Abstract
Objective
To evaluate survival and associated comorbidities in inclusion body myositis (IBM) in a population-based, case-control study.
Methods
We utilized the expanded Rochester Epidemiology Project medical records-linkage system, including 27 counties in Minnesota and Wisconsin, to identify patients with IBM, other inflammatory myopathies (IIM), and age/sex-matched population-controls. We compared the frequency of various comorbidities and survival among groups.
Results
We identified 50 IBM patients, 65 IIM controls and 294 population controls. Dysphagia was most common in IBM (64%) patients. The frequency of neurodegenerative disorders (dementia/parkinsonism) and solid cancers was not different between groups. Rheumatoid arthritis was the most common rheumatic disease in all groups. A total of 36% of IBM patients had a peripheral neuropathy, 6% had Sjögren’s syndrome and 10% had a haematologic malignancy. T-cell large granular lymphocytic leukaemia was only observed in the IBM group. None of the IBM patients had hepatitis B or C, or HIV. IBM patients were 2.7 times more likely to have peripheral neuropathy, 6.2 times more likely to have Sjögren’s syndrome and 3.9 times more likely to have a haematologic malignancy than population controls. IBM was associated with increased mortality, with a 10-year survival of 36% from index, compared with 67% in IIM and 59% in population controls. Respiratory failure or pneumonia (44%) was the most common cause of death.
Conclusions
IBM is associated with lower survival, and higher frequency of peripheral neuropathy, Sjögren’s syndrome and haematologic malignancies than the general population. Close monitoring of IBM-related complications is warranted.
Keywords: inclusion body myositis, case-control study, large granular lymphocytic leukemia, peripheral neuropathy, Sjögren’s syndrome
Rheumatology key messages.
Compared to population controls, patients with inclusion body myositis (IBM) have lower survival.
Peripheral neuropathy, Sjögren’s syndrome, and T-cell large granular lymphocytic leukemia are more common in IBM.
The frequency of neurodegenerative disorders and solid cancers was similar to population controls.
Introduction
Inclusion body myositis (IBM) is a progressive muscle disease associated with distinctive clinical and histopathological characteristics. IBM histopathological findings include a combination of an inflammatory exudate invading non-necrotic muscle fibres, and disrupted protein homeostasis as witnessed by the presence of rimmed vacuoles and congophilic inclusions [1, 2]. The congophilic inclusions consist of a wide spectrum of protein aggregates reminiscent of neurodegenerative diseases of ageing, such as amyloid-β peptides, ubiquitin, phosphorylated tau and TDP-43 [3–5]. Despite these common histopathological features, the prevalence of neurodegenerative diseases in IBM patients has not been evaluated in comparison to the general population. Interestingly, more than expected frequency of peripheral neuropathy has been reported in IBM patients, and it remained unclear whether this was purely related to age or comorbidities [6, 7]. Given the inflammatory component on muscle biopsy and the poorly understood disease pathogenesis, several studies evaluated the association of IBM with various disorders: immune-mediated such as rheumatic diseases, infectious such as hepatitis C and HIV, and neoplastic such as chronic T-cell large granular lymphocytic (T-LGL) leukaemia [8–12]. However, the majority of the literature derives from cross-sectional retrospective studies performed at referral centres, as most population-based studies focussed on evaluating the prevalence of the disease [13–19]. Reported IBM prevalence varies widely between studies, with an estimated overall prevalence of 24.8–45.6 per million according to a meta-analysis [13–20]. Furthermore, most previous studies reported that IBM does not affect longevity, by comparing to life expectancy in general population databases [21, 22]. A head-to-head comparison in a case-control population-based study is lacking.
The Rochester Epidemiologic Project (REP), started about half a century ago, is a comprehensive medical records linkage system for all residents of Olmsted County, Minnesota, USA [23]. Mayo Clinic and Olmsted Medical Center being the only healthcare systems in Olmsted county makes the REP an ideal setting for population-based studies including calculation of incidence and prevalence of diseases. This resulted in several epidemiologic projects on various diseases including inclusion body myositis [14, 24]. In the most recent study, we identified 20 patients with IBM who lived in Olmsted county (population of about 150 000) between 1980 and 2019 [24]. In that study, we reported the highest prevalence of IBM to date (182 per million in people 50 years of age or older). The long-term follow-up over 40 years enabled us to provide detailed information about the natural history of the disease. We also found shorter survival in IBM patients; however, the study was limited by the low number of patients given the small population of Olmsted county. This also precluded a comprehensive evaluation for associated comorbidities.
In 2010, the REP population was expanded (expanded REP) to include 27 counties in Southern Minnesota and Western Wisconsin, to capture a population of >700 000 [25]. The large expanded REP population facilitated population-based studies on rare diseases such as IBM, and allowed us to identify 50 patients with IBM seen between 2010 and 2019, only five of whom were included in previous Olmsted county studies [14, 24]. In this study, we aimed to evaluate the association of IBM with the various aforementioned neurological, immune-mediated, infectious and neoplastic disorders, in a large case-control, population-based study. We also aimed to evaluate whether IBM affects survival, by head-to-head comparison with age and sex-matched controls. In addition, we aimed to compare the findings in IBM patients to a group of patients with other idiopathic inflammatory myopathies (IIM).
Methods
Study population
The study was approved by the Institutional Review Boards of the Mayo Clinic and of Olmsted Medical Center. The study was considered minimal risk by both Institutional Review Boards; therefore, the requirement for informed consent was waived. However, records of any patient who had not provided authorization for their medical records to be used for research, as per Minnesota statute 144.335, were not reviewed.
Case ascertainment
We searched the medical records–linkage system of the expanded REP to identify participants who had the diagnosis codes for IBM using ICD9 and ICD10 codes (359.71, G72.41), polymyositis (710.4, M33.20, M33.21, M33.22, M33.29) and dermatomyositis (710.3, M33.10, M33.11, M33.12, M33.13, M33.19). We then performed a manual chart review and review of muscle biopsy reports of the identified patients to classify patients into IBM vs IIM. We included in the IBM group patients fulfilling European Neuromuscular Centre (ENMC) 2011 diagnostic criteria for clinico-pathologically defined, clinically-defined, or probable IBM [26]. We included in the IIM groups patients with the following entities: dermatomyositis, overlap myositis, antisynthetase syndrome, immune-mediated necrotizing myopathy and nonspecific myositis [27, 28]. Patients with myositis were excluded from the IIM control group if there was clinical suspicion for IBM (not fulfilling ENMC diagnostic criteria), or if the available information was not sufficient to exclude IBM. We also excluded patients with limited information regarding the diagnosis in their chart.
Matching
Index date consisted of the first time the patient was coded for the diagnosis of IBM. Each IBM patient was individually matched by age (within a year) and sex, with six population controls from the REP database seen during the same calendar year. As we expected the number of individuals with other IIM to be limited and the IIM population to be younger, each IBM patient was individually matched by age (±15 years) and sex to one or two IIM cases.
Data collection
The medical records of cases and controls were electronically searched to extract patients’ demographics, tobacco use and education level at index date. For IBM disease characteristics, we searched diagnostic codes for dysphagia, gastrostomy tube, impaired gait and wheelchair use. Patients’ prescribed medication lists were electronically pulled and manually reviewed to abstract data regarding the use of immunosuppressant drugs. For IBM patients, we performed a manual chart review to determine the indication for corticosteroid treatment.
Regarding the associated conditions, we electronically pulled data by diagnostic codes for the following entities of interest: neurodegenerative disorders (any form of dementia, Parkinson’s disease, or other Parkinsonian disorders), peripheral neuropathy, peripheral neuropathy-associated conditions (diabetes mellitus, vitamin B12 deficiency, thyroid dysfunction and monoclonal gammopathy), rheumatic diseases (RA, lupus erythematous, Sjögren syndrome and scleroderma), malignancy of any type, haematologic cancers, and infections (hepatitis B and C, and HIV). Infectious etiologies were also searched for by serological testing data. We also retrieved mortality data including cause of death and death certificates. All diagnostic codes were reviewed for accuracy. We then performed a manual chart review of the following variables that cannot be ascertained solely based on diagnostic codes: peripheral neuropathy, Sjögren syndrome, malignancy of any type (including haematologic) and cause of death (chart review and death certificate review). For malignancies, we included the malignancies that occurred within 5 years preceding match date or any time afterwards. For recurring cancers (such as skin cancers), they were only included if they occurred for the first time during the defined time frame.
Statistics and data analysis
Descriptive summaries are presented as frequency and percentage for categorical variables, and mean (s.d.) for continuous variables. Statistical analysis was carried out using JMP® 13 and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) statistical software. Comparison among the three groups was performed using Kruskall–Wallis, χ2 or Exact test as appropriate. All of the tests were two sided. When the P-value for an outcome comparing the three groups reached statistical significance (<0.05), groups were then compared in a pairwise manner using Wilcoxon Rank Sum, χ2 or Exact test as appropriate. This was followed by logistic regression to evaluate for the association between the outcomes of interest (comorbidities with P < 0.05) and the groups, while adjusting for age at index; reported as odds ratios and [95% CI]. Time-to-death as an end point was analyzed using Kaplan–Meier method and Cox proportional hazard regression.
Results
Demographics and baseline characteristics
Fifty patients fulfilled the 2011 ENMC criteria for IBM and included 31 clinicopathologically-defined, 16 clinically-defined and three probable IBM. We identified 69 IIM controls: 32 with dermatomyositis, 13 with antisynthetase syndrome, five with overlap myositis, 17 with nonspecific myositis and two with necrotizing autoimmune myopathy. Demographic data is shown in Table 1. Patients in the IIM group were on average 6.5 years younger and more commonly females. All IBM patients were white, one of them Hispanic; racial and ethnic distribution of IBM patients are not different from those of the catchment area. There was no difference in the use of tobacco or educational level between groups. Dysphagia was most common in IBM group, as IBM patients were 7.6 [3.8, 14.9] times more likely to have dysphagia than population controls (P < 0.0001). There was a trend for IBM patients to have more dysphagia than IIM patients; however, this became less evident adjusting for age: odds ratio of 1.6 [0.7, 3.6] (P = 0.23). In contrast, IBM patients were 3.7 [1.5, 9] times more likely to have impaired gait compared with the IIM group (P = 0.0036), and 4.2 [2.1, 8.7] times compared with the population controls (P < 0.0001). A small number of patients were coded for the use of a wheelchair; nevertheless, this was most common in the IBM group. Regarding medication exposure during the study period, 22 (44%) IBM patients and 29 (44.6%) IIM patients were treated with corticosteroids for their condition. Comparing the group of IBM patients who were treated with corticosteroids vs the ones who were not, there was no statistically significant difference in age at index (mean: 71.5 vs 74.4 years, respectively), sex (50% males vs 64.3%), the presence of dysphagia (68.2% vs 60.7%), gait difficulty (77.3% vs 71.4%) or follow-up duration (40.8 vs 58.1 months, P = 0.074). Patients were maintained on varying doses of oral prednisone. They were usually started on a high dose of oral prednisone followed by a slow taper. The mean reached high dose was 43.4 mg/day (s.d. 19.5). Duration of treatment was highly variable with a mean of 5.5 years (s.d. 7.2). 25 (38.5%) IIM and 17 (34%) IBM patients were treated with an immunosuppressant drug (IIM: MTX, AZA, MMF, ciclosporin, or rituximab; IBM: MTX or MMF).
Table 1.
Demographics and baseline characteristics of patients
| Variable | IBM (n = 50) | IIM (n = 65) | Population controls (n = 294) |
|---|---|---|---|
| Demographics | |||
| Age (years)* | 73.5 (10.39) | 67.4 (10.98)** | 73.0 (10.44) |
| Sex: male* | 29 (58) | 25 (38.4)* | 173 (58.8) |
| Race, n (%) | |||
| Black | 0 (0.0%) | 2 (3.1) | 1 (0.3) |
| Asian | 0 (0.0%) | 1 (1.5) | 12 (4.1) |
| Other/mixed | 0 (0.0%) | 3 (4.6) | 3 (1.0) |
| White | 50 (100) | 59 (90.8) | 277 (94.2) |
| Unknown | 0 0 | 0 0 | 1 (0.3) |
| Hispanic, n (%) | 1 (2) | 1 (1.5) | 8 (2.7) |
| Weight (kg) | 80.1 (14.54) | 77.6 (20.25) | 79.0 (17.69) |
| Height (cm) | 170.1(10.88) | 165.9 (9.86) | 167.7 (9.72) |
| Tobacco use | |||
| Current or former smoker | 7 (17.1) | 6 (11.5) | 31 (16.1) |
| Unknown | 9 | 13 | 101 |
| Education level | |||
| Primary | 1 (2.2) | 0 0 | 8 (2.9) |
| Secondary | 16 (34.8) | 24 (38.1) | 89 (32.1) |
| Tertiary | 29 (63) | 39 (61.9) | 180 (65.5) |
| Unknown | 4 | 2 | 17 |
| Disease characteristics | |||
| Dysphagia**** | 32 (64) | 30 (46.2)# | 61 (20.8)**** |
| Gastrostomy tube* | 3 (6) | 5 (7.7) | 5 (1.7) |
| Impaired gait**** | 37 (74) | 24 (36.9)**** | 128 (43.5)**** |
| Wheelchair use** | 5 (10) | 1 (1.5)* | 5 (1.7)* |
Categorical variables are displayed as n (percentage), and continuous variable as mean (s.d.). The P-value corresponding to comparison of the three groups is shown in the first column, P-value for IBM vs IIM group is shown in ‘IIM’ column, and P-value for IBM vs population controls is shown in ‘Population controls’ column.
0.01≤P-value < 0.05;
P-value <0.01;
P-value <0.001;
P-value <0.0001;
P-value = 0.057.
IBM: inclusion body myositis; IIM: inflammatory myopathy.
Association with neurological conditions
Results are shown in Table 2. There was no statistically significant difference in the frequency of Alzheimer’s or Parkinson’s diseases between the groups. Peripheral neuropathy was more common in IBM and IIM patients than population controls. IBM patients were 2.7 [1.4, 5.3] times more likely to have a peripheral neuropathy than population controls (P = 0.0033). Diabetes mellitus, thyroid dysfunction and vitamin B12 deficiency were not more common in IBM group to explain the increased frequency of peripheral neuropathy. There was a trend for increased frequency of a monoclonal gammopathy of uncertain significance in the IBM group that did not reach statistical significance (could be due to small sample size).
Table 2.
Inclusion body myositis-associated conditions
| IBM (n = 50) |
IIM (n = 65) |
Controls (n = 294) |
||
|---|---|---|---|---|
| Neurological | ||||
| Dementia | 3 (6) | 7 (10.8) | 31 (10.5) | |
| Parkinson’s disease | 2 (4) | 2 (3.1) | 17 (5.8) | |
| Peripheral neuropathy** | 18 (36) | 15 (23.1) | 51 (17.4)** | |
| Conditions that may cause a peripheral neuropathy: | ||||
|
16(32) | 19(29) | 129(44) | |
|
11(22) | 31(48)** | 87(30) | |
|
3(6) | 3(5) | 8(3) | |
|
4(8) | 1(2) | 8(3)# | |
| Rheumatological | ||||
| Lupus erythematous*** | 0 | 6(9)* | 1(<1) | |
| Systemic scleroderma*** | 1(2) | 7(11) | 0 | |
| Sjögren syndrome* | 3(6) | 2(3) | 3(1)* | |
| RA* | 4(8) | 8(12) | 13(5) | |
| Any of the above*** | 8(16) | 19(29) | 15(5)** | |
| Malignancies | ||||
| Malignancy of any type | 20 (40) | 23 (35.4) | 92 (31.3) | |
| Solid cancers | 16 (32) | 21 (32.3) | 88 (29.9) | |
| Haematologic malignancies* | 5 (10) | 3 (4.6) | 8 (2.7)* | |
|
Diffuse large B cell (1) Marginal zone (1) |
Hodgkin’s (1) Waldenstrom (1) Diffuse large B cell (1) |
Difffuse large B cell (4) Marginal zone (1) |
|
|
TLGL(2) | 0 | CLL(1) | |
|
Multiple myeloma (1) | 0 | 0 | |
|
0 | 0 | 2 | |
| Infectious | ||||
| Hepatitis B | 0 0 | 0 0 | 2 (0.7) | |
| Hepatitis C | 0 0 | 1 (1.5) | 2 (0.7) | |
| HIV | 0 0 | 00 | 00 |
P = comparison of the three groups; NS: not significant with P >0.05, P1 = IBM vs IIM, P2 = IBM vs Categorical variables are displayed as n (percentage). The P-value corresponding to comparison of the three groups is shown in the first column, P-value for IBM vs IIM group is shown in ‘IIM’ column, and P-value for IBM vs population controls is shown in ‘population controls’ column.
0.01≤P-value < 0.05;
P-value <0.01;
P-value <0.001;
P-value <0.0001;
P-value = 0.06.
IBM: inclusion body myositis; IIM: inflammatory myopathy.
Association with rheumatic diseases
Rheumatic diseases were more common in IBM and IIM patients than population controls (Table 2). RA was the most common rheumatic disease in all groups. IBM patients were 6.4 [1.3, 33.2] times more likely to have Sjögren’s syndrome than population controls (P = 0.026).
Association with malignancies
A total of 135 patients developed 155 malignancies within 5 years preceding match date or any time afterwards. Among the 20 patients with more than one malignancy, 15 had skin cancer. There was no difference in the frequency of patients with solid cancers between the groups as shown in Table 2. The distribution of various cancer types per group is shown in Supplementary Fig. S1, available at Rheumatology online. However, haematologic malignancies were most common in IBM group, and IBM patients were 3.9 [1.2, 12.6] times more likely to have a haematologic malignancy than population controls (P = 0.021). Although IBM patients were 2.8 [0.6, 12.9] times more likely to have a haematologic malignancy than IIM patients, the difference did not reach statistical significance (P = 0.18), which may be due to limited sample size given the wide 95% CI. Chronic T-cell large granular lymphocytic leukaemia was only observed in IBM group. In both patients, the diagnosis of T-LGL leukaemia preceded the diagnosis of IBM. The first patient presented with lymphocytosis, neutropenia and elevated lactate dehydrogenase level, and had bone marrow involvement. She was diagnosed with T-LGL leukaemia 3 years prior to the diagnosis of IBM. The patient passed away from respiratory failure attributed to IBM, 13 years after IBM symptom onset. The second patient presented with neutropenia and had bone marrow involvement. She was diagnosed with T-LGL leukaemia, and then a year and a half later she was diagnosed with IBM. Haematologic malignancies in IIM group were all B-cell lymphomas. The most common haematologic malignancy in population controls was diffuse large-B-cell lymphoma. None of the IBM patients and one of the IIM patients with haematologic malignancies was exposed to an immunosuppressant (AZA).
Association with infectious etiologies
The number of patients with infectious diseases of interest was low in all groups, with no difference in frequency between groups. None of the IBM patients had hepatitis B, hepatitis C or HIV. We then evaluated the test positivity rates (number of patients with positive serology/total number of tested patients ×100) for participants with available serological testing. For hepatitis B, the positivity rate was 0% (0/8) for IBM, 0% (0/29) for IIM and 6.45% (2/31) for population controls (P > 0.05). For hepatitis C, the positivity rate was 0% (0/9) for IBM, 2.9% (1/34) for IIM and 1.8% (1/57) for population controls (P > 0.05). The second patient with hepatitis C was identified by ICD code. For HIV, the number of tested patients was 10 in IBM, 20 in IIM and 38 in population controls.
Survival outcomes
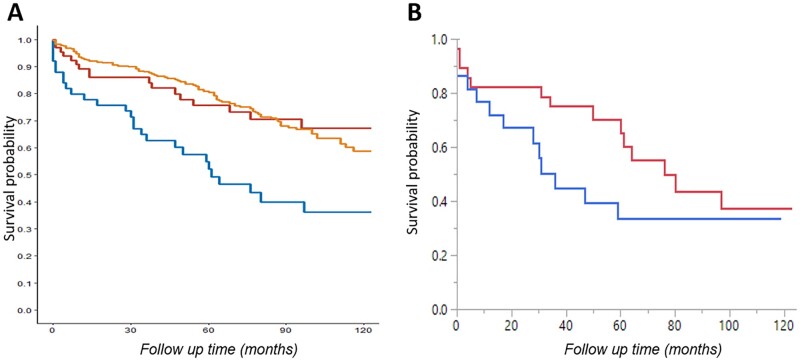
Mean follow up time from index was 51 (39.3) months for IBM, 65 (42.2) for IIM and 71 (37.3) for population controls. Mean age at death was 79.3 (8.5) for IBM patients, 77.5 (9.12) for IIM patients and 83.6 (6.68) for population controls (P = 0.021). Patients with IBM had lower survival than IIM patients and population controls as shown in (Fig. 1) (P < 0.0001). Survival for IBM patients, IIM patients and population controls respectively was as follows: 75%, 86% and 90% at 2 years, 52%, 76% and 81% at 5 years, and 36%, 67% and 59% at 10 years. The most common causes of death were respiratory failure or pneumonia (44.4%), cancer (14.8%), and old age and medical comorbidities (14.8%) in IBM group; cancer (47%), respiratory failure (17.6%) and cardiovascular (11.8%) in IIM group; and cancer (24.1%), cardiovascular (24.1%) and respiratory failure or pneumonia (13.8%) in population controls. We then evaluated whether any of the following univariables were predictors of mortality in IBM patients: age at index, sex, dysphagia or any of the associated comorbidities (peripheral neuropathy, Sjögren’s syndrome, rheumatic disease of any type, haematologic malignancy, and malignancy of any type). Age at index and probably dysphagia were the only predictors, with a hazard ratio per 1 year increase of 1.06 [1.01, 1.11] (P = 0.025) for age, and a hazard ratio of 2.6 [0.98, 6.97] for dysphagia but with a P-value of 0.054. Given that IIM patients were younger and more commonly females, we compared survival between the three groups with group, age and sex as multivariables. IBM and IIM were associated with increased mortality risk compared with population controls, with hazard ratio per 1 year increase of 2.69 [1.74, 4.15] (P < 0.0001) for IBM, and 2.11 [1.22, 3.65] (P = 0.0078) for IIM. However, IBM was not associated with increased mortality risk compared with IIM. We then compared survival between patients treated with corticosteroids vs not (Fig. 1). Patients treated with corticosteroids had a median survival of 31 months compared with 76 months in the untreated group. However, this did not reach statistical significance (P = 0.2), which could be due to the small sample size and low event rate as shown in Fig. 1. There was no difference in survival based on treatment with corticosteroids in the IIM group, or based on treatment with other immunosuppressant drugs in either group (data not shown).
Fig. 1.
Survival outcomes
(A) Kaplan–Meier survival curves comparing IBM patients (blue line), inflammatory myopathy patients (red line) and population controls (orange line). (B) Clear separation in survival curves for IBM patients treated with corticosteroids (blue line) compared with untreated IBM patients (red line). IBM: inclusion body myositis.
Discussion
Herein, we demonstrate in a population-based, case-control study, the increased frequency of peripheral neuropathy, haematologic malignancies and Sjögren sydnrome in IBM. Furthermore, we show that IBM patients have lower survival compared with population controls.
Despite the similarities between IBM and other neurological diseases of ageing, the frequency of dementia and Parkinson’s disease in IBM was not different from population controls. However, IBM patients were 2.7 times more likely to have peripheral neuropathy than population controls. Peripheral neuropathy in IBM is considered independent of the myopathic process, as witnessed by the lack of correlation with disease severity or duration, and the lack of inflammation or tubulofilamentous aggregates on nerve biopsy [29, 30]. Interestingly, the presence of peripheral neuropathy has been reported to be associated with treatment unreponsiveness in patients with polymyositis [6]. While this finding could be because peripheral neuropathy in these patients is simply a marker of inclusion body myositis, which is inherently refractory to treatment, nerve involvement in IBM and its contribution to the clinical phenotype and disease course is yet to be explored [31]. In constrast to previous studies, diabetes mellitus was not more common in IBM or IIM patients compared with population controls [32, 33].
While rheumatic diseases are expected to be more prevalent in IIM patients, we demonstrate they are also more common in IBM, namely Sjögren syndrome. Sjögren syndrome has been reported in patients with IBM; however, the nature of the relationship between the two disorders is not well understood [16]. It may be due to underlying genetic predisposition, as seen in a study from Western Australia, where patients with IBM and Sjögren syndrome were more likely to be carriers of HLA-DR3 and the 8.1 MHC ancestral haplotype [34]. The frequency of Sjögren syndrome in our study is relatively similar to previously reported in most recent studies (10–12%) [16, 34].
Inclusion body myositis pathologic hallmark is the invasion of nonnecrotic muscle fibres by a well-orchestrated inflammtory infiltrate, with highly differentiated, cytotoxic T cells in the front line [35, 36]. When prospectively screened, 58% of IBM patients had aberrant populations of large granular T cells [36]. However, the presence of a T-LGL clone (T-LGL lymphocytosis) does not necessarily mean that the patient has T-LGL leukaemia. The demarcation line between the two entities remains blurry [37]. T-LGL leukaemia is a very rare lymphoproliferative disorder and little is known regarding its pathogenesis, dysregulated signalling pathways, and treatment options [38]. Our two patients were diagnosed with leukaemia based on the presence of associated cytopenias and bone marrow involvement. It was postulated that chronic antigenic stimulation in IBM may result in clonal expansion of a T-LGL population, or vice versa, the T-LGL clone may invade muscle tissue and cause IBM [36, 39]. However, in both of our patients, the diagnosis of T-LGL leukaemia preceded that of IBM, and preceded the onset of muscle weakness in at least one paitent (details are unclear for the second patient). It is noteworthy that similar association with T-LGL clonal expansion has been observed in certain immune-mediated disorders such as RA and Sjögren syndrome [40, 41].
Given the low prevalence of hepatitis B, C and HIV in the local population as witnessed by the low prevalence in the control group, and given the lack of any IBM patient in our cohort with any of these disorders, we cannot draw any conclusions regarding the association with IBM based on our study results. However, hepatitis C seropositivity is reported in 28% of Japanese patients with IBM, which is less likely to be the case in our IBM population as none of the tested nine patients was seropositive; these results are relatively similar to those reported in a Brazilian study [42].
Traditionally, IBM is not considered to affect longevity, and survival is reported to be similar to the life expectancy of the general population in France and the Netherlands [21, 22]. We demonstrate in our study that survival is reduced in IBM patients when compared with age- and sex-matched controls. IBM patients died on average 3 years younger than population controls. These results are similar to those reported in Noway and Olmsted county [16, 24]. Our reported 10-year survival of 36% from match date (mean age at match date was 73.5 years) was relatively similar to the 42% 10-year survival from diagnosis (mean age at diagnosis was 69.9 years) reported by Dobloug et al. [43]. The risk of death in our study was influenced by age at match and probably by the presence of dysphagia. In previous reports, survival was mainly influenced by age at diagnosis [21, 43]. Similar to previous reports, the death in IBM patient was most commonly related to respiratory complications including respiratory failure or pneumonia [24, 43–47]. In contrast, the most common cause of death in IIM group was cancer, similar to population controls. Lastly, there was a trend for lower survival in IBM patients treated with corticosteroids, which is intriguing and has not been previously reported. Benveniste et al. reported that IBM patients treated with glucocorticosteroids or other immunosuppressants needed a walking aid sooner than untreated patients, with no difference in the rate of progression to a wheelchair [21]. In our study, there was no clear difference baseline characteristics between the treated and untreated patients. It remains unclear whether the difference in survival is a direct effect of the use of corticosteroids, vs other confounding factors. Nevertheless, there is no evidence that treatment with corticosteroids has any benefit in IBM patients, and thus it should be avoided.
Our study has several limitations. The rarity of IBM and IIM limited our sample size, and our ability to further stratify by sex. Likewise, some of the comorbidities of interest such as various rheumatic diseases were uncommon as well. The vast majority of our patients and controls were white, limiting generalizability to other races. Additionally, it is possible that some subjects were diagnosed with one of the diseases of interest at a medical facility outside of the records-linkage system and were never coded for such diagnosis in our system. It is noteworthy that the first time a patient is coded for a condition may not necessarily reflect the diagnosis date. However, given the large amount of data in epidemiologic studies, any search method has to be based on diagnosis codes. Nevertheless, we performed a chart review to confirm the accuracy of various diagnoses as mentioned in the methods section. Additional limitations are inherent to the retrospective design of the study and the variability in the work-up obtained in each study participant. Lastly, having a control group would help mitigate to some extent some of the study limitations.
Supplementary Material
Acknowledgements
The authors acknowledge their colleagues in neurology and rheumatology who contributed to the care of the published patients. The authors also acknowledge the Rochester Epidemiologic Project team members for their technical help with study design, and data acquisition and interpretation.
Funding: The study was funded by the small grant program of the Center for Clinical and Translational Sciences (CCATS) at Mayo Clinic (grant number UL1TR002377), and was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
Data not provided in the article and additional information on methods and materials may be shared upon request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Greenberg SA. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol 2019;15:257–72. [DOI] [PubMed] [Google Scholar]
- 2. Naddaf E, Barohn RJ, Dimachkie MM.. Inclusion body myositis: update on pathogenesis and treatment. Neurotherapeutics 2018;15:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salajegheh M, Pinkus JL, Taylor JP. et al. Sarcoplasmic redistribution of nuclear TDP-43 in inclusion body myositis. Muscle Nerve 2009;40:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Askanas V, Engel WK.. Inclusion-body myositis, a multifactorial muscle disease associated with aging: current concepts of pathogenesis. Curr Opin Rheumatol 2007;19:550–9. [DOI] [PubMed] [Google Scholar]
- 5. Mendell JR, Sahenk Z, Gales T, Paul L.. Amyloid filaments in inclusion body myositis. Novel findings provide insight into nature of filaments. Arch Neurol 1991;48:1229–34. [DOI] [PubMed] [Google Scholar]
- 6. Amato AA, Gronseth GS, Jackson CE. et al. Inclusion body myositis: clinical and pathological boundaries. Ann Neurol 1996;40:581–6. [DOI] [PubMed] [Google Scholar]
- 7. Lindberg C, Oldfors A, Hedström A.. Inclusion body myositis: peripheral nerve involvement. Combined morphological and electrophysiological studies on peripheral nerves. J Neurol Sci 1990;99:327–38. [DOI] [PubMed] [Google Scholar]
- 8. Badrising UA, Schreuder GM, Giphart MJ. et al. Associations with autoimmune disorders and HLA class I and II antigens in inclusion body myositis. Neurology 2004;63:2396–8. [DOI] [PubMed] [Google Scholar]
- 9. Rojana-udomsart A, Needham M, Luo YB. et al. The association of sporadic inclusion body myositis and Sjögren's syndrome in carriers of HLA-DR3 and the 8.1 MHC ancestral haplotype. Clin Neurol Neurosurg 2011;113:559–63. [DOI] [PubMed] [Google Scholar]
- 10. Uruha A, Noguchi S, Hayashi YK. et al. Hepatitis C virus infection in inclusion body myositis: a case-control study. Neurology 2016;86:211–7. [DOI] [PubMed] [Google Scholar]
- 11. Lloyd TE, Pinal-Fernandez I, Michelle EH. et al. Overlapping features of polymyositis and inclusion body myositis in HIV-infected patients. Neurology 2017;88:1454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greenberg SA, Pinkus JL, Kong SW. et al. Highly differentiated cytotoxic T cells in inclusion body myositis. Brain 2019;142:2590–604. [DOI] [PubMed] [Google Scholar]
- 13. Oflazer PS, Deymeer F, Parman Y.. Sporadic-inclusion body myositis (s-IBM) is not so prevalent in Istanbul/Turkey: a muscle biopsy based survey. Acta Myol 2011;30:34–6. [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson FC, Ytterberg SR, St Sauver JL, Reed AM.. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J Rheumatol 2008;35:445–7. [PubMed] [Google Scholar]
- 15. Tan JA, Roberts-Thomson PJ, Blumbergs P. et al. Incidence and prevalence of idiopathic inflammatory myopathies in South Australia: a 30-year epidemiologic study of histology-proven cases. Int J Rheum Dis 2013;16:331–8. [DOI] [PubMed] [Google Scholar]
- 16. Dobloug GC, Antal EA, Sveberg L. et al. High prevalence of inclusion body myositis in Norway; a population-based clinical epidemiology study. Eur J Neurol 2015;22:672–e41. [DOI] [PubMed] [Google Scholar]
- 17. Needham M, Corbett A, Day T. et al. Prevalence of sporadic inclusion body myositis and factors contributing to delayed diagnosis. J Clin Neurosci 2008;15:1350–3. [DOI] [PubMed] [Google Scholar]
- 18. Badrising UA, Maat-Schieman M, van Duinen SG. et al. Epidemiology of inclusion body myositis in the Netherlands: a nationwide study. Neurology 2000;55:1385–7. [DOI] [PubMed] [Google Scholar]
- 19. Callan A, Capkun G, Vasanthaprasad V, Freitas R, Needham M.. A systematic review and meta-analysis of prevalence studies of sporadic inclusion body myositis. J Neuromuscul Dis 2017;4:127–37. [DOI] [PubMed] [Google Scholar]
- 20. Felice KJ, North WA.. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine 2001;80:320–7. [DOI] [PubMed] [Google Scholar]
- 21. Benveniste O, Guiguet M, Freebody J. et al. Long-term observational study of sporadic inclusion body myositis. Brain 2011;134:3176–84. [DOI] [PubMed] [Google Scholar]
- 22. Cox FM, Titulaer MJ, Sont JK. et al. A 12-year follow-up in sporadic inclusion body myositis: an end stage with major disabilities. Brain 2011;134:3167–75. [DOI] [PubMed] [Google Scholar]
- 23. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shelly S, Mielke MM, Mandrekar J, Milone M. et al. Epidemiology and natural history of inclusion body myositis: a 40-year population-based study. Neurology 2021;96:e2653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rocca WA, Grossardt BR, Brue SM. et al. Data resource profile: expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol 2018;47:368–j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rose MR; ENMC IBM Working Group. 188th ENMC International Workshop: inclusion Body Myositis, 2-4 December 2011, Naarden, The Netherlands. Neuromuscul Disord 2013;23:1044–55. [DOI] [PubMed] [Google Scholar]
- 27. Lundberg IE, Tjärnlund A, Bottai M. et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol 2017;69:2271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benveniste O, Stenzel W, Allenbach Y.. Advances in serological diagnostics of inflammatory myopathies. Curr Opin Neurol 2016;29:662–73. [DOI] [PubMed] [Google Scholar]
- 29. Hermanns B, Molnar M, Schröder JM.. Peripheral neuropathy associated with hereditary and sporadic inclusion body myositis: confirmation by electron microscopy and morphometry. J Neurol Sci 2000;179:92–102. [DOI] [PubMed] [Google Scholar]
- 30. Lee JH, Boland-Freitas R, Liang C, Howells J, Ng K.. Neuropathy in sporadic inclusion body myositis: a multi-modality neurophysiological study. Clin Neurophysiol 2020;131:2766–76. [DOI] [PubMed] [Google Scholar]
- 31. Eisen A, Berry K, Gibson G.. Inclusion body myositis (IBM): myopathy or neuropathy? Neurology 1983;33:1109–14. [DOI] [PubMed] [Google Scholar]
- 32. Keshishian A, Greenberg SA, Agashivala N, Baser O, Johnson K.. Health care costs and comorbidities for patients with inclusion body myositis. Curr Med Res Opin 2018;34:1679–85. [DOI] [PubMed] [Google Scholar]
- 33. Limaye VS, Lester S, Blumbergs P, Roberts-Thomson PJ.. Idiopathic inflammatory myositis is associated with a high incidence of hypertension and diabetes mellitus. Int J Rheum Dis 2010;13:132–7. [DOI] [PubMed] [Google Scholar]
- 34. Rojana-Udomsart A, Bundell C, James I. et al. Frequency of autoantibodies and correlation with HLA-DRB1 genotype in sporadic inclusion body myositis (s-IBM): a population control study. J Neuroimmunol 2012;249:66–70. [DOI] [PubMed] [Google Scholar]
- 35. Engel AG, Arahata K.. Monoclonal antibody analysis of mononuclear cells in myopathies. II: phenotypes of autoinvasive cells in polymyositis and inclusion body myositis. Ann Neurol 1984;16:209–15. [DOI] [PubMed] [Google Scholar]
- 36. Greenberg SA, Pinkus JL, Amato AA, Kristensen T, Dorfman DM.. Association of inclusion body myositis with T cell large granular lymphocytic leukaemia. Brain 2016;139:1348–60. [DOI] [PubMed] [Google Scholar]
- 37. Ohgami RS, Ohgami JK, Pereira IT. et al. Refining the diagnosis of T-cell large granular lymphocytic leukemia by combining distinct patterns of antigen expression with T-cell clonality studies. Leukemia 2011;25:1439–43. [DOI] [PubMed] [Google Scholar]
- 38. Leblanc F, Zhang D, Liu X, Loughran TP.. Large granular lymphocyte leukemia: from dysregulated pathways to therapeutic targets. Future Oncol 2012;8:787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hohlfeld R, Schulze-Koops H.. Cytotoxic T cells go awry in inclusion body myositis. Brain 2016;139:1312–4. [DOI] [PubMed] [Google Scholar]
- 40. Sun H, Wei S, Yang L.. Dysfunction of immune system in the development of large granular lymphocyte leukemia. Hematology 2019;24:139–47. [DOI] [PubMed] [Google Scholar]
- 41. Friedman J, Schattner A, Shvidel L, Berrebi A.. Characterization of T-cell large granular lymphocyte leukemia associated with Sjogren's syndrome-an important but under-recognized association. Semin Arthritis Rheum 2006;35:306–11. [DOI] [PubMed] [Google Scholar]
- 42. Alverne AR, Marie SK, Levy-Neto M. et al. Inclusion body myositis: series of 30 cases from a Brazilian tertiary center. Acta Reumatol Port 2013;38:179–85. [PubMed] [Google Scholar]
- 43. Dobloug GC, Garen T, Brunborg C, Gran JT, Molberg Ø.. Survival and cancer risk in an unselected and complete Norwegian idiopathic inflammatory myopathy cohort. Semin Arthritis Rheum 2015;45:301–8. [DOI] [PubMed] [Google Scholar]
- 44. Peng A, Koffman BM, Malley JD, Dalakas MC.. Disease progression in sporadic inclusion body myositis: observations in 78 patients. Neurology 2000;55:296–8. [DOI] [PubMed] [Google Scholar]
- 45. Teixeira A, Cherin P, Demoule A. et al. Diaphragmatic dysfunction in patients with idiopathic inflammatory myopathies. Neuromuscul Disord 2005;15:32–9. [DOI] [PubMed] [Google Scholar]
- 46. Oh TH, Brumfield KA, Hoskin TL, Kasperbauer JL, Basford JR.. Dysphagia in inclusion body myositis: clinical features, management, and clinical outcome. Am J Phys Med Rehabil 2008;87:883–9. [DOI] [PubMed] [Google Scholar]
- 47. Voermans NC, Vaneker M, Hengstman GJ. et al. Primary respiratory failure in inclusion body myositis. Neurology 2004;63:2191–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data not provided in the article and additional information on methods and materials may be shared upon request.