Abstract
Background
Anlotinib, an oral small molecule tyrosine kinase inhibitor targeting VEGFR 1/2/3, FGFR 1-4, PDGFR a/β, and c-kit, had demonstrated prolonged progression-free survival (PFS) in refractory metastatic colorectal cancer (mCRC). This multicenter, single-arm, phase II, exploratory study was conducted to evaluate the efficacy and safety of anlotinib combined with capecitabine and oxaliplatin as first-line treatment for unresectable RAS/BRAF wild-type mCRC.
Methods
Patients aged 18–75 with RAS/BRAF wild-type unresectable mCRC, without prior systemic treatment, and ECOG performance status ≤1 were enrolled. Eligible patients received capecitabine (850 mg/m2, p.o., bid, on day 1–14 every 21 days), oxaliplatin (130 mg/m2, i.v., on day 1 every 21 days), and anlotinib (12 mg, p.o., qd, on days 1–14 every 21 days) as induction therapy. Following 6 cycles of therapy, patients who achieved response or stable disease received capecitabine and anlotinib as maintenance therapy until tumor progression. The primary endpoint was objective response rate (ORR) according to RECIST (version: 1.1), and the secondary endpoints were PFS, disease control rate (DCR), duration of response (DOR), and safety.
Results
Between November 2019 and February 2021, 31 patients were enrolled. One patient was excluded for refusing treatment. The primary endpoint of ORR was 76.7% (95% CI, 57.7–90.1) with 1 patient achieving a complete response and 22 patients partial response. DCR was 93.3% (95% CI, 77.9–99.2). At a median follow-up of 14.1 months (95% CI, 9.9–18.3), median PFS was 11.3 months (95% CI, 7.1–14.1), and DOR was 7.9 months (95% CI, 5.5–12.7). Twenty-five (83.3%) patients experienced grade 3 or 4 treatment-emergent adverse events (TEAEs). No grade 5 TEAE was reported. The most common grade 3 or 4 TEAEs (>10%) were hypertension (15/30; 50%), neutrophil count decreased (8/30; 26.7%), and diarrhea (4/30; 13.3%). A total of 18 (60%) patients had TEAEs that resulted in dose reduction, interruptions, or delays.
Conclusions
Anlotinib combined with capecitabine and oxaliplatin showed considerable ORR, DCR, PFS, and DOR in the first-line therapy of mCRC with manageable toxicity profiles.
Trial registration
Keywords: Metastatic colorectal cancer, Anlotinib, Capecitabine, Oxaliplatin, First-line therapy
Background
Colorectal cancer (CRC) is the third most prevalent malignancy worldwide and is ranked as the second largest contributor to fatalities of patients after lung cancer. In 2020, more than 1.9 million CRC cases were diagnosed globally, with expected 935,000 deaths [1]. At the time of diagnosis, up to 20% of patients had metastatic disease. As the disease progressed, 40% of individuals with CRC had developed metastases [2]. In the case of metastatic colorectal cancer (mCRC), the prognosis is unsatisfied, with a 5-year survival rate of below 20% [3].
The conventional therapy for mCRC includes irinotecan, fluoropyrimidine, or oxaliplatin plus anti-vascular endothelial growth factor (VEGF) monoclonal antibodies or anti-epidermal growth factor receptor (EGFR) monoclonal antibodies, including bevacizumab, panitumumab, or cetuximab—based on Ras/Raf status. These treatment regimens significantly prolonged patients’ overall survival (OS) from 6 to ~20 months [4–7]. Immune therapy is used only for a small proportion of mCRC patients (4%) with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) [8].
Anlotinib is an orally administered small molecule tyrosine kinase inhibitor (TKI) that targeted tyrosine kinases, including VEGF receptor 1/2/3, the fibroblast growth factor receptor (FGFR) 1–4, platelet-derived growth factor receptor (PDGFR) α/β, and c-Kit, and has a broad spectrum of inhibitory effects on tumor angiogenesis and growth [9, 10]. Clinical studies have confirmed the effectiveness of anlotinib in several advanced malignant cancers, including non-small cell lung cancer, small cell lung cancer, soft tissue sarcoma, medullary thyroid cancer, and renal cell carcinoma [11–15]. The phase III trial ALTER-0703 demonstrated the efficacy and tolerability of anlotinib monotherapy in mCRC patients who failed to achieve remission after standard treatment [16]. Anlotinib significantly prolonged the progression-free survival (PFS) of mCRC patients over placebo (4.1 months versus 1.5 months; HR = 0.34; P < 0.0001), while the median OS in the anlotinib group and placebo group was similar (8.6 months versus 7.2 months; HR = 1.02; p = .870). The subgroup analysis demonstrated the OS benefit of anlotinib in patients with KRAS/NRAS/BRAF wild-type mCRC (HR = 0.68, 0.47–0.99). Therefore, patients with RAS/BRAF wild-type mCRC might be potential candidates for anlotinib therapy.
A phase I/II study was conducted to investigate the safety and efficacy of anlotinib plus irinotecan in patients with advanced CRC who had received initial treatment. This trial demonstrated that the combination of anlotinib and irinotecan was a promising second-line therapy for advanced CRC with a manageable safety profile [17].
The safety and efficacy of anlotinib in combination with chemotherapy as a first-line treatment for mCRC have not been investigated previously as far as we know. The objective of the present study was to determine the efficacy and tolerability of anlotinib combined with oxaliplatin and capecitabine as a first-line therapy for RAS/BRAF wild-type mCRC.
Methods
Study design and participants
The ALTER-C-002 trial was a multicenter, single-arm, phase II, exploratory study (NCT04080843) conducted at 3 centers in Zhejiang, China. Each center had independent ethics committee that granted approval for the research protocol. The trial was performed in accordance with the principles of Good Clinical Practice and Declaration of Helsinki [18], as well as all relevant regulations and laws in the applicable countries. Before participating in the trial, all patients gave written informed consent.
Eligible patients should be aged between 18 and 75 with histologically or cytologically confirmed colon and rectum adenocarcinoma and RAS/BRAF wild-type. Patients were confirmed to have unresectable lesions or to have a metastatic disease without potentially resectable disease. RAS/BRAF wild-type mCRC patients who did not receive previous systemic therapy for mCRC or who received neoadjuvant/adjuvant chemotherapy for stages I–III CRC relapsed more than 6 months from the last administration of peri-operation chemotherapy were included. Other inclusion criteria were patients that had at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [19], Eastern Cooperative Oncology Group performance status of 0 or 1, adequate bone marrow, liver, and renal function, and a minimum of three-month predicted survival time duration. Primary exclusion criteria included previous treatment with anti-VEGF therapy or TKIs, and uncontrolled hypertension (systolic blood pressure >150 mm/Hg or diastolic blood pressure >90 mm/Hg) despite adequate care.
Procedures
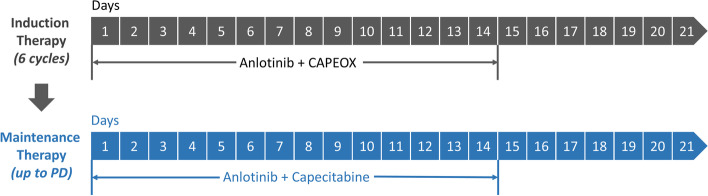
Enrolled patients received capecitabine (850 mg/m2, p.o., bid, on days 1–14, every 21 days), oxaliplatin (130 mg/m2, i.v., on day 1, every 21 days), and anlotinib (12 mg, p.o., qd. on days 1–14, every 21 days). Patients who achieved response or stable disease (SD) were then administered with capecitabine and anlotinib as maintenance treatment after completing 6 cycles of induction therapy. An overview of the therapy procedures is shown in Fig. 1.
Fig. 1.
Therapy procedures (21-day cycle). Oxaliplatin:130mg/m2, ivgtt, d1; anlotinib: 12mg, po, qd, d1–14; capecitabine: 850mg/m2, po, bid, d1–14
The medication was continued until tumor progression (according to RECIST version 1.1), unacceptable toxicity, withdraw of patients’ consent, death, or investigator’s decision to discontinue the treatment. Patients could continue to receive the remaining medications even if one of the drugs had to be discontinued due to toxicity. Dose modifications were conducted for any drug of this combination regimen in order to manage drug-related toxicities. Dosage of anlotinib could be reduced to 10 mg or even 8 mg. Dosage of oxaliplatin could be modified to 75% of the initial one, while that of capecitabine was allowed to be altered to 75% or even 50% of the initial one.
Endpoints
The primary endpoint was objective response rate (ORR) based on investigator’s assessment according to RECIST version 1.1. ORR was defined as the proportion of patients achieved a best overall tumor response of complete response (CR) or partial response (PR). Secondary endpoints included PFS, disease control rate (DCR; refers to the proportion of patients with response and stable disease), duration of response (DOR), safety, and tolerability. PFS was defined as the time from the day when patients received the first dose of treatment regimen to the date of first documented progression or death from any cause, which ever firstly occurred, including induction and maintenance therapy. DOR was defined as the duration from the day when patients firstly had response to the day they had progressive disease. Severity of adverse events (AEs) was assessed according to the Common Terminology Criteria Adverse Events (NCI CTC AE version 4.03).
Assessments
Tumor evaluation was performed by computed tomography or magnetic resonance imaging during the screening period and every two cycles throughout the study until disease progression. Survival evaluation was performed every 2 months until death or withdrawal of consent. Safety was recorded continuously until 30 days after the end of treatment.
Statistical analysis
The objective of the study was to explore whether anlotinib combined with oxaliplatin and capecitabine could substantially enhance the ORR when compared to that of other studies of chemotherapies. The expected sample size was calculated according to the alternative hypothesis that the ORR with anlotinib plus oxaliplatin and capecitabine would be 61% or higher (H1=61%) and the null hypothesis that the ORR would be 31% or lower (H0=31%) [20–25]. With α of 5% and power of 90%, 27 cases would be recruited using the Clopper-Pearson method (PASS version 15, NSCC, LLC). Considering an approximate drop-out incidence of 10%, a total of 30 patients would be recruited.
The full analysis set (FAS) and safety analysis set (SAS), both of which comprised patients receiving a minimum of one dosage of anlotinib, were used to conduct the efficacy and safety evaluations, respectively. ORR and DCR were analyzed based on the Clopper-Pearson method. To assess the median value and 95% confidence interval (CI) of PFS, DOR, duration of treatment (DOT), duration of maintenance therapy, and follow-up duration, a Kaplan-Meier (KM) analysis was performed. All statistical analyses were carried out using SAS version 9.4 (SAS Institute Inc).
Results
Patient demographics and characteristics
Patient recruitment was initiated in November 2019 and ended in February 2021 when the desired number of patients was reached.
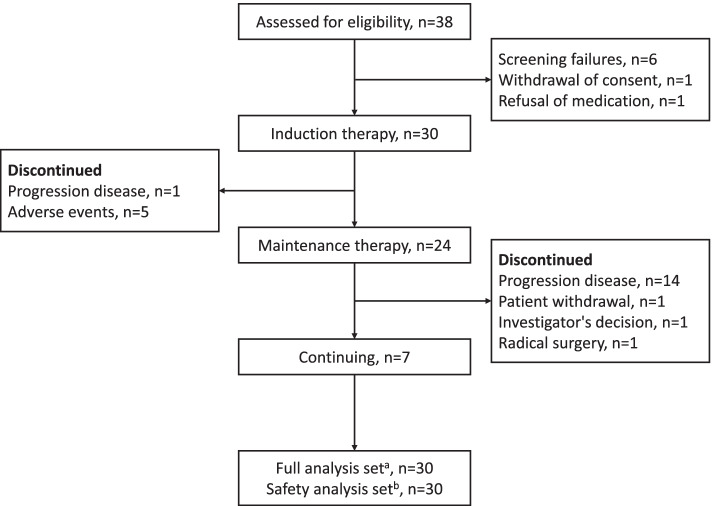
Thirty eight patients were screened. Among these patients, 31 met the inclusion criteria with 1 patient excluded for refusing treatment. A total of 30 patients were included in FAS and SAS (Fig. 2). Patient demographic and baseline characteristics are presented in Table 1. Patients’ age ranged between 32 and 72 years with the median age being 60 years. The ratio of males to females was 26 (86.7%): 4 (13.3%). Three patients (10.0%) had an ECOG PS score of 0, while 27 patients (90.0%) had a score of 1. The left colon and rectum were found as primary tumor locations in 26 individuals (86.7% ). The majority of participants were found to have liver metastases (25; 83.3% ) and 25 patients (83.3%) had synchronous metastasis. Nineteen patients (63.3%) had primary tumors resected; among them, 5 received radical surgery.
Fig. 2.
CONSORT diagram. 30 patients had ≥1 dose of the treatment regimen. b 30 patients received ≥1 dose of treatment regimen and safety had been recorded after administration. c The data cutoff date was October 15, 2021
Table 1.
Patient demographic and baseline characteristics (full analysis set, N=30)
| Characteristics | n | % |
|---|---|---|
| Male/female ratio | 26/4 | 86.7:13.3 |
| Age (years) | ||
| Median (range) | 60 (32–72) | |
| > 65 | 5 | 16.7 |
| ECOG performance status | ||
| 0 | 3 | 10.0 |
| 1 | 27 | 90.0 |
| TNM stage | ||
| IV | 30 | 100 |
| CEA(ng/ml) | ||
| < 5 | 2 | 6.7 |
| 5–200 | 16 | 53.3 |
| > 200 | 10 | 33.3 |
| Unknown | 2 | 6.7 |
| Primary disease site | ||
| Colon | 13 | 43.3 |
| Rectum | 16 | 53.3 |
| Colon and rectum | 1 | 3.3 |
| Primary tumor location | ||
| Right | 4 | 13.3 |
| Left colon and rectum | 26 | 86.7 |
| Primary tumor resected | ||
| Yes | 19 | 63.3 |
| No | 11 | 36.7 |
| Metastatic sites | ||
| Liver only | 16 | 53.3 |
| Liver + other | 9 | 30 |
| Other only | 5 | 16.7 |
| Metastatic status | ||
| Synchronous | 25 | 83.3 |
| Metachronous | 5 | 16.7 |
| Diameter of the largest target lesions (mm) | ||
| median (range)a | 45 (16–122) | |
| MSI/MMR statusb | ||
| MSS/pMMR | 25 | 83.3 |
| MSI-H/dMMR | 0 | 0 |
| Unknow | 5 | 16.7 |
ECOG, Eastern Cooperative Oncology Group; TNM, tumor, node, metastasis; CEA, carcinoembryonic antigen; MSI, micro satellite instability; MMR, mismatch repair
aThe median is determined by IQR
bThirteen patients received the MSI test by PCR and MMR test by immunohistochemistry, and 9 patients only received the MMR test by immunohistochemistry and 3 patients only received MSI test by PCR
Twenty-four patients who achieved response or SD after induction therapy subsequently received maintenance therapy. Seven patients were still treated with maintenance therapy at the data cut-off day (15 October 2021), and the median follow-up duration was 14.1 months (95% CI, 9.9–18.3).
Efficacy
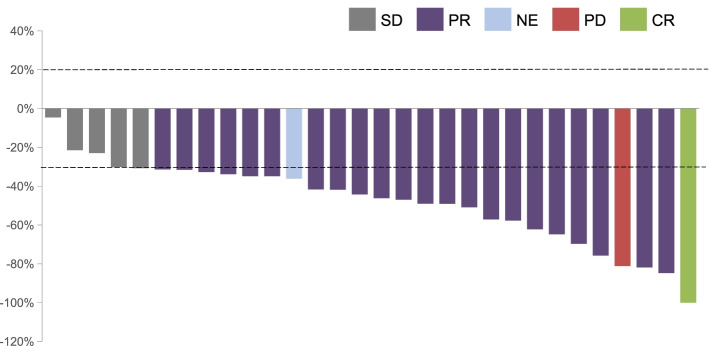
Of 30 patients in the FAS, a confirmed ORR was observed in 76.7% (95% CI, 57.7–90.1) of patients (23/30; 1 patient achieved confirmed CR and 22 patients achieved confirmed PR), while DCR was 93.3% (95% CI, 77.9–99.2); 1 patient had progressive disease (PD) in the second evaluation and 1 patient had inevaluable (NE) (PR in the first evaluation; however, the patient failed to take the second evaluation) (Table 2). All 30 patients had tumor shrank and the best change in target lesion diameter from baseline is shown in Fig. 3.
Table 2.
Investigator-assessed response utilizing RECIST (version: 1.1)
| Best overall response | Anlotinib + oxaliplatin + capecitabine (n = 30) |
|---|---|
| CR, n (%) | 1 (3.3) |
| PR, n (%) | 22 (73.3) |
| SD, n (%) | 5 (16.7) |
| PD, n (%) | 1 (3.3) |
| NE, n (%) | 1 (3.3) |
| ORRa, n (%, 95%CI) | 23 (76.7,57.7–90.1) |
| DCRb, n (%, 95%CI) | 28 (93.3, 77.9–99.2) |
RECIST, Response Evaluation Criteria in Solid Tumors; ORR, objective response rate; DCR, disease control rate; CI, confidence interval
aORR was defined as the proportion of patients with a best overall tumor response, CR, or PR
bDCR refers to the proportion of patients with response and stable disease
Fig. 3.
The best change in target lesion diameter from baseline (full analysis set, N = 30)
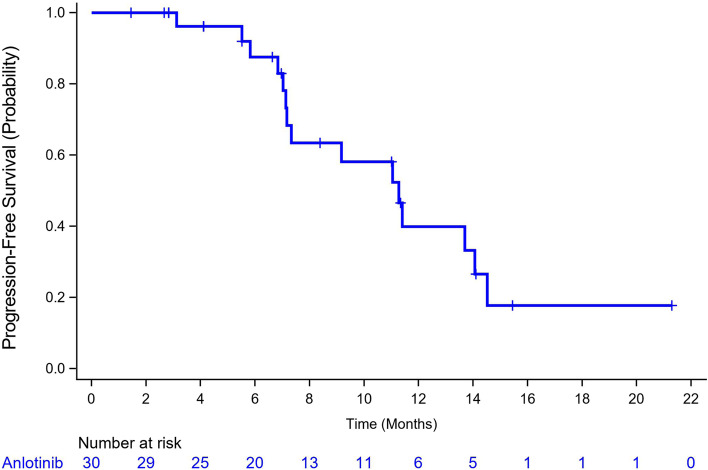
The median PFS according to Kaplan–Meier estimations was 11.3 months (95% CI, 7.1–14.1) (Fig. 4). The median PFS in responders (n = 23) was 13.7 months (95% CI, 7.2–14.5), while those with stable and progressive disease (n = 6) had a median PFS of 7.3 months (95% CI, 3.1–11.4). The PFS of maintenance treatment was also analyzed, which was named as PFS2 and defined as the time from maintenance treatment to disease progression. The median PFS2 was 7.1 months (95% CI, 4.5–9.8).
Fig. 4.
Estimates of the progression-free survival using Kaplan-Meier analysis (full analysis set, N = 30)
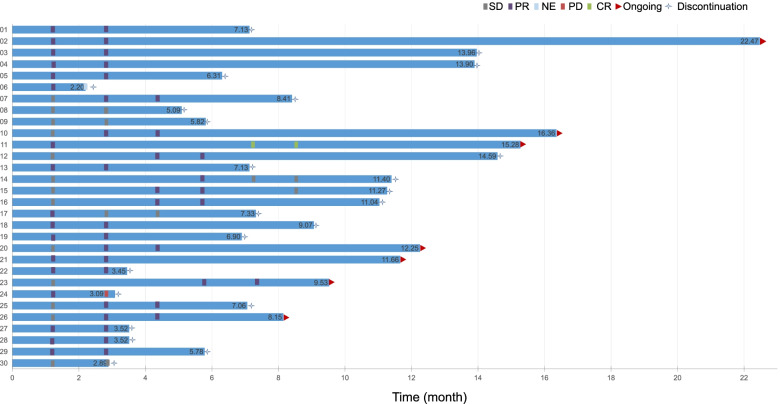
The median DOR was 7.9 months (95% CI, 5.5–12.7). Despite the fact that it was not a pre-defined outcome, we assessed the investigator-reported DOT. The median DOT was 7.9 months (95% CI, 6.3–11.4) and 1 patient has received this regimen for at least 22 months (Fig. 5). The median duration of maintenance treatment was 6.9 months (95% CI, 3.0–9.8) (Table 3).
Fig. 5.
Swimmer plots of patients. Patients who received anlotinib plus oxaliplatin and capecitabine as first-line therapy in RAS/BRAF wild-type unresectable mCRC
Table 3.
Treatment duration and maintenance treatment duration
| Treatment duration | N = 30 |
|---|---|
| Months, median (95% CI) | 7.9 (6.3–11.4) |
| Cycles, median (range) | 11 (3–32) |
| Patients on treatment >6 months, n (%) | 21 (70) |
| Patients on treatment >1 year, n (%) | 7 (23.3) |
| Maintenance treatment duration | N = 24 |
| Months, median (95% CI) | 6.9 (3.0–9.8) |
| Patients on maintenance treatment >6 months, n (%) | 11 (36.7) |
| Patients on maintenance treatment >1 year, n (%) | 2 (6.7) |
By post hoc analysis, factors including primary tumor location, primary tumor resected, metastatic sites, and serum carcinoembryonic antigen (CEA) level before chemotherapy were not associated with PFS.
Safety
At least 1 treatment-emergent adverse event (TEAE) occurred in each of the 30 patients receiving the experimental treatment over the course of the trial. The most frequent TEAEs (≥10%) were presented in Table 4. A total of 25 (83.3%) patients experienced grade 3 or 4 TEAEs. Grade 5 TEAEs were not reported. The most frequent grade 3 or 4 TEAEs (≥10%) were hypertension (15/30; 50%), neutrophil count decreased (8/30; 26.7%), diarrhea (4/30; 13.3%), aspartate aminotransferase increased (3/30; 10.0%), platelet count decreased (3/30; 10%), hypertriglyceridemia (3/30,10%), and palmar-plantar erythrodysesthesia syndrome (3/30; 10%). The most common anlotinib-related TEAE of hypertension can be controlled by optimal management and does not lead to dose reduction. The peripheral neurotoxicity and hematotoxicity could be ascribed to oxaliplatin, while palmar-plantar erythrodysesthesia syndrome was the most frequent AE due to capecitabine. Only 1 case required anlotinib interruption due to the palmar-plantar erythrodysesthesia syndrome. Serious TEAEs (SAEs) including abnormal liver function, acute appendicitis, electrolyte disturbance, bone marrow suppression, intestinal obstruction, and colonic perforation occurred in 6 (20.0%) patients, while 4 patients (13.3%) were reported to have experienced drug-related SAEs. Four patients’ SAEs alleviated or recovered, while the others did not.
Table 4.
Treatment-emergent adverse events occurring in ≥10% of patients (safety population, N = 30)
| TEAE, n (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any grade |
|---|---|---|---|---|---|
| Hypertension | 11 (36.7%) | 15 (50%) | 26 (86.7%) | ||
| Palmar-plantar erthrodysesthesia syndrome | 10 (33.3%) | 9 (30%) | 3 (10%) | 22 (73.3%) | |
| White blood cell decreased | 12 (40%) | 7 (23.3%) | 2 (6.7%) | 21 (70%) | |
| Neutrophil count decreased | 7 (23.3%) | 5 (16.7%) | 8 (26.7%) | 20 (66.7%) | |
| Vomiting | 11 (36.7%) | 2 (6.7%) | 2 (6.7%) | 15 (50%) | |
| Decreased appetite | 11 (36.7%) | 4 (13.3%) | 15 (50%) | ||
| Nausea | 11 (36.7%) | 2 (6.7%) | 13 (43.3%) | ||
| Malaise | 11 (36.7%) | 1 (3.3%) | 12 (40%) | ||
| Platelet count decreased | 7 (23.3%) | 2 (6.7%) | 1 (3.3%) | 2 (6.7%) | 12 (40%) |
| Diarrhea | 4 (13.3%) | 2 (6.7%) | 4 (13.3%) | 10 (33.3%) | |
| Peripheral neurotoxicity | 8 (26.7%) | 1 (3.3%) | 1 (3.3%) | 10 (33.3%) | |
| Alanine aminotransferase increased | 5 (16.7%) | 3 (10%) | 1 (3.3%) | 9 (30.0%) | |
| Aspartate aminotransferase increased | 4 (13.3%) | 2 (6.7%) | 3 (10.0%) | 9 (30.0%) | |
| Constipation | 4 (13.3%) | 2 (6.7%) | 6 (20%) | ||
| Toothache | 5 (16.7%) | 1 (3.3%) | 6 (20%) | ||
| Fever | 5 (16.7%) | 5 (16.7%) | |||
| Abdominal pain | 3 (10%) | 1 (3.3%) | 1 (3.3%) | 5 (16.7%) | |
| Thyroid-stimulating hormone increased | 3 (10%) | 1 (3.3%) | 4 (13.3%) | ||
| Hypertriglyceridemia | 1 (3.3%) | 1 (3.3%) | 2 (6.7%) | 4 (13.3%) | |
| Dizziness | 4 (13.3%) | 4 (13.3%) | |||
| Proteinuria | 2 (6.7%) | 1 (3.3%) | 3 (10%) | ||
| Hypokalemia | 2 (6.7%) | 1 (3.3%) | 3 (10%) | ||
| Hyponatremia | 1 (3.3%) | 1 (3.3%) | 1 (3.3%) | 3 (10%) | |
| Cough | 2 (6.7%) | 1 (3.3%) | 3 (10%) | ||
| Upper respiratory infection | 3 (10%) | 3 (10%) | |||
| Weight loss | 1 (3.3%) | 2 (6.7%) | 3 (10%) | ||
| Headache | 3 (10%) | 3 (10%) |
A total of 18 (60%) patients had TEAEs that resulted in dose reduction, interruptions, or delays. Five patients discontinued therapy at the induction stage due to TEAEs. Reasons for discontinuations were abnormal liver function (grade 4), bone marrow suppression (grade 3), palmar-plantar erythrodysesthesia syndrome (grade 2), and colonic perforation (grade 4).
During the study, 4 patients underwent surgery after anlotinib administration, including 2 patients who underwent surgery for intestinal obstruction after anlotinib discontinuation for 7 or 10 days, and the other 2 received radical surgery considering the benefits of patients after anlotinib discontinuation for 8 or 10 days. No bleeding and anastomotic fistula were observed during the perioperative period.
Discussion
The ALTER-C-002 study reached its primary endpoint, with patients treated with anlotinib, oxaliplatin, and capecitabine showing an improved ORR compared with those receiving conventional treatment. It had been seen that the ORR of chemotherapy alone was 35%–55% while the ORR in this trial was 76.7% [26, 27]. The addition of anlotinib to oxaliplatin and capecitabine resulted in tumor shrinkage in all patients. Although it was a non-head-to-head study, anlotinib combined with oxaliplatin and capecitabine therapy correlated with a longer median PFS (11.3 months) when compared with CAPEOX alone (8.0 months) [26, 27], suggesting synergistic action of anlotinib, oxaliplatin, and capecitabine.
Moreover, the ORR and median PFS in our study were comparable to historical data from patients with RAS/BRAF wild-type disease who received chemotherapy in combination with bevacizumab (ORR 55.2%; median PFS 10.6 months) or cetuximab (ORR 59.6%; median PFS 10.5 months) [28].
The ALTER-C-002 study was designed in such a way that any component of the therapy might be modified in order to manage AEs. Under the guidance of this customized strategy, we discovered that this combined regimen seemed to have a manageable tolerability profile when used as first-line therapy for patients with RAS/BRAF wild-type mCRC. Overall, the AE profile of the combined anlotinib, oxaliplatin, and capecitabine therapy seemed to be perfectly compatible, without unexpected AEs. Moreover, the incidence of palmar-plantar erythrodysesthesia syndrome did not increase when anlotinib was added. Besides chemotherapy-related TEAEs, some anlotinib-related TEAEs, including hypertension and proteinuria, were identified. In our study, we reduced the dosage of capecitabine to 850 mg/m2 to minimize palmar-plantar erythrodysesthesia syndrome. Ten patients required dose reduction of capecitabine for TEAEs like palmar-plantar erythrodysesthesia syndrome, platelet count decreased, abnormal liver function, and weight loss. Besides, 10 patients took oxaliplatin dose reduction due to peripheral neurotoxicity, hematotoxicity, vomiting, weight loss, and abnormal liver function. Four patients required dose reduction of anlotinib due to palmar-plantar erythrodysesthesia syndrome, platelet count decreased, gum bleeding, and proteinuria.
Interestingly, although the treatment duration was not a pre-specified endpoint, 7 patients received the study medication for longer than 1 year, with the median DOT of 7.9 months (95% CI, 6.3–11.4). Moreover, 1 patient has received this regimen for at least 22 months. The extended DOT reported, even though in a phase II research, differed from findings reported in phase III trials of other first-line treatments for mCRC, where the DOT was seldom longer than 6 months [26, 27].
Maintenance treatment is very important after the initiation of induction therapy, and a variety of drugs have been evaluated for maintenance therapy. Although progress has been made in recent years, the best maintenance regimen that balances effectiveness with safety and costs has not been developed. Following completion of a phase III study, it was discovered that maintenance treatment with a single agent of capecitabine might be an effective treatment alternative since the PFS in the capecitabine maintenance group was statistically significantly prolonged as opposed to that in the observation group (6.43 months versus 3.43 months; HR = 0.54, P < 0.001) [29]. Comparing the current trial to earlier research, the median PFS2 was 7.1 months, which is comparable to fluoropyrimidine with bevacizumab (6.3 months) or capecitabine with bevacizumab (8.5 months) [30, 31]. However, the difference is that anlotinib is an oral, small molecule, multi-target TKI. The participants could avoid central venous catheterization for the treatment, which not only reduced a series of catheter-related complications [32, 33], such as venous thrombosis and infection, but also reduced the length of stay in treatment facility. Moreover, during maintenance treatment, patients do not need any intravenous infusion, which heavily improved patients’ medical compliance and quality of life, especially in the Covid-19 era. In the study, 13 patients received FOLFIRI plus cetuximab therapy after PD. Among these patients, all received more than one tumor evaluation, with 8 PR, 3 SD, and 2 PD cases. This indicated that anlotinib usage does not influence the efficacy of cetuximab plus chemotherapy, which may benefit the OS.
The ALTER-C-002 trial has several limitations, including a single-arm design, a limited sample size, and the absence of an OS analysis. In addition, this non-global trial was only carried out in Zhejiang province in China. In spite of the limitations, the ORR and PFS in this research were substantially better than those obtained in regimens incorporating other TKIs [34–37]. It may be possible to determine whether this theorized effect translates into prolonged OS once data are available from longer-term follow-up of patients in ALTER-C-002.
Conclusions
In conclusion, anlotinib combined with oxaliplatin and capecitabine achieved considerable ORR, DCR, and PFS and showed potential efficacy as first-line therapy for mCRC with manageable toxicity profiles. No new safety signals were identified with anlotinib when combined with capecitabine and oxaliplatin. Our findings could also provide a framework for additional insight into the application of anlotinib with capecitabine as a maintenance medication in patients with RAS/BRAF wild-type mCRC who have obtained clinical benefits from induction therapy. The efficacy of this treatment is comparable to standard treatment in mCRC. We are launching a randomized, multicenter, phase 3 trial (NCT04854668) to further assess the efficacy of this regimen.
Acknowledgements
All the co-authors would like to thank the patients and their families, the study investigators, coordinators, and research staff. Anlotinib was provided by Chia Tai Tianqing Pharmaceutical Group CO. Ltd.
Abbreviations
- CEA
Carcinoembryonic antigen
- CI
Confidence interval
- CRC
Colorectal cancer
- CR
Complete response
- DCR
Disease control rate
- DOR
Duration of response
- DOT
Duration of treatment
- ECOG PS
Eastern Cooperative Oncology Group performance status
- FAS
Full analysis set
- FGFR
Fibroblast growth factor receptor
- KM
Kaplan-Meier
- mCRC
Metastatic CRC
- NE
Inevaluable
- NCI CTC AE
National Cancer Institute Common Terminology Criteria for Adverse Events
- OS
Overall survival
- ORR
Objective response rate
- PD
Progressive disease
- PDGFR
Platelet-derived growth factor receptor
- PFS
Progression-free survival
- PR
Partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- SAEs
Serious TEAEs
- SD
Stable disease
- SAS
Safety analysis set
- TKIs
Tyrosine kinase inhibitors
- TEAE
Treatment-emergent adverse event
- VEGFR
Vascular endothelial growth factor receptor
Authors’ contributions
KFD and YL were responsible for trial conception and design. YL and QX analyzed the data and drafted the manuscript. QX, JJH, JLD, JQC, ZL, JPW, LFS, DX, JL, XJL, JWW, YBC, CC, ZKJ, and YY managed patients and collected data; LHW evaluated images; YL had drafted the work; and KFD and QX substantively revised it. All authors critically reviewed drafts of the manuscript and approved the final manuscript.
Funding
This study was funded by grants from the National Natural Science Foundation of China (No. 81902818 to Y Liu, No.82072360 to Q Xiao, and No.82072624 to KF Ding), the Natural Science Foundation of Zhejiang Province (LBY20H160002 to Q Xiao), and Project of the regional diagnosis and treatment center of the Health Planning Committee (No. JBZX-201903 to KF Ding). Funding agencies had no role in the design of the study; collection, analysis, and interpretation of data; and writing the manuscript.
Availability of data and materials
The datasets generate and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Human Ethics Committee of The Second Affiliated Hospital of Zhejiang University School of Medicine (IR2019001114, initial approval: 11/08/2019), Zhejiang Cancer Hospital (IRB-2019-159, initial approval: 08/11/2019), and Zhejiang University Jinhua Hospital, (2020-186-001, initial approval: 07/05/2020).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yue Liu and Qian Xiao contributed equally to this work.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 4.Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 5.Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 6.Guo Y, Xiong BH, Zhang T, Cheng Y, Ma L. XELOX vs. FOLFOX in metastatic colorectal cancer: an updated meta-analysis. Cancer Invest. 2016;34(2):94–104. doi: 10.3109/07357907.2015.1104689. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G) Ann Oncol. 2016;27(8):1539–1546. doi: 10.1093/annonc/mdw206. [DOI] [PubMed] [Google Scholar]
- 8.Grothey A. Pembrolizumab in MSI-H-dMMR advanced colorectal cancer - a new standard of care. N Engl J Med. 2020;383(23):2283–2285. doi: 10.1056/NEJMe2031294. [DOI] [PubMed] [Google Scholar]
- 9.Syed YY. Anlotinib: First Global Approval. Drugs. 2018;78(10):1057–1062. doi: 10.1007/s40265-018-0939-x. [DOI] [PubMed] [Google Scholar]
- 10.Lin B, Song X, Yang D, Bai D, Yao Y, Lu N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRbeta and FGFR1. Gene. 2018;654:77–86. doi: 10.1016/j.gene.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. doi: 10.1001/jamaoncol.2018.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L, et al. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled Phase 2 study. Br J Cancer. 2021;125(3):366–371. doi: 10.1038/s41416-021-01356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res. 2018;24(21):5233–5238. doi: 10.1158/1078-0432.CCR-17-3766. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Chi Y, Chen X, Ge M, Zhang Y, Guo Z, et al. Anlotinib in locally advanced or metastatic medullary thyroid carcinoma: a randomized, double-blind phase IIB Trial. Clin Cancer Res. 2021;27(13):3567–3575. doi: 10.1158/1078-0432.CCR-20-2950. [DOI] [PubMed] [Google Scholar]
- 15.Zhou AP, Bai Y, Song Y, Luo H, Ren XB, Wang X, et al. Anlotinib versus sunitinib as first-line treatment for metastatic renal cell carcinoma: a randomized phase II clinical trial. Oncologist. 2019;24(8):e702–e708. doi: 10.1634/theoncologist.2018-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi Y, Shu Y, Ba Y, Bai Y, Qin B, Wang X, et al. Anlotinib monotherapy for refractory metastatic colorectal cancer: a double-blinded, placebo-controlled, randomized phase III trial (ALTER0703) Oncologist. 2021;26(10):e1693–e1703. doi: 10.1002/onco.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B, Wang X, Zhu L, Li Q, Huang J. Combination of anlotinib and irinotecan in the second-line treatment of patients with advanced colorectal cancer: An open-label, multicenter phase I/II study. J Clin Oncol. 2020;38(15_suppl):e16036. doi: 10.1200/JCO.2020.38.15_suppl.e16036. [DOI] [Google Scholar]
- 18.World Medical A World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Ning J, Jiao Y, Li M, Liu P, Ma T, Xiong F, et al. Efficacy and safety of s-1 and oxaliplatin (SOX) as first-line chemotherapy for metastatic colorectal cancer. Anhui Med Pharm J. 2017;21(9):1669–1673. [Google Scholar]
- 21.Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31(16):1931–8. doi: 10.1200/JCO.2012.44.8308. [DOI] [PubMed] [Google Scholar]
- 22.Bokemeyer C, Bondarenko I, Hartmann JT, Braud F, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22(7):1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 23.Cutsem EV, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. New Engl J Med. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 24.Guan ZZ, Xu JM, Luo RC, Feng FY, Wang LW, Shen L, et al. Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trial. Chin J Cancer. 2011;30(10):682–689. doi: 10.5732/cjc.011.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ducreux M, Bennouna J, Hebbar M, et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer. 2010;128:682–690. doi: 10.1002/ijc.25369. [DOI] [PubMed] [Google Scholar]
- 26.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 27.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27(5):663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 28.Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392–2401. doi: 10.1001/jama.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo HY, Li YH, Wang W, Wang ZQ, Yuan X, Ma D, et al. Single-agent capecitabine as maintenance therapy after induction of XELOX (or FOLFOX) in first-line treatment of metastatic colorectal cancer: randomized clinical trial of efficacy and safety. Ann Oncol. 2016;27(6):1074–1081. doi: 10.1093/annonc/mdw101. [DOI] [PubMed] [Google Scholar]
- 30.Hegewisch-Becker S, Graeven U, Lerchenmuller CA, Killing B, Depenbusch R, Steffens CC, et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015;16(13):1355–1369. doi: 10.1016/S1470-2045(15)00042-X. [DOI] [PubMed] [Google Scholar]
- 31.Simkens LH, van Tinteren H, May A, ten Tije AJ, Creemers GJ, Loosveld OJ, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843–1852. doi: 10.1016/S0140-6736(14)62004-3. [DOI] [PubMed] [Google Scholar]
- 32.Underwood J, Marks M, Collins S, Logan S, Pollara G. Intravenous catheter-related adverse events exceed drug-related adverse events in outpatient parenteral antimicrobial therapy. J Antimicrob Chemother. 2019;74(3):787–790. doi: 10.1093/jac/dky474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mielke D, Wittig A, Teichgraber U. Peripherally inserted central venous catheter (PICC) in outpatient and inpatient oncological treatment. Support Care Cancer. 2020;28(10):4753–4760. doi: 10.1007/s00520-019-05276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrato A, Swieboda-Sadlej A, Staszewska-Skurczynska M, Lim R, Roman L, Shparyk Y, et al. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: a randomized, phase III trial. J Clin Oncol. 2013;31(10):1341–1347. doi: 10.1200/JCO.2012.45.1930. [DOI] [PubMed] [Google Scholar]
- 35.Hecht JR, Trarbach T, Hainsworth JD, Major P, Jager E, Wolff RA, et al. Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol. 2011;29(15):1997–2003. doi: 10.1200/JCO.2010.29.4496. [DOI] [PubMed] [Google Scholar]
- 36.Schmoll HJ, Cunningham D, Sobrero A, Karapetis CS, Rougier P, Koski SL, et al. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study (HORIZON III) J Clin Oncol. 2012;30(29):3588–3595. doi: 10.1200/JCO.2012.42.5355. [DOI] [PubMed] [Google Scholar]
- 37.Argiles G, Saunders MP, Rivera F, Sobrero A, Benson A, 3rd, Guillen Ponce C, et al. Regorafenib plus modified FOLFOX6 as first-line treatment of metastatic colorectal cancer: A phase II trial. Eur J Cancer. 2015;51(8):942–949. doi: 10.1016/j.ejca.2015.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generate and analyzed during the current study are available from the corresponding author on reasonable request.