Abstract
The activity of ABT-773 was studied against extracellular and intracellular Legionella pneumophila and for the treatment of guinea pigs with L. pneumophila pneumonia. The ABT-773 MIC at which 50% of isolates are inhibited (MIC50) for 20 different Legionella sp. strains was 0.016 μg/ml, whereas the MIC50s of clarithromycin and erythromycin were 0.032 and 0.125 μg/ml, respectively. ABT-773 (1 μg/ml) was bactericidal for two L. pneumophila strains grown in guinea pig alveolar macrophages. In contrast, erythromycin and clarithromycin had easily reversible static activity only. Therapy studies of ABT-773 and erythromycin were performed with guinea pigs with L. pneumophila pneumonia. When ABT-773 was given to infected guinea pigs by the intraperitoneal route (10 mg/kg of body weight), mean peak levels in plasma were 0.49 μg/ml at 0.5 h and 0.30 μg/ml at 1 h postinjection. The terminal half-life phase of elimination from plasma was 0.55 h, and the area under the concentration-time curve from 0 to 24 h (AUC0–24) was 0.65 μg · h/ml. For the same drug dose, mean levels in the lung were 15.9 and 13.2 μg/g at 0.5 and 1 h, respectively, with a half-life of 0.68 h and an AUC0–24 of 37.0 μg · h/ml. Ten of 15 L. pneumophila-infected guinea pigs treated with ABT-773 (15 mg/kg/dose given intraperitoneally once daily) for 5 days survived for 9 days post-antimicrobial therapy, as did 14 of 15 guinea pigs treated with erythromycin (30 mg/kg given intraperitoneally twice daily) for 5 days. All of the ABT-773-treated animals that died appeared to do so because of drug-induced peritonitis rather than overwhelming pneumonia. None of 12 animals treated with saline survived. ABT-773 is as effective as erythromycin against L. pneumophila in infected macrophages and in a guinea pig model of Legionnaires' disease. These data support studies of the clinical effectiveness of ABT-773 for the treatment of Legionnaires' disease.
ABT-773 is a novel ketolide antimicrobial agent with high levels of activity against human respiratory tract and oral bacteria, including erythromycin-sensitive and -resistant Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Chlamydia pneumoniae, Mycobacterium avium, Toxoplasma gondii, common isolates from animal and human bites, and some Staphylococcus aureus strains (1, 3, 4, 20, 22, 25; R. Jung, D. H. Li, S. L. Pendland, and L. H. Danziger, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2146, 1999; Z. Ma, R. F. Clark, S. Wang, A. M. Nilius, R. K. Flamm, and Y. S. Or, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2133, 1999). Previous studies have shown that the drug has good activity against Legionella sp. bacteria in vitro (Jung et al., 39th ICAAC; Ma et al., 39th ICAAC; K. Sens, A. Mietzner, A. Sagnimeni, J. E. Stout, and V. L. Yu, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2159, 2000). Limited studies have also shown that the compound appeared to be active against Legionella pneumophila grown in HL-60 cells (Jung et al., 39th ICAAC; Sens et al., 40th ICAAC). This study was designed to further define the extracellular and intracellular activities of ABT-773 against L. pneumophila, as well as to determine the in vivo activity of the drug for the treatment of infection in a guinea pig model of Legionnaires' disease. We demonstrate that ABT-773 is as active as erythromycin in the animal models of L. pneumophila infection and more active than erythromycin against the intracellular and extracellular bacterium.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Twenty clinical isolates of Legionella sp. bacteria were used to determine the in vitro activities of the study compounds. These bacteria were low-passage-number strains that we had isolated and comprised 13 strains of L. pneumophila (9 serogroup 1 strains and 1 strain each of serogroups 2, 4, 6, and 9); 2 strains each of Legionella micdadei, Legionella longbeachae, and Legionella dumoffii; and 1 strain of Legionella bozemanii. We have previously used these 20 strains in other studies of antimicrobial activity against Legionella spp. (11, 13, 15, 17). Included among these strains were L. pneumophila strains F889 and F2111, which have been extensively studied in a cell model of L. pneumophila infection. We have also used strain F889 extensively in a well-validated guinea model of L. pneumophila pneumonia (5–7, 9, 17). S. aureus ATCC 29213 was used as the control organism for susceptibility testing. To obtain inocula for susceptibility testing, legionellae were grown on morpholinepropanesulfonic acid (MOPS)-buffered charcoal yeast extract medium supplemented with 0.1% α-ketoglutarate (BCYEα) that was made in our laboratory (14), and nonlegionellae were grown on commercial tryptic soy agar containing 5% sheep blood (14). Incubation of all media was at 35°C in humidified air for 24 to 48 h, depending on the organism and the growth rate.
Antimicrobials.
Standard powders of ABT-773, clarithromycin, and erythromycin were obtained from Abbott Pharmaceuticals. For susceptibility studies, ABT-773 powder was dissolved in methanol and then phosphate buffer (0.1 M; pH 6.5); subsequent dilutions were made in tissue culture medium, Mueller-Hinton broth, or buffered yeast broth, as appropriate. ABT-773 used in animal treatment and pharmacokinetic studies was dissolved in sterile water for injection, USP, in enough 1 N HCl to produce a pH of 4.4, which was about 5.5 mM HCl. The erythromycin standard powder was first dissolved in 95% ethanol and was then diluted in tissue culture medium or bacterial culture broths, as described above. The clarithromycin standard powder was dissolved identically to the ABT-773 powder used in susceptibility studies. Final dilutions of the dissolved compounds were sufficient to remove the possibility that the solubilizing agents would have antimicrobial activity. Erythromycin lactobionate for intravenous administration was prepared according to the manufacturer's instructions and was diluted so that the volume administered was 1 ml. The ABT-773 was used for the pharmacokinetic and treatment studies at a concentration of 4 mg/ml, and ABT-773 at this concentration was prepared within 1 h of injection.
Antimicrobial susceptibility testing.
Broth microdilution susceptibility testing was performed with N-(2-acetamido)-2-aminoethanesulfonic acid (ACES)-buffered yeast extract broth supplemented with 0.1% α-ketoglutarate (BYEα) (Legionella bacteria) or Mueller-Hinton broth (non-Legionella bacteria), with a final volume of 100 μl and a final bacterial concentration of 5 × 10 5 CFU/ml (16). The Legionella and non-Legionella bacteria were incubated for 48 and 24 h, respectively, both at 35°C. The BYEα was made in our laboratory.
Growth inhibition in alveolar macrophages.
Guinea pig pulmonary alveolar macrophages were harvested and purified as described previously (11). The final concentration of macrophages was approximately 105 cells per well. Incubation conditions for all macrophage studies were 5% CO2 in air at 37°C.
L. pneumophila strains F889 and F2111 grown overnight on BCYEα agar were used to infect the macrophages. Approximately 104 bacteria were added to each well. The bacteria were incubated with macrophages for 1 h in a shaking incubator and then for 1 day in stationary culture, as described previously (11). One set of replicate wells was washed (500 μl) three times with tissue culture medium and was then sonicated at low energy to release the intracellular bacteria, which were quantified with BCYEα agar. Antimicrobials were then added to the washed nonsonicated wells; no antimicrobial was added to several wells, which served as growth controls. The infected tissue cultures were then incubated for 2 days, after which supernatants were taken for quantitative culture. The antimicrobials were then removed by washing, and the experiment was continued for 4 more days, with daily quantification of the L. pneumophila isolates in the supernatants of the wells. All experiments were carried out in duplicate or triplicate, and quantitative plating was done in duplicate. All wells were observed microscopically daily to detect macrophage infection and to roughly quantify the numbers of macrophages in the wells. To determine whether ABT-773 had toxicity for macrophages, control wells that contained macrophages, tissue culture medium, and antimicrobial agents, but no bacteria, were set up. Prior studies have demonstrated that neither erythromycin nor clarithromycin causes macrophage toxicity (9, 11). In this system there is no extracellular growth of L. pneumophila, so all increases in bacterial concentrations in the supernatants are the result of intracellular growth.
Guinea pig pneumonia model.
Specific-pathogen-free male Hartley strain guinea pigs (weight, ≈300 g; Charles River Laboratories, Wilmington, Mass.) were used for the pneumonia model, as described previously (7). Animals were observed for illness 1 week prior to infection; in the case of the animals used for the treatment study, temperatures and weights were obtained during the preinfection period. The guinea pigs were infected with L. pneumophila serogroup 1, strain F889, which was administered by the intratracheal route, as described previously (7). The bacterial inocula administered were 8.4 × 10 6 and 7.7 × 10 6 CFU for the pharmacokinetic and treatment studies, respectively.
Pharmacokinetic study.
Plasma and lung specimens were obtained from guinea pigs with L. pneumophila pneumonia for determination of ABT-773 concentrations, as described previously (6, 18). The drug was given in a single intraperitoneal dose (4.0 mg/ml at 10 mg/kg of body weight, with the injection volume dependent on the individual animal's weight) to guinea pigs 1 day after infection; the mean guinea pig weight was 296 g. At timed intervals after drug injection, anesthetized (ketamine, xylazine, and lidocaine) animals in groups of two to three animals each were exsanguinated by removal of heart blood under direct vision, followed by lung removal. Heart blood was collected with a syringe and needle, transferred immediately to heparinized tubes (Vacutainer; Becton-Dickinson, Rutherford, N.J.), and immediately refrigerated (5°C). Within 1 h, the plasma was separated from the cellular blood components by centrifugation at 5,000 × g at 5°C for 10 min and was then stored frozen at −70°C until it was shipped to Abbott Laboratories on dry ice. Following removal, the lungs were rinsed in phosphate-buffered saline, drained on sterile cotton gauze, weighed, and ground in a known amount of high-pressure liquid chromatography-grade water; the final volume of the homogenate was measured to determine the lung weight per volume of final homogenate. Negative controls included guinea pig lung homogenates and plasma that had been collected as described above from healthy guinea pigs given identical anesthesia but no antimicrobial. A second study estimated plasma ABT-773 concentrations in two L. pneumophila-infected guinea pigs 1 h after intraperitoneal administration of 15 mg/kg.
Drug assay.
Plasma and lung homogenates were assayed for ABT-773 by Abbott Pharmaceuticals. ABT-773 and a proprietary internal standard, A-207257 (Abbott Pharmaceuticals), were selectively removed from the plasma or lung homogenate by liquid-liquid extraction. The lung tissue was homogenized with 2 volumes of saline. The extraction method combined an aliquot of plasma or lung tissue homogenate (sample or spiked standard) with an internal standard, 0.5 M Na2CO3 and ethyl acetate-hexane (1:1, by volume). The samples were vigorously vortexed, followed by centrifugation. The organic layer was transferred and evaporated to dryness with a gentle stream of nitrogen at room temperature. The samples were reconstituted by vortexing with the mobile phase. The parent drug and internal standard were separated from coextracted contaminants on a Kromasil C18 column (50 by 3 mm; particle size, 5 μm; Keystone Scientific, Bellfonte, Pa.) with an acetonitrile-trifluoroacetic acid (40:60, by volume) mobile phase at a flow rate of 0.25 to 0.3 ml/min with a 10- to 25-μl injection volume. The compounds of interest were quantified by selective ion monitoring detection (m/z 766.3) with a turbo ion spray source on an API 2000 mass spectrometer (Applied Biosystems|MDS Sciex, Foster City, Calif.). Triplicate plasma standards at each of eight separate concentrations over a concentration range of 0.028 to 6.47 μg/ml were characterized by good accuracy (86 to 120% of the theoretical accuracy) and reproducibility (coefficient of variation, 3.1 to 10.5%), with an estimated limit of quantitation of ≈20 ng/ml. Triplicate lung homogenate standards at each of eight separate concentrations over the same concentration range were characterized by good accuracy (86 to 115% of the theoretical accuracy) and reproducibility (coefficient of variation, 1.0 to 13.3%), with an estimated limit of quantitation of ≈0.1 μg/g. The extraction efficiencies for both ABT-773 and the internal standard were greater than 90%. The lung homogenate and plasma samples were analyzed as a single batch.
Animal treatment study.
Guinea pigs that survived surgery were randomized into three treatment groups 1 day after infection. Starting on that day, treatment was given once or twice daily (9 a.m. and 5 p.m.) for 5 days. All injections were given by the intraperitoneal route in a 1.0-ml volume. One group of 15 animals received ABT-773 (15 mg/kg) once daily; another group of 15 animals received erythromycin (30 mg/kg/dose) twice daily. A third group of 12 animals received saline solution once daily (1 ml). Dosing of the antimicrobial agents was designed to roughly emulate expected peak concentrations in the serum of humans, as determined by pharmacokinetic studies with the animals and published studies with humans, without regard to different drug clearances in the two different species (7; R. S. Pradhan, L. E. Gustavson, D. D. Londo, Y. Zhang, J. Zhang, and M. M. Paris, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2135, 2000). Animal weights and temperatures were taken periodically during the 13-day postinfection observation period; the measurements were taken about 2 h after drug administration on all treatment days. Necropsies and quantitative lung cultures were performed on all animals that died. All animals surviving for 13 days postinfection were killed with pentobarbital (150 mg/kg, given intraperitoneally). Necropsies, lung histopathology, and quantitative lung cultures were performed on all 10 survivors in the ABT-773 treatment group and the 10 lowest-weight survivors in the erythromycin treatment group (7). The lower limit of detection of L. pneumophila in the lung was about 100 CFU/g. All animal studies were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Statistical analysis.
All data analyses were performed with the use of either Prism (version 3.02) or InStat (version 2.01) software (GraphPad, San Diego, Calif.). Prism software was also used to calculate pharmacokinetic parameters. A P value of ≤0.05 was predefined as significant. Lung histologic scores were analyzed by using a paired Student's t test, after first testing for normality of the population distributions. Body weight and temperature comparisons were analyzed by the methods specified for each result. Lungs with no detectable bacteria were arbitrarily assigned a value of 10 CFU/g for calculation and comparison of group mean values. Plasma and lung samples containing no detectable drug were arbitrarily assigned a value of 0.0 μg/ml or 0.0 μg/g, respectively, to allow their graphical representation and calculation of the pharmacokinetic parameters.
RESULTS
Broth dilution susceptibility.
The ABT-773 MIC at which 50% of isolates are inhibited (MIC50), MIC90, and MIC range for the Legionella spp. tested were 0.016, 0.064, and 0.004 to 0.125 μg/ml, respectively. The erythromycin MIC50, MIC90, and MIC range were 0.125, 0.25, and 0.064 to 0.5 μg/ml, respectively. Clarithromycin MICs were 0.032 μg/ml for all isolates tested. The highest ABT-773 MIC observed, 0.125 μg/ml, was for one L. dumoffii strain; the ABT-773 MIC for the other L. dumoffii strain studied was 0.032 μg/ml. In contrast, the highest erythromycin MIC observed, 0.5 μg/ml, was for the single L. bozemanii strain studied and for both L. micdadei strains studied. No inhibition of the test drugs by the broth medium was observed. The MICs of the three drugs tested for the control S. aureus strain obtained with BYEα broth were within 1 doubling of the result obtained with Mueller-Hinton broth.
Antimicrobial inhibition of intracellular growth.
All three drugs tested significantly inhibited both L. pneumophila strains grown in guinea pig alveolar macrophages (Fig. 1). The activities of all drugs tested were nearly identical when they were tested at a concentration of 0.25 μg/ml. There was no enhanced activity of either erythromycin or clarithromycin at a higher concentration (1 μg/ml). In contrast, ABT-773 (1 μg/ml) caused a several-day postantibiotic effect that was not observed for either erythromycin or clarithromycin. ABT-773 showed no evidence of toxicity for macrophages in drug-only control wells.
FIG. 1.
Growth of L. pneumophila serogroup 1 strain F889 in guinea pig alveolar macrophages versus day of incubation after initiation of infection. The data for strain F2111 were nearly identical and are not shown. All points represent the means for triplicate wells counted in duplicate; error bars represent 95% confidence intervals, which, unless shown otherwise, were smaller than the height of the symbol representing the mean. (A) Data for drug concentrations of 0.25 μg/ml; (B) data for drug concentrations of 1 μg/ml; data for the no-drug control are shown in both panels for greater clarity. □, no-drug control; ▵, ABT-773; ○, erythromycin; ⋄, clarithromycin.
Pharmacokinetic study.
ABT-773 administration (10 mg/kg intraperitoneally) to L. pneumophila-infected guinea pigs gave the highest mean measured concentrations in plasma: 0.49 and 0.30 μg/ml at 0.5 and 1.0 h, respectively (Fig. 2). The highest mean measured lung ABT-773 concentrations were 15.9 and 13.2 μg/g, measured at 0.5 and 1 h, respectively. Plasma samples taken at 4 and 6 h postinjection contained no detectable drug in one of three and one of two animals, respectively. A two-phase exponential decay model gave the best fit for the data and was used to calculate the half-lives. The terminal-phase (β-phase) half-lives of elimination from plasma and lung were calculated to be 0.55, and 0.68 h, respectively. The area under the concentration-time curve from 0 to 24 h (AUC0–24) for plasma was calculated to be 0.65 μg · h/ml, and that for the lung was 37.0 μg · h/ml. Lung and plasma ABT-773 concentrations 1 h after administration of the drug (at 15 mg/kg intraperitoneally) to two guinea pigs were 19.4 and 14.2 μg/g and 1.05 and 0.29 μg/ml, respectively.
FIG. 2.
Mean plasma (A) and lung (B) ABT-773 concentrations in guinea pigs with L. pneumophila pneumonia. Animals were given a single 10-mg/kg dose administered by the intraperitoneal route at time zero. For each time point except the 6-h time point, samples were obtained from three animals; samples were obtained from two animals for the 6-h time point. One plasma sample taken at 3, 4, and 6 h each contained no detectable drug. The vertical bars represent the ranges for each time point. The dotted line shows the one-component exponential decay regression curve for each data set (r 2 = 0.66 for plasma and 0.76 for the lungs). The horizontal dashed line in panel A shows the lower limit of detection.
Therapy in guinea pigs.
Ten of 15 guinea pigs treated with ABT-773 survived for 13 days postinfection, as did 14 of 15 erythromycin-treated animals (P = 0.17 by Fisher's exact test). This was in contrast to 100% deaths in the 12 guinea pigs receiving saline alone, a significant difference from the outcomes for the two treatment groups (P = 0.0001 by the chi-square test) (Fig. 3). Necropsies of the five ABT-773-treated animals that died before day 13 showed that all had peritonitis, with the severity being inversely proportional to the duration since the time of administration of the last dose of the drug. The peritonitis was most severe in the right lower quadrant, the site of drug injection, but in some cases it was generalized throughout the peritoneum. No visible drug precipitates were observed in the peritoneums of the ABT-773-treated animals with peritonitis. Histologic examination of the peritoneum from one of these animals demonstrated findings of acute peritonitis, manifested by infiltration of neutrophils; histologic examination of the peritoneum from an erythromycin-treated animal showed that it was normal. The lungs from these animals were much smaller and less consolidated than the lungs from saline-treated animals; histologic examination showed little residual lung consolidation (see below). All 10 ABT-773-treated guinea pigs that survived to day 13 had evidence of healed peritonitis at necropsy, manifested exclusively by intra-abdominal adhesions in the right lower quadrant. No erythromycin-treated or saline-treated animal had peritonitis at necropsy.
FIG. 3.
Percent survival of guinea pigs with L. pneumophila pneumonia versus day postinfection. Animals were treated on postinfection days 1 to 5 with saline (▪), ABT-773 (▾), or erythromycin (▵).
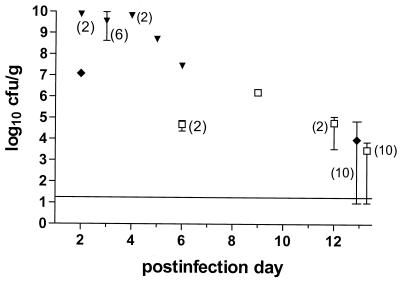
Lung L. pneumophila concentrations were significantly lower in the animals in the ABT-773 treatment group that died before day 13 than in the animals in the saline treatment group (P = 0.002 by the Mann-Whitney test), although the comparison does not account for the day of death (Fig. 4). Lung culture and necropsy results for all saline-treated animals were diagnostic of fatal L. pneumophila pneumonia; the mean concentration of L. pneumophila was 9.3 log10 CFU/g of lung, with a range of 7.5 to 10.0 log10 CFU/g. One of the 10 lungs examined from the survivors in the ABT-773 treatment group was negative (<2.0 log10 CFU/g) for L. pneumophila; for the other 9 lungs the median, average, and range of L. pneumophila concentrations were 3.3, 3.5, and 1.0 to 3.9 log10 CFU/g, respectively. Three of 10 lungs examined from the survivors in the erythromycin treatment group were negative for L. pneumophila; for the other seven lungs the median, average, and range of L. pneumophila concentrations were 3.2, 4.0, and 1.0 to 4.9 log10 CFU/g. There was no significant difference in L. pneumophila counts in the lungs or L. pneumophila positivity rates between the surviving animals in the ABT-773 and erythromycin treatment groups (P > 0.6 by the Mann-Whitney test and Fisher's exact test, respectively).
FIG. 4.
Lung L. pneumophila concentrations for animals that had necropsies. The surviving animals were killed on day 13 postinfection, so data for days before day 13 are for animals that died. The numbers of animals represented by each datum point are one unless indicated otherwise in parentheses. Error bars represent the range, which in some cases is smaller than the height of the symbol. The horizontal solid line shows the lower limit of detection of the assay. ▾, saline; ♦, erythromycin; □, ABT-773.
Weights but not temperatures differed significantly between the animals in the active treatment groups on day 9 only (data not shown). ABT-773-treated animals weighed significantly less than the erythromycin-treated animals on day 9 but not on the other days (mean difference, 24.9 g; 95% confidence interval [CI] for the difference, 2 to 48 g [P = 0.03 by the unpaired t test]). This was attributed to the presence of peritonitis in the ABT-773 treatment group.
Histologic examination of lungs from the survivors in the active treatment groups showed no significant difference in the amount of lung consolidation (n = 10 in each group [P = 0.3 by the Student t test]). The median amount of lung consolidation in the ABT-773 treatment group was 10%, with a 95% CI of 6 to 28%; for the erythromycin treatment group the median value was 22% and the 95% CI was 11 to 45%. The five ABT-773-treated animals that died before day 13 had a median amount of consolidation of 5%, with a range of 5 to 15%. In comparison, the amount of consolidation was much greater in the saline treatment group, with a median value of 60% and a 95% CI of 50 to 74%.
DISCUSSION
These studies confirm that ABT-773 is very active against both extracellular and intracellular Legionella bacteria and that the drug is also active in an animal model of Legionnaires' disease. ABT-773 appears to be about as active as erythromycin in the animal model, even though it was given at a substantially lower dose.
Our in vitro susceptibility testing results show that ABT-773 is about as active as clarithromycin against Legionella bacteria and that both drugs are considerably more active than erythromycin against the bacterium. These results appear to be in concordance with those of prior studies (Jung et al., 39th ICAAC; Ma et al. 39th ICAAC; Sens et al., 40th ICAAC). We have previously shown that erythromycin is less active against Legionella spp. other than L. pneumophila than it is against L. pneumophila and now demonstrate this to be the case for ABT-773 (8). Of interest, the clarithromycin MICs for the Legionella bacteria tested were unimodal, in contrast to the results obtained for erythromycin and ABT-773. We may have underestimated the in vitro activity of clarithromycin, as its 14-OH metabolite is known to have activity against L. pneumophila, but it is unlikely that the parent drug was hydrolyzed under our test conditions (21, 23).
ABT-773 inhibited the growth of intracellular L. pneumophila and at a higher concentration (1 μg/ml) had bactericidal activity against the bacterium. This is in contrast to the solely inhibitory activities of erythromycin and clarithromycin at the same concentration. We have previously shown in the same model that erythromycin never demonstrates intracellular bactericidal activity, even at extracellular concentrations as high as 5 μg/ml (12). ABT-773 appears to have about the same activity against intracellular L. pneumophila as another ketolide, HMR3004, and greater activity than HMR3647 (telithromycin), based on a historical comparison in the same model system (9).
ABT-773 was rapidly cleared from guinea pig plasma, a feature of most drugs studied in the guinea pig model. In contrast to the half-life in guinea pig plasma of 0.55 h, the half-lives in the plasma of humans dosed with a single oral dose range from 3.6 to 6.7 h (Pradhan et al., 40th ICAAC). The maximum plasma ABT-773 concentrations that we measured and the AUC0–24 for guinea pigs are similar to those obtained in humans receiving a single oral 100-mg dose (Pradhan et al., 40th ICAAC). Despite its rapid clearance from plasma and its low maximum concentrations in plasma, ABT-773 achieved high concentrations in the lungs, with a median concentration in lung:concentration in plasma ratio of 57:1, which is severalfold higher than observed in other animals (L. Hernandez, N. Sadrzadeh, S. Krill, Z. Ma, and K. Marsh, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2148, 1999). A prior study with the same animal model showed that erythromycin given by the same route and dose achieved concentrations in serum of 9.9 to 14.4 and 5.1 μg/ml at 30 and 60 min postinjection, respectively (7). In the same study, lung erythromycin levels were 18 to 36 and 17 to 21 μg/g at 30 and 60 min postinjection, respectively. In a similar guinea pig model, the half-life of erythromycin in serum was about 0.4 h (19). In humans the half-life of erythromycin in serum is about 1.4 h, the levels in the lungs are about fivefold greater than those in serum, and levels in serum 1 h after receipt of 1 g intravenously are about 10 μg/ml (2, 26).
Higher concentrations of azithromycin are found in the lungs of guinea pigs with L. pneumophila pneumonia than in healthy animals, probably because the drug is delivered to the lung by inflammatory cells containing azithromycin at high concentrations (24). Whether the same mechanism occurs with ABT-773 requires further study, including side-by-side comparisons of drug pharmacokinetics in infected and uninfected animals. The meaning of AUC0–24 measurements is unknown for the animal model of L. pneumophila pneumonia or human Legionnaires' disease.
In the animal model of L. pneumophila pneumonia, ABT-773 was as active as erythromycin, and both drugs were substantially more effective than the saline control therapy. ABT-773 therapy resulted in a decreased mortality rate compared to that with saline treatment and also resulted in substantial clearing of the bacterium from the lungs. For the 1 day on which both saline- and ABT-773-treated animals died, there was an ≈2.5 log 10 lower concentration of L. pneumophila in the ABT-773-treated animals.
Strong evidence supports peritonitis rather than pneumonia as a cause of the early deaths in the ABT-773-treated animals. This evidence includes the finding of severe peritonitis in the animals, the absence of severe pneumonia on gross and microscopic examination of the lungs, and the relatively low L. pneumophila concentrations in the lungs. In addition, three of the five deaths occurred much later than the times of death for saline-treated animals, and the deaths are more in line with what is observed for deaths due to erythromycin or rifampin gastrointestinal tract toxicity for guinea pigs in this animal model (7). The peritonitis was most likely chemical in nature. Bowel perforation during drug injection or L. pneumophila peritonitis is a most unlikely explanation for peritonitis in the ABT-773 treatment group, as none of the animals in the other treatment groups had evidence of peritonitis. Also, L. pneumophila peritonitis has not been observed in this animal model. Chemical peritonitis due to intraperitoneal injection of dalfopristin-quinupristin or injection of a drug-solubilizing agent, Capmul MCM, has been observed in this guinea pig model, but it has not been observed after injection of multiple other compounds (10; P. Edelstein, unpublished data). Peritonitis is not of concern in humans, as the drug will not be administered to humans by the intraperitoneal route.
Neither erythromycin nor ABT-773 completely eliminated L. pneumophila bacteria from the lungs of animals that survived for 8 days after therapy. This is the result of either incomplete bacterial clearance or relapse after drug discontinuation. Such a finding is in accord with the relapses observed in humans with Legionnaires' disease treated with erythromycin and with the reversible inhibition of bacterial growth in cell models of infection (5). That ABT-773 did not effect a higher bacterial clearance rate in lungs is surprising in view of the high concentrations of the drug in the lungs and the intracellular bactericidal capacity of the drug at high extracellular concentrations. Whether ABT-773 would have a greater bactericidal effect against L. pneumophila in humans is unknown, but it is possible, based on the more favorable drug pharmacokinetics in humans.
The guinea pig model of L. pneumophila pneumonia is an effective predictor of drug efficacy for Legionnaires' disease, especially when combined with supportive evidence of the good in vitro and intracellular activities of the drug against the bacterium (7). All three of these measures are supportive of the good activity of ABT-773 against human Legionnaires' disease and give good evidence to support the performance of clinical trials of ABT-773 for the treatment of Legionnaires' disease.
ACKNOWLEDGMENTS
This study was funded by Abbott Pharmaceuticals.
Takashi Shinzato provided excellent technical assistance. Kennan Marsh performed assays of ABT-773 in the plasma and lungs.
REFERENCES
- 1.Brueggemann A B, Doern G V, Huynh H K, Wingert E M, Rhomberg P R. In vitro activity of ABT-773, a new ketolide, against recent clinical isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother. 2000;44:447–449. doi: 10.1128/aac.44.2.447-449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brun Y, Forey F, Gamondes J P, Tebib A, Brune J, Fleurette J. Levels of erythromycin in pulmonary tissue and bronchial mucus compared to those of amoxycillin. J Antimicrob Chemother. 1981;8:459–466. doi: 10.1093/jac/8.6.459. [DOI] [PubMed] [Google Scholar]
- 3.Cynamon M H, Carter J L, Shoen C M. Activity of ABT-773 against Mycobacterium avium complex in the beige mouse model. Antimicrob Agents Chemother. 2000;44:2895–2896. doi: 10.1128/aac.44.10.2895-2896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies T A, Ednie L M, Hoellman D M, Pankuch G A, Jacobs M R, Appelbaum P C. Antipneumococcal activity of ABT-773 compared to those of 10 other agents. Antimicrob Agents Chemother. 2000;44:1894–1899. doi: 10.1128/aac.44.7.1894-1899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edelstein P H. Antimicrobial chemotherapy for Legionnaires' disease: a review. Clin Infect Dis. 1995;21:S265–S276. doi: 10.1093/clind/21.supplement_3.s265. [DOI] [PubMed] [Google Scholar]
- 6.Edelstein P H. The guinea-pig model of Legionnaires' disease. In: Zak O, Sande M A, editors. Handbook of animal models of infection. London, United Kingdom: Academic Press; 1999. pp. 303–314. [Google Scholar]
- 7.Edelstein P H, Calarco K, Yasui V K. Antimicrobial therapy of experimentally induced Legionnaires' disease in guinea pigs. Am Rev Respir Dis. 1984;130:849–856. doi: 10.1164/arrd.1984.130.5.849. [DOI] [PubMed] [Google Scholar]
- 8.Edelstein P H, Edelstein M A. In vitro activity of SCH 27899 (Ziracin) against Legionella species. Diagn Microbiol Infect Dis. 1999;33:59–62. doi: 10.1016/s0732-8893(98)00106-0. [DOI] [PubMed] [Google Scholar]
- 9.Edelstein P H, Edelstein M A. In vitro activity of the ketolide HMR 3647 (RU 6647) for Legionella spp., its pharmacokinetics in guinea pigs, and use of the drug to treat guinea pigs with Legionella pneumophila pneumonia. Antimicrob Agents Chemother. 1999;43:90–95. doi: 10.1128/aac.43.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelstein P H, Edelstein M A. In vitro activity of quinupristin/dalfopristin (Synercid, RP 59500) against Legionella spp. Diagn Microbiol Infect Dis. 2000;36:49–52. doi: 10.1016/s0732-8893(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 11.Edelstein P H, Edelstein M A C. WIN 57273 is bactericidal for Legionella pneumophila grown in alveolar macrophages. Antimicrob Agents Chemother. 1989;33:2132–2136. doi: 10.1128/aac.33.12.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelstein P H, Edelstein M A C. In vitro activity of azithromycin against clinical isolates of Legionella species. Antimicrob Agents Chemother. 1991;35:180–181. doi: 10.1128/aac.35.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelstein P H, Edelstein M A C. In vitro activity of Ro 23-9424 against clinical isolates of Legionella species. Antimicrob Agents Chemother. 1992;36:2559–2561. doi: 10.1128/aac.36.11.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelstein P H, Edelstein M A C. Comparison of three buffers used in the formulation of buffered charcoal yeast extract medium. J Clin Microbiol. 1993;31:3329–3330. doi: 10.1128/jcm.31.12.3329-3330.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelstein P H, Edelstein M A C. In vitro activity of RP 74501-RP 74502, a novel streptogramin antimicrobial mixture, against clinical isolates of Legionella species. Antimicrob Agents Chemother. 1993;37:908–910. doi: 10.1128/aac.37.4.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edelstein P H, Edelstein M A C, Lehr K H, Ren J. In-vitro activity of levofloxacin against clinical isolates of Legionella spp, its pharmacokinetics in guinea pigs, and use in experimental Legionella pneumophila pneumonia. J Antimicrob Chemother. 1996;37:117–126. doi: 10.1093/jac/37.1.117. [DOI] [PubMed] [Google Scholar]
- 17.Edelstein P H, Edelstein M A C, Ren J J, Polzer R, Gladue R P. Activity of trovafloxacin (CP-99,219) against Legionella isolates: in vitro activity, intracellular accumulation and killing in macrophages, and pharmacokinetics and treatment of guinea pig with L. pneumophila pneumonia. Antimicrob Agents Chemother. 1996;40:314–319. doi: 10.1128/aac.40.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelstein P H, Edelstein M A C, Weidenfeld J, Dorr M B. In vitro activity of sparfloxacin (CI-978; AT-4140) for clinical Legionella isolates, pharmacokinetics in guinea pigs, and use to treat guinea pigs with L. pneumophila pneumonia. Antimicrob Agents Chemother. 1990;34:2122–2127. doi: 10.1128/aac.34.11.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson D H, Fitzgeorge R B. Persistence in serum and lungs of guinea pigs of erythromycin, gentamicin, chloramphenicol and rifampicin and their in-vitro activities against Legionella pneumophila. J Antimicrob Chemother. 1983;12:235–244. doi: 10.1093/jac/12.3.235. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein E J, Citron D M, Merriam C V, Warren Y, Tyrrell K. Comparative in vitro activities of ABT-773 against aerobic and anaerobic pathogens isolated from skin and soft-tissue animal and human bite wound infections. Antimicrob Agents Chemother. 2000;44:2525–2529. doi: 10.1128/aac.44.9.2525-2529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones R N, Erwin M E, Barrett M S. In vitro activity of clarithromycin (TE-031, A-67268) and 14OH-clarithromycin alone and in combination against Legionella species. Eur J Clin Microbiol Infect Dis. 1990;9:846–848. doi: 10.1007/BF01967390. [DOI] [PubMed] [Google Scholar]
- 22.Khan A A, Araujo F G, Craft J C, Remington J S. Ketolide ABT-773 is active against Toxoplasma gondii. J Antimicrob Chemother. 2000;46:489–492. doi: 10.1093/jac/46.3.489. [DOI] [PubMed] [Google Scholar]
- 23.Martin S J, Pendland S L. Bactericidal activity and postantibiotic effect of clarithromycin and 14-hydroxyclarithromycin, alone and in combination, against Legionella pneumophila. J Antimicrob Chemother. 1998;41:643–648. doi: 10.1093/jac/41.6.643. [DOI] [PubMed] [Google Scholar]
- 24.Stamler D A, Edelstein M A C, Edelstein P H. Azithromycin pharmacokinetics and intracellular concentrations in Legionella pneumophila-infected and uninfected guinea pigs and their alveolar macrophages. Antimicrob Agents Chemother. 1994;38:217–222. doi: 10.1128/aac.38.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strigl S, Roblin P M, Reznik T, Hammerschlag M R. In vitro activity of ABT 773, a new ketolide antibiotic, against Chlamydia pneumoniae. Antimicrob Agents Chemother. 2000;44:1112–1113. doi: 10.1128/aac.44.4.1112-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Washington J A, Wilson W R. Erythromycin: a microbial and clinical perspective after 30 years of clinical use (First of two parts) Mayo Clin Proc. 1985;60:189–203. doi: 10.1016/s0025-6196(12)60219-5. [DOI] [PubMed] [Google Scholar]