Abstract
Objective
Continuing care, which is an extension of post-discharge care, is recognized as a crucial element of high-quality health services and is essential to patients. This systematic review aims to identify the effectiveness of continuing care for patients with stomas.
Methods
PubMed, EMBASE, Cochrane Trial Register and Web of Science databases were searched. Study selection and quality appraisal were performed independently by two reviewers. We calculated the mean differences (MD) or the relative risk (RR) with 95% confidence intervals and assessed heterogeneity.
Results
Nine studies (1134 participants) met the inclusion criteria. This meta-analysis revealed that, in the continuous care group, the stoma self-efficacy (MD = 6.46; 95% CI = 3.81–9.11; P < 0.001; I2 = 0%), and the quality of life (MD = 7.48; 95% CI = 5.13–9.82; P < 0.001; I2 = 0%) increased significantly 1 month after discharge; stoma adjustment and care satisfaction also showed a trend toward improvement while stoma complications (RR = 0.71; 95% CI = 0.58–0.87; P = 0.001; I2 = 25%) decreased significantly. Continuing care did not decrease hospital readmission rates or medical costs.
Conclusions
Continuing care showed beneficial effects in improving health outcomes and care satisfaction for patients with stomas compared with routine care. We proposed an integrated continuing care program with different elements and recommendations for its implementation.
Keywords: Colostomy, Continuing care, Meta-analysis, Nursing, Stoma nurse, Systematic review
Introduction
In patients with colorectal malignancies and inflammatory bowel disease, a stoma surgery is a frequently applied treatment to allow recovery of the more distal and diseased parts of the bowel, accompanied by the possibility of a temporary or permanent stoma.1 Although the operation is a life-saving procedure that provides a new artificial pathway for waste elimination, it leads to a dramatic change in both lifestyle and habits.2,3 As healthcare providers, how do we help patients successfully overcome these challenges and resume a normal life? This has been the focus of our research.4,5
The development of stoma therapy as a specialized nursing role closely follows the advances made by colorectal surgeons in this area of care.6 This specialised role benefits the patient by providing specialist knowledge to the client and help deal with any ongoing difficulties.7,8 As a well-established specialty, a stoma nurse needs to establish cooperative relationships with surgeons, other health care professionals, and patients. From the moment of a diagnosis leading to stoma creation, patient care is handled mainly by nursing professionals, who provide comprehensive care throughout the care process.2 Due to the particularity of the structure and the physiology involved in enterostomy, the aftercare for patients with stoma lasts throughout the patient's life.9
In the current health care setting (e.g., the turnover rate of hospital beds, the use of minimally invasive technology, and the national medical insurance system), patients are quickly discharged after short-term hospitalization surgery and enter the long-term care stage at home.10,11 In fact, in the case of limited medical resources and imperfect community health care institutions, the provision of health-related specialized care to patients is mostly limited to the period of hospitalization, but two-thirds of discharged patients still need nursing services, especially for permanent enterostomy patients with a keen desire for stoma care.12,13 Nowadays, routine discharge care mainly includes health education on medication, diet, mobility, and stoma care instruction before discharge. In addition, a single form of health education and care instruction is often guided by a passive acceptance model, ignoring the diverse needs of patients at different disease stages after discharge. In other words, it is essential for stoma patients to promptly receive post-discharge guidance and support from healthcare practitioners who are well trained and have current information about the patient's goals, preferences, and clinical status.14
Continuing care is a series of actions designed to ensure that the patients can continue to use professional care services even after they are transferred from the hospital to their families and communities.14 As an extension of post-discharge care, continuing care is recognized as a crucial element among high quality health services and is believed to be essential to patients.15,16 In our current medical setting, continuing care, which is usually provided by the stoma therapy nurse, includes all elements of stoma care that facilitate the patients to live independently after discharge and resume a normal life in a shorter period. Patients can receive comprehensive and continuous care from specialized nurses throughout the postoperative recovery period by reading self-management manuals, using telemedicine, participating in intensive monitoring, communicating using a telephone, and accepting home visit follow-ups.
In the existing research on the continuous care of patients with stomas, multiple isolated and integrated continuing care interventions have been described in a wide variety of formats and modalities. These interventions, based on continuous care, have shown degrees of effectiveness;17, 18, 19 however, there is a lack of certainty over the best modality and the most suitable level of intensity of continuous care intervention for patients with stomas. Furthermore, there are no standardized protocols for continuous care in existing literature, and there is a lack of a systematic analysis of research on these continuing care interventions for people with stomas.
It is helpful for stoma therapy nurses to determine whether the provided care is beneficial to the patient based on the available evidence for the effectiveness and efficiency of care interventions. The purpose of a systematic review is to summarize the effectiveness, strengths, and weaknesses of reported findings and point out the areas in which there is a lack of information. With the advent of evidence-based health care, systematic reviews encourage healthcare providers to base their decision-making on current best evidence in regards to the care of individual patients. Therefore, this systematic review aims to analyse the best available evidence to evaluate the effectiveness of continuing care provided by stoma nurses for patients with stomas. It could offer insight to stoma nurses into how to effectively organize and perform continuing care for patients with stomas after they have finished being provided with their care in the hospitalization phase.
Methods
Study design
The systematic review and meta-analysis were conducted according to the Cochrane Handbook for Systematic Reviews of Interventions.20 They were reported based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidance for systematic reviews.21
Search strategy
To identify studies published up to July 2020, a sensitive search was conducted in four electronic databases (PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and Web of Science; Appendix S1 shows the complete search strategy). Trials registers (Current Controlled Trials, included at http://clinicaltrials.gov/) were searched to identify ongoing trials. We formed a PICO (Participants, Intervention, Comparison, and Outcome) question and used it to establish the eligibility criteria.22 Studies were considered eligible if they met the following criteria: (1) participants are adults (aged over 18 years old) who have undergone colostomy or ileostomy surgery; (2) the intervention is continuing care provided by a stoma nurse (enterostomal therapist [ET] or wound, ostomy, and continence certified nurse [WOCN] or gastrointestinal surgery nurse); (3) the comparison includes groups described as “usual care” (preoperative preparation and postoperative rehabilitation guidance in regards to diet, medication, rest, mobility, and ostomy care [note that after discharge, the usual care also included outpatient follow-up visits and telephone calls initiated by the patient to the nurses if needed but without a set time and mode]); (4) considering the outcomes, the meta-analysis examined the studies that have reported health variables, including quality of life for stoma patients, stoma care self-efficacy, stoma complications, and other variables, and the reported outcomes also included care satisfaction, hospital readmission rates, and medical costs; and finally, (5) the study's design (S) is a randomized controlled trial (RCT). The exclusion criteria were (1) studies that include patients who received urostomy and (2) studies that are not published in English.
Study selection
Studies retrieved from the search were imported to Endnote X9 23. After removing duplicates, two independent reviewers examined and screened the remaining studies for further assessment. The studies were selected in two phases. First, we screened the titles and abstracts of the studies retrieved in the initial literature search. Second, full texts were screened for eligibility against the inclusion and exclusion criteria described above, and the reasons for exclusion in each study were recorded. After the studies were selected independently by the two reviewers, the studies were gathered and any discrepancies in the extracted data were resolved by discussion.
Quality appraisal
Two reviewers independently assessed the quality of each study's methodology that fit the eligibility criteria. The Cochrane risk-of-bias tool was used to assess the studies' risks of bias.20 Seven domains of bias were assessed as either having a high, low, or unclear risk of bias: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other possible biases. Any discrepancies between the two reviewers were settled through discussion. The results of the quality assessment were reported using Review Manager 5.1 (software).
Data extraction
Data extraction was conducted by one reviewer and independently checked by a second reviewer, and any disagreement was settled by discussion, and a consensus was reached. The data extracted were documented on pre-designed forms (Microsoft Excel). Data that were extracted include the following: study authors, publication year, sample size, participant characteristics (age and gender), inclusion criteria, intervention strategies, control strategies, main variables, time points of assessment, and study outcomes.
Data synthesis
The outcome data of the studies were extracted and entered into Revman 5.3. Eligible studies were analyzed using the means and standard deviations (SDs) to measure the change from the baseline to the endpoint during each intervention period. To calculate the effect sizes of the main outcomes, the mean differences (MD) with 95% confidence intervals (CIs), the standardized mean differences (SMD) with 95% confidence intervals (CIs), or relative risk (RR) with 95% confidence intervals (CIs) were selected.24 Due to differences in participants and interventions, different studies resulted in different effect sizes, and heterogeneity was expected. According to Borenstein et al.,25 the random-effects model is generally a more plausible match; therefore, the random-effects model was used throughout the analysis. A narrative synthesis, which encompasses characteristics of the selected studies, was presented for the studies that were not pooled. The heterogeneity of the selected studies was evaluated with Cochran's Q (χ2 test). Based on the test statistics, the values 0%, 25%, 50%, and 75% indicate no heterogeneity, low heterogeneity, moderate heterogeneity, and high heterogeneity, respectively.26
Results
Search outcomes
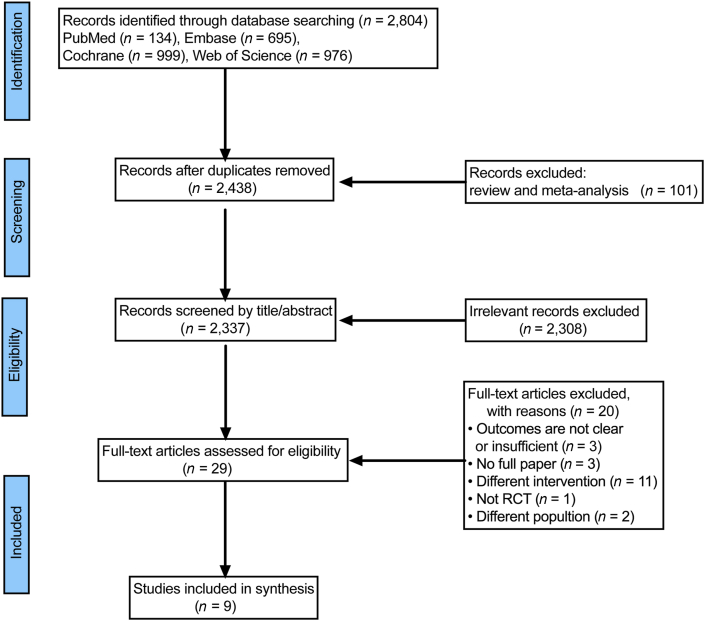
As illustrated in the PRISMA flow chart (Figure 1), the search identified 2804 studies, from which 366 duplicates and 101 reviews and meta-analysis studies were removed. After screening the titles and abstracts of the included articles, 2308 articles were excluded. Twenty more articles were excluded after a full text screening, and the main reasons for exclusion were as follows: a full article contained a different population (n = 2); it contained a different intervention (n = 11); it was not an RCT (n = 1); it was not a full article (n = 3); and it had an incomplete outcome (n = 3) (Appendix S2 shows all the excluded studies and the reasons). In the end, nine eligible studies were identified for this systematic review.
Figure 1.
Flow diagram of the selection process. RCT, Randomized controlled trial.
Quality assessment
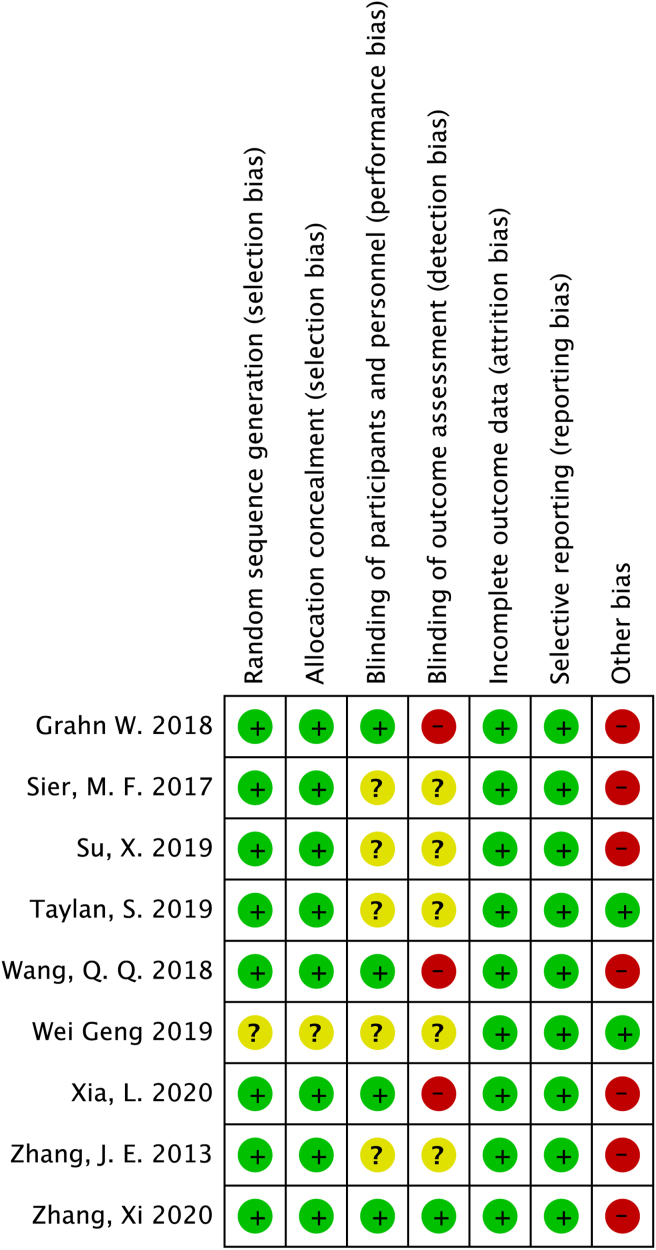
Eight studies illustrated the process of randomization and allocation concealment in detail, indicating that they had a lower risk of bias on the randomization procedure. When we consider the blinding of the studies, only one of the studies was a double-blind study. We did not obtain adequate information about the blinding procedures of participants and outcome assessors in five studies, and we considered this condition to be an unclear risk of bias. In the remaining three studies, participants were blinded to their group allocation, but the outcome assessors were not blinded, thus leading to a high risk of bias because of insufficient blinding. Although the outcome data were incomplete in three studies and might contain a high risk of bias, there was a low risk of attrition bias in all the studies based on our assessment; this is because the missing data were balanced between the groups and the reasons for dropouts were similar. In addition, the nine included studies had a low risk of reporting bias because all the outcomes were reported in their result sections. For other sources of risk, seven studies received financial support for performing the study and thus had a high risk of bias. Furthermore, insufficient studies were available to assess publication bias by funnel plotting. Of the included studies, two studies were graded as having a low risk of bias and seven studies were graded as having a high risk of bias (Figure 2).
Figure 2.
Risk of bias summary.
Description of included studies
Most studies were conducted in China (n = 6), and the rest were conducted in other countries: USA (n = 1), Turkey (n = 1), and the Netherlands (n = 1). The characteristics of the nine included studies are listed in Table 1. In total, 1135 patients were studied; note that if a study27 has an adult group followed by a pediatric group, we only included the adult group (n = 60) based on the inclusion criteria of this systematic review. The characteristics among the study population in the individual studies were similar; however, the interventions and outcome measures showed pronounced heterogeneity.
Table 1.
Characteristics of included studies: participants.
| Country | Setting | Sample size (n) | Age (years), Mean ± SD | Gender male (%) | Stoma status (%) | Stoma type (%) |
|---|---|---|---|---|---|---|
| China28 | University Hospital | 155 | EP: 48.15% > 50y CP: 51.35% > 50y | 53.55 | Permanent: 100 | Colostomy: 100 |
| China29 | 4 general tertiary hospitals | 107 | EP: 56.98 ± 14.66 CP: 59.11 ± 12.93 | 62.62 | Temporary: 100 | Ileostomy: 75.70 Colostomy: 24.30 |
| Turkey30 | University Hospital | 70 | EP: 53.00 ± 11.18 CP: 50.74 ± 13.72 | 52.86 | Permanent: 27.14 Temporary: 72.86 |
Ileostomy: 55.71 Colostomy: 44.29 |
| United states31 | Hospital | 100 | EP: 34.69% > 65y CP: 25.49% > 65y | 45 | Permanent: - Temporary: - |
Ileostomy: 100 |
| Netherlands32 | 3 hospitals | 218 | EP: 63.70 ± 10.50 CP: 60.80 ± 13.40 | 64.68 | Permanent: 61.01 Temporary: 37.16 |
Colostomy: 58.26 Ileostomy: 39.91 |
| China33 | 6 hospitals and 1 cancer center | 103 | EP: 52.90 ± 13.30 CP: 55.30 ± 13.70 | 65.05 | Permanent: 100 | Colostomy: 100 |
| China27 | University Hospital | 60 | EP: 45.37 ± 13.28 CP: 45.37 ± 13.28 | 58.33 | Permanent: - Temporary: - |
Colostomy: 50 Ileostomy: 50 |
| China34 | 10 tertiary general hospitals | 203 | EP: 56.95 ± 14.88 CP: 59.18 ± 14.13 | 63.55 | Permanent: 100 | Colostomy: 76.85 Ileostomy: 17.24 Other: 5.91 |
| China35 | 3 medical centers | 119 | EP: 58.63 ± 8.64 CP: 59.41 ± 7.90 | 68.07 | Permanent: 100 | Colostomy: 100 |
Note: -, not reported; EP, experimental group; CP: control group.
Participants
Most studies included a sufficient number of participants, and the sample size ranged from 60 to 203. In our protocol, we decided to include participants with both temporary stoma and permanent stoma, without specifying the disease stage and the reason for the stoma creation. Four of the studies included only patients with permanent stomas,33,28, 34, 35 one included only patients with temporary stomas,29 and the remaining four included patients with both permanent and temporary stomas.32,27,31,30 Of the participants, 60.35% were male and 39.65% were female. For the age distribution in the studies (n = 7), the mean age of participants was 57.40 (SD 13.47) years, and two studies only reported the percentage of participants aged 50–65 years. The details are listed in Table 1.
Interventions
Interventions varied in duration (1–6 months), intervention frequency, and type of continuing care (Table 2). The intervention providers were professional nursing staff with extensive experience and trained in stoma care. The details are listed in Table 2.
Table 2.
Characteristics of included studies: intervention.
| Study | Continuing care strategies | Intervention provider | Duration of intervention | Frequency and timing of intervention |
|---|---|---|---|---|
| 28 | Continuous care model of information-based hospital–family integration | Colostomy therapist | 3 months | Self-management manual: - Colostomy care video: once (the day before operation) Education program on stoma care: once (the day before operation) Social software communication: any time, including off work home visits: - |
| 29 | Evidence-based continuing care bundle | Enterostomal therapist or wound, ostomy, and continence certified nurse | 3 months | Self-management manual: 3 times (24 h of admission to the hospital; 24 h after surgery; day of discharge) Telephone follow-up: 4 times (within 3–7; 14 to 20; 27 to 30; and 87–90 days after discharge, respectively) Outpatient follow-up: once (the fourth week after surgery) |
| 30 | Telephone counseling | Certified stoma nurse | 3 months | 5 times (at postop weeks 1, 2, and 4 and once a month after discharge) |
| 31 | Telephone surveillance and prompting | Advanced practice provider; stoma inpatient and outpatient nurse | 1 month | 30 times (once a day) |
| 32 | Home visits | Enterostomal therapist | 3 months | Home visit: once (3 weeks before hospital admission) Outpatient examine: once (2 weeks after surgery) Further home visits: 2 times (4–6 weeks and 12 weeks after discharge) |
| 33 | Telephone follow-up | Enterostomal therapist | 3 months | 2–3 times (3–7 days after discharge/14 to 20 days after discharge/23 to 27 after discharge (if stoma self-care ability was still lower than 5 on the Stoma Self Care Scale (range, 0–10) |
| 27 | Continuous nursing | Stoma professional nursing staff | 6 months | Customization of care plan: - Nursing training for families: once (the day before operation) Mental health care: - Nursing supervision and telephone follow-up: 24 times (once a week). |
| 34 | Smartphone app follow-up | Enterostomal therapist | 6 months | 7 times (the first month after discharge: once a week/the next two months after discharge: once every two weeks/the next three months after discharge: once a month. |
| 35 | Hospital–family holistic care model | Gastrointestinal surgery nurse | 4 months | Phone call: 15 times (the first month: twice a week/the second month: once a week/the third month: once every 2 weeks/the fourth month: once a month. Clinic visit: 6 times (the first month: once every 2 weeks/the second month: once every 2 weeks/the third month: once every 3 weeks/the fourth month: once a month. |
Note: -, not reported.
Telephone calls
Three studies used telephone calls for their intervention group,33,31,30 but their actual implementation of these calls differed substantially.
Taylan and Akil30 carried out counselor-initiated, once-a-month telephone counseling to provide patients with general support. Based on the patient's request, the telephone counselor focused on the sexual problems experienced due to the existence of the stoma, without using any special counseling techniques.
Grahn et al.31 used a monitoring protocol, which uses repeated phone calls to encourage patients to report their daily ileostomy volumes and symptoms of dehydration. During the monitoring task, the study personnel did not have any patient contact, and they called and prompted the inpatient nurse to assess the patients’ progress using educational tools before their discharge, and after their discharge, the personnel called the outpatient nurse or an advanced practice provider to prompt the providers to perform a telephone follow-up with the patient.
Zhang et al.33 used nurse-initiated calls at predetermined times to improve patient ostomy adjustment. The telephone follow-up protocol consists of three parts: assessment, management options, and evaluation. The ET called patients 2–3 times to manage and support the patients during the follow-up period.
Home visits
One study32 used home visits as a new form of care pathway and compared it with a standard care pathway. The home visits care pathway included a detailed plan and content, including three home visits by ET before admission and after discharge. Based on the patient's request, home visits were supplemented with one outpatient visit after surgery, and a 24/7 telephone contact plan was arranged.
Mobile application
One study34 used a mobile app as a diagnostic and consulting tool so that patients could receive stoma care from the ET through this app at home. Participants provided photos of their stomas and also essential medical information through this app at predetermined times, and the ET nurse would make a diagnosis of the stoma condition, and finally, the patients would obtain directions online from the ET nurse.
Integrated continuous care model
Four studies adopted an integrated continuous care model, which is the combined use of multiple modes of continuous care and differs from the studies that adopted a single continuous care model.26 27, 28, 29, 35
Xia et al.28 used a continuous care model of information-based, hospital-and-family integration, which includes hospital aspects (self-management manuals, colostomy care videos, and education programs on colostomy care) and family aspects (home caregiver training, contact with a colostomy therapist in real time via social software, and home visits). In addition to the interaction between the stoma therapist and patient, it is worth noting that in this integrated continuous care model, family caregivers were also involved and made the protocol more extensive and more operable.
Su et al.29 used an evidence-based continuing care bundle (CCB) to improve the health outcomes of patients with temporary stomas. The CCB included a self-management manual, telephone follow-ups, and stoma outpatient follow-ups that together form a package of interventions, which were performed collectively. This study also included a detailed formal care plan that was performed based on Bandura's self-efficacy theory by an ET or a WOCN.
In the study of Geng et al.27 a continuous care nursing team, which was established by professional nursing staff, employed continuous care measures in the following forms: a six-month continuing care plan tailored to each patient's situation, nursing training for families of patients, nursing supervision, and telephone follow-ups. In addition, mental health care was also one of the measures of continuous care that was used during regular return visits.
Zhang et al.35 adopted “timing it right (TIR)” as the study's theoretical framework and used a hospital-and-home holistic care model at different disease stages and at predetermined points in time. It includes two parts: in-hospital (one-on-one and face-to-face communications, lectures, bedside instructions and demonstrations, and videos) and out-of-hospital (internet tools, outpatient follow ups, and telephone follow-ups).
Effects of interventions
The main findings of the included studies are summarized in Table 3. Of the nine included studies, seven studies27, 28, 29, 30, 33, 34, 35 showed a significant trend in favor of the intervention group, and two studies31, 32 reported significantly better results for the intervention group on some but not all outcomes. We highlighted several findings from different outcome measures in more detail below. Most studies included in this systematic review used different outcome variables to measure the effect of intervention, and even if the same outcome variables were measured, the types of data collected were variable in nature since these studies used different tools. Therefore, a meta-analysis could only be performed for a limited number of outcome variables, and other data were reported in a narrative format.
Table 3.
Characteristics of included studies: outcomes and findings.
| Study | Time points of assessment | Outcome measures | Tool | Findings |
|---|---|---|---|---|
| 28 | At time of discharge 1 month after discharge 3 months after discharge |
State and trait anxiety Stoma self-efficacy Stoma complication Stoma quality of life Care satisfaction |
STAI SSES SCPC Stoma–QOL CS |
EP had less anxiety; better self-efficacy; fewer complications; better quality of life scores and were more satisfied with the care. |
| 29 | At time of discharge 4 weeks after discharge 12 weeks after discharge |
Stoma self-efficacy Stoma quality of life Stoma complication Stoma reversal Care satisfaction |
SSES Stoma–QOL SCPC OSR CS |
EP had significantly improved self-efficacy; quality of life; significantly lower stoma complications. The stoma reversal in EP was more likely to occur and to occur earlier. EP reporting higher satisfaction. |
| 30 | At time of discharge 6 weeks after discharge 12 weeks after discharge |
Stoma-related data Sexual satisfaction |
QIIS GRISS |
EP had significantly improved the GRISS scores. |
| 31 | Within 30 days of discharge (satisfaction was measured at 2–3 months after discharge) | Hospital readmissions Acute kidney injury Care satisfaction Medical costs |
– – CS – |
EP did not reduce hospital readmissions or readmissions for Acute Kidney Injury. EP was neither costly nor cost-saving, and it did not increase patient satisfaction. |
| 32 | 2 weeks of discharge 4 weeks after discharge 12 weeks after discharge |
Stoma complications Quality of life Outpatient frequency Medical costs |
– Stoma–QOL – – |
In EP more patients had stoma complications/QoL were significantly better. There was no difference between groups in frequency of outpatient and medical costs. |
| 33 | At time of discharge 1 month after discharge 3 months after discharge |
Stoma adjustment Stoma self-efficacy Care satisfaction Stoma complications |
OAS SSES CS SCPC |
EP had significantly better stoma adjustment; higher stoma self-efficacy; higher satisfaction with care; less stoma complications. |
| 27 | 6 months after discharge | Anxiety Depression Stoma quality of life Stoma complications Care satisfaction Stoma knowledge |
SAS SDS Stoma–QOL – CS – |
In EP: SAS and SDS scores were significantly lower; QOL score was significantly higher; stoma complications were significantly lower; care satisfaction was significantly higher; and knowledge of stoma care was significantly higher. |
| 34 | At time of discharge 1 month after discharge 3 months after discharge |
Ostomy adjustment Stoma self-efficacy Stoma complications |
OAI-23 SSES SCPC |
In EP: adjustment and stoma self-efficacy score were significantly higher; stoma complications were tending to reduce. |
| 35 | At time of discharge 3 month after discharge 6 months after discharge |
Resilience Self-care agency Quality of Life Stoma complications |
CD-RISC ESCA Stoma–QOL SCPC |
In EP: the psychological resilience, self-care ability and quality of life were significantly better; the complications were significantly lower. |
Abbreviations: CD-RISC, Connor Davidson resilience scale; CS, care satisfaction (Likert 5 grade score); EP, experimental group; ESCA, exercise of self-care agency scale; GRISS, Golombok–Rust inventory of sexual satisfaction; QIIS, Questionnaire for Individuals with Intestinal Stoma; OAI-23, Ostomy Adjustment Inventory-23; OAS, Ostomy adjustment scale; OSR: outcomes of stoma reversal; SAS, self-rating anxiety scale; SCPC, stoma complication preset checklist; SDS, self-rating depression scale; SSES, stoma self-efficacy scale; STAI, State-Trait Anxiety Inventory; Stoma-QOL, stoma quality of life scale; -, not reported.
Stoma care self-efficacy
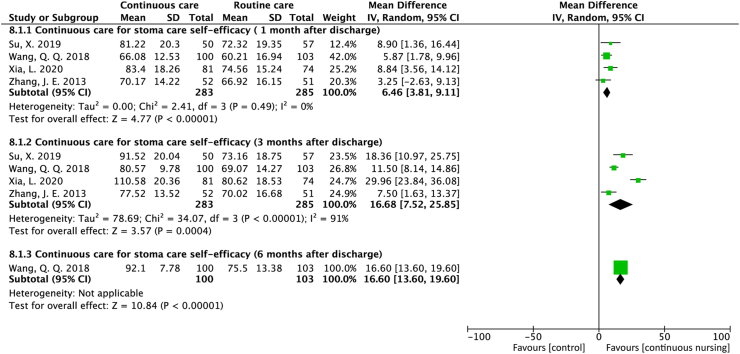
A total of four studies reported stoma self-efficacy. All four studies28, 29, 33, 34 reported a significant increase in the stoma self-efficacy scores in the continuous care group at 1 month (MD = 6.46; 95% CI = 3.81 to 9.11; P < 0.001; I2 = 0%) and 3 months (MD = 16.68; 95% CI = 7.52 to 25.85; P < 0.001; I2 = 91%) after discharge. In addition, one study34 reported that the stoma self-efficacy scores continued to increase at six months after discharge (MD = 16.60; 95% CI = 13.60 to 19.00; P < 0.001) (Figure 3).
Figure 3.
Forest plot of studies that assessed the effect of stoma care self-efficacy.
Quality of life in patients with stomas
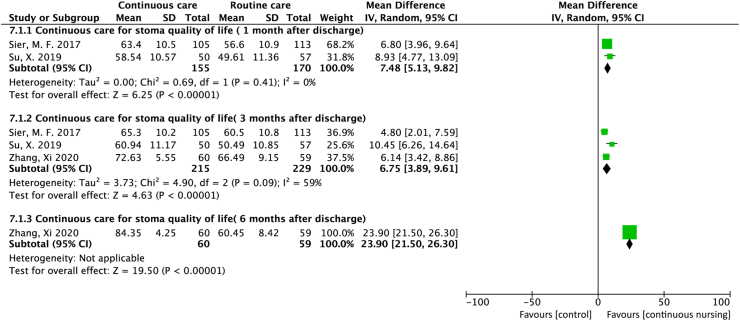
The quality of life in patients with stomas was reported in three studies. Among these three studies, two reported32,29 the outcome 1 month after discharge, and the pooled result indicates an improved quality of life in the continuous care group (MD = 7.48; 95% CI = 5.13 to 9.82; P < 0.001; I2 = 0%). All three studies32,35,29 reported this outcome three months after discharge, and the pooled result of continuous care indicates improved quality of life (MD = 6.75; 95% CI = 3.89 to 9.61; P < 0.001; I2 = 59%). Furthermore, one study35 measured this outcome 6 months after discharge and also found an improved quality of life (MD = 23.90; 95% CI = 21.50 to 26.30; P < 0.001) (Figure 4).
Figure 4.
Forest plot of studies that assessed the effect of stomas on the quality of life.
Stoma adjustment
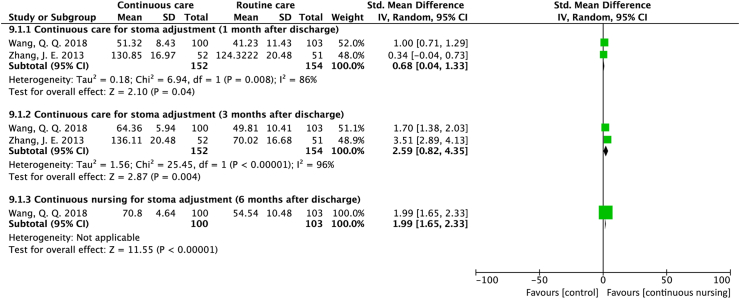
Two studies used different scales to assess stoma adjustment at three endpoints after discharge. The pooled results based on two studies33,34 suggest that continuous care improved stoma adjustment 1 month (SMD = 0.68; 95% CI = 0.04 to 1.33; P = 0.04; I2 = 86%) and 3 months (SMD = 2.59; 95% CI = 0.82 to 4.35; P = 0.004; I2 = 96%) after discharge. The result from one study34 suggests that stoma adjustment continued to improve at six months after discharge (SMD = 1.99; 95% CI = 1.65 to 2.33; P < 0.001) (Figure 5).
Figure 5.
Forest plot of studies assessing the effect of stoma adjustment.
Stoma complications
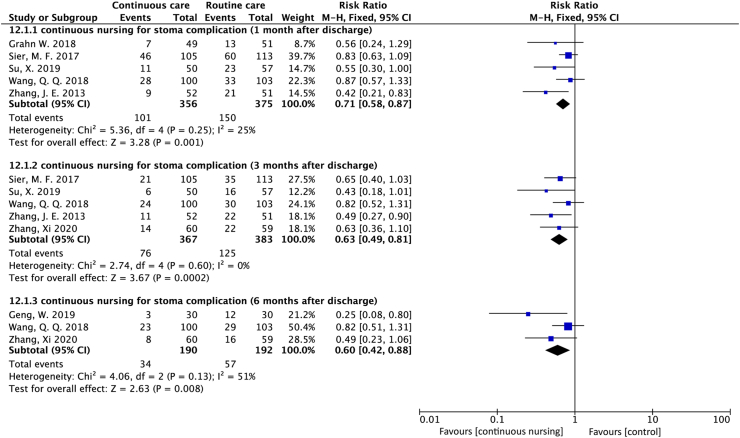
Eight studies27, 28, 29, 31, 32, 33, 34, 35 measured stoma complication but only seven reported the complication incidence rates. Among these seven studies, five reported29, 31, 33, 34, 35 a significant decreasing trend in the incidence rate of complications with continuous care at one month (RR = 0.71; 95% CI = 0.58 to 0.87; P = 0.001; I2 = 25%) and three months (RR = 0.63; 95% CI = 0.49 to 0.81; P < 0.001; I2 = 0%) after discharge. Three studies27, 34, 35 also reported a decreased incidence of stoma complication in the continuous care group at six months after discharge (RR = 0.60, 95% CI = 0.42 to 0.88; P = 0.008; I2 = 51%) (Figure 6).
Figure 6.
Forest plot of studies that assessed the effect of stoma complications.
Psychological status
Of the included studies, only three studies involved psychological variables: anxiety, depression, and psychological resilience. One study27 reported the results of anxiety and depression, and it showed that anxiety and depression scores of the intervention group were significantly lower than those of the control group after six months after the operation (51.07 ± 5.82 vs. 28.67 ± 5.18). One study28 reported the state anxiety and trait anxiety, and its result also proves that both scores of state anxiety and trait anxiety in the intervention group were significantly lower than the scores of the control group (state anxiety: 27.21 ± 6.88 vs. 38.02 ± 6.33; trait anxiety: 31.50 ± 7.16 vs. 39.65 ± 9.47). One study35 measured psychological resilience and found that the psychological resilience of the two groups of patients increased significantly with observation time, especially in the intervention group, which improved fastest in the period from 3 to 6 months after discharge (F = 92.03, P < 0.05).
Sexual satisfaction
Regarding the sex life of patients with a bowel stoma, only one study30 measured sexual satisfaction using the Golombok–Rust inventory of sexual satisfaction (GRISS). The result shows that the GRISS total and several subscale scores of both men and women in the intervention group increased in the 12th week after the operation and were statistically significant (P < 0.001).
Care satisfaction
Although five studies measured care satisfaction, only two studies28, 29 were pooled due to different data types. The pooled result suggests that, in terms of satisfaction, continuous care may be superior to routine care; however, it did not achieve statistical significance (MD = 0.41, 95% CI = −0.09 to 0.91; P = 0.11; I2 = 87%). Two studies reported the results as a percentage; one study showed that care satisfaction of the continuous care group was slightly higher, but there was no statistical significance (33% vs. 23%, P > 0.05), while another study27 showed that continuous care significantly increased care satisfaction (96.67% vs. 53.33%, P < 0.001). The remaining study33 only reported average values, and the result also shows a significant increase in care satisfaction in the continuous care group (1.45 vs. 2.04, P < 0.001) (Figure 7).
Figure 7.
Forest plot of the studies' effect of care satisfaction.
Hospital readmission
One study 31 measured the number of unplanned hospital readmissions within 30 days of discharge and found no difference in the overall number of hospital readmissions between the intervention and control groups (20.4% vs. 19.6%; P = 1.00), as well as the number of readmissions due to dehydration (8.2% vs. 5.9%; P = 0.71).
Medical costs
Two studies reported medical costs, including the costs of medication, the costs of post-discharge outpatient clinical visits, the costs of specialist and nursing home care, and the costs of hospital readmissions. In the study by Grahn et al.,31 the direct cost total was slightly lower for the cost of the intervention compared with the cost of usual care ($23,404 vs. $26,083). In the study by;32 there was no significant difference in medical costs between the control group and the intervention group ($10,119 vs. $10,277) because more patients in the intervention group received chemotherapy and radiation therapy, resulting in higher costs.
Discussion
Continuous care, as an extension of post-discharge care, has been widely used in patients with stomas after discharge. Although there is a large body of literature that reported on the various formats and modalities of continuing care, the effectiveness and optimal intervention for continuing care have yet to be determined. This systematic review and meta-analysis aimed to identify, appraise, extract, and synthesize the best available evidence for the effectiveness of continuous care provided by stoma therapy nurses to patients with stomas. Of the twenty-five studies that were considered relevant based on a detailed review, sixteen were screened out, and the nine that met the inclusion criteria were subjected to critical appraisal and analysis.
Limited evidence available
Our review shows that few high-quality studies on continuing care for patients with stomas are available. We noted several available studies that included a mixed population of patients with colostomy, ileostomy and urostomy. Broadening our participant eligibility criteria to this population would have allowed for a much larger sample of studies. However, for the reasons we will discuss below, we decided during the protocol stage to include only the studies that included a population with colostomy and ileostomy without urostomy. Although enterostomy and urostomy are surgical procedures that open new passages for feces or urine, both groups seem to differ with regards to postoperative care, complications, and the severity of impact on life.36, 37, 38 Therefore, in order to most reliably compare the continuing care interventions, we strictly defined the studied population and excluded studies that recruited a mixed population with urostomy.
For the outcome variables in the included studies, most studies only selected stoma-related variables (stoma self-efficacy, quality of life, and complications), as well as satisfaction in continuous care. However, we found that few studies examined outcomes such as readmission rate, medical costs, and the psychological status of patients. To evaluate the effectiveness of a nursing intervention more comprehensively, researchers should not only consider the improvement of patients' physiological factors, but also include psychological factors and social functions and the potential role interventions play in reducing medical burden and costs.
Although we attempted to maximize the homogeneity between the populations of the nine included studies, differences evolved in regards to the status of the participants' stomas (permanent or temporary), continuing care intervention modality, endpoints of observation, and reported outcomes. The results of some studies still indicate significant heterogeneity. Among these differences, the permanent stomas need to be followed up throughout a patient's life, whereas the temporary stomas' follow-up period lasts 3–6 months; the participants' stoma statuses may be the main reason for this heterogeneity. We strongly recommend future researchers to separate permanent and temporary stoma patients in their studies.
Intervention effect
Based on the available data, we conclude that continuing care provided by stoma therapy nurses can improve health outcomes in patients with stomas. The transition from hospital to home has always been an important part of the care process. Continuing care differs from routine care in many aspects. As a client-driven collaborative process, continuing care interventions bring treatment and care more proactively to the patient and are usually performed on a more patient-convenient mode. They include the use of high-quality health and support services with a focus on patient empowerment and meet ongoing requirements, whereas routine care consists mainly of symptomatic treatment and supportive counseling without a set mode. Finally, continuing care interventions also target the functioning of the patients within their family networks via phone communication and improve coordination between the patient and different healthcare services through the effective and efficient use of resources.
In addition to health outcomes, we also observed the effectiveness of continuous care in improving care satisfaction. The pooled result of the two studies suggests that continuous care may be superior to routine care in terms of satisfaction; however they did not achieve statistical significance. We speculate that the effect is not significant due to an insufficient sample size; RCTs with larger samples should be recommended in future studies. However, from the collective results of five of the included studies, we can still draw a preliminary conclusion that continuous care can effectively improve patients' satisfaction towards care.
Compared to routine care, there was no difference in medical costs and hospital readmission rates after continuing care was provided according to our analysis. The reason for this result may be related to the limited number of included studies, or the disease characteristics of the included subjects; for example, more patients in the intervention group received chemotherapy and radiation therapy, which lead to higher costs. Therefore, we need more research that examines not only the effectiveness of care interventions, but also the effectiveness of different interventions for specific persons with different characteristics at different stages of the illness. Studies must include measurements of medical costs as well as costs of interventions, thus examining expenditures from all points of view.
We compared our findings with the findings from existing literature. We noticed that our main conclusion agrees with the findings of several systematic reviews of continuing care for patients with chronic diseases (i.e. osteoporosis,39; persons with dementia,40; alcohol use disorders,16). At present, several single and integrated continuing care interventions have been implemented. In clinical practice, using a single continuing care intervention is still controversial. For example, telemedicine can be accessed quickly, but patients remain concerned about its effectiveness; telephone follow-ups can save patients' time and money, but they cannot clearly show the local status of the stoma; and outpatient follow-ups can provide post-discharge care, but they do not always address patients' psychosocial and informational needs.12,41,42 For integrated continuing care interventions, the types and content were rich and diverse, but the development and implementation of these multi-form interventions require a theoretical basis. Due to differences in medical resources, health systems, and cultures, different regions and hospitals have different medical settings, which have led to a variety of continuing care interventions and corresponding intervention effects. However, in this systematic review, single continuing care and integrated continuing care were both significant for improving patients’ health outcomes and nursing satisfaction.
Of the included studies, five studies used single continuous care, and four studies used integrated continuous care. We attempted to compare the effects of the interventions between them; however, due to the limited number of studies, we were unable to make comparisons. We can still speculate that integrated continuous care with a detailed nursing plan and with a theoretical base appears to be more effective than single continuous care. For the integrated care of chronic diseases, an integrated care program based on Wagner's chronic care model can be used during the phase of continuous care for patients with stomas. This model relies on the concept of continuous, integrated care and encourages the interaction between patients and a well-prepared and proactive practice team.43,44 The integrated care program includes five key elements: patient centeredness, continuity of care, evidence-based practice, multi-professional teamwork, and continuous quality improvement.45 Its aim is to promote autonomy and enhance the functionality of the dependent person who is regaining functional independence through rehabilitation, re-adaptation, and family and social reintegration. At present, the integrated continuing care (ICC) has been developed and implemented in Germany, Spain, and Brazil, and other countries, and has achieved satisfactory results.15,46
Implications for clinical practice and research
Based on current evidence, continuing care has shown promising results, especially when used to improve the health outcomes and care satisfaction in patients with stomas. However, rigorous research methods are still needed, especially the use of RCT, to compare the intervention with the control groups who receive usual care. For continuous care interventions, we believe that compared with a single continuous care, an integrated continuous care could better satisfy the complex demands of patients with stomas. We advocate an integrated continuing care program (ICCP), which can be used in the continuous care setting of patients with stomas. The program may include the following:43 patients use a mobile application to upload their stoma photos and stoma-related problems regularly, and stoma therapy nurses complete a diagnosis or evaluation based on the photos and provide online guidance; patients could also use the notepad function to record the difficulties they encountered, the problems they want to solve, and the goals they expect to reach. In addition, a telephone follow-up may be initiated by stoma therapy nurse within a predetermined time. The calls may include monitoring the patient's tasks and discussing the notes from the notepads, as well as coordinating care with gastrointestinal surgeons, psychiatrists, and other care providers. Mobile applications and phone calls could be supplemented with stoma-related outpatient follow-ups or home visits if the patient or the stoma nurse believes they are necessary. Excessive follow-up time should be avoided when we take into account its burden on the patient, its feasibility, and its cost-effectiveness of the intervention; therefore, we suggest a weekly intervention in the beginning. The intensity, frequency, and duration of interventions should be continuously adapted to the needs of the patients. In addition, we strongly recommend the use of more homogeneous and comprehensive outcome measures. Self-reported data and data from other sources should be combined, which means combining the data related to the evaluation of patients' health outcomes, care satisfaction, and costs of care from the hospital and from the patient's family.
Strengths and limitations
This is the first systematic analysis of continuing care research for patients with stomas. Findings from this research not only provide practical guidance for the care of patients with stomas but also provide a solid basis for further research. Furthermore, these findings could offer insight to stoma nurses into how to effectively organize and provide continuing care to patients with stomas. Even though the current findings offer numerous benefits, several limitations need to be overcome to provide avenues for future research. First, this review is based on a limited number of studies, with various outcome-related measurement tools. The heterogeneity between studies affected the strength of the conclusions. Second, seven of the included studies contain a high risk of bias. Due to the type of interventions, it was generally not possible to perform blinding on the participants, personnel, and outcome assessors, and this could have engendered a certain degree of performance and detection bias. Most studies also received financial support, which may have led to publication bias. Finally, this review included only studies published in English and some relevant studies in other languages might have been missed.
Conclusions
In this systematic review, existing evidence demonstrates that continuing care provided by stoma therapy nurses is more effective than routine care in improving health outcomes in patients with stomas. The results also confirmed that evidence-based, integrated continuing care with a theoretical basis may be more effective than single continuing care. In stoma care, stoma therapy nurses are important, and it is clear that their role is valuable and indispensable to the individual patient and society. For patients with stomas, continuous care may be required throughout the patient's life; therefore, stoma nurses need to explore ways to better support the needs of patients during postoperative care.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Asia-Pacific Journal of Oncology Nursing at https://doi.org/10.1016/j.apjon.2021.12.006.
Data availability statement
This is a systematic review and all the data were extracted from public article. All results were provided within this review.
Declaration of competing interest
None declared.
Funding
Nil.
Supplementary material
The following is the Supplementary data to this article:
References
- 1.Jayarajah U., Samarasekera D.N. A cross-sectional study of quality of life in a cohort of enteral ostomy patients presenting to a tertiary care hospital in a developing country in South Asia. BMC Res Notes. 2017;10:75. doi: 10.1186/s13104-017-2406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capilla-Díaz C., Black P., Bonill-de Las Nieves C., Luis Gómez-Urquiza J., Hernández Zambrano S., Montoya-Juárez R., et al. The patient experience of having a stoma and its relation to nursing practice: implementation of qualitative evidence through clinical pathways. Gastrointest Nurs. 2016;14:39–46. doi: 10.12968/gasn.2016.14.3.39. [DOI] [Google Scholar]
- 3.de La Encarnacion O.C. Impact on quality of life and importance of stoma care in patients with ostomy complications. Rev Rol Enfermeria. 2019;42:60–63. [Google Scholar]
- 4.Giordano V., Nicolotti M., Corvese F., Vellone E., Alvaro R., Villa G. Describing self-care and its associated variables in ostomy patients. J Adv Nurs. 2020;76:2982–2992. doi: 10.1111/jan.14499. [DOI] [PubMed] [Google Scholar]
- 5.Jin Y.F., Ma H.M., Jimenez-Herrera M. Self-disgust and stigma both mediate the relationship between stoma acceptance and stoma care self-efficacy. J Adv Nurs. 2020;76:2547–2558. doi: 10.1111/jan.14457. [DOI] [PubMed] [Google Scholar]
- 6.Doughty D. History of WOC(ET) nursing education. J Wound, Ostomy Cont Nurs. 2013;40:127–129. doi: 10.1097/WON.0b013e3182850764. [DOI] [PubMed] [Google Scholar]
- 7.Burch J. Care of patients undergoing stoma formation: what the nurse needs to know. Nurs Stand. 2017;31:40–45. doi: 10.7748/ns.2017.e10177. [DOI] [PubMed] [Google Scholar]
- 8.Finlay B., Sexton H., Mcdonald C. Care of patients with stomas in general practice. Aust J Gen Pract. 2018;47:362–365. doi: 10.31128/ajgp-12-17-4430. [DOI] [PubMed] [Google Scholar]
- 9.Folguera-Arnau M., Gutierrez-Vilaplana J.M., Gonzalez-Maria E., Moreno-Casbas M.T., Obarrio-Fernández S., Lorente-Granados G., et al. Implementation of best practice guidelines for ostomy care and management: care outcomes. Enfermeria Clin. 2020;30:176–184. doi: 10.1016/j.enfcli.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Wu A.W., Gu J., Wang J., Ye S.W., An Q., Yao Y.F., et al. Results after change of treatment policy for rectal cancer--report from a single hospital in China. Eur J Surg Oncol. 2007;33:718–723. doi: 10.1016/j.ejso.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Wang H.Y., Chen Y.K., Chen J.J., Song C., Gu J., et al. Current status of surgical treatment of rectal cancer in China. Chin Med J. 2020;133:2703–2711. doi: 10.1097/CM9.0000000000001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaver K., Latif S., Williamson S., Procter D., Sheridan J., Heath J., et al. An exploratory study of the follow-up care needs of patients treated for colorectal cancer. J Clin Nurs. 2010;19:3291–3300. doi: 10.1111/j.1365-2702.2010.03407.x. [DOI] [PubMed] [Google Scholar]
- 13.Black P. Stoma care nursing management: cost implications in community care. Br J Community Nurs. 2009;14:352–355. doi: 10.12968/bjcn.2009.14.8.43515. [DOI] [PubMed] [Google Scholar]
- 14.Coleman E.A., Boult C., American Geriatrics Society Health Care Systems Committee Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51:556–557. doi: 10.1046/j.1532-5415.2003.51186.x. [DOI] [PubMed] [Google Scholar]
- 15.Da Costa L.P., Pinheiro E.A., Siqueira R.M.D., Giacon-Arruda B.C.C., Amorim M.D., Sato D.M., et al. Integrated continuous care: implementation in Mato Grosso do Sul, Brazil. Biosci J. 2020;36:628–635. doi: 10.14393/BJ-v36n2a2020-42311. [DOI] [Google Scholar]
- 16.Lenaerts E., Mathei C., Matthys F., Zeeuws D., Pas L., Anderson P., et al. Continuing care for patients with alcohol use disorders: a systematic review. Drug Alcohol Depend. 2014;135:9–21. doi: 10.1016/j.drugalcdep.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Li X.X., Du X.W., Song W., Lu C., Hao W.N. Effect of continuous nursing care based on the IKAP theory on the quality of life of patients with chronic obstructive pulmonary disease A randomized controlled study. Medicine. 2020;99:e19543. doi: 10.1097/md.0000000000019543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabrizi F.M., Rajabzadeh H., Eghtedar S. Effects of the continuous care model on the health-promoting lifestyle in breast cancer survivors: a randomized clinical trial. Holist Nurs Pract. 2020;34:221–233. doi: 10.1097/hnp.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 19.Zakeri M.A., Khoshnood Z., Dehghan M., Abazari F. The effect of the Continuous Care Model on treatment adherence in patients with myocardial infarction: a randomised controlled trial. J Res Nurs. 2020;25:54–65. doi: 10.1177/1744987119890666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J.P.T., Altman D.G., Gotzsche P.C., Jüni P., Moher D., Oxman A.D., et al. Cochrane Bias Methods, G. Cochrane Stat Methods, G The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schardt C., Adams M., Owens T., Keitz S., Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bramer W., Bain P. Updating search strategies for systematic reviews using EndNote. J Med Libr Assoc JMLA. 2017;105:285–289. doi: 10.5195/jmla.2017.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedges L.V. Distribution theory for glass's estimator of effect size and related estimators. J Educ Stat. 1981;6:107–128. doi: 10.3102/10769986006002107. [DOI] [Google Scholar]
- 25.Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 26.Pigott T.D. Springer; 2012. Advances in Meta-Analysis. [Google Scholar]
- 27.Geng W., Tao N., Wang T., Zhang Y., Wang Y. Continuous nursing reduces postoperative complications and improves quality of life of patients after enterostomies. Int J Clin Exp Med. 2019;12:5895–5901. [Google Scholar]
- 28.Xia L. The effects of continuous care model of information-based hospital-family integration on colostomy patients: a randomized controlled trial. J Cancer Educ: Off J Am Assoc Cancer Educ. 2020;35:301–311. doi: 10.1007/s13187-018-1465-y. [DOI] [PubMed] [Google Scholar]
- 29.Su X., Zhong M.H., Ye X.M., Zhen L., Yin X.X., Qin F., et al. Effects of evidence-based continuing care bundle on health outcomes in rectal cancer patients with temporary stomas: a multicenter randomized controlled trial. Cancer Nurs. 2021;44:223–234. doi: 10.1097/NCC.0000000000000762. [DOI] [PubMed] [Google Scholar]
- 30.Taylan S., Akil Y. The effect of postoperative telephone counseling on the sexual life of patients with a bowel stoma: a randomized controlled trial. Wound Manag Prev. 2019;65:14–29. doi: 10.25270/wmp.2019.6.1429. [DOI] [PubMed] [Google Scholar]
- 31.Grahn S.W., Lowry A.C., Osborne M.C., Melton G.B., Gaertner W.B., Vogler S.A., et al. System-wide improvement for transitions after ileostomy surgery: can intensive monitoring of protocol compliance decrease readmissions? A randomized trial. Dis Colon Rectum. 2019;62:363–370. doi: 10.1097/dcr.0000000000001286. [DOI] [PubMed] [Google Scholar]
- 32.Sier M.F., Oostenbroek R.J., Dijkgraaf M.G.W., Veldink G.J., Bemelman W.A., Pronk A., et al. Home visits as part of a new care pathway (iAID) to improve quality of care and quality of life in ostomy patients: a cluster-randomized stepped-wedge trial. Colorectal Dis. 2017;19:739–749. doi: 10.1111/codi.13630. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J.E., Wong F.K., You L.M., Zheng M.C., Li Q., Zhang B.Y., et al. Effects of enterostomal nurse telephone follow-up on postoperative adjustment of discharged colostomy patients. Cancer Nurs. 2013;36:419–428. doi: 10.1097/NCC.0b013e31826fc8eb. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q.Q., Zhao J., Huo X.R., Wu L., Yang L.F., Li J.Y., et al. Effects of a home care mobile app on the outcomes of discharged patients with a stoma: a randomised controlled trial. J Clin Nurs. 2018;27:3592–3602. doi: 10.1111/jocn.14515. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X., Gao R., Lin J.L., Chen N., Lin Q., Huang G.F., et al. Effects of hospital-family holistic care model on the health outcome of patients with permanent enterostomy based on the theory of 'Timing it Right. J Clin Nurs. 2020;29:2196–2208. doi: 10.1111/jocn.15199. [DOI] [PubMed] [Google Scholar]
- 36.Davis S., Magliato B. A multidisciplinary approach to ensuring preoperative stoma site marking and education of elective colostomy, ileostomy, and urostomy patients. J Wound, Ostomy Cont Nurs. 2019;46:S41–S42. [Google Scholar]
- 37.Jansen F., van Uden-Kraan N., Braakman A., Verdonck-de Leeuw I. The generic and stoma-specific quality of life of cancer patients with a colostomy, ileostomy or urostomy. Psycho Oncol. 2013;22:208–209. [Google Scholar]
- 38.Salvadalena G. Incidence of complications of the stoma and peristomal skin among individuals with colostomy, ileostomy, and urostomy: a systematic review. J Wound, Ostomy Cont Nurs. 2008;35:596–607. doi: 10.1097/01.Won.0000341473.86932.89. [DOI] [PubMed] [Google Scholar]
- 39.Murphy E., Tan K.M., O'Keefe J., Smyth B., Mc Glynn J., Cogan L. A review of osteoporosis treatment in a continuing care setting. Ir J Med Sci. 2012;181 S279-S279. [Google Scholar]
- 40.Roberts J., Browne G., Gafni A., Varieur M., Loney P., de Ruijter M. Specialized continuing care models for persons with dementia: a systematic review of the research literature. Can J Aging-Revue Canad Du Vieillissement. 2000;19:106–126. doi: 10.1017/s0714980800016615. [DOI] [Google Scholar]
- 41.Bohnenkamp S.K., Mcdonald P., Lopez A.M., Krupinski E., Blackett A. Traditional versus telenursing outpatient management of patients with cancer with new ostomies. Oncol Nurs Forum. 2004;31:1005–1010. doi: 10.1188/04.ONF.1005-1010. [DOI] [PubMed] [Google Scholar]
- 42.Taylor C. Reviewing the follow−up care of colorectal cancer patients. Gastrointest Nurs. 2008;6:29–34. [Google Scholar]
- 43.Bodenheimer T., Wagner E.H., Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 44.Bodenheimer T., Wagner E.H., Grumbach K. Improving primary care for patients with chronic illness - the chronic care model, part 2. J Am Med Assoc. 2002;288:1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 45.Ouwens M., Wollersheim H., Hermens R., Hulscher M., Grol R. Integrated care programmes for chronically ill patients: a review of systematic reviews. Int J Qual Health Care. 2005;17:141–146. doi: 10.1093/intqhc/mzi016. [DOI] [PubMed] [Google Scholar]
- 46.Comas-Herrera A., Costa-Font J., Gori C., Maio A., Patxot C., Pickard L. European Study of Long-Term Care Expenditure: Investigating the Sensitivity of Projections of Future Long-term Care Expenditure in Germany, Spain, Italy and the United Kingdom to Changes in Assumptions About Demography, Dependency, Informal Care, Formal Care and Unit Costs. London School of Economics; 2003. 2003. The long-term care projection models and base case projections.http://ec.europa.eu/employment_social/soc-prot/healthcare/ltc_study_en Report to the European Commission, Employment and Social Affairs DG. [Online] [Accessed 2021] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This is a systematic review and all the data were extracted from public article. All results were provided within this review.