Abstract
Objective
To describe risk factors for IBD development in a cohort of children with JIA.
Methods
JIA patients who developed IBD were identified from the international Pharmachild register. Characteristics were compared between IBD and non-IBD patients and predictors of IBD were determined using multivariable logistic regression analysis. Incidence rates of IBD events on different DMARDs were calculated, and differences between therapies were expressed as relative risks (RR).
Results
Out of 8942 patients, 48 (0.54% ) developed IBD. These were more often male (47.9% vs 32.0%) and HLA-B27 positive (38.2% vs 21.0%) and older at JIA onset (median 8.94 vs 5.33 years) than patients without IBD development. They also had more often a family history of autoimmune disease (42.6% vs 24.4%) and enthesitis-related arthritis (39.6% vs 10.8%). The strongest predictors of IBD on multivariable analysis were enthesitis-related arthritis [odds ratio (OR): 3.68, 95% CI: 1.41, 9.40] and a family history of autoimmune disease (OR: 2.27, 95% CI: 1.12, 4.54). Compared with methotrexate monotherapy, the incidence of IBD on etanercept monotherapy (RR: 7.69, 95% CI: 1.99, 29.74), etanercept with methotrexate (RR: 5.70, 95% CI: 1.42, 22.77) and infliximab (RR: 7.61, 95% CI: 1.27, 45.57) therapy was significantly higher. Incidence on adalimumab was not significantly different (RR: 1.45, 95% CI: 0.15, 13.89).
Conclusion
IBD in JIA was associated with enthesitis-related arthritis and a family history of autoimmune disease. An increased IBD incidence was observed for etanercept therapy regardless of concomitant methotrexate use.
Keywords: IBD, JIA, etanercept, enthesitis-related arthritis
Rheumatology key messages.
Inflammatory bowel disease (IBD) significantly impairs quality of life in juvenile idiopathic arthritis (JIA).
IBD in JIA was associated with enthesitis-related arthritis and a family history of autoimmune disease.
Incidence of IBD was raised on etanercept therapy, regardless of whether it was combined with methotrexate.
Introduction
JIA is the most frequent chronic rheumatic disease in childhood with a reported prevalence varying from 16 to 150 per 100 000 children [1]. As per ILAR criteria, seven mutually exclusive categories of JIA with distinct clinical features can be identified [2]. Treatment of JIA is mainly targeted towards reducing disease activity and commonly used drugs are NSAIDs, steroids, conventional synthetic (cs)DMARDs and biologic (b)DMARDs whose administration is now subject to several recommendations and guidelines [3].
A rare comorbidity in JIA is presented by IBD, a chronic inflammatory condition of the gastrointestinal tract that comprises ulcerative colitis, Crohn’s disease and indeterminate colitis [4]. Entheropathic arthritis may also present as an extra-intestinal manifestation prior to gastrointestinal symptoms in IBD [5, 6]. A previous study reported an incidence of 1.31/1000 patient-years for IBD in a registry of 3071 JIA patients treated with and without bDMARDs [7]. This figure is much higher than the reported incidence of paediatric-onset IBD in the general population, which varies globally up to 0.23/1000 person-years [8]. Reported prevalence of IBD in Western countries varies up to 200 per 100 000 children [9–12]. It is known that IBD has a significant negative impact on quality of life besides complicating the therapeutic approach to JIA [13].
Due to sparse data, there is currently limited knowledge about the characteristics of JIA patients who develop IBD and risk factors for its development. Furthermore, based on limited numbers of IBD cases, several studies suggest an association with IBD and etanercept (ETN) therapy [14] and it has also been proposed that MTX is effective in preventing or treating IBD in JIA [7, 15].
The aim of this study is to describe characteristics of JIA patients who develop IBD in comparison with those who did not, determine predictors for the development of IBD and establish a possible association between drug therapy and IBD in the largest existing pharmacovigilance cohort of JIA patients worldwide. We wanted to explore a possible protective effect of MTX and hypothesized based on literature that IBD is associated with enthesitis-related arthritis (ERA) and ETN therapy [7, 16].
Methods
Patients
Pharmachild is an ongoing international observational registry that started in 2011 and contains both retrospective and prospective clinical, laboratory and demographic data from JIA patients treated in 85 member centres of the Paediatric Rheumatology INternational Trials Organisation (PRINTO) from 31 countries worldwide [17]. Key objectives of Pharmachild are to capture adverse events (AEs) in JIA patients developing under cs- or bDMARDs and to determine efficacy of these therapies. Inclusion criteria are children with JIA as defined by ILAR criteria who receive NSAIDs, steroids, cs- or bDMARDs prescribed by their treating physician. Further details of the Pharmachild registry are available elsewhere [18, 19]. All patients from the Pharmachild database were included in the present study. Data lock occurred on 3 May 2019.
For every patient, the ever occurrence of IBD (yes/no) was determined from different sources in Pharmachild. AEs in Pharmachild are reported using version 22 of the Medical Dictionary of Regulatory Activities (MedDRA) hierarchy [20]. Furthermore, IBD was one of the 23 events of specific interest for which extra specific information such as performed tests and medical history was reported in cases in which IBD was diagnosed. All preferred terms (PTs) of AEs, with a three-level monitoring check for consistency [19] [treating physician, medical monitor (J.S.) and PRINTO certified MedDRA coders], were screened. Definite IBD cases (Crohn’s disease, ulcerative colitis or IBD), possible IBD cases (e.g. proctitis) and tests or procedures possibly related to IBD were selected (e.g. colonoscopy). Of the latter two, free-text AE descriptions were screened for further information on IBD diagnosis. IBD after JIA onset could also be mentioned as a free-text comorbidity when the IBD was already established at the moment of inclusion into the registry. Cases were retrieved by two authors (J.S., R.K.) and then checked for correctness by a third reader (J.vS.).
Characteristics collected for all patients were demographics, a history of autoimmune disease(s) in first and second degree relatives, ILAR category and ANA, HLA B27 and RF status. For ANA positivity only one positive test was required. For a positive RF status, two positive tests at least 3 months apart were required as per ILAR classification criteria.
Statistical analysis
Patient characteristics
Descriptive statistics of patient characteristics at last visit were summarized for patients who developed IBD (both with and without available onset date) and patients who did not. Categorical variables were compared between these patients by χ2 test or Fisher’s exact test if appropriate. Continuous variables were compared by Mann–Whitney U-test. All tests were performed two-sided and results were considered statistically significant in cases of a P-value of <0.05. Subsequently, all statistically significant variables were entered as independent variables in a multivariable logistic regression based on complete case analysis in order to develop a prediction model for the outcome variable IBD, defined as the ever occurrence of IBD. ILAR categories were treated as separate dichotomous variables in order to avoid fitting an overfit model. Odds ratios with 95% confidence intervals (CI) of predictor variables in the model were reported and their joint ability to predict IBD was assessed by the area under the receiver operating characteristic curve (AUC). Linearity of continuous variables with the logit outcome was assessed using the Box–Tidwell test.
Drug therapy
In order to establish a possible association between IBD and medication, incidence rates of IBD were determined for MTX, ETN, sulfasalazine, leflunomide, adalimumab (ADA) and infliximab (IFX) therapy. For these analyses, only IBD events with available onset date were included. Incidence rates for ETN were calculated for monotherapy (ETN without MTX) and combination therapy with MTX. Incidence rates for MTX were calculated for monotherapy only (MTX without any biologic). An event of IBD was assigned to a particular drug therapy if this therapy was received within the last 3 months prior to IBD onset [15], and also if the therapy was started or stopped within this interval. Incidences were also calculated for an at-risk window of 6 and 12 months. As a sub-analysis, we repeated all analyses for only ERA patients. Drug therapy received after onset of IBD was censored in all analyses. For all drug therapies, relative risks (i.e. incidence rate ratios) compared with MTX monotherapy were calculated. If the 95% confidence interval of the relative risk did not contain 1, this was considered statistically significant.
All analyses were performed with IBM SPSS Statistics version 25 (IBM Corp., Armonk, NY, USA) and the stats, pROC and epitools packages for R version 3.6.3 [21].
Ethics
Pharmachild and all participating centres obtained approval from their respective ethics committees and were conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent/assent based on existing national regulations.
Results
Patient characteristics
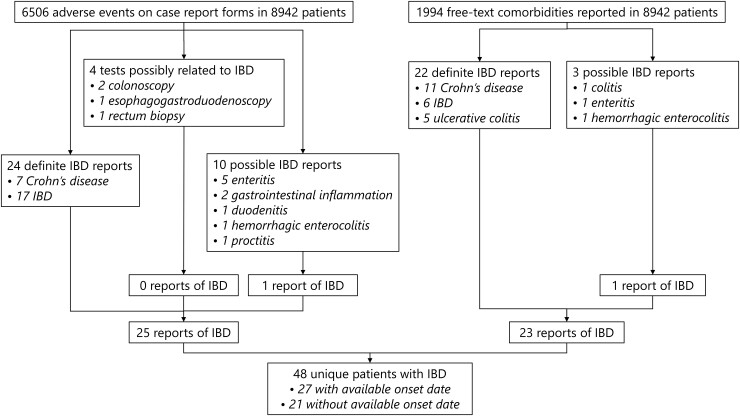
The number of patients included in this study was 8942, from which 48 (0.54%) unique cases of IBD were identified from 6506 AEs and 1994 free‐text comorbidities (Fig. 1). These included 13 cases (27%) of ulcerative colitis, 22 cases (46%) of Crohn’s disease and 13 cases (27%) of indeterminate colitis. Date of onset could not be retrieved for 21/48 (44%) IBD cases (Supplementary Table S1, available at Rheumatology online). Characteristics of patients who developed IBD and those who did not are presented in Table 1. The total observation time from JIA onset to last visit of all included patients was 56 138 years with a median of 5.4 years [interquartile range (IQR): 2.7–8.9]. It was observed that patients who developed IBD were significantly more often male (47.9%), HLA-B27 positive (38.2%) and older at JIA onset than patients who did not develop IBD. Furthermore, they had significantly more often a family history of autoimmune disease(s) (42.6%) and ERA (39.6%). The most commonly reported autoimmune diseases in first and second degree relatives were psoriasis (25%), rheumatoid arthritis (18%) and Hashimoto’s thyroiditis (10%). Other ILAR categories, ANA status and RF status did not differ significantly between IBD and non-IBD patients. No RF positive patients developed IBD. For cases of IBD with available onset date, the median time from JIA onset to IBD onset was 4.2 years (IQR: 2.3–7.7) and the median age at IBD onset was 13.7 years (IQR: 11.7–15.9).
Fig. 1.
Flowchart of identified IBD cases
Table 1.
Demographic and clinical characteristics of Pharmachild patients included for analysis
| Total cohort | No IBD | IBD | P-value | |
|---|---|---|---|---|
| (n = 8942) | (n = 8894) | (n = 48) | ||
| Demographics, % (n) | ||||
| Female | 67.9 (6072) | 68.0 (6047) | 52.1 (25) | 0.03* |
| Family history of autoimmune disease(s)a | 24.5 (2117) | 24.4 (2097) | 42.6 (20) | <0.01* |
| (n = 8648) | (n = 8601) | (n = 47) | ||
| Clinical characteristics | ||||
| Age at JIA onset, median (IQR), years | 5.34 (2.38–9.96) | 5.33 (2.38–9.95) | 8.94 (5.95–11.53) | <0.01* |
| ILAR category, % (n) | ||||
| Persistent oligoarthritis | 25.2 (2250) | 25.2 (2245) | 10.4 (5) | 0.49 |
| Extended oligoarthritis | 12.7 (1132) | 12.7 (1128) | 8.3 (4) | 0.49 |
| Systemic JIA | 10.7 (956) | 10.7 (953) | 6.3 (3) | 0.48 |
| RF− polyarticular JIA | 26.4 (2364) | 26.5 (2354) | 20.8 (10) | 0.47 |
| RF+ polyarticular JIA | 4.0 (357) | 4.0 (357) | 0.0 0 | 0.26 |
| Psoriatic JIA | 3.4 (301) | 3.4 (300) | 2.1 (1) | 1 |
| Enthesitis-related JIA | 10.9 (977) | 10.8 (958) | 39.6 (19) | <0.01* |
| Undifferentiated JIA | 6.8 (605) | 6.7 (599) | 12.5 (6) | 0.14 |
| Laboratory characteristics, % (n) | ||||
| ANA positive | 41.7 (3491) | 41.7 (3471) | 43.5 (20) | 0.92 |
| (n = 8375) | (n = 8329) | (n = 46) | ||
| RF positive | 4.3 (343) | 4.4 (343) | 0.0 0 | 0.41 |
| (n = 7906) | (n = 7868) | (n = 38) | ||
| HLA-B27 positive | 21.1 (1141) | 21.0 (1128) | 38.2 (13) | 0.02* |
| (n = 5402) | (n = 5368) | (n = 34) |
Percentages listed are column percentages. aOnly taking into account first and second-degree relatives. *Statistically significant. IQR: interquartile range.
Predictors of IBD
When combining all statistically significant variables into a multivariable prediction model, only ERA (OR: 3.68, 95% CI: 1.41, 9.40) and a family history of autoimmune disease(s) (OR: 2.27, 95% CI: 1.12, 4.54) remained significantly associated with IBD at a significance level of 5% (Table 2). This model included 5272 patients with 33 IBD cases due to missing information for predictor variables. The AUC of the model was 0.74 (95% CI: 0.66, 0.82). The median predicted probability for IBD in the dataset was 0.4% (range: 0.2–4.6%).
Table 2.
Risk factors for IBD on multivariable logistic regression analysis (n = 5272)
| Variable | OR | 95% CI |
|---|---|---|
| Female | 0.70 | 0.33, 1.48 |
| Family history of autoimmune disease(s)a | 2.27 | 1.12, 4.54* |
| Age at JIA onset | 1.05 | 0.96, 1.15 |
| Enthesitis-related JIA | 3.68 | 1.41, 9.40* |
| HLA-B27 positive | 0.81 | 0.33, 2.02 |
Area under the receiver operating characteristic curve = 0.74 (95% CI: 0.66, 0.82). aOnly taking into account first and second-degree relatives. *Statistically significant. OR: odds ratio.
Drug therapy
Of the 27 patients with known onset date of IBD, 13 (48.1%) used ETN (with or without MTX) within the last 3 months prior to IBD onset (Table 3). For these patients, the median duration of ETN use to IBD onset was 382 days (IQR: 275–853). This duration was positively correlated with JIA disease duration until IBD onset (r = 0.8, P < 0.005). For six cases (23%), no DMARD therapy was received within the 3 months at-risk window. Of these cases, four (67%) had previously stopped MTX therapy and two (33%) had stopped ETN (58 and 4 months before IBD onset). It was observed that incidence rates of IBD were significantly higher for combination therapy with ETN and MTX (six events during 5236 exposure years), ETN monotherapy (seven events during 4524 exposure years) and IFX (two events during 1306 exposure years) compared with MTX monotherapy (three events during 14 913 exposure years). No significant difference was found for ADA therapy (one event during 3440 exposure years). The same effects were observed when taking at-risk windows of 6 and 12 months (Supplementary Tables S2 and S3, available at Rheumatology online). Incidence rates of IBD on drug therapy in ERA patients were higher compared with the total cohort. Nevertheless, no significant differences were found between drug therapies in this sub-analysis of ERA patients. Drug therapy details for each IBD case with available onset date are presented in Supplementary Data S1, available at Rheumatology online.
Table 3.
Incidence rates of IBD events with available onset date on drug therapy
| All patients (n = 8921) |
ERA patients (n = 967) |
|||||
|---|---|---|---|---|---|---|
| Drug therapya | IBD events, % (n) (n = 27)b | Incidence ratec (95% CI) | RR (95% CI) | IBD events, % (n) (n = 9)d | Incidence ratec (95% CI) | RR (95% CI) |
| csDMARDs | ||||||
| MTX mono | 11.1 (3) | 0.02 | Reference | 22.2 (2) | 0.22 | Reference |
| (0.00, 0.06) | (0.03, 0.79) | |||||
| Sulfasalazine | 3.7 (1) | 0.07 | 3.68 | 11.1 (1) | 0.15 | 0.68 |
| (0.00, 0.41) | (0.38, 35.40) | (0.00, 0.83) | (0.06, 7.51) | |||
| Leflunomide | 3.7 (1) | 0.14 | 6.96 | 0.0 (0) | — | — |
| (0.00, 0.78) | (0.72, 66.87) | |||||
| bDMARDs | ||||||
| ETN mono | 25.9 (7) | 0.15 | 7.69 | 33.3 (3) | 0.67 | 3.07 |
| (0.06, 0.32) | (1.99, 29.74)* | (0.14, 1.97) | (0.51, 18.37) | |||
| ETN + MTX | 22.2 (6) | 0.11 | 5.70 | 11.1 (1) | 0.27 | 1.24 |
| (0.04, 0.25) | (1.42, 22.77)* | (0.01, 1.51) | (0.11, 13.66) | |||
| Infliximab | 7.4 (2) | 0.15 | 7.61 | 11.1 (1) | 0.53 | 2.44 |
| (0.02, 0.55) | (1.27, 45.57)* | (0.01, 2.98) | (0.22, 26.89) | |||
| Adalimumab | 3.7 (1) | 0.03 | 1.45 | 0.0 (0) | — | — |
| (0.00, 0.16) | (0.15, 13.89) | |||||
Percentages listed are column percentages. aDrug therapy was received within the last 3 months prior to IBD onset. bFor six cases, no DMARD therapy was received within the last 3 months prior to IBD onset. cNumber of IBD events per 100 exposure years. dFor one case, no DMARD therapy was received within the last 3 months prior to IBD onset. *Statistically significant. bDMARD: biologic DMARD; csDMARD: conventional synthetic DMARD; ERA: enthesitis-related arthritis; ETN: etanercept; RR: relative risk.
Discussion
In this study, IBD patients were older at JIA onset and more often male and HLA-B27 positive than non-IBD patients. Furthermore, they had more often a family history of autoimmune disease and ERA. On multivariable analysis, ERA and a family history of autoimmune disease were the strongest predictors of IBD in JIA. The incidence of IBD on therapy with both ETN and MTX, ETN monotherapy and IFX was significantly higher compared with MTX monotherapy.
The prevalence of IBD in our cohort was 0.54%. This a priori risk is higher than reported prevalence rates varying up to 0.02% in the paediatric population of Western countries [9–12], in which the burden of IBD is known to be highest worldwide [4, 8]. Indeed, IBD and JIA share common genetic features [7] and it has been hypothesized that asymptomatic gut wall inflammation can be present in certain patients with JIA [16]. Following our prediction model, the highest predicted probability of IBD in our cohort was 4.6%. Although this risk may not seem high, the implications are huge and screening for underlying IBD is quite simple, cheap and harmless by performing a faecal calprotectin test [22, 23]. Therefore, this screening might be appropriate in relatively high-risk patients who according to our analysis would be male ERA patients with older age at JIA onset and a family history of autoimmune disease. We found a median age at IBD onset of 13.7 years, which is in line with other studies that reported cases of IBD in JIA [7, 16, 24].
The strongest predictor of IBD in our cohort was ERA, which largely explains why patients with IBD were on average older and more often male and HLA-B27 positive than patients without IBD in our cohort [1]. Moreover, ERA resembles spondyloarthropathy in adults [16], a rheumatic disease known to be associated with development of IBD [25]. A previous study by Barthel et al. also reported a higher percentage of ERA among JIA patients who developed IBD (2/11; 18.2%) compared with the total cohort (425/3071; 13.8% ) [7]. The authors of the same study also hypothesized that JIA patients with psoriatic arthritis and extended oligoarthritis were at an increased risk of IBD, which we cannot confirm with the results of our current study. The other statistically significant predictor of IBD in our multivariable analysis was a family history of one or more autoimmune diseases. This is consistent with existing knowledge on the pathophysiology of IBD, which suggests that both genetic and environmental factors play a role [4]. In addition, familial IBD is more common in children than in adult cases [26]. Corresponding to most studies [14, 27, 28], no RF positive polyarthritis patients developed IBD in the present study, although IBD in RF positive polyarticular JIA has been reported [29].
Compared with MTX monotherapy, the incidence of IBD on ETN therapy was significantly higher. Several studies have also reported a potential association between ETN and IBD in JIA [14, 15]. In our study, this association was observed even when taking different at-risk windows. In fact, for cases where ETN was received within the last 3 months prior to IBD onset, the median duration from the start of ETN to IBD onset was little over a year. Due to this rather long duration, the authors believe there is not much bias in the reported IBD events that were attributed to ETN therapy, which would not be the case if patients had only switched to ETN therapy shortly before IBD onset. Other studies confirm this long duration of ETN therapy until IBD onset [7, 27]. The observed positive correlation between ETN therapy duration and duration until IBD onset might provide some evidence for a dose–response relationship, given that IBD events that still occurred after the median duration of JIA until IBD onset also had a longer exposure to ETN. Data from the BiKeR register with 14 included IBD cases has suggested that MTX is protective against IBD in JIA, even when combined with ETN [15]. The rate of incident IBD on ETN and MTX combination therapy in the BiKeR study was higher than the rate on MTX monotherapy (0.1 vs 0.03 events per 100 person-years), but this was not a statistically significant difference. In the present study, we found a similar effect, which was statistically significant probably due to the larger number of IBD cases included. In our study, ETN was associated with IBD in JIA, regardless of concomitant use of MTX. It remains questionable, however, whether or not this is a causal relationship. It has been suggested that in JIA patients under ETN therapy, a pre-existing clinically silent IBD can manifest since ETN is ineffective in the treatment of Crohn’s disease [27]. One could even argue that patients with silent IBD and arthropathy, misdiagnosed as JIA patients, will not benefit from ETN therapy for their joints either. Hence these patients will be switched to a more effective treatment such as ADA, thereby preventing the symptomatic occurrence of IBD. Nevertheless, IBD onset after ETN therapy has also been reported in patients with long-lasting definite JIA without previous abdominal complaints [30]. In a systematic literature review of 53 cases of IBD in JIA, Bieber et al. describe that most cases that developed under ETN therapy improved after discontinuation of ETN, suggesting a causal link [14]. Several hypotheses about the biological mechanism behind a possible relationship between ETN and IBD development exist [7, 14, 16, 27].
Unlike ETN, IFX and ADA have been proven effective in the treatment of IBD [31–33]. We observed that the incidence of IBD on ADA was not significantly higher than MTX monotherapy, but, surprisingly, incidence on IFX was. For two cases of IBD found in our study, IFX was given within the last 3 months prior to IBD onset, and for one additional case within the last 12 months. However, two of these three cases had previously also received ETN. Tarkiainen et al. also reported a case of IBD following IFX therapy and suggested that IBD can develop in JIA patients at a low IFX dosage, which is associated with the formation of anti-IFX antibodies that is linked to a reduced treatment response in Crohn’s disease [24]. It is indeed the case that the IFX dosage recommended for JIA is 3 mg/kg body weight, while this is 5 mg/kg body weight for IBD. A randomized placebo-controlled trial of IFX in JIA also reported an increased risk for development of antibodies to IFX at a dosage of 3 mg/kg compared with 6 mg/kg [34]. Development of IBD following IFX treatment has also been described in an adult spondyloarthropathy patient [35].
We did not find significant differences in rates of incident IBD between different therapies within the subset of ERA patients due to few events. However, the higher absolute incidence rates for all therapies in this sub-analysis compared with our main analysis indicate that ERA patients run a higher overall risk of developing IBD. This confirms our multivariable analysis results.
This study has strengths and limitations. Due to missing onset dates, drug therapy prior to onset could not be retrieved for all IBD cases. Moreover, because of this, drug therapy could not be studied as an independent variable for IBD in addition to the current variables in our complete case logistic regression model. This would have resulted in exclusion of the majority of IBD cases and an overfit model. Missing onset dates of IBD events have influenced absolute incidence rates reported in this study, but the authors do not believe this has caused significant bias in relative risks between drug therapies given the likely missing completely at random (MCAR) nature of the missing observations. The mentioned limitations are partially covered by the relatively large sample size. Although it might be difficult to draw firm conclusions based on 48 cases of IBD, this is the most comprehensive single international registry of IBD in JIA and its characteristics and risk factors. Also, ERA was excluded as a confounding variable in our incidence analyses by performing a sub-analysis in ERA patients only. In this sub-analysis, similar effects were observed as in the main analysis, but these effects were not statistically significant due to a considerably reduced number of observations.
For the future, it would be ideal if a larger number of IBD cases from different national and international cohorts/registries could be combined, and associations with risk factors including drug therapy could be confirmed using multivariable analysis in a case–control study. Next to clinical and genetic features, also dietary and environmental factors should be considered as predictors for IBD in JIA. A 2013 population based study in Denmark revealed that among others high sugar intake and urban residency were risk factors for the development of paediatric-onset IBD [36]. Lastly, more experimental work on the biological mechanism behind a possible causal relationship between ETN and IBD (in the presence of MTX) is required.
To conclude, this study has highlighted several risk factors for IBD in patients with JIA, of which the most important are ERA and a family history of autoimmune disease. Moreover, we found that compared with MTX monotherapy the incidence of IBD was higher on therapy with ETN (regardless of concomitant use of MTX) and IFX. Hence, it might be suggested to consider ADA as the biologic of choice for treatment of ERA patients with a family history of autoimmune disease.
Acknowledgements
The authors would like to thank all PRINTO centres for their contribution to the data collection. A full list of PRINTO members can be found in Supplementary Data S2, available at Rheumatology online. The authors would also like to express their acknowledgements to the European Reference Network for Immunodeficiency, Autoinflammatory, Autoimmune and Paediatric Rheumatic diseases (ERN-RITA). Finally, we thank all patients and their parents for consenting to this research.
Funding: This work was supported by a research grant from FOREUM Foundation for Research in Rheumatology. Pharmachild has been supported by a grant from the European Union (grant 260353) and by funding from the Italian public hospital IRCCS Istituto Giannina Gaslini.
Disclosure statement: M.C. reports personal fees from AbbVie, outside the submitted work. P.D. reports non-financial support from Pfizer, non-financial support from Roche, personal fees and non-financial support from AbbVie, personal fees and non-financial support from Novartis, personal fees and non-financial support from Sobi, personal fees from Eli Lilly and personal fees and non-financial support from MEDAC, outside the submitted work. N.R. reports personal fees from AstraZeneca–Medimmune, personal fees from Ablynx, personal fees from Biogen, personal fees from Boehringer, grants and personal fees from Hoffman-La Roche, grants and personal fees from Pfizer, grants and personal fees from Novartis, personal fees from Takeda, grants and personal fees from Eli Lilly, grants and personal fees from GSK, grants and personal fees from Janssen, personal fees from EMD Serono, personal fees from Merck, personal fees from R-Pharma, personal fees from Sanofi, personal fees from Servier, personal fees from Sinergie, grants and personal fees from Sobi, personal fees from Aurinia, personal fees from Centrical Global, personal fees from Domain Therapeutics, personal fees from Idorsia and grants and personal fees from Bristol Myers and Squibb, outside the submitted work. The remaining authors have declared no conflicts of interest.
Data availability statement
All relevant data are reported in the article. Additional details can be provided by the corresponding author upon reasonable request. The Pharmachild registry is registered at Clinicaltrials.gov (NCT01399281) and at the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP; http://www.encepp.eu/encepp/viewResource.htm?id=19362).
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Ravelli A, Martini A.. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. [DOI] [PubMed] [Google Scholar]
- 2. Petty RE, Southwood TR, Manners P, et al. ; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 3. Beukelman T, Patkar NM, Saag KG. et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken) 2011;63:465–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 2006;12(Suppl 1):S3–9. [DOI] [PubMed] [Google Scholar]
- 5. Cardile S, Romano C.. Current issues in pediatric inflammatory bowel disease-associated arthropathies. World J Gastroenterol 2014;20:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voulgari PV. Rheumatological manifestations in inflammatory bowel disease. Ann Gastroenterol 2011;24:173–80. [PMC free article] [PubMed] [Google Scholar]
- 7. Barthel D, Ganser G, Kuester R-M. et al. Inflammatory bowel disease in juvenile idiopathic arthritis patients treated with biologics. J Rheumatol 2015;42:2160–5. [DOI] [PubMed] [Google Scholar]
- 8. Sýkora J, Pomahačová R, Kreslová M. et al. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol 2018;24:2741–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ye Y, Manne S, Treem WR, Bennett D.. Prevalence of inflammatory bowel disease in pediatric and adult populations: recent estimates from large national databases in the United States, 2007–2016. Inflamm Bowel Dis 2020;26:619–25. [DOI] [PubMed] [Google Scholar]
- 10. Rosen MJ, Dhawan A, Saeed SA.. Inflammatory bowel disease in children and adolescents. JAMA Pediatr 2015;169:1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benchimol EI, Bernstein CN, Bitton A. et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol 2017;112:1120–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gearry RB, Richardson A, Frampton CMA. et al. High incidence of Crohn’s disease in Canterbury, New Zealand: results of an epidemiologic study. Inflamm Bowel Dis 2006;12:936–43. [DOI] [PubMed] [Google Scholar]
- 13. Knowles SR, Graff LA, Wilding H. et al. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses—part I. Inflamm Bowel Dis 2018;24:742–51. [DOI] [PubMed] [Google Scholar]
- 14. Bieber A, Fawaz A, Novofastovski I, Mader R.. Antitumor necrosis factor-α therapy associated with inflammatory bowel disease: three cases and a systematic literature review. J Rheumatol 2017;44:1088–95. [DOI] [PubMed] [Google Scholar]
- 15. Klotsche J, Niewerth M, Haas J-P. et al. Long-term safety of etanercept and adalimumab compared to methotrexate in patients with juvenile idiopathic arthritis (JIA). Ann Rheum Dis 2016;75:855–61. [DOI] [PubMed] [Google Scholar]
- 16. van DTD, Vastert SJ, Gerloni VM. et al. Development of inflammatory bowel disease in patients with juvenile idiopathic arthritis treated with etanercept. J Rheumatol 2011;38:1441–6. [DOI] [PubMed] [Google Scholar]
- 17. Ruperto N, Martini A.. Networking in paediatrics: the example of the Paediatric Rheumatology International Trials Organisation (PRINTO). Arch Dis Child 2011;96:596–601. [DOI] [PubMed] [Google Scholar]
- 18. Swart J, Giancane G, Horneff G. et al. ; Paediatric Rheumatology International Trials Organisation (PRINTO), BiKeR and the board of the Swedish Registry. Pharmacovigilance in juvenile idiopathic arthritis patients treated with biologic or synthetic drugs: combined data of more than 15,000 patients from Pharmachild and national registries. Arthritis Res Ther 2018;20:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giancane G, Swart JF, Castagnola E. et al. ; Paediatric Rheumatology International Trials Organisation (PRINTO). Opportunistic infections in immunosuppressed patients with juvenile idiopathic arthritis: analysis by the Pharmachild Safety Adjudication Committee. Arthritis Res Ther 2020;22:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merrill GH. The MedDRA paradox. AMIA Annu Symp Proc 2008;2008:470–4. [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. https://www.R-project.org/ (27 May 2021, date last accessed). [Google Scholar]
- 22. Ferrara G, Pastore S, Sancin L. et al. Fecal calprotectin to detect inflammatory bowel disease in juvenile idiopathic arthritis. J Rheumatol 2018;45:1418–21. [DOI] [PubMed] [Google Scholar]
- 23. Aalto K, Lahdenne P, Kolho K-L.. Fecal calprotectin in juvenile idiopathic arthritis patients related to drug use. Pediatr Rheumatol Online J 2017;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tarkiainen M, Tynjälä P, Vähäsalo P, Lahdenne P.. Occurrence of inflammatory bowel disease in four patients with juvenile idiopathic arthritis receiving etanercept or infliximab. Scand J Rheumatol 2011;40:150–2. [DOI] [PubMed] [Google Scholar]
- 25. Fragoulis GE, Liava C, Daoussis D. et al. Inflammatory bowel diseases and spondyloarthropathies: from pathogenesis to treatment. World J Gastroenterol 2019;25:2162–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oliveira SB, Monteiro IM.. Diagnosis and management of inflammatory bowel disease in children. BMJ 2017;357:j2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dallocchio A, Canioni D, Ruemmele F. et al. ; SOFREMIP. Occurrence of inflammatory bowel disease during treatment of juvenile idiopathic arthritis with etanercept: a French retrospective study. Rheumatology (Oxford) 2010;49:1694–8. [DOI] [PubMed] [Google Scholar]
- 28. Windschall D, Müller T, Becker I, Horneff G.. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis. Clin Rheumatol 2015;34:61–9. [DOI] [PubMed] [Google Scholar]
- 29. Verazza S, Davì S, Consolaro A. et al. ; Italian Pediatric Rheumatology Study Group. Disease status, reasons for discontinuation and adverse events in 1038 Italian children with juvenile idiopathic arthritis treated with etanercept. Pediatr Rheumatol Online J 2016;14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gerloni V, Pontikaki I, Gattinara M. et al. Focus on adverse events of tumour necrosis factor alpha blockade in juvenile idiopathic arthritis in an open monocentric long-term prospective study of 163 patients. Ann Rheum Dis 2008;67:1145–52. [DOI] [PubMed] [Google Scholar]
- 31. Akobeng AK. Crohn’s disease: current treatment options. Arch Dis Child 2008;93:787–92. [DOI] [PubMed] [Google Scholar]
- 32. Van den Brande J, Braat H, van den Brink GR, et al. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn’s disease. Gastroenterology 2003;124:1774–85. [DOI] [PubMed] [Google Scholar]
- 33. Corica D, Romano C.. Biological therapy in pediatric inflammatory bowel disease: a systematic review. J Clin Gastroenterol 2017;51:100–10. [DOI] [PubMed] [Google Scholar]
- 34. Ruperto N, Lovell DJ, Cuttica R. et al. ; Pediatric Rheumatology Collaborative Study Group. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum 2007;56:3096–106. [DOI] [PubMed] [Google Scholar]
- 35. Fouache D, Goëb V, Massy-Guillemant N. et al. Paradoxical adverse events of anti-tumour necrosis factor therapy for spondyloarthropathies: a retrospective study. Rheumatology (Oxford) 2009;48:761–4. [DOI] [PubMed] [Google Scholar]
- 36. Jakobsen C, Paerregaard A, Munkholm P, Wewer V.. Environmental factors and risk of developing paediatric inflammatory bowel disease—a population based study 2007–2009. J Crohn’s Colitis 2013;7:79–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are reported in the article. Additional details can be provided by the corresponding author upon reasonable request. The Pharmachild registry is registered at Clinicaltrials.gov (NCT01399281) and at the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP; http://www.encepp.eu/encepp/viewResource.htm?id=19362).