Abstract
Objective
Increasing psoriasis severity has been associated with comorbidities including cardiovascular disease. The objective of this study was to examine the association of psoriasis severity with the development of PsA.
Methods
A prospective population-based cohort study was performed within The Health Improvement Network, a UK medical record database. Patients aged 25–60 years with a code for psoriasis were randomly selected between 2008 and 2011. Questionnaires were sent to their general practitioners to confirm the diagnosis of psoriasis and provide the patient’s approximate body surface area (BSA). Incidence of PsA was calculated by BSA, and Cox proportional hazard ratios were used to examine the risk of developing PsA by BSA category after adjusting for other covariates.
Results
Among 10 474 questionnaires sent, 9987 (95%) were returned, 9069 (91%) had confirmed psoriasis, and BSA was provided for 8881 patients: 52% had mild psoriasis, 36% moderate psoriasis and 12% severe psoriasis. The mean age was 46, and 49% were female. Mean follow-up time was 4.2 years (s.d. 2.1); the incidence of PsA was 5.4 cases per 1000 person-years. After adjusting for age and sex, BSA >10% [hazard ratio (HR) 2.01, 95% CI: 1.29, 3.13], BSA 3–10% (HR 1.44, 95% CI: 1.02, 2.03), obesity (HR 1.64, 95% CI: 1.19, 2.26) and depression (HR 1.68, 95% CI: 1.21, 2.33) were associated with incident PsA.
Conclusions
In this large prospective cohort study, BSA assessed by general practitioners was a strong predictor of developing PsA, and obesity and depression were additive risk factors.
Keywords: psoriatic arthritis, psoriasis, epidemiology, obesity, depression
Rheumatology key messages.
Obesity, depression and psoriasis severity may be risk factors for PsA among those with psoriasis.
This large, prospective, population-based cohort study examined combined effects of common risk factors for PsA.
General practitioner assessment of body surface area of psoriasis is a strong predictor of developing PsA.
Introduction
Psoriasis is a common inflammatory skin condition associated with systemic inflammation that affects up to 2% of adults in North America and Europe [1]. As the total amount of psoriasis increases, as measured by body surface area (BSA), the risk for other chronic diseases such as coronary artery disease [2, 3] and diabetes, as well as the risk for mortality, also increases [4, 5]. Thus, measuring psoriasis severity in clinical practice can help risk-stratify patients who may be at increased risk for cardiometabolic disease. However, PsA is among the most common and impactful concomitant conditions in patients with psoriasis, affecting 10–30% of patients [6, 7]. PsA is a chronic inflammatory arthritis that can cause significant disability. The early identification of PsA is important to improving long-term outcomes, including decreased radiographic damage and improved response to therapy [8, 9]. However, early identification remains an important challenge [10]. Compared with other rheumatic diseases, PsA has a substantial advantage: most patients with PsA first developed psoriasis [6]. If general practitioners (GPs) could risk-stratify patients with psoriasis and integrate that knowledge into the assessment of joint complaints in this patient population, they may decrease the prolonged time to diagnosis in PsA [11–13].
As with cardiometabolic disease, psoriasis severity has been identified as a potential risk factor for PsA. Only two cohort studies have addressed whether psoriasis severity increases the risk for PsA: one retrospective cohort study used number of sites of psoriasis as a measure of severity, and the other studied a dermatology patient population and used the Psoriasis Area and Severity Index (PASI), a measure that is too complex to implement into primary care clinical practice [14, 15]. No prospective population-based cohort studies have fully addressed the risk for PsA by psoriasis severity as measured by BSA [6].
Beyond psoriasis severity, one of the consistently identified risk factors for PsA is obesity [16–19]. Metabolic conditions, including hyperlipidaemia, have also been associated with the development of PsA [20]. Obesity and metabolic conditions are known to be associated with more severe psoriasis and thus may be potential confounders in the relationship between psoriasis severity and PsA [21]. It remains unclear whether obesity and psoriasis severity are independent risk factors for PsA that each contribute to risk. The objective of this paper is to examine the association of BSA (an objective measure of psoriasis severity), obesity and other potential risk factors with the development of PsA in a prospective cohort of patients with psoriasis.
Methods
Study design
This was a prospective population-based cohort study.
Patient population
The Health Improvement Network (THIN) is a population-based electronic medical record database that collects data from GP practices in the UK. Over 7 million lives are covered in this dataset including ∼130 000 patients with psoriasis. This study was performed within the Incident Health Outcomes and Psoriasis Events (iHOPE) cohort, a prospective cohort study embedded within THIN that has been previously described [4, 22, 23]. Between 2008 and 2011, patients with at least one code for psoriasis who were aged 25–60 years in THIN were randomly selected from among all patients with psoriasis who were followed in participating practices. Questionnaires were sent to their GPs to ascertain severity of psoriasis. This cohort (iHOPE) was then followed prospectively for the development of incident cardiovascular disease and PsA. The date of questionnaire receipt was the index date. Patients with psoriasis were matched on age, category, sex and practice to up to 10 general population controls to serve as a reference. This reference population helps in understanding the risk for collider bias (i.e. the risk that we identify risk factors for PsA among patients with psoriasis that may not be risk factors for PsA in the general population) [24, 25]. Patients with PsA, RA, reactive arthritis and ankylosing spondylitis (AS) prior to index date were excluded. Data through 2015 were used in the current analysis.
This study complies with the Declaration of Helsinki. This protocol was approved by THIN Scientific Review Committee and is considered exempt by the University of Pennsylvania Institutional Review Board. This study utilized de-identified data. All questionnaires went through a third party so that the investigators never received identifiable information.
Outcome
The primary outcome of interest was the development of incident PsA, which was defined as the presence of a single code (Supplementary Table S1, available at Rheumatology online). The code for PsA was previously validated and found to have a positive predictive value (PPV) of 85% [26]. In a sensitivity analysis, we examined the alternative outcomes of RA, AS, gout and OA, all previously studied disease states in THIN [27–33]. The goal of these analyses was to understand whether risk factors identified for PsA among patients with psoriasis were unique to PsA or common to other types of arthritis (suggesting potential misclassification).
Exposures
GPs were asked to confirm the diagnosis of psoriasis and to provide the patient’s approximate BSA that the patient typically demonstrates using the language from the Patient Report of Extent of Psoriasis Involvement (PREPI) tool [34]. BSA categories were also included on the survey and categorized based on categories that are commonly used for epidemiological studies: <3%, 3–10%, and >10%, representing mild, moderate, and severe, respectively [4].
Covariates
Covariates were defined prior to the index date. BMI was obtained nearest to the index date and divided into World Health Organization categories (underweight <18.5, normal 18.5–24.9, overweight 25–29.9 and obese >30). We also examined smoking, alcohol intake and other previously defined risk factors, including depression, uveitis, inflammatory bowel disease, metabolic comorbidities (i.e. hypertension, hyperlipidaemia, diabetes), thyroid disease, anxiety and diarrhoeal illness [6, 35]. Covariates were defined by the presence of a READ code [36, 37].
Statistical analysis
After excluding patients with PsA at baseline, the incidence of PsA among patients with psoriasis was calculated. The prevalence of covariates at the index date was descriptively reported and compared with matched population controls for reference. Cox proportional hazard ratios were used to examine the risk of developing PsA among patients with mild (<3%), moderate (3–10%) and severe (>10%) psoriasis. Other covariates were tested in univariable models. We used a purposeful selection modelling approach [38]. Factors significant at the univariable level (P < 0.1) were included in multivariable models and removed if they were not statistically significant (P > 0.05) or did not change the main effects (i.e. BSA coefficient). We also decided to a priori test biologically relevant covariates in the final model regardless of the initial P-value (i.e. age, sex and obesity).
We additionally tested a statistical interaction between BSA and obesity, and we present both stratified and unstratified results. Finally, a similar process was used to generate multivariable Cox models for the outcomes of RA, AS, gout and OA.
Results
Among 10 474 questionnaires sent, 9987 (95%) were returned. Of those, 9069 (91%) had confirmed psoriasis. The mean age was 46, and 49% were female (Table 1). Aside from age, sex and menopause, most comorbidities were more common in patients with psoriasis compared with matched general population controls without psoriasis. BSA was provided for 8881 patients, of whom 50% had BSA <3% (mild psoriasis), 35% had BSA 3–10% (moderate psoriasis) and 12% had BSA >10% (severe psoriasis). A total of 45% of patients with psoriasis were followed by a dermatologist. Patients with a previous diagnosis of RA (n = 94), reactive arthritis (n = 15), AS (n = 29) and general inflammatory arthritis codes (n = 43) were excluded in further analyses.
Table 1.
Baseline characteristics
| Characteristic | Controls | Psoriasis | SMD |
|---|---|---|---|
| (n = 90 547) | (n = 9056) | ||
| Female | 47 887 (53) | 4478 (49) | 0.00 |
| BSA | |||
| Mild | 4531 (50) | −0.10 | |
| Moderate | 3129 (35) | 1.49 | |
| Severe | 1087 (12) | 1.50 | |
| Missing BSA | 309 (3) | 0.94 | |
| Obese | 19 674 (22) | 2424 (27) | 0.08 |
| Missing BMI | 10 589 (12) | 1005 (11) | 0.16 |
| Smoker | |||
| Never | 46 115 (51) | 3531 (39) | −0.01 |
| Current | 21 788 (24) | 2828 (31) | −0.27 |
| Past | 20 747 (23) | 2572 (28) | 0.16 |
| Missing smoker | 1897 (2) | 125 (1) | 0.42 |
| Alcohol | |||
| Never | 12 288 (14) | 1118 (12) | −0.02 |
| Current | 65 108 (72) | 6647 (73) | −0.03 |
| Past | 2027 (2) | 247 (3) | 0.10 |
| Missing alcohol | 11 124 (12) | 1044 (12) | 0.02 |
| Fracture | 21 755 (24) | 2404 (27) | −0.02 |
| COPD | 1203 (1) | 176 (2) | 0.20 |
| Hypertension | 13 293 (15) | 1464 (16) | 0.02 |
| Diabetes | 5896 (7) | 711 (8) | 0.06 |
| Hyperlipidaemia | 7829 (9) | 909 (10) | 0.05 |
| Liver disease | 1307 (1) | 162 (2) | 0.11 |
| Anxiety | 16 227 (18) | 1850 (20) | 0.01 |
| Depression | 23 284 (26) | 2634 (29) | 0.06 |
| Substance abuse | 1469 (2) | 175 (2) | 0.26 |
| Menopausea | 8027 (17)a | 813 (18)a | 0.01 |
| Thyroid disease | 4188 (5) | 458 (5) | 0.01 |
| Pharyngitis | 22 135 (24) | 2430 (27) | 0.01 |
| Diarrhoea | 15 264 (17) | 1798 (20) | 0.06 |
| Crohn's disease | 350 (0) | 47 (1) | 0.45 |
| Ulcerative colitis | 513 (1) | 60 (1) | 0.02 |
| Uveitis | 676 (1) | 90 (1) | 0.01 |
| PsA | 4 (0) | 637 (7) | 0.01 |
| Ankylosing spondylitis | 186 (0) | 29 (<1) | 1.37 |
| RA | 585 (1) | 94 (1) | 0.01 |
| Reactive arthritis | 95 (0) | 15 (<1) | 0.11 |
| Inflammatory arthritis | 148 (0) | 43 (<1) | 0.01 |
| Gout | 1923 (2) | 296 (3) | 0.02 |
| OA | 6331 (7) | 753 (8) | 0.04 |
Values are shown as n (%). Menopause proportion is among women only. Italicized text in the table refers to missing data to help the reader understand the proportion of missing data for each variable. BSA: body surface area; COPD: chronic obstructive pulmonary disease; SMD: standardized mean difference.
Mean follow-up time was 4.2 years (s.d. 2.1); the incidence of PsA was 5.4 cases per 1000 person years (Table 2). Among those with BSA >10%, 35 (3.9%) developed PsA during the observation period, compared with 73 (1.7%) and 75 (3.0%) with BSA <3% and BSA 3–10%, respectively. New diagnosis of PsA was uncommon in the general population patients without psoriasis (0.03%).
Table 2.
Incidence of PsA
| Psoriasis (all) | Mild psoriasis (BSA < 3%) | Moderate psoriasis (BSA 3–10%) | Severe psoriasis (BSA >10%) | Controls | |
|---|---|---|---|---|---|
| New PsA diagnoses, n | 190 | 73 | 75 | 35 | 29 |
| Time, mean, years | 4.2 | 4.2 | 4.0 | 4.2 | 4.2 |
| Total, n | 8323 | 4310 | 2836 | 899 | 89 598 |
| PYs | 34 915 | 18 164 | 12 041 | 3606 | 374 616 |
| Incidence/1000 PYs | 5.4 | 4.0 | 6.2 | 9.7 | 0.08 |
BSA: body surface area; PYs: person-years.
Univariable and multivariable hazard ratios are shown in Table 3. After adjusting for age and sex, the final model included psoriasis severity with BSA >10% vs BSA <3% (HR 2.01, 95% CI: 1.29, 3.13), BSA 3–10% vs BSA <3% (HR 1.44, 95% CI: 1.02, 2.03), obesity (HR 1.64, 95% CI: 1.19, 2.26) and depression (HR 1.68, 95% CI: 1.21, 2.33). This model suggests that psoriasis severity, obesity and depression are independent risk factors in defining risk for PsA. To address the concern that patients with moderate to severe psoriasis and/or comorbidities were followed more closely for the outcome, a sensitivity analysis including only patients followed at least annually during the study period (to allow for adequate detection of the outcome) produced similar results to the final model.
Table 3.
Univariable and multivariable models for incident PsA
| HR (95% CI) |
||||
|---|---|---|---|---|
| Univariable | Multivariable (model 1) | Multivariable (model 2) | Sensitivity analysis (followed yearly, n = 5975) | |
| Age | 0.99 (0.98, 1.00) | 0.99 (0.97, 1.01) | 0.99 (0.98, 1.00) | 0.98 (0.97, 1) |
| Sex | 0.80 (0.60, 1.06) | 0.75 (0.53, 1.07) | 0.72 (0.52, 1.00) | 0.63 (0.46, 0.87) |
| BMI | 1.05 (1.02, 1.07) | |||
| Obese | 1.76 (1.29, 2.40) | 1.49 (1.05, 2.13) | 1.64 (1.19, 2.26) | 1.49 (1.08, 2.06) |
| Smoker | ||||
| Never | Ref | |||
| Current | 0.93 (0.65, 1.33) | |||
| Past | 1.29 (0.92, 1.81) | |||
| BSA | ||||
| Continuous BSA (per 1% unit) | 1.02 (1.01, 1.03) | |||
| Continuous BSA (per 5% unit) | 1.09 (1.04, 1.16) | 1.08 (1.01, 1.14) | ||
| Mild (<3%) | Ref | Ref | ||
| Moderate (3–10%) | 1.55 (1.12, 2.14) | 1.44 (1.02, 2.03) | 1.38 (0.98, 1.95) | |
| Severe (>10%) | 2.41 (1.61, 3.6) | 2.01 (1.19, 3.13) | 1.82 (1.17, 2.84) | |
| Anxiety | 1.00 (0.70, 1.42) | |||
| Depression | 1.45 (1.08, 1.95) | 1.73 (1.21, 2.47) | 1.68 (1.21, 2.33) | 1.54 (1.12, 2.13) |
| Diarrhoeal illness | 1.38 (1.00, 1.92) | |||
| Duration 5+ years (vs <5) | 1.37 (1.01, 1.87) | |||
| Hyperlipidaemia | 1.03 (0.65, 1.63) | |||
| Hypertension | 1.14 (0.78, 1.65) | |||
| Diabetes | 0.75 (0.41, 1.38) | |||
| Liver disease | 0.92 (0.30, 2.89) | |||
| COPD | 0.89 (0.28, 2.78) | |||
| Fracture | 1.02 (0.74, 1.41) | |||
| Anxiety | 1.00 (0.70, 1.42) | |||
| Substance abuse | 0.85 (0.27, 2.65) | |||
| Thyroid disease | 1.15 (0.63, 2.12) | |||
| Pharyngitis | 1.19 (0.87, 1.63) | |||
| Crohn’s disease | 1.05 (0.15, 7.52) | |||
Multivariable models include those covariates with a HR in the respective column. Model 1 includes BSA as a continuous variable (per increase of 5%), obesity (BMI ≥30 vs BMI <30), and depression (binary) and adjusts for age and sex. Model 2 includes BSA by category (mild, moderate and severe), obesity and depression and adjusts for age and sex. Psoriasis duration was not statistically significant in the multivariable model. BSA: body surface area; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; Ref: reference group.
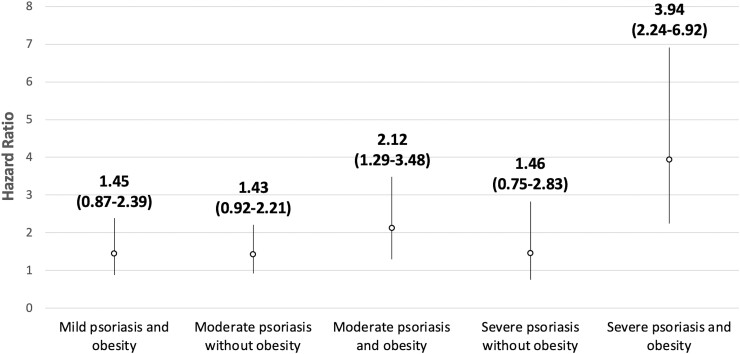
In the final multivariable model, we tested an interaction term between obesity (binary) and psoriasis severity (three categories), with mild psoriasis without obesity as the reference group. The stratified results are shown visually in Fig. 1. Patients with moderate psoriasis and obesity had a HR of 2.12 (95% CI: 1.29, 3.48) compared with patients with mild psoriasis and no obesity. The HR for severe psoriasis without obesity and severe psoriasis with obesity were 1.46 (95% CI: 0.75, 2.83) and 3.94 (95% CI: 2.24, 6.92), respectively. The interaction term was not statistically significant (P = 0.34).
Fig. 1.
Psoriasis severity–obesity interaction
While there is a qualitative increase in the risk for PsA in patients who are both obese and have moderate or severe psoriasis, the interaction term is not statistically significant. Mild psoriasis without obesity is the reference group (1.0). Error bars represent the 95% CIs.
While the effect size (or HR) of each of these individual variables was high, the incidence of developing PsA when having one or more of these factors was still overall low. Thus, the positive predictive value for these predictors is low; the PPV of having all three conditions was 8.5% (Supplementary Table S2, available at Rheumatology online). For reference, this PPV is similar to the risk estimates in the ACC/AHA 2013 atherosclerotic cardiovascular disease risk algorithm used in cardiovascular prevention where 7.5% 10-year risk is considered intermediate risk [39].
To examine the specificity of these risk factors for PsA, we additionally examined the risk for other types of arthritis in psoriasis including RA, OA, AS and gout (Supplementary Table S3, available at Rheumatology online). Obesity was not associated with new RA in patients with psoriasis but was associated with the development of gout and OA. Psoriasis severity was not associated with these other outcomes. Depression was associated with the diagnosis of OA but not associated with RA or gout (Supplementary Table S4, available at Rheumatology online).
Discussion
In this large prospective cohort study, we found that BSA is a strong predictor of developing PsA, and obesity and depression are additive risk factors among patients with psoriasis. Knowledge of the patient’s BSA may help GPs assess the patient’s risk for PsA as well as the patient’s risk for cardiometabolic outcomes [2, 3, 40]. One of the key advantages of BSA is that it can be easily assessed by GPs, as demonstrated by this study, and by patients themselves, as demonstrated previously [34].
This study adds to the literature the combined effects of key risk factors in an adequately powered study and has several strengths. To our knowledge, this is the first prospective population-based cohort of patients with confirmed psoriasis with known BSA with follow-up of 3–7 years. The large number allowed for testing risk factors of relatively low prevalence for a relatively rare outcome. Patients were followed prospectively and without specific surveillance for the outcome of interest. One note of caution is that there may be underdiagnosis or misdiagnosis of these outcomes [10]. This misclassification should be non-differential (i.e. similar between groups) and thus would not have a significant impact on the outcome. On the other hand, it may be that patients with severe psoriasis are more likely to be diagnosed with PsA because the skin symptoms are more apparent.
There are important considerations and some limitations in interpreting the results of this study. First, a limited number of risk factors (defined a priori) were tested due to the relatively small number of outcomes. Second, GPs, rather than dermatologists, assessed BSA. This is assumed to be valid as patients are able to validly quantify BSA [34]. While developed as a patient assessment, this is a simple tool that explains quantification of BSA that could be used by providers as well and is a pragmatic way to monitor BSA in primary care practice. Third, as noted above, detection bias may have played a role in the identification of PsA among patients with more severe psoriasis. Obese and/or depressed patients and those with more comorbidities may also see their GP more often. We addressed this in a sensitivity analysis among patients who had seen their GP at least yearly, and the results were similar. Psoriasis BSA and other risk factors were measured at a single time point, and the cumulative effect of psoriasis may be important. Time-varying analysis may have led to different effect sizes but would also answer a different question. Fourth, the effect sizes for interaction between obesity and psoriasis severity are provocative but not statistically significant, potentially due to insufficient sample size in the smallest subgroups. This should be tested again in future larger studies. Additionally, more follow-up time may allow for the greater power to detect a difference. Finally, we did not account for the treatment of psoriasis in this algorithm. This was intentional as confounding by indication (being prescribed a medication hinges on having sufficiently severe psoriasis to warrant it) prevents the interpretation of the model outcomes [6, 25].
The results of this study have external validity and are consistent with the results seen in previous studies that addressed psoriasis severity as a risk factor for PsA. Obesity and depression have previously been described as risk factors with nearly identical hazard ratios in population-based cohorts [16, 18, 19, 35]. Only one cohort study, conducted by Eder et al., found that a PASI score of >20 compared with <10 was associated with the development of PsA among patients with psoriasis who were enrolled from phototherapy and dermatology clinics (HR 5.39, 95% CI: 1.64, 17.7) after adjusting for education and retinoid use, the only two other variables in the final model [14]. Notably, PASI, a commonly used psoriasis severity index in dermatology, is less feasible for use in primary care practice than a simple BSA.
This study demonstrates that a combination of relatively common risk factors can be used to approximate risk for PsA in patients with psoriasis in primary care clinical practice. These data inform which patients may be at highest risk for PsA such that a patient’s presentation with joint complaints may warrant further work-up for inflammatory arthritis. Furthermore, these data suggest that there is an opportunity to identify patients with PsA earlier and open the opportunity for interventions targeting prevention of PsA. Additionally, these data underscore the importance of both obesity and depression, treatable conditions, in patients with psoriasis.
Supplementary Material
Acknowledgements
We thank Tori Fischer for assistance in medical editing and administrative assistance.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained. In writing this manuscript, we have adhered to the Strengthening The Reporting of OBservational studies in Epidemiology (STROBE) guidelines [41].
Funding: This work was supported by the National Institutes of Health/the National Institute of Arthritis and Musculoskeletal and Skin Diseases [grant number K23 AR063764 to A.O.]; and the National Institutes of Health/the National Heart, Lung, and Blood Institute [grant number R01 HL089744 to J.M.G.]. The funding source (National Institutes of Health) had no involvement in study design, execution or interpretation. Researchers were independent from funders, and all authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure statement: No commercial entities provided support for the work in the submitted manuscript. A.O.: grants from Novartis (to Penn), Pfizer (To Penn), Amgen (To Forward/NDB); Consulting for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Eli Lilly and Company, Gilead, Janssen, Novartis, Pfizer, UCB and Takeda. T.J.L: served as a consultant for Celgene. J.G: served as a consultant for BMS, Boehringer Ingelheim, Lilly, Janssen Biologics, Novartis Corp, UCB (DSMB), Neuroderm (DSMB), Dr Reddy’s Labs, Pfizer Inc. and Sun Pharma, receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Boehringer Ingelheim, Janssen, Novartis Corp, Celgene, Ortho Dermatologics and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Lilly, Ortho Dermatologics and Novartis. J.M.G. is a co-patent holder of resiquimod for treatment of cutaneous T cell lymphoma, and is a Deputy Editor for the Journal of Investigative Dermatology, receiving honoraria from the Society for Investigative Dermatology, and is a member of the Board of Directors for the International Psoriasis Council, receiving no honoraria. D.S. has declared no conflicts of interest.
Data availability tatement
Data were obtained from The Health Improvement Network. Requests for data can be processed through IQVIA. Code lists, stata do-files and de-identified patient survey data can be obtained from the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Springate DA, Parisi R, Kontopantelis E. et al. Incidence, prevalence and mortality of patients with psoriasis: a U.K. population-based cohort study. Br J Dermatol 2017;176:650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wan MT, Shin DB, Hubbard RA. et al. Psoriasis and the risk of diabetes: a prospective population-based cohort study. J Am Acad Dermatol 2018;78:315–22.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noe MH, Shin DB, Wan MT, Gelfand JM.. Objective measures of psoriasis severity predict mortality: a prospective population-based cohort study. J Invest Dermatol 2018;138:228–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeung H, Takeshita J, Mehta NN. et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol 2013;149:1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takeshita J, Grewal S, Langan SM. et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol 2017;76:377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scher JU, Ogdie A, Merola JF, Ritchlin C.. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol 2019;15:153–66. [DOI] [PubMed] [Google Scholar]
- 7. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:2095–6. [DOI] [PubMed] [Google Scholar]
- 8. McHugh NJ. Verna Wright lecture: Psoriatic arthritis: the need for early intervention. J Rheumatol Suppl 2015;93:10–3. [DOI] [PubMed] [Google Scholar]
- 9. McHugh NJ. Early psoriatic arthritis. Rheum Dis Clin North Am 2015;41:615–22. [DOI] [PubMed] [Google Scholar]
- 10. Mease PJ, Gladman DD, Papp KA. et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol 2013;69:729–35. [DOI] [PubMed] [Google Scholar]
- 11. Haroon M, Gallagher P, FitzGerald O.. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis 2015;74:1045–50. [DOI] [PubMed] [Google Scholar]
- 12. Ogdie A, Nowell WB, Applegate E. et al. Patient perspectives on the pathway to psoriatic arthritis diagnosis: results from a web-based survey of patients in the United States. BMC Rheumatol 2020;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boehncke WH, Horvath R, Dalkilic E. et al. Association between clinical specialty setting and disease management in patients with psoriatic arthritis: results from LOOP, a cross-sectional, multi-country, observational study. J Eur Acad Dermatol Venereol 2020;34:2035–43. [DOI] [PubMed] [Google Scholar]
- 14. Eder L, Haddad A, Rosen CF. et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol 2016;68:915–23. [DOI] [PubMed] [Google Scholar]
- 15. Wilson FC, Icen M, Crowson CS. et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum 2009;61:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Green A, Shaddick G, Charlton R. et al. ; PROMPT study group. Modifiable risk factors and the development of psoriatic arthritis in people with psoriasis. Br J Dermatol 2020;182:714–20. [DOI] [PubMed] [Google Scholar]
- 17. Soltani-Arabshahi R, Wong B, Feng BJ. et al. Obesity in early adulthood as a risk factor for psoriatic arthritis. Arch Dermatol 2010;146:721–6. [DOI] [PubMed] [Google Scholar]
- 18. Love TJ, Zhu Y, Zhang Y. et al. Obesity and the risk of psoriatic arthritis: a population-based study. Ann Rheum Dis 2012;71:1273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li W, Han J, Qureshi AA.. Obesity and risk of incident psoriatic arthritis in US women. Ann Rheum Dis 2012;71:1267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu S, Li WQ, Han J, Sun Q, Qureshi AA.. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol 2014;66:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jensen P, Skov L.. Psoriasis and obesity. Dermatology 2016;232:633–9. [DOI] [PubMed] [Google Scholar]
- 22. Seminara NM, Abuabara K, Shin DB. et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol 2011;164:602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ogdie A, Langan S, Love T. et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford) 2013;52:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cole SR, Platt RW, Schisterman EF. et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol 2010;39:417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogdie A. The preclinical phase of PsA: a challenge for the epidemiologist. Ann Rheum Dis 2017;76:1481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ogdie A, Alehashemi S, Love TJ. et al. Validity of psoriatic arthritis and capture of disease modifying antirheumatic drugs in the health improvement network. Pharmacoepidemiol Drug Saf 2014;23:918–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez LA, Tolosa LB, Ruigomez A, Johansson S, Wallander MA.. Rheumatoid arthritis in UK primary care: incidence and prior morbidity. Scand J Rheumatol 2009;38:173–7. [DOI] [PubMed] [Google Scholar]
- 28. Thomas SL, Edwards CJ, Smeeth L, Cooper C, Hall AJ.. How accurate are diagnoses for rheumatoid arthritis and juvenile idiopathic arthritis in the general practice research database? Arthritis Rheum 2008;59:1314–21. [DOI] [PubMed] [Google Scholar]
- 29. Dubreuil M, Peloquin C, Zhang Y. et al. Validity of ankylosing spondylitis diagnoses in The Health Improvement Network. Pharmacoepidemiol Drug Saf 2016;25:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeng C, Bennell K, Yang Z. et al. Risk of venous thromboembolism in knee, hip and hand osteoarthritis: a general population-based cohort study. Ann Rheum Dis 2020;79:1616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zeng C, Dubreuil M, LaRochelle MR. et al. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA 2019;321:969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cea Soriano L, Rothenbacher D, Choi HK, Garcia Rodriguez LA.. Contemporary epidemiology of gout in the UK general population. Arthritis Res Ther 2011;13:R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu N, Dubreuil M, Zhang Y. et al. Gout and the risk of Alzheimer's disease: a population-based, BMI-matched cohort study. Ann Rheum Dis 2016;75:547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dommasch ED, Shin DB, Troxel AB, Margolis DJ, Gelfand JM.. Reliability, validity and responsiveness to change of the Patient Report of Extent of Psoriasis Involvement (PREPI) for measuring body surface area affected by psoriasis. Br J Dermatol 2010;162:835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lewinson RT, Vallerand IA, Lowerison MW. et al. Depression is associated with an increased risk of psoriatic arthritis among patients with psoriasis: a population-based study. J Invest Dermatol 2017;137:828–35. [DOI] [PubMed] [Google Scholar]
- 36. Garcia Rodriguez LA, Perez Gutthann S.. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol 1998;45:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL.. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 2007;16:393–401. [DOI] [PubMed] [Google Scholar]
- 38. Bursac Z, Gauss CH, Williams DK, Hosmer DW.. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S49–73. [DOI] [PubMed] [Google Scholar]
- 40. Gelfand JM, Neimann AL, Shin DB. et al. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296:1735–41. [DOI] [PubMed] [Google Scholar]
- 41. von Elm E, Altman DG, Egger M. et al. ; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data were obtained from The Health Improvement Network. Requests for data can be processed through IQVIA. Code lists, stata do-files and de-identified patient survey data can be obtained from the corresponding author.