Abstract
Aims
Using bilateral internal thoracic arteries (BITAs) for coronary artery bypass grafting (CABG) has been suggested to improve survival compared to CABG using single internal thoracic arteries (SITAs) for patients with advanced coronary artery disease. We used data from the Arterial Revascularization Trial (ART) to assess long-term cost-effectiveness of BITA grafting compared to SITA grafting from an English health system perspective.
Methods and results
Resource use, healthcare costs, and quality-adjusted life years (QALYs) were assessed across 10 years of follow-up from an intention-to-treat perspective. Missing data were addressed using multiple imputation. Incremental cost-effectiveness ratios were calculated with uncertainty characterized using non-parametric bootstrapping. Results were extrapolated beyond 10 years using Gompertz functions for survival and linear models for total cost and utility. Total mean costs at 10 years of follow-up were £17 594 in the BITA arm and £16 462 in the SITA arm [mean difference £1133 95% confidence interval (CI) £239 to £2026, P = 0.015]. Total mean QALYs at 10 years were 6.54 in the BITA arm and 6.57 in the SITA arm (adjusted mean difference −0.01 95% CI −0.2 to 0.1, P = 0.883). At 10 years, BITA grafting had a 33% probability of being cost-effective compared to SITA, assuming a cost-effectiveness threshold of £20 000. Lifetime extrapolation increased the probability of BITA being cost-effective to 51%.
Conclusions
BITA grafting has significantly higher costs but similar quality-adjusted survival at 10 years compared to SITA grafting. Extrapolation suggests this could change over lifetime.
Keywords: Healthcare economics, Coronary artery disease, Coronary artery disease surgery, Revascularization, Bypass
Introduction
The treatment of coronary artery disease places a large economic burden on healthcare systems, with a substantial proportion of that cost arising from coronary artery bypass grafting (CABG).1 CABG using a single left internal thoracic artery (SITA) has been found to improve long-term survival and quality of life (QoL) and to be cost-effective in comparison to the alternative of drug-eluting stents-percutaneous coronary intervention (PCI) for patients with severe coronary disease and patients with diabetes in a Dutch context.2
The success of SITA grafting has raised interest in the use of bilateral internal thoracic arteries (BITAs) grafting. A recent meta-analysis of 29 observational studies comparing BITA and SITA found BITA was associated with significantly improved long-term survival (hazard ratio 0.78).3 However, no previous study has reported a comparison of quality of life, resource use and costs between SITA and BITA.
The Arterial Revascularization Trial (ART) was the first large randomized controlled trial to compare BITA grafting with SITA grafting and was designed with an integrated economic evaluation. Clinical outcomes of ART have recently been published, reporting no significant difference between the two groups for the primary outcome of death from any cause at 10 years of follow-up.4 Interim analyses of costs from index admission to 1 year of follow-up and 5 years of follow-up have been published previously,5,6 finding that BITA grafting was associated with 9% higher costs after 1 year, primarily due to longer time in theatre and hospital stay and higher costs associated with sternal wound problems. No further cost differences were found from years 2 to 5, with healthcare costs increasing by approximately £700 per annum in each trial arm. An interim analysis of quality of life scores at 5 years found no significant differences between trial arms in the EQ-5D-3L, SF-36, or Shortened WHO Rose Angina Questionnaire.7
The current report presents a cost-effectiveness analysis comparing BITA grafting with SITA grafting for the 3102 patients in ART. Comparison of the two treatments is made in quality-adjusted survival, resource use, and associated costs across 10 years of follow-up. This is the first study to report a randomized comparison of costs and QoL between SITA and BITA grafting.
Methods
ART randomized patients from 28 centres across seven countries between 2004 and 2007, allocating patients to either SITA or BITA. The trial complied with the Declaration of Helsinki. Ethics approval for UK centres was obtained from the Multi-Centre Research Ethical Committee (MREC), reference number 04/3/006. Prior ethics approval was obtained at each non-UK participating centre and every patient was required to provide written informed consent. Patients were eligible for the trial if they had multi-vessel coronary artery disease and were scheduled to undergo CABG as part of their routine care plan (this included patients requiring urgent surgery, but not those with evolving myocardial infarction). Patients requiring only single grafts or concomitant valve surgery, as well as those with a history of CABG, were excluded. Full details of the trial design including inclusion and exclusion criteria and sample size calculations can be found in the trial protocol.8
Quality of life
Quality of life data were collected at baseline and each follow-up time point using the EuroQol EQ-5D-3L9 and the shortened World Health Organization Rose angina questionnaire.10 At baseline, 5 years and 10 years the Medical Outcomes Study 36-Item Short-Form Health Survey version 2 (SF-36) was also administered.11 The primary analysis makes use of responses from the EQ-5D-3L. EQ-5D-3L values were calculated using the UK population tariff with a score of 1 indicating ‘full health’, 0 death and negative values states worse than death.12 Quality-adjusted life years (QALYs) for each patient were derived by combining survival and quality of life data and then calculating area under the curve after linear interpolation between time points.
Resource use and costs
The perspective of the cost analysis was the English healthcare system, and other costs were not systematically collected. We follow the methods used in several other international trials,13,14 by applying a common set of unit costs to all patients, hence results are reported in pounds sterling using 2017–2018 prices, adjusted where necessary by the GDP deflator index. This avoids the multiple difficulties of collecting sets of unit costs from many countries and then attempting to standardise them using aggregate measures of purchasing power parity.15 Mean total costs were derived using the cost of the initial hospital admission combined with annual costs of healthcare contacts and medications across 10 years of follow-up. The costing methodology followed that used in the analyses to 1 year and 5 years of follow-up.5,6 This methodology assigned detailed costs to the initial hospital admission, including the total cost of surgery, post-operative costs, and any in-hospital adverse events (myocardial infarction, cerebrovascular accident, further CABG, further PCI, revascularization with catheter, major bleed, and the cost of hospital stay associated with other adverse events and death). Unit costs were obtained from NHS reference costs where available or from the finance department of one participating UK hospital. Supplementary material online, Table S1 documents all unit costs and their sources. Reference costs were adjusted for clinical events occurring during the index admission to avoid double counting the cost of stay in hospital.
Resource use data over the 10-year follow-up were collected from case record forms and from a short questionnaire at annual follow-up. Data were collected on numbers of general practitioner (GP) and practice nurse visits, outpatient clinic attendances, cardiac rehabilitation attendance, hospital admission bed days, medication usage, and resource use associated with severe adverse events. GP and nurse visits were costed using estimates from the Personal Social Services Research Unit while NHS reference costs provided unit costs for all recorded hospital outpatient clinic, cardiac rehabilitation clinic visits and costs associated with severe adverse events. The cost of adverse events classed as ‘other’ and death were assumed to be captured by costing the length of stay of the associated hospital admission. An emergency department attendance was assumed where participants were admitted for an event but no overnight stay was reported. The cost of hospital bed days was adjusted to avoid double counting those associated with ‘other’ adverse events and death. Individual medication usage was costed using unit costs from the NHS electronic Market Information Tool (eMIT).
Cost-effectiveness analyses
The primary analysis compared patients as randomized on an intention to treat basis. Mean resource use items and associated costs, and mean total costs (cost of initial hospital admission plus all healthcare contacts and medications across 10 years of follow-up) were compared using two-sample t-tests, while Poisson regression models were used to compare non-zero counts of adverse events. Standard errors were adjusted to account for clustering at the hospital level. Differences in mean QALYs were compared using a linear regression model and were adjusted for imbalances in baseline QoL.16 Future QALYs and costs were discounted to present values at an annual rate of 3.5%. Incremental cost-effectiveness ratios (ICERs) were then calculated from the mean difference in QALYs and costs. Cost-effectiveness was evaluated assuming a willingness to pay of £20 000 per QALY and at other levels.17 The uncertainty surrounding the ICER estimate was characterized using non-parametric bootstrap replications of the mean difference in QALYs and costs.18 Bootstrap samples were taken independently within each treatment group and with resampling at the hospital level. These replications were used to plot the cost-effectiveness plane19 and to construct cost-effectiveness acceptability curves, which show the likelihood that the intervention is cost-effective as the willingness to pay changes.20
Around a quarter of patients assigned to the SITA arm of the trial received an additional radial-artery graft, while 14% of the BITA group actually underwent SITA grafting. Evidence has grown since the trial was designed that radial-artery grafts are associated with better clinical outcomes in comparison to saphenous-vein grafts,21 and so a non-randomized comparison of patients receiving multiple arterial grafts (MAGs) or single arterial grafts (SAGs) was also made. These groups were well balanced in terms of baseline characteristics.4 Nonetheless, one-to-one propensity score matching was used to adjust total costs for imbalances in baseline covariates.
The cost-effectiveness of BITA compared to SITA arms beyond the 10 years of the trial was estimated using a Markov cohort model. Survival in each arm of the trial was estimated using Gompertz functions. The first year of trial data was excluded as this improved the fit of the functions to the data. Costs and utility were estimated beyond 10 years with linear models adjusting for age using data from the last 5 years of the trial. This allowed for variation in mean costs and mean utility by age. Patients begin the model at a mean age of 74 years in line with trial participants at 10 years of follow-up, and face an annual probability of death as determined by the survival functions. QALYs and costs in the extrapolation were discounted at an annual rate of 3.5%. Probabilistic sensitivity analysis was conducted to characterize the uncertainty around the extrapolation results using 1000 bootstrap samples from each imputed dataset. The output from the sensitivity analysis is presented using cost-effectiveness acceptability curves.
Missing data
The rate of missing data was low overall but increased over time, with between 3% and 37% of some resource use items missing at different time points, and between 9% and 38% of QoL data. Missing rates of both resource use and EQ-5D-3L data were similar for BITA and SITA groups. Seventy-one patients had missing vital status, and 279 patients had incomplete adverse event data. Where clinical outcome data were missing, it was assumed an event had not occurred. Logit models of missing total costs and utility across 10-year follow-up on baseline variables showed missing data to be associated with baseline hospital and being a smoker or former smoker at baseline.
Imputation was implemented separately by randomized treatment allocation. EQ-5D-3L data were missing at baseline for 159 (5.1%) patients: these were imputed using mean imputation. All other missing values for both resource use and EQ-5D-3L value data were imputed using chained equations and predictive mean matching.22 These equations used baseline hospital, age, sex, baseline Canadian Cardiovascular Society (CCS) class, diabetes, smoking status, peripheral arterial disease, and baseline EQ-5D-3L index. The procedure was repeated to produce 50 imputed datasets with Rubin’s Rule used to summarize across imputations.23 The non-parametric bootstrap approach used to construct the estimates for the cost-effectiveness plan and CEAC drew 1000 samples for each imputed dataset.24
Sensitivity analyses
The sensitivity of the results to the missing at random assumption was investigated using a pattern mixture model.25 Imputed values were adjusted by a multiplicative scale parameter. The included values of the sensitivity parameter varied both imputed costs and QoL to −20% of their original value at 5% point intervals. The different missing not at random scenarios were then compared in terms of the probability of BITA being cost-effective at a willingness to pay of £20 000 per QALY.
Subgroup analyses
The cost-effectiveness of BITA compared to SITA was estimated for all patients and for pre-specified patient subgroups: diabetic and non-diabetic, age ≥70 years vs. <70 years, on-pump vs. off-pump, prior myocardial infarction (MI) vs. no prior MI, New York Heart Association (NYHA) class I and II vs. NYHA class III and IV, and Canadian Cardiovascular Society (CCS) class 0, I and II vs. CCS class III and IV. Comparison was also made in each of the three countries (UK, Poland, and Australia) which recruited more than 100 patients to the trial. Linear models with interaction terms between subgroup and treatment allocation were used to test for significant differences in treatment between subgroups, with standard errors again being adjusted for clustering at the hospital level.
Results
Table 1 shows resource use, the frequency of adverse events and associated mean cost and QALYs at 10 years for the two trial arms. BITA grafting was associated with significantly larger total mean costs at 10 years of follow-up. This was primarily the result of the significantly higher index admission cost in the BITA group. The BITA group also had significantly higher mean costs associated with outpatient clinic visits and sternal wound problems. There were no significant differences in costs associated with any other healthcare contacts, medication usage or adverse events.
Table 1.
Resource use, costs, quality-adjusted life years, and cost-effectiveness at 10 year follow-up (intention-to-treat analysis)
| Mean resource use/adverse events at year 10 |
Mean total cost at year 10 |
|||||
|---|---|---|---|---|---|---|
| SITA (n = 1554) | BITA (n = 1548) | BITA vs. SITA Mean difference (95% CI, P-value) | SITA (n = 1554) | BITA (n = 1548) | BITA vs. SITA Mean difference (95% CI, P-value) | |
| Initial surgery | ||||||
| Index admission | 8819 | 9475 | 656 (101, 1212; 0.023) | |||
| Discharge cost | 562 | 532 | −30 (−353, 292; 0.848) | |||
| Healthcare contacts | ||||||
| GP visits | 32 | 31 | −0.67 (−2, 1; 0.461) | 1424 | 1405 | −19 (−100, 61; 0.627) |
| Nurse visits | 14 | 14 | 0.26 (−1, 2; 0.772) | 231 | 234 | 3 (−19, 25; 0.784) |
| Outpatient clinic visits | 10 | 11 | 0.84 (−1, 2; 0.222) | 1538 | 1683 | 145 (19, 271; 0.026) |
| Cardiac rehabilitation visits | 10 | 10 | −0.58 (−3, 2; 0.659) | 779 | 736 | −43 (−316, 230; 0.750) |
| Number of nights in hospitala | 2 | 2 | 0.42 (−0, 1; 0.196) | 716 | 875 | 159 (−163, 481; 0.318) |
| All health care contacts | 4689 | 4934 | 245 (−103, 594; 0.159) | |||
| Medications | ||||||
| Total medication | 35 | 35 | −0.09 (−1, 1; 0.881) | 218 | 222 | 5 (−6, 16; 0.383) |
| SAE treatmentb | ||||||
| Myocardial infarction | 53 | 52 | 0.98 (0.7, 1.4; 0.938) | 74 | 67 | −7 (−36, 22; 0.633) |
| Cerebrovascular accident | 63 | 45 | 0.72 (0.5, 1.1; 0.088) | 129 | 87 | −42 (−94, 10; 0.111) |
| Further CABG | 0 | 3 | 0 | 14 | 14 (−4, 31; 0.127) | |
| Further PCI | 157 | 154 | 0.98 (0.8, 1.2; 0.892) | 302 | 300 | −2 (−76, 73; 0.965) |
| Revascularization with catheter | 82 | 79 | 0.97 (0.7, 1.3; 0.832) | 100 | 107 | 7 (−46, 60; 0.784) |
| Sternal wound problems | 39 | 72 | 1.85 (1.3, 2.7; 0.002) | 101 | 276 | 175 (34, 316; 0.017) |
| Major bleeding | 11 | 10 | 0.91 (0.4, 2.1; 0.834) | 48 | 70 | 22 (−49, 93; 0.537) |
| Other AEs (cost of hospital stay only) | 1506 | 1749 | 1.17 (1.1, 1.2; 0.000) | 2201 | 2377 | 176 (−322, 675; 0.474) |
| Death (cost of hospital stay only) | 314 | 297 | 0.95 (0.8, 1.1; 0.522) | 405 | 363 | −41 (−262, 179; 0.703) |
| All adverse event costs | 3360 | 3662 | 302 (−290, 894; 0.304) | |||
| All costs | 17 647 | 18 825 | 1178 (204, 2152; 0.020) | |||
| Discounted total cost | 16 462 | 17 594 | 1133 (239, 2026; 0.015) | |||
| Life years | 9.03 | 9.05 | 0.02 (−0.15, 0.20; 0.801) | |||
| QALYsc | 6.57 | 6.54 | −0.01 (−0.2, 0.1; 0.883) | |||
| Incremental cost-effectiveness ratio: | BITA dominated [more expensive, less effective (non-significant)] | |||||
| Probability of cost-effectiveness at willingness to pay threshold | ||||||
| £13 000 | 29% | |||||
| £20 000 | 33% | |||||
| £30 000 | 36% | |||||
The cost of index admission includes the total cost of surgery [time in theatre (min), duration-related theatre costs and staff, duration-related anaesthetic costs, time on bypass (min), and other surgery costs (consumables, blood products, aprotinin), post-operative costs (ventilation time, intra-aortic balloon pump, inotropic support, renal support therapy, haemofiltration)] and any in hospital adverse events (myocardial infarction, cerebrovascular accident, further CABG, further PCI, revascularization with catheter, major bleed, other AEs (cost of hospital stay only), death (cost of hospital stay only)].
Number of nights in hospital exclusive of those associated with an ‘other’ adverse event or death.
SAE treatment is that occurring in the follow-up period only. The cost of SAEs which occurred during the index admission is included in the cost of index admission.
Estimated differences in QALYs are adjusted for baseline EQ-5D-3L index.
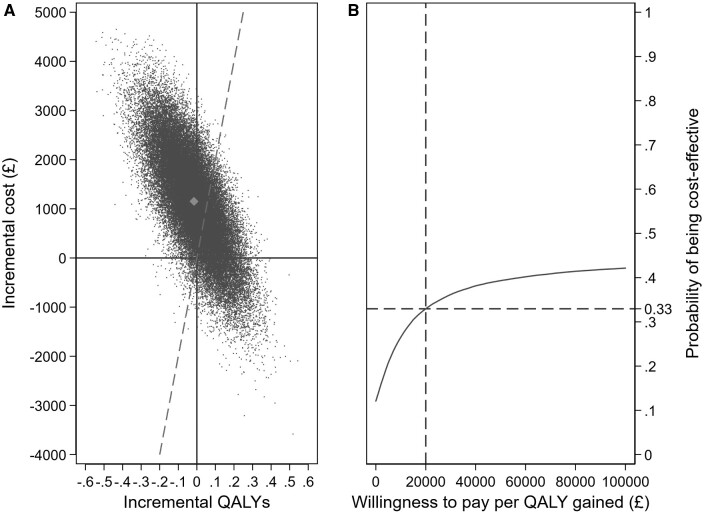
Mean EQ-5D-3L values initially increased following surgery for both treatment groups but then decreased as patients aged (Supplementary material online, Tables S2, S3). No differences were found between the two groups at any time point during the trial (Supplementary material online, Tables S2, S3). This was also the case using both the SF-36 and Rose Angina Questionnaire (Supplementary material online, Tables S4 and S5, respectively). By 10 years differences between the two groups in life years and QALYs were small and not statistically significant. Combining the cost [mean difference £1133 95% confidence interval (CI) £239 to £2026, P = 0.015] and QALY (adjusted mean difference −0.01 95% CI −0.2 to 0.1, P = 0.883) differences, BITA is dominated (more expensive, less effective) than SITA. The probability of BITA grafting being cost-effective compared to SITA (that is, of having a cost per QALY gained of less than £20 000) was 33% (Figure 1A and B).
Figure 1.
(A) Cost-effectiveness plane and (B) cost-effectiveness acceptability curve at 10 years of follow-up. (A) Plots a series of simulations showing the likelihood that BITA yields more or fewer quality-adjusted life years than SITA (X-axis), and costs more or less than SITA (Y-axis). Co-ordinates to the left and above the dashed line have a cost-effectiveness ratio above £20 000 per QALY gained; those to the right and below the line are less than £20 000 per QALY gained. (B) The probability that BITA is cost-effective compared to SITA as the willingness to pay for each QALY gained is varied from £0 to £100 000, that is, as the dashed line is rotated around the origin.
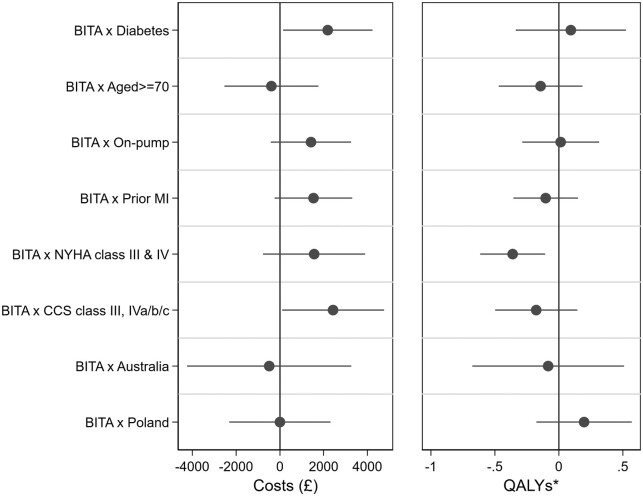
Table 2 shows summary results on costs, QALYs and cost-effectiveness for each pre-specified patient subgroup, and Figure 2 shows these subgroup analyses when tested for interaction between treatment allocation and cost or QALY differences. Concerning costs, a significant interaction with treatment allocation was found for patients with diabetes and for patients with a higher baseline severity CCS class, in both cases the cost difference being larger. A significant interaction was also found between treatment allocation and NYHA class, with classes III and IV being associated with a lower QALY gain: in this group 6.29 QALYs had been accumulated by 10 years in the SITA group compared to 6.01 in the BITA group, a difference of −0.29 (95% CI −0.57, −0.01, P = 0.044). Combining these costs and QALY differences, SITA dominated BITA (that is, less costly, more health benefit) in six subgroup analyses. Only in the groups with off-pump surgery, no prior MI, less severe NYHA, and CCS classes and in Poland was the cost of BITA per QALY gained lower than the threshold of £20 000, and in none of these groups was the test for interaction between treatment allocation and either costs or QALYs significant. Further details of these subgroup analyses are provided in Supplementary material online, Tables S7–S13 and Figure S2.
Table 2.
Total costs, QALYs, and cost-effectiveness at 10 year follow-up by patient subgroup
| Total cost (£) |
QALYs |
|||||||
|---|---|---|---|---|---|---|---|---|
| SITA | BITA | BITA vs. SITA Mean difference (95% CI, P-value) | SITA | BITA | BITA vs. SITAa Mean difference (95% CI, P-value) | ICER | Pr CE | |
| No history of diabetes (n = 2368) | 16 202 | 16 803 | 601 (–453, 1656); 0.251) | 6.67 | 6.61 | −0.032 (−0.19, 0.13; 0.688) | Dominatedb | 34% |
| Diabetes (n = 734) | 17 315 | 20 105 | 2790 (1064, 4516; 0.003) | 6.23 | 6.32 | 0.061 (−0.35, 0.47; 0.760) | 45 642 | 39% |
| Aged <70 years (n = 2271) | 15 276 | 16 563 | 1287 (576, 1998; 0.001) | 6.76 | 6.78 | 0.018 (−0.16, 0.19; 0.836) | 72 840 | 39% |
| Aged ≥70 years (n = 831) | 19 601 | 20 495 | 894 (–1282, 3070; 0.402) | 6.06 | 5.89 | −0.135 (−0.44, 0.17; 0.365) | Dominatedb | 14% |
| Off-pump (n = 1259) | 17 204 | 17 639 | 435 (–578, 1448; 0.379) | 6.51 | 6.52 | 0.027 (−0.20, 0.26; 0.805) | 13 903 | 51% |
| On-pump (n = 1819) | 16 067 | 17 781 | 1714 (297, 3130; 0.020) | 6.65 | 6.64 | −0.002 (−0.21, 0.21; 0.986) | Dominatedb | 32% |
| No prior MI (n = 1800) | 16 410 | 16 898 | 488 (–655, 1631; 0.387) | 6.65 | 6.65 | 0.026 (−0.18, 0.23; 0.794) | 18 903 | 49% |
| Prior MI (n = 1300) | 16 534 | 18 591 | 2057 (686, 3428; 0.005) | 6.46 | 6.38 | −0.079 (−0.28, 0.12; 0.430) | Dominatedb | 19% |
| NYHA class I and II (n = 2431) | 16 451 | 17 237 | 786 (–102, 1674; 0.080) | 6.64 | 6.70 | 0.071 (−0.08, 0.22; 0.338) | 11 496 | 56% |
| NYHA class III and IV (n = 669) | 16 494 | 18 839 | 2346 (51, 4640; 0.046) | 6.29 | 6.01 | −0.290 (−0.57, −0.01; 0.044) | Dominatedb | 15% |
| CCS class 0, I, II (n = 2143) | 16 565 | 16 938 | 372 (–742, 1486; 0.497) | 6.65 | 6.67 | 0.046 (−0.13, 0.22; 0.592) | 8758 | 56% |
| CCS class III, IVa/b/c (n = 959) | 16 225 | 19 029 | 2805 (898, 4711; 0.006) | 6.40 | 6.27 | −0.132 (−0.42, 0.16; 0.353) | Dominatedb | 20% |
| UK (n = 2053) | 17 173 | 18 181 | (−439, 2476; 0.154) 1008 (–385, 2402; 0.141) | 6.40 | 6.40 | 0.024 (−0.19, 0.24; 0.810) | 42 481 | 40% |
| Poland (n = 606) | 14 615 | 15 623 | (−72, 2084; 0.061) 1007 (–65, 2080; 0.060) | 6.79 | 6.96 | 0.202 (−0.06, 0.46; 0.091) | 4986 | 65% |
| Australia (n = 192) | 18 430 | 18 948 | (−2073, 3240; 0.665) 518 (–2165, 3202; 0.704) | 6.59 | 6.54 | −0.058 (−0.65, 0.53; 0.846) | Dominatedb | 39% |
Estimated differences in QALYs are adjusted for baseline EQ-5D-3L index.
BITA is more expensive but less effective.
Figure 2.
Interaction between treatment allocation and cost or QALY differences by selected subgroups. *Estimated differences in QALYs are adjusted for baseline EQ-5D-3L value.
Sensitivity analyses in which the assumption that missing data were missing at random was replaced with an assumption that missing cost and QALY data might be systematically different in either arm of the trial, found that the probability of BITA being cost-effective was considerably more sensitive to systematic differences in imputed QALYs than in total costs. For example, a reduction of 10% in the imputed costs in the BITA arm increased the probability of BITA being cost-effective from 33% to 39%. In contrast, a decrease in imputed QALYs of the same magnitude in the SITA arm increased the probability of BITA being cost-effective to 69%. Further results from the sensitivity analysis are shown in Supplementary material online, Figure S1.
Results from the non-randomized comparison of multiple vs. SAGs are shown in Table 3. MAGs were associated with significantly higher costs in several resource use categories including the index procedure, GP visits, and treatment for sternal wound problems. As a result, total costs were significantly higher over the 10 years of follow-up. However, MAGs were also associated with longer survival over 10 years (9.14 years vs. 8.94, average treatment effect 0.10, CI −0.09, 0.29, P = 0.306), and slightly more QALYs were accrued in the MAG group, so that the ICER was £16 412 per QALY gained, with a probability of being cost-effective (less than £20 000) of 54%.
Table 3.
Resource use, costs, quality-adjusted life years, and cost-effectiveness at 10 year follow-up (non-randomized comparison of multiple vs. single arterial graft)
| Mean resource use/n of adverse events at year 10 |
Mean total cost at year 10 |
|||||
|---|---|---|---|---|---|---|
| SAG (n = 1330) | MAG (n = 1690) | SAG vs. MAG Mean difference (95% CI, P-value) | SAG (n = 1330) | MAG (n = 1690) | SAG vs. MAG Average treatment effect (95% CI, P-value) | |
| Initial surgery | ||||||
| Index admission | 8818 | 9509 | 641 (195, 1088; 0.005) | |||
| Discharge cost | 647 | 480 | −132 (−418, 153; 0.363) | |||
| Healthcare contacts | ||||||
| GP visits | 30 | 33 | 3 (1, 5; 0.001) | 1348 | 1466 | 115 (28, 202; 0.010) |
| Nurse visits | 14 | 13 | −1 (−2, 1; 0.521) | 239 | 226 | −18 (−47, 12; 0.240) |
| Outpatient clinic visits | 11 | 11 | 0 (−1, 2; 0.771) | 1597 | 1632 | 28 (−198, 254; 0.808) |
| Cardiac rehabilitation visits | 9 | 10 | 1 (−1, 4; 0.308) | 698 | 805 | 98 (−106, 301; 0.347) |
| Number of nights in hospitala | 2 | 2 | 0.4 (−0, 1; 0.222) | 711 | 870 | 193 (−92, 478; 0.184) |
| All healthcare contacts | 4593 | 4998 | 416 (−38, 870; 0.073) | |||
| Medications | ||||||
| Total medication | 35 | 36 | 2 (0, 3; 0.017) | 218 | 222 | 4 (−10, 17; 0.589) |
| SAE treatmentb | ||||||
| Myocardial infarction | 38 | 64 | 1.33 (0.9, 2.0; 0.169) | 62 | 78 | 31 (−4, 67; 0.086) |
| Cerebrovascular accident | 55 | 52 | 0.74 (0.5, 1.1; 0.126) | 122 | 101 | −25 (−86, 37; 0.433) |
| Further CABG | 0 | 3 | . | 0 | 12 | 14 (−7, 34; 0.181) |
| Further PCI | 141 | 160 | 0.89 (0.7, 1.1; 0.327) | 315 | 288 | −34 (−140, 72; 0.530) |
| Revascularization with catheter | 75 | 78 | 0.82 (0.6, 1.1; 0.215) | 106 | 99 | −9 (−60, 43; 0.740) |
| Sternal wound problems | 34 | 73 | 1.69 (1.1, 2.5; 0.012) | 95 | 264 | 45 (−153, 244; 0.656) |
| Major bleeding | 9 | 11 | 0.96 (0.4, 2.3; 0.931) | 53 | 65 | −9 (−90, 71; 0.824) |
| Other AEs (cost of hospital stay only) | 1283 | 1871 | 1.15 (1.1, 1.2; 0.000) | 2013 | 2504 | 567 (−227, 1360; 0.162) |
| Death (cost of hospital stay only) | 298 | 293 | 0.77 (0.7, 0.9; 0.002) | 436 | 352 | −58 (−299, 183; 0.637) |
| All adverse event costs | 3201 | 3763 | 523 (−458, 1504; 0.296) | |||
| All costs | 17 478 | 18 972 | 1451 (99, 2803; 0.035) | |||
| Discounted total cost | 16 367 | 17 681 | 1251 (63, 2438; 0.039) | |||
| Life years | 8.94 | 9.14 | 0.10 (−0.09, 0.29; 0.306) | |||
| QALYsc | 6.51 | 6.67 | 0.08 (−0.1, 0.2; 0.380) | |||
| ICER | 16 412 | |||||
| Probability of cost-effectiveness | 54% | |||||
Number of nights in hospital exclusive of those associated with an ‘other’ adverse event or death.
SAE treatment is that occurring in the follow-up period only. The cost of SAEs which occurred during the index admission is included in the cost of index admission.
Estimated differences in QALYs are adjusted for baseline EQ-5D-3L index.
Table 4 and Supplementary material online, Figure S4 show the results from the extrapolation model. Costs and QoL observed in the trial in each group were extrapolated over an expected lifetime, with competing risks in line with extrapolated survival. The between-group difference in costs remained similar to the 10-year result but the QALY gain associated with BITA grafting showed a small increase, primarily attributable to slightly lower mortality risk in the BITA arm. This resulted in an ICER of £12 962 per QALY and increased the probability of BITA being cost-effective from 33% to 51% at a willingness to pay of £20 000.
Table 4.
Lifetime cost-effectiveness (extrapolation) of BITA vs. SITA (intention to treat analysis)
| SITA (n = 1554) | BITA (n = 1548) | BITA vs. SITA | |
|---|---|---|---|
| Cost (£) | £21 829 | £22 707 | £1165 |
| QALYs | 12.52 | 12.61 | 0.09 |
| Life years | 17.65 | 17.89 | 0.24 |
| ICER | 12 962 | ||
| Probability of cost-effectiveness | 51% | ||
Discussion
This is the first study to report a randomized comparison of costs and quality-adjusted survival between SITA and BITA grafting. We found the higher initial cost of BITA, previously observed at 1 year and at 5 years,5,6 was maintained to 10-year follow-up while no significant differences were observed in QALYs over the same time period. As a result, the probability of BITA being cost-effective at a willingness to pay of £20 000 per QALY gained was only around 33%. There were no significant differences in QoL at any time point across 10 years of follow-up.
Subgroup analyses
In our analyses of pre-specified subgroups, we found a significant interaction between treatment allocation and difference in costs for patients with diabetes or who were in a higher baseline severity CCS class, both of which were associated with a larger cost difference in favour of SITA. Similarly, we found a significant interaction between treatment allocation and difference in QALYs for patients in NYHA classes III and IV, such that those allocated to SITA accumulated significantly more QALYs over 10 years than those in the BITA group. As a result, we found some evidence that BITA grafting was more cost-effective in groups with less severe baseline conditions and less cost-effective in groups with more severe baseline conditions. Compared to SITA with or without radial artery use, BITA grafting requires sequential (as opposed to simultaneous) harvesting of the conduits and increases the trauma to the chest wall. One hypothesis could therefore be that recovery from the increased surgical trauma is more prolonged and less complete in patients with marginal functional status at baseline, resulting in higher costs and lower quality-adjusted survival compared to SITA in this group.
Single vs. multiple artery grafts
The non-randomized comparison of patients who received a SAG with those receiving multiple grafts found a survival advantage over 10 years in the MAG group and the resulting cost per QALY gained was well below conventional bounds of willingness to pay for health benefit. These results are suggestive that the use of multiple grafts is preferable to single grafts from an economic perspective as well as from a clinical perspective.
Longer-term costs and benefits
The costs and benefits of BITA vs. SITA beyond 10 years of follow-up remain an important question. Previously, Buttar et al.3 conducted a meta-analysis which reviewed both long-term and short-term clinical outcomes following BITA and SITA grafting, and reported overall survival out to more than 20 years after surgery. However, their finding of a significantly lower hazard ratio for the bilateral internal mammary artery group was based on observational studies and is not confirmed by ART at 10 years. Therefore we chose to extrapolate outcomes and costs beyond the end of the trial using a Markov model driven by some fairly simple assumptions on how survival, quality of life and costs would evolve over the remaining lifetime of the trial participants, drawing on ART data. This analysis indicated that the probability of BITA grafting being cost-effective increased to 51%. Continuing follow-up of ART participants beyond 10 years would provide valuable data to test the validity of these assumptions.
Limitations
The overall level of attrition in the ART trial was exceptionally low,4 but the economic analysis, drawing on many different aspects of trial data including questionnaires, case record forms, quality of life and resource use measures and all time points, did face a degree of missing data. We followed best practice in relying primarily on multiple imputation methods to deal with this, and tested the methods using sensitivity analysis. This indicated that the results were robust to large changes in the level of the imputed cost data, but were more sensitive to changes in the values of imputed quality of life data. This is a common result in sensitivity analysis of imputed data in trial-based cost-effectiveness analysis25 and highlights the importance of minimizing missing QoL data.
ART was an international study, enrolling patients from seven countries. We conducted this analysis from the perspective of the UK only, applying UK unit costs and quality of life valuations to all observed data. This can be justified on the grounds that the approach has been adopted in a number of other international trial analyses,13 two-thirds of all ART patients were recruited in the UK, and the subgroup analyses found no significant interactions between treatment allocation and country for costs or QALYs. However, it remains possible that the results were influenced by variations in treatment pathways between countries.
Although our subgroup analyses were suggestive that BITA grafting was more cost-effective in groups with less severe baseline conditions, it is possible that these results arise from confounding: for example, approximately 22% of patients in NYHA classes I and II had a radial artery graft compared to 17% in classes III and IV. Similarly, the proportion of patients with ejection fraction <50% was 26% in NYHA classes I and II compared with 31% in classes III and IV.
More generally, the results of the intention-to-treat analysis of ART may have been affected by important confounders such as the high crossover rate and the high use of the radial artery in both groups.26
Finally, while the results of our as-treated analysis support use of multiple arterial over single grafts from an economic as well as a clinical perspective, we acknowledge that the limitations of non-randomized comparisons may have biased this result. A randomized comparison of multiple arterial and single grafts will be provided by the ROMA trial.27
Conclusions
We found that BITA grafting incurs higher costs than SITA during the initial procedure which are not offset by cost savings in later years. There are no significant differences in quality-adjusted survival at 10 years and so the likelihood that BITA is cost-effective compared to SITA at 10 years is low. However, our extrapolation suggested that BITA may become more cost-effective over a lifetime horizon. Uncertainty surrounding our extrapolation results can best be reduced by continuing to follow-up ART patients for as long as possible.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
Acknowledgements
We thank all patients who participated in this trial worldwide; investigators at participating centres, steering and data and safety monitoring committee members, clinical-events adjudicators, and Dr Jeremy Pearson (British Heart Foundation) and Dr Mark Pitman (UK Medical Research Council), for support throughout; Ms Emma Haines, Ms Sarah Dutton, Mr Edmund Wyatt, Surgical Intervention Trials Unit, University of Oxford, Oxford, UK and the late Ms Eva Matesanz, Mr Wajid Aslam and Ms Fiona Nugara, Clinical Trials and Evaluation Unit, Royal Brompton and Harefield NHS Foundation Trust, London, UK for data co-ordination and management; Professor Lukasz Krzych, Medical University of Silesia, Katowice, Poland for help in co-ordinating and managing the trial in Poland; Dr Surjeet Singh and Mr Damien Hayward, Surgical Intervention Trials Unit, University of Oxford, Oxford, UK for trial support; and Dr Helen Campbell, Dr Oliver Rivero and Professor Emma McIntosh for contributions to the economic analysis over the course of the trial.
Funding
This work was supported by the British Heart Foundation, London (SP/03/001), the UK Medical Research Council, London (G0200390), and the National Institute of Health Research Efficacy and Mechanism Evaluation Programme, Southampton (09/800/29). A.M.G. is partly funded by the NIHR Biomedical Research Centre, Oxford, UK.
Conflict of interest: none declared.
Data availability
Requests for access to the data underlying this article can be sent to the corresponding author, and will be considered by the ART trial principal investigator and Trial Management Group.
Patient and public involvement
Patient and public involvement in this study was in line with the recommendations of the relevant funding bodies from the time the study was conceived and designed onwards. A patient lay member was a full member of the Trial Steering Committee and provided advice on all aspects of the study. The TSC consisted of: Professor P. Sleight, Emeritus Professor of Cardiovascular Medicine, Oxford, UK (Chairman); the late Professor D. Altman, Professor of Statistics in Medicine, Oxford, UK; Professor K. Channon, Professor of Cardiovascular Medicine, Oxford, UK; Professor J. Dark, Professor of Cardiac Surgery, Newcastle, UK; Ms B. Farrell, Trials Director, National Perinatal Epidemiology Unit, Oxford; Professor M. Flather, Professor of Medicine and Clinical Trials, Norwich, UK; Professor A. Gray, Professor of Health Economics, Oxford, UK; Professor J. Pepper, Professor of Cardiac Surgery, London, UK; Dr R. Stables, Consultant Cardiologist, Liverpool, UK; Professor D. Taggart Consultant Cardiac Surgeon, Oxford, UK (Chief Investigator); the late Professor G. Vermes, Emeritus Professor of Hebrew Studies, Oxford, UK (Patient Lay Member).
Contributor Information
Matthew Little, Nuffield Department of Population Health, Health Economics Research Centre, University of Oxford, Old Road Campus, Headington, Oxford OX3 7LF, UK.
Alastair M Gray, Nuffield Department of Population Health, Health Economics Research Centre, University of Oxford, Old Road Campus, Headington, Oxford OX3 7LF, UK.
Douglas G Altman, Nuffield Department of Orthopaedics, Rheumatology & Musculoskeletal Sciences, Centre for Statistics in Medicine, Botnar Research Centre, University of Oxford, Oxford OX3 7LD, UK.
Umberto Benedetto, School of Clinical Sciences, University of Bristol and Bristol Royal Infirmary, 69 St Michael's Hill, Bristol BS2 8DZ, UK.
Marcus Flather, Norwich Medical School, University of East Anglia and Norfolk and Norwich University Hospital, Norwich Research Park, Norwich, Norfolk NR4 7TJ, UK.
Stephen Gerry, Nuffield Department of Orthopaedics, Rheumatology & Musculoskeletal Sciences, Centre for Statistics in Medicine, Botnar Research Centre, University of Oxford, Oxford OX3 7LD, UK.
Belinda Lees, Nuffield Department of Surgical Sciences, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DU, UK.
Jacqueline Murphy, Nuffield Department of Population Health, Health Economics Research Centre, University of Oxford, Old Road Campus, Headington, Oxford OX3 7LF, UK.
Mario Gaudino, Department of Cardiothoracic Surgery, Weill Cornell Medicine, New York Presbyterian Hospital, 525 E 68th St, New York, NY 10065, USA.
David P Taggart, Nuffield Department of Surgical Sciences, University of Oxford, John Radcliffe Hospital, Oxford OX3 9DU, UK.
References
- 1. Wilkins E, Wilson L, Wickramasinghe K, Bhatnagar P, Leal J, Luengo-Fernandez R et al. European Cardiovascular Disease Statistics 2017. Brussels: European Heart Network; 2017. [Google Scholar]
- 2. Osnabrugge RL, Magnuson EA, Serruys PW, Campos CM, Wang K, van Klaveren D et al. Cost-effectiveness of percutaneous coronary intervention versus bypass surgery from a Dutch perspective. Heart 2015;101:1980–1988. [DOI] [PubMed] [Google Scholar]
- 3. Buttar SN, Yan TD, Taggart DP, Tian DH. Long-term and short-term outcomes of using bilateral internal mammary artery grafting versus left internal mammary artery grafting: a meta-analysis. Heart 2017;103:1419–1426. [DOI] [PubMed] [Google Scholar]
- 4. Taggart DP, Benedetto U, Gerry S, Altman DG, Gray AM, Lees B et al. Bilateral versus single internal-thoracic-artery grafts at 10 years. N Engl J Med 2019;380:437–446. [DOI] [PubMed] [Google Scholar]
- 5. Gray AM, Murphy J, Altman DG, Benedetto U, Campbell H, Flather M et al. One-year costs of bilateral or single internal mammary grafts in the Arterial Revascularisation Trial. Heart 2017;103:1719–1726. [DOI] [PubMed] [Google Scholar]
- 6. Little M, Gray A, Altman D, Benedetto U, Flather M, Gerry S et al. Five-year costs from a randomised comparison of bilateral and single internal thoracic artery grafts. Heart 2019;105:1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taggart DP, Altman DG, Gray AM, Lees B, Gerry S, Benedetto U et al. Randomized trial of bilateral versus single internal-thoracic-artery grafts. N Engl J Med 2016;375:2540–2549. [DOI] [PubMed] [Google Scholar]
- 8. Taggart DP, Lees B, Gray A, Altman DG, Flather M, Channon K et al. ; the ART Investigators. Protocol for the Arterial Revascularisation Trial (ART). A randomised trial to compare survival following bilateral versus single internal mammary grafting in coronary revascularisation. Trials 2006;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The Euroqol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 10. Lawlor DA, Adamson J, Ebrahim S. Performance of the WHO Rose angina questionnaire in post-menopausal women: are all of the questions necessary? J Epidemiol Community Health 2003;57:538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247–263. [DOI] [PubMed] [Google Scholar]
- 12. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095–1108. [DOI] [PubMed] [Google Scholar]
- 13. Reed SD, Li Y, Leal J, Radican L, Adler AI, Alfredsson J et al. ; for the TECOS Study Group. Longitudinal medical resources and costs among type 2 diabetes patients participating in the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS). Diabetes Obes Metab 2018;20:1732–1739. [DOI] [PubMed] [Google Scholar]
- 14. Becker F, Dakin H, Reed SD, Li Y, Leal J, Gustavson S et al. Lifetime cost-effectiveness simulation of exenatide once-weekly in type 2 diabetes: evidence from the EXSCEL trial (abstr). Diabetologia 2018;61:S366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Organisation for Economic Co-operation and Development. Purchasing power parities for GDP. http://stats.oecd.org/Index.aspx?datasetcode=SNA_TABLE4 (14 August 2020).
- 16. Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487–496. [DOI] [PubMed] [Google Scholar]
- 17. NICE. Developing NICE guidelines: the manual. https://www.nice.org.uk/process/pmg20/chapter/incorporating-economic-evaluation (5 June 2019).
- 18. Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219–3236. [DOI] [PubMed] [Google Scholar]
- 19. Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212–214. [DOI] [PubMed] [Google Scholar]
- 20. Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves—facts, fallacies and frequently asked questions. Health Econ 2004;13:405–415. [DOI] [PubMed] [Google Scholar]
- 21. Gaudino M, Benedetto U, Fremes S, Biondi-Zoccai G, Sedrakyan A, Puskas JD et al. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N Engl J Med 2018;378:2069–2077. [DOI] [PubMed] [Google Scholar]
- 22. White IR, Horton NJ, Carpenter J, Statistics RIMAS, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342:d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 2004, p33. [Google Scholar]
- 24. Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med 2018;37:2252–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leurent B, Gomes M, Faria R, Morris S, Grieve R, Carpenter JR et al. Sensitivity analysis for not-at-random missing data in trial-based cost-effectiveness analysis: a tutorial. Pharmacoeconomics 2018;36:889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaudino MFL, Taggart DP, Fremes SE. The ROMA trial: why it is needed. Curr Opin Cardiol 2018;33:622–626. [DOI] [PubMed] [Google Scholar]
- 27. Gaudino M, Alexander JH, Bakaeen FG, Ballman K, Barili F, Calafiore AM et al. Randomized comparison of the clinical outcome of single versus multiple arterial grafts: the ROMA trial—rationale and study protocol. Eur J Cardiothorac Surg 2017;52:1031–1040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for access to the data underlying this article can be sent to the corresponding author, and will be considered by the ART trial principal investigator and Trial Management Group.