Abstract
Introduction
Celiac disease (CD) has been described before in Saudi Arabia (SA) to be at the range of 1%–2% in the general population, but the association of celiac disease and cystic fibrosis (CF) has never been described before in the Middle East.
Objectives
To describe the prevalence of the association of CD and CF in patients with gastrointestinal symptomatology in a tertiary care center.
Method
ology: A retrospective charts review of all confirmed CD and CF patients for the years 1989–2018.
Results
In a total of 391 confirmed CF patients, 74 of them (19%) had celiac screening due to their symptomatology in the form of (abdominal pain and distension, vomiting, diarrhea despite adequate pancreatic enzyme replacements, and had high antigliadin antibodies and anti-transglutaminase IgA (tTGA). Thirty-five of the 74 patients were male (47.3%, and 39 (52.7%) were female patients. The mean age at diagnosis of CD was 6.1 (3.9), and the mean age at follow up was 7 (5 years). Only 2 of the 74 patients (3%) had bowel biopsies with the typical pathological findings of CD with villous atrophy. Both patients were placed on a gluten-free diet and showed marked improvement in symptomatology and weight gain.
Conclusion
CD screening should be considered in all CF patients despite the absence of symptoms. The prevalence of CD in CF patients in SA is similar to or slightly higher than that of the general population. A further study to screen the whole CF population is needed to delineate the actual prevalence, particularly in nonsymptomatic CF.
Keywords: Cystic fibrosis, Celiac disease, CFTR, Arabs
1. Introduction
Cystic fibrosis (CF) is a fatal, multisystem debilitating autosomal recessive disease [1]. Pulmonary disease, however, remains the predominant cause of morbidity and mortality [2]. It is a chronic disease that affects 70,000 individuals worldwide [1,2]. The incidence of CF in Saudi Arabia (SA) has been reported at 1 in 4243 live births [[2], [3], [4], [5]]. Cystic fibrosis and celiac disease (CD) were thought to be a single entity dating back to the 1930s due to the fact that they present with similar gastrointestinal symptoms, such as poor weight gain, fatty stools, abdominal pain and fatigue [6]. Prevalence rates of concomitanty CF (CF) and CD has been reported to be 1.2% of CF population in Scandinavia and Poland, which is three times higher than the general population [7]. A predilection with CF and other diseases such as congenital adrenal hyperplasia (CAH), and Marfan's Syndrome has been established [5].
CD is a chronic auto-immune disease, which can develop in genetically susceptible people throughout life. In the US and other developed countries, the prevalence of CD in the general population is approximately 1% [[6], [7], [8]]. The incidence of developing CD in patients with CD is estimated to be 1:2,000,000–1:5,900,000 if there were no overlap in pathogenetic mechanisms [9].
Reviewing the literature of association for CF and CD showed that in 1973 Goodchild analyzed two case reports of patients initially diagnosed as CF WHO then later developed CD concluded that These two disease occurring in one patient appeared to be rare but these two disease could predispose to one another on the basis of common HL-A loci, Goodchild failed to compare these two patients to the literature and only based her findings on the presence of HL-A1 and 8 within her patients [10]. Fluge based her studies of patients with CF and CD in Scandinavia and concluded that patients with the two diseases likely had a prevalence of 1:220. Their tests included Transglutaminase-IgA (tTGA), endomysium-IgA (EMA) and total IgA, and a subset of subjects also underwent duodenal biopsies. However, these authors failed to correlate human leukocyte antigen HLA-DQ with disease [7]. Walkowiak concluded that despite the close relationship between the two diseases there was not a difference between the two in genetically predisposed individuals as compared to the general population. However, they proposed that having CF would leave the patient at risk of developing CD. Their study was limited to the Polish population. Their methods of conducting the research was both thorough and concise, using genetic testing, tissue transglutaminase antibodies (TGA), EMA and biopsy specimens to evaluate the extent of the CD [11]. Frpm other studies, there appears to be a strong association of CD with HLA-DQ genes located on chromosome 6p21 [12]. 90–95% of the patients are carriers of the DQ2 variant, only a minority carry the DQ8 allele [11]. Concerningly, these aforementioned articles did not compare their findings to other populations and other research in the field.
The co-incidence of CD and CF might be explained by the increased intestinal permeability caused by the intestinal inflammation and pancreatic exocrine insufficiency common in CF leading to a higher antigen load presented to the host immune system caused by incompletely digested dietary products [6,7]. Gluten peptides cross the epithelium and are presented by DQ2+ or DQ8+ antigen presenting cells to pathogenic CD4+ cells after deamination by tissue transglutaminase [3]. The clinical features of the two conditions are similar, despite the difference in pathogenesis. Impaired growth, muscle wasting, abnormal stools, abdominal distension can be seen in both CF and CD. Thus, CD in patients with CF may easily be missed [8].
The association between CF and CD has never been reported in the Middle East. We therefore provide the first report on this association in the largest CF referral center.
1.1. Objectives
To report on the prevalence of the association of CD and CF with classic CD symptomatology in a tertiary care center.
2. Methodology
A retrospective chart review on all confirmed CF patients based on elevated sweat chloride results (more than 60 mmol/l), and/or CFTR mutations in both alleles for the period 1989–2018 at King Faisil Specialty Hospital and Research Centre. CF patients with CD symptomatology as abdominal pain and distension, failure to thrive, and persistent loose stool despite adequate pancreatic enzyme replacement therapy were labelled as possible CD diagnosis and were included in
the study. Within that group, patients with high level Tissue transglutaminase (TGA levels), and high Antigliadin IgG and IgA antibodies were subsequently considered for duodenal biopsy for confirmation.
2.1. Definitions
Normal values for antibodies were defined as the following: Anti-gliadin IgG as (0–18 mg/dl), Anti-gliadin IgA as (0-3 mg/dl), Anti-Tissue Transglutaminase IgA (tTGA) (0-20 mg/dl).
Three values of serological testing were determined: The first serological testing at suspicion of CD diagnosis, at mid period of follow up for each patient, and then at last follow up visit (Table 1).
Table 1.
Antibody levels of CF/CD patients (total of 74 patients).
| Antibodies tests | # | 1st antibodies level (SD) | Mid period of follow-up levels (SD) | Last follow-up levels (SD) |
|---|---|---|---|---|
| Anti-gliadin IgG | Abn | 73.1 (47) (N = 36) | 67.6 (53.2) (N = 22) | 36.8 (17.8) (N = 5) |
| Norm | 8.2 (5.5) (N = 38) | 7.6 (5.2) (N = 17) | 7.8 (8.1) (N = 6) | |
| All | 41.1 (46.7) (N = 74) | 41.5 (49.9) (N = 39) | 21 (19.7) (N = 11) | |
| Anti-gliadin IgA | Abn | 4.8 (1.4) (N = 4) | 7 (5.2) (N = 5) | 6.4 (2.9) (N = 3) |
| Norm | 1.2 (0.6) (N = 70) | 1.1 (0.4) (N = 34) | 1.1 (0.4) (N = 8) | |
| All | 1.4 (1) (N = 74) | 1.9 (2.6) (N = 39) | 2.5 (2.8) (N = 11) | |
| Anti tTGA IgA | Abn | 106.8 (103) (N = 2) | 102.3 (93.2) (N = 2) | – |
| Norm | 5.5 (3.6) (N = 72) | 5.3 (2.3) (N = 38) | – | |
| All | 11 (31.3) (N = 74) | 16.6 (42.7) (N = 40) | 4.6 (1.5) (N = 15) | |
| Anti endomysial IgA | Abn | Positive (N = 3) | Positive (N = 2) | – |
| Norm | Negative (N = 71) | Negative (N = 43) | – | |
| All | (N = 74) | (N = 45) | – |
| Anti-reticulin IgA | ||||||
|---|---|---|---|---|---|---|
| First |
Second |
Third |
||||
| Frequency | Percentage | Frequency | Percentage | Frequency | Percentage | |
| Negative | 72 | 97.3 | 43 | 95.5 | 15 | 100 |
|
Positive |
2 |
2.7 |
2 |
4.5 |
– |
– |
| Blood tests | ||||||
| First | Second | Third | ||||
|
N |
Mean (SD) |
N |
Mean (SD) |
N |
Mean (SD) |
|
| IgA level | 41 | 1.98 (1.9) | 29 | 1.69 (0.9) | 14 | 1.4 (0.6) |
| Hb | 64 | 122.8 (16.4) | 0 | – | 0 | – |
| Albumin | 63 | 41.7 (6.9) | 0 | – | 0 | – |
| ALT test | 66 | 34.3 (23.4) | 41 | 31.3 (21) | 14 | 31.1 (20.1) |
| AST test | 65 | 40.3 (17.7) | 40 | 37.1 (17.7) | 14 | 31.6 (7.7) |
Legend Table 1.
N = Number of patients.
SD = Standard deviation.
Norm = Normal.
Abn = Abnormal.
SD = Standard Deviation.
Anti TGA = Anti-transglutaminase.
Hb = Hemoglobin.
IgA = Immunoglobulin A level.
IgA = Immunoglobulin A level.
ALT = alanine aminotransferase.
AST = aspartate aminotransferase.
Normal range: Anti-gliadin IgG (0–18 mg/dl).
Anti-gliadin IgA (0-3 mg/dl).
Anti-Tissue Transglutaminase IgA (0-20 mg/dl).
2.2. Ethical considerations and statistical method
After obtaining the ethical approval by the research advisory committee. The Declaration of Helsinki and good clinical practice guidelines were followed. Data collection and data entry were supervised by the principal investigator. All data needed were obtained by a retrospective chart review. All data were stored in the pediatrics research unit, accessed only by the principle investigator and the assigned Clinical Research Coordinator. The entire patient's information kept strictly confidential. Each patient was given a study number, and all patients' data were entered into the designated data sheet (EXCEL) without any patient identification. The department of Biostatistics Epidemiology and Scientific Computing (BESC) carried out statistical analysis of the data.
2.3. Statistical analysis
For continuous variables, mean, standard deviation and median were calculated using T-Test. Results were presented at a level of significance of P < .05. All values were expressed in mean ± standard deviation (SD).
3. Results
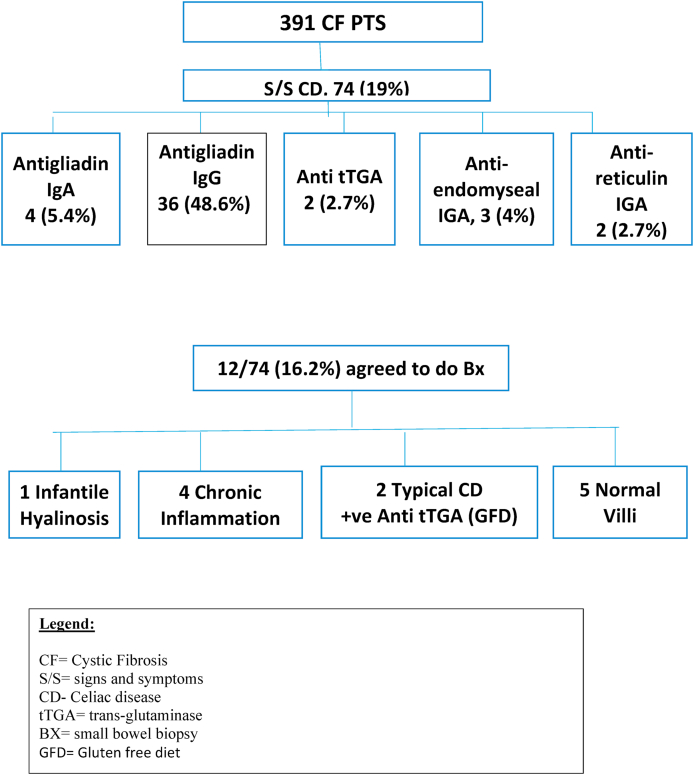
A total of 391 confirmed CF patients, 74 of them (19%) had celiac screening due to symptomatology in the form of (abdominal pain in 15 (20%), abdominal distension in 12 (16%), vomiting in 23 (33%), diarrhea despite adequate pancreatic enzymes replacements in 28 (38%), constipation in 37 (50%), and failure to thrive in 31 (42%). Thirty-five of the 74 patients were males (47.3%, and 39 (52.7%) were females. The mean age at diagnosis of suspected CD was 6.1 years (3.9), the mean age at follow up was 7 years (5). Geographic origins were: 32 (43.2%) from the Eastern province, 4 (5.4%) from the Western province, 12 (16.2%) from the Central, 15 (20.3%) from the Northern, and 11 (14.9%) form the Southern provinces.
Antigliadin IgG level was high in 36/74 (48.6%) patients and continued to be high through the follow-up period (Table 1). Anti-gliadin IgA was high in 4/74 (5.4%), Anti tTGA in 4/74 (5.4%), Anti-endomyseal IgA was positive in 3/74 (4%), anti-reticulin IgA was positive in 2 (2.7%) of patients. The mean immunoglobulin A level (IgA) was 1.98 (1.9 mg/dl). Liver enzymes levels remained normal through the follow-up periods (Table 1).
Twelve patients out of 74 (16.2%) agreed to bowel biopsy, 2/12 patients had the typical pathological findings of CD with villous atrophy (Table 2)
Table 2.
CF/CD patients biopsy results, total 2 patients.
| Anti-Gliadin IgG | Anti-Gliadin IgA | Anti-tTGA | Anti-Endomysial IgA | Anti-Reticulin IgA | IgA Level | ALT | AST | Duodenal Biopsy Result | |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 86.30 (H) | 1.21 | 254.0 (H) | positive | positive | 0.46 | 40 | 37 | flattening of villi associated with mild inflammation |
| P2 | 70 (H) | 1.24 | 149 (H) | positive | positive | 2.6 (H) | 13.9 | 16 | flattening of villi associated with mild inflammation |
Legends Table 2.
P1–P6 = Patients with positive bowel biopsy.
Anti-tTGA = Anti-transglutaminase.
IgA = Immunoglobulin A level.
ALT = alanine aminotransferase.
AST = aspartate aminotransferase.
H = High level.
(Fig. 1), another 4 patients showed partial villous atrophy possibly due to chronic inflammation with negative serology. 5 patients showed normal villi and negative serology, one patient showed infantile systemic hyalinosis. Both patients were placed on a gluten-free diet and showed marked improvement in symptomatology and weight gain.
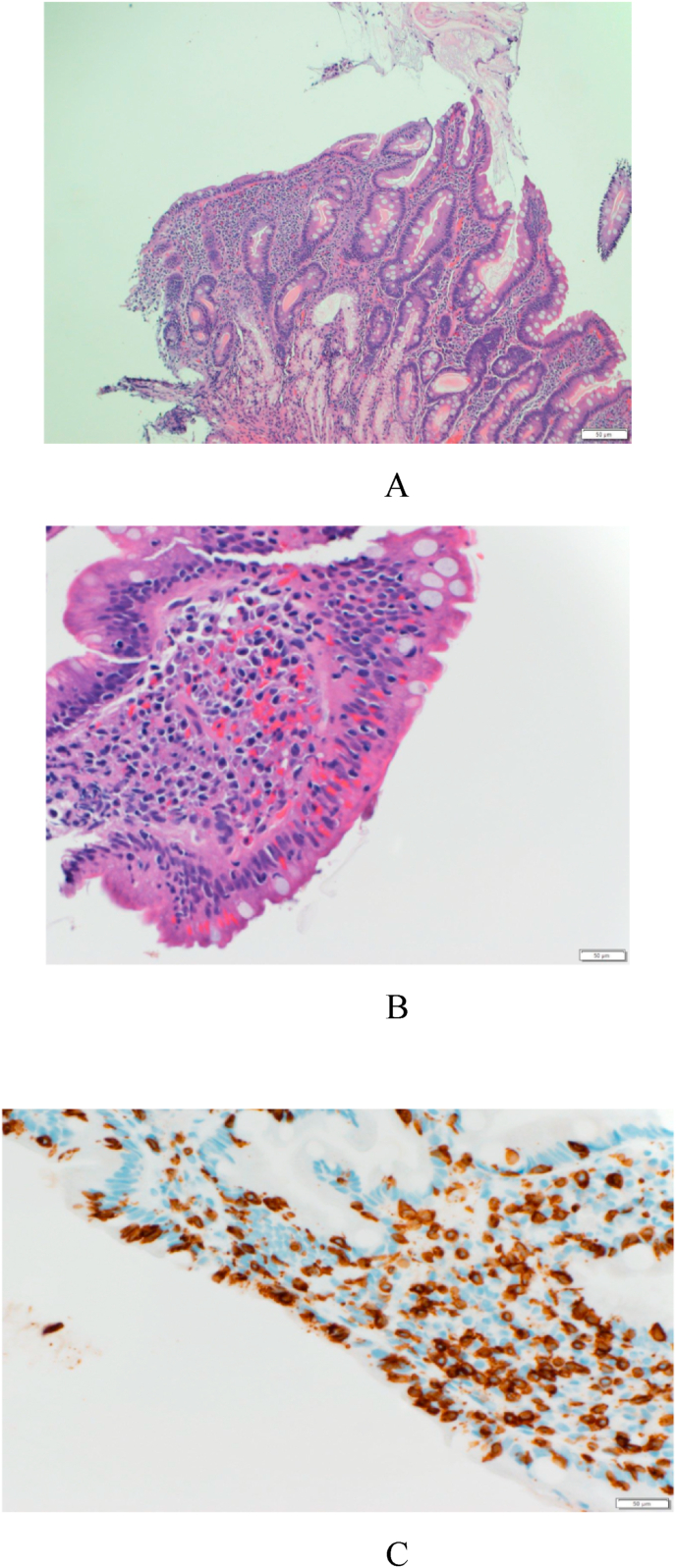
Fig. 2.
Biopsy result of the patient in case report showing villous atrophy
LegendFig. 2:
Fig. 2A = hematoxylin-eosin staining showing: Destructive type or atrophic lesion with moderate villous atrophy and diffuse increase in intra epithelial lymphocytes (IELs).
Fig. 2B = Flat lesion with total villous atrophy and diffuse increase in IELs
Fig. 2C = An immunohistochemical stain for CD3 which is positive in the infiltrating lymphocytes.
Fig. 1.
Flow Diagram for Cystic fibrosis patients with Celiac disease.
The following case report is a case presentation of one of the 2 patients that was typical for confirmed CF/CD on bowel biopsy.
3.1. Case report (patient #1)
A two-month-old newborn female, of Saudi ethnicity delivered normally as a full-term pregnancy normal vaginal delivery with a birth weight of 2.9 kg, and no history of perinatal complications. On the first day of life, she developed abdominal distention with bilious vomiting and was unable to pass meconium until the twelfth hour of life. She was later operated on for intestinal obstruction due to meconium ileus. An ileostomy and mucus fistula were performed in the local hospital and she was observed for two weeks. After the second week of life, she continued to have abdominal distention, the distended segment of the ileum was resected with a primary anastomosis and closure of the prior stoma. At one month of age, the patient was noted to have icterus of the skin and sclera with poor appetite and loose bowel movements and minimal weight gain. At this point, she was referred to our center for further investigation and management.
On examination the patient was breathing comfortably but she looked pale with icteric discoloration of the skin and sclera. Her weight and height were at the fifth percentile for age. She had sunken eyes, sunken fontanelles and abnormal skin turgor. Chest examination showed normal bilateral air entry without adventitious sounds. The abdomen was distended with prominent anterior abdominal veins. Liver was 3 cm below costal margin and the spleen was not palpable. Investigations showed low levels A, D, E, and K. Her hemoglobin was low with a value of 7.8 g/dl, serum albumin: 30 mg/dl, Total Bilirubin: 153 mmol/1, Direct Bilirubin: 55.2 mmol/l. A sweat chloride test showed: 88 mmol/L (normal < 29 mmol/L). Her cystic fibrosis transmembrane conductance regulator (CFTR) result showed homozygous known mutation 711 + 1 G to A in intron 5. This mutation is leading to an abnormal splicing between exon 5 and 6.
After the diagnosis of CF was confirmed, pancreatic enzymes were started in addition to Vitamins A, D, E, K supplements with proper nutritional rehabilitation, along with Iv hydration, Iron supplementation and electrolytes (sodium and potassium) replacement. Parents were taught airway clearance technique to be done twice a day. Her follow up showed improvement of her growth to the 25th percentile for age with improvement of hemoglobin and electrolytes.
At five years of age, she developed increased sputum production with cough and sputum culture revealed mucoid Pseudomonas aeruginosa. Her pulmonary function test (PFT): forced vital capacity (FVC) = 44%, forced expiratory volume in 1 s (FEV1) = 50%, forced expiratory volume in 1 s divided by forced vital capacity (FEV1/FVC) = 117, mid maximal expiratory flow (MMEF) = 43%, functional residual capacity (FRC) = 170%, residual volume (RV) = 276%, total lung capacity (TLC) = 103%, residual volume divided by total lung capacity (RV/TLC) = 72. These results show severe obstructive lung disease with marked air trapping. She was started on oral ciprofloxacin and nebulized tobramycin with a good clinical response. Her weight was stationary and she had increased flatulence along with increased bowel movements to five times per day despite an increase in pancreatic enzyme supplementation. A suspicion of CD was entertained.
A screening test for CD showed Anti Gliadin IgG was high with a value of 86. 30 mg/dl. Tissue transglutaminase IgA antibodies of 254 mg/dl. Duodenal biopsy showed focal villous blunting with an increase in intestinal lymphoepithelial infiltrate, consistent with celiac disease (Fig. 1). Gastric biopsy showed mild chronic inactive gastritis, no helicobacter pylori or intestinal metaplasia. Esophageal biopsy showed no significant pathology. A pediatric gastroenterologist consulted and started a gluten free diet with good response in her bowel movement pattern, weight gain, somatic growth and development.
Similarly, the second patient with CF/CD showed similar bowel biopsy findings with a good response to gluten-free diet in regards to symptomatic improvement and weight gain (Table 2).
The estimated prevalence of CD in our CF symptomatic population is estimated to be 2:74 or 3%, which is similar or slightly higher than the general population [note to authors: is this calculation based on the two patients alone? What do you make of the other patients who had positive testing or who responded to gluten-free diets? Is CD an all-or-none diagnosis in CF patients?
Save this statement for the discussion section. We think the prevalence is under-estimated due to under screening of the other non-symptomatic CF patients.
4. Discussion
CF in itself is a disease that affects multiple systems with pulmonary dysfunction being the predominant cause of morbidity and mortality.
Aljebreen et al. [13] suggested that the sero-prevalence of CD in Saudi Arabia might be the highest in the world. His study was conducted by taking healthy students ranging from grade 10 to 12 in December 2007 in the regions of Aseer, Al-Qassim and Madinah and venous blood samples were tested for Immunoglobulin A and Immunoglobulin G endomysial antibody by indirect immunofluorescence. Out of a sample of 1167 students across the regions, 26 students had a seropositive result for CD, which calculates to a prevalence of CD of 2.2% [13].
Alhatlani et al. [14] concluded that: the prevalence of CD was estimated to be about 1% among symptom-free children from the public schools of the military campus of National Guard in the Eastern Province of Saudi Arabia. This study was conducted between 2012 and 2014. Serum samples were collected from students between the age of 6–18 years. Blood samples were tested for anti-transglutaminase Ig A and Ig G antibodies; individuals with positive results were then offered intestinal biopsy for further confirmation [14].
Ten of the 32 students accepted to undergo endoscopy and biopsies; all the 10 students had duodenal biopsies consistent with CD.
Alajlan et al. [15] studied the prevalence of CD among healthy individuals and individuals with irritable bowel syndrome within the Kingdom of Saudi Arabia. The population studied was native of Riyadh in particular, those that are either in university or within the health field working in hospitals within the capital (Riyadh). The screening method used was blood samples looking for serological markers for CD, i.e., tissue transglutaminase and endomysial autoantibody. The prevalence of CD amongst the screened population was determined to be 1.9% within healthy individuals and 9.6% within the Irritable bowel syndrome population. The author concluded that CD can be underdiagnosed due to the similarity in symptomatology with irritable bowel syndrome and also that irritable bowel syndrome is over-diagnosed. He suggested that irritable bowel syndrome patients should be screened for CD [15].
Both CF and CD can cause low bone mineral density (BMD) and fractures. Celiac disease may occur at a higher frequency in patients with CF than the general population, and symptoms of these conditions may overlap. Because adherence to a gluten-free diet may improve BMD in patients with CD, this could have important implications for treatment. Clinicians should consider screening for CD in patients with CF who have low BMD, worsening BMD in the absence of other risk factors, and/or difficult to treat vitamin D deficiency.
From our study, 391 confirmed CF cases and 74 of them (19%) had CD symptomatology. Six of the 74 cases (8%) had significantly high serology (Anti-gliadin antibodies, tissue transglutaminase tTGA) and 6 of them agreed to do bowel biopsy and had typical findings of celiac disease on histopathology. They were placed on a gluten-free diet and showed significant improvement in symptomatology and weight gain. The combination of CF and CD diseases has never been reported in the Middle East. The estimated prevalence of celiac disease in our CF population is 6:74 (8% or 8:100) which is higher than the general population. Its prevalence could be higher if all 391 CF patients had a routine celiac screening in the absence of CD symptomatology as the disease could have a silent course. Therefore, we recommend routine CD screening for all CF patients.
5. Conclusion
CD screening should be considered in all CF patients despite absence of symptoms. The prevalence of CD in CF patient in Saudi Arabia appears to be similar to the general population. A further study to screen the whole CF population is needed to delineate the actual prevalence especially in asymptomatic CF patients.
Ethical statement
-
1)
This material has not been published in whole or in part elsewhere;
-
2)
the manuscript is not currently being considered for publication in another journal; and
-
3)
all authors have been personally and actively involved in substantive work that led to the writing of this manuscript and will hold themselves jointly and individually responsible for its content.
Author statement
We are pleased to submit an original article entitled as “The First report on The Association of Celiac Disease and Cystic Fibrosis in a tertiary care center in Saudi Arabia” for your kind review and consideration for publication in the International Journal of Pediatrics and Adolescent Medicine.
In this manuscript, we obtained the incidence and the prevalence of celiac disease in CF patients and its relation to other clinical, laboratory, and radiological data. We believe that we have the largest cystic fibrosis population in the Kingdom of Saudi Arabia as it is the main referral center for such disease, and this is the first paper to report on it prevalence and its relation to the CFTR mutations.
We declare that this manuscript is original, has never been published, or under the consideration for publication elsewhere (in part or in whole). We also declare that the corresponding author and all of the co-authors have actively participated in this manuscript.
We confirm that none of the authors have any conflicts of interest associated to this manuscript. In addition, we confirm that this manuscript has been approved by all authors to be published in the International Journal of Pediatrics and Adolescent Medicine, and all of the copyright is transferred to the International Journal of Pediatrics and Adolescent Medicine in case of acceptance.
Declaration of competing interest
None.
Acknowledgment
We express our gratitude to Ibrahim AlMogarri MD., Sami AlHaider MD., and Imran Nizami, MD. for providing the patients population.
Sara Alkaf, Dhefaf AlAbdaly, and Manal Al Shike Biostatistics, Epidemiology, and scientific computing Department, (KFSHRC), Riyadh for helping in Data entry.
We would like to acknowledge the financial support received from Vertex Pharmaceuticals (Europe) Limited:
• Company Number (if required): 02907620.
• Address: 2 Kingdom Street, London, United Kingdom.
• Postcode: W2 6BD.
• Tel: +44 2032 045100.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpam.2021.05.001.
Visual abstract
The following is the supplementary data related to this article:
References
- 1.Banjar H., Angyalosi G. The road for survival improvement of cystic fibrosis patients in Arab countries. Int. J Pediatr. Adolesc. Med. June 2015;2(Issue 2):47–58. doi: 10.1016/j.ijpam.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becq E. Cystic fibrosis transmembrane conductance regulator modulators for personalized drug treatment of cystic fibrosis. Drug. 2010;70(3) doi: 10.2165/11316160-000000000-00000. 241-59. [DOI] [PubMed] [Google Scholar]
- 3.CFF Patient Registry annual data report. 2012. http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient- Registry.pdf [accessed 01.09.15] Available from: [Google Scholar]
- 4.Banjar H. Overview of cystic fibrosis: patients aged 1-12 years in a tertiary care center in Saudi Arabia. Middle East Pediatr. 1999;4(2):44e9. [Google Scholar]
- 5.Banjar H.H. Cystic Fibrosis: presentation with other diseases, the experience in Saudi Arabia. J Cyst Fibros. 2003 Sep;2(3):155–159. doi: 10.1016/S1569-1993(03)00058-4. [DOI] [PubMed] [Google Scholar]
- 6.Ramos A.T., Figueiredo M.M., Aguiar A.P., Almeida Cde G., Mendes P.S., Souza E.L. Celiac disease and cystic fibrosis: challenges to differential diagnosis. Folia Med (Plovdiv) 2016;58(2):141–147. doi: 10.1515/folmed-2016-0020. [DOI] [PubMed] [Google Scholar]
- 7.Fluge G., Olesen H.V., Gilljam M., Meyer P., Pressler T., Storrösten O.T., et al. Co- morbidity of cystic fibrosis and celiac disease in Scandinavian cystic fibrosis patients. J Cyst Fibros. 2009;8(3):198–202. doi: 10.1016/j.jcf.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Rubio-Tapia A., Murray J. Celiac disease. Current Opin Gastroenterl. 2010;26:116–122. doi: 10.1097/MOG.0b013e3283365263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venuta A., Bertolani P., Casarini R., Ferrari F., Guaraldi N., Gaeretti E. Coexistence of cystic fibrosis and celiac disease. Description of a clinical case and review of the literature. Pediatr Med e Chir. 1999;21:223–226. [PubMed] [Google Scholar]
- 10.Goodchild Mary C., Nelson R., Anderson Charlotte M. Cystic fibrosis and coeliac disease: coexistence in two children. Arch Dis Child. 1973;48(9):684–691. doi: 10.1136/adc.48.9.684. Archives of Disease in Childhood. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walkowiak J., Blask-Osipa A., Lisowska A., Oralewska B., Pogorzelski A., Cichy W., Sapiejka E., Kowalska M., Korzon M., Szaflarska-Poplawska A. Cystic fibrosis is a risk factor for celiac disease. Acta Biochim Pol. 2010;57:115–118. [PubMed] [Google Scholar]
- 12.Kaukinen K., Partanen J., Maki M., Collin P. HLA-DQ typing in the diagnosis of celiac disease. Am J Gastroenterol. 2002;97:695–699. doi: 10.1111/j.1572-0241.2002.05471.x. [DOI] [PubMed] [Google Scholar]
- 13.Aljebreen Abdulrahman M., et al. Seroprevalence of celiac disease among healthy adolescents in Saudi Arabia. World J Gastroenterol. 2013;19(15):2374–2378. doi: 10.3748/wjg.v19.i15.2374. [World Journal of Gastroenterology. Web] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Hatlani M.M. Prevalence of celiac disease among symptom-free children from the Eastern Province of Saudi Arabia. Saudi J Gastroenterol. 2015;21:367–371. doi: 10.4103/1319-3767.170952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aljebreen A.M., Almadi M.A., Alhammad A., Al Faleh F.Z. Seroprevalence of celiac disease among healthy adolescents in Saudi Arabia. World J Gastroenterol. 2013;19(15):2374–2378. doi: 10.3748/wjg.v19.i15.2374. http://www.wjgnet.com/1007-9327/full/v19/i15/2374.htm Available from: URL: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.