Abstract
Garcinia kola belongs to the Garcinia genus of the Clusiaceae family and Malpighiales order. It contains more than 180 members all over the globe. It is found all over Asia and in tropical African countries. In Africa, traditionally, G kola is used to manage and treat cancer, diabetes, malaria, analgesics, hypertension, and other numerous ailments. This review aimed to comprehensively update relevant information regarding the pharmacological potential of Garcinia kola. Electronic databases such as ScienceDirect, PubMed, Wiley, Google Scholar, Hindawi, and Springer extracted valuable information from original scientific research papers. Inclusion Criteria. Antioxidant, antimicrobial, antidiabetic, antibacterial, medications, antiviral, traditional medicine, ethnopharmacology, toxicity, cytotoxic action, chemical composition, mineral elements, GCMS analysis, and any other related phrases were used as filters to find studies. Exclusion Criteria. Data from questionable online sources, as well as thesis reports and review publications, were excluded from this investigation. The investigation revealed that seeds of G. kola are very efficient as antioxidant, antimicrobial, antidiabetic, antihypertension, antianalgesic, and anti-inflammatory. The study also found that too much consumption of the seeds caused low fertility and toxicity. However, the safety and efficacy of G. kola have not been wholly assessed in humans, and further well-designed clinical trials are needed to corroborate preclinical findings. The mechanism of action of the seed extract should be examined. The standard dose and safety of the seed should be established.
1. Introduction
Traditional medicines produced from plants have become more important as alternative medicines in treating a broad spectrum of ailments, and researchers are continuing to pay attention to the use of plant materials in the treatment of many afflictions [1, 2]. The majority of the developing world believes that these plant-based products are safer and more cost-effective [3]. With the emergence of new diseases and microorganism resistance, the usage of these plant products has increased in developed, developing, and underdeveloped countries [4, 5]. Ethnopharmacology and medication discovery employing plant-based products are still critical in healthcare delivery worldwide. Garcinia kola is regarded as a miracle plant because every component has medicinal use. The following reviews aimed to update and do a comprehensive review regarding the biological potential of G. kola.
2. Methodology
Electronic databases such as ScienceDirect, PubMed, Wiley, Google Scholar, Hindawi, and Springer extracted valuable information from original scientific research papers. Inclusion criteria: Antioxidant, antimicrobial, antidiabetic, antibacterial, medications, antiviral, traditional medicine, ethnopharmacology, toxicity, cytotoxic action, chemical composition, mineral elements, GCMS analysis, and any other related phrases were used as filters to find studies. Exclusion criteria: Data from questionable online sources, as well as thesis reports and review publications, were excluded from this investigation.
3. Results and Discussion
3.1. Taxonomy, Distribution, and Morphology of Garcinia kola
The Garcinia genus includes Garcinia kola from the Clusiaceae family and Malpighiales order [6]. It contains more than 180 members all over the globe. Synonym names are; Garcinia akawaensis Spirlet, Garcinia giadidii De Wild, and Garcinia bergheana Spirlet. G. kola is a sub-Saharan African forest tree that has been dubbed a “wonder plant” since practically every portion of it has been proven to have medicinal value. It grows natively from Sierra Leone to Southern Nigeria, then on to Zaire and Angola, but it has been widely spread by man and is frequently found growing near communities. It is a tree grown in the Central and West Africa coastal rain forests. It is found all over Asia and in tropical African countries. It reaches a height of about 30 m. The orange-sized fruit is smooth and reddish yellow, with peach-like skin and yellow flesh, and three or four seeds with a brown seed coat. The seed is a nut that may be eaten. The seed coat is dark with branching lines, while the kernels are pale and punctured with resin pockets (Figure 1). Fruits are yellow, reddish, and orange-sized, with a yellow-orange, sometimes reddish pulp. The greenish-white flowers have a reddish indumentum [6].
Figure 1.

Leaf and fruits (a) [7] and seeds (b) of Garcinia kola.
3.2. Biological Evaluation
Alternative medicine is based on medicinal plants, which has led to the development of many novel pharmaceuticals [8]. More than 80% of medicine was derived from plants in the nineteenth century. The scientific revolution led to the development of the pharmaceutical business, where manufactured pharmaceuticals became more prominent [9]. There is greater usage of medicinal plants in treating ailments because they are regarded as safe and effective pharmaceuticals, have fewer side effects, and cost less than other drugs [10]. Garcinia kola was subjected to several biological tests (Table 1).
Table 1.
Biological evaluation.
| S/N | Activity | Method | Extract | Major findings | Reference | |
|---|---|---|---|---|---|---|
| 1 | Antioxidant | In vivo | Seeds | In flies fed a diet enriched with higher G. kola seed inclusions, GST and catalase activities were dramatically boosted, whereas NO content was significantly reduced compared to controls. | [42] | |
| In vivo | Seed | Petroleum ether | Kolaviron appears to operate as an in vivo natural antioxidant and an effective hepatoprotective agent in the current investigation. It is as effective as BHA. | [12] | ||
| In vivo | Seeds | Ethanol | These findings showed that seeds could be useful by reducing the oxidative damage produced by chronic ethanol treatment in Wistar rats' livers. | [43] | ||
| In vivo | Seed | Methanol | Significant rise in total white blood cell count with no increase in hemoglobin at p > 0.05. These findings imply that the seeds have immune-stimulatory properties, which could support ethno-medicinal efficacy claims. | [44] | ||
| DPPH, FRAP | Seed | Ethanol and aqueous | Using radical trapping test and the ion conversion method revealed that Ci 50 (65.86, 1.17 g/mL) and the reducing power of the ferric ion (125. 4 4. 91 mg/mL) are statistically significant. | [14] | ||
| DPPH | Seed/EO | Hexane | The highest scavenging activity was recorded at 85.6%. | [45] | ||
| DPPH | Seeds | Methanol, chloroform, and ethyl acetate | Antioxidant activity through radical scavenging activity was found to be 46.00. In terms of antioxidant activity, the total polyphenols showed a significant level of association (r2 = 0.927). | [18] | ||
| DPPH, FRAP and FTC | Seeds | Petroleum ether, acetone, and ethanol | The results of the three test methods revealed that all extracts, regardless of the solvent employed for extraction, had strong antioxidant activity starting at 0.5 mg/mL. | [46] | ||
| Seed | Ethanol | This discovery suggested that the extracts may include antioxidants and hence can scavenge free radicals, thereby preventing oxidative stress. This may support their use in treating hepatic dysfunction and stress-related disorders on a local level. | [47] | |||
| In vivo and in vitro | Root | According to the biological evaluation, the saponin extract from the root has scavenging actions against free radicals. The root has the potential to be used as a natural antioxidant source. | [48] | |||
| Seeds | Methanol | The ME4 had the highest level of activity. The ME4 fraction was also significantly reduced nitric oxide generation in lipopolysaccharide-activated macrophage U937 cells. | [49] | |||
| In vivo | Leaf | Cold 70% ethanol | The extract inhibits the most in both liver and brain homogenates at the same concentration (26.7 g/mL), with the percent inhibition of 64.1% and 38.25, respectively. | |||
| DPPH, FRAP and Fe2+ chelating | Seed | Aqueous and ethanol | At p > 0.05, the ethanolic extract had considerably better characteristics. Given this, the usage of the plant in traditional medicines for the treatment of cough and liver diseases could be linked to its phytochemical composition. | [50] | ||
| DPPH, FRAP | Seeds | Aqueous | It exhibited significant antioxidant activity at varying doses, which might be attributed to diverse phenolic components in the plants. | [51] | ||
| In Vivo | Seeds | Compared to the control group, prolonged administration had no negative effects on spermatozoa features but considerably increased testosterone concentration. Malondialdehyde levels in the liver, testes, and spermatozoa of rats were much lower as antioxidant systems improved. When compared to controls, prolonged administration of G. kola had no effect on the liver and testes at all doses, according to histological analysis. | [52] | |||
| The antioxidant regarding the phenolic content was found between 10–21 mg·g−1. The scavenging at 26%–55% was high, showing that it could be a good source of natural antioxidants and employed as food supplements. | [13] | |||||
| DPPH | Seed oil | n-hexane | The highest scavenging activity was recorded at 91.05 ± 0.12 mg/mL. | [53] | ||
| DPPH | Seed | Ethanol | The antioxidant studies revealed a dose-dependent substantial (p > 0.05) increase in its ability to scavenge free radicals. The findings of this investigation suggested that the seed could be used to treat free radical-mediated illnesses. | [26] | ||
| In vivo | Seed | Ethanol | On day 7, the 500 mg/kg extract-treated group had a 49.70% drop in blood glucose levels compared to the positive control group (45.03%). The findings of this investigation suggested that the seed could be used to treat illnesses and diabetic management. | [26] | ||
| Linoleic acid system | Seed | Petroleum ether | Seeds overall antioxidant activity on lipid peroxidation might be ascribed to their ability to scavenge free radicals and active oxygen species. It could be linked to the inhibition of in vivo lipid peroxidation propagation. | [54] | ||
| In vivo | Seed | Ethanol | When compared to rats in group 2, the glutathione concentration of the group was significantly lower (p < 0.05). Compared to rats in group 1 and the treatment group, the vitamin C level in group 2 was significantly lower (p < 0.05). | [55] | ||
| 2 | Agar well diffusion | Seeds | Acetone | The synergistic efficacy of bitter kola and fantastic kola exhibited superior antibacterial activities. The positive results for both Gram negative (Escherichia coli, Pseudomonas sp.) and Gram-positive (S. aureus and Bacillus sp.) bacteria indicate that they could be employed as broad-spectrum antibiotics. | [56] | |
| Agar well diffusion | Ethan, aqueous | At a 30 mg/mL of ethanol and aqueous (hot water) dosage, extracts showed higher antibacterial activity, with zones of inhibition ranging from 17 to 23 mm for ethanol. | [19] | |||
| Agar dilution method | Seed | Methanol and aqueous | This study found that G. kola extracts have good antifungal activity against clinical isolates of Fusobacterium nucleatum and its connection with periodontal infections. | [57] | ||
| Seed | Ethanol | The extract was the most effective against the test organisms, with a mean inhibition zone of 15.33 mm. As a result, it can be deduced that bitter kola, kola nut, and avocado seeds exhibit antibacterial action, with the kind of extracting solvent having a significant impact on the level of antimicrobial activity. This means that an antibacterial seed or herb extract should be made in the most appropriate solvent for maximum efficiency. | [58] | |||
| Disc diffusion method | Leaf | Methanol and aqueous | The extracts ranged from 25 mgL−1 to 50 mgL−1. The findings suggest that these plants' leaves could be utilized to treat ailments caused by the test organisms. The bioactive components of the leaves would be characterised further using crude extracts. | [59] | ||
| Agar-well diffusion method | Seed | Methanol | At a 20 mg/mL final dosage, the extract showed considerable inhibitory effect against all examined bacteria except four. The inhibition zones varied from 10 to 23 mm, while the typical antibiotics' zones of inhibition ranged from 15 to 25 mm; 12 and 25 mm, respectively. | [60] | ||
| Tube dilution susceptibility | Leaves (combine with other plants) | Aqueous | The findings imply that the formulation has high in vitro antibacterial activity against common wound isolates and could be used for routine wound and sepsis treatment instead of antibiotic chemotherapy. | [61] | ||
| In Vivo | Seed | n-hexane, hot aqueous and ethanol | The best antibacterial activity was found in the n-hexane extract, followed by ethanol and finally hot water. According to MIC, the inhibitory zone diameter of n-hexane clove extract was the biggest, followed by bitter kola extract, and finally tobacco extract. | [62] | ||
| Test tubes bottles | Seed oil | n-hexane | The oil was discovered to have broad-spectrum activity against gram-positive and gram-negative bacteria isolates, which was concentration-dependent. | [63] | ||
| Agar-well diffusion method | Seeds | Methanol, aqueous | At the same dose of 2.5 mg/mL, the extract had bactericidal activity against Klebsiella pneumoniae and Shigella species. As a result, pharmaceutical companies should examine extracts that have been demonstrated to be effective against test organisms. | [64] | ||
| In vivo | Seed | Aqueous | The findings show that both uncoated and coated bitter cola has medical promise as a lead toxicity reducer and alternative antibacterial. Furthermore, it could be a two-edged sword for treating lead toxicity and subsequent infections caused by lead poisoning. | [15] | ||
| Microdilution broth method | Leaf (EO) | The oil contains several chemicals that were active against the bacteria tested, with minimum inhibitory concentrations ranging from 50 to 400 g/mL and might be used to produce plant-based medications. | [65] | |||
| Agar diffusion method | Seeds | Petroleum ether, 70% ethanol and aqueous | The presence of a polyisoprenyl benzophenone (Kolanone) in the petroleum ether extract and the hydroxy biflavanonols in the ethyl acetate fraction was found to be responsible for the observed activity. | [66] | ||
| MIC | Seed | Ethanol | The extract had a broad spectrum of activity, whereas the fractions had a narrow spectrum of activity because they were only active against S. aureus, E. coli, and Pseudomonas aeruginosa. These findings could explain why G. kola seeds are useful in treating microbial diseases. | [67] | ||
| Methanol | Compared to chloramphenicol [standard medicine], which had MICs of 14.31–31.62 g/mL, the minimum inhibitory concentration (MIC) for bacteria. | [20] | ||||
| Agar disc diffusion method | Seed | Aqueous | As a result, the findings imply that biogenic AgNPs have potential biological applications and might potentially be used as a key component in the development of innovative nanopaints against the tested bacterial strains. | [68] | ||
| Disc diffusion | Seed | Aqueous, ethanol and methanol | This study reveals that seeds extracts from these plants have antibacterial characteristics and could be utilized as an alternative to antibiotics. | [69] | ||
| Checkerboard technique | Seeds | Methanol | The extract's MICs against microorganisms were found to be 1.562 and 3.125 mg/mL, respectively. | [70] | ||
| Disk | Seeds | The ethanol seeds extract was found to have significantly higher activity (p < 0.001) than the aqueous preparation. The presence of several pharmacokinetic substances could explain the activity. | [71] | |||
| Agar-well diffusion method | Seeds (black nanocrystal of silver nanoparticles) | Aqueous | All of the bacteria examined showed that the produced silver had good antibacterial action. Green nanoparticles can be employed in a variety of medicinal applications. | [72] | ||
| Agar diffusion method | Seed | Methanol and aqueous | There was a higher level of activity with the hot water seeds extract. The findings supported herbalists' historical usage of botanicals in treating bacterial illnesses. | [73] | ||
| Agar-well diffusion method | Seed | Petroleum ether | Antimicrobial activity against a broad spectrum of microorganisms has been observed in the isolated chemical. | [74] | ||
| Disc | Seeds | Aqueous and ethanol | At p < 0.05, the results were significant. Against the bacterial isolates, the extracts demonstrated different levels of inhibition. | [75] | ||
| Disk | Seeds | Aqueous and ethanol | At p < 0.05, the results were significant. Against the fungal isolates, the extracts demonstrated different levels of inhibition. | [75] | ||
| Agar-well diffusion method | Leaves | Cold aqueous, hot aqueous, ethanol and methanol | The MIC was evaluated at different concentrations of 25 and 12.5 mg/mL and showed efficacy. The findings support the plant's long-standing use in Nigerian rural communities to treat infectious disorders. | [76] | ||
| Agar well diffusion | Seeds | Methanol, chloroform, and ethyl acetate | Antibacterial activity was found in the plant seed extracts against the test strains. However, at a low dose of 1.25 mg/mL, the maximum spectrum activity was observed against S. mutans and B. subtilis (l4 and l2 mm). The findings suggested that using these plant extracts as nutraceuticals could help to minimize the risk of microbial infections. | [18] | ||
| Agar well diffusion | Seeds | 100% (neat) | With a zone diameter of 22.0 mm and above, the extract showed significant inhibition against the strains. | [77] | ||
| Seeds | Methanol | Except for E. coli, all studied bacterial strains have an inhibition zone diameter of roughly 20 0.91 mm. At p > 0.05, the standard used (tetracycline) had a larger zone of inhibition. Antibacterial characteristics were present in the extract. | [78] | |||
| Fractional inhibitory concentration | Seeds | Acetone | Combinations against gram-positive species yielded mostly synergistic interactions (FIC index of 0.52—0.875), while combinations against gram-negatives yielded more antagonistic interactions (FIC indices of 2.0–5.0). We infer that the seed extract could be a source of broad-spectrum antibiotic resistance-modifying chemicals. | [79] | ||
| Disc diffusion | Seed | Ethanol (70%) | The extracts showed an inhibitory effect on the test isolates, likely due to the high tannin and flavonoid content. Above all, our research indicates that the seed possessed antimicrobial properties. According to the findings, consuming the seed in a controlled manner may help to prevent bacterial infections in the intestine. | [16] | ||
| Agar well diffusion | Seed | Ethanol and aqueous | The effects of various concentrations were studied. It was discovered that a synergistic blend of aqueous and honey seed extracts was more effective than using the extracts separately in suppressing the growth of the bacterial strain. | [80] | ||
| Disc | Seeds | Petroleum ether, acetone and ethanol | Antibacterial sensitivity testing revealed that the extracts reduced the growth of the test isolates, as evidenced by measured zones of inhibition, which differed between species. | [46] | ||
| Ager well | Seeds | Ethyl acetate, ethanol, methanol, acetone and aqueous | The extracts had inhibitory zone widths ranging from 0–24 1.1 mm, with MIC and MBC values of 0.04–1.25 mg/mL and 0.081–2.5 mg/mL, respectively. The findings of this study support the use of this plant in traditional medicine and provide a lead for the creation of new and powerful antimicrobials. | [81] | ||
| Bottles of molten agar | Seeds | Methanol | The antibacterial activity against all isolates was significantly lower than the standard antibiotic, gentamicin 4 mg/mL, at p > 0.05. Similarly, the activity was dose-dependent, with greater inhibition zones corresponding to higher concentrations at p > 0.05. | [82] | ||
| In vivo | Seeds | Aqueous | At 1 and 2.5 h, the interaction was antagonistic, but at 4 h, it became potentiated. The actual mechanism that causes the observed biphasic interaction is unknown. | [83] | ||
| Agar diffusion method | Seeds | Aqueous, ethanol, and methanol | The crude extracts' sensitivity patterns of inhibition zones revealed a proportionate degree of inhibitory activity against the tested bacterial strain. | [84] | ||
| Agar well diffusion | Seeds | Ethanol | The findings of this investigation revealed that the extract had inhibitory activity against the bacterial isolates tested at various concentrations, with a greater inhibitory effect on E. coli at a concentration of 300 mg/mL shows that as the concentration of the extract against the bacteria increases, the zones of inhibition expand. | [85] | ||
| Cup plate method and broth dilution methods | Seeds | Ethanol | Fraction of hexane: ethyl acetate 70 : 30 had the highest activity against S. mutans and S. viridans, with MICs of 1.50 mg·mL−1 and 0.33 mg·mL−1, respectively, | [17] | ||
| Agar dilution method | Seeds | Ethanol and aqueous | S. aureus and K. pneumonia had minimal bactericidal concentrations of 1.00 mg/ml and 0.50 mg/mL, respectively. | [86] | ||
| Disc method | Seeds | Ethanol | The plant seeds should be recommended for treating E. coli diarrhoea and all B. cereus diseases based on this research; nevertheless, more research is needed to isolate the medicinal chemicals, explore their mode of action, and the effect of the same in the in vivo environment. | [87] | ||
| Disc diffusion | Seed | Aqueous, acetone, methanol and ethanol | The findings demonstrated that methanol extract has the highest inhibitory activity at various doses against all tested bacterial strains, with S. aureus having the maximum zone of inhibition (p > 0.05). | [88] | ||
| Agar-well diffusion | Seed | Methanol and aqueous | It is concluded that secondary metabolites included in the extract are responsible for the bacteria inhibition reported in this investigation; consequently, the test plant could be used to make medications to treat illnesses caused by the test organisms. | [89] | ||
| Seed | Methanol | These seeds extracts' antibacterial properties could be effective in treating multidrug-resistant Acinetobacter baumannii. The aqueous fraction outperformed the other fractions in terms of activity. | [90] | |||
| Agar-well diffusion | Seeds and leaves | Aqueous, ethanol, and methanol | Both the leaves and seeds extracts had a substantial antibacterial impact on S. aureus. | [91] | ||
| Cork-borer | Seed | Aqueous, ethanol, and methanol | On the other hand, the extracts had a stronger antibacterial activity, with a ZOI of 8.66 0.42 mm (5.30.4–13.50.4) compared to 6.36 0.36 mm (3.90.06–8.906 mm). | [92] | ||
| Disc | Bark | Aqueous | The antibacterial screening of the biosynthesised AgNPs revealed that they had inhibitory potential and could hinder microorganisms' growth. | [93] | ||
| Tube dilution | Leaves | Aqueous | The findings imply that the formulation has high in vitro antibacterial activity against common wound isolates and could be used for routine wound and sepsis treatment instead of antibiotics and chemotherapy. | [94] | ||
| Agar well method | Bark and seeds | Ethanol | The extract inhibited all tested bacterial strains in a zone of inhibition ranging from 12 to 23 mm. | [95] | ||
| Agar well diffusion | More research is needed to determine the sort of antimicrobial activity they exhibit (bactericidal or bacteriostatic), as well as the active components contained in the vinegar samples that allow them to exhibit such activities. | [96] | ||||
| Disc | Seed | Ethanol | Antibacterial activity tests revealed that all three eluates had cumulative bactericidal activity against five of the ten species tested. The pyridine/pyrimidine moiety in Eluate 2 suppressed the development of K. aerogenes in a way that the other eluates and the broad-spectrum antibiotic levofloxacin did not. | [97] | ||
| Agar diffusion method | Seeds | Aqueous and ethanol | The various test plant extracts moderately inhibited the standard bacteria E. coli NCTC 10418 and S. aureus NCTC 6571, with inhibition zones ranging from 8 mm to 20 mm. The antibacterial properties of these plants are revealed in this investigation. | [98] | ||
| Agar well diffusion method | Seeds | Methanol | The extract exhibited strong activity against the tested strains. | [99] | ||
| Agar diffusion | Seed oil | n-hexane | Antibacterial tests revealed a high susceptibility to all germs examined. Salmonella typhi was the most susceptible of the bacteria tested, with an inhibition of 27 mm, while E. coli had the least, with an inhibition of 12 mm, at a dose of 100 mg/mL. | [53] | ||
| Polyphenolic | IR, 1H, and 13C-NMR spectroscopy were used to characterize the fraction with the strongest antibacterial potential. The molecule could be Catechin, methyl-dl-tyrosine, p-naphtholbenzein—or Naringin, according to the combined spectroscopic data. | [100] | ||||
| Agar well diffusion | Leaves | Ethanol, methanol, hot and cold aqueous | The findings revealed that of the 96 wound swabs collected, 15 (21.7%) bacteria pathogens were identified in the following order: E. coli 9 (60%), P. aeruginosa 4 (26.6%), Klebsiella spp 1 (6.6%), and S. aureus 1 (6.6%). | [101] | ||
| Agar well diffusion | Seeds | Methanol, ethanol, and aqueous | Methanolic and ethanolic seed extracts were found to have antibacterial action against gram-positive and gram-negative bacteria. | [102] | ||
| 3 | Antifungal | Agar well diffusion | Seeds | Methanol, ethanol, and aqueous | No activity. | [102] |
| Agar well diffusion | Seeds | Ethanol and aqueous (cold and hot) | Antifungal activity was also found in the seed extracts against A. niger. Compared to the usual antibiotics employed in the study, the results indicate strong antifungal capabilities. | [19] | ||
| Seeds | Methanol lead acetate | On two beer spoilage microorganisms, Candida vini and Lactobacillus delbruckii, exhibited significant activity. | [103] | |||
| Agar diffusion method | Seeds | Petroleum ether, 70% ethanol and aqueous | The extract has significant activity against C. albicans and A. flavus in a fungistatic manner. | [66] | ||
| MIC | Seed | Ethanol | It was also effective against fungi such as Penicillium notatum and A. niger. | [67] | ||
| TTest tubes bottles | Seed oil | n-hexane | The oil was discovered to have broad-spectrum activity against fungal isolates examined in the following study, which was concentration-dependent. | [63] | ||
| Agar well diffusion | Fruit mesocarp | Methanol | Ketoconazole [standard medicines], which had MICs of 2.66–2.99 g/mL, fungi ranged from 275.4 to 691/mL and from 346.7 to 318.2/mL, respectively. These findings show that the extract could be a source of chemicals that can be employed to fight microbial infection | [20] | ||
| Microdilution broth method | Leaf (essential oil) | The oil contains several chemicals that were active against the fungi tested, with minimum inhibitory concentrations ranging from >400 to 50 g/mL and might be used to produce plant-based medications. | [65] | |||
| In vivo | Seed | Aqueous | According to this study, seeds of G. kola are good against candida infection. In resource-constrained regions. | [104] | ||
| Agar-well diffusion method | Seeds (black nanocrystal of silver nanoparticles) | Aqueous | The fungal examined showed that the produced silver had good antibacterial action. Green nanoparticles can be employed in a variety of medicinal applications. | [72] | ||
| Agar Disc diffusion method | Seed | Aqueous | As a result, the findings imply that biogenic AgNPs have potential biological applications and might potentially be used as a key component in the development of innovative nanopaint against the tested fungal strains. | [68] | ||
| Disc | Seeds | C. albicans had no response to various concentrations used in the water extract. The ethanol extract was found to have significantly higher activity (p > 0.001) than the aqueous preparation. The presence of several pharmacokinetic substances could explain the activity. | [71] | |||
| Agar well diffusion | Seeds | Ethanol | On the fungal isolates, the extract has no inhibitory effect. | [85] | ||
| Checkerboard assay | Seeds | Ethanol | In comparison to their separate activities, the combined activities of the two extracts demonstrated a significant improvement in anti-Candida activity. The findings suggest using the ethanolic seeds extracts' with individual bioactive ingredients and combining them to create viable antifungal medicines. | [105] | ||
| 5 | Antiviral | In vivo | Aqueous | This research has found that the extract's ability to immediately remedy the patient's ocular symptoms and indicators is obvious and encouraging. | [21] | |
| 5 | Antihypertension | In vivo | Chloroform, methanol | In the third week, rats fed G. kola enriched diet showed a significant drop in blood pressure (p > 0.05). Finally, Garcinia kola includes a vasoactive component that can help to decrease blood pressure. However, the exact mechanism of action is still unknown. | [106] | |
| In vivo | After histaminergic blockage, however, there was a substantial (p < 0.05) decrease in the extract effect. According to this study, the alcohol extract of G. kola has a vasoactive component that lowers blood pressure. | [107] | ||||
| In vivo | The intraocular pressure of healthy young people was reduced by 21% after taking it orally. Patients with POAG or ocular hypertension in low-income settings may benefit from this effect. | [34] | ||||
| 6 | Anti-inflammatory | In vivo | Seed | When compared to aspirin, the anti-inflammatory potency of acetylsalicylic acid demonstrated rather good anti-inflammatory action. The greatest edema inhibition achieved in rats pretreated with 100 mg/kg kolaviron (59.52% ± 4.65) is comparable to that obtained with 150 mg/kg Aspirin (62.05 ± %3.75). | [108] | |
| Cell proliferation assay | Seed | G kola seeds appears to have the capacity to reduce mitogen-activated vascular cell proliferation as well as inflammatory responses. | [24] | |||
| In vivo | Seed | 70% methanol | In albino Wistar rats, the extract at dosages of 500, 1000, and 1500 mg/kg exhibited a statistically significant (p < 0.01) dose-dependent decrease of brewer's yeast-induced pyrexia. The findings reveal that the extracts had a strong antipyretic effect, indicating that their ethnomedicinal use is justified. | [109] | ||
| In vivo | Seeds | G. kola seeds appeared to provide clinically significant analgesic/anti-inflammatory benefits in knee osteoarthritis patients. | [110] | |||
| MTT assay | Seeds | Methanol | Treatment with 25, 50, and 100 g/mL inhibited cell proliferation in a dose- and time-dependent manner. The inclusion of chemicals with anti-inflammatory characteristics contributed to the study's findings | [23] | ||
| 7 | Antidiabetic | In vivo | Seeds | Ethanol | Compared to the controls, there was no significant difference (p > 0.05) in single-dose glucose levels, long-term HDL levels, or body weights. However, glucose (mmol/L) levels in the four-week treated rats were considerably lower (16.2 ± 2.9, 21.6 ± 3.6, 86.8 ± 18.2, 29.8 ± 10.9; p > 0.05) than in the controls (21.63.6), and LDL levels in the treated group were significantly lower by 66% (p > 0.01; 86.818.2 against 29.810.9). | [111] |
| In vivo | Kolaviron | At a dose of 100 mg kg1, kolaviron-linked biflavonoids effectively reduced hypoglycemic symptoms in normal and alloxan diabetic rabbits. | [112] | |||
| 8 | Analgesic | In vivo | Seeds | Ethanol | At all doses, there was a reduction in the number of writhes compared to control animals at p < 0.05. The seed has antianalgesic properties. | [27] |
| In vivo | Seed | The findings reveal that the chemical has antinociceptive activity against acetic acid-induced abdominal constriction in mice in a dose-dependent manner. | [108] | |||
| 9 | Antipneumonia | Seeds | With a drop in the concentration of seeds, anti-Klebsiella pneumonia activity increased. The seed was effective at 500 mg/kg and exhibited significance at p < 0.0001. | [28] | ||
| 10 | Antiobesity | In vivo | Seeds | Ethanol | The results revealed a considerable rise in the counts of RBCS in both tested animals, as well as a reduction in their weight. Very low-level density lipoprotein in the plasma was reduced in the dependent-dose approach, while the level of chylomicrons increased in a dependent-dose approach. Low levels of high-density lipoproteins and an increase in low-density lipoproteins play a role in cardiovascular disease. | [113] |
| 11 | Fertility evaluation | In vivo | Seeds | For 28 days, animals were grouped into 4. 100, 200, and 400 mg/kg body extracts were given in groups 2, 3, and 4, respectively. A solution of normal saline was given to the control group. When comparing serum levels of LH and testosterone in rats treated with bitter kola extract to those in the control group, a dose-dependent drop was detected at p < 0.05. On the other hand, the extract did not affect FSH levels in the blood at any of the amounts tested. This finding revealed that bitter kola could lower fertility in male Wistar rats. | [33] | |
| In vivo | Ethanol | Experimental models were grouped into three: groups 1 and 2 were given extracts orally at doses of 400 and 200 mg for 28 days, respectively, while group 3 was the control. Group 1 exhibited modest interstitial congestion, disorientation of the cells, whereas group 2 had a normal interstitial space with the regeneration of the germinal epithelium and a small number of matured spermatozoa, according to the study. As a result, this research implies that excessive intake may have a more negative impact on sperm parameters and testis shape. | [32] | |||
| In vivo | Seed | Ethanol | The extract has been shown to have an antispermatogenic effect in male Wistar rats. It may be harmful to male reproductive health, necessitating managing its intake rate. | [114] | ||
| In vivo | Seed | Aqueous | In a dose-dependent manner, the extract reduced sperm motility, concentration, and viability and affected normal sperm cell morphology. | [115] | ||
| In vitro | Seeds | Methanol | The seeds extract had dose-dependent effects on induced cholinergic contractions and spasms generated by cumulatively raised concentrations of barium chloride and acetylcholine. | [116] | ||
| 12 | Antitrypanosoma | In vivo | Seeds | 50 and 100% and methanol | Except for the group given 600 mg/kg body weight per day of 50% of the extract, which had a very low parasite count for nearly four months after treatment was terminated, but all treated died. | [35] |
| In vivo | Seeds | Ethanol | This study found the extract and its alkaloid, flavonoid, and saponin fractions, at 50 and 100 mg/kg, have anti-Trypanosoma brucei activity. | [117] | ||
| Anticancer | Seed/essential oil | n-hexane | At an 8.3 mg/mL dosage, this essential oil showed significant anticancer activity against MCF-7, A549, and Hela cell lines, with inhibition of 96, 0.9, 98, 0.5, and 94%, respectively. | [45] | ||
| 13 | Ingestion | In vivo | Seeds | Ethanol | The findings revealed erythrocyte count, PCV, and hemoglobin concentration values had all reduced dramatically. This demonstrates that it is an active ingredient and has no long-term toxicological implications when tested on mammalian erythrocytes. | [118] |
| 14 | Geotactic behavior | In vivo | Seeds | In flies fed a diet enriched with higher G kola seed inclusions, GST and catalase activities were dramatically boosted, whereas NO content was significantly reduced compared to controls. | [42] | |
| 15 | Steroid hormones | In vivo | Seed | 70% Ethanol | These findings suggest that they play a role in regulating cortisol, potassium, and sodium secretion control. Despite the possible benefits, it should be used with caution because it is a depressive. | [37] |
| 16 | Growth promoter | In vivo | Contain chemicals that reduce feed intake and growth performance. The effect appears to get stronger as the concentration gets higher. However, because the RBC and WBC levels increased, it is recommended that G. kola seeds be used in small doses or sporadically rather than continuously. | [119] | ||
| In vivo | Seeds | The inclusion of G. kola in the feed resulted in the highest feed efficacy. The study found no evidence of mortality. | [120] | |||
| In vivo | Seeds | There were no variations in the moisture, protein, or ash content of the fish carcasses between the treatments (p > 0.05). The findings imply that feeding C. gariepinus fingerlings G. kola seed powder increased growth rate, feed utilization, and survival. | [121] | |||
| In vivo | Seed | Ethanol | The growth parameters and the food conversion ratio showed significant differences at p > 0.05. The fish fed a 1.0 g/kg ethanolic extract diet gained the most weight compared to the other treatments. This supports the probiotic benefits of the plant as a growth promoter. | [122] | ||
| In vivo | Seeds | There are no differences in any performance indicators assessed between birds treated with BK 5 and those treated with BK 10. Birds on BK 5 showed greater FW, WG, and ADWG (p > 0.05). At a 5 g/kg diet, sun-dried ground bitter kola could be utilized as a supplement in broiler diets. | [123] | |||
| 17 | Liver injury | In vivo | 70% ethanol | The findings showed that combining the two plants had a therapeutic effect on the wounded liver's repair. This supported its long-standing use in the treatment of liver-infected patients. | [124] | |
| 18 | Haematological evaluation | In vivo | Seed | Aqueous | These data imply that it has no negative effects on the liver's activity and may have a favorable effect, as evidenced by its ability to reduce serum total cholesterol content and boost WBC count significantly. | [41] |
| In vivo | Seeds | Ethanol | As a result, this extract has a minor erythropoietic impact, but a moderate leucopenia characterized by lymphocytosis but a decrease in all other WBC lines. | [125] | ||
| In vivo | Seed | The result revealed that the meal increases the number of lymphocytes in rabbit bucks, which lead to an increase in total white blood cell count. Serum biochemical features revealed possible modest organ degeneration, as evidenced by a substantial (p > 0.05) increase in aspartate amino transaminase (AST) and alanine amino transaminase (ALT) in rats fed Garcinia kola seed meal diets. | [126] | |||
| In vivo | Seed | Ethanol | The extract reduced the volume of the cell, mean cell, and hemoglobin cell mean in the animals' plasma substantially (p > 0.05). As a result of its antioxidant properties, the ethanolic extract of G. kola seed has hematological, stimulating, and boosting effects. | [40] | ||
| In vivo | Seed | Ethanol | White blood cells proliferated significantly in this study, with a p > 0.05. Given the critical function that white blood cells play in the body's immune defense mechanism in organisms, this most likely explains the antibacterial activity of ethanolic extracts of plants. | [122] | ||
| 19 | Cytotoxicity | In vivo | Seeds | Compared to control flies, the high concentration of the plant in the diet dramatically reduced the survival rate of the experimental model. These findings could be linked to the bioactivity of G. kola seed compounds, including saponins and glycosides, which are hazardous at high concentrations. As a result of this research, it appears that excessive use of G. kola seeds may have toxicological consequences and that moderate consumption is therefore advised. | [42] | |
| Median lethal dose (LD50) | Stem bark | Methanol | The extract did not appear to have any significant toxicological effects on the erythrocytes, although it did exhibit a propensity to increase the number of erythrocytes over time. | [127] | ||
| In vivo | Seed | CdCl2 dramatically reduced the number of spermatozoa in the seminiferous tubules, resulting in decreased spermatogenesis, sperm counts, and histopathology. | [128] | |||
| In vivo | Seeds | Ethanol, aqueous | Compared to the control, the results demonstrated that neither of the medicinal plant extracts had any significant deleterious effects on total protein or glutamate pyruvic transaminase at p > 0.05. | [86] |
Note. MIC: Minimum Inhibitory Concentration, MBC: Minimum Bacterial Concentration, DPPH: 2,2-diphenyl-1-picrylhydrazyl, FRAP: Ferric Reducing Antioxidant Power (FRAP) Assay.
3.3. Antioxidant
The presence of free radicals and reactive oxygen species refers to oxidative stress, which is produced under normal human physiological activity but are harmful when not removed [11]. Kolaviron appears to be as effective as BHA as an in vivo natural antioxidant and an effective hepatoprotective agent in the current study [12]. These data suggested that G. kola seeds might be beneficial in minimizing the oxidative damage caused by chronic ethanol therapy in the livers of Wistar rats. The phenolic content of the antioxidant was found to be between 10 and 21 mg·g−1, and the scavenging was found to be between 26% and 55%, indicating that it will serve as a reservoir of natural antioxidants and be used as food enhancers [13]. Using the radical trapping test and the ion conversion method, we revealed that Ci 50 (65.86–1.17 g/mL) and the reducing power of the Ferric ion (125.4–4.91 mg/mL) are statistically significant [14]. There was a substantial increase in total white blood cell count but not in hemoglobin (p > 0.05). These data suggest that the seeds have immune-stimulatory capabilities, which could support the claims of ethnomedicinal efficacy (Table 1). All antioxidant biological evaluations carried out were found to exhibit significant activity irrespective of the method (Table 1).
3.4. Antibacterial
Consistent use of synthetic antibiotics is the leading cause of resistance in bacteria, which can be connected with biological phenomena such as membrane permeability, mutations, physiochemical changes, and efflux dynamics in target microorganisms [15]. In comparison to other microbes, bacterial strains have the genetic potential to rapidly acquire and transfer resistance to routinely used antibiotics [15]. Antibacterial medication resistance is becoming a critical global problem, prompting researchers to look for novel compounds with antibacterial properties and the potential to be used as raw materials in developing new treatments [16]. Some bacterial strains were isolated from tooth caries; therefore, the fraction of ethyl acetate hexane had the highest inhibitory activity against Streptococcus viridans and Streptococcus mutans at 0.33 and 0.33 mg·mL−1, respectively [17]. It is commonly used to treat toothache and prevent dental cavities, proving the traditional herbalist's claim [17]. The extracts showed an inhibitory effect on the test isolates, likely due to the high tannin and flavonoid content (Table 1). The test strains were shown to have antibacterial activity. The highest spectrum activity was seen against S. mutans and Bacillus subtilis at a low dosage of 1.25 mg/mL. (l4&l2 mm) [18]. Above all, our research indicates that the seed possessed antimicrobial properties. According to the findings, consuming the seed in a controlled manner may help to prevent bacterial infections in the intestine (Table 1). According to the review, the antibacterial potentials of plant extracts have been widely investigated (Table 1). The ethnobotanical research showing the traditional therapeutic potential of plant parts were confirmed in this review. According to the reported research in the following studies, all extracts tested against the tested bacterial strains, whether from human, animal, or other sources, strongly inhibited growth at a high inhibition zone (Table 1).
3.5. Antifungal
The utilization of plant extracts as sources for developing novel antifungal medicines has long been practiced. Plant-based medicines have considerably enhanced human health and well-being. The extracts also had antifungal efficacy against Aspergillus niger. Compared to the standard antibiotics used in the investigation, the data show that the compound has substantial antifungal properties [19]. In a fungistatic approach, the seed extract exhibits high action against Candida albicans and Aspergillus flavus (Table 1). The MICs of ketoconazole [standard medications], which had a range of 275–691/mL and 346–318/mL, respectively, the fungus ranged from 275–691/mL and 346–318/mL. These findings suggest that the extract may include compounds that can combat microbial illness [20].
3.6. Antiviral
This research has found that the extract's ability to immediately remedy a patient's ocular symptoms and indicators is obvious and encouraging (Table 1). Given the lack of a particular antiadenoviral medication on the market, this could be a game-changer in treating these viral infections [21]. According to this study, G kola is effective against viral infection and in areas where resources are scarce (Table 1).
3.7. Antihypertension
Hypertension, well-known as high blood pressure, is considered by persistently excessive blood pressure in the arteries [22]. High blood pressure can damage arteries supplying blood to the kidneys, heart, brain, and eyes [22]. The blood pressure of rats fed G kola enriched meals dropped significantly by the third week at p < 0.05. Finally, G. kola contains a vasoactive component that can reduce blood pressure. However, the actual method of action is still unknown (Table 1). Traditional medicinal practitioners have always advocated for using G. kola parts to treat hypertension. The findings of the following studies bring up new research options for new antihypertensive drugs or herbal formulations. Plant-based treatments are considered effective.
3.8. Anti-Inflammatory
Inflammation is the body's natural response to damage or foreign irritation. Inflammation, marked by pain, has been known to humanity since the dawn of time. Since the dawn of time, humans have been looking for ways to reduce and manage inflammation, including using plants [1]. Treatment with 25, 50, and 100 g/mL inhibited cell proliferation in a dosage and time-dependent approach. The inclusion of chemicals with anti-inflammatory characteristics contributed to the study's findings [23]. It could be beneficial in conditions marked by cellular proliferation and inflammatory reactions [24].
3.9. Antidiabetic
Diabetes mellitus is a metabolic condition characterized by hyperglycemia, the most prevalent symptom. Its chronic stage impacts blood vessels, kidneys, the heart, and nerves [25]. Diabetes affects 463 million people worldwide, and that number is expected to rise to 578 million by 2030 [25]. At a dose of 100 mg·kg1, kolaviron linked bioflavonoids effectively reduced hypoglycemic symptoms in normal and alloxan diabetic rabbits (Table 1). Compared to the controls, there was no significant change (p > 0.05) in single-dose glucose levels, long-term HDL levels, or body weight. However, glucose (mmol/L) levels in the four-week treated rats were significantly lower (16.22.9; p > 00.05) than in the controls (21.63.6), and LDL levels were 66% lower in the treated group (p < 0.01; 86.818.2 against 29.810.9) (Table 1). On day 7, the 500 mg/kg ethanolic seeds extract-treated group had a 49.70% drop in blood glucose levels compared to the positive control group (45.03%). The findings of this investigation suggested that the seed could be used to treat illnesses and diabetic management [26]. The results mentioned above validate the usage of the plants in the traditional medicinal system to treat diabetes by traditional practitioners.
3.10. Antianalgesic
Controlling acute and chronic pain has become a serious concern, particularly among the elderly. Pain is a nonspecific symptom of many diseases that lead to unpleasant emotional and sensory experiences. The findings show that the chemical possesses dose-dependent antinociceptive properties against acetic acid-induced abdominal constriction in mice (Table 1). At all doses, there was a reduction in the number of writhes compared to control animals at p < 0.05. The seed has antianalgesic properties [27]. The studies examined in the following study found the extract from bitter kola exhibited strong antianalgesic properties.
3.11. Antipneumonia
Pneumonia is an inflammatory, infectious lung disease condition that affects the mucosal parts of the lungs and can be acute and persistent [28]. Fungi, bacteria, and viruses cause the disorder. Anti-Klebsiella pneumonia activity rose when kolaviron concentrations dropped. Kolaviron was efficacious at 500 mg/kg and showed a significant difference at p < 0.0001. Bitter kola can treat pneumonia because it contains antimicrobial properties (Table 1).
3.12. Antiobesity
Obesity is a complicated health condition classified as a chronic disease that has a detrimental impact on the human body [29]. Obesity raises the risk of diabetes, hypertension, heart disease, and other serious illnesses. Obesity cases are increasing at an alarming rate worldwide [30]. There are currently more than 300 million obese people on the planet [31]. The results revealed a considerable rise in the counts of RBCS in both tested animals, as well as a reduction in their weight. Very low-level density of lipoprotein in the plasma was reduced in the approach of dependent dose, while the level of chylomicrons increased in a dependent-dose approach. Low levels of high-density lipoproteins and an increase in low-density lipoproteins play a role in cardiovascular diseases (Table 1).
3.13. Fertility Evaluation
Medicinal plants have long been used to boost or manage fertility. The experimental model was divided into three groups: groups 1 and 2 received the extracts orally at doses of 400 and 200 mg for 28 days, respectively, while group 3 served as a control group. According to the study, group 1 had slight interstitial congestion disorientation of the cells, whereas group 2 had a normal interstitial space with germinal epithelium regeneration and a small number of matured spermatozoa. As a result, this study suggests that a high-calorie diet could have a deleterious impact on sperm parameters and testis shape [32]. This discovery demonstrated that bitter kola could reduce fertility in male Wistar rats [33]. The extract has been proven to have an antispermatogenic effect. It can damage the male reproductive organs, necessitating controlling the amount consumed (Table 1).
3.14. Antiglaucoma
Everywhere across the globe, glaucoma is the most common cause of permanent blindness [34]. The most prevalent kind of primary open-angle glaucoma (POAG) is characterized by progressive optic nerve degeneration and affects over 60 million individuals worldwide. In the African continent, 15% of blindness was due to glaucoma [34]. After taking it orally, healthy young people's intraocular pressure was lowered by 21%. In low-income settings, patients with POAG or ocular hypertension may benefit from such an effect (Table 1).
3.15. Antitrypanosome
Humans and animals are both affected by trypanosomiasis, a parasite disease. Trypanosoma is a parasite species that causes the disease. More than 50 million individuals and more than 50 million animals are infected worldwide [35]. Only the experimental model that received the dose of 600 mg/kg per day of their body weight, which got a very minimal parasite total for nearly four months after therapy, was terminated. Yet, all those who were on it died (Table 1).
3.16. Ingestion
The results revealed that the erythrocyte count, PCV, and hemoglobin concentration had all dropped significantly. When evaluated on mammalian erythrocytes, this shows that the active component has no long-term toxicological effects (Table 1).
3.17. Geotactic Behavior
All living species have an inbuilt behavioral response called geotaxis, defined by motor actions toward or away from the Earth. Flying animals, in particular, have a lot of negative geotaxis against Earth's gravity [36]. In flies fed a diet enriched with higher G. kola seed inclusions, GST, and catalase activities were dramatically boosted, whereas no content was significantly reduced compared to controls (Table 1).
3.18. Steroid Hormones
These data imply that the seed extract plays a function in cortisol, potassium, and sodium secretion regulation (Table 1). Despite its potential benefits, it should be used with caution because it is a depressive drug [37]. These data imply that plays a function in cortisol, potassium, and sodium secretion regulation. It should be used with caution because it is a depressant (Table 1).
3.19. Growth Performance
The moisture, protein, and ash content of the fish carcasses did not differ across the treatments (p > 0.05). The data suggest that feeding G. kola seed powder to Clarias gariepinus fingerlings boosted growth rate, feed utilization, and survival (Table 1). At p > 0.05, there were significant variations in the growth metrics and the food conversion ratio. Compared to the other treatments, the fish given 1.0 g/kg ethanolic seed extract diets gain the most weight. This supports the plant's probiotic advantages as a growth promoter (Table 1).
3.20. Healing of Liver Injury
The liver is a vital organ in our body responsible for most metabolic and secretory functions. As a result, it appears to be a sensitive target for drugs that modulate biotransformation [38]. The duration or persistence of a liver injury is arbitrarily split into acute and chronic liver injury in clinical practice [39]. The researchers discovered that combining the two plants had a therapeutic effect on the healing of the injured liver. This backed up its long-standing usage in treating individuals with liver infections (Table 1). The plant has the potential to be utilized in the development of drugs for liver treatment.
3.21. Hematological Analysis
As a result, the aqueous seed extract has a minimal erythropoietic effect but causes moderate leucopenia with lymphocytosis and a decrease in all other WBC lines (Table 1). The extract significantly decreased the volume of the cell mean cell and hemoglobin cell means in the plasma of the animals (p < 0.05). The ethanolic extract of G kola seed has hematological, stimulating, and enhancing effects due to its antioxidant qualities [40]. These findings suggest that it has no harmful effects on the liver's function and may have a beneficial effect, as indicated by its capacity to drastically lower total serum cholesterol and increase WBC count [41].
3.22. Cytotoxicity
Many plant-derived chemicals have now been shown to have antibacterial, anticancer, and other biological properties [129]. Parts of medicinal plants are considered the reservoir of a novel compound with a therapeutic potential to treat a wide array of diseases compared to the synthetic drugs available [130]. Many studies have proven that medicinal plants contain a wide array of compounds that have a positive biological effect [8, 11]. These components are only beneficial if they are confirmed to be nontoxic or have minimal toxicity. Quite a number of studies have been carried out on the toxicity of G. kola parts (Table 1) both in vivo and in vitro. Higher dietary intake of G. kola seeds drastically lowered the survival rate of D. melanogaster compared to control flies [42]. These findings could be linked to the bioactivity of G. kola seed components such saponins and glycosides, both of which are hazardous in large doses. The extract did not appear to have any substantial toxicological effects on erythrocytes, although it did tend to increase erythrocyte amount over time [127]. The results showed that neither medicinal plant extract had any significant negative effects on total protein or glutamate pyruvic transaminase at p > 0.05 compared to the control [86]. Garcinia kola has modest toxicity, with an oral 50% fatal dose of over 5000 mg/kg bw [52]. Based on the study's findings, excessive usage of G. kola seeds may have toxicological implications, and moderate use is consequently recommended.
3.23. Chemical Compounds Responsible for the Biological Activity
Due to the presence of tannin in the plant, it could be used to cure burns and wounds [131]. The plant's high alkaloid and flavonoid content suggest that they have antioxidant potential and explain their medicinal activities, which might be exploited in drug formulation [131]. The presence of large levels of flavonoids in all plant parts demonstrated that the plants perform biological tasks such as protecting against allergies, free radicals, microbes, ulcers, inflammation, hepatotoxins, and viruses (Figure 2). Natural compounds, including garcinoic acid, garcinol, and tocotrienol extracted from the seed of G. kola from Nigeria, have 1.5 times the antioxidant activity of a-tocopherol [52]. The ME4 fraction was chromatographically fractionated and spectroscopically analyzed, revealing the presence of some compounds: Garcinia biflavonoids 1, Garcinol and Garcinoic acid (Figure 2). These findings suggest that these four chemicals are responsible for some of G. kola seeds' high antioxidant activity. This adds to the evidence of G. kola's nutraceutical and medicinal potentials [132]. The ability of a plant extract to inhibit bacteria, particularly those with substantial health implications, is mainly dependent on essential phytochemical components having antimicrobial activity [53]. The presence of a wide range of chemicals in extracts from various plant sections has been linked to their pharmacological properties [53]. The following compounds were reported present in the essential oil extracted from the seed 9-Octadecenoic acid methyl ester, 9,12-Octadecadienoic acid (Z, Z), Stearic acid methyl ester, and Hexadecanoic acid methyl ester; they are reported to be responsible for antibacterial, antioxidant, and many more pharmacological properties (Figure 2). Research uncovered G. kola was discovered to possess numerous chemical components that have antioxidant properties [133]. Benzophenones, flavonoids, and xanthenes are among the components found in G. kola (Figure 2). They are known to have antiparasitic, anti-inflammation, antibacterial, and antiviral activities [110]. The anti-inflammatory action of the seed is considered due to the presence of flavonoids and benzophenone [134].
Figure 2.
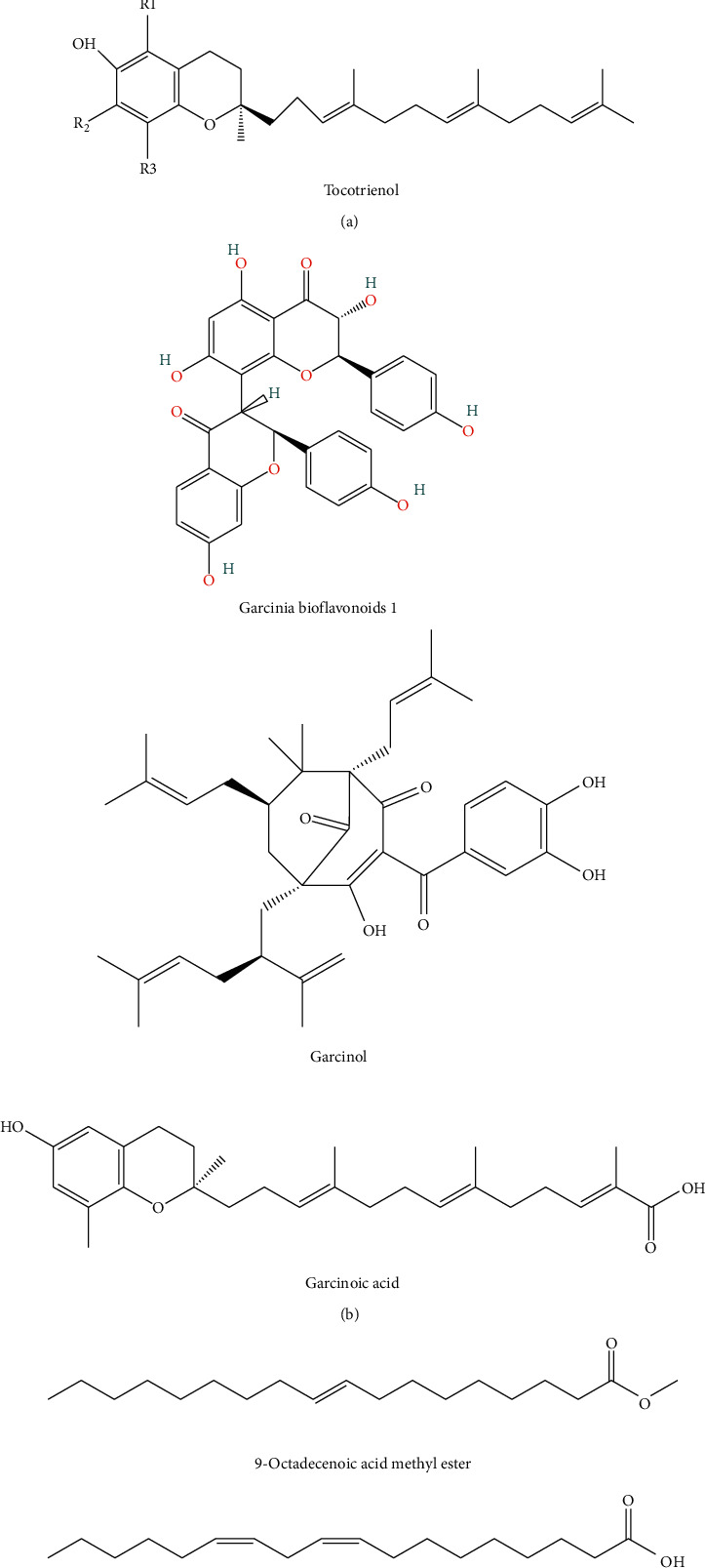
Some of the chemical structures found in G. kola are responsible for its biological activity.
4. Conclusion and Future Recommendations
Research into the pharmacological benefits of medicinal plants provides us with critical knowledge for better organizing current and future studies to address a variety of human illnesses. G. kola is a remarkable medicinal plant with a variety of traditional usage that has been documented since antiquity. Preclinical investigations have already been conducted on a variety of biological activities. The seeds were found to have significant biological activity, and this is due to the G. kola containing nutritionally and pharmacologically essential compounds. Research into the mechanisms behind the bioactivity of the constituent chemical components is required. As a result, well-designed clinical trials are recommended to obtain more conclusive evidence about the usefulness of G. kola seeds.
Acknowledgments
Heartfelt gratitude and admiration is expressed to Professor Dr. Nashriyah Mat and Professor Dr. Abdul Manaf Ali for their support and guidance in my life.
Data Availability
Data are available within the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Mahmoud Dogara Abdulrahman, Saber W. Hamad, Harmand A. Hama, Sarwan W. Bradosty, Soran Kayfi, Sawsan S. Al-Rawi, and Abubakar Abdullahi Lema contributed equally to data search, analysis of the retrieved data, and drafting of the manuscripts.
References
- 1.Mahmoud A. D., Abba A. Ethnomedicinal survey of plants used for management of inflammatory diseases in Ringim local government, Jigawa state, Nigeria. Ethnobotany Research and Applications . 2021;22:1–27. [Google Scholar]
- 2.Dogara A., Labaran I., Hamad S. W., Lema A. A., Jakada B. H. Traditional medicinal plants used for the treatment of cancer in Mubi, Adamawa state, Nigeria. Al-Qadisiyah Journal of Pure Science . 2021;26(4):258–268. doi: 10.29350/qjps.2021.26.4.1423. [DOI] [Google Scholar]
- 3.Dogara A., Hamad S. W., Usman M., Tahir S. M., Sunusi N., Yunusa A. Therapeutic plants used for typhoid fever treatment in Kaduna state, Nigeria. Al-Qadisiyah Journal of Pure Science . 2021;26(3):9–21. doi: 10.29350/qjps.2021.26.4.1432. [DOI] [Google Scholar]
- 4.Kayfi S., Abdulrahman M. D. Ethnopharmacology of plants in Choman, the Kurdistan region of Iraq. Applied Biological Research . 2021;23(4):322–330. doi: 10.5958/0974-4517.2021.00042.2. [DOI] [Google Scholar]
- 5.Abdulrahman M. D., Ali A. M., Fatihah H., Khandaker M. M., Mat N. Traditional medicinal knowledge of Malays in Terengganu, Peninsular Malaysia. Malayan Nature Journal . 2018;70(3):349–364. [Google Scholar]
- 6.Huft M. The world flora online. 2021. https://www.worldfloraonline.org/taxon/wfo-4000038758 .
- 7.Useful tropical plants database. 2019. https://tropical.theferns.info .
- 8.Abdulrahman M. Antioxidant, alpha-glucosidase and antibacterial evaluation of Syzygium mytifolium (Roxb.) Walp. Plant Science Today . 2021;8(2):410–415. doi: 10.14719/pst.2021.8.2.1113. [DOI] [Google Scholar]
- 9.Ullah R., Alqahtani A. S., Noman O. M. A., Alqahtani A. M., Ibenmoussa S., Bourhia M. A review on ethno-medicinal plants used in traditional medicine in the Kingdom of Saudi Arabia. Saudi Journal of Biological Sciences . 2020;27(10):2706–2718. doi: 10.1016/j.sjbs.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odhav B., Thangaraj K., Khumalo N., Baijnath H. Screening of African traditional vegetables for their alpha-amylase inhibitory effect. Journal of Medicinal Plants Research . 2013;4(14):1502–1507. [Google Scholar]
- 11.Abdulrahman M. D., Hasan Nudin N., Khandaker M. M., Ali A. M., Mat N. In vitro biological investigations on Syzygium polyanthum cultivars. International Journal of Agriculture and Biology . 2019;22(6):1399–1406. [Google Scholar]
- 12.Farombi E. O., Tahnteng J. G., Agboola A. O., Nwankwo J. O., Emerole G. O. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron-A Garcinia kola seed extract. Food and Chemical Toxicology . 2000;38(6):535–541. doi: 10.1016/s0278-6915(00)00039-9. [DOI] [PubMed] [Google Scholar]
- 13.Onyekwelu J. C., Oyewale O., Stimm B., Mosandl R. Antioxidant, nutritional and anti-nutritional composition of Garcinia kola and Chrysophyllum albidum from rainforest ecosystem of Ondo state, Nigeria. Journal of Forestry Research . 2015;26(2):417–424. doi: 10.1007/s11676-015-0068-2. [DOI] [Google Scholar]
- 14.Ban O. V. A., Djyh B. N., Bahi C., Adama D. B. K. Phytochemical screening and study of in vitro antioxidant activities of the aqueous extract and the alcoholic (koutoukou extract) of Garcinia kola seeds (Guttiferea) collected in Abidjan (Ivory coast) Journal of Applied Biosciences . 2019;147:15108–15116. [Google Scholar]
- 15.Osemwegie O. O., Nwonuma C. O., Oluyori A. P., et al. In vitro antimicrobial and in vivo lead acetate poison abatement study of Garcinia kola Heckel. Journal of Taibah University for Science . 2017;11(6):883–894. doi: 10.1016/j.jtusci.2017.06.001. [DOI] [Google Scholar]
- 16.Uhunmwangho E., Okhia O., Blackies H., Eruotor O., Uhunmwangho A. Antibacterial activities of bitter kola (Garcinia kola) on upper respiratory tract isolates from students of Ambrose Alli university students, Ekpoma, Nigeria. International Journal of Herbs and Pharmacological Research . 2014;3(4):80–83. [Google Scholar]
- 17.Ajayi T., Moody J., Adeyemi T., Fakeye T., Ngere L. Antimicrobial activities of Garcinia kola seeds extracts on dental caries-causing microorganisms. Planta Medica . 2008;74(9):74–257. doi: 10.1055/s-0028-1084255. [DOI] [Google Scholar]
- 18.Ugwuowo B., Ahmed A., Oluwasola H., Ukoha P. Comparative assessment of phytochemicals, antioxidant activity and antimicrobial activity of Cola acuminata, Garcinia kola and Vernonia amygdalina. Journal of Chemical Society of Nigeria . 2021;46(4):698–710. [Google Scholar]
- 19.Babandoko A. M., Ojo K. R. M., Elizabeth A. A., Kamoldeen A. A. Antimicrobial effects of Garcinia kola (bitter kola) on some selected pathogens from university of Ilorin teaching hospital Ilorin, Nigeria. Journal of Asian Scientific Research . 2012;2(4):159–169. [Google Scholar]
- 20.Jude A. I., Chekwube A. C., Hannah O. N. Phytochemical screening and antimicrobial activity of methanol extract of Garcinia Kola Heckle fruit mesocarp. Journal of Medicinal Plants Research . 2020;14(11):579–582. [Google Scholar]
- 21.Adefule-Ositelu A., Adefule A., Omilabu S. Clinical evaluation of ocular antiviral effect of Garcinia kolanut water extract in epidemic haemorrhagic keratoconjunctivitis in Lagos. Nigerian Quarterly Journal of Hospital Medicine . 2004;14(3):270–276. [Google Scholar]
- 22.Baharvand-Ahmadi B., Bahmani M., Tajeddini P., Rafieian-Kopaei M., Naghdi N. An ethnobotanical study of medicinal plants administered for the treatment of hypertension. Journal of Renal Injury Prevention . 2016;5(3):123–128. doi: 10.15171/jrip.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adedapo A., Omobowale T., Oyagbemi A., Yakubu M., Oyekan A. The methanol extract of Garcinia kola seed blunts lipopolyssacharide (LPS)-and angiotensin II-induced cell proliferation as well as nitric oxide production in in vitro vascular smooth muscle cells (VSMC) assay. FASEB Journal . 2015;29:773–786. doi: 10.1096/fasebj.29.1_supplement.773.6. [DOI] [Google Scholar]
- 24.Adedapo A. A., Omobowale T. O., Oyagbemi A. A., Yakubu M. A. The methanol seed extract of Garcinia kola attenuated angiotensin II- and lipopolyssacharide-induced vascular smooth muscle cell proliferation and nitric oxide production. Macedonian Veterinary Review . 2016;39(2):153–158. doi: 10.1515/macvetrev-2016-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdulrahman M. Ethnobotany of medicinal plants with antidiabetic potentials in Northern Nigeria. Eurasian Journal of Science and Engineering . 2021;7(1):46–58. [Google Scholar]
- 26.Omodamiro O. D., Ajah O., Ewa-Ibe C. Evaluation of antioxidant potential and anti-diabetic effect of ethanol seed extract of Garcinia kola (bitter kola) in albino rat. Journal of Medicinal Herbs and Ethnomedicine . 2020;6(2020):56–60. doi: 10.25081/jmhe.2020.v6.6091. [DOI] [Google Scholar]
- 27.Iniaghe L. O., Onyemaonyeoru A. I. Evaluation of the analgesic property of the ethanolic extract of Garcinia kola Heckel (Guttiferae) seeds in mice. Journal of Science and Practice of Pharmacy . 2015;2(1):46–50. [Google Scholar]
- 28.Calista D., Dozie N., Kingsley U., Catherine O., Felicia O. Effects of kolaviron on pneumonia-like infection induced in albino Wistar rats. Anti-inflammatory & anti-allergy agents in medicinal chemistry. Medicinal Chemistry . 2020;20(2):219–227. doi: 10.2174/1871523019666200915085729. [DOI] [PubMed] [Google Scholar]
- 29.Wyatt S. B., Winters K. P., Dubbert P. M. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. American Journal of the Medical Sciences . 2006;331(4):166–174. doi: 10.1097/00000441-200604000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Hossain P., Kawar B., El Nahas M. Obesity and diabetes in the developing world—a growing challenge. New England Journal of Medicine . 2007;356(3):213–215. doi: 10.1056/nejmp068177. [DOI] [PubMed] [Google Scholar]
- 31.Seidell J. C. Obesity, insulin resistance and diabetes—a worldwide epidemic. British Journal of Nutrition . 2000;83(S1):S5–S8. doi: 10.1017/s000711450000088x. [DOI] [PubMed] [Google Scholar]
- 32.Chikere O. U., Gloria O. C., Nnabuihe E. D., Uchechi E. E., Jesse A. C. The effect of ethanolic extract of Garcinia kola on the sperm parameters and histology of the testis of male Wistar rats. Advances in Life Science and Technology . 2015;33:18–25. [Google Scholar]
- 33.Airaodion A. I., Ekenjoku J. A., Ngwogu A. C., Ngwogu K. O., Megwas A., Ime A. Antaphrodisiac potential of bitter kola (Garcinia kola) seeds in male Wistar rats. International Journal of Bio-Science and Bio-Technology . 2020;12(3):36–43. [Google Scholar]
- 34.Ilechie A. A., Jeduah M. M., Abraham C. H., et al. Oral consumption of Garcinia kola (bitter kola) lowers intraocular pressure. Acta Ophthalmologica . 2020;98(8):1028–1033. doi: 10.1111/aos.14440. [DOI] [PubMed] [Google Scholar]
- 35.Ogbadoyi E. O., Kabiru A. Y., Omotosho R. F. Preliminary studies of the antitrypanosomal activity of Garcinia kola nut extract in mice infected with Trypanosoma brucei. Journal of Medicine and Medical Sciences . 2011;2(1):628–631. [Google Scholar]
- 36.Bae J. E., Bang S., Min S., et al. Positive geotactic behaviors induced by geomagnetic field in Drosophila. Molecular Brain . 2016;9(1):55–13. doi: 10.1186/s13041-016-0235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falana O., Smith O., Gazal O., et al. Effects of bitter cola (Garcinia cola) extract on steroid hormones and selected electrolytes in West African dwarf bucks. Indian Journal of Animal Research . 2013;47(4):273–282. [Google Scholar]
- 38.Roy A., Bhoumik D., Sahu R., Dwivedi J. Medicinal plants used in liver protection—a review. Pharmaceutical and Biosciences Journal . 2014;2(1):23–33. doi: 10.20510/ukjpb/2/i1/91143. [DOI] [Google Scholar]
- 39.Malhi H., Gores G. J. Cellular and molecular mechanisms of liver injury. Gastroenterology . 2008;134(6):1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atsukwei D., Daniel E., Odeh S., Adams M., Malgwi I., Olih P. Efficacy of Garcinia kola seed ethanolic extract on haematological parameters in male Wistar rats. Journal of Advances in Medical and Pharmaceutical Sciences . 2015;3(1):10–23. doi: 10.9734/jamps/2015/15305. [DOI] [Google Scholar]
- 41.Tamuno-Emine D., Ben-Chioma A., Uwakwe A. Effects of Garcinia kola seed on some haematological and serum biochemical parameters of Wistar albino rats. Pyrex Journal of Biomedical Research . 2015;1(4):29–32. [Google Scholar]
- 42.Oboh G., Ogunsuyi O. B., Ojelade M. T., Akomolafe S. F. Effect of dietary inclusions of bitter kola seed on geotactic behavior and oxidative stress markers in Drosophila melanogaster. Food Sciences and Nutrition . 2018;6(8):2177–2187. doi: 10.1002/fsn3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adaramoye O. A., Awogbindin I., Okusaga J. O. Effect of kolaviron, a biflavonoid complex from Garcinia kola seeds, on ethanol-induced oxidative stress in liver of adult Wistar rats. Journal of Medicinal Food . 2009;12(3):584–590. doi: 10.1089/jmf.2008.0138. [DOI] [PubMed] [Google Scholar]
- 44.Bukar B. B., Dayom D. W., Okpeke O. S., Ior L., Falang K. D., Uguru M. O. Evaluation of some metabolic activities and immuno-stimulatory potential of methanolic seed extract of Garcinia kola (Heckel) in female albino Wistar rats. African Journal of Pharmacy and Pharmacology . 2017;11(37):470–474. doi: 10.5897/ajpp2017.4839. [DOI] [Google Scholar]
- 45.Olatunde O. Z., Tian D., Yong J., Lu C. Chemical compositions of the essential oil extracted from the seeds of Garcina kola, and its biological activities. Biomedical and Pharmacology Journal . 2021;14(2):607–621. doi: 10.13005/bpj/2163. [DOI] [Google Scholar]
- 46.Aqanbi E., Kadiri H. E., Okoro I. O., Joel K., Aqboje S. E., Eze F. N. Antimicrobial and antioxidant properties of extracts of garcinia kola and ocimum gratissimum. Nigerian Journal of Life Sciences . 2017;7(2):16–28. [Google Scholar]
- 47.Joshua P. E., Ukegbu C. Y., Eze C. S., et al. Comparative studies on the possible antioxidant properties of ethanolic seed extracts of Cola nitida (kola nut) and Garcinia kola (bitter kola) on hydrogen peroxide induced oxidative stress in rats. Journal of Medicinal Plants Research . 2017;12(22):367–372. doi: 10.5897/jmpr2017.6346. [DOI] [Google Scholar]
- 48.Smith Y. A., Adanlawo I. In vitro and in vivo antioxidant activity of saponin extracted from the root of Garcinia kola (bitter Kola) on alloxan-induced diabetic rats. World Journal of Pharmacy and Pharmaceutical Sciences . 2014;3(7):8–26. [Google Scholar]
- 49.Okoko T. In vitro antioxidant and free radical scavenging activities of Garcinia kola seeds. Food and Chemical Toxicology . 2009;47(10):2620–2623. doi: 10.1016/j.fct.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 50.Ogunmoyole T., Olalekan O., Fatai O., Makun J., Kade I. Antioxidant and phytochemical profile of aqueous and ethanolic extract of Garcinia kola. Journal of Pharmacognosy and Phytotherapy . 2012;4(5):66–74. [Google Scholar]
- 51.Abubakar A., Olorunkemi M. R., Musa B., Hamzah R. U., Abdulrasheed-Adeleke T. Comparative in vitro antioxidant activities of aqueous extracts of Garcinia kola and Buchholzia coriacea seeds. Tanzania Journal of Science . 2020;46(2):498–507. [Google Scholar]
- 52.Farombi E. O., Adedara I. A., Oyenihi A. B., Ekakitie E., Kehinde S. Hepatic, testicular and spermatozoa antioxidant status in rats chronically treated with Garcinia kola seed. Journal of Ethnopharmacology . 2013;146(2):536–542. doi: 10.1016/j.jep.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 53.Stephen F., Awoyinka A., Oluchi O., et al. Evaluation of the nutraceutical potential of Garcinia kola seed oil. Journal of Pharmacognosy and Phytochemistry . 2017;6(5):1894–1901. [Google Scholar]
- 54.Farombi E. O., Akanni O. O., Emerole G. O. Antioxidant and scavenging activities of flavonoid extract (kolaviron) of Garcinia kola seeds. Pharmaceutical Biology . 2002;40(2):107–116. doi: 10.1076/phbi.40.2.107.5838. [DOI] [Google Scholar]
- 55.Nworah I., Umeaku C. Antimicrobial activities of Vernonia amygdalina, Ocimum gratissimum and Garcinia kola. International Journal of Research in Medical and Basic Sciences . 2016;6(2):1–12. [Google Scholar]
- 56.Kigigha L. T., Selekere R. E., Izah S. C. Antibacterial and synergistic efficacy of acetone extracts of Garcinia kola (bitter kola) and Buchholzia coriacea (wonderful kola) Journal of Basic Pharmacology and Toxicology . 2018;2(1):13–17. [Google Scholar]
- 57.Nwaokorie F., Coker A., Ogunsola F., et al. Antimicrobial activities of Garcinia kola on oral Fusobacterium nucleatum and biofilm. African Journal of Microbiology Research . 2010;4(7):509–514. [Google Scholar]
- 58.Obire O., Ogbonna S. I. Antimicrobial activity of some seed extracts on bacteria isolated from Maize slurry (Akamu) in Port Har-court metropolis. Science and Technology . 2017;4(1):188–202. [Google Scholar]
- 59.Ewelike N. C., Okammadu J. C., Ogwudire V. E., Nnadozie R. I. In-vitro antimicrobial activity of methanolic and aqueous leaf extracts of Chrysophyllum albidum (African star apple) and Garcinia kola (bitter kola) GSC Biological and Pharmaceutical Sciences . 2021;14(3):249–253. doi: 10.30574/gscbps.2021.14.3.0092. [DOI] [Google Scholar]
- 60.Adegboye M., Akinpelu D., Okoh A. The bioactive and phytochemical properties of Garcinia kola (Heckel) seed extract on some pathogens. African Journal of Biotechnology . 2008;7(21):3934–3938. [Google Scholar]
- 61.Mboto C., Eja M., Adegoke A., et al. Phytochemical properties and antimicrobial activities of combined effect of extracts of the leaves of Garcinia kola, Vernonia amygdalina and honey on some medically important microorganisms. African Journal of Microbiology Research . 2009;3(9):557–559. [Google Scholar]
- 62.Uju D. E., Obioma N. P. Anticariogenic potentials of clove, tobacco and bitter kola. Asian Pacific journal of Tropical Medicine . 2011;4(10):814–818. doi: 10.1016/s1995-7645(11)60200-9. [DOI] [PubMed] [Google Scholar]
- 63.Aderibigbe S. A. Antimicrobial activities of Garcinia kola seed oil against some clinical microbial isolates. International Research Journal of Pharmacy . 2012;2(3):68–72. [Google Scholar]
- 64.Sabo I., Adamu S., Imarenezor E., Oko I. Antibiogram of Garcinia kola seeds extract on some selected enteric bacteria. Trends in Science and Technology Journal . 2020;5(3):809–812. [Google Scholar]
- 65.Okhale S. E., Buba C. I., Oladosu P., et al. Chemical constituents and antimicrobial activity of the leaf essential oil of Garcinia kola Heckel (Clusiaceae) from Nigeria. Chemical Science International Journal . 2016;13(5):1–7. doi: 10.9734/acsj/2016/24019. [DOI] [Google Scholar]
- 66.Madubunyi I. I. Antimicrobial activities of the constituents of Garcinia kola seeds. International Journal of Pharmacognosy . 1995;33(3):232–237. doi: 10.3109/13880209509065369. [DOI] [Google Scholar]
- 67.Akerele J., Obasuyi O., Ebomoyi M., Oboh I. Antimicrobial activity of the ethanol extract and fractions of the seeds of Garcinia kola Heckel (Guttiferae) African Journal of Biotechnology . 2008;7(2):169–172. [Google Scholar]
- 68.Adelere I. A., Babayi H., Aboyeji D. O., Adabara N. U., Jagaba A., Sunday H. Z. Antimicrobial activities and evaluation of biogenic silver nanoparticles as antimicrobial additive in paint. Journal of Science, Technology, Mathematics and Education . 2021;17(1):24–35. [Google Scholar]
- 69.Ugwu C., Ezeonu I., Mbah-Omeje K., Agu C., Onuorah S. Evaluation of the antimicrobial effects of Syzygium aromticum (clove) and Garcinia kola (bitter kola) extracts singly and in combination, on some bacteria. World Journal of Pharmacy and Pharmaceutical Sciences . 2017;6(12):1–13. [Google Scholar]
- 70.Ofokansi K., Mbanefo A., Ofokansi M., Esimone C. Antibacterial interaction of crude methanol extract of Garcinia kola seed with gatifloxacin. Tropical Journal of Pharmaceutical Research . 2008;7(4):1159–1165. doi: 10.4314/tjpr.v7i4.14702. [DOI] [Google Scholar]
- 71.Chukwuezi Fabian O., Ugwu Okechukwu P. Antimicrobial effects of bitter kola (Garcinia kola) nut on Staphylococcus aureus, Eschererichia coli and Candida alibicans. Journal of Dental and Medical Sciences . 2014;13(4):29–32. [Google Scholar]
- 72.Hassan L. A., Elijah A. T., Ojiefoh O. C., et al. Biosynthesis of silver nanoparticles using Garcinia kola and its antimicrobial potential. African Journal of Pure and Applied Chemistry . 2016;10(1):1–7. [Google Scholar]
- 73.Indabawa I., Arzai A. Antibacterial activity of Garcinia kola and Cola nitida seed extracts. Bayero Journal of Pure and Applied Sciences . 2011;4(1):52–55. doi: 10.4314/bajopas.v4i1.11. [DOI] [Google Scholar]
- 74.Hussain R., Owegby A., Parimoo P., Waterman P. Kolanone, a novel polyisoprenylated Benzophenone with antimicrobial properties from the fruit of Garcinia kola. Planta Medica . 1982;44(02):78–81. doi: 10.1055/s-2007-971406. [DOI] [PubMed] [Google Scholar]
- 75.Ebana R., Edet U., Ekanemesang U., et al. Comparison of antimicrobial activity and phytochemical screening of seeds and testas of Dacryodes edulis and Garcinia kola. Journal of Advances in Microbiology . 2016;1(3):1–7. doi: 10.9734/jamb/2016/31173. [DOI] [Google Scholar]
- 76.Emmanuel N., Ifeanyichukwu I., Chika E., Emmanuel E., Chinwe N. Inhibitory effects of neem (Azadirachta indica Linn.) and bitter kola (Garcinia kola Heckel) leaves on selected pathogenic bacteria. African Journal of Pharmacy and Pharmacology . 2013;7(41):2763–2767. doi: 10.5897/ajpp2013.3852. [DOI] [Google Scholar]
- 77.Amala S. E., Nweke S. N., Nwalozie R., Monsi T. P. Antimicrobial properties and phytochemical composition of Garcinia kola, Bryophyllum pinnatum, and Allium sativum juices on some clinical pathogens. Advances in Bioscience and Biotechnology . 2021;12(11):388–406. doi: 10.4236/abb.2021.1211025. [DOI] [Google Scholar]
- 78.Salome A. C., Momoh M., Onyishi V., Abonyi A., Godswill C. O., Attama A. Evaluation of properties of Garcinia kola (Heckel) seed extract in lipospheres based on fat from Capra hircus: an antimicrobial study. Journal of Current Pharma Research . 2014;4(4):1274–1280. [Google Scholar]
- 79.Sibanda T., Okoh A. In vitro evaluation of the interactions between acetone extracts of Garcinia kola seeds and some antibiotics. African Journal of Biotechnology . 2008;7(11):1274–1280. doi: 10.5897/ajb08.924. [DOI] [Google Scholar]
- 80.Akinnibosun F., Itedjere E. Evaluation of the antibacterial properties and synergistic effect of Garcinia kola Heckel (Family: guttiferae) seed extract and honey on some bacteria. African Journal of Microbiology Research . 2013;7(3):174–180. [Google Scholar]
- 81.Seanego C. T., Ndip R. N. Identification and antibacterial evaluation of bioactive compounds from Garcinia kola (Heckel) seeds. Molecules . 2012;17(6):6569–6584. doi: 10.3390/molecules17066569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bukar B., Adurogboye J., Falang K. A Study of the effect of fractionation on phytochemical composition and in vitro antimicrobial activity of methanol extract of Garcinia kola (Heckel) seeds on some bacterial isolates. Scientific Research Journal . 2019;7(5):14–28. doi: 10.31364/scirj/v7.i5.2019.p0519649. [DOI] [Google Scholar]
- 83.Esimone C. O., Nwafor S. V., Okoli C. O., et al. In vivo evaluation of interaction between aqueous seed extract of Garcinia kola Heckel and ciprofloxacin hydrochloride. American Journal of Therapeutics . 2002;9(4):275–280. doi: 10.1097/00045391-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 84.Omwirhiren E. M., Abass A. O., James S. A. The phytochemical constituents and relative antimicrobial activities against clinical pathogens of different seed extracts of Cola nitida (Vent.), Cola acuminata (Beauvoir) and Garcinia kola (Heckel) grown in South West, Nigeria. Journal of Pharmacognosy and Phytochemistry . 2017;6(1):493–501. [Google Scholar]
- 85.Yahaya A. M., Abdullahi A., Bala A. M., Garba R. Antibacterial and antifungal activities of Garcinia kola. 2021. https://repository.futminna.edu.ng:8080/xmlui/bitstream/handle/123456789/9432/PUBLICATION%204.pdf?sequence=1&isAllowed=y .
- 86.Duro D., Hafsat B., Odeh O., Yahaya T., Salawu O. Investigating the toxicity and antimicrobial activity of Garcinia kola extracts. World Journal of Pharmaceutical Research . 2015;4(4):57–67. [Google Scholar]
- 87.Jackie O., Swamy T. A., Mutuku N. C. Preliminary phytochemical and in vitro control of selected pathogenic organisms by ethanolic extract of Garcinia kola seeds. International Journal of Current Microbiology and Applied Sciences . 2014;3(4):183–196. [Google Scholar]
- 88.Okwulehie I., Alozie V., Ikechukwu G. C., Nwokeocha O. Effect of extraction solvents on bioactive compounds and antimicrobial activities of two varieties Garcinia kola (Heckel) OBOWO 02 (soft and less bitter) and OBOWO 03 (hard and very bitter) Pharmaceutical and Biosciences Journal . 2017;5(6):20–25. doi: 10.20510/ukjpb/5/i6/166563. [DOI] [Google Scholar]
- 89.Unegbu V. N., Okey-Ndeche F. N., Obum-Nnadi C. N., Egwuatu P. I. Phytochemical and antibacterial properties of Garcinia kola seeds (bitter kola) on Escherichia coli and Staphylococcus aureus. Global Science Independent Journal . 2020;1(1):1–9. [Google Scholar]
- 90.Badger-Emeka L. I., Khalil H. E., Emeka P. M. Evaluation of different fractions of Garcinia kola extracts against multidrug resistant clinical bacterial and fungal isolates. Pharmacognosy Journal . 2018;10(5):1055–1060. [Google Scholar]
- 91.Opara J.-K., Onwuliri F., Agumah N. B., Njoku O., Onwuliri E. Evaluation antibacterial effects of Garcinia kola and Vernonia amygdalina on Staphylococcus aureus isolated from residents of Abuja, Nigeria. World Journal of Pharmaceutical Research . 2017;6(15):95–104. [Google Scholar]
- 92.Fredrick A. C. Garcinia kola (Heckel) [bitter kola] seed. International Journal of Research in Pharmacy and Chemistry . 2014;4(2):237–242. [Google Scholar]
- 93.Akintelu S. A., Olugbeko S. C., Folorunso F. A., Oyebamiji A. K., Folorunso A. S. Characterization and pharmacological efficacy of silver nanoparticles biosynthesized using the bark extract of Garcinia kola. Journal of Chemistry . 2020;2020:7. doi: 10.1155/2020/2876019.2876019 [DOI] [Google Scholar]
- 94.Akeh M. Phytochemical properties and antimicrobial activities of combined effect of extracts of the leaves of Garcinia kola, Vernonia amygdalina and honey on some medically important microorganisms. African Journal of Microbiology Research . 2009;3(9):557–559. [Google Scholar]
- 95.Ogunjobi A. A., Ogunjobi T. Comparative study of antibacterial activities of ethanol extracts of the bark and seeds of Garcinia kola and Carica papaya. African Journal of Biomedical Research . 2011;14(2):147–152. [Google Scholar]
- 96.Ikechukwu N., Orji-Udezuka A. Antibacterial effect of vinegar produced from Garcina kola and Artocarpus heterophyllus. Asian Journal of Microbiology and Biotechnology . 2021;6(1):36–44. [Google Scholar]
- 97.Duru C. E., Duru I. A., Ibe F. C., Achinihu I. O., Ukiwe L. Functional group analysis and antibacterial studies of column chromatography eluates from the fruit of Garcinia kola. IOSR Journal of Applied Chemistry . 2015;8(9):35–38. [Google Scholar]
- 98.Ogbulie J., Ogueke C., Nwanebu F. Antibacterial properties of Uvaria chamae, Congronema latifolium, Garcinia kola, Vemonia amygdalina and Aframomium melegueta. African Journal of Biotechnology . 2007;6(13):1549–1553. [Google Scholar]
- 99.Piu A. O., Ruth A. I., Olayinka O. I., Iyadunn A. O., Oguntope A. S. Susceptibility of multi drug resistant bacteria associated with respiratory tract infection to methanolic extract of Garcinia kola Heckel (bitter kola) Advances in Biological Research . 2015;9(6):424–435. [Google Scholar]
- 100.Ejele A., Iwu I., Enenebeaku C., Ukiwe L., Okolue B. Bioassay-guided isolation, purification and partial characterization of antimicrobial compound from basic metabolite of Garcinia Kola. Journal of Emerging Trends in Engineering and Applied Sciences . 2012;3(4):668–672. [Google Scholar]
- 101.Chidinma I., Ogbonnaya O., Chika E., Ifeanyichukwu I., Emmanuel N., Agabus N. Comparative analysis on the antimicrobial activity of herbal extracts (Garcinia kola and Azadirachta indica) and conventional antibiotics against bacterial pathogens isolated from orthopedic wound infections. International Journal of Biology, Pharmacy and Allied Sciences . 2016;5(6):1468–1476. [Google Scholar]
- 102.Jegede J., Aruma J.-F., Ihejirika N. O., Chikwem J. O. Comparison of antimicrobial effect of methanolic, ethanolic and aqueous extracts of Garcinia kola, Cola acuminata and Cola nitida. Lincoln University Journal of Science . 2018;7:6–16. [Google Scholar]
- 103.Aniche G. N., Uwakwe G. U. Potential use of Garcinia kola as hop substitute in lager beer brewing. World Journal of Microbiology and Biotechnology . 1990;6(3):323–327. doi: 10.1007/bf01201305. [DOI] [PubMed] [Google Scholar]
- 104.Abah A., Agbelusi G., Odukoya O., Ayanbadejo P., Adebiyi K. The use of “Garcinia kola” in the treatment of oral candida infection in HIV patients: the use of “Garcinia Kola” in the treatment of oral candida. African Journal of Oral and Maxillofacial Pathology and Medicine . 2015;1(1):27–33. [Google Scholar]
- 105.Chinwuko G. O., Okezie U. M., Nwakile C. D., Rowaiye A. B., Okeke I. J., Oli A. N. Preliminary study on the synergistic interaction of Garcinia kola and Vernonia amygdalina against Candida albicans. GSC Biological and Pharmaceutical Sciences . 2021;15(3):206–211. doi: 10.30574/gscbps.2021.15.3.0159. [DOI] [Google Scholar]
- 106.Naiho A., Ugwu A. Blood pressure reducing effect of bitter kola (Garcinia kola, Heckel) in Wistar rats. African Journal of Biomedical Research . 2009;12(2):131–134. [Google Scholar]
- 107.Naiho A., Ugwu A. Blood pressure reducing effect of Garcinia kola in Wistar rats. African Journal of Tropical Medicine and Biomedical Research . 2011;2(1):61–107. [Google Scholar]
- 108.Olaleye S., Farombi E., Adewoye E., Owoyele B., Onasanwo S., Elegbe R. Analgesic and anti-inflammatory effects of kaviiron (a Garcinia kola seed extract) African Journal of Biomedical Research . 2000;3(3):171–174. [Google Scholar]
- 109.Falang K., Uguru M. O., Nnamonu N. L. Anti-pyretic activity of Garcinia kola seed extract. European Journal of Medicinal Plants . 2014;4(5):511–521. doi: 10.9734/ejmp/2014/4484. [DOI] [Google Scholar]
- 110.Adegbehingbe O. O., Adesanya S. A., Idowu T. O., Okimi O. C., Oyelami O. A., Iwalewa E. O. Clinical effects of Garcinia kola in knee osteoarthritis. Journal of Orthopaedic Surgery and Research . 2008;3(1):34–10. doi: 10.1186/1749-799X-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Duze B., Sewani-Rusike C., Nkeh-Chungag B. Effects of an ethanolic extract of Garcinia kola on glucose and lipid levels in Streptozotocin induced diabetic rats. African Journal of Biotechnology . 2012;11(33):8309–8315. doi: 10.5897/ajb11.2980. [DOI] [Google Scholar]
- 112.Iwu M. M., Igboko O. A., Okunji C. O., Tempesta M. S. Antidiabetic and aldose reductase activities of biflavanones of Garcinia kola. Journal of Pharmacy and Pharmacology . 1990;42(4):290–292. doi: 10.1111/j.2042-7158.1990.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 113.Oluyemi K. A., Omotuyi I. O., Jimoh O. R., Adesanya O. A., Saalu C. L., Josiah S. J. Erythropoietic and anti-obesity effects of Garcinia cambogia (bitter kola) in Wistar rats. Biotechnology and Applied Biochemistry . 2007;46(1):69–72. doi: 10.1042/BA20060105. [DOI] [PubMed] [Google Scholar]
- 114.Okafor E., Onuka A., Umoh I., Ugwu F. Ethanolic seed extract of Garcinia kola reduces epididymal sperm count and some serum reproductive hormone concentrations in adult male albino Wistar rats. Asian Journal of Research in Medical and Pharmaceutical Sciences . 2018;4(2):1–5. doi: 10.9734/ajrimps/2018/42640. [DOI] [Google Scholar]
- 115.Abu A., Amuta P., Buba E., Inusa T. Evaluation of antispermatogenic effect of Garcinia kola seed extract in Albino rats. Asian Pacific Journal of Reproduction . 2013;2(1):15–18. doi: 10.1016/s2305-0500(13)60108-6. [DOI] [Google Scholar]
- 116.Udia P. M., Braide V. B., Owu D. U. Antispasmodic and spasmolytic effects of methanolic extract from seeds of Garcinia kola on isolated rat small intestine. Nigerian Journal of Physiological Sciences: Official Publication of the Physiological Society of Nigeria . 2009;24(2):111–116. doi: 10.4314/njps.v24i2.52912. [DOI] [PubMed] [Google Scholar]
- 117.Johnson T., Omoniwa B. In vivo trypanocidal activity of ethanolic crude extract and phytochemical fractions of Garcinia kola seeds. Annual Research and Review in Biology . 2014;4(1):212–222. doi: 10.9734/arrb/2014/5716. [DOI] [Google Scholar]
- 118.Esomonu U. G., El-Taalu A. B., Anuka J. A., Ndodo N. D., Salim M. A., Atiku M. K. Effect of ingestion of ethanol extract of Garcinia kola seed on erythrocytes in Wistar rats. Nigerian Journal of Physiological Sciences: Official Publication of the Physiological Society of Nigeria . 2005;20(1):30–32. [PubMed] [Google Scholar]
- 119.Ebenebe C. I., Nwankwor A. O., Izukanne R. O., Ufele A. N. Performance and haematological parameters of rabbits fed graded levels of bitter kola (Garcinia kola) International Journal of Livestock Production . 2016;7(12):128–133. doi: 10.5897/ijlp2016.0298. [DOI] [Google Scholar]
- 120.Adedeji O., Farimi G., Ameen S., Olayemi J. Effects of bitter kola (Garcinia kola) as growth promoter in broiler chicks from day old to four weeks old. Journal of Animal and Vetinary Advances . 2006;5(3):191–193. [Google Scholar]
- 121.Dada A. A., Oviawe N. E. The use of bitter kola Garcinia kola dry seed powder as a natural growth-promoting agent for African sharp tooth catfish Clarias gariepinus fingerlings. African Journal of Aquatic Science . 2011;36(1):97–100. doi: 10.2989/16085914.2011.559703. [DOI] [Google Scholar]
- 122.Dada A., Ikuerowo M. Effects of ethanolic extracts of Garcinia kola seeds on growth and haematology of catfish (Clarias gariepinus) broodstock. African Journal of Agricultural Research . 2009;4(4):344–347. [Google Scholar]
- 123.Mohammed A. A., Malik M. A. A. Effect of bitter kola (Garcinia kola) as a dietary additive on the performance of broiler chicks. Journal of Environment and Ecology . 2013;4(2):95–104. [Google Scholar]
- 124.Emeji R., Gabriel T.-E. D., Ndokiari B. Therapeutic effects of Viscum album combined with Garcinia kola against CCl4 induced liver injury in albino rats. Asian Journal of Biochemistry, Genetics and Molecular Biology . 2019;2(3):1–8. doi: 10.9734/ajbgmb/2019/v2i330060. [DOI] [Google Scholar]
- 125.Ekpenyong C. E., Akpan U., Ben E., Nwama E., Ibu J. Hematological effect of chronic administration of ethanolic extract of Garcinia conruana seed on rat. Journal of Natural Products . 2011;4:173–176. [Google Scholar]
- 126.Iwuji T., Herbert U. Haematological and serum biochemical characteristics of rabbit bucks fed diets containing Garcimiola kola seed meal. Advances in Agriculture, Sciences and Engineering Research . 2012;2(8):299–305. [Google Scholar]
- 127.Kagbo H., Ejebe D. Phytochemistry and preliminary toxicity studies of the methanol extract of the stem bark of Garcinia kola (Heckel) Internet Journal of Toxicology . 2010;7(2):1–18. [Google Scholar]
- 128.Uwagie-Ero E. A., Nwaehujor C. O. Effects of Garcinia hydroxybiflavanonol-1 (GB1) isolated from Garcinia kola Heckel (Guttiferae) seeds on reproductive toxicity induced with cadmium chloride (CdCl2) in male Wistar rats. Nigerian Journal of Environmental Sciences and Technology . 2020;4(1):21–30. doi: 10.36263/nijest.2020.01.0166. [DOI] [Google Scholar]
- 129.Soltanian S., Sheikhbahaei M., Mohamadi N. Cytotoxicity evaluation of methanol extracts of some medicinal plants on P19 embryonal carcinoma cells. Journal of Applied Pharmaceutical Science . 2017;7(7):142–149. [Google Scholar]
- 130.Latif A., Amer H. M., Hamad M. E., Alarifi S. A. R., Almajhdi F. N. Medicinal plants from Saudi Arabia and Indonesia: in vitro cytotoxicity evaluation on Vero and Hep-2 cells. Journal of Medicinal Plants Research . 2014;8(34):1065–1073. [Google Scholar]
- 131.Eleazu C., Eleazu K., Awa E., Chukwuma S. Comparative study of the phytochemical composition of the leaves of five Nigerian medicinal plants. Journal of Biotechnology and Pharmaceutical Research . 2012;3(2):42–46. [Google Scholar]
- 132.Okoko T. Chromatographic characterisation, in vitro antioxidant and free radical scavenging activities of Garcinia kola seeds. African Journal of Biotechnology . 2009;8(24):7133–7137. doi: 10.1016/j.fct.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 133.Yété P., Ndayishimiye V., Agbangnan P., Djènontin S., Wotto V., Sohounhloué D. Chemical composition of the seeds and the defatted meal of Garcinia kola Heckel (Guttifferae) from Benin. Chemistry Journal . 2014;4(5):13–19. [Google Scholar]
- 134.Elekwa I. Preliminary phytochemical screening and gas chromatographic FID evaluation of Garcinia kola seed extracts. Journal of Pharmacognosy and Phytochemistry . 2014;2(6):115–119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available within the manuscript.


