Abstract
Objective
To estimate the occurrence and relative risks of first-ever-incident non-cutaneous cancer overall and for 16 sites in patients with RA treated with biologic and targeted synthetic DMARDs (b/tsDMARDs), by time since treatment start, attained age, and duration of active treatment.
Methods
This is an observational nationwide and population-based cohort study of patients with RA (n = 69 308), treated with TNF inhibitors (TNFi; adalimumab, certolizumab, etanercept, golimumab, infliximab) or other b/tsDMARDs (abatacept, rituximab, baricitinib, tofacitinib and tocilizumab) compared with RA patients not treated with b/tsDMARDs, and matched general population referents (n = 109 532), 2001–2018. The study was based on prospectively collected data from the Swedish Rheumatology Quality Register and from other registers, linked to the national Swedish Cancer Register. Incidence rates and hazard ratios were estimated via Cox regression adjusted for co-morbidities and other health characteristics.
Results
Based on 8633 incident cancers among RA patients, the overall relative risk of cancer with TNFi [hazard ratio (HR) = 1.0] was neither increased nor did it change with time since treatment start, duration of active treatment, or attained age, when compared with b/tsDMARD-naïve RA. For other b/tsDMARDs, we noted no consistent signal of increased overall risks (HRs ranged from 1.0 to 1.2), but there were statistically significant estimates above 1 for abatacept with 2–5 years of active treatment, for older age groups, and between several of the bDMARDs and urinary tract cancer.
Conclusion
TNFis, as used long term in clinical practice against RA, are not linked to increased risks for cancer overall. For other b/tsDMARDs, and for site-specific risks, our results are generally reassuring but contain signals that call for replication.
Keywords: rheumatoid arthritis, b/tsDMARD, cancer, Sweden, register, cohort
Rheumatology key messages.
We found no increased risk for cancer overall in RA patients treated with TNFis, anti-CD20 or anti-IL6.
For RA patients treated with abatacept, we noted a potential signal of increased risk for cancer overall.
A potential signal for increased risk for urinary tract cancer for RA patients treated with TNFis, rituximab, abatacept and tocilizumab was identified.
Introduction
Biologic and targeted synthetic DMARDs [b/tsDMARDs; including tumor necrosis factor inhibitors (TNFis), abatacept, anti-CD20 and anti-IL6 treatments, and Janus Kinase inhibitors (JAKis)] have been approved for treatment of RA, and for a growing number of other chronic inflammatory diseases. Since b/tsDMARDs interfere with the immune system, and in light of the role of immune competence in cancer development and outcome [1], concerns have been raised that b/tsDMARDs might impede host immune-surveillance against emergent cancers, or accelerate the growth of existing cancers, and have short-as well as longer-term effects on cancer risks [2]. For TNFis, early meta-analyses of pivotal trials suggested a possible increase in the risk of cancer already within months [3]. Later meta-analyses [4] and most observational studies have not found evidence of an increase in the overall risk of cancer with TNFis [5–8].
Studies on cancer risks with TNFis have been based on relatively short follow-up (although a few studies had a medium follow-up of more than 3 years), so this has left questions on longer-term risks essentially unanswered [9–11]. This is concerning, as from a cancer induction and surveillance point of view, relevant time-frames may be much longer. For non-TNFi bDMARDs, the available safety data are based on data from clinical trial programs and (a few) observational cohorts, with even shorter follow-up times than those available for TNFis. So far, the output from these studies has been either reassuring [12, 13] or pointed to signals of potential risk increases [9, 14]. For tsDMARDs, because of their later introduction, data on cancer risks from their use in clinical practice are scarce [15].
Further, the risk of cancer in older age groups, where the background incidence is higher and the distribution of risks for site-specific cancers is different, remains less well understood. With b/tsDMARDs increasingly used in elderly patients, such information is critical.
For all of the above reasons, the primary aim of this study was to assess the association between b/tsDMARDs and short- and longer-term cancer incidence in patients with RA. A secondary aim was to study the occurrence and relative risks of cancer in these populations by attained age and duration of treatment. A third aim was to put the observed incidences in relation to those expected in the general population [16, 17].
Methods
Study design and data sources
We performed an observational, nationwide cohort study in patients with RA using data from the Swedish Rheumatology Quality Register (SRQ), enriched through linkage with other national registers, including the National Patient Register (NPR), Prescribed Drug Register (PDR), Total Population Register, and Cancer Register (Supplementary Table S1, available at Rheumatology online). The study was approved by the Swedish Ethical Review Authority (2015–1844-31/2).
Study population
All individuals with RA above 18 years of age were identified using the SRQ and the NPR (Supplementary Table S2, available at Rheumatology online). In the NPR, all individuals with two or more visits in non-primary outpatient care listing a main diagnosis of RA between 2001 and 2018 were identified. At least one of these visits had to be from an internal medicine or rheumatology department. The second of the two visits served as date of inclusion. Through this, we identified virtually all patients with RA alive in Sweden at the beginning of our study period (1 January 2001) or diagnosed with RA before the end of our study period (31 December 2018). For each unique individual with RA, five general population subjects from the Total Population Register were matched.
Exposure
Through the SRQ and PDR, we identified all dispensing of oral or s.c. administered b/tsDMARDs. In addition, through SRQ, we identified all registered i.v. bDMARD treatment starts. We defined exposure cohorts: (i) any TNFi drug (adalimumab, certolizumab pegol, etanercept, golimumab, infliximab), (ii) rituximab, (iii) abatacept, (iv) tocilizumab, (v) any JAKi (baricitinib or tofacitinib) and (vi) b/tsDMARD-naïve RA (Supplementary Table S3, available at Rheumatology online), and (vii) the general population referents. One individual could contribute to more than one cohort, each with its own baseline-data pertaining to the time-point of entry into the cohort in question.
Follow-up
To minimize associations not related to the treatment, follow-up for cohorts (i)–(v) began 3 months after the initiation of the drug in question. We used an ever-treated approach, in which each patient was followed until the occurrence of the outcome, death, emigration from Sweden, or end of the study period, whichever came first, irrespective of any discontinuation of the b/tsDMARD, or start of a new b/tsDMARD. Patients who started one TNFi and later switched to a second TNFi were considered as contributing twice to the TNFi cohort, but patients who switched from an originator product to its biosimilar(s) were regarded as remaining on the same therapy.
Outcomes
Through linkage to the Swedish Cancer Register, we identified incident cancers before and during the study period, using International Classification of diseases (ICD) codes (1): overall first-ever invasive non-cutaneous (main outcome) (2), prostate (3), testicular (4), female breast (5), haematopoietic (leukaemias, immunoproliferative-, myeloproliferative-, lymphoproliferative disease and lymphomas) (6), renal (7), lung (8), colorectal (9), ovarian (10), cervical (11) urinary tract (bladder, urethra, ureter) (12), CNS (13), uterus (14), ear–nose–throat (15), digestive tract (esophagus, ileum and jejunum) (16), pancreas, and (17) liver-gallbladder cancers (Supplementary Table S4, available at Rheumatology online). We did not include cutaneous cancers, because their pattern of occurrence and thus the desired analytical approach is different from that of most other cancers. Patients who had a history of any invasive cancer were excluded. Individuals who, during follow-up, developed cancer of another type than the one under study were not censored.
Covariates
Through linkage to the patient and prescribed drug registers, information on comorbidities (Supplementary Table S5, available at Rheumatology online) was obtained. Through linkages to the Swedish Social Insurance Agency’s register or to the Longitudinal Database for Insurance and Labour Market Studies, information on work ability, sick leave and disability was obtained. Through linkages to the Total Population Register, demographics and socio-economic characteristics was obtained (see Table 1).
Table 1.
Baseline characteristics of the study cohorts of Swedish patients with RA, by treatment status
| Cohort | TNFi | Rituximab | Abatacept | Tocilizumab | JAKi | B/tsDMARD-naïve | General population |
|---|---|---|---|---|---|---|---|
| Individuals (n) | 21 365 | 4123 | 3306 | 2689 | 1289 | 58 233 | 109 532 |
| Observations (n) | 33 609 | 4367 | 3558 | 2895 | 1435 | 58 233 | 215 592 |
| Age, mean (SD) | 56 (46–65) | 62 (52–70) | 60 (50–68) | 58 (47–66) | 59 (49–69) | 63 (52–73) | 57 (46–65) |
| Male (%) | 23% | 23% | 19% | 20% | 18% | 30% | 22% |
| Cohort entry median (IQR) | 2011 (2006–2015) | 2012 (2009–2015) | 2014 (2012–2016) | 2014 (2012–2016) | 2018 (2017–2018) | 2009 (2006–2014) | 2012 (2008–2016) |
| Years of follow-up, median (IQR)a | 6.6 (3.1–10.9) | 5.4 (2.7–8.4) | 3.8 (1.9–5.8) | 3.9 (2.1–6.5) | 0.7 (0.3–1.1) | 6.6 (3.3–10.9) | 5.9 (2.7–10.0) |
| Educational level (%) | |||||||
| Below 9 years (%) | 24% | 27% | 23% | 22% | 19% | 35% | 21% |
| 10–12 years (%) | 47% | 47% | 49% | 48% | 50% | 43% | 45% |
| >12 years (%) | 30% | 26% | 28% | 30% | 32% | 22% | 34% |
| Smoker (%) | 54% | 62% | 57% | 55% | 62% | DU | DU |
| Comorbidities (%)b | |||||||
| Joint replacement surgery (%) | 14% | 20% | 19% | 18% | 20% | 12% | 3% |
| Diabetes mellitus (%) | 6% | 10% | 10% | 8% | 9% | 7% | 4% |
| Hypertension (%) | 15% | 25% | 25% | 21% | 27% | 18% | 10% |
| IHD (%) | 6% | 10% | 10% | 7% | 9% | 9% | 4% |
| CHD (%) | 2% | 4% | 5% | 3% | 4% | 4% | 1% |
| COPD (%) | 3% | 6% | 6% | 4% | 6% | 4% | 2% |
| Renal insufficiency (%) | 1% | 2% | 2% | 2% | 2% | 1% | 1% |
| Disease duration, median (IQR) | 9 (3–17) | 12 (6–21) | 12 (6–21) | 11 (5–19) | 13 (7–22) | 1 (0–7) | NA |
| HAQ median (IQR) | 1.1 (0.8–1.6) | 1.3 (0.9–1.9) | 1.3 (0.9–1.8) | 1.3 (0.9–1.8) | 1.3 (0.8–1.8) | DU | NA |
| DAS28-CRP median (IQR) | 4.7 (3.9–5.5) | 4.8 (4.0–5.6) | 4.7 (3.9–5.4) | 4.8 (4.1–5.6) | 4.5 (3.7–5.2) | DU | NA |
| Tender joint count median (IQR) | 6 (3–10) | 6 (3–11) | 6 (3–10) | 7 (3–12) | 6 (3–10) | DU | NA |
| Swollen joint count median (IQR) | 6 (3–10) | 6 (3–10) | 5 (2–8) | 6 (3–10) | 4 (2–7) | DU | NA |
| ESR (mm/h) | 22 (11–40) | 28 (14–46) | 23 (11–40) | 26 (12–46) | 20 (10–35) | DU | NA |
| CRP (mg/dl) | 10 (4–26) | 12 (5–30) | 8 (3–22) | 11 (4–29) | 5 (2–16) | DU | NA |
| RF-positive RA (%) | 77% | 88% | 79% | 78% | 77% | 69% | NA |
| VAS pain median (IQR) | 60 (40–75) | 61 (42–77) | 65 (45–78) | 65 (46–79) | 63 (42–78) | DU | NA |
| VAS patient global median (IQR) | 60 (40–76) | 62 (43–78) | 65 (47–79) | 65 (46–79) | 64 (45–78) | DU | NA |
| VAS physician global median (IQR) | 40 (25–50) | 40 (30–60) | 45 (30–60) | 50 (34–60) | 40 (30–50) | DU | NA |
| MTX (%) | 62% | 53% | 51% | 48% | 37% | 50% | 0% |
| Non-MTX csDMARDs (%) | 21% | 22% | 16% | 14% | 14% | 14% | 0% |
| CSs (%) | 56% | 70% | 67% | 66% | 64% | 43% | 1% |
| Disability pension (%) | 2% | 2% | 2% | 2% | 2% | 1% | 1% |
| Sick-leave (%) | 18% | 14% | 17% | 18% | 17% | 10% | 8% |
Extended information on baseline characteristics in Supplementary Table S15, available at Rheumatology online.
Follow-up calculated as time from treatment start, until end of follow-up on 31 December 2018, migration date, death date or diagnosis of another rheumatic disease, whichever occurred first.
Co-morbidities, registered up to 5 years before start of follow-up. RA disease characteristics at start of b/tsDMARD median (IQR).
COPD: chronic obstructive pulmonary disease; IHD: ischaemic heart disease; CHD: coronary heart disease; csDMARD: conventional synthetic DMARD; TNFi: TNF inhibitor; NA: not applicable; DU: data unavailable; IQR: interquartile range; VAS: visual analogue scale.
Statistics
For each cohort, we assessed the number of incident cancers, person-time of follow-up and crude incidence rates per 1000 person-years. We used stratified and adjusted Cox regression to estimate hazard ratios (HRs), comparing rates across cohorts, using attained age as the time-scale and accommodating the number of previous biologics (0, 1, 2, 3, 4, 5+). This guaranteed that an individual’s person-time appearing more than once would not be compared with itself. We estimated HRs from models adjusting for calendar year (2001–2004, 2005–2009, 2010–2014, 2015–2018) and sex (except for sex-specific cancers) (HRa). Additional Cox models were adjusted for comorbid conditions, disability and sick leave, socio-economic data, and concomitant medications (NSAIDs and steroids) (HRb). We also performed analyses stratified by attained age, by time since treatment start, and by time on active b/tsDMARD treatment. Cluster robust standard errors were used to account for a subject’s potential contribution to more than one exposure category in the same analysis. We refrained from assessing relative risks for sites for which the number of observed cancers was below five. Our initial plan was to assess HRs, but for the above reason (less than five incident cancers), only the descriptive data on JAKis are presented (Table 1).
As sensitivity analyses, we assessed the impact of three alternative b/tsDMARD-naïve cohort definitions requiring current csDMARD use (Supplementary Table S3, available at Rheumatology online), changed the exposure window by censoring at the start of another b/tsDMARD, additionally adjusted for disease activity (DAS28 and HAQ), stratified for seropositive vs unknown/seronegative RA, and assessed HRs specifically among patients with vs without previous b/tsDMARD treatments.
Results
Characteristics of the study population
A total of 69 308 unique patients with RA without a prior cancer diagnosis were included: 21 365 patients contributed to the TNFi cohort, 4123 to rituximab, 3306 to abatacept, 2689 to tocilizumab, 1289 to JAKi, and 56 233 contributed to the b/tsDMARD-naïve cohort. The general population cohort comprised 109 532 individuals (Table 1).
Occurrence of cancer overall
During a total of 658 589 person-years of follow-up of all individuals with RA, 8633 incident cancers occurred, compared with 13 205 cancers during a total follow-up of 1 335 994 person-years in the general population cohort. The median (IQR) follow-up times were as follows: TNFis 6.6 years (3.1–10.9), rituximab 5.4 years (2.7–8.4), abatacept 3.8 years (1.9–5.8), tocilizumab 3.9 years (2.1–6.5), JAKis 0.7 years (0.3–1.1). Tables 1 and 2 display the number of patients, person-time of follow-up, incident cancers and crude incidence rates.
Table 2.
Number of patients, events, crude incidences, age- and sex-adjusted hazard ratios (HRas), and fully adjusted hazard ratios (HRbs) for overall invasive cancer, excluding skin cancer, in Swedish patients with RA treated or untreated with b/tsDMARDs, with b/tsDMARD-naïve RA and the general population as references
| Cohort | Patient episodes | Events | Person-years of follow-up | Crude incidence per 1000 | HRa (95% Cl) B/tsDMARD-naïve | HRb (95% Cl) | HRa (95% CI) general population |
|---|---|---|---|---|---|---|---|
| TNFi | 33 609 | 2395 | 224 661.2 | 10.7 | 1.0 (0.9, 1.0) | 1.0 (0.9, 1.0) | 1.1 (1.0, 1.2)a |
| RTX | 4367 | 294 | 22 846.9 | 12.9 | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) | 1.1 (1.0, 1.3)b |
| ABT | 3558 | 180 | 13 604.6 | 13.6 | 1.2 (1.0, 1.4)c | 1.2 (1.0, 1.3)d | 1.3 (1.1, 1.6) |
| TCZ | 2895 | 119 | 11 572.3 | 11.6 | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.2) | 1.1 (0.9, 1.3) |
| B/tsDMARD-naïve | 58 233 | 5642 | 38 5173.5 | 14.6 | 1.0 (reference) | 1.0 (reference) | 1.2 (1.1, 1.2)e |
| General population | 215 592 | 13 205 | 1 335 994.4 | 9.9 | 0.9 (0.8, 0.9) | NA | 1.0 (reference) |
HR = 1.10 (1.04, 1.16).
HR = 1.14 (1.00, 1.29).
HR = 1.16 (1.00, 1.36).
HR = 1.15 (0.98, 1.34).
HR = 1.15 (1.11, 1.19).
TNFi: TNF inhibitor; RTX: rituximab; ABT: abatacept; TCZ: tocilizumab; HR: hazard ratio; HRa: hazard ratio adjusted for attained age, year of start of follow-up, and sex; HRb, as for HRa, plus adjustment for selected comorbidities, NSAID use, steroid use, educational level, sick leave and disability as defined at baseline, i.e. fully adjusted; NA: not applicable; ref.: reference.
Relative risk in each of the RA cohorts vs the general population
Fig. 1A and Table 2 display the adjusted HRs of overall cancer across cohorts, using the general population cohort as the reference. Overall, b/tsDMARD-naïve RA were at increased risk of cancer (HR = 1.2, 95% CI: 1.1, 1.2). Similar levels of increased risks were observed for all RA treatment cohorts.
Fig. 1.
Relative risks for cancer overall in RA, by bDMARD treatment status
(A) In each of the RA treatment cohorts vs. the general population. (B) In bDMARD-treated RA vs. bDMARD-naïve RA.
TNFi: TNF inhibitor; b/tsDMARD: biologic and targeted synthetic DMARD.
Relative risks in b/tsDMARD-treated RA vs b/tsDMARD-naïve RA
Fig. 1B and Table 2 display the numbers of events and the fully adjusted HRs for cancer overall in the b/tsDMARD-treated cohorts, using the b/tsDMARD-naïve cohort as a reference. We noted no statistically significantly increased or decreased incidences for TNFi (HRb = 1.0, 95% CI: 0.9, 1.0), rituximab (HRb = 1.0, 95% CI: 0.9, 1.1) or tocilizumab (HRb = 1.0, 95% CI: 0.8, 1.2). For abatacept the HRb was 1.2, 95% CI: 1.0, 1.3.
Relative risks in b/tsDMARD-treated RA vs b/tsDMARD-naïve RA, by time since treatment start
Fig. 2A (Supplementary Table S6, available at Rheumatology online) presents the numbers of events and fully adjusted HRs of cancer stratified by time since treatment start. For TNFis, we did not find evidence of any trend towards increasing or decreasing HRb over time. For instance, the HRb 10 or more years after treatment start was 1.0 (95% CI: 0.8, 1.2). For rituximab, tocilizumab and abatacept, we did not observe any clear evidence of any trends with increasing or decreasing HRbs.
Fig. 2.
Relative risks for cancer overall in bDMARD treated RA vs. bDMARD naïve RA, stratified by time and attained age
(A) By time since treatment start (years). (B) By time on active treatment (years). (C) By attained age.
TNFi: TNF inhibitor; b/tsDMARD: biologic and targeted synthetic DMARD.
Relative risks in b/tsDMARD-treated RA vs b/tsDMARD-naïve RA, by time on active treatment
When we assessed relative risks by time on active treatment as a time-varying exposure variable, counting from the start of each drug until its first registered stop date, and disregarding any later starts/stops of the same drug, we noted no evidence of any trend towards increasing or decreasing HRb with increasing accrued time on active treatment (Fig. 2B and Supplementary Table S7, available at Rheumatology online), but a statistically significantly increased HRb of 1.8 (95% CI: 1.2, 2.6) for abatacept for 2–5 years of active treatment.
Relative risks in b/tsDMARD-treated RA vs b/tsDMARD-naïve RA, by attained age
For all drugs, we noted a trend of point estimates below 1 for the younger age groups, and point estimates around or above 1 for the older age groups, but no statistically significant trend for either drug (Fig. 2C and Supplementary Table S8, available at Rheumatology online).
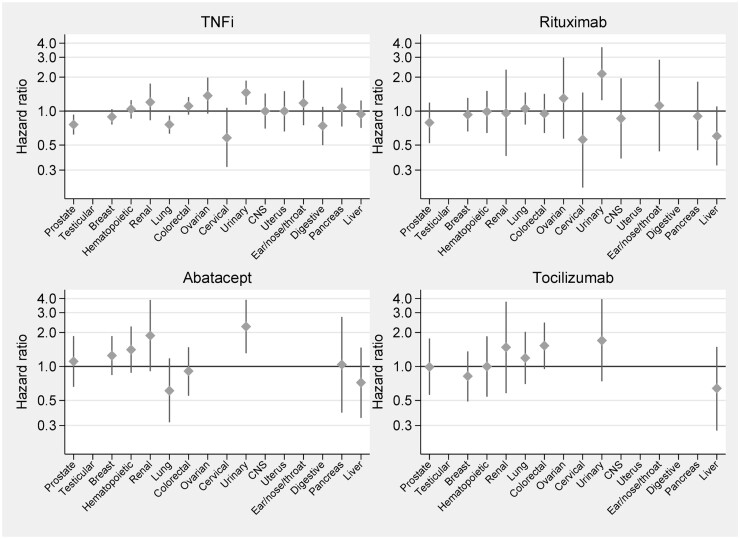
Site-specific relative risks for cancer with b/tsDMARDs-treated vs b/tsDMARD-naïve RA
Fig. 3 and Supplementary Table S9 (available at Rheumatology online) display the numbers of events and HRb for each of the 16 site-specific cancer outcomes. Of the total of 80 tests (16 cancer outcomes in 5 cohorts), we noted statistically significant associations between several b/tsDMARD treatments and risk of urinary tract cancers as follows: TNFis (HRb = 1.5, 95% CI: 1.1, 1.9), rituximab (HRb = 2.1, 95% CI: 1.3, 3.7) and abatacept (HRb = 2.3, 95% CI: 1.3, 3.9); for tocilizumab, the corresponding HR was 1.7 (95% CI: 0.7, 4.0). Additionally, for colorectal cancer the HRb for tocilizumab was 1.5 (95% CI 0.95, 2.45), and for ovarian cancer the HRb for TNFi was 1.4 (95% CI: 0.95, 1.98). For all other site-specific cancers, we noted no statistically significantly increased hazard ratios.
Fig. 3.
Site-specific relative risks for cancer with bDMARD-treated vs bDMARD-naïve RA
TNFi: TNF inhibitor.
Sensitivity analyses
The impact of replacing the b/tsDMARD-naïve cohort with either of three alternative cohorts based on csDMARD exposures was small (Supplementary Table S10, available at Rheumatology online). Changing the main analysis ‘ever since treatment start‘ to a ‘most recent drug’ window resulted in a marginal reduction in the person-time of follow-up and of incident events but had little impact on the estimated HRs for cancer overall (Supplementary Table S11, available at Rheumatology online). Adjusting for disease activity (DAS28 and HAQ) at treatment start did not materially change the HRs from the main analysis, although the difference in risk for cancer overall between abatacept and TNFis reached statistical significance (Supplementary Table S12, available at Rheumatology online). When stratified by RA serostatus, HRs in the two subsets were similar (Supplementary Table S13, available at Rheumatology online). When stratified by previous b/tsDMARD treatment, we noted HRs (HRa and HRb) similar to our main analyses (Supplementary Table S14, available at Rheumatology online).
Discussion
In this large observational study with, to our knowledge, the hitherto longest average follow-up of cancer risks in patients with RA treated with b/tsDMARD in clinical practice, we did not observe any increase in the overall occurrence of cancer with TNFis, nor any trends with time since treatment start, time on active treatment, or attained age, compared with b/tsDMARD-naïve patients. For other bDMARDs, we noted no consistent signal of any increased overall cancer risks, even if certain relative risk estimates were (statistically significantly) above 1. With respect to relative risks for the 16 specific cancer sites, we noted several statistically significant associations for urinary tract cancer with the TNFis rituximab and abatacept.
Few observational studies on cancer risks with b/tsDMARDs and a mean follow-up of over 3 years have been performed. Mercer et al., from the UK Biologics Register in RA, reported no elevated cancer risk with TNFis but limited the presentation of time-specific risks to ‘3 or more years’ from start of follow-up [11]. In two previous reports from our group, based on a subset of our current study population and follow-up, we reported no increase in the overall cancer risk associated with TNFi treatment nor with time since start of treatment [9, 10]. Our current analyses represent a considerable expansion of the study population and follow-up time, with 61% more individuals, longer average follow-up, and doubled numbers of person-years and events.
Our first observation, that the overall cancer risk, excluding that of skin cancers, with TNFis was not higher or lower than among patients with RA treated otherwise, is in keeping with previous studies [9–11]. Our present study extends these results by demonstrating the absence of any trend of increasing risks with even longer follow-up times, and by demonstrating the absence of any increased risk by attained age or time on active treatment. Collectively, these results add to the safety profile of TNFis as used in clinical practice, at least in RA.
Our second observation, that the overall cancer risk, excluding that of skin cancers, with b/DMARDs other than TNFis was not higher than in RA treated otherwise, is also in keeping with the existing data [6, 9, 12, 13]. For abatacept, our main analysis for cancer overall indicated a relative risk estimate above 1, but did not reach formal statistically significance, and the analyses of cancer overall by time on active treatment indicated a statistically significant increased risk in one latency interval (2–5 years on active treatment). Also, in the analyses of overall cancer in which we compared b/tsDMARDs with TNFis and adjusted for disease severity, we noted an elevated overall cancer incidence with abatacept. These results must, however, be interpreted in light of the exploratory nature of our analyses, which represent a safety monitoring rather than a testing of a defined biological hypothesis regarding cancer risks with, in this case, abatacept. Previous reports on overall cancer risks with abatacept compared with other bDMARDs show conflicting results. Montastruc et al. [14] found a statistically significant increased risk of cancer overall (HR 1.17; 95% CI: 1.06, 1.30), and Simon et al. [18] reported a small increase in risk (HR 1.09; 95% CI: 1.02, 1.16). No increased risk for overall cancer was noted in the study by de Germay et al. [19] (OR 0.98; 95% CI: 0.91, 1.05), and Ozen et al. [20] found a non-statistically significant HR (1.89; 95% CI: 0.93, 3.84). Our results must also be viewed in light of the observational design and risk of confounding by indication. Despite extensive adjustments, we cannot exclude residual or unmeasured confounding. Thus, at this stage, further monitoring of the safety of abatacept (in light of our results) and other bDMARDs (in light of the still limited precision) seems warranted, even if neither in their current shape would call for changes to clinical practice.
Our third observation, that there was a statistically significantly increased risk of urinary tract cancer with the TNFis rituximab and abatacept, has not been reported previously. Urinary tract cancer has only been studied as a defined end point in a limited number of studies, with few events of bladder cancer and risk ratios not being statistically significant, with point estimates being under or around 1 [8, 21]. Again, our results must be interpreted with caution and in light of the absence of any defined hypothesis specific to urinary tract cancer. Since the signal of an increased risk for urinary tract cancer was observed for all bDMARDs, one alternative explanation might be that rather than the rates in bDMARD-exposed patients being elevated, it may be that the rate in the bDMARD-naïve comparator in our study was unusually low (even if we note that this rate was still higher than in the general population). Again, our finding calls for replication, but would not at this stage call, for example, for general bladder cancer screening before starting bDMARDs in RA patients.
Furthermore, we found that b/tsDMARD-naïve RA patients had an increased risk of cancer compared with the general population. This is in line with other studies and adds to the external validity of our study, and strengthens the notion of an association between RA and malignancies independently of b/tsDMARD exposures [8, 22].
Our study has limitations. We compared incidences in the treatment-defined RA sub-cohorts with those among all patients defined by the absence of previous or ongoing b/tsDMARD treatment, for whom start of follow-up did not correspond to any specific clinical action-point. To this end, we identified additional control groups and employed sensitivity analyses, including active treatment comparators, which did not alter the results. The TNFi cohort, on average, had a less severe disease than the other b/tsDMARD cohorts. As mentioned, we performed a sensitivity analysis (with adjustment for DAS28-CRP and HAQ) in which we noted a tendency towards higher cancer incidence in the abatacept cohort compared with the TNFi cohort, the nature of which remains to be explored. Also, despite representing a considerable extension of previously reported follow-up times, our follow-up time for the non-TNFis were shorter compared with the follow-up time for TNFis. Furthermore, for JAKis, the number of observed cancer events was too low to allow for meaningful interpretations. Also, whereas most RA patients receive more than one b/tsDMARD, our primary aim was to study risks with individual bDMARDs. Although previous bDMARD exposure was accounted for in our analyses, further work is required in order to assess the impact, if any, on cancer risks with particular b/tsDMARD treatment sequences. Also, and as indicated above, our safety assessment was not based on any specific hypothesis regarding any particular drug–cancer type association, but encompassed close to 100 statistical tests for significance. We thus cannot dismiss chance alone as an explanation for the handful of statistically significant HRs observed. At the same time, being a study on drug safety, every such signal needs to be weighed against available evidence (or calls for replication).
Strengths of our study include our use of nationwide, population-based prospective registers with high coverage and validity, which ensured the inclusion of the vast majority of all patients with RA treated with the drugs under study, and their incident cancers, maximizing both internal and external validity. Further, and as attribution of events to individual exposures is inherently complicated for long-term risks such as cancer, we noted a high level of consistency between the two risk-windows used. The large sample sizes ensured good statistical precision in the main analyses. Most point estimates were close to 1, if anything in the direction of a protective effect for several drugs, with confidence limits narrow enough to rule out large risk increases for overall cancer in the first 5–10 years (non-TNFis), and for TNFis also beyond 10 years.
To conclude, from a scientific point of view our results extend the safety profile of bDMARDs in RA, but also contain signals that call for replication in further studies, although at this stage, neither the consistency nor the magnitude of these signals currently seems to warrant changes to clinical practice.
Supplementary Material
Acknowledgements
We would like to thank all Swedish RA patients and rheumatologists for entering data into the Swedish Rheumatology Quality Register. V.H., H.B., H.W., T.F. and J.A. had full access to all the data in the study and participated in the design of the study. H.B. conducted the statistical analyses. All authors participated in designing the analyses and in the interpretation of the results. V.H. and J.A. contributed to the drafting of the manuscript.
Funding: This work was supported by funding from the Karolinska Institute Region Stockholm funds (ALF). Funders had no impact on the design or interpretation of the study or its results.
Disclosure statement: V.H., H.W., H.B. and T.F. have no competing interests to declare. J.A. previously had or currently has research agreements with Abbvie, Astra-Zeneca, BMS, Eli Lilly, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi, and UCB, mainly in the context of safety monitoring of biologics via the ARTIS/Swedish Biologics Register.
Data availability statement
For reasons related to the legal framework governing the raw data used for this study, individual-level data cannot be freely shared. For requests for study data please contact the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
Contributor Information
the ARTIS group:
Gerd-Marie Ahlenius, Eva Baecklund, Katerina Chatzidionysiou, Nils Feltelius, Helena Forsblad-d’Elia, Alf Kastbom, Lars Klareskog, Elisabet Lindqvist, Ulf Lindström, Carl Turesson, Christopher Sjöwall, and Johan Askling
References
- 1. de Visser KE, Eichten A, Coussens LM.. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 2006;6:24–37. [DOI] [PubMed] [Google Scholar]
- 2. Lebrec H, Ponce R, Preston BD. et al. Tumor necrosis factor, tumor necrosis factor inhibition, and cancer risk. Curr Med Res Opin 2015;31:557–74. [DOI] [PubMed] [Google Scholar]
- 3. Bongartz T, Sutton AJ, Sweeting MJ. et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275–85. [DOI] [PubMed] [Google Scholar]
- 4. Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA. et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. Jama 2012;308:898–908. [DOI] [PubMed] [Google Scholar]
- 5. Strangfeld A, Hierse F, Rau R. et al. Risk of incidence or recurrent malignancies among patients with rheumatoid arthritis exposed to biologic therapy in German biologics register RABBIT. Arthritis Res Ther 2010;12:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aaltonen KJ, Joensuu JT, Virkki L. et al. Rates of serious infections and malignancies among patients with rheumatoid arthritis receiving either tumor necrosis factor inhibitor or rituximab therapy. J Rheumatol 2015;42:372–8. [DOI] [PubMed] [Google Scholar]
- 7. Askling J, Fored CM, Brandt L. et al. Risks of solid cancers in patients with rheumatoid arthritis and after treatment with tumour necrosis factor antagonists. Ann Rheum Dis 2005;64:1421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dreyer L, Mellemkjær L, Andersen AR. et al. Incidences of overall and site specific cancers in TNFα inhibitor treated patients with rheumatoid arthritis and other arthritides – a follow-up study from the DANBIO Registry. Ann Rheum Dis 2013;72:79–82. [DOI] [PubMed] [Google Scholar]
- 9. Wadstrom H, Frisell T, Askling J; Anti-Rheumatic Therapy in Sweden (ARTIS) Study Group. Malignant neoplasms in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors, tocilizumab, abatacept, or rituximab in clinical practice: a nationwide cohort study from Sweden. JAMA Intern Med 2017;177:1605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Askling J, van Vollenhoven RF, Granath F. et al. Cancer risk in patients with rheumatoid arthritis treated with anti–tumor necrosis factor α therapies: does the risk change with the time since start of treatment? Arthritis Rheum 2009;60:3180–9. [DOI] [PubMed] [Google Scholar]
- 11. Mercer LK, Lunt M, Low AL. et al. ; BSRBR Control Centre Consortium. Risk of solid cancer in patients exposed to anti-tumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2015;74:1087–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Vollenhoven RF, Fleischmann RM, Furst DE, Lacey S, Lehane PB.. Longterm safety of rituximab: final report of the rheumatoid arthritis global clinical trial program over 11 years. J Rheumatol 2015;42:1761–6. [DOI] [PubMed] [Google Scholar]
- 13. Slimani S, Lukas C, Combe B, Morel J.. Rituximab in rheumatoid arthritis and the risk of malignancies: report from a French cohort. Joint Bone Spine 2011;78:484–7. [DOI] [PubMed] [Google Scholar]
- 14. Montastruc F, Renoux C, Hudson M. et al. Abatacept initiation in rheumatoid arthritis and the risk of serious infection: a population-based cohort study. Semin Arthritis Rheum 2019;48:1053–8. [DOI] [PubMed] [Google Scholar]
- 15. Sivaraman P, Cohen SB.. Malignancy and Janus Kinase inhibition. Rheum Dis Clin N Am 2017;43:79–93. [DOI] [PubMed] [Google Scholar]
- 16. Grivennikov SI, Greten FR, Karin M.. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baecklund E, Iliadou A, Askling J. et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 2006;54:692–701. [DOI] [PubMed] [Google Scholar]
- 18. Simon TA, Boers M, Hochberg M. et al. Comparative risk of malignancies and infections in patients with rheumatoid arthritis initiating abatacept versus other biologics: a multi-database real-world study. Arthritis Res Ther 2019;21:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Germay S, Bagheri H, Despas F, Rousseau V, Montastruc F.. Abatacept in rheumatoid arthritis and the risk of cancer: a world observational post-marketing study. Rheumatology (Oxford) 2020;59:2360–7. [DOI] [PubMed] [Google Scholar]
- 20. Ozen G, Pedro S, Schumacher R, Simon TA, Michaud K.. Safety of abatacept compared with other biologic and conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: data from an observational study. Arthritis Res Ther 2019;21:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolfe F, Michaud K.. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum 2007;56:2886–95. [DOI] [PubMed] [Google Scholar]
- 22. Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S.. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther 2015;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For reasons related to the legal framework governing the raw data used for this study, individual-level data cannot be freely shared. For requests for study data please contact the corresponding author.