Abstract
Rhesus monkey bone marrow expresses a cathelicidin whose C-terminal domain comprises a 37-residue alpha-helical peptide (RL-37) that resembles human LL-37. Like its human counterpart, RL-37 rapidly permeabilized the membranes of Escherichia coli ML-35p and lysed liposomes that simulated bacterial membranes. When tested in media whose NaCl concentrations approximated those of extracellular fluids, RL-37 was considerably more active than LL-37 against staphylococci. Whereas human LL-37 contains five acidic residues and has a net charge of +6, rhesus RL-37 has only two acidic residues and a net charge of +8. Speculating that the multiple acidic residues of human LL-37 reduced its efficacy against staphylococci, we made a peptide (LL-37 pentamide) in which each aspartic acid of LL-37 was replaced by an asparagine and each glutamic acid was replaced by a glutamine. LL-37 pentamide's antistaphylococcal activity was substantially greater than that of LL-37. Thus, although the precursor of LL-37 is induced in human skin keratinocytes by injury or inflammation, its insufficiently cationic antimicrobial domain may contribute to the success of staphylococci in colonizing and infecting human skin.
Cathelin is a porcine leukocyte peptide with 96 amino acid residues. Its name is an acronym for cathepsin L inhibitor and reflects an early belief that it was a cysteine-protease inhibitor (28). Subsequently, the cathelin sequence was recognized as a conserved domain in the precursors of many mammalian antimicrobial peptides that are now collectively known as “cathelicidins” (42).
Cathelin-associated antimicrobial peptides are structurally diverse (14). Many have an amphipathic alpha-helical structure, while others are β-sheet peptides with intramolecular cystine disulfide bonds and some have numerous proline or tryptophan residues. The bovine cathelicidin peptides include a cyclic dodecapeptide (29), a tryptophan-rich tridecapeptide called indolicidin (8, 31), two proline- and arginine-rich bactenecins (10, 30), and at least three alpha-helical bovine myeloid antimicrobial peptides (BMAPs) (13, 33). Porcine cathelicidin peptides include three alpha-helical porcine myeloid antimicrobial peptides (PMAPs) (35, 38, 43), five β-sheet protegrins, (20, 44), and several proline-rich molecules (2, 15, 28). Sheep and goats also possess multiple cathelicidins (3, 17, 24, 32).
In contrast to the above, only a single cathelicidin, human cationic antimicrobial peptide of 18 kDa (hCAP-18), is currently known to exist in humans. This propeptide, whose C-terminal domain constitutes the 37-residue antimicrobial peptide called LL-37, is produced constitutively by precursors of neutrophils in the bone marrow and is stored within the secondary (specific) granules of the neutrophil (33). hCAP-18 is also produced constitutively by epididymal epithelial cells, so that large concentrations of hCAP-18 are present in normal seminal plasma and the peptide coats the surfaces of normal spermatozoa (25). Recent evidence suggests that certain human lymphocyte populations also express LL-37 (1).
The gene for hCAP-18 contains putative interleukin-6-responsive promoter elements (12), and in vivo hCAP-18 expression is induced in normal skin keratinocytes after infection, inflammation, or injury (11). Squamous epithelia of the human mouth, tongue, esophagus, cervix, and vagina also express hCAP-18 mRNA and peptide, which suggests a more general role for the peptide in protecting surface epithelia (12). A recent study compared human LL-37 to the alpha-helical cathelicidin peptides of several other mammals and found that the human peptide was much less potent than rabbit CAP-18 or sheep SMAP-29 (39). In this group of alpha-helical cathelicidin peptides, activity appeared to correlate with net positive charge and the presence of a hydrophobic gradient along the peptide backbone.
Because rhesus macaques (Macacca mulatta) are widely used in experimental studies, we sought homologues of human hCAP-18 in rhesus bone marrow. Anticipating that the human and rhesus cathelin domains would be similar, we used the previously described human cathelin sequence to probe for the corresponding rhesus cathelicidin mRNA. We isolated a single molecular species that encoded the alpha-helical peptide whose properties are described herein.
MATERIALS AND METHODS
cDNA cloning.
Rhesus bone marrow was obtained from macaques that were euthanatized at the California Regional Primate Center, Davis, for reasons unrelated to this study. Total RNA was purified by the Tri-Reagent procedure according to the protocol of the manufacturer (Molecular Research Center, Cincinnati, Ohio). Briefly, fresh bone marrow was mixed with 3.75 ml of Tri-Reagent BD and after phase separation occurred, and the aqueous phase was transferred to a fresh tube and reserved for RNA purification. Rapid amplification of 3′ cDNA ends (3′RACE) was done with a kit (Gibco BRL, Gaithersburg, Md.), using 1 μg of total monkey RNA and 1 μl of adapter primer (10 μl) to obtain first-strand cDNA. A sense primer (5′ CGGCCATGAAGACCCAAAGGAATGG) that corresponded to nucleotides 7 to 31 of human LL-37 was synthesized. PCR was done in a 50-μl volume, using 10% of the above cDNA product, 10 pmol of each abridged universal amplification primer (AUAP; Gibco Life Technologies, Rockville, Md.), and sense primer. The reaction was performed for 32 cycles in a GeneAmp PCR System 2400 (Perkin-Elmer, Palo Alto, Calif.) with the following temperatures and times: 94°C, 20 s; 55°C, 20 s, 72°C, 40 s. After electrophoresis on 0.8% agarose gels, a 600-bp product was purified and cloned into PCR2.1 vector (Invitrogen, Carlsbad, Calif.). Sequencing was performed with an Applied Biosystem 373 DNA Sequencer (Perkin-Elmer) at the UCLA DNA Sequencing Facility, using the fluorescein-labeled dideoxynucleotide terminator method.
Peptide synthesis and purification.
The peptides (Table 1) were synthesized at a 0.25-mmol scale with a Perkin-Elmer ABI 431 A synthesizer, using prederivatized polyethylene glycol polystyrene serine resin (PerSeptive Biosystems, Framingham, Mass.), FastMoc chemistry, and single coupling for all residues. After purification by reverse-phase high-performance liquid chromatography, each peptide appeared homogeneous, and for each the mass as measured by electrospray ionization (ESI) mass spectrometry agreed well with its theoretical mass.
TABLE 1.
Peptides used in this study
| Peptide | Primary sequencea | Avg mass (Da) | Net charge |
|---|---|---|---|
| RL-37 | RLGNFFRKVKEKIGGGLKKVGQKIKDFLGNLVPRTAS | 4,100.9 | +8 |
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFFRNLVPRTES | 4,527.3 | +6 |
| LL-37 pentamide | LLGNFFRKSKQKIGKQFKRIVQRIKNFFRNLVPRTQS | 4,522.3 | +11 |
The five amides that were introduced into the LL-37 pentamide sequence are shown in boldface type. Residues of RL-37 that are identical to those in LL-37 are underlined.
Antibacterial assays.
The antimicrobial properties of RL-37 were tested by a two-stage radial diffusion assay (22, 34). Briefly, approximately 4 × 106 CFU of mid-logarithmic-phase organisms was dispersed into 10 ml of a molten (43°C) underlay gel mixture consisting of 10 mM sodium phosphate (pH 7.4), Trypticase soy broth power (0.3 mg/ml; Difco, Detroit, Mich.), and 1% (wt/vol) Sigma A6013 agarose, with or without additional 100 mM NaCl. The mixture was vortexed and decanted into a petri dish. Multiple sample wells were punched in this underlay gel after it had solidified.
Serial peptide dilutions containing 250, 79.1, 25, 7.91, 2.5, and 0.79 μg of peptide per ml were prepared in 0.01% acetic acid, to which 0.1% human serum albumin had been added to minimize adsorptive peptide loss. Aliquots (8 μl) of these dilutions were applied to the wells. After 3 h of incubation at 37°C, overlay gels that contained 60 mg of Trypticase soy broth powder/ml in 1% (wt/vol) agarose were poured atop the underlay gels. After the plates were incubated overnight at 37°C, the resulting clear zones were measured to the nearest 0.1 mm after overnight incubation and were expressed in units (1 mm = 10 U) after subtracting the well diameter (3.2 mm). We then either plotted the log10 peptide concentration (X axis) against the zone diameter (Y axis) or performed a linear regression analysis of the data in order to determine the X intercept, whose value represented the minimal effective concentration (MEC). The procedure and its rationale are fully described elsewhere (34) and were compared to conventional NCCLS-type procedures in an earlier paper published in this journal (40).
LPS binding.
Quantitative chromogenic Limulus amoebocyte lysate assays were performed with a QCL-1000 kit (Bio Whittaker, Walkersville, Md.). Incubation were done in flat-bottom, nonpyrogenic 96-well tissue culture plates (catalog no. 3596; Costar, Cambridge, Mass.). Peptides were prepared in endotoxin-free, acidified water (0.01% acetic acid) and serially diluted in this vehicle. In step 1, the peptide of interest was incubated at 37°C for 30 min with 0.5 endotoxin units/ml of E. coli 0111:B4 lipopolysaccharide (LPS) in a volume of 50 μl. Then (step 2), 50 μl of Limulus amebocyte lysate was added, and the mixture was incubated for 10 min at 37°C. Finally (step 3), 100 μl of the chromogenic substrate (acetyl-Ile-Glu-Ala-Arg-p-nitroanilide) was added and the incubation was continued for 30 min. During this time, the liberation of p-nitroaniline was monitored every 60 s at 405 nm, with a SpectraMax 250 Kinetic Microplate Spectrophotometer (Molecular Devices, Sunnyvale, Calif.). The change in optical density (ΔOD) between 11 and 17 min was calculated for an LPS-free control sample that contained the peptide, and this value was subtracted from the ΔOD between 11 and 17 min of the experimental sample, which contained the peptide and LPS. The percent binding (inhibition) was calculated from the quotient (Q) of the ΔOD with peptide divided by the ΔOD peptide-free controls, with the formula: (1 − Q) × 100. Performing the assays kinetically allowed us to monitor spontaneous procoagulant activation and verify that the peptide did not activate Limulus procoagulant directly when LPS was absent and that the peptide was not contaminated with LPS. In the absence of peptide, the ΔOD was a linear function of the amount of LPS added in step 1, between 0.05 and 1.0 endotoxin units of LPS.
Bacterial membrane permeabilization.
To assess the ability of RL-37 to permeabilize the inner and outer membranes of Escherichia coli ML-35p, we modified a previously described spectrophotometric procedure (21) for fluorescent substrates, because PADAC, the chromogenic β-lactamase substrate [7-(thieny-2-acetamido)-3-(2-(4-N,N-dimethylaminophenylazo)-pyrididiummethyl)-3-cephem-4-carboxylic acid] was no longer commercially available. Its fluorogenic replacement was CCF2-free acid (mass, 864 Da) was purchased from Aurora Bioscience (San Diego, Calif.). This molecule's cephalosporin core links a 7-hydroxycoumarin residue to a fluorescein moiety, such that fluorescence resonance energy transfer occurs when the coumarin is excited (45). Cleavage of the cephalosporin's β-lactam ring results in spontaneous elimination of the 3′ fluorescein, with an attendant decrease in fluorescence resonance energy transfer. We replaced ONPG (O-nitrophenyl-β-d-galactopyranoside), a chromogenic β-galactosidase substrate, with DiFMUG (6,8 difluoro-4-methylumbelliferyl β-d-galactopyranoside), which was purchased from Molecular Probes (Eugene, Oreg.). Both fluorogenic substrate stock solutions (200 μM) were prepared in 10 mM sodium phosphate buffer (pH 7.4), and the assays were performed in black, 96-well plates with lids (Corning, Corning, N.Y.). Their hydrolysis products were detected with an f-max fluorescence microplate reader (Molecular Devices Corp.), using SOFTmaxPRO software supplied by the manufacturer. An excitation wavelength of 380 nm and emission wavelength of 460 nm was suitable for both substrates.
The final incubation medium contained 10 mM sodium phosphate buffer, 100 mM NaCl, and 0.3 mg of Trypticase soy broth powder per ml. Incubation wells (final volume, 200 μl) also contained 10 μM substrate (CCF2 or DiFMUG); 2.5 × 107 CFU of washed, stationary-phase E.coli ML-35p cells; and various concentrations of the peptide of interest or an equivalent volume of acidified water (negative controls). Assays were run at 37°C, with 10 s of shaking every minute. Reactions were started by adding the bacteria.
Liposome studies.
Lipids were purchased from Avanti Polar Lipids (Alabaster, Ala.). A cationic thiadicarbocyanine dye—N,N′-di(3-trimethylammoniumpropyl)thiadicarbocyanine tribromide—was from Molecular Probes. Liposomes simulating E. coli membranes were prepared from 1-palmitoyl-2-oleoyl-sn- glycero-3-phosphatidylethanolamine (POPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylglycerol (POPG), and cardiolipin (CL) in a 3:1:0.44 molar ratio (POPE-POPG-CL) Liposomes that simulated Staphylococcus aureus membranes were prepared by combining POPG and CL in a 3:1 ratio (16). Thiadicarbocyanine dye-encapsulated lipid vesicles were prepared by extrusion. For each preparation, around 20 mg of the lipid mixture was dissolved in 2 ml of chloroform in a glass tube. The solvent was removed under streaming argon and further dried for 2 h under vacuum. The lipids were hydrated and then dispersed into 2 ml of 10 mM phosphate buffer, pH 7.4, containing 0.5 mg of the dye per ml. This dispersion was treated by 10 cycles of freezing in liquid nitrogen followed by thawing in a 50°C water bath. The milky product was passed seven times through a 100-nm polycarbonate filter mounted in an Avanti Mini-extruder. Unencapsulated dye was removed by passing the liposomes through a Sephadex G-50 column. The final concentration of the lipid was 67 μg/ml.
Graded amounts of RL-37, LL-37, and PG-1 were added to each well to obtain final peptide concentrations between 3 to 40 μM. The 96-well plate was incubated at room temperature for 10 min. Fluorescence measurements were made with a SpectraMAX Gemini XS Microplate Spectrophotometer (Molecular Devices), with excitation at 653 nm and emission at 674 nm. The no-lysis control contained liposomes in phosphate buffer without peptide. The 100% lysis control contained liposomes with 0.1% Triton X-100. The percentage of peptide-induced liposome lysis was calculated using the following equation, where F represents the fluorescence intensity of samples that contained peptides, F0 is the fluorescence in the absence of peptides, and Ft is the fluorescence in the presence of 0.1% Triton X-100: (F − F0)/(Ft − F0) × 100.
CD spectroscopy.
Circular dichroism (CD) spectra were taken at 25°C on a model 62DS spectropolarimeter (AVIV Associates, Lakewood, N.J.) in a rectangular 0.1-mm-path-length cell that contained either 10 mM phosphate buffer, pH 7.4; 50% trifluoroethanol; sodium dodecyl sulfate; unilamellar liposomes (∼100 nm diameter) in 10 mM phosphate buffer, pH 7.4; or an LPS dispersion (40). The instrument was calibrated with (+)-10-camphorsulfonic acid (18). Extruded liposomes that simulated bacterial membranes were prepared with a LipoSoFast extruder (Avestin, Ottawa, Canada) as previously described (16). The helical content of the peptide in various solvents was estimated from its mean residue ellipticity by the following equation6: % helix = [θ]MRE222/(−39,500 [1 − (2.57/n)]) deg cm2 dmol−1.
Nucleotide sequence accession number.
The RL-37 sequence was deposited in GenBank, as accession no. AF181954.
RESULTS
cDNA cloning of the rhesus cathelicidin.
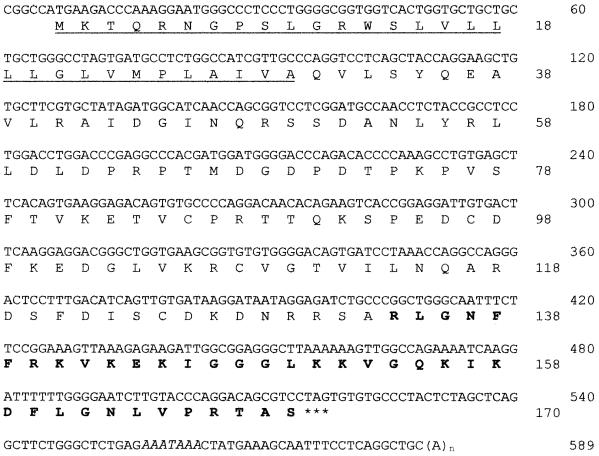
The primary nucleotide and inferred protein sequences of the rhesus cathelicidin peptide, preproRL-37, are shown in Fig. 1. The cDNA sequence contained a 510-bp open reading frame that encoded a 170-residue prepropeptide with a mass of 18,861 Da and an isoelectric point of 10.06. Its 30-amino-acid signal peptide was followed sequentially by a 103-residue cathelin domain and the 37-residue RL-37 domain. Overall, the rhesus cDNA sequence was 92% identical to that of human hCAP-18. The signal sequences differed at only 5 bp (5.6%), the cathelin domains differed at only 16 bp (5.2%), and the antimicrobial peptide domains, differed at only 16 bp (14.4%).
FIG. 1.
cDNA sequence of the rhesus monkey cathelicidin, RL-37. The precursor has 170 residues, a mass of 18,861 Da, and a pI of 10.06. The predicted signal sequence (33 residues) is underlined, and the expected mature peptide is shown in bold face type. The stop codon is indicated by asterisks, and the polyadenylation site is indicated by italics.
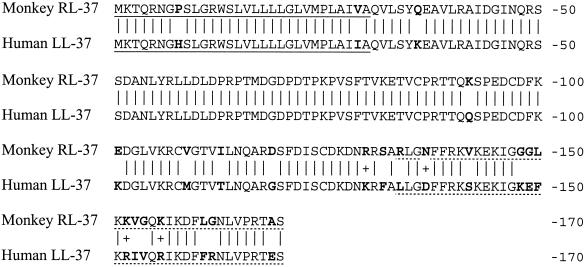
Rhesus preproRL-37 and human preproLL-37 peptide sequences are shown in Fig. 2. Overall, 146 of the 170 residues (85.9%) are identical. In the signal sequences, 28 of 30 (93.3%) of the residues are identical, as are 94 of 103 of the residues in the cathelin domains. Only 25 of the 37 residues (67.6%) in the respective antimicrobial peptide domains are identical. RL-37 has 10 positively charged residues (three arginines and seven lysines) and two negatively charged residues (one aspartic acid and one glutamic acid), giving it a net charge of +8. Human LL-37 contains 11 positively charged residues (five arginines and six lysines), but also has five negatively charged ones (two aspartic and three glutamic acids), making its net charge + 6.
FIG. 2.
Comparison of rhesus and human cathelicidin peptides. Both signal peptides (underlined) have 30 residues, of which 28 (93.3%) are identical. Both cathelin domains have 101 residues, of which 93 (92%) are identical. The mature domains (dotted underlined) of RL-37 and LL-37 both contain 37 residues, of which 25 (67.6%) are identical. Mature rhesus RL-37 has a mass of 4,100.9 Da, a theoretical pI of 11.20, and a net charge of +8. Mature human LL-37 has a mass of 4527.34, a pI of 10.61, and a net charge of +6. A vertical line connects identical residues, and a plus sign identifies conservative substitutions. Residues that differ in the human and rhesus peptides are shown in boldface type.
Antimicrobial activity.
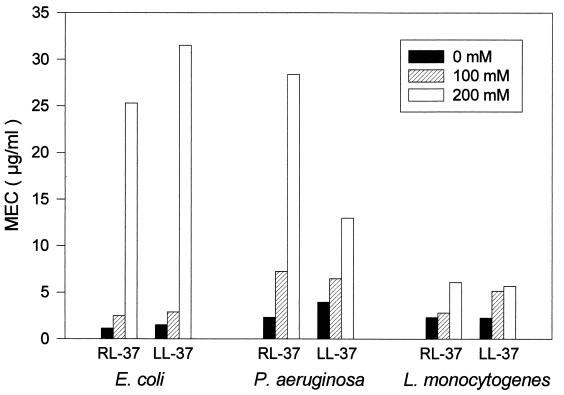
When we compared the antimicrobial activities of RL-37 and LL-37, their relative activities depended both on the organism being tested and the salinity of the test medium. Neither peptide was active (MEC >250 μg/ml) against Candida albicans, even when tested under our least-stringent conditions, without added NaCl. The human and rhesus peptides showed similar potency against E. coli ML-35p, Pseudomonas aeruginosa, and Listeria monocytogenes, and both retained substantial activity against these organisms even in underlay gels supplemented with 200 mM NaCl (Fig. 3).
FIG. 3.
Activity against E. coli, P. aeruginosa, and L. monocytogenes. Radial diffusion assays were performed in underlay gels that contained different amounts of NaCl (0 mM, 100 mM, or 200 mM), in addition to their common basic ingredients (10 mM sodium phosphate buffer, pH 7.4; Trypticase soy broth powder, 0.3 mg/ml; and 1% [wt/vol] agarose).
The most notable differences between the peptides were evident when we tested staphylococci in the presence of 100 or 175 mM NaCl. With each of the four test strains (two S. aureus, one Staphylococcus epidermidis and one methicillin resistant S. aureus), RL-37 was significantly more potent than LL-37 under such conditions. This difference disappeared when the peptides were studied under low-salt conditions (no added NaCl) and was magnified when the NaCl concentration of the underlay gel was raised to 175 mM (Table 2).
TABLE 2.
Effect of NaCl on activity against staphylococci
| Peptide | Mean MEC (μg/ml) ± SEMa
|
|||
|---|---|---|---|---|
| S. aureus 930918-3 | S. aureus 710A | MRSA ATCC 33591 | S. epidermidis ATCC 49741 | |
| 0 mM NaCl | ||||
| LL-37 | 1.39 ± 0.31 | 1.25 ± 0.11 | 1.72 ± 0.33 | 1.77 ± 0.28 |
| mRL-37 | 0.98 ± 0.08 | 1.00 ± 0.10 | 1.33 ± 0.02 | 1.39 ± 0.12 |
| LL-37 pentamide | 2.42 ± 0.09 | 2.30 ± 0.27 | 2.52 ± 0.17 | 1.88 ± 0.22 |
| 100 mM NaCl | ||||
| LL-37 | 48.2 ± 10.6 | 16.1 ± 0.29 | 186 ± 16.7 | 23.6 ± 1.22 |
| mRL-37 | 2.81 ± 0.11∗ | 2.48 ± 0.04∗∗∗ | 4.17 ± 0.30∗∗∗ | 2.40 ± 0.09∗∗∗ |
| LL-37 pentamide | 3.47 ± 0.84∗ | 2.75 ± 0.20∗∗∗ | 3.41 ± 0.40∗∗∗ | 2.14 ± 0.10∗∗∗ |
| 175 mM NaCl | ||||
| LL-37 | >250 | 193 ± 57.0 | >250 | 110 ± 42.2 |
| mRL-37 | 6.49 ± 0.30∗∗∗ | 5.76 ± 0.27∗∗∗ | 11.6 ± 0.90∗∗∗ | 6.92 ± 0.38∗∗∗ |
| LL-37 pentamide | 4.63 ± 0.47∗∗∗ | 4.82 ± 1.03∗∗∗ | 4.69 ± 1.32∗∗∗ | 4.31 ± 0.75∗∗∗ |
n = 3. The MECs for LL-37 pentamide or RL-37 were compared with those for LL-37 by the t test. Symbols for significance: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
Table 2 also includes our results with LL-37 pentamide, a peptide whose primary sequence (Table 1) was identical to that of LL-37 except that each aspartic acid of LL-37 was replaced by asparagine, and each glutamic acid in LL-37 was replaced by a glutamine. The pentamide variant was significantly more effective than LL-37, and its potency equaled or exceeded that of RL-37. The activity of LL-37 and LL-37 pentamide against several additional organisms is shown in Table 3. These studies were done in 100 mM NaCl. Although RL-37 was somewhat more effective than LL-37 against Klebsiella pneumoniae and P. aeruginosa, the differences between the peptides were not nearly as striking as those seen with staphylococci. Surprisingly, RL-37 was considerably less active than LL-37 against group B streptococci.
TABLE 3.
Uffect of amidation on antimicrobial activitya
| Organism | Strain | P | Mean MEC (mM) ± SEM
|
||
|---|---|---|---|---|---|
| LL-37 | LL-37pam | RL-37 | |||
| Group B strep | A12973 | 0.019 | 8.37 ± 2.09 (8) | 2.27 ± 0.41 (7) | >79.0 (3) |
| L. acidophilus | A4356 | NS | 18.6 ± 6.1 (4) | 9.0 ± 1.7 (4) | NT |
| K. pneumoniae | 2270 | 0.02 | 1.81 ± 0.02 (9) | 0.76 ± 0.14 (7) | 0.73 ± 0.06 (3) |
| P. aeruginosa | MR3007 | 0.012 | 1.28 ± 0.18 (10) | 0.73 ± 0.05 (9) | 1.26 ± 0.08 (3) |
| E. coli | ML-35p | NS | 0.66 ± 0.07 (9) | 0.61 ± 0.04 (6) | 0.57 ± 0.01 (3) |
| E. coli | A33780 | NS | 5.57 ± 0.15 (3) | 6.47 ± 0.39 (3) | 5.74 ± 0.39 (3) |
Radial diffusion assays were performed with underlay gels that contained 100 mM NaCl; 10 mM sodium phosphate buffer, pH 7.4; and Trypticase soy broth powder, 30 mg/ml. P values (for LL-37 versus LL-37 PAM) were calculated by the t test. Abbreviations: NT, not tested; NS, not significant.
Mechanism of activity.
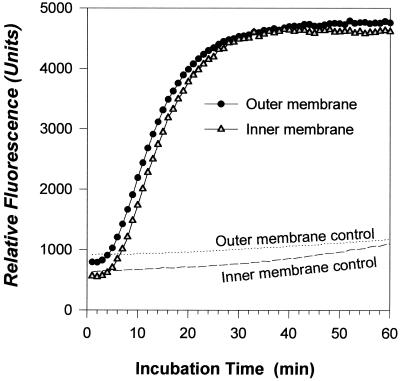
Many antimicrobial peptides act, at least in part, by permeabilizing the membranes of their microbial targets. This property can be tested conveniently in intact bacteria by monitoring the ability of normally impermeant substrates to reach the periplasmic β-lactamase and cytoplasmic β-galactosidase enzymes of E. coli ML-35p. Figure 4 shows that 2.5 μg of RL-35 per ml rapidly permeabilized both the outer and inner membranes of this organism.
FIG. 4.
Membrane permeabilization. Stationary-phase E. coli ML-35P (2.5 × 107 CFU/ml) was suspended in 10 mM sodium phosphate buffer, pH 7.4, containing 100 mM NaCl, 0.3 mg of Trypticase soy broth powder per ml, and fluorogenic substrates for β-lactamase and β-galactosidase. Fluorescence monitoring began immediately after the addition of 2.5 μg of RL-37. Controls were incubated under identical conditions but in the absence of the peptide.
Liposome lysis assay.
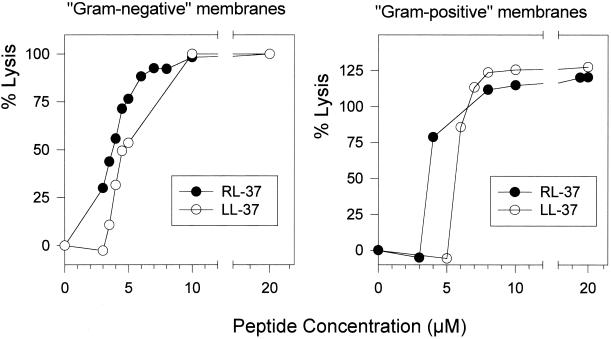
The ability of RL-37 and LL-37 to lyse phospholipid liposomes whose compositions simulated the membranes of gram-negative and gram-positive bacteria is illustrated in Fig. 5. For both types of liposomes, RL-37 was more potent than LL-37. Neither peptide was hemolytic for human erythrocytes, even when tested at a maximal concentration of 80 μg/ml (data not shown).
FIG. 5.
Liposome lysis. A cationic thoadicarbocyanine dye was encapsulated in liposomes formulated to resemble gram-positive and gram-negative membranes. Fluorescence measurements were made 10 min after the addition of peptides.
LPS binding.
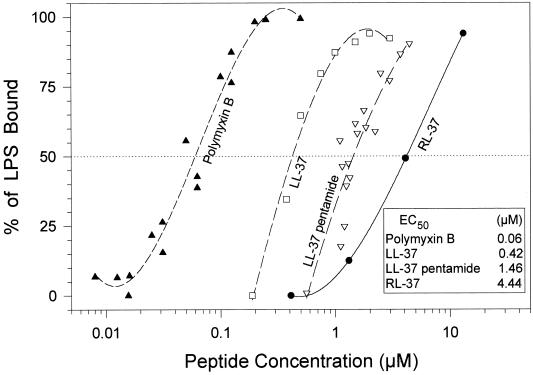
The ability of the study peptides (LL-37, RL-37, and LL-37 pentamide) to bind E. coli 0111:B4 LPS is shown in Fig. 6. Polymyxin B, a lipopeptide antibiotic well known to bind LPS, served as a positive control. The respective concentrations of these peptides that bound half of the added LPS (the 50% effective concentration [EC50]) is an index of their binding affinity for this ligand. Thus, LL-37 (EC50, 420 nM) had an approximately 3-fold greater affinity for LPS than did LL-37 pentamide (EC50, 1.46 μM) and a 10-fold greater affinity than did RL-37 (EC50, 4.44 μM). The affinity of polymyxin B for LPS (EC50, 60 nM) was ∼7-fold higher than that of LL-37, ∼25-fold higher than that of LL-37 pentamide, and ∼75-fold higher than that of RL-37.
FIG. 6.
LPS binding. We used a chromogenic Limulus amoebocyte assay to obtain binding isotherms for E. coli 0111:B4 lipopolysaccharide. The peptides examined included polymyxin B, LL-37, LL-37 pentamide, RL-37, and rabbit CAP-18 (another LPS binding cathelicidin). The EC50 (i.e., the peptide concentrations that bound 50% of the LPS) are shown and provide an approximate binding constant.
Secondary structural analysis.
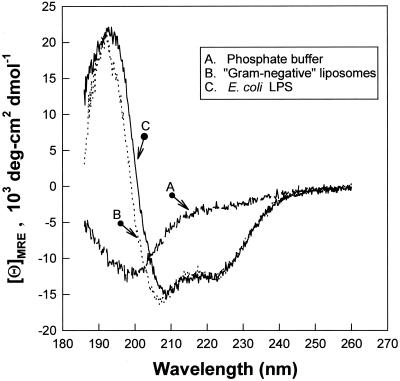
CD measurements were done in several systems, and representative spectra are shown in Fig. 7. RL-37 showed very little helical structure when dissolved in aqueous buffer (7.9% helix) or in normal saline solution (8% helix). In trifluoroethanol (a structure promoting solvent) detergent micelles, or dispersed phospholipids or lipopolysaccharides, changes characteristic of a helical conformation appeared: dichroic minima at 222 and 208 nm with a well-defined maximum at 193 nm. RL-37 was about 40% helix (5, 41) in trifluoroethanol-containing buffer, 43.2% helix in sodium dodecyl sulfate micelles, 34.6% helix in phospholipid dispersions that simulated gram-negative bacterial membranes, and 35.5% helical in LPS dispersions.
FIG. 7.
CD. Spectra labeled A, B, and C are shown. Spectrum A was obtained in 10 mM sodium phosphate buffer, pH 7.4. Spectrum B was obtained in liposomes that contained POPE-POPG-cardiolipin in a 3:1:0.44 molar ratio that simulated gram-negative membranes. These liposomes were dispersed in 10 mM sodium phosphate buffer, pH 7.4. Spectrum C was obtained in a dispersion of diphosphoryl lipid A from E. coli F583 (Sigma) in 10 mM sodium phosphate buffer. In each experiment, the peptide concentration was 120 μM and the lipid-to-peptide molar ratio was 20:1.
DISCUSSION
The content of antimicrobial peptides in mammalian leukocytes shows considerable interspecies variation. For example, α-defensins are abundant in the neutrophils of humans, rats, rabbits, and guineas pigs, but only β-defensins occur in bovine neutrophils. Furthermore, in our previous studies of neutrophils from laboratory mice, horses, sheep, goats and pigs, we have noted that these cells apparently lack defensins completely (7, 9, 20, 32). There is similar interspecies variation with respect to cathelicidins. Whereas the granulocytes of cattle, pigs, sheep, and goats contain many different cathelicidin molecules, human neutrophils contain only one—hCAP-18, the cathelicidin that carries the 37-residue peptide called LL-37.
It was recently reported that the bone marrow of M. mulatta, the rhesus monkey, expresses multiple α-defensins (36) as well as a remarkable circular peptide (RTD-1) derived from two truncated and spliced α-defensin precursors (37). Nothing has been reported about the cathelicidins of this species, except for a recent paper, which described a rhesus cathelicidin that was identical to human hCAP-18 at the nucleotide and peptide level (4). Like this report, we found that only a single cathelicidin, the precursor of RL-37, was expressed in the bone marrow of M. mulatta. However, as discussed above, only 146 of the 170 residues (85.9%) in the rhesus and human sequences were identical.
The signal sequence and cathelin domain of RL-37 showed the typical sequence conservation characteristic of cathelicidins. The amino acid sequence of RL-37′s cathelin domain revealed 66 to 78% identity to the sequences of horse cathelicidin 2 (78%), rabbit CAP-18 (70%), sheep or goat bactenecin-5 (69%), and porcine prophenin-2 (66%). Even RL-37 signal sequence was 70 to 77% identical to cathelicidins from horse, sheep, and goat.
Although RL-37 generally resembled LL-37 in its size, sequence, and ability to adopt an alpha-helical structure, we were intrigued by its greater potency against staphylococci and sought to learn why this occurred. Because electrostatic interactions between cationic antimicrobial peptides and the negatively charged surface molecules (e.g., lipoteichoic acid and/or acidic phospholipids) of staphylococci are likely to influence their antimicrobial properties, we modified LL-37 in a manner that simultaneously increased its net positive charge and eliminated its five acidic residues, without greatly changing its overall configuration (Table 1). The resulting peptide, LL-37 pentamide, had greatly improved potency against S. aureus and methicillin-resistant S. aureus (Table 2).
These findings were consistent with the recent findings of Peschel et al. on staphylococcal dlt gene mutants (27). These investigators reported that the teichoic acids of these mutants were deficient in d-alanine, causing these bacterial macromolecules to have an increased negative surface charge and to show increased binding of cationic (positively charged) proteins relative to wild-type bacteria. These dlt mutants were more sensitive to human α-defensins HNP 1 to 3 and to other cationic antimicrobial peptides. Wild-type strains with additional copies of the dlt operon were less sensitive to these antimicrobial peptides, presumably because their teichoic acids bound the peptides less well. We speculate that the altered charge balance of LL-37 pentamide allowed it to bind (lipo)teichoic acid and anionic phospholipids more effectively and that one or both of these properties was responsible for its increased effectiveness against staphylococcus. This is entirely consistent with Peschel's recent description of a mutant Staphylococcus strain that was hypersensitive to host defense peptide due to its inability to modify phosphatidylglycerol with L-lysine—a reaction that reduces the negative membrane surface charge in wild-type S. aureus (26). They suggested that intrinsic MprF-mediated peptide resistance was most likely based on repulsion of the cationic peptides and that mprF inactivation led to increased binding of antimicrobial peptides by the bacteria.
Skin certainly must be the saltiest surface of the body, since sweat continually delivers salt to the body surface, where its NaCl content undergoes concentration by evaporation (23). Staphylococci normally reside on the skin surface, and most grow well in the presence of high salt concentrations. Normal human skin can express at least two different classes of antimicrobial peptides, β-defensins (HBDs) and the cathelicidin hCAP-18. Whereas the HBD-1 is expressed constitutively, HBD-2 and hCAP-18 are expressed after induction by signals associated with inflammation, injury, and infection. Since defensins (19) and LL-37 (40) are not very effective against staphylococci in the presence of the concentrations of NaCl found in extracellular fluids or at the skin surface, it is not surprising that S. epidermidis can colonize human skin and that recruitment of neutrophils is frequently needed to deal with staphylococcal invasion. Neutrophils are effective because they can expose ingested staphylococci to high concentrations of oxidants as well as to very high concentrations of α-defensins and other antimicrobial molecules that are translocated to its phagocytic vacuoles.
Because we saw a pronounced inhibitory effect of salt on the antimicrobial properties of LL-37 only in our studies with staphylococci, the accumulation of LL-37 in and between viable keratinocytes should still form an effective chemical barrier that would protect against invasion by many microorganisms. Our data clearly show that a high-salt ionic environment, like that found in bulk extracellular fluid, is inimical to the antistaphylococcal activity of LL-37. However, if LL-37 were to accumulate extracellularly in the spaces between keratinocytes, this polycationic peptide might lower the ambient sodium and chloride concentrations of this interstitial microenvironment via a Donnan equilibrium, possibly augmented by active keratinocyte-mediated ion transport. While these musings about interstitial microenvironments are purely speculative, they might eventually prove to be accurate. If so, then LL-37 or the similarly polycationic- and salt-sensitive human β-defensins might play a larger part in deterring staphylococcal invasion than our data would otherwise suggest.
ACKNOWLEDGMENTS
We thank Jennifer Guerrero for assistance in performing antimicrobial assays.
This work was supported, in part, by National Institutes of Health grants AI 22839 and AI 43934.
REFERENCES
- 1.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, Gudmundsson G H. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 2.Agerberth B, Lee J Y, Bergman T, Carlquist M, Boman H G, Mutt V, Jornvall H. Amino acid sequence of PR-39. Isolation from pig intestine of a new member of the family of proline-arginine-rich antibacterial peptides. Eur J Biochem. 1991;202:849–854. doi: 10.1111/j.1432-1033.1991.tb16442.x. [DOI] [PubMed] [Google Scholar]
- 3.Bagella L, Scocchi M, Zanetti M. cDNA sequences of three sheep myeloid cathelicidins. FEBS Lett. 1995;376:225–228. doi: 10.1016/0014-5793(95)01285-3. [DOI] [PubMed] [Google Scholar]
- 4.Bals R, Lang C, Weiner D J, Vogelmeier C, Welsch U, Wilson J M. Rhesus monkey (Macaca mulatta) mucosal antimicrobial peptides are close homologues of human molecules. Clin Diagn Lab Immunol. 2001;8:370–375. doi: 10.1128/CDLI.8.2.370-375.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruch M D, Dhingra M M, Gierasch L M. Side chain-backbone hydrogen bonding contributes to helix stability in peptides derived from an alpha-helical region of carboxypeptidase A. Proteins. 1991;10:130–139. doi: 10.1002/prot.340100206. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y H, Yang J T, Chau K H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974;13:3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- 7.Couto M A, Harwig S S, Cullor J S, Hughes J P, Lehrer R I. Identification of eNAP-1, an antimicrobial peptide from equine neutrophils. Infect Immun. 1992;60:3065–3071. doi: 10.1128/iai.60.8.3065-3071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Sal G, Storici P, Schneider C, Romeo D, Zanetti M. cDNA cloning of the neutrophil bactericidal peptide indolicidin. Biochem Biophys Res Commun. 1992;187:467–472. doi: 10.1016/s0006-291x(05)81517-7. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer P B, Lehrer R I. Mouse neutrophils lack defensins. Infect Immun. 1992;60:3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank R W, Gennaro R, Schneider K, Przybylski M, Romeo D. Amino acid sequences of two proline-rich bactenecins. Antimicrobial peptides of bovine neutrophils. J Biol Chem. 1990;265:18871–18874. [PubMed] [Google Scholar]
- 11.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson G H. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 12.Frohm N M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Stahle-Backdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gennaro R, Scocchi M, Merluzzi L, Zanetti M. Biological characterization of a novel mammalian antimicrobial peptide. Biochim Biophys Acta. 1998;1425:361–368. doi: 10.1016/s0304-4165(98)00087-7. [DOI] [PubMed] [Google Scholar]
- 14.Gennaro R, Zanetti M. Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Biopolymers. 2000;55:31–49. doi: 10.1002/1097-0282(2000)55:1<31::AID-BIP40>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Harwig S S, Kokryakov V N, Swiderek K M, Aleshina G M, Zhao C, Lehrer R I. Prophenin-1, an exceptionally proline-rich antimicrobial peptide from porcine leukocytes. FEBS Lett. 1995;362:65–69. doi: 10.1016/0014-5793(95)00210-z. [DOI] [PubMed] [Google Scholar]
- 16.Harwig S S, Waring A, Yang H J, Cho Y, Tan L, Lehrer R I. Intramolecular disulfide bonds enhance the antimicrobial and lytic activities of protegrins at physiological sodium chloride concentrations. Eur J Biochem. 1996;240:352–357. doi: 10.1111/j.1432-1033.1996.0352h.x. [DOI] [PubMed] [Google Scholar]
- 17.Huttner K M, Lambeth M R, Burkin H R, Burkin D J, Broad T E. Localization and genomic organization of sheep antimicrobial peptide genes. Gene. 1998;206:85–91. doi: 10.1016/s0378-1119(97)00569-6. [DOI] [PubMed] [Google Scholar]
- 18.Johnson W C., Jr Protein secondary structure and circular dichroism: a practical guide. Proteins. 1990;7:205–214. doi: 10.1002/prot.340070302. [DOI] [PubMed] [Google Scholar]
- 19.Kohashi O, Ono T, Ohki K, Soejima T, Moriya T, Umeda A, Meno Y, Amako K, Funakosi S, Masuda M. Bactericidal activities of rat defensins and synthetic rabbit defensins on Staphylococci, Klebsiella pneumoniae (Chedid, 277, and 8N3), Pseudomonas aeruginosa (mucoid and nonmucoid strains), Salmonella typhimurium (Ra, Rc, Rd, and Rd of LPS mutants) and Escherichia coli. Microbiol Immunol. 1992;36:369–380. doi: 10.1111/j.1348-0421.1992.tb02036.x. [DOI] [PubMed] [Google Scholar]
- 20.Kokryakov V N, Harwig S S, Panyutich E A, Shevchenko A A, Aleshina G M, Shamova O V, Korneva H A, Lehrer R I. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327:231–236. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- 21.Lehrer R I, Barton A, Ganz T. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J Immunol Methods. 1988;108:153–158. doi: 10.1016/0022-1759(88)90414-0. [DOI] [PubMed] [Google Scholar]
- 22.Lehrer R I, Rosenman M, Harwig S S, Jackson R, Eisenhauer P. Ultrasensitive assays for endogenous antimicrobial polypeptides. J Immunol Methods. 1991;137:167–173. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- 23.Lim J K, Saliba L, Smith M J, McTavish J, Raine C, Curtin P. Normal saline wound dressing—is it really normal? Br J Plast Surg. 2000;53:42–45. doi: 10.1054/bjps.1999.3246. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney M M, Lee A Y, Brezinski-Caliguri D J, Huttner K M. Molecular analysis of the sheep cathelin family reveals a novel antimicrobial peptide. FEBS Lett. 1995;377:519–522. doi: 10.1016/0014-5793(95)01390-3. [DOI] [PubMed] [Google Scholar]
- 25.Malm J, Sorensen O, Persson T, Frohm-Nilsson M, Johansson B, Bjartell A, Lilja H, Stahle-Backdahl M, Borregaard N, Egesten A. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect Immun. 2000;68:4297–4302. doi: 10.1128/iai.68.7.4297-4302.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peschel A, Jack R W, Otto M, Collins L V, Staubitz P, Nicholson G, Kalbacher H, Nieuwenhuizen W F, Jung G, Tarkowski A, van Kessel K P, van Strijp J A. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with 1-lysine. J Exp Med. 2001;193:1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peschel A, Otto M, Jack R W, Kalbacher H, Jung G, Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 28.Pungercar J, Strukelj B, Kopitar G, Renko M, Lenarcic B, Gubensek F, Turk V. Molecular cloning of a putative homolog of proline/arginine-rich antibacterial peptides from porcine bone marrow. FEBS Lett. 1993;336:284–288. doi: 10.1016/0014-5793(93)80821-b. [DOI] [PubMed] [Google Scholar]
- 29.Romeo D, Skerlavaj B, Bolognesi M, Gennaro R. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J Biol Chem. 1988;263:9573–9575. [PubMed] [Google Scholar]
- 30.Scocchi M, Wang S, Zanetti M. Structural organization of the bovine cathelicidin gene family and identification of a novel member. FEBS Lett. 1997;417:311–315. doi: 10.1016/s0014-5793(97)01310-0. [DOI] [PubMed] [Google Scholar]
- 31.Selsted M E, Novotny M J, Morris W L, Tang Y Q, Smith W, Cullor J S. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J Biol Chem. 1992;267:4292–4295. [PubMed] [Google Scholar]
- 32.Shamova O, Brogden K A, Zhao C, Nguyen T, Kokryakov V N, Lehrer R I. Purification and properties of proline-rich antimicrobial peptides from sheep and goat leukocytes. Infect Immun. 1999;67:4106–4111. doi: 10.1128/iai.67.8.4106-4111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorensen O, Arnljots K, Cowland J B, Bainton D F, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 34.Steinberg D, Lehrer R I. Designer assays for antimicrobial peptides: disputing the “one size fits all” theory. In: Shafer W M, editor. Methods in molecular biology. Totowa, N.J: Humana Press; 1997. pp. 169–187. [DOI] [PubMed] [Google Scholar]
- 35.Storici P, Scocchi M, Tossi A, Gennaro R, Zanetti M. Chemical synthesis and biological activity of a novel antibacterial peptide deduced from a pig myeloid cDNA. FEBS Lett. 1994;337:303–307. doi: 10.1016/0014-5793(94)80214-9. [DOI] [PubMed] [Google Scholar]
- 36.Tang Y Q, Yuan J, Miller C J, Selsted M E. Isolation, characterization, cDNA cloning, and antimicrobial properties of two distinct subfamilies of alpha-defensins from rhesus macaque leukocytes. Infect Immun. 1999;67:6139–6144. doi: 10.1128/iai.67.11.6139-6144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Y Q, Yuan J, Osapay G, Osapay K, Tran D, Miller C J, Ouellette A J, Selsted M E. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 38.Tossi A, Seocchi M, Zanetti M, Storici P, Gennaro R. PMAP-37, a novel antibacterial peptide from pig myeloid cells. cDNA cloning, chemical synthesis and activity. Eur J Biochem. 1995;228:941–946. doi: 10.1111/j.1432-1033.1995.tb20344.x. [DOI] [PubMed] [Google Scholar]
- 39.Travis S M, Anderson N N, Forsyth W R, Espiritu C, Conway B D, Greenberg E P, McCray P B, Jr, Lehrer R I, Welsh M J, Tack B F. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun. 2000;68:2748–2755. doi: 10.1128/iai.68.5.2748-2755.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner J, Cho Y, Dinh N N, Waring A J, Lehrer R I. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woody R W. Circular dichroism of peptides. In: Blout E R, Bovey F A, Goodman M, Lotan N, editors. The peptides. Vol. 7. New York, N.Y: Academic Press; 1985. pp. 15–114. [Google Scholar]
- 42.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 43.Zanetti M, Storici P, Tossi A, Scocchi M, Gennaro R. Molecular cloning and chemical synthesis of a novel antibacterial peptide derived from pig myeloid cells. J Biol Chem. 1994;269:7855–7858. [PubMed] [Google Scholar]
- 44.Zhao C, Liu L, Lehrer R I. Identification of a new member of the protegrin family by cDNA cloning. FEBS Lett. 1994;346:285–288. doi: 10.1016/0014-5793(94)00493-5. [DOI] [PubMed] [Google Scholar]
- 45.Zlokarnik G, Negulescu P A, Knapp T E, Mere L, Burres N, Feng L, Whitney M, Roemer K, Tsien R Y. Quantitation of transcription and clonal selection of single living cells with beta-lactamase as reporter. Science. 1998;279:84–88. doi: 10.1126/science.279.5347.84. [DOI] [PubMed] [Google Scholar]