Abstract
This was a randomized, double-blind, placebo-controlled parallel study in human immunodeficiency virus type 1 (HIV-1)-uninfected healthy subjects to investigate the pharmacokinetic interaction between indinavir (IDV) and ritonavir (RTV). Subjects were allocated to treatment groups of IDV given with RTV in the following milligram doses twice daily: 800 mg of IDV-100 mg of RTV (800-100 mg), 800-200, 800-400, and 400-400 mg, placebo of IDV with RTV doses of 100, 200, and 400 mg, and placebo of both IDV and RTV. Doses of both drugs were administered for 14 days with a low-fat meal and one dose on day 15 with a high-fat meal. Blood was obtained for drug concentration measurements on days 14 and 15. Seventy-three volunteers enrolled in the study: 29 men and 44 women. Fifty-three volunteers completed the study. When compared to standard historical data for 800 mg of IDV every 8 h (q8h), the IDV area under the concentration-time curve for 24 h (AUC24) of IDV-RTV regimens 400-400, 800-100, and 800-200 mg were at least 1.4, 2.3, and 3.3 times higher, respectively, regardless of meal. The concentrations at the end of the dosing interval were 10 to 25 times higher than that observed in the standard regimen of 800 mg of IDV q8h for IDV-RTV 800-100 and 800-200 mg regimens, respectively. RTV at 200 mg maximally enhanced the IDV profile. Improved tolerability was associated with IDV-RTV 800-100 mg versus IDV-RTV 800-200, 800-400, and 400-400 mg q12h. The advantages of IDV-RTV twice daily over 800 mg of IDV q8h include no food restrictions and twice-daily dosing. Also, the regimens achieve levels of IDV that may be helpful in suppressing strains of HIV-1 that have reduced susceptibility to IDV or other protease inhibitors.
Pharmacokinetic drug-drug interactions have the potential to enhance drug exposure of protease inhibitors for human immunodeficiency virus (HIV) infection. Indinavir (IDV) plus ritonavir (RTV) is a combination that appears to have a very favorable pharmacokinetic interaction. The metabolic interaction of these drugs results in augmented IDV plasma levels that may prove useful in more convenient dosing intervals and removal of food restrictions. The high IDV levels may be also be active against virus strains with genotypic mutations or phenotypic profiles associated with decreased sensitivity to protease inhibitors at conventional drug concentrations. Thus, a combination regimen of IDV with RTV may be useful in antiretroviral treatment-naive patients, as well as in rescue regimens.
A current regimen combines 400 mg of IDV with 400 mg of RTV twice daily (6, 9), but tolerability to RTV is sometimes difficult (NORVIR package circular, Abbott Laboratories, Abbott Park, Ill.). RTV at lower doses is being studied to see if they will provide sufficient metabolic inhibitory activity to permit dosing IDV in a twice-a-day (b.i.d.) regimen and to assess tolerability. The present study was undertaken to characterize the pharmacokinetic profiles of a wider array of dose combinations of IDV plus RTV at steady state (2 weeks), with doses administered with both a low-fat meal and a high-fat meal, and to assess relative tolerability in a double-blind, randomized study of HIV-1-uninfected healthy volunteers.
(Preliminary results were presented at the 6th Conference on Retroviruses and Opportunistic Infections, Chicago, Ill., 1999.)
MATERIALS AND METHODS
Study design.
This was a randomized, double-blind, placebo-controlled parallel study of healthy volunteers. The protocol was IRB approved by Western IRB and conducted by Phoenix International Life Sciences, Inc., Cincinnati, Ohio. Doses of both drugs were administered for days 1 to 14 with a low-fat meal (2 slices of toast, 2 teaspoons [tsp.] of jelly, 6 oz. of apple juice, 1 cup of coffee, 2 tablespoons of skim milk, 2 tsp. of sugar), and one dose was administered on day 15 with a high-fat meal (2 scrambled eggs, 2 strips of bacon, 2 slices of toast, 2 pats of butter, 4 oz. of hash brown potatoes, and 8 oz. of whole milk). IDV capsules were given with RTV capsules twice daily to parallel groups in doses of 800 mg of IDV-100 mg of RTV (800-100 mg), 800-200, 800-400, and 400-400 mg; a placebo of IDV was given with RTV capsules of 100, 200, and 400 mg; and placebos of both IDV and RTV were given.
Determination of IDV and RTV concentrations.
Blood for measurement of IDV and RTV levels was obtained on day 14 after a low-fat meal and on day 15 after a high-fat meal. All subjects were required to consume 1.5 liters of water per day. Blood samples were drawn at 0, 0.5, 1.0, 1.5, 2, 3, 4, 6, 8, and 12 h postdose. Plasma concentrations of IDV and RTV were determined using a validated electrospray liquid chromatography coupled to triple quadruple mass spectrometry method (IDV limit of quantitation, 50 mg/liter) by BAS Analytics, West Lafayette, Ind. IDV concentrations were converted to a molar basis using a molecular weight of 613.81.
Statistical analysis.
The protocol had two primary pharmacological hypotheses encompassing three comparisons. The first was that after 2 weeks of administration of either of two RTV-sparing regimens (800 mg of IDV every 12 h [q12h] with 100 mg of RTV q12h, or 800 mg of IDV q12h with 200 mg of RTV q12h), the area under the concentration-time curve (AUC) of IDV would be at least comparable to that achieved with the comparator regimen (400 mg of IDV q12h with 400 mg of RTV q12h). Specifically, the geometric mean ratio (RTV-sparing regimen/comparator regimen) will be at least 0.80. The second hypothesis was that the geometric mean ratio of the IDV AUC for the 800-400 mg regimen over the 400-400 mg regimen will be at least 1.50. All power calculations were based on the minimal number of subjects to be enrolled in the study (10 subjects per combination regimen). With 10 subjects per regimen and assuming that the true geometric mean ratio is 1, there was at least an 80% probability that the one-sided 96.7% confidence interval for the geometric mean ratio AUC for the 800-100 or 800-200 mg regimen over the 400-400 mg combination would be greater than 0.81. There is 80% power to detect a 25% difference between the 800-400 mg and 400-400 mg regimens (geometric mean ratio, ≥1.25).
For each IDV and RTV plasma concentration profile, the AUC from 0 to 12 h (AUC0–12) was calculated by a modified trapezoidal rule using piecewise cubic polynomials (11), and the maximum concentration of drug in serum (Cmax), trough concentration of drug in serum (C12), and time to maximum concentration of drug in serum (Tmax) were determined directly from the concentration-time data. Summary statistics for the estimated AUC24 were calculated by multiplying the respective AUC by the number of doses per day.
Statistical analysis was done to compare the IDV AUC24 of each of the IDV-RTV doses, 800-100, 800-200, and 800-400 mg, to the 400-400 mg combination. An analysis of variance (ANOVA) model was used to estimate and test the difference in log-transformed AUC between the groups. A Bonferroni adjustment for multiple comparisons was used across the three resulting hypothesis tests leading to an adjusted α of 0.0167 (α = 0.05/3). Adverse events were enumerated for each regimen.
RESULTS
Study sample.
Seventy-three volunteers (29 men and 44 women) enrolled in the study. Fifty-three volunteers completed the study; 10 discontinued because of adverse events and 10 withdrew consent. The mean age of those who completed the study was 29 years (range, 18 to 45); 62% were white, 36% were African-American, and 2% were Asian.
Pharmacokinetic profiles. (i) IDV.
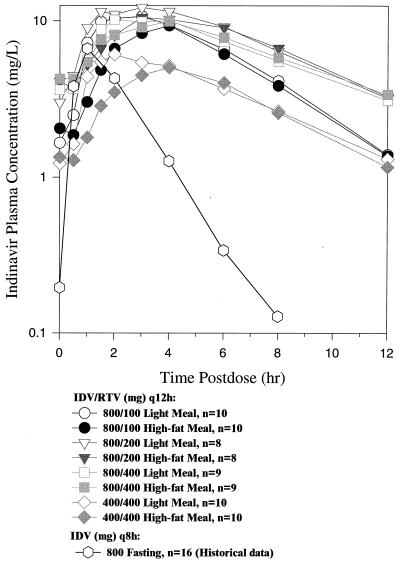
The plasma pharmacokinetic profiles of the regimens with the low-fat and the high-fat meals are shown in Fig. 1, and pharmacokinetic parameters are summarized in Table 1.
FIG. 1.
Mean concentrations of IDV in plasma over the dosing interval using IDV plus RTV q12h over all active panels when administered with a low-fat meal (day 14) and with a high-fat meal (day 15) as well as historical data showing mean concentrations of IDV in plasma from using 800 mg of IDV q8h.
TABLE 1.
Pharmacokinetic profile of IDV in combination with RTV taken with a low-fat meal after 2 weeks of dosing and with a high-fat meal on day 15a
| Dose | n |
Cmax (mg/liter)
|
AUC24 (estimateda) (mg · h/liter)
|
Ctrough (mg/liter)d
|
|||
|---|---|---|---|---|---|---|---|
| Low fat | High fat | Low fat | High fat | Low fat | High fat | ||
| IDV-RTV, q12h | |||||||
| 800-100 mg | 10 | 11.7 (10.4, 13.1) [9.6 to 15.2] | 9.6 (8.2, 11.2) [6.2 to 14.8] | 142.5c (120.4, 168.6) [112.8 to 239.4] | 119.7c (97.3, 147.2) [77.4 to 244.7] | 1.396 (0.979, 1.991) [0.816 to 4.701] | 1.371 (0.923, 2.037) [0.578 to 5.934] |
| 800-200 mg | 8 | 12.9 (11.4, 14.6) [8.5 to 16.1] | 11.6 (9.6, 13.9) [7.0 to 17.9] | 187.5c (156.6 224.5) [114.8 to 263.3] | 173.8c (136.9, 220.6) [80.5 to 299.5] | 3.119 (2.133, 4.559) [1.019 to 6.039] | 3.281 (2.076, 5.182) [0.919 to 6.791] |
| 800-400 mg | 9 | 10.9 (9.6, 12.4) [8.4 to 13.8] | 10.3 (8.7, 12.2) [8.6 to 13.4] | 163.6c (139.5, 195.9) [112.5 to 263.4] | 161.6c(129.6, 201.6) [126.5 to 217.3] | 3.105 (2.124, 4.538) [1.754 to 8.687] | 3.392 (2.222, 5.179) [1.321 to 5.536] |
| 400-400 mg | 10 | 7.0 (6.2, 7.9) [5.3 to 11.7] | 5.4 (4.6, 6.3) [3.4 to 7.9] | 85.8 (72.5, 101.5) [50.6 to 131.2] | 74.0 (60.2, 90.9) [38.6 to 113.3] | 1.308 (0.917, 1.865) [0.334 to 3.390] | 1.161 (0.782, 1.725) [0.315 to 2.874] |
| 800 mg of IDV q8h Merck 021, without food; Day 15 as monotherapy | 16 | 7.3 (6.3, 8.6) [3.4 to 12.6] | 52.9 (44.6, 62.7) [21.8 to 91.6] | 0.127 (0.098, 0.168) [0.047 to 0.446] | |||
Statistical significance testing was performed for AUC only. Data shown are ANOVA model-based geometric means (95% confidence intervals) [range]. Historical data for 800 of IDV q8h without food is provided for comparison.
AUC0–τ × doses/day.
Data were significantly different from data of the IDV-RTV 400-400 mg regimen at P values of 0.004 (low-fat meal) and <0.005 (high-fat meal).
Data used were C12 for IDV-RTV regimens and C8 for historical data for 800 mg of IDV q8h alone.
Administration of the IDV-RTV combination regimens of 800-100 mg q12h, 800-200 mg q12h, and 800-400 mg q12h resulted in higher plasma exposure to IDV than the IDV-RTV regimen of 400-400 mg q12h (Fig. 1 and Table 1). There were no notable differences between IDV plasma concentrations achieved with the 800-200 mg q12h and 800-400 mg q12h regimens. The 800-100 mg q12h regimen achieved high concentrations of IDV in plasma, with geometric means of C12 in excess of 1.2 mg/liter (2,000 nM) after low-fat or high-fat meals; however, these C12 concentrations were lower than those achieved with the 800-200 mg q12h and 800-400 mg q12h regimens. To provide perspective on the effect of dosing IDV with RTV compared to IDV alone, historical pharmacokinetic data for 800 mg of IDV q8h (Protocol 021, Merck document, Merck, West Point, Pa.) are provided in Table 1 and Fig. 1. Dosing IDV q12h with RTV q12h compared to IDV alone q8h (historical data) had a modest effect on the early postdose IDV time points and a much more dramatic effect on the latter part of the dosing interval (Fig. 1, 800-100 and 800-200 mg plots). This corresponded to very large increases in IDV Ctrough values. The concentrations at the end of the dosing interval were 10 to 25 times higher for the IDV-RTV 800-100 and 800-200 mg regimens, respectively, than for the 800 mg q8h regimen (Table 1). In contrast, when compared to historical data for 800 mg of IDV q8h, the geometric mean AUC24s of the IDV-RTV 800-100 and 800-200 mg regimens were only 2.3 to 3.5 times higher, respectively (Table 1), and increases in Cmax were less than twofold.
One objective of this protocol was to compare the effect of high-fat and low-fat meals on IDV concentrations during dosing of IDV-RTV regimens. High-fat meals decrease AUC of IDV by 80% compared to low-fat meals when IDV is administered alone (12). In the IDV-RTV regimens, dosing with high-fat meals on day 15 after 2 weeks of dosing IDV-RTV with low-fat meals resulted in roughly comparable IDV plasma concentration profiles (Fig. 1 and Table 1). The arithmetic mean Tmax for the IDV-RTV regimens with 800-mg doses of IDV with a low-fat meal ranged from 2.4 to 3.3 h and with the high fat meal ranged from 3.4 to 3.6 h. Tmax for the standard regimen of 800 mg q8h without food is 0.8 h (12).
(ii) RTV.
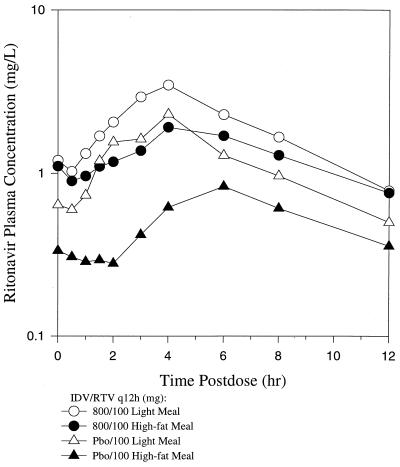
The pharmacokinetic profiles for RTV in the combinations are shown in Table 2. It can be seen that IDV increased RTV concentrations in plasma at the lower doses of RTV, i.e., 100 and 200 mg twice daily. The concentration-time plot of 100 mg of RTV given with IDV showed less of a food effect, a longer terminal half-life, and a higher concentration at the end of the dosing interval than 100 mg of RTV given with placebo IDV (Fig. 2). These effects were not seen with the 400-mg dose of RTV. Addition of IDV to the 400-mg dose of RTV did not result in a higher AUC24, longer terminal half-life, or higher concentrations at the end of the dosing interval than RTV given with IDV placebo.
TABLE 2.
Pharmacokinetic profile of RTV in combination with IDV taken with a low-fat meal after 2 weeks of dosing and with a high-fat meal on day 15a
| IDV-RTV dose given q12h | n |
Cmax (mg/liter)
|
AUC24 (estimated) (mg · h/liter)
|
C12 (mg/liter)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low fat | High fat | GMR (95% CI) | Low fat | High fat | GMR (95% CI) | Low fat | High fat | GMR (95% CI) | ||
| 800-100 | 10 | 3.64 (2.81, 4.70) [2.55 to 5.68] | 2.07 (1.55, 2.76) [1.39 to 3.26] | 0.57 (0.47, 1.13) | 46.8 (68.8, 56.5) [30.8 to 63.0] | 31.9 (25.1, 40.5) [18.7 to 58.1] | 0.68 (0.60, 0.77) | 0.78 (0.52, 1.17) [0.36 to 1.33] | 0.76 (0.48, 1.20) [0.26 to 1.65] | 0.97 (0.79, 1.19) |
| Pbo-100 | 3 | 2.26 (1.28, 4.00) [1.56 to 3.21] | 0.78 (0.37, 1.63) [0.46 to 1.14] | 0.39, (0.18, 0.84) | 23.4 (11.1, 49.0) [16.6 to 42.6] | 11.9 (4.3, 33.0) [7.8 to 17.6] | 0.51 (0.31, 0.83) | 0.48 (0.20, 1.18) [0.30 to 0.65] | 0.35 (0.11, 1.13) [0.25 to 0.44] | 0.79 (0.59, 1.06) |
| 800-200 | 8 | 7.22 (5.48, 9.51) [3.27 to 12.65] | 6.18 (4.41, 8.65) [2.78 to 8.51] | 0.78 (0.59, 1.02) | 120.0 (94.3, 152.7) [47.9 to 158.5] | 98.9 (68.2, 143.5) [58.0 to 211.8] | 0.82 (0.70, 0.97) | 2.45 (1.59, 3.78) [0.90 to 4.75] | 2.78 (1.62, 4.76) [0.86 to 4.87] | 1.00 (0.81, 1.23) |
| Pbo-200 | 5 | 6.07 (3.89, 9.46) [3.97 to 9.20] | 3.67 (2.22, 6.04) [1.85 to 11.80] | 0.60 (0.33, 1.10) | 55.3 (33.8, 90.3) [31.6 to 78.9] | 41.3 (20.0, 85.1) [21.0 to 102.0] | 0.75 (0.44, 1.27) | 0.52 (0.24, 1.14) [0.26 to 0.91] | 0.53 (0.24, 1.17) [0.23 to 0.89] | 0.92 (0.85, 0.99) |
| 800-400 | 9 | 19.0 (14.46, 25.07) [8.06 to 32.60] | 13.8 (10.15, 18.84) [9.50 to 26.65] | 0.73 (0.48, 1.11) | 240.4 (186.9, 309.3) [132.1 to 300.9] | 225.1 (181.6, 279.0) [166.5 to 378.4] | 0.94 (0.71, 1.24) | 6.10 (3.95, 9.42) [3.28 to 18.21] | 5.79 (3.53, 9.50) [1.95 to 9.41] | 0.95 (0.66, 1.378) |
| 400-400 | 10 | 19.70 (15.25, 25.45) [12.15 to 38.16] | 14.42 (10.81, 19.22) [8.07 to 20.54] | 0.73 (0.60, 0.89) | 240.6 (179.6, 322.4) [119.7 to 435.0] | 199.0 (142.9, 277.1) [93.2 to 336.8] | 0.83 (0.70, 0.98) | 5.64 (3.76, 8.44) [1.09 to 11.38] | 4.39 (2.77, 6.96), [0.86 to 10.06] | 0.78 (0.57, 1.06) |
| Pbo-400 | 5 | 20.41 (13.09, 31.83) [10.69 to 29.77] | 14.77 (8.96, 24.34) [7.99 to 25.88] | 0.72 (0.46, 1.13) | 258.4 (158.0, 422.8) [150.6 to 416.2] | 207.9 (122.7, 352.3) [132.9 to 358.7] | 0.80 (0.60, 1.08) | 5.41 (2.68, 10.91) [3.31 to 13.07] | 4.65 [2.33 to 9.31] | 0.86 (0.61, 1.22) |
Data shown are ANOVA model-based geometric means (95% confidence intervals) [range]. GMR, geometric mean ratio; Pbo, IDV placebo. AUC24 was estimated as AUC0-τ × doses/day.
FIG. 2.
Mean concentrations of RTV in plasma over the dosing interval by using 800 mg of IDV plus 100 mg of RTV q12h and placebo to IDV plus 100 mg of RTV q12h when administered with a low-fat meal and with a high-fat meal.
Analysis of IDV and RTV levels by gender and by body mass index (BMI) showed no associations between IDV or RTV concentrations and either gender or BMI (data not shown).
Safety and tolerability.
In general, adverse events were mild to moderate in severity and consisted mostly of upper gastrointestinal symptoms. Two subjects had severe adverse events; both occurred in the IDV-RTV 800–200 mg q12h arm. One was a vaso-vagal fainting episode that may have been due to blood drawing, and the other was an episode of nephrolithiasis. Two subjects had hematuria recorded as clinical adverse events; one was the individual with nephrolithiasis. No laboratory adverse events of hematuria or increased red blood cells in urine were recorded. Also, no subject had crystalluria noted as an adverse event.
There were 10 discontinuations from the study due to clinical adverse events. One subject had the nephrolithiasis episode mentioned above. The remaining nine subjects had one or more adverse experiences of chest pain, asthenia/fatigue, nausea and/or vomiting, headache, and paresthesia.
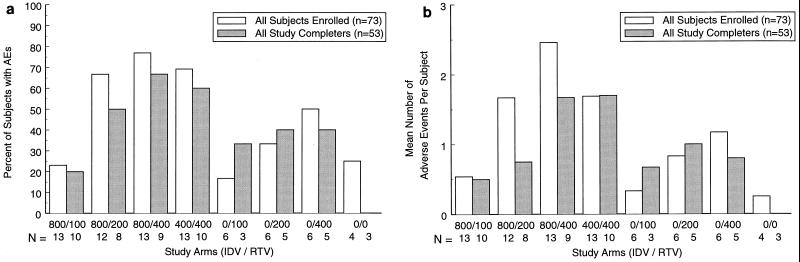
Figure 3a shows the proportion of volunteers who had at least one clinical adverse experience considered by the investigator to be of either moderate or severe intensity. Figure 3b shows the mean number of moderate and severe clinical adverse events per volunteer. When clinical adverse events were calculated on the basis of number of subjects in each treatment arm, the IDV-RTV combinations of 800-100, 800-200, 800-400, and 400-400 mg produced the following numbers of adverse events per subject: 0.54, 1.67, 2.46, and 1.69 in all patients who enrolled in the study, respectively. The odds ratio for the occurrence of clinical adverse events of moderate or severe intensity was significantly higher for the IDV-RTV 400-400 mg regimen than for the 800-100 mg regimen (P = 0.021, Cochran-Mantel-Haenszel Chi-square test) but was not statistically significantly different between the IDV-RTV 400-400 mg regimen and the 800-200 and 800-400 mg regimens (Table 3). Figure 3b suggests that tolerability to a higher dose of RTV alone is reduced by the presence of IDV.
FIG. 3.
(a) Percent of participants with at least one moderate or severe adverse experience out of all enrolled participants and those who completed the study, by treatment group. (b) Mean number of adverse experiences of moderate or severe intensity per subject in all enrolled participants and those who completed the study, by treatment group.
TABLE 3.
Odds ratio that a subject experienced a clinical adverse event of moderate or severe intensity by specific regimens
| IDV-RTV doses | Odds ratio (OR) | 95% CI | P |
|---|---|---|---|
| 400-400 over 800-100 | |||
| All participants enrolled | 7.50 | 1.36, 41.31 | 0.021 |
| All participants completing studya | 6.0 | 0.83, 43.17 | 0.075 |
| 400-400 over 800-200 | |||
| All participants enrolled | 1.13 | 0.20, 6.26 | 0.893 |
| All participants completing studya | 1.50 | 0.22, 10.31 | 0.680 |
| 400-400 over 800-400 | |||
| All participants enrolled | 0.68 | 0.11, 3.99 | 0.665 |
| All participants completing studya | 0.75 | 0.11, 5.15 | 0.770 |
Excluding participants who discontinued the study prematurely.
DISCUSSION
The potential for beneficial pharmacokinetic drug-drug interactions when two HIV protease inhibitors are dosed in combination has been increasingly recognized. For the combination of IDV and RTV specifically, published reports have shown that twice-daily dosing can achieve much higher, more sustained, and less variable IDV concentrations that are not affected by food compared to IDV alone dosed every 8 h without food (12; A. Hsu, R. Granneman, and M. Heath-Chiozzi, Abstr. 12th World AIDS Conf., abstr. 22361, 1998). Among the objectives of the present study were to characterize the multiple-dose pharmacokinetics and tolerability to various IDV-RTV combination regimens and to confirm the lack of a meaningful food effect.
In the present study, the pharmacokinetics of and tolerability to two lower-dose RTV regimens (IDV-RTV regimens 800-100 and 800-200 mg) and one high-dose RTV regimen (IDV-RTV regimen 800-400 mg) were compared to that of the IDV-RTV 400-400 mg regimen after 14.5 days of coadministration. The low-dose RTV regimens were investigated to assess whether improved tolerability over the IDV-RTV 400-400 mg regimen twice daily could be achieved while maintaining IDV concentrations. These regimens rely predominantly on IDV for antiretroviral effect.
IDV AUCs for the two low-dose RTV regimens and the high-dose RTV regimen were all statistically significantly higher than the AUC for the IDV-RTV 400-400 mg regimen. IDV pharmacokinetic parameters for the IDV-RTV 800-400 and 800-200 mg regimens were similar, indicating that 200 mg of RTV is sufficient to maximize inhibition of IDV metabolism. IDV pharmacokinetic parameters from patients receiving the IDV-RTV 400-400 mg regimen in the present study were substantially higher than in another study (7). The most likely explanation is the difference in duration of IDV and RTV coadministration between the two studies, 1 day versus 14.5 days.
As expected based on published reports (6, 7), a comparison with historical IDV pharmacokinetic data for the standard regimen of 800 mg of IDV dosed alone q8h shows that IDV concentrations were higher and more sustained for all of the combination regimens despite the reduction in dosing frequency and, therefore, total daily dose. The increase in trough concentrations was much greater than the increase in peak concentration. For example, the geometric mean of Cmax was 11.7 mg/liter (19.0 μM) and that of C12 was 1.40 mg/liter (2,274 nM) for the 800-100 mg combination dosed q12h with a low-fat meal versus Cmax of 7.3 mg/liter (11.1 μM) and C8 of 0.13 mg/liter (208 nM) for 800 mg of IDV dosed alone q8h when fasting (Protocol 021, Merck document). These results suggest that 100 mg of RTV substantially inhibits clearance of IDV, presumably via inhibition of CYP3A-mediated metabolism of IDV (2) or, possibly, inhibition of p-glycoprotein (8).
A comparison of the IDV pharmacokinetic profile following drug administration with a high-fat meal on day 15 and a low-fat meal on day 14 showed that there were no meaningful differences for any of the IDV-RTV combination regimens. The 95% confidence interval about the geometric mean ratios (high-fat meal/low-fat meal) of the IDV pharmacokinetic parameters generally fell within the range of 0.6 to 1.74. The effect of a high-fat meal on IDV pharmacokinetics when dosed in combination with RTV is consistent with previously published studies (7) and is in contrast to the marked reduction in IDV concentration when IDV alone is administered with a high-fat meal (12).
RTV concentrations in plasma were moderately decreased by a high-fat meal at the 100-mg RTV dose when dosed with either 800 mg of IDV or placebo. There appears to have been a trend for IDV to reduce the effect of the high-fat meal on RTV pharmacokinetics; however, the confidence intervals are too wide to discern a difference, primarily due to the small number of subjects in the RTV-alone group (n = 3). The high-fat meal had a less clear effect on 200-mg RTV doses and had no apparent effect on 400-mg RTV doses. The absence of a food effect on RTV pharmacokinetics at higher doses is consistent with previous studies described in the RTV package circular (NORVIR package circular, Abbott Laboratories). The food effect at lower doses does not appear to have been reported previously.
Dual protease inhibitor therapy is being used more commonly, especially in salvage regimens. In the IDV-RTV 800-200 mg regimen, the high IDV AUC and trough concentrations offer the potential for activity against viral strains that may be resistant to standard levels of IDV, as well as wild-type strains. IDV plasma concentrations for both the IDV-RTV 800-100 and 800-200 mg regimens exceed the 95% inhibitory concentration (IC95) of HIV strains with multiple resistance mutations against IDV in the protease gene region (3). The concentration of IDV at the end of the dosing interval was more than 1.2 mg/liter (2,000 nM) for the IDV-RTV 800-100 mg twice-daily regimen and was more than 3 mg/liter (5,000 nM) for the IDV-RTV 800-200 mg twice-daily regimen. By comparison, the IC95 for wild-type virus is approximately 0.03 mg/liter (50 nM) and for resistant virus can be 0.2 to 2.0 mg/liter (300 to 3,000 nM) or higher. Such data appear to provide the opportunity to rescue some patients with protease inhibitor resistance, as well as treating relatively therapy-naïve patients. Additionally, Condra et al. (4) have shown that correcting for protein binding in the IDV-RTV 800-200 mg regimen results in trough IDV blood levels 79 times higher than the IC95 of wild-type virus; the level is 33 times higher for the trough of the 800-100 mg regimen. These high IDV exposure data are directly relevant to interpreting phenotypic or genotypic HIV-1 resistance data because the currently used drug concentrations for IDV susceptibility may be obsolete. The current interpretive criteria for HIV-1 resistance testing should be re-examined as more clinical viral suppression data become available from well-characterized strains of HIV-1, i.e., strains considered resistant at routine concentrations of IDV. Additionally, IDV has a high level of penetration into cerebrospinal fluid (5) and semen (S. Taylor, D. Back, S. Drake, S. Gibbons, H. Reynolds, D. White, and D. Pillay, Abstr. 7th Conf. Retrovir. Opportun. Infect., abstr. 318, 2000) among the protease inhibitors, and RTV increases the concentration of IDV in cerebrospinal fluid (10).
Clinical data showing that the IDV-RTV 800-200 mg regimen has activity against strains from patients who have experienced protease inhibitor failure are accumulating [R. E. Campo, G. A. Suarez, N. Miller, J. Moreno, M. Kolber, D. J. Holder, M. Shivaprakash, D. M. DeAngelis, J. L. Wright, K. Holmes, W. A. Schleif, E. A. Emini, and J. H. Condra, Proc. 3rd Int. Workshop Salvage Theory HIV Infect., abstr. 7, Antivir. Ther. 5(Suppl. 2):6, 2000; H. Grossman, A. Luber, D. Butcher, D. Purdom, P. Duong, and L. Markson, Proc. 3rd Int. Workshop Salvage Theory HIV Infect., abstr. 27, Antivir. Ther. 5(Suppl. 2):23, 2000; W. A. O'Brien, T. L. Atkinson, X. Han, M. Sova, and J. East, Abstr. 37th Annu. Meet. Infect. Dis. Soc. Am., abstr. 355, 1999]. With patients in whom a regimen containing at least one protease inhibitor had failed, Grossman et al. (Proc. 3rd Int. Workshop Salvage Theory HIV Infect.) found that 17 of 30 (57%) patients with 24-week data and 10 of 16 (63%) patients with 36-week data had HIV RNA counts below 400 copies/ml. Campo et al. (Proc. 3rd Int. Workshop Salvage Theory HIV Infect.) found 13 of 17 (76%) adherent patients responded with HIV RNA counts below 400 copies/ml, including patients with phenotypic resistance to IDV and RTV. O'Brien et al. (Abstr. 37th Annu. Meet. Infect. Dis. Soc. Am.) used both IDV-RTV 800-100 and 800-200 mg regimens with 20 anti-retroviral-therapy experienced patients, of whom six had HIV RNA counts suppressed below 50 copies/ml and four others had at least a 1 log decline. Frequency of nephrolithiasis ranged from none in 68 patients (Campo et al., Proc. 3rd Int. Workshop Salvage Theory HIV Infect.; Grossman et al., Proc. 3rd Int. Workshop Salvage Theory HIV Infect.) to 6 of 20 patients (30%) (O'Brien et al., Abstr. 37th Annu. Meet. Infect. Dis. Soc. Am.).
The combination of IDV with low-dose RTV is generally well tolerated (1). That experience was also seen here in this double-blind study. The odds ratio for a patient experiencing adverse experiences of moderate or severe intensity is 7.5 when comparing the IDV-RTV 400-400 mg regimen to the IDV-RTV 800-100 mg regimen for all subjects who enrolled. Similarly, Hsu et al. (A. Hsu, A. Zolopa, N. Shulman, D. Havlir, J. Gallant, E. Race, P. Jiang, S. Boller, J. Swerdlow, C. Renz, O. Jasinsky, A. J. Japour, D. Kempf, and E. Sun, Abstr. 8th Conf. Retrovir. Opportun. Infect., abstr. G1012P, 2001) used the IDV-RTV 400-400 mg regimen and found 16 of 37 (43%) patients discontinuing because of tolerability difficulties or plasma biochemical toxicity. Down-dosing RTV to 300 mg q12h was available to study participants, but the number of individuals who received reduced doses of RTV was not reported in the presentation.
The total number of clinical adverse experiences of moderate and severe intensity by treatment shows that higher doses of RTV, when coadministered with IDV, are associated with more frequent adverse experiences in all subjects enrolled and in those subjects who completed the study. The differences between groups are more striking in those who completed the study. In order to control for the differing numbers of participants in each treatment group, especially the RTV-only and double-placebo groups, the mean numbers of moderate and severe adverse experiences per subject were calculated. In these data, the relative tolerability of 100 mg of RTV q12h in the presence of IDV is good. Patients should maintain adequate hydration during therapy with IDV-RTV to minimize the risk of nephrolithiasis.
Overall, the pharmacokinetic data of IDV-RTV combinations using 800-100 and 800-200 mg regimens twice daily support further research in protease inhibitor-naïve populations and in those requiring rescue therapy due to virological failure.
REFERENCES
- 1.Burger D, Hugen P, Aarnoutse R, Dieleman J, Prins J, van der Poll T, ten Veen J, Mulder J, Meenhorst P, Blok W, van der Meer J, Reiss P, Lange J. A retrospective, cohort-based survey of patients using twice-daily indinavir + ritonavir combinations: pharmacokinetics, safety, and efficacy. J Acquir Immune Defic Syndr. 2001;26:218–224. doi: 10.1097/00042560-200103010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Chiba M, Hensleigh M, Nishime J A, Balani S K, Lin J H. Role of cytochrome P450 3A4 in human metabolism of MK-639, a potent human immunodeficiency virus protease inhibitor. Drug Metab Dispos. 1996;24:307–314. [PubMed] [Google Scholar]
- 3.Condra J H, Holder D J, Schleif W A, Blahy O M, Danovich R M, Gabryleslki L J, Graham D J, Laird D, Quintero J C, Rhodes A, Robbins H L, Roth E, Shivaprakash M, Yang T, Chodakewitz J A, Deutsch P J, Leavitt R Y, Massari F E, Mellors J W, Squires K E, Steigbigel R T, Teppler H, Emini E A. Genetic correlates of invivo viral resistance to indinavir, a human immunodeficiency virus type 1 protease inhibitor. J Virol. 1996;12:8270–8276. doi: 10.1128/jvi.70.12.8270-8276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condra J H, Petropoulos C J, Ziermann R, Schleif W A, Shivaprakash M, Emini E A. Drug resistance and predicted virologic responses to human immunodeficiency virus type 1 protease inhibitor therapy. J Infect Dis. 2000;182:758–765. doi: 10.1086/315782. [DOI] [PubMed] [Google Scholar]
- 5.Haas D W, Stone J, Clough L A, Johnson B, Spearman P, Harris V L, Nicotera J, Johnson R H, Raffanti S P, Zhong L, Bergqwist P, Chamberlin S, Hoagland V, Ju W D. Steady-state pharmacokinetics of indinavir in cerebrospinal fluid and plasma among adults with human immunodeficiency virus type 1 infection. Clin Pharmacol Ther. 2000;68:367–374. doi: 10.1067/mcp.2000.109391. [DOI] [PubMed] [Google Scholar]
- 6.Hsu A, Granneman G R, Witt G, Locks C, Denissen J, Molla A, Valdes J, Smith J, Erdman K, Lyons N, Niu P, Decourt J P, Four-tillan J B, Girault J, Leonard J M. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 1997;41:898–905. doi: 10.1128/aac.41.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu A, Granneman G R, Cao G, Carothers L, Japour A, El-Shourbagy T, Dennis S, Berg J, Erdman K, Leonard J M, Sun E. Pharmacokinetic interaction between ritonavir and indinavir in healthy volunteers. Antimicrob Agents Chemother. 1998;42:2784–2791. doi: 10.1128/aac.42.11.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Profit L, Eagling V, Back D. Modulation of P-glycoprotein function in human lymphocytes and Caco-2 cell monolayers by HIV-1 protease inhibitors. AIDS. 1999;13:1623–1627. doi: 10.1097/00002030-199909100-00004. [DOI] [PubMed] [Google Scholar]
- 9.Rockstroh J K, Bergmann F, Wiesel W, Rieke A, Thiesen A, Fatkenheuer G, Oette M, Carls H, Fenske S, Nadler M, Knechten H. Efficacy and safety of twice daily first-line ritonavir/indinavir plus double nucleoside combination therapy in HIV-infected individuals. German ritonavir/indinavir Study Group. AIDS. 2000;14:1181–1185. doi: 10.1097/00002030-200006160-00015. [DOI] [PubMed] [Google Scholar]
- 10.van Praag R, Weverling G, Portegies P, Jurriaans S, Zhou X, Foisy M, Sommadossi J P, Burger D, Hoetelmans R, Lange J, Prins J. Enhanced penetration of indinavir in cerebrospinal fluid and semen after the addition of low-dose ritonavir. AIDS. 2000;14:1187–1194. doi: 10.1097/00002030-200006160-00016. [DOI] [PubMed] [Google Scholar]
- 11.Yeh K, Small R D. Pharmacokinetic evaluation of stable piecewise cubic polynomials as numerical integration functions. J Pharmacokinet Biopharm. 1989;17:721–740. doi: 10.1007/BF01062126. [DOI] [PubMed] [Google Scholar]
- 12.Yeh K C, Deutsch P J, Haddix H, Hesney M, Hoagland V, Ju W D, Justice S J, Osborne B, Sterrett A T, Stone J A, Woolf E, Waldman S. Single-dose pharamcokinetics of indinavir and the effect of food. Antimicrob Agents Chemother. 1998;42:332–338. doi: 10.1128/aac.42.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]