Abstract
Objectives
The use of biologic and targeted synthetic (b/ts) DMARDs in the treatment of RA is increasing. Therefore, prevention of b/tsDMARDs-induced infection is important. Here we describe a prophylaxis protocol for preventing pneumocystis pneumonia (PCP) in RA patients treated with b/tsDMARDs.
Methods
The study subjects were 3787 RA patients from the FIRST registry. They were divided into cohort 1 (n = 807, requiring prophylaxis against PCP based on physicians’ assessment at the point of new treatment with or switch to b/tsDMARDs) and cohort 2 (n = 2980, receiving strategic PCP prophylaxis). The incidence and risk factors for PCP were investigated.
Results
Twenty-six PCP cases were observed throughout the study. After the introduction of strategic PCP prophylaxis, PCP incidence diminished from 0.51/100 person-years (PYs) to 0.21/100 PYs (risk ratio = 0.42). Sulfamethoxazole and trimethoprim in combination (SMX–TMP) showed greater efficacy in the prevention of PCP than pentamidine inhalation (P <0.0001). The prophylaxis rate increased chronologically despite the falls in the average SMX–TMP dose and in the incidence of PCP. Subanalysis of the data for 929 patients from both groups who did not receive prophylaxis showed that old age, high BMI, coexisting lung diseases, low lymphocyte count, and low serum IgG levels increased the risk of PCP development. Development of PCP could be predicted (using an equation based on these variables) in patients not treated with glucocorticoids [area under the curve (AUC) = 0.910)], but less accurately in those on glucocorticoids (AUC = 0.746).
Conclusions
Our study clarified the risk factors for PCP in RA patients on b/tsDMARDs treatment and highlighted and defined the criteria for effective prophylaxis against PCP.
Keywords: pneumocystis pneumonia, b/tsDMARDs, prophylaxis
Rheumatology key messages.
Old age, obesity, coexisting lung diseases, neutropenia, and low serum IgG levels can predict pneumocystis pneumonia (PCP) in RA patients on b/tsDMARDs treatment.
Sulfamethoxazole and trimethoprim in combination (SMX–TMP) was more efficacious in prevention of PCP than pentamidine inhalation.
A low dose of SMX–TMP (<560 mg TMP/week) can be considered.
Introduction
Biologics and targeted synthetic DMARDs (b/tsDMARDs) are indispensable in the treatment of RA [1, 2]. b/tsDMARDs are clinically efficacious, though special attention should be paid with regard to the potential of occurrence of infectious diseases, including opportunistic infections [3–11]. Despite accumulating evidence and clinical information about b/tsDMARDs treatment, there is currently no clear strategy for preventing opportunistic infections in patients treated with b/tsDMARDs [12, 13].
Pneumocystis pneumonia (PCP) is a common and sometimes lethal opportunistic infection in patients with HIV and in patients treated with antitumor agents or immunosuppressants [14]. While the incidence of PCP is lower in non-HIV patients than in HIV patients [14], the reported mortality in RA patients treated with b/tsDMARDs (28.9–30.8% [15, 16]) is higher than that in HIV patients (10–20%) [14]. In both groups of patients, there is no doubt that PCP is associated with a high risk of death. In this setting, there is extensive work on PCP in both HIV and non-HIV patients [14, 17]. Sulfamethoxazole and trimethoprim in combination (SMX–TMP), pentamidine inhalation and atovaquone are widely used in HIV patients as well as in non-HIV patients to suppress PCP; however, all these groups of drugs can cause side effects and thus preclude their use for PCP prophylaxis in at least some patients at risk of PCP [17].
The reported incidence of PCP in RA patients treated with b/tsDMARDs varies from one country to another, e.g. 0.1–0.4% in Japan [3–5, 10, 11] and 0.02–0.04% in Western countries [6, 9]. Japanese post-marketing surveillance indicates that all modes of action of b/tsDMARDs can trigger PCP development [3–5, 10, 11]. Of note, an increase in the incidence of PCP has been reported in developing countries with rising gross domestic product [18], where an expansion of the b/tsDMARDs market is also expected. Therefore, it is critical that a prophylaxis strategy against PCP in RA patients under b/tsDMARDs treatment be formulated, and preferable that this strategy is mentioned in international guidelines.
Hence, the present study was designed to establish an appropriate prophylaxis strategy against PCP in RA patients on b/tsDMARDs treatment. The study included 3787 RA patients treated with b/ts DMARDs in a real-world setting and re-evaluated the criteria for prophylaxis that we have reported previously [19]. We provide here revised criteria that reflect the change in patients’ characteristics over time and the increased choice of b/tsDMARDs on the market.
Patients and methods
Data source and study design
Data was collected from the registry of RA patients (the FIRST registry [20–25], available at The First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, Japan and its affiliated facilities). In this registry, all registered RA patients were enrolled in a long-term observational study at the point of new (or switching to) a prescription of b/tsDMARDs. Clinical information, including patients’ demographics, RA disease activity, results of laboratory tests, patient-reported outcomes, treatment details and adverse events were collected at the time of enrolment, after 2 weeks, 3 months, 6 months, 1 year and then every year during the period of observation. Patients were censored when the b/tsDMARDs treatment was discontinued. To evaluate the effect of the number of failed b/ts DMARDs and the mechanism of action of b/ts DMARDs, participants who underwent b/tsDMARDs switch prescription were censored and registered again as new participants (i.e. analysis on a per-registration basis).
Two subcohorts from the FIRST registry were included in the present study, and the incidence of PCP under two different prophylaxis strategies was estimated. From August 2003 to September 2009, use of PCP prophylaxis was considered solely based on physician’s assessments at the point of new or switch prescription of b/tsDMARDs, and took into consideration various factors such as participant’s age, ability to carry out the activities of daily living, liver function tests, renal function tests, pulmonary status, and past history of infectious diseases (cohort 1). After October 2009, participants underwent PCP prophylaxis based on published prophylaxis criteria [19] plus physicians’ assessment (= strategic PCP prophylaxis, cohort 2). In the present study, patient follow-up was undertaken at the PCP onset, and 90 days after discontinuation of b/tsDMARDs or at the point of loss to follow-up.
End points
The primary end point was the incidence of PCP in each cohort. The secondary end points were the rate of severe adverse events due to PCP prophylaxis and the rate of PCP prophylaxis in each cohort.
PCP prophylaxis
PCP prophylaxis includes either oral SMX–TMP at 80–560 mg/week, pentamidine 300 mg inhalation per visit or oral atovaquone 1500 mg once daily. The prophylaxis regimen was decided by the attending physician and tailored to the participant’s clinical conditions, such as drug allergy, liver dysfunction, and chronic kidney disease. Strategic prophylaxis for PCP systemically placed the participants in cohort 2 if they fulfilled two or more of the following criteria: (i) age ≥65 years, (ii) presence of lung disease, (iii) concomitant use of oral glucocorticoids (GCs) [19]. Lung diseases were defined as interstitial pneumonitis, pleuritis, diffuse panbronchiolitis, bronchiectasis, old tuberculosis or inflammatory nodules detected on chest X-ray/CT. PCP prophylaxis was not applied for some participants, despite their fulfilling the above prophylaxis criteria (e.g. those who did not consent to receive prophylaxis).
Diagnosis of PCP
We used the PCP diagnostic approach described in detail previously [19]. Briefly, participants with progressive hypoxaemia and interstitial pneumonia were diagnosed with PCP based on the presence of elevated serum β-D glucan and/or positivity for Pneumocystis jirovecii PCR [26] in the sputum or broncho-alveolar lavage fluid.
Statistical analysis
JMP Pro 15 software (SAS Institute, Cary, NC) was used for statistical analysis. Descriptive statistics (mean, S.D., median, interquartile range, percentage) were used to examine differences at enrolment. The Student’s t test or Mann–Whitney U test were used in the comparison of two groups. The χ2 test was used for comparing categorical variables unless otherwise indicated. The Log rank test was used for comparison of the incidence of PCP between the two cohorts. The incidence of PCP was estimated by the Cox proportional hazards model and logistic regression in an intention-to-treat analysis (with regard to PCP prophylaxis). Age, concomitant GC use, coexisting lung diseases, BMI, estimated glomerular filtration rate, peripheral blood neutrophil count, peripheral blood lymphocytes count, serum albumin, and serum IgG were tested as independent variables for univariable and multivariable analysis. Missing data was recovered by the low rank matrix approximation method. The bootstrapping technique was used for the internal validation of PCP incidence estimation for 1000 replications. All reported P values were two-sided and not adjusted for multiple testing. P values <0.05 denoted the presence of statistically significant difference.
Ethical considerations
This study complied with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of the University of Occupational and Environmental Health School of Medicine (#04–23). Informed consent was obtained from all participants in appropriate methods based on the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Japanese Ministry of Health, Labour and Welfare in April 2015. In practice, written informed consent was obtained from the participants enrolled into the study after April 2015. Otherwise, written or verbal informed consent was obtained from the participants.
Results
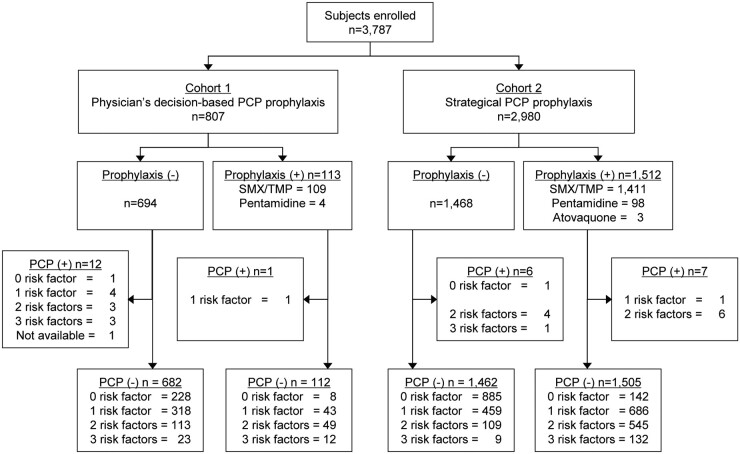
A total of 3787 individuals were enrolled in this study. Cohort 1 included 807 participants, and 113 underwent PCP prophylaxis (SMX–TMP n = 113, pentamidine n = 4). Thirteen PCP cases were observed in this cohort. PCP developed in one case despite the administration of the prophylaxis at study baseline, and in 12 who did not receive prophylaxis. In cohort 2, PCP prophylaxis was administrated to 1512 participants (SMX–TMP n = 1411, pentamidine n = 98, atovaquone n = 3) and 13 PCP cases were observed from this cohort [7 from the group who were administered prophylaxis at baseline, and 6 from the non-prophylaxis group (Fig. 1)]. The PCP mortality rate was 11.5% (3/26) in this study.
Fig. 1.
Study flow chart
RA patients were enrolled in the study at the time of administration of biologics/targeted synthetic DMARDs. Prophylaxis against PCP was considered based on physician’s decision in cohort 1. In cohort 2, prophylaxis was based on certain criteria (≥2 risk factors, including age ≥ 65, concomitant glucocorticoid use, coexisting lung disease. Background information about PCP risk factors was not available in one PCP case in cohort 1 (indicated as ‘Not available‘). PCP: Pneumocystis pneumonia; Prophylaxis (+); participants received PCP prophylaxis, PCP (+); participants developed PCP. SMX–TMP: sulfamethoxazole and trimethoprim in combination.
The background characteristics of the participants of the two cohorts varied widely (Table 1). Briefly, participants with disadvantages with respect to susceptibility to PCP, such as old age [58 (14) vs 62 (14), P <0.0001] and more complications [e.g. past history of pneumonia (no/yes) 3.0% vs 8.7%, P <0.0001, past history of malignancy (no/yes) 6.2% vs 10.9%, P <0.0001, or past history of bone fracture (no/yes) 6.7% vs 14.4%, P <0.0001, coexisting lung disease (no/yes) 18.2% vs 27.0%, P <0.0001] were treated with b/tsDMARDs earlier and in less severe disease state [disease duration 71 months vs 59 months P = 0.01, clinical disease activity index (CDAI) 29.5 (14.2) vs 25.4 (12.8), P <0.0001] in cohort 2. Cohort 1 included patients with treatment including TNF inhibitors and IL-6 receptor inhibitors, whereas cohort 2 included patients treated in addition with cytotoxic lymphocyte antigen 4 (CTLA4)-Ig (n = 630) and janus kinase (JAK) inhibitors (n = 263).
Table 1.
Background characteristics of study participants
| Cohort 1 | Cohort 2 | ||
|---|---|---|---|
| Aug 2003–Sept 2009 | Oct 2009–Dec 2019 | P-value | |
| Number of patients | 807 | 2980 | |
| Age | 58 (14) | 62 (14) | <0.0001 |
| ≥65 (years) | 35.4 | 48.3 | <0.0001 |
| Sex, female (%) | 83.1 | 80.4 | 0.08 |
| RA disease backgrounds | |||
| Disease duration (month) | 71 (21, 168) | 59 (14, 145) | 0.01 |
| Stage | <0.0001 | ||
| I (%) | 14.0 | 23.8 | |
| II (%) | 41.4 | 43.9 | |
| III (%) | 20.3 | 17.7 | |
| IV (%) | 24.3 | 14.6 | |
| RF, positive (%) | 85.4 | 78.0 | <0.0001 |
| ACPA, positive (%) | 80.9 | 75.1 | 0.10 |
| Treatment of RA | |||
| b/ts-DMARDs | <0.0001 | ||
| TNF inhibitors | 700 | 1483 | |
| IL-6R inhibitors | 106 | 604 | |
| CTLA4-Ig | 0 | 630 | |
| JAK inhibitors | 0 | 263 | |
| Others | 1 | 0 | |
| Number of past b/ts-DMARDs use | 0.2 (0.5) | 0.7 (1.0) | <0.0001 |
| b/ts-DMARDs naïve (%) | 83.9 | 57.5 | <0.0001 |
| Concomitant MTX use (%) | 81.9 | 73.7 | <0.0001 |
| MTX dose (mg/week) | 8 (8, 10) | 12 (8, 16) | <0.0001 |
| Concomitant GC use (%) | 46.9 | 22.1 | <0.0001 |
| GC dose, prednisone equivalent (mg/day) | 5.0 (2.5, 5) | 5.0 (2.5, 7.5) | 0.0002 |
| RA disease activity | |||
| CRP (mg/dl) | 1.70 (0.50, 4.08) | 0.88 (0.17, 2.94) | <0.0001 |
| ESR (mm/h) | 52 (31, 77) | 44 (22, 75) | <0.0001 |
| MMP-3 (pg/ml) | 201 (101, 377) | 135 (63, 296) | <0.0001 |
| CDAI | 29.5 (14.2) | 25.4 (12.8) | <0.0001 |
| HAQ | 1.330 (0.819) | 1.314 (0.845) | 0.35 |
| Past history and comorbidity | |||
| Pneumonia (%) | 3.0 | 8.7 | <0.0001 |
| Malignancy (%) | 6.2 | 10.9 | <0.0001 |
| Fracture (%) | 6.7 | 14.4 | <0.0001 |
| Coexisting lung disease (%) | 18.2 | 27.0 | <0.0001 |
| Other clinical features | |||
| BMI (kg/m2) | 21.5 (3.5) | 22.3 (4.0) | <0.0001 |
| eGFR (ml/min/1.73m2) | 90.1 (29.1) | 81.9 (28.3) | <0.0001 |
| Alb (g/dl) | 3.7 (0.5) | 3.7 (0.6) | 0.055 |
| IgG (mg/dl) | 1575 (509) | 1510 (491) | 0.001 |
| Neutrophils (/μl) | 5300 (2200) | 4600 (2100) | <0.0001 |
| Lymphocytes (/μl) | 1300 (500) | 1400 (600) | 0.06 |
| FBS (mg/dl) | 100 (25) | 95 (20) | 0.01 |
| HbA1c (%) | 5.8 (0.9) | 5.8 (0.7) | 0.27 |
| KL-6 (U/ml) | 227 (182, 312) | 233 (174, 334) | 0.83 |
| PCP prophylaxis | |||
| Risk factors (%) | 0.004 | ||
| 0 | 237 (29.4) | 1028 (34.5) | |
| 1 | 366 (45.4) | 1146 (38.4) | |
| 2 | 165 (20.5) | 664 (22.3) | |
| 3 | 38 (4.7) | 142 (4.8) | |
| Fulfilling prophylaxis criteria (%) | 25.2 | 27.1 | 0.29 |
| PCP prophylaxis at baseline (%) | 113 (14.0) | 1512 (50.7) | <0.0001 |
| PCP onset | |||
| Number of PCP onset (%) | 13 (1.6) | 13 (0.4) | 0.004 |
| - in participants administrated prophylaxis at baseline (%) | 1 (0.9) | 7 (0.5) | 0.44 |
| - in participants receiving prophylaxis at onset (%) | 1 (0.9) | 4 (0.3) | 0.25 |
Data are mean (s.d.), median (1st IQR, 4th IQR) or %. P values by unpaired t test, Mann–Whitney U test or χ2 test. Cohort 1: subjects who did not receive strategic prophylaxis against PCP. Cohort 2: subjects who received strategic PCP prophylaxis. CDAI, clinical disease activity index. IL-6R; IL 6 receptor, CTLA4; cytotoxic lymphocyte antigen 4, JAK; janus kinase, GC; glucocorticoid, CDAI; clinical disease activity index, eGFR; estimated glomerular filtration rate. FBS; fasting blood sugar; PCP: pneumocystis pneumonia.
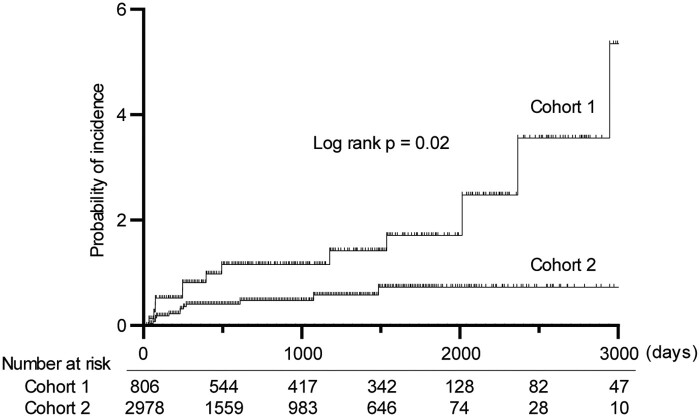
For cohorts 1 and 2, 25.2% and 27.1% (P =0.29) of the participants fulfilled the prophylaxis criteria, respectively, and PCP prophylaxis was provided to more participants of cohort 2 (50.7%) (Table 1). However, no severe adverse events due to PCP prophylaxis were observed in either cohort 1 (observation period: 2559 person-years; PYs) or cohort 2 (6048 PYs). Predictably, the PCP incidence was suppressed from 0.51/100 PYs to 0.21/100 PYs by strategic PCP prophylaxis (risk ratio = 0.42, 95% CI 0.20, 0.91, Log rank P =0.02. Fig. 2) during the observational period (up to 5251 days, median, 617 days). This result was reproducible in the per-person analysis: the PCP rate fell from 0.48/100 PYs to 0.20/100 PYs (risk ratio = 0.43, 95% CI 0.20, 0.92, Log rank P =0.01, data not shown).
Fig. 2.
Strategic prophylaxis reduced the incidence of pneumocystis pneumonia
Kaplan-Meier curves for time to pneumocystis pneumonia (PCP)-onset. P-value was calculated by log rank test under intention-to-treat analysis.
Supplementary Table S1 (available at Rheumatology online) provides details on the PCP prophylaxis profile at the study baseline. The PCP incidence increased with increased number of coexisting risk factors from 0.2% (no risk factor) to 11.1% (3 risk factors); however, PCP prophylaxis suppressed the risk factors–dependent increase in PCP incidence in both cohorts (no risk factor, 0.0%; 1 risk factor, 0.3%; 2 risk factors, 1.0%; 3 risk factors, 0.0%; Supplementary Table S1, available at Rheumatology online).
Of note, 8 participants throughout the study developed PCP despite having received prophylaxis at baseline (Fig. 1 and Table 1). Furthermore, each was being treated with either pentamidine inhalation prophylaxis (n = 5) or untreated with any prophylaxis (n = 3) at the time of PCP onset. On the other hand, no PCP was observed in participants treated with SMX–TMP prophylaxis (P <0.0001, Supplementary Table S2, available at Rheumatology online), suggesting that SMX–TMP is the preferable prophylaxis. However, SMX–TMP is known to be associated with a considerable rate of adverse events. Indeed, 11.4% of participants discontinued the regular prophylaxis dose of SMX–TMP (560 mg TMP/week) due to adverse events in another cohort study conducted at our hospital (data not shown). Therefore, we de-escalated the prophylaxis dose of SMX–TMP from 2012 with reference to a new regimen using a lower dose SMX–TMP known to be associated with less adverse events [27]. The incidence of PCP in this study decreased chronologically from 3.4/100 PYs in 2004 to 0/100 PYs in 2019, although the SMX–TMP dose in the prophylaxis was reduced from 454 mg/week in 2004 to 275 mg/week in 2019 on average (Supplementary Fig. S1, available at Rheumatology online). These data suggest that a lower dose of SMX–TMP can be effective in preventing PCP in RA patients treated with b/tsDMARDs.
The current criteria for PCP prophylaxis are based on (i) age ≥65 years, (ii) existing lung disease, and (iii) GC use. Under this system, patients on GCs are eligible for prophylaxis if they fulfil either condition (i) or (ii), whereas those not treated with GCs have to fulfil both criteria (i) and (ii). However, a considerable proportion of the RA patients in cohort 2 were not treated with GCs, and thus would have been considered ineligible for prophylaxis. Does this underestimate the risk? In our study, there was a considerable difference in GC usage between the two cohorts (cohort 1, 46.9%; cohort 2, 22.1%; P <0.0001, Table 1). To analyse the importance of GCs on the prophylaxis protocol, we pooled together the data for 122 participants in cohort 2 who were not on prophylaxis despite their eligibility to be on prophylaxis with that for 807 subjects of cohort 1 for further analysis. Three models were generated by backward multivariable Cox proportional hazards regression: model 1, which included age, serum albumin and serum IgG; model 2, which included age, coexisting lung disease, BMI, peripheral blood lymphocyte count and serum IgG; and model 3, which included age only. To avoid any selection bias for the 929 participants, we used the bootstrapping resampling method, which added support to the robustness of the three models (Table 2). To estimate the risk of PCP in the individual patient, we used logistic regression analysis, with the independent variables selected for each model (Table 3). Receiver operating characteristic (ROC) analysis showed that model 2 was the best in predicting PCP onset [sensitivity = 1.00, specificity = 0.805 and area under the curve (AUC) = 0.910 for the GC (−) group; sensitivity = 0.778, specificity = 0.722 and AUC = 0.746 for GC (+) group]. Notably, all three models were less effective in the prediction of PCP onset in the GC (+) group (Table 4 and Supplementary Fig. S2, available at Rheumatology online). The cut-off values for PCP risk estimation with model 2 calculated from the ROC curves were; 0.0176 for GC (–) and 0.0250 for GC (+).
Table 2.
Predictors of pneumocystis pneumonia according to treatment with glucocorticoids
| Predictors | Total (n = 929) | GC (–) (n = 496) | GC (+) (n = 433) |
|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Univariable analysis | |||
| Age | 1.085* (1.035, 1.146) | 1.103* (1.025, 1.212) | 1.073* (1.010, 1.115) |
| Concomitant GC use | 1.247* (0.480, 3.241) | – | – |
| Coexisting lung disease | 3.979* (1.517, 10.43) | 5.582* (1.315, 23.68) | 3.043* (0.805, 11.50) |
| b/ts-DMARDs, mechanism of action | P value = 0.67 | P value = 0.34 | P value = 0.91 |
| Number of past b/ts-DMARDs use | 1.177* (0.470, 2.157) | 1.699* (0.635, 3.112) | 0.521* (0.032, 1.880) |
| BMI (kg/m2) | 1.033* (0.912, 1.117) | 1.194* (1.003, 1.392) | 0.902* (0.728, 1.074) |
| eGFR (ml/min/1.73 m2) | 0.989* (0.974, 1.006) | 0.971* (0.947, 0.996) | 1.003* (0.982, 1.026) |
| Neutrophil count (/μl) | 1.000* (1.000, 1.000) | 1.000* (1.000, 1.001) | 1.000* (1.000, 1.000) |
| Lymphocyte count (/μl) | 0.999* (0.998, 1.000) | 0.999* (0.997, 1.000) | 1.000* (0.998, 1.001) |
| Serum albumin (g/dl) | 0.279* (0.121, 0.691) | 0.322* (0.095, 1.325) | 0.250* (0.078, 0.869) |
| Serum IgG (mg/dl) | 0.998* (0.997, 0.999) | 0.997* (0.995, 0.999) | 0.999* (0.997, 1.000) |
| Multivariable analysis | Model 1 | Model 2 | Model 3 |
| Age | 1.060* (1.008, 1.121) | 1.092* (1.005, 1.206) | 1.073* (1.010, 1.150) |
| Concomitant GC use | |||
| Coexisting lung disease | 5.128* (1.028, 25.57) | ||
| BMI (kg/m2) | 1.251* (1.064, 1.459) | ||
| eGFR (ml/min/1.73 m2) | |||
| Neutrophil count (/μl) | |||
| Lymphocyte count (/μl) | 0.998* (0.996, 0.999) | ||
| Serum albumin (g/dl) | 0.270* (0.108, 0.734) | ||
| Serum IgG (mg/dl) | 0.998* (0.997, 0.999) | 0.997* (0.995, 0.999) | |
| Bootstrapping resampling | Model 1 | Model 2 | Model 3 |
| Age | 1.062* (1.021, 1.117) | 1.114* (1.002, 1.190) | 1.077* (1.022, 1.133) |
| Concomitant GC use | |||
| Coexisting lung disease | 9.360* (1.080, 49.40) | ||
| BMI (kg/m2) | 1.268* (1.098, 1.451) | ||
| eGFR (ml/min/1.73 m2) | |||
| Neutrophil count (/μl) | |||
| Lymphocyte count (/μl) | 0.998* (0.997, 0.999) | ||
| Serum albumin (g/dl) | 0.296* (0.129, 0.664) | ||
| Serum IgG (mg/dl) | 0.998* (0.997, 0.999) | 0.997* (0.995, 0.999) |
Values are risk ratio (RR) and 95% confidential interval. *P <0.05. Cox proportional hazard regression analysis using data for all participants and participants with/without concomitant glucocorticoid (GC) treatment. Variables were measured at baseline. GFR, glomerular filtration rate.
Table 3.
Results of multivariable logistic regression formulae for each model
| Model 1 |
Model 2 |
Model 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, Alb, IgG |
Age, lung disease, BMI, Lymph, IgG |
Age |
|||||||||
| GC (–) |
GC (+) |
||||||||||
| GC (−) | GC (+) | Lung disease |
Lung disease |
GC (−) | GC (+) | ||||||
| (−) | (+) | (−) | (+) | ||||||||
| α | 2.5605 | −1.6494 | −7.9920 | −6.8932 | −3.7464 | −3.0594 | −9.9273 | −7.3780 | |||
| β1 | (Age) | 0.0742 | 0.0375 | (Age) | 0.0756 | 0.0513 | (Age) | 0.0885 | 0.0556 | ||
| β2 | (Alb) | −1.7572 | −0.9972 | (BMI) | 0.2044 | −0.1176 | |||||
| β3 | (IgG) | −0.0038 | −0.0008 | (IgG) | −0.0028 | −0.0007 | |||||
| β4 | (Lymph) | −0.0020 | −0.0001 | ||||||||
The incidence of pneumocystis pneumonia (p) was calculated using the formula: Ln(α + β1 × Age + β2 × Alb + β3 × IgG. GC, concomitant glucocorticoid use; Alb, serum albumin (g/dl); IgG, serum IgG (mg/dl); Lymph, lymphocyte count (/μl).
Table 4.
Comparison of current and revised criteria for prophylaxis against pneumocystis pneumonia in RA patients treated with b/ts-DMARDs
| Current criteria |
Model 1 |
Model 2 |
Model 3 |
|||||
|---|---|---|---|---|---|---|---|---|
| Age ≥65, lung disease, GC use |
Age, Alb, IgG |
Age, lung disease, BMI, Lymph, IgG |
Age |
|||||
| GC (−) | GC (+) | GC (−) | GC (+) | GC (−) | GC (+) | GC (−) | GC (+) | |
| Sensitivity | 0.625 | 0.444 | 1.000 | 0.667 | 1.000 | 0.778 | 0.875 | 0.556 |
| Specificity | 0.803 | 0.896 | 0.750 | 0.642 | 0.805 | 0.722 | 0.557 | 0.755 |
| AUC | 0.755 | 0.655 | 0.908 | 0.724 | 0.910 | 0.746 | 0.755 | 0.678 |
Power of prediction was calculated by the multivariable (Models 1, 2) or univariable (Model 3) logistic regression analysis and post-hoc ROC analysis. Independent variables entered in each model are shown in Table 2. AUC, area under the curve; GC, concomitant glucocorticoid use; Alb, serum albumin (g/dl); IgG, serum IgG (mg/dl); Lymph; lymphocyte count (/μl).
Discussion
Biologic DMARDs usage carries a well-known risk of life-threatening opportunistic infections. For example, whereas the incidence of PCP in non-HIV patients is relatively low, the mortality is high [14–16]. Several post-marketing clinical surveillances in Japan have shown a considerable risk of PCP in RA patients, with 0.4% for infliximab [3], 0.2% for etanercept [4], 2.62/100 PYs for tocilizumab [5], 0.1% for abatacept [10], and 0.4% for tofacitinib [11]. These reports clearly show that the risk of PCP should be considered seriously in all RA patients treated with b/tsDMARDs. The global market size of b/tsDMARDs has been increasing; therefore, a prevention strategy against PCP should be established.
In this study, we showed that the prophylaxis criteria that we have recommended previously [19] are less than ideal today (Table 4 and Supplementary Fig. S2, available at Rheumatology online). These criteria were based on age, coexisting lung disease and concomitant use of oral GCs. However, clear changes in the characteristics of patients on prophylaxis were noted in this study. The rate of concomitant use of GCs was significantly lower than in the past, presumably due to accumulating evidence on the efficacy and safety of b/tsDMARDs, and recommendations about RA treatment have prompted health care providers to select b/tsDMARDs instead of oral GCs for the treatment of RA. We assumed that the decrease in GC use suppressed the power of the prophylaxis criteria estimated by Katsuyama et al. (the sensitivity decreased from 0.778 in their report to 0.444–0.625) [19]. (See also Table 4.) Therefore, we decided to revise the criteria, using a model that incorporated age, coexisting lung disease, BMI, peripheral blood lymphocyte count and serum IgG. This model is appropriate because all of the model variables are known risk factors for infection.
The concomitant use of GCs was not a predictor of PCP in this study. The confounding effect of age, IgG level, and lymphocytes count on GC use could explain this phenomenon. Another possible explanation is that b/tsDMARDs facilitate dose de-escalation of GCs during the treatment. A decrease in the GC dose may be followed by decrease in BMI, serum IgG recovery, lymphocyte count correction, and subsequently a potential decrease in the risk of PCP. The existence of unknown confounding factors cannot be excluded at this stage in view of the change in participants’ background characteristics. It is noteworthy that the concomitant use of GCs was not a predictor of PCP (Table 2); however, GC use blocked all the revised criteria with respect to precise PCP risk estimation (Table 4 and Supplementary Fig. S2, available at Rheumatology online). This finding suggests that rheumatologists should consider the use of treatments other than prioritizing GCs in RA patients.
Historically, SMX–TMP and pentamidine have been the drugs most widely used for PCP prophylaxis [16]. In the present study, SMX–TMP was found to be significantly more efficacious than pentamidine inhalation in the prevention of PCP (Supplementary Table S2, available at Rheumatology online). Adverse events relating to SMX–TMP seem to be common; however, our results suggested the efficacy of a reduced dosage of SMX–TMP in the prophylaxis (Supplementary Fig. S1, available at Rheumatology online). This suggests that de-escalation of SMX–TMP, instead of administration of / switching to pentamidine inhalation, should be considered in patients who show inadequate response to SMX–TMP. In addition, PCP prophylaxis was administered to an additional 23.6% of the participants in cohort 2 based on physicians’ assessment (Fig. 1, Table 1). This was presumably because the formulation of the strategic PCP prophylaxis by Katsuyama et al. [19] further encouraged physicians to recommend PCP prophylaxis. Importantly, no severe adverse events due to prophylaxis were observed. This phenomenon also suggests that beyond-necessity provision of PCP prophylaxis with dose de-escalation is acceptable and seems a better clinical practice. Moreover, different cut-off values for PCP risk estimation with model 2 were calculated for the GC (−) and GC (+) groups. If we adopt the cut-off value of 0.0176, which was calculated for the GC (−) group, as the PCP risk estimation for GC (+) group using model 2, the prediction specificity drops from 0.722 to 0.583. However, in view of the efficacy and safety of PCP prophylaxis with dose de-escalation, together with the generally accepted view that GC increase risks of infection, this adaptation appears to be feasible.
Our study had certain limitations. First, pooling the data for 929 participants for the revision of the criteria for prophylaxis probably introduced selection bias. However, we used the bootstrap resampling method to compensate for any such selection bias. Ideally, verification of the results obtained in this study would involve a cohort free of any bias and free from any PCP prophylaxis consideration. Second, the PCP rate varies globally. For this study, we estimated the cut-off values for the PCP risk estimation above using model 2; however, we stress that these cut-off values cannot be applied in other countries, because they depend to a large extent on the PCP rate in each population. Therefore, fine tuning of the variables and the cut-off values of the identified criteria should be considered separately for each population.
In conclusion, our study clarified the risk factors for PCP in RA patients on b/tsDMARDs treatment, and highlighted and defined the criteria for effective prophylaxis against PCP. Our study also showed the effectiveness of PCP prophylaxis in RA patients treated with tsDMARDs. The adverse effects of SMX–TMP can be suppressed prophylactically according to the prophylaxis criteria, as demonstrated in this study. De-escalation of the dose of prophylaxis would be helpful. Taken together, our study provides a better management concept for RA patients treated with b/tsDMARDs, especially with regard to the control of potential infectious adverse events.
Supplementary Material
Acknowledgements
The authors thank the medical staff of all participating institutions for providing the study data, with special thanks to Mses. Hiroko Yoshida, Yoko Saito and Ayumi Maruyama from the FIRST registry for their excellent data management. We also thank Dr Kazuyoshi Saito at Tobata General Hospital; Drs Kentaro Hanami and Shunsuke Fukuyo from Wakamatsu Hospital of the University of Occupational and Environmental Health; Dr Keisuke Nakatsuka from Fukuoka Yutaka Cenrtal Hospital; and all staff members of Kitakyushu General Hospital and Shimonoseki Saiseikai Hospital for their engagement in data collection from the FIRST registry. We also thank Dr Sho Sasaki from Iizuka Hospital for his excellent advice on statistical analysis.
Funding: This work was supported in part by a Grant- In- Aid for Scientific Research from the University of Occupational and Environmental Health, Japan, through the University of Occupational and Environmental Health, Japan, (UOEH) for Advanced Research (#19K17919).
Disclosure statement: K.N. has received speaking fees from Bristol-Myers, Sanofi, AbbVie, Eisai, Eli Lilly, Chugai, Pfizer, Takeda, and Mitsubishi-Tanabe, and research grants from Mitsubishi- Tanabe and Eisai. S.N. has received speaking fees from Bristol-Myers, Pfizer, GlaxoSmithKline, Sanofi, Chugai, Astellas, Asahi-kasei, and Boehringer Ingelheim and has research grants from Mitsubishi-Tanabe, Novartis and MSD. Y.T. has received speaking fees and/or honoraria from Daiichi-Sankyo, Eli Lilly, Novartis, YL Biologics, Bristol-Myers, Eisai, Chugai, Abbvie, Astellas, Pfizer, Sanofi, Asahi-kasei, GSK, Mitsubishi-Tanabe, Gilead and Janssen, and research grants from Abbvie, Mitsubishi-Tanabe, Chugai, Asahi-Kasei, Eisai, Takeda and Daiichi-Sankyo, and consultant fees from Eli Lilly, Daiichi-Sankyo, Taisho, Ayumi, Sanofi, GSK and Abbvie. The other authors have declared no conflicts of interest.
Data availability statement
Data cannot be shared for ethical/privacy reasons.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Tanaka Y. Rheumatoid arthritis. Inflamm Regener 2020;40:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smolen JS, Aletaha D, McInnes IB.. Rheumatoid arthritis. Lancet 2016;388:2023–38. [DOI] [PubMed] [Google Scholar]
- 3. Takeuchi T, Tatsuki Y, Nogami Y. et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis 2008;67:189–94. [DOI] [PubMed] [Google Scholar]
- 4. Koike T, Harigai M, Inokuma S. et al. Postmarketing surveillance of safety and effectiveness of etanercept in Japanese patients with rheumatoid arthritis. Modern Rheumatol 2011;21:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koike T, Harigai M, Inokuma S. et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 2011;70:2148–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baddley JW, Winthrop KL, Chen L. et al. Non-viral opportunistic infections in new users of tumour necrosis factor inhibitor therapy: results of the SAfety Assessment of Biologic ThERapy (SABER) study. Ann Rheum Dis 2014;73:1942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh JA, Cameron C, Noorbaloochi S. et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet 2015;386:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winthrop KL, Park SH, Gul A. et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis 2016;75:1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bruce ES, Kearsley-Fleet L, Watson KD, Symmons DP, Hyrich KL.. Risk of Pneumocystis jirovecii pneumonia in patients with rheumatoid arthritis treated with inhibitors of tumour necrosis factor alpha: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology 2016;55:1336–7. [DOI] [PubMed] [Google Scholar]
- 10. Harigai M, Ishiguro N, Inokuma S. et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Modern Rheumatol 2016;26:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mimori T, Harigai M, Atsumi T. et al. AB0431 Post-marketing surveillance of tofacitinib in Japanese patients with rheumatoid arthritis: an interim report of safety data. Ann Rheum Dis 2017;76(suppl 2):1200–1. [Google Scholar]
- 12. Smolen JS, Landewe RBM, Bijlsma JWJ. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 13. Holroyd CR, Seth R, Bukhari M. et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis—executive summary. Rheumatology 2019;58:220–6. [DOI] [PubMed] [Google Scholar]
- 14. Thomas CF, Limper AH.. Pneumocystis pneumonia. New Engl J Med 2004;350:2487–98. [DOI] [PubMed] [Google Scholar]
- 15. Ward MM, Donald F.. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis Rheum 1999;42:780–9. [DOI] [PubMed] [Google Scholar]
- 16. Ishikawa Y, Nakano K, Tokutsu K. et al. Estimation of treatment and prognostic factors of pneumocystis pneumonia in patients with connective tissue diseases. RMD Open 2021;7:e001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stern A, Green H, Paul M, Vidal L, Leibovici L.. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev 2014;2014:CD005590. [DOI] [PubMed] [Google Scholar]
- 18. Oladele RO, Otu AA, Richardson MD, Denning DW.. Diagnosis and management of pneumocystis pneumonia in resource-poor settings. J Health Care Poor Underserved 2018;29:107–58. [DOI] [PubMed] [Google Scholar]
- 19. Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y.. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther 2014;16:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kubo S, Nakayamada S, Nakano K. et al. Comparison of the efficacies of abatacept and tocilizumab in patients with rheumatoid arthritis by propensity score matching. Ann Rheum Dis 2016;75:1321–7. [DOI] [PubMed] [Google Scholar]
- 21. Ochi S, Saito K, Mizoguchi F, Kato S, Tanaka Y.. Insensitivity versus poor response to tumour necrosis factor inhibitors in rheumatoid arthritis: a retrospective cohort study. Arthritis Res Ther 2020;22:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawabe A, Nakano K, Kubo S, Asakawa T, Tanaka Y.. Differential long-term retention of biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis by age group from the FIRST registry. Arthritis Res Ther 2020;22:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ochi S, Mizoguchi F, Nakano K, Tanaka Y.. Similarity of response to biologics between elderly-onset rheumatoid arthritis (EORA) and non-EORA elderly patients: from the FIRST registry. J Rheumatol 2021; Advance Access published 15 February 2021, doi: 10.3899/jrheum.201135 [DOI] [PubMed] [Google Scholar]
- 24. Ochi S, Mizoguchi F, Nakano K, Tanaka Y.. Difficult-to-treat rheumatoid arthritis with respect to responsiveness to biologic/targeted synthetic DMARDs: a retrospective cohort study from the FIRST registry. Clin Exp Rheumatol 2021; Advance Access published 15 February 2021. [DOI] [PubMed] [Google Scholar]
- 25. Miyazaki Y, Nakano K, Nakayamada S. et al. Efficacy and safety of tofacitinib versus baricitinib in patients with rheumatoid arthritis in real clinical practice: analyses with propensity score–based inverse probability of treatment weighting. Ann Rheum Dis 2021;80:1130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saito K, Nakayamada S, Nakano K. et al. Detection of Pneumocystis carinii by DNA amplification in patients with connective tissue diseases: re-evaluation of clinical features of P. carinii pneumonia in rheumatic diseases. Rheumatology 2004;43:479–85. [DOI] [PubMed] [Google Scholar]
- 27. Utsunomiya M, Dobashi H, Odani T. et al. Optimal regimens of sulfamethoxazole–trimethoprim for chemoprophylaxis of Pneumocystis pneumonia in patients with systemic rheumatic diseases: results from a non-blinded, randomized controlled trial. Arthritis Res Ther 2017;19:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared for ethical/privacy reasons.