (Supplementary Fig. S3, available at Rheumatology online). The primary objective was to demonstrate a difference in mean change from baseline to week 12 between secukinumab and placebo groups related to PDUS synovitis response using GLOESS (sum of the affected joints out of 48 joints). In addition, change between secukinumab and placebo from baseline to week 12 in the core components (SH and PD signal) of GLOESS was analysed exploratory. The clinical exploratory outcome measures presented here include the proportion of patients achieving ACR70, the mean change from baseline in HAQ-DI score, and distribution of joints by ultrasound and clinical assessment at baseline.
Data presented for the secukinumab group were pooled data from 300 mg and 150 mg. The primary analysis was performed using a mixed-effect model repeated measures (MMRM; valid under the ‘missing at random’ assumption), with treatment regimen, centre and analysis visit as factors and weight and baseline GLOESS as continuous covariates. Treatment by analysis visit was included as an interaction term in the model. An unstructured covariance structure was assumed for this model. The significance of the treatment effect for secukinumab was determined using the comparisons performed between the secukinumab and placebo arms at week 12. Missing values were imputed as non-response [non-responder imputation (NRI)] for binary variables via logistic regression, with study treatment as a factor and baseline weight as a covariate. Odds ratio and relative risk (for binary variables) or differences in adjusted mean change (for continuous variables) and 95% CI are presented comparing secukinumab vs placebo. A ‘null zone’ derived from the CI around the difference, obtained from the MMRM analysis, was plotted for continuous variables [17]. It shows the area where the means are located when there is no significant difference between the groups at the P < 0.05 level.
Safety analyses included all patients who received ≥1 dose of study medication. AEs were reported as absolute frequencies over the placebo-controlled period, referring to the cumulative treatment period (i.e. events started after the first dose of study treatment or events present before the first dose of study treatment but increased in severity based on preferred term and on or before the last dose plus 84 days). The clinical and ultrasound response on enthesitis that were secondary and exploratory objectives are not included in the present report.
Results
Patient disposition and baseline characteristics
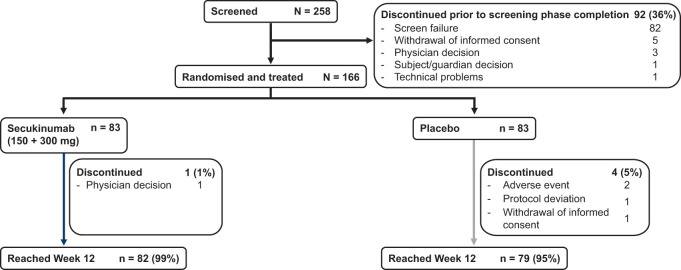
Overall, 258 patients were screened, of whom 82 were ineligible for the study and 10 were not included for other reasons (Fig. 1). Out of 166 patients (64%) enrolled, 161 (97%) completed the first 12 weeks (secukinumab, 99%; placebo, 95%; Fig. 1). The proportion of patients with at least one protocol deviation was 15% (secukinumab, 16%; placebo, 13%; Supplementary Table S3, available at Rheumatology online). Demographics and baseline clinical characteristics were comparable between the treatment groups (Table 2). The mean age was 47 years, median disease duration was 4 years, and 55% were women. Patients had active disease at baseline with a mean number of 14 tender joints, nine swollen joints and four clinically active enthesitis, as well as a mean Psoriasis Area and Severity Index score of 10.
Fig. 1.
Patient disposition through week 12
Screen failures are those who were screened but failed to meet the inclusion or met the exclusion criteria or met eligibility but did not move into treatment period 1 (i.e. the patient was not randomized; percentage is computed using the number of screened patients as the denominator). n: total number of patients.
Table 2.
Baseline demographic and clinical characteristics
| Characteristicsa | Secukinumab | Placebo |
|---|---|---|
| (300 mg + 150 mg) (n = 83) | (n = 83) | |
| Age (years) | 47 (12) | 47 (12) |
| Female, n (%) | 45 (54) | 46 (55) |
| Caucasian, n (%) | 75 (90) | 75 (90) |
| Time since diagnosis of PsA (years) | 6 (7) | 7 (7) |
| TJC (78 joints) | 13 (8) | 15 (12) |
| SJC (76 joints) | 10 (8) | 9 (9) |
| Patient pain (VAS) | 59 (21) | 59 (24) |
| Global assessment of disease activity (VAS) | ||
| Patient | 60 (23) | 60 (23) |
| Physician | 56 (18) | 52 (22) |
| HAQ-DI score | 1.3 (0.6) | 1.2 (0.7) |
| hsCRP level (mg/l), median (min–max) | 7 (1‒77) | 5 (0‒102) |
| PsOb, n (%) | 36 (43) | 33 (40) |
| PASI scoreb | 9 (6) | 11 (9) |
| GLOESSc | 24 (16) | 27 (17) |
| SH | 24 (16) | 27 (17) |
| PD | 8 (8) | 7 (7) |
| Number of joints with PDUS synovitis | 9 (5) | 10 (5) |
| Concomitant corticosteroids, n (%) | 13 (16) | 19 (23) |
| Concomitant MTX, n (%) | 35 (42) | 34 (41) |
mean (s.d.) unless otherwise specified; bcalculated only for patients with BSA ≥3%; c24 paired joints.
BSA: body surface area; GLOESS: Global EULAR-OMERACT Synovitis Score; HAQ-DI: HAQ disability index; hsCRP: high sensitivity CRP; N: total number of randomized patients; PASI: Psoriasis Area and Severity Index; PD: Power Doppler; PDUS: Power Doppler Ultrasonography; PsO: psoriasis; SH: Synovial hypertrophy; SJC: swollen joint count; TJC: tender joint count; VAS: visual analogue scale (range, 0–100).
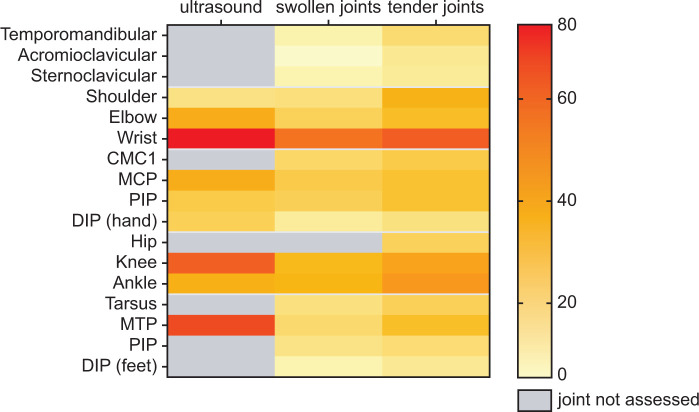
The average time spent on PDUS assessments at baseline for the evaluation of the pre-specified set of 24 paired joints was 39 min, for both the secukinumab and placebo arms. The distribution of PDUS synovitis revealed that wrists, knees, MCPs and MTPs were the more frequently affected joints. A similar distribution was observed on clinical examination of swollen or tender joints with lower frequency. These data are presented in a heat map in Fig. 2 and Supplementary Fig. S4, Tables S4 and S5 (available at Rheumatology online), respectively.
Fig. 2.
Distribution of synovitis detected by ultrasound and tender and swollen joints detected by clinical assessment at baseline
The distribution of synovitis detected by ultrasound and distribution of tender and swollen joint detected by clinical examination at baseline side by side. Frequency of distribution varies from 0 to 80% (highest proportion of patients with ultrasound detected synovitis on wrist) and is visualized by a code of colour from yellow to red shown on the right bar. Grey colour means ultrasound did not assess synovitis of these joints. CMC: carpometacarpal; DIP: distal interphalangeal; MCP: metacarpophalangeal; MTP: metatarsophalangeal; PIP: proximal interphalangeal.
PDUS efficacy
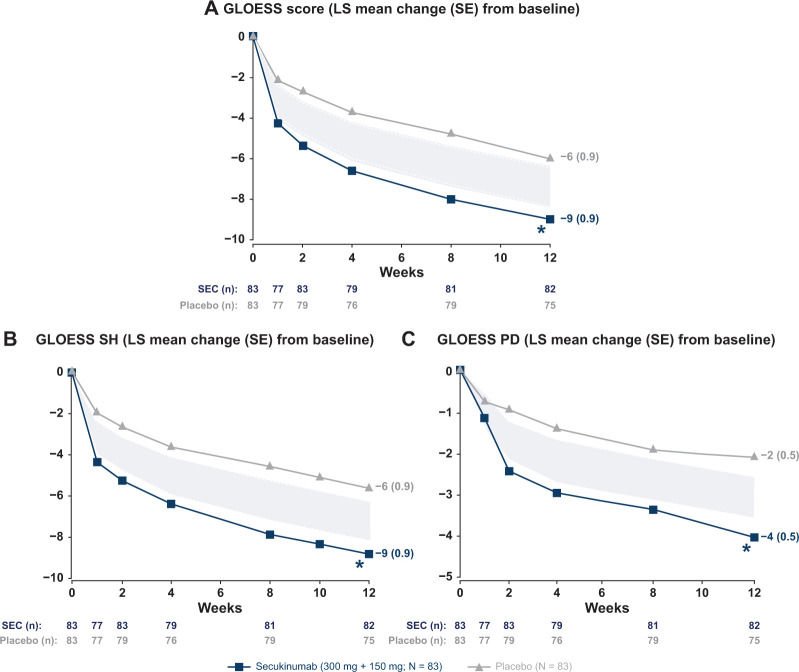
The primary end point was met at week 12 (Fig. 3); the adjusted mean (s.e.) change in GLOESS was significantly higher in the secukinumab vs placebo [−9 (0.9) vs −6 (0.9), difference (95% CI): −3 (−6, −1); one-sided P = 0.004]. A markedly significant difference between secukinumab and placebo was observed as early as 1 week after treatment initiation. The mean (s.e.) change from baseline to week 12 in SH (secukinumab vs placebo) was −9 (0.9) vs −6 (0.9) and in PD was −4 (0.5) vs −2 (0.5), with significance as early as week 1 for SH and week 2 for PD signal (Fig. 3).
Fig. 3.
PDUS efficacy outcomes through week 12
*P < 0.05 vs placebo. (A) primary endpoint GLOESS [MMRM, difference (95% CI): –3 (–6, –1), P =0.004] at Week 12; (B) GLOESS SH [MMRM, difference (95% CI): –3 (–6, –1), P =0.004]; and (C) GLOESS PD [MMRM, difference (95% CI): –2 (–3, –1), P =0.001]. The ‘null zone’ presented GLOESS scores was derived from the CI around the difference, which was obtained from the MMRM. It shows the area where the means are located when there is no significant difference between the groups at the P <0.05 level. GLOESS: Global OMERACT-EULAR Synovitis Score; LS: least squares; MMRM: mixed-effect model repeated measures; N: total number of randomized patients; n: number of evaluable patients; PD: Power Doppler; SEC: secukinumab; SH: synovial hypertrophy.
Clinical efficacy
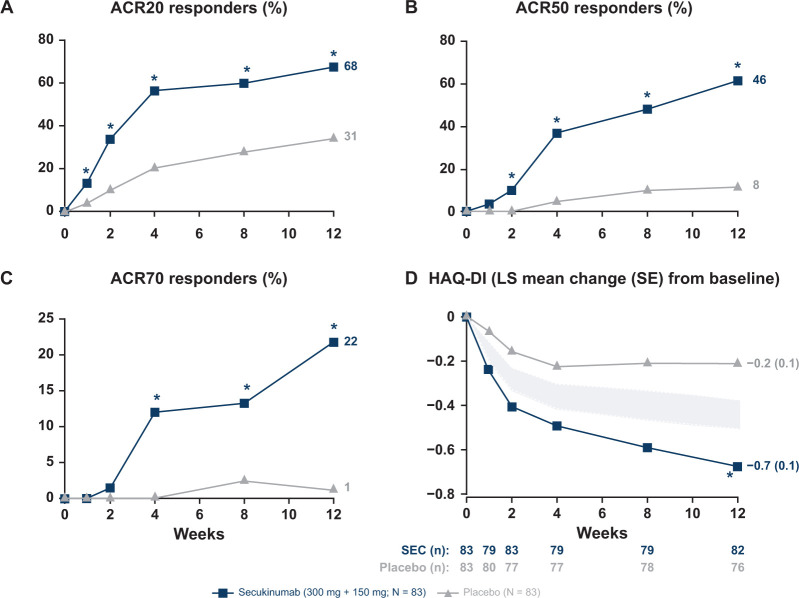
ACR20 and ACR50 responses were met and favored secukinumab-treated patients against placebo at week 12, with significant improvements observed as early as week 1 for ACR20 and week 2 for ACR50 compared with placebo (Fig. 4). Significantly higher responses were observed in secukinumab-treated patients for the exploratory endpoints (ACR70 response and HAQ-DI score) at week 12 compared with placebo (Fig. 4). The mean changes from baseline to week 12 in ACR core components are presented in Supplementary Table S6, available at Rheumatology online.
Fig. 4.
Clinical efficacy outcomes through week 12
*P < 0.05 vs placebo. (A) ACR20 response [NRI, odds ratio (95% CI): 5 (2, 9), P <0.0001, relative risk: 2]; (B) ACR50 response [NRI, odds ratio (95% CI): 10 (4, 24), P <0.0001, relative risk: 5]; (C) ACR70 response [NRI, odds ratio (95% CI): 23 (3, 178), P =0.0013, relative risk: 18]; and (D) HAQ-DI score [MMRM, difference: ‒0.5 (‒0.6, ‒0.3); P <0.0001]. The ‘null zone’ presented HAQ-DI score was derived from the CI around the difference, which was obtained from the MMRM. It shows the area where the means are located when there is no significant difference between the groups at the P <0.05 level. HAQ-DI: Health Assessment Questioner Disability Index; LS: least squares; MMRM: mixed-effect model repeated measures; N: total number of randomized patients; n: number of evaluable patients; NRI: non-responder imputation; SEC: secukinumab.
Safety
Overall, the incidence of treatment-emergent AEs up to week 12 was 58% for the secukinumab group and 57% for the placebo group. The most frequent treatment-emergent AEs in terms of crude incidence rates up to week 12 were nasopharyngitis, hypertension, diarrhoea, headache and latent tuberculosis in either secukinumab or placebo group. No serious AEs were reported in the secukinumab group. No deaths, serious infections, neutropaenia, major adverse cardiovascular events, inflammatory bowel disease or malignancies were reported in either treatment group. Safety data are presented separately for individual treatment groups (secukinumab and placebo) in Supplementary Table S7, available at Rheumatology online.
Discussion
ULTIMATE is the first randomized, placebo-controlled, PDUS Phase III study in PsA that primarily aimed to address the effects of biological DMARDs on synovitis detected by a validated ultrasound outcome measurement instrument as a primary end point. The primary efficacy data of the ULTIMATE study showed a significant effect of secukinumab treatment compared with placebo in reducing active synovitis in PsA. This effect was observed as early as 1 week after the initiation of treatment and continued to improve at each time point of evaluation until week 12. The ultrasound approach also allowed assessment of which aspects of synovitis improved first. Thus, the SH component showed the response as early as 1 week and the PD component as early as 2 weeks after treatment initiation, highlighting a fast onset of efficacy of secukinumab in controlling inflammation in PsA.
To date, only one small observational study has suggested that DMARDs have an effect on synovitis in PsA [18]. Large controlled studies aiming to assess the direct effect of DMARDs on synovitis are lacking, despite the availability of objective instruments to measure such effects. The ULTIMATE study revealed that the activity of synovitis in PsA can be scored at patient level using a validated ultrasound scoring system (GLOESS). Moreover, the study showed that reliable assessment of synovitis in PsA is feasible across different centres. Thus, GLOESS was sensitive to detect decrease in synovitis across different ultrasound devices and examiners even without excluding patients with protocol deviations. The absence of a true reliability exercise among the examiners may be considered as a limitation. However, potential variability in ultrasound assessment related to expertise was minimized using a rigorous ultrasound training, an atlas with reference images and central reading of images of the first patient enrolled across all sites. Possible remaining variability did not detract from the high sensitivity to change of GLOESS, which was developed to be sensitive across examiners and machines. Hence, these data suggest that assessment of synovitis by GLOESS is a reliable method to address the direct effect of DMARDs on synovitis in PsA.
The observed improvement in the signs and symptoms of PsA upon exposure to secukinumab confirmed its known clinical efficacy and was in accordance with earlier studies. Higher ACR responses were observed with secukinumab in the current study than in the secukinumab FUTURE 2 and FUTURE 5 studies [19, 20], possibly because of the uniquely rigorous combined clinical and ultrasound inclusion criteria on joints, and the stringent monitoring in this study over the initial 3 months. Treatment with secukinumab was well tolerated and the safety profile was consistent with the established safety profile across approved indications [21].
In conclusion, ULTIMATE is the first randomized study that evaluated the effect of DMARDs on PDUS measured synovitis as the primary end point. It demonstrated that secukinumab rapidly and significantly decreased synovitis, indicating a direct effect of IL-17 inhibition on the synovium in patients with PsA. As synovitis is critical for cartilage and bone destruction in PsA [1, 3, 4], these data also provide the basis for the observed protection of joint structure by secukinumab in patients with PsA.
Acknowledgements
The authors thank the patients and investigators who participated in the study. The authors also thank John Gallagher (Novartis Pharmaceuticals UK Ltd, UK) and Corine Gaillez (Novartis Pharma AG, Basel, Switzerland) for medical guidance and editorial support. The study was designed by the scientific steering committee and Novartis personnel. The first draft of the manuscript was written by medical writers, employed by the study sponsor (Niladri Maity and Gurleen Kaur, Novartis Healthcare Pvt. Ltd, Hyderabad, India), under the guidance of the authors. Statistical analyses were performed by statisticians employed by the study sponsor (Novartis Pharma AG, Basel, Switzerland). All authors had access to the data, contributed to the interpretation, and collaborated in the development of the manuscript. All authors critically reviewed and provided feedback on subsequent versions for important intellectual content. All authors approved the final version of the manuscript to be submitted for publication and vouch for the accuracy and completeness of the data and fidelity of this report to the study protocol.
The study protocol was reviewed and approved by the Independent Ethics Committee or Institutional Review Board for each participating centre. The study was conducted according to the ICH E6 guideline for Good Clinical Practice that has its origin in the Declaration of Helsinki. Written informed consent was obtained from all enrolled patients.
Study conception and design: M.A.D’A., G.S., A.-M.D., P.G., M.B. and C.G. Acquisition of data: M.A.D’A., G.S., A.L.-R., L.Š., K.F., R.B.-V., J.M.-C., E.N., P.C. and M.B. Analysis and interpretation of data: M.A.D’A., G.S., A.L.-R., L.Š., K.F., R.B.-V., J.M.-C., E.N., P.C., A.-M.D., P.G., M.B. and C.G.
Funding: The study (NCT02662985) was supported by Novartis Pharma AG, Basel, Switzerland.
Disclosure statement: M.A.D’A. has received honoraria for consulting or speaking from Sanofi, Novartis, BMS, Janssen, Celgene, AbbVie, UCB pharma, and Eli Lilly. G.S. has received honoraria for speaking from AbbVie, BMS, Celgene, Gilead, Janssen, Eli Lilly, Novartis, Roche and UCB pharma. A.L.-R. has received honoraria for consulting or speaking from Roche, Eli Lilly, Novartis, BMS and Neovacs and research grant from those companies. L.Š. has received research grants from AbbVie; honoraria for speaking from AbbVie, Amgen, BMS, Celgene, Eli Lilly, Gilead, MSD, Mylan, Novartis, Pfizer, Roche, Sanofi, Sandoz and UCB pharma; expenses for attendance at advisory board meeting from AbbVie, BMS, Celgene, MSD, Novartis, Pfizer, Roche and UCB pharma; and honoraria for clinical trials from AbbVie, Amgen, BMS, Celgene, Novartis, Pfizer, Takeda and UCB. K.F. declares no competing interest. R.B.-V. has received honoraria for speaking from Novartis. J.M.-C. has received honoraria for speaking or consulting from Pfizer, MSD, Sanofi-Aventis, Novartis, BMS, Roche, Boehringer Ingelheim, Schering-Plough, Abbott, UCB, Eli Lilly and Gilead and principal investigator in clinical trials for those companies. E.N. has received honoraria for speaking from AbbVie, Roche, BMS, Pfizer, UCB, Eli Lilly, Novartis, Janssen and Celgene; honoraria for clinical trials from AbbVie, Novartis and BMS; and research grants from Eli Lilly. P.C. has received research grants from UCB, MSD and Pfizer; honoraria for speaking or consulting from Pfizer, MSD, Novartis, BMS, AbbVie, UCB, Eli Lilly, Gilead and Celgene. A.-M.D. and P.G. are employees of Novartis. M.B. has received honoraria for consulting from BMS, Novartis, Pfizer and GSK. C.G. is an employee of Novartis and owns stock from Novartis and BMS.
Data availability statement
The datasets generated during and/or analysed during the current study are not publicly available. Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. The data may be requested from the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1.Day MS, Nam D, Goodman S, Su EP, Figgie M.. Psoriatic arthritis. J Am Acad Orthop Surg 2012;20:28–37. [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P.. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64(Suppl 2):ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 4.Veale DJ, Fearon U.. The pathogenesis of psoriatic arthritis. Lancet 2018;391:2273–84. [DOI] [PubMed] [Google Scholar]
- 5.Coates LC, Kavanaugh A, Ritchlin CT; GRAPPA Treatment Guideline Committee. Systematic review of treatments for psoriatic arthritis: 2014 update for the GRAPPA. J Rheumatol 2014;41:2273–6. [DOI] [PubMed] [Google Scholar]
- 6.Uson J, Loza E, Möller I. et al. Recommendations for the use of ultrasound and magnetic resonance in patients with spondyloarthritis, including psoriatic arthritis, and patients with juvenile idiopathic arthritis. Reumatol Clin 2018;14:27–35. [DOI] [PubMed] [Google Scholar]
- 7.D'Agostino MA, Aegerter P, Bechara K. et al. How to diagnose spondyloarthritis early? Accuracy of peripheral enthesitis detection by power Doppler ultrasonography. Ann Rheum Dis 2011;70:1433–40. [DOI] [PubMed] [Google Scholar]
- 8.D'Agostino MA, Terslev L, Aegerter P. et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce-Part 1: definition and development of a standardised, consensus-based scoring system. RMD Open 2017;3:e000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandl P, Navarro-Compán V, Terslev L. et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015;74:1327–39. [DOI] [PubMed] [Google Scholar]
- 10.Terslev L, Naredo E, Aegerter P et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce-Part 2: reliability and application to multiple joints of a standardised consensus-based scoring system. RMD Open 2017;3:e000427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakefield RJ, D'Agostino MA, Iagnocco A. et al. The OMERACT Ultrasound Group: status of current activities and research directions. J Rheumatol 2007;34:848–51. [PubMed] [Google Scholar]
- 12.Mease PJ, Kavanaugh A, Reimold A. et al. Secukinumab in the treatment of psoriatic arthritis: efficacy and safety results through 3 years from the year 1 extension of the randomised phase III FUTURE 1 trial. RMD Open 2018;4:e000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McInnes IB, Mease PJ, Kivitz AJ. et al. Long-term efficacy and safety of secukinumab in patients with psoriatic arthritis: 5-year (end-of-study) results from the phase 3 FUTURE 2 study. Lancet Rheumatol 2020;2:e227–35. [DOI] [PubMed] [Google Scholar]
- 14.van der Heijde D, Mease PJ, Landewe RBM. et al. Secukinumab provides sustained low rates of radiographic progression in psoriatic arthritis: 52-week results from a phase 3 study, FUTURE 5. Rheumatology 2020;59:1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 16.D'Agostino MA, Wakefield RJ, Berner-Hammer H. et al. Value of ultrasonography as a marker of early response to abatacept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results from the APPRAISE study. Ann Rheum Dis 2016;75:1763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boers M. Null bar and null zone are better than the error bar to compare group means in graphs. J Clin Epidemiol 2004;57:712–5. [DOI] [PubMed] [Google Scholar]
- 18.Kampylafka E, d'Oliveira I, Linz C. et al. Resolution of synovitis and arrest of catabolic and anabolic bone changes in patients with psoriatic arthritis by IL-17A blockade with secukinumab: results from the prospective PSARTROS study. Arthritis Res Ther 2018;20:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McInnes IB, Mease PJ, Kirkham B. et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137–46. [DOI] [PubMed] [Google Scholar]
- 20.Mease P, van der Heijde D, Landewe R. et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis 2018;77:890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deodhar A, Mease PJ, McInnes IB. et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther 2019;21:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available. Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. The data may be requested from the corresponding author.