Abstract
The orally administered neuraminidase (NA) inhibitor RWJ-270201 was tested in parallel with zanamivir and oseltamivir against a panel of avian influenza viruses for inhibition of NA activity and replication in tissue culture. The agents were then tested for protection of mice against lethal H5N1 and H9N2 virus infection. In vitro, RWJ-270201 was highly effective against all nine NA subtypes. NA inhibition by RWJ-270201 (50% inhibitory concentration, 0.9 to 4.3 nM) was superior to that by zanamivir and oseltamivir carboxylate. RWJ-270201 inhibited the replication of avian influenza viruses of both Eurasian and American lineages in MDCK cells (50% effective concentration, 0.5 to 11.8 μM). Mice given 10 mg of RWJ-270201 per kg of body weight per day were completely protected against lethal challenge with influenza A/Hong Kong/156/97 (H5N1) and A/quail/Hong Kong/G1/97 (H9N2) viruses. Both RWJ-270201 and oseltamivir significantly reduced virus titers in mouse lungs at daily dosages of 1.0 and 10 mg/kg and prevented the spread of virus to the brain. When treatment began 48 h after exposure to H5N1 virus, 10 mg of RWJ-270201/kg/day protected 50% of mice from death. These results suggest that RWJ-270201 is at least as effective as either zanamivir or oseltamivir against avian influenza viruses and may be of potential clinical use for treatment of emerging influenza viruses that may be transmitted from birds to humans.
Influenza is a leading cause of morbidity, mortality, and economic loss throughout the world (22, 32). Prevention and treatment of influenza currently rely on inactivated vaccines and antiviral agents. Although vaccines are considered the best option for control of influenza, at least 6 months is needed to produce vaccines based on the surface glycoproteins of an epidemic virus strain (9). The efficacy of such antiviral drugs as amantadine and rimantadine is limited by their inapplicability to influenza B viruses and to the rapid emergence and transmission of drug-resistant variants (15, 16).
Synthesis of the neuraminidase (NA) inhibitors was a significant milestone in antiviral influenza therapy (23, 44). Influenza virus NA is located on the surface of the virus particle and plays an important role in the spread of virus from cell to cell and within the respiratory tract (24, 27). The genetic stability of the NA enzymatic active center among all influenza viruses (8) makes it a promising target for antiviral drugs that would offer protection against any influenza virus that might emerge in humans. Sialic acid analogs, such as zanamivir and oseltamivir (23, 26, 44), were synthesized after the crystal structures of influenza NA complexes with sialic acid and the sialic acid derivative 2-deoxy-2,3-dehydro-N-acetyl-neuraminic acid were determined (4, 42). Inhaled zanamivir and orally administered oseltamivir were effective in the prophylaxis and early treatment of influenza in experimentally infected volunteers (17, 18, 20) and were effective and well tolerated in adults treated for natural influenza infection (32, 33).
The novel, potent, selective, and orally active influenza NA inhibitor RWJ-270201 is a recent product of structure-based drug design (1). Crystallographic studies have shown that RWJ-270201 is structurally unlike existing NA inhibitors: it is a cyclopentane derivative with a negatively charged carboxylate group, a positively charged guanidino group with an orientation unlike that in zanamivir, and lipophilic side chains (1). The different structures of the three NA inhibitors suggest that they may differ in their antiviral activity and in their susceptibility to the emergence of mutant variants. In fact, RWJ-270201 has been shown to retain its inhibitory activity against the zanamivir-resistant Glu-119 variant of influenza A virus NA (L. V. Gubareva, D. Schallon, and F. G. Hayden, 2nd Int. Symp. Influenza Other Respir. Viruses, abstr. P24, 1999).
No studies have assessed the effectiveness of the NA inhibitors under pandemic conditions, although antiviral drugs can be crucial for prophylaxis and therapy in the absence of effective vaccines. The direct transmission of avian H5N1 and H9N2 influenza viruses to humans in Hong Kong in 1997 and 1999 (34, 41) suggested that interspecies transmission of all 15 hemagglutinin (HA) subtypes of influenza virus is possible. Although the large-scale slaughter of poultry eradicated the highly lethal H5N1 virus, its precursors continue to circulate in poultry in Asia (6). Zanamivir protects mice against lethal challenge with A/HK/156/97 (H5N1) influenza virus (14) and protects chickens from A/chick/Victoria/1/85 (H7N7), a highly pathogenic virus (13), but it has failed to protect chickens from other highly virulent viruses of the NA subtypes N1, N2, N3, N7, and N8 (13, 29). Oral administration of oseltamivir is effective for treating infections caused by H5N1 and H9N2 influenza viruses in mice (25). However, the efficacy of these drugs has not been compared with that of RWJ-270201 against all of the NA subtypes, either in vitro or in vivo.
In this study, we evaluated the potential usefulness of the NA inhibitor RWJ-270201 in a preparedness plan for pandemic influenza. We tested RWJ-270201's inhibition of NA activity and of replication in tissue culture of a panel of avian influenza viruses representing all nine NA subtypes. We then investigated the efficacy of orally administered RWJ-270201 against highly pathogenic avian influenza A/HK/156/97 (H5N1) and A/quail/HK/G1/97 (H9N2) viruses in a mouse model. These studies were conducted as parallel experiments that compared the effects of RWJ-270201 on the enzymatic and cellular levels with those of zanamivir and oseltamivir carboxylate and, in the animal model, with those of oseltamivir, the orally active prodrug form of oseltamivir carboxylate.
MATERIALS AND METHODS
NA inhibitors.
RWJ-270201 {[1S,2S,3R,4R,1′S]-3-[1′-acetylamino-2′-ethyl]butyl-4-[(aminoimino)-methyl]amino-2-hydroxycyclopentane-1-carboxylic acid; BCX-1812}, zanamivir (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid; GG167), GS4104 (oseltamivir phosphate, or oseltamivir), and GS4071 (oseltamivir carboxylate, the active metabolite of oseltamivir: [3R,4R,5S]-4-acetamido-5-amino-3-[1-ethylpropoxy]-1-cyclohexane-1-carboxylic acid) were synthesized by BioCryst Pharmaceuticals (Birmingham, Ala.) by procedures reported previously (23, 26, 44) and were provided by R.W. Johnson Pharmaceutical Research Institute (Raritan, N.J.). The compounds were provided as lyophilized powder and were maintained at 4°C. They were mixed with tissue culture medium for in vitro studies and with sterile phosphate-buffered saline (PBS), pH 7.4, for in vivo experiments.
Cells.
Madin-Darby canine kidney (MDCK) cells obtained from the American Type Culture Collection (Manassas, Va.) were grown in minimal essential medium supplemented with 5% fetal calf serum, 5 mM l-glutamine, sodium bicarbonate, 100 U of penicillin per ml, 100 μg of streptomycin sulfate per ml, and 100 μg of kanamycin sulfate per ml in a humidified atmosphere of 5% CO2.
Viruses.
Avian influenza A viruses were obtained from the repository at St. Jude Children's Research Hospital and were propagated in the allantoic cavities of 10-day-old embryonated chicken eggs. The histories of isolation and passage of the influenza A/Hong Kong/156/97 (A/HK/156/97) (H5N1) and A/quail/Hong Kong/G1/97 (A/quail/HK/G1/97) (H9N2) viruses used in this study were described previously (10, 12, 25). All experiments with highly pathogenic avian H5N1 and H9N2 viruses were conducted in a biosafety level 3 facility approved for studies of these viruses.
NA activity assay.
NA activity was determined by using 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUN; Sigma Chemical Co., St. Louis, Mo.) as a substrate, as described previously (2). Viruses used in the NA assay were grown in embryonated chicken eggs and were obtained from the allantoic fluid after centrifugation at 2,000 × g for 10 min. The NA activity of each virus was determined before it was used in NA inhibition tests. Briefly, 10 μl of each of a series of twofold virus dilutions was mixed with 10 μl of enzyme buffer [33 mM 2-(N-morpholino)ethanesulfonic acid (MES), pH 6.5, and 4 mM CaCl2] and 30 μl of substrate in enzyme buffer to give a final MUN concentration of 100 μM. The reaction mixtures were incubated on a shaker at 37°C for 30 min. The reactions were then stopped by addition of 150 μl of 0.014 N NaOH in 83% ethanol to each well. The fluorescence of the released 4-methylumbelliferone was quantified in a Fluoroskan II (Labsystems, Helsinki, Finland) spectrophotometer (excitation wavelength, 355 nm; emission wavelength, 460 nm).
NA inhibition was assayed by determining the drug concentration required to reduce NA activity to 50% of control NA activity (IC50). Fourfold dilutions ranging from 8 μM to 0.125 nM were made of the appropriate compound, and 10 μl of each dilution was incubated with 10 μl of virus-containing allantoic fluid at a standard amount of NA activity (100 to 150 relative fluorescence units). The mixture was shaken at 37°C for 30 min to allow interaction of drug and virus. The enzymatic reaction was initiated by adding 30 μl of substrate in enzyme buffer at a final concentration of 100 μM. The reaction was stopped after 1 h of incubation at 37°C. Standard curves were constructed by plotting the percentage of fluorescence inhibition relative to the activity of controls against the logs of inhibitor concentrations. The IC50s were obtained from the graphs by extrapolation, and the means were calculated on the basis of three independent experiments.
Virus neutralization assay in tissue culture.
The antiviral activities of the compounds were assessed by a modified microneutralization assay followed by enzyme-linked immunosorbent assay (ELISA) (3) to measure expression of viral nucleoprotein (NP) in infected cells, as described elsewhere (25). Briefly, a confluent monolayer of MDCK cells was overlaid with 100 μl of minimal essential medium containing 2.5 μg of N-tosyl-l-phenylalanine chloromethyl ketone-treated trypsin (Sigma Chemical Co.)/ml and 100 μl of RWJ-270201, zanamivir, or oseltamivir carboxylate at concentrations of 1 μM to 150 μM. After incubation for 30 min at 37°C, the cells were infected with influenza virus at a multiplicity of infection of 0.01 to 0.1 PFU/cell. The infected cells were cultured in the presence of the drug for 18 h at 37°C. The 50% effective concentration (EC50) of the drug was determined by plotting the percent inhibition of virus replication after correction for background values (obtained from uninfected cultures) as a function of compound concentration calculated from the dose-response curve. Data were expressed as the means of the EC50s.
Drug efficacy in vivo.
Female BALB/c mice (weight, 18 to 20 g; Jackson Laboratories, Bar Harbor, Maine) were anesthetized by inhalation of methophane and inoculated intranasally with 100 μl of infectious virus. The dose of virus lethal to 50% of mice (MLD50) was determined for each experiment by infecting groups of mice (four per group) with serial 10-fold dilutions of virus. The MLD50 was calculated after a 16-day observation period. RWJ-270201 and oseltamivir were administered to groups of 6 to 12 mice at dosages of 0.01, 0.1, 1.0, 10, and 100 mg per kg of body weight per day by oral gavage twice daily for 5 days. Control (infected untreated) animals received sterile PBS on the same schedule. Four hours after the first dose of drug, mice were inoculated with 5 MLD50s of A/HK/156/97 (H5N1) or mouse-adapted A/quail/HK/G1/97 (H9N2) influenza virus. Mice were observed daily for 16 days for clinical signs of infection and for survival. The mean days of survival were calculated by using the log-hazard scale. The mice were weighed on days 0, 4, 7, 9, 11, 14, and 16 after infection, and the loss or gain of weight was calculated for each mouse as a percentage of its weight on day 0 before virus inoculation. Reported values are average percent changes in weight ± standard errors (SEs). As controls for toxicity, six mice were given each dosage of each drug (0.01, 0.1, 1.0, 10, or 100 mg/kg/day) and were observed daily for survival and for overt toxic effects. These mice were weighed before treatment began and on days 4, 7, 9, 11, 14, and 16 of therapy.
The effects of delayed treatment were tested in parallel experiments with RWJ-270201 and oseltamivir. BALB/c mice (9 or 10 animals per group) were infected intranasally with 10 MLD50s of influenza A/HK/156/97 (H5N1) virus and treated with RWJ-270201 or oseltamivir at a dosage of 10 mg/kg/day by oral gavage twice daily for 5 days. The treatment began 24, 36, 48, or 60 h after virus inoculation. The mice were observed daily for clinical signs of infection or death.
Determination of virus titers in lungs and brain.
On days 3, 4, and 7 after infection with either influenza A/HK/156/97 (H5N1) or mouse-adapted A/quail/HK/G1/97 (H9N2) virus, three mice from each experimental and control group were killed. The brains and then the lungs were removed and were thoroughly rinsed with sterile PBS to remove cellular debris and red blood cells. The organs were homogenized and suspended in 1 ml of cold PBS. The suspensions were also cleared of cellular debris by centrifugation at 2,000 × g for 10 min, and then 0.1 ml of the supernatants was injected into the allantoic cavity of 10-day-old embryonated chicken eggs to determine the 50% egg infective dose (EID50). Virus titers in mouse lungs and brain were calculated as the mean log10 EID50/0.1 ml ± SE.
Statistical analysis.
The Kaplan-Meier method was used to estimate the probability of survival, and the log-rank test was used for pairwise comparisons of the control and treatment groups over the period of 16 days (43). Mean survival time was estimated by the Kaplan-Meier method. Fisher's exact test was used to analyze differences between groups in survival rates when there were no censored observations present. Linear mixed-effects models were used to analyze weight changes in the animals. This technique accommodates individual variations through the random effects but ties different animals together through the fixed effects, allowing for nonconstant correlation among the observations. The second-degree polynomial was chosen to model fixed effects of the dosage and day after infection on the virus titers in the lungs and brains of the animals. The regression models were compared for all dosage groups on different days after infection. The hypothesis testing was done as two-tailed. Statistical significance was estimated if P was <0.05.
RESULTS
RWJ-270201 inhibition of NA activity and replication of avian influenza A viruses in MDCK cells.
Inhibition of the NA activity of avian influenza A viruses by RWJ-270201, zanamivir, and oseltamivir carboxylate was tested in parallel (Table 1). Two strains of each of the nine NA subtypes, representing both Eurasian and American lineages, were included. RWJ-270201 was effective in inhibiting the NA activity of influenza viruses of all NA subtypes, with mean IC50s of 0.9 to 4.3 nM. The mean IC50s obtained with RWJ-270201 were usually below those for zanamivir (2.2 to 30.1 nM) and oseltamivir carboxylate (1.9 to 69.2 nM). The various influenza strains tested, which were isolated from different geographic regions and in different years, did not differ appreciably in their sensitivities to RWJ-270201. In contrast, the viruses of the different NA subtypes varied in their sensitivities to zanamivir and oseltamivir carboxylate (Table 1). Zanamivir was more efficacious in inhibiting NA activity in N2, N3, N4, N6, and N7 subtypes than in N5 and N9 subtypes. Oseltamivir carboxylate was very effective in inhibiting enzymatic activities of the N2 and N3 subtypes, with IC50s comparable to those of RWJ-270201, whereas at least 10-fold-higher concentrations of the drug were required to reduce the NA activity by 50% in the N1, N5, and N8 subtypes (Table 1).
TABLE 1.
Inhibition of the NA activity of avian influenza A viruses by RWJ-270201, zanamivir, and oseltamivir carboxylate
| NA subtype | Virusb | Mean IC50 ± SE (nM)a
|
||
|---|---|---|---|---|
| RWJ-270201 | Zanamivir | Oseltamivir carboxylate | ||
| N1 | A/teal/Hong Kong/W312/97 (H6N1) | 2.6 ± 0.4 | 19.7 ± 4.2 | 36.1 ± 8.3 |
| A/duck/Alberta/35/76 (H1N1) | 3.4 ± 1.0 | 5.8 ± 2.1 | 53.2 ± 10.3 | |
| N2 | A/chicken/NY/13307-3/95 (H7N2) | 2.7 ± 1.1 | 7.4 ± 0.4 | 2.7 ± 1.1 |
| A/turkey/Wisconsin/66 (H9N2) | 2.0 ± 0.6 | 4.7 ± 0.7 | 1.9 ± 0.2 | |
| N3 | A/duck/Singapore/3/97 (H5N3) | 3.8 ± 1.4 | 4.9 ± 0.8 | 2.9 ± 0.7 |
| A/duck/Germany/1215/73 (H2N3) | 3.1 ± 0.6 | 5.9 ± 0 | 3.3 ± 1.2 | |
| N4 | A/red knot/DE/254/94 (H8N4) | 2.3 ± 0.6 | 4.5 ± 1.3 | 47.0 ± 0 |
| A/turkey/Ontario/6118/68 (H8N4) | 3.2 ± 0.6 | 2.2 ± 0.9 | 5.5 ± 2.4 | |
| N5 | A/mallard duck/Astrakhan/263/82 (H14N5) | 2.7 ± 1.1 | 13.6 ± 1.9 | 36.8 ± 5.2 |
| A/shearwater/Australia/1/72 (H6N5) | 1.2 ± 0.1 | 29.7 ± 1.6 | 33.2 ± 1.9 | |
| N6 | A/shorebird/DE/224/97 (H13N6) | 1.3 ± 0.8 | 7.2 ± 0.6 | 4.9 ± 1.0 |
| A/duck/Czechoslovakia/56 (H4N6) | 3.1 ± 0.5 | 3.4 ± 2.1 | 3.2 ± 0.1 | |
| N7 | A/chicken/Jena/1816/87 (H7N7) | 1.5 ± 0.3 | 5.9 ± 1.5 | 4.4 ± 0.9 |
| A/duck/Germany/“N”/49 (H10N7) | 4.3 ± 1.8 | 7.2 ± 0.6 | 8.2 ± 2.1 | |
| N8 | A/duck/Hong Kong/Y264/97 (H4N8) | 1.2 ± 0.3 | 30.1 ± 2.9 | 69.2 ± 3.4 |
| A/quail/Italy/1117/65 (H10N8) | 1.1 ± 0.3 | 5.3 ± 0.6 | 29.7 ± 1.4 | |
| N9 | A/duck/Hong Kong/P50/97 (H11N9) | 0.9 ± 0.1 | 11.5 ± 4.6 | 17.7 ± 4.9 |
| A/duck/Memphis/546/74 (H11N9) | 1.6 ± 0.1 | 10.4 ± 4.6 | 9.5 ± 1.6 | |
| Range (N1–N9) | 0.9–4.3 | 2.2–30.1 | 1.9–69.2 | |
MUN (final concentration of 100 μM) was used as the substrate. Values are means ± SEs of at least three independent determinations.
The second strain in each pair is the reference strain of influenza A virus used for the antigenic subtyping of the NA of the new influenza isolates.
Because influenza A/HK/156/97 (H5N1) virus is highly pathogenic and has been transmitted to humans, we tested the efficacy of RWJ-270201 against N1 NA in A/teal/Hong Kong/W312/97 (H6N1) influenza virus. This virus strain possesses a NA gene very closely related to that of the highly pathogenic H5N1 virus (21). RWJ-270201 efficiently inhibited the enzymatic activity of that virus, with IC50s as low as 2.6 nM. Influenza A/teal/Hong Kong/W312/97 virus was 7 to 14 times more sensitive to RWJ-270201 than to zanamivir and oseltamivir carboxylate (Table 1).
The effects of RWJ-270201, zanamivir, and oseltamivir carboxylate on influenza virus replication in MDCK cells were determined in parallel by an ELISA method that assayed expression of the viral NP in infected cells (Table 2). None of the three agents showed any signs of cellular toxicity at concentrations as high as 150 μM. RWJ-270201 effectively inhibited the replication of influenza viruses of all nine NA subtypes, with mean EC50s that ranged from 0.5 to 11.8 μM. The inhibitory activity of RWJ-270201 was greatest against viruses of the N2, N3, N7, and N8 NA subtypes, for which concentrations of <2.7 μM were required to produce a 50% reduction in the surface expression of NP protein. Viruses of the other NA subtypes varied approximately 1.5- to 4.5-fold in their sensitivity to RWJ-270201. These findings were consistent with the inhibition of NA in viruses of all NA subtypes by similar concentrations of RWJ-270201. The sensitivities of the influenza viruses to zanamivir and oseltamivir carboxylate varied among the NA subtypes and within one subtype (Table 2). Zanamivir was more potent against some (but not all) of the recent isolates (A/teal/Hong Kong/W312/97, A/chicken/NY/13307-3/95, A/duck/Hong Kong/Y264/97) than against reference strains. Oseltamivir carboxylate was highly effective against both strains tested of the N2, N3, N7, and N8 NA subtypes (EC50, 1.0 to 6.8 μM), although EC50s varied approximately 11-fold within the N9 NA subtype. The mean EC50s required to inhibit replication of influenza A/teal/Hong Kong/W312/97 (H6N1) virus in MDCK cells were comparable for all three drugs (Table 2). Thus, as shown by the inhibition of NA activity and viral replication in MDCK cells, avian influenza A viruses of all nine NA subtypes were at least as sensitive to RWJ-270201 as to zanamivir and oseltamivir carboxylate.
TABLE 2.
Inhibition of replication of avian influenza A viruses in MDCK cells by RWJ-270201, zanamivir, and oseltamivir carboxylate
| NA subtype | Virusb | Mean EC50 ± SE (μM)a
|
||
|---|---|---|---|---|
| RWJ-270201 | Zanamivir | Oseltamivir carboxylate | ||
| N1 | A/teal/Hong Kong/W312/97 (H6N1) | 5.0 ± 1.8 | 9.4 ± 0.7 | 8.1 ± 1.6 |
| A/duck/Alberta/35/76 (H1N1) | 1.1 ± 0.4 | 11.3 ± 2.4 | 11.9 ± 0.5 | |
| N2 | A/chicken/NY/13307-3/95 (H7N2) | 1.1 ± 0.3 | 6.9 ± 0.8 | 1.0 ± 0.3 |
| A/turkey/Wisconsin/66 (H9N2) | 0.9 ± 0.4 | 19.7 ± 0.9 | 1.0 ± 0.1 | |
| N3 | A/duck/Singapore/3/97 (H5N3) | 1.0 ± 0.5 | 10.3 ± 0.2 | 1.6 ± 0.5 |
| A/duck/Germany/1215/73 (H2N3) | 1.4 ± 0.2 | 32.3 ± 1.5 | 6.8 ± 1.2 | |
| N4 | A/red knot/DE/254/94 (H8N4) | 8.5 ± 2.5 | 17.5 ± 0.5 | 17.9 ± 0.1 |
| A/turkey/Ontario/6118/68 (H8N4) | 2.6 ± 1.4 | 12.0 ± 1.8 | 6.2 ± 2.2 | |
| N5 | A/mallard duck/Astrakhan/263/82 (H14N5) | 11.8 ± 0.5 | 12.0 ± 0.3 | 13.0 ± 1.0 |
| A/shearwater/Australia/1/72 (H6N5) | 2.7 ± 0.9 | 19.3 ± 3.0 | 3.5 ± 1.4 | |
| N6 | A/shorebird/DE/224/97 (H13N6) | 11.0 ± 0.8 | 11.2 ± 0.6 | 10.2 ± 0.6 |
| A/duck/Czechoslovakia/56 (H4N6) | 8.7 ± 1.7 | 42.0 ± 1.5 | 10.7 ± 0.9 | |
| N7 | A/chicken/Jena/1816/87 (H7N7) | 0.5 ± 0.1 | 12.3 ± 1.5 | 4.7 ± 0.9 |
| A/duck/Germany/“N”/49 (H10N7) | 1.7 ± 0.5 | 27.0 ± 1.2 | 3.8 ± 0.8 | |
| N8 | A/duck/Hong Kong/Y264/97 (H4N8) | 2.3 ± 0.8 | 4.0 ± 1.0 | 3.5 ± 2.5 |
| A/quail/Italy/1117/65 (H10N8) | 2.7 ± 0.4 | 55.0 ± 8.8 | 1.0 ± 0.1 | |
| N9 | A/duck/Hong Kong/P50/97 (H11N9) | 7.9 ± 1.7 | 10.8 ± 0.6 | 3.7 ± 3.3 |
| A/duck/Memphis/546/74 (H11N9) | 11.7 ± 1.8 | 58.3 ± 1.7 | 42.0 ± 1.5 | |
| Range (N1–N9) | Range of values | 0.5–11.8 | 4.0–58.3 | 1.0–42.0 |
Antiviral activity was assessed in MDCK cells by a modified microneutralization assay followed by ELISA. Values are means ± SEs for at least three independent determinations.
The second strain in each pair is the reference strain of influenza A virus used for the antigenic subtyping of the NA of the new influenza isolates.
RWJ-270201 and oseltamivir efficacy in protecting mice against lethal challenge with H5N1 and H9N2 influenza viruses.
To evaluate the efficacy of RWJ-270201 in an animal model and to compare its efficacy with that of the other orally administered NA inhibitor, oseltamivir, we administered these drugs at 0.01, 0.1, 1.0, and 10 mg/kg/day to BALB/c mice. Four hours after initiation of treatment, the mice were infected with 5 MLD50s of highly pathogenic influenza A/HK/156/97 (H5N1) or mouse-adapted A/quail/HK/G1/97 (H9N2) viruses. Table 3 shows the results of the survival analysis and the estimated mean duration of survival. There were no signs of toxicity in control uninfected animals after treatment with as much as 100 mg of either drug/kg/day. All control animals died 4 to 9 days after infection with H5N1 and 7 to 10 days after infection with H9N2 influenza virus. Treatment of H5N1-infected mice with RWJ-270201 was significantly protective at all dosages tested. A dosage as low as 0.1 mg/kg/day doubled the duration of survival and prevented death in 70% of animals (P = 0.0002). Complete protection of H5N1-infected mice was achieved at a dosage of 10 mg of RWJ-270201/kg/day. Although oseltamivir provided complete protection against H5N1 virus at a dosage of only 0.1 mg/kg/day, survival rates produced by RWJ-270201 at 0.1 mg/kg/day and by oseltamivir at 0.1 mg/kg/day did not differ significantly (P = 0.154).
TABLE 3.
RWJ-270201 and oseltamivir protect mice against lethal infection with H5N1 and H9N2 influenza viruses
| Virus | Treatmenta | Dosage (mg/kg/day) | No. of survivors/total | Survival rate (%) | Mean survival ± SE (no. of days)b | P valuec |
|---|---|---|---|---|---|---|
| A/HK/156/97 (H5N1) | RWJ-270201 | 0.01 | 3/10 | 30.0 | 9.9 ± 1.4 | 0.014 |
| 0.1 | 7/10 | 70.0 | 13.4 ± 1.3 | 0.0002 | ||
| 1.0 | 9/10 | 90.0 | 15.0 ± 1.0 | 0.00003 | ||
| 1.0 | 9/9 | 100.0 | 16.0 ± 0 | <0.0001 | ||
| Oseltamivir | 0.01 | 2/6 | 33.3 | 11.3 ± 1.5 | 0.003 | |
| 0.1 | 6/6 | 100.0 | 16.0 ± 0 | 0.0001 | ||
| 1.0 | 5/5 | 100.0 | 16.0 ± 0 | 0.0001 | ||
| PBS (control) | 0 | 0/11 | 0 | 6.3 ± 0.4 | ||
| A/quail/HK/G1/97 (H9N2) | RWJ-270201 | 0.01 | 3/12 | 25.0 | 9.3 ± 1.2 | 0.684 |
| 0.1 | 4/11 | 36.4 | 11.1 ± 1.2 | 0.022 | ||
| 1.0 | 12/12 | 100.0 | 16.0 ± 0 | <0.0001 | ||
| 10 | 11/11 | 100.0 | 16.0 ± 0 | <0.0001 | ||
| Oseltamivir | 0.01 | 5/11 | 45.5 | 12.2 ± 1.2 | 0.018 | |
| 0.1 | 4/11 | 36.4 | 11.9 ± 1.0 | 0.007 | ||
| 1.0 | 11/11 | 100.0 | 16.0 ± 0 | <0.0001 | ||
| 10 | 12/12 | 100.0 | 16.0 ± 0 | <0.0001 | ||
| PBS (control) | 0 | 0/11 | 0 | 8.0 ± 0.4 |
RWJ-270201 and oseltamivir were administered to BALB/c mice by twice-daily oral gavage for 5 days begining 4 h before viral infection. Control (infected untreated) animals received sterile PBS on the same schedule.
Mean survival time was estimated by the Kaplan-Meier method.
Differences in survival between the treatment and the control groups were analyzed by using the log-rank test.
RWJ-270201 significantly increased the survival of mice infected with A/quail/HK/G1/97 (H9N2) virus, starting at a dosage as low as 0.1 mg/kg/day (Table 3). A dosage of 1.0 mg/kg/day resulted in complete protection in a group of mice infected with H9N2 virus. The pairwise comparisons of the survival curves did not show either of the two drugs to be superior for treating either A/HK/156/97 (H5N1) or A/quail/HK/G1/97 (H9N2) influenza virus infection in mice.
Fisher's exact test was used to analyze differences in survival rates between groups of mice that received specific combinations of virus and drug dosage. There was insufficient evidence at the 5% level of significance to conclude that the survival rate depended on either the NA inhibitor administered (RWJ-270201 or oseltamivir) or the influenza virus used for challenge (A/HK/156/97 or A/quail/HK/G1/97). However, our findings suggested that low dosages of RWJ-270201 provided more effective protection against lethal infection with A/HK/156/97 (H5N1) than with A/quail/HK/G1/97 (H9N2) virus (Table 3).
Efficacy of RWJ-270201 and oseltamivir in reducing virus titers in the lungs and brains of infected mice.
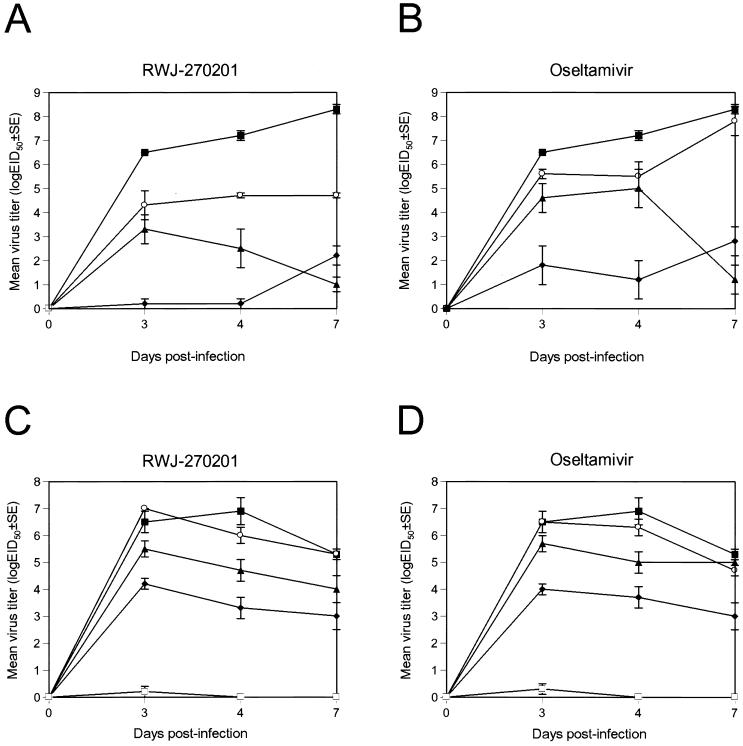
The influenza A/HK/156/97 (H5N1) and A/quail/HK/G1/97 (H9N2) viruses used in this study replicate systemically and are neurotropic in mice (11, 28). We compared the efficacy of RWJ-270201 and oseltamivir in reducing virus titers in the lungs (Fig. 1) and preventing the spread of virus to the brains of infected mice. On each of the days tested, virus titers in the lungs of mice infected with A/HK/156/97 virus and treated with RWJ-270201 (0.01 and 0.1 mg/kg/day) were at least 100-fold lower than those in infected untreated animals (P < 0.001). Virus titers in the lungs of mice treated with oseltamivir at the dosage of 0.01 mg/kg/day did not differ from those of controls (Fig. 1B). Administration of RWJ-270201 at 1.0 mg/kg/day resulted in virus titers 6.1 to 7.0 logs lower than those of the control group (P < 0.0001), and treatment at 10 mg/kg/day completely eliminated virus in the lungs of mice infected with H5N1 virus (Fig. 1A). In mice infected with H9N2 virus, RWJ-270201 and oseltamivir at 0.01 mg/kg/day each failed to reduce virus titers. At 0.1, 1.0, and 10 mg/kg/day, the two drugs demonstrated similar efficacies (Fig. 1C and D), markedly reducing virus titers in the lungs of mice infected with H9N2 virus (P, 0.026 to <0.0001).
FIG. 1.
Effect of oral treatment with RWJ-270201 and oseltamivir on virus titers in the lungs of mice infected with influenza H5N1 and H9N2. RWJ-270201 and oseltamivir at dosages of 0.01 (○), 0.1 (▴), 1.0 (⧫), and 10 (□) mg/kg/day were administered to BALB/c mice by twice-daily oral gavage for 5 days beginning 4 h before viral infection. Mice were infected with 5 MLD50s of A/HK/156/97 (H5N1) (A and B) or mouse-adapted A/quail/HK/G1/97 (H9N2) (C and D) influenza viruses. Values shown are mean virus titers determined from three animals (log10 EID50/0.1 ml). Control untreated animals (▪) received sterile PBS on the same schedule.
We also investigated whether the A/HK/156/97 and A/quail/HK/G1/97 influenza viruses became resistant to RWJ-270201 and oseltamivir during the treatment of mice by comparing the drug sensitivity of the challenge viruses with that of viruses obtained from murine lungs after treatment. We used cell ELISA to test the viruses used to infect the mice and the viruses obtained on the seventh day after infection and treatment with 1.0 mg of the drugs per kg per day. After treatment, the mean EC50s required to inhibit replication of A/HK/156/97 in MDCK cells were 5.2 μM for RWJ-270201 and 7.3 μM for oseltamivir carboxylate. Replication of A/quail/HK/G1/97 influenza virus was inhibited at 7.7 and 8.2 μM, respectively. The mean EC50s of the challenge viruses differ by 0.2 to 0.5 μM from those obtained after treatment. Thus, the viruses isolated after administration of the NA inhibitors were as sensitive to the drugs as were the viruses used to infect animals.
In the brains of untreated mice, virus titers on days 3, 4, and 7 after infection with A/HK/156/97 or A/quail/HK/G1/97 influenza virus ranged from 0.7 to 2.5 log10 EID50s. On days 3 and 4 after infection with H5N1 virus, virus titers were reduced 32-fold in the brains of mice treated with RWJ-270201 at 0.01 mg/kg/day (P = 0.001) and 10-fold in the brains of mice treated with oseltamivir at 0.01 mg/kg/day (P = 0.011) (results not shown). The A/HK/156/97 (H5N1) virus was undetectable in the brains of mice after treatment with either RWJ-270201 or oseltamivir at a dosage of 0.1 mg/kg/day or more. The spread of A/quail/HK/G1/97 (H9N2) influenza virus to the brains of infected mice was reduced 32-fold at a dosage of either drug of 0.1 mg/kg/day (P < 0.0001) and was eliminated at a dosage of either drug of 1.0 mg/kg/day.
Among animals infected with H5N1 virus, those treated with RWJ-270201 tended to have lower lung virus titers than those treated with oseltamivir (P = 0.077); no difference was observed in the reduction of virus titers in the brain. In mice infected with H9N2 virus, no statistically significant difference was seen between the two drugs in reduction of virus titers in the lungs and brains.
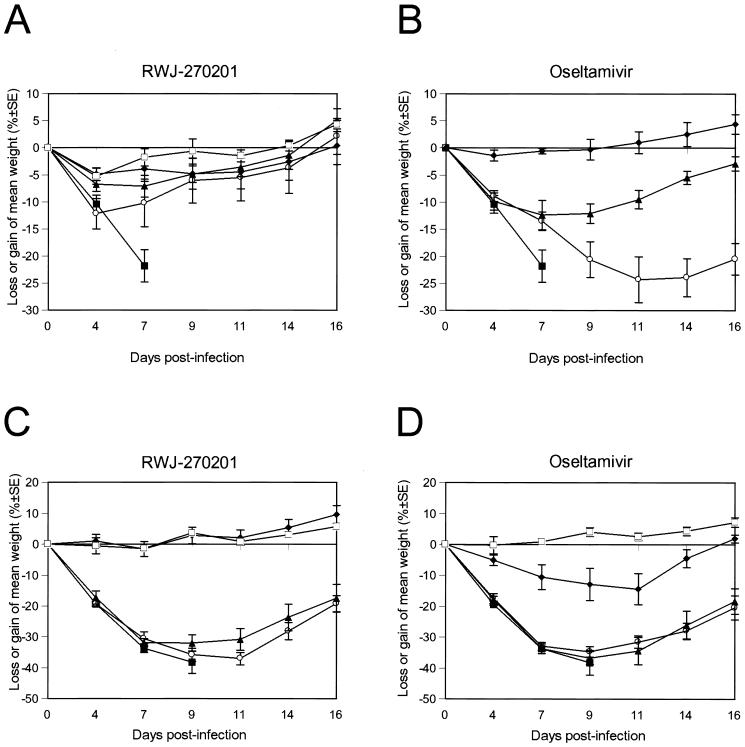
Effect of RWJ-270201 and oseltamivir on loss or gain of weight in mice infected with H5N1 and H9N2 influenza viruses.
Weight change is a useful tool for assessing the morbidity of mice after challenge with influenza viruses. We weighed the animals before infection and again on days 4, 7, 9, 11, 14, and 16 after infection with H5N1 (Fig. 2A and B) and H9N2 (Fig. 2C and D) influenza viruses.
FIG. 2.
Effect of treatment with RWJ-270201 and oseltamivir on mean loss or gain of weight in mice infected with H5N1 and H9N2 influenza viruses. RWJ-270201 and oseltamivir at dosages of 0.01 (○), 0.1 (▴), 1.0 (⧫), and 10 (□) mg/kg/day were administered to BALB/c mice by twice-daily oral gavage for 5 days beginning 4 h before viral infection. Mice were infected with 5 MLD50s of A/HK/156/97 (H5N1) (A and B) or mouse-adapted A/quail/HK/G1/97 (H9N2) (C and D) influenza viruses. Control untreated animals (▪) received PBS. The mice were weighed on day 0 (before inoculation) and days 4, 7, 9, 11, 14, and 16 after inoculation. Loss or gain of weight was calculated for each mouse as a percentage of its weight on day 0. Values are the averages for each group.
Mice infected with H5N1 virus and treated with RWJ- 270201 lost weight consistently for the first 4 days after infection (day 4 weight was −5.3% to −12.2% of baseline weight). The animals began to regain weight by day 7 after infection (day 7 weight, −1.8% to −10.2% of baseline) and had regained most of their weight by day 14. There were significant differences between the weight changes of control animals and those given RWJ-270201 at dosages of 0.1 (P ≤ 0.01), 1.0 (P ≤ 0.002), and 10 (P ≤ 0.002) mg/kg/day. The pattern of weight change in H5N1-infected mice treated with oseltamivir at 1.0 mg/kg/day was similar to that seen with RWJ-270201 (Fig. 2B). However, at low dosages (0.01 and 0.1 mg/kg/day), RWJ-270201 reduced weight loss more effectively than oseltamivir. Statistical analysis of the different combinations of viruses, drugs, dosages, and number of days after infection showed that at a dosage of 0.01 mg/kg/day, RWJ-270201 was more effective than oseltamivir in reducing the weight loss of mice infected with H5N1 virus on days 9 to 14 after infection (P < 0.01).
Among mice infected with A/quail/HK/G1/97 virus, those treated with low dosages (0.01 and 0.1 mg/kg/day) of the two drugs did not differ from the control group in weight on days 4, 7, and 9 after infection (Fig. 2C and D). However, those treated with higher dosages (1.0 and 10 mg/kg/day) had significantly increased weight on days 4, 7, and 9 (P < 0.001). On days 7, 9, and 11, the weights of mice infected with A/quail/HK/G1/97 virus and treated with either RWJ-270201 or oseltamivir differed only at a dosage of 1.0 mg/kg/day (Fig. 2C and D). Animals treated with RWJ-270201 tended to regain weight more rapidly than those treated with oseltamivir (P, 0.012 to 0.039).
RWJ-270201, administered at a dosage of 10 mg/kg/day, prevents mean weight loss in mice infected with either H5N1 or H9N2 influenza viruses. This dosage of RWJ-270201 was at least as effective as administration of another oral NA inhibitor, oseltamivir in preventing weight loss and other signs of infection.
Effect of delayed treatment with RWJ-270201 or oseltamivir on H5N1 virus infection in mice.
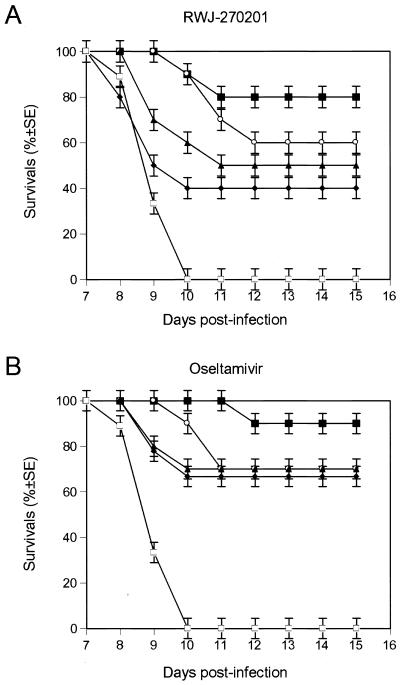
To assess the potential therapeutic, rather than prophylactic, usefulness of RWJ-270201 for treatment of influenza virus infection, we examined the efficacy of the drug when given late in the infection. Mice infected with 10 MLD50s of A/HK/156/97 virus began receiving RWJ-270201 or oseltamivir at 10 mg/kg/day 24, 36, 48, or 60 h after infection (Fig. 3). All untreated control animals died between days 8 and 10 after infection. Oral administration of either RWJ-270201 or oseltamivir increased the survival rates of mice in all treatment groups. No deaths were observed until day 7 after infection in mice treated with RWJ-270201 and until day 8 after infection in mice treated with oseltamivir. A significant antiviral effect was observed when therapy with RWJ-270201 began 24 h after virus inoculation (80% of animals survived) or 36 h after virus inoculation (60% of animals survived) (Fig. 3). When therapy with RWJ-270201 was delayed until 48 h after infection, 50% of animals survived. When treatment with oseltamivir was given 24 h after inoculation, 90% of mice survived. Even when given as late as 60 h after infection, oseltamivir protected more than 65% of mice from lethal infection with H5N1 virus. The survival rates of mice treated with oseltamivir were higher than those of mice treated with RWJ-270201 at all postinfection time intervals tested; however, these differences were not statistically significant.
FIG. 3.
Effect of delayed treatment with RWJ-270201 or oseltamivir on survival rates of mice infected with A/HK/156/97 (H5N1) influenza virus. BALB/c mice (n = 9 to 10 per group) were infected intranasally with 10 MLD50s of influenza A/HK/156/97 (H5N1) virus and treated with RWJ-270201 or oseltamivir at 10 mg/kg/day by twice-daily oral gavage for 5 days. Treatment began 24 (▪), 36 (○), 48 (▴), or 60 (⧫) hours after virus inoculation. Control untreated animals (□) received PBS.
DISCUSSION
We found that the novel oral NA inhibitor RWJ-270201 was effective in inhibiting the NA activity and in vitro replication of all nine NA subtypes of influenza virus. In vitro, the avian influenza viruses were at least as sensitive to RWJ-270201 as to zanamivir and oseltamivir carboxylate. Administration of 10 mg of RWJ-270201/kg/day completely protected mice against lethal infection with the highly pathogenic H5N1 and H9N2 influenza viruses that were transmitted from birds to humans in Hong Kong (34, 41).
The inhibitory effects of RWJ-270201 on the nine NA subtypes were distributed within a narrow range of concentrations. The NA activity of all nine subtypes was inhibited by 0.9 to 4.3 nM RWJ-270201, and replication was inhibited in all nine subtypes by 0.5 to 11.8 μM RWJ-270201. The other NA inhibitors tested, zanamivir and oseltamivir carboxylate, had less consistent inhibitory effects among the different NA subtypes. There was an approximately 15-fold difference between the minimum and maximum IC50s determined for zanamivir and an approximately 35-fold difference for oseltamivir carboxylate. Although there are few available reports on RWJ-270201, it has been observed that the in vitro potency of RWJ-270201 is comparable or superior to that of zanamivir and oseltamivir carboxylate (39). Other authors have noted varying sensitivities to zanamivir and oseltamivir carboxylate among influenza viruses of different NA subtypes (13, 30, 37).
To some extent, differences in NA-inhibitory activity of zanamivir and oseltamivir carboxylate among the influenza virus strains can be attributed to amino acid substitutions surrounding the enzyme active center of the NA. X-ray crystallographic studies of different compounds bound to influenza NA have shown that approximately 6 to 8 amino acid residues play the most important roles in these interactions, although as many as 30 amino acid residues can be involved (23, 26, 44). Although the enzyme active site of the NA is highly conserved among all influenza NA subtypes (8), there are some sequence variations that can influence the interaction of the inhibitor with the enzyme. The balance between HA and NA activities is an important factor in the explanation of some biological properties of the influenza viruses (31). This balance can play an essential role in the different activities of any one drug against different influenza viruses expressing various HA and NA proteins. The lower the affinity of HA for sialic acid receptors, the less dependent it is on NA functions for release from cells or from other viruses. This reduced dependence on NA provides an advantage under the pressure of the inhibitor.
Another factor in the different effects of NA inhibitors is chemical structure. The structure of RWJ-270201 differs from those of zanamivir and oseltamivir (1). Three active chemical groups (negatively charged carboxylate, positively charged guanidine, and lipophilic side chains) have been identified in the crystal structure of complexes of RWJ-270201 bound to the active center of the influenza virus NA (1). It is possible that the chemical structure of RWJ-270201 gives it an energetic advantage or a more favorable fit within the enzyme active center than other NA inhibitors have. Any or all of the mechanisms discussed above may underlie the diverse antiviral effects of the NA inhibitors against different virus strains. However, the nature of the NA inhibitor appears most worthy of further consideration.
Another aim of this study was to evaluate the in vivo efficacy of RWJ-270201 against naturally occurring highly pathogenic influenza A/HK/156/97 (H5N1) and A/quail/HK/G1/97 (H9N2) viruses. The H5N1 influenza viruses caused an outbreak of influenza among humans in 1997, are highly pathogenic in chickens (36, 40), and replicate systemically (including in the brain) in mice without prior adaptation (14, 28, 36). Since the early 1990s, H9N2 influenza viruses have become widespread in domestic chickens in Asia, and they caused an outbreak of influenza in Hong Kong in 1999 (34). The high amino acid homology between the internal genes of H9N2, H6N1, and H5N1 influenza viruses indicates that these subtypes are able to exchange their internal genes and are therefore a potential source of pandemic virus (21). Virus genes carried by the H5N1 Hong Kong viruses continue to circulate in poultry in mainland China (6). Thus, therapies effective against these viruses are of considerable interest.
Oral administration of RWJ-270201 at a dosage of 10 mg/kg/day completely protected mice against lethal infection with both influenza A/HK/156/97 (H5N1) and A/quail/HK/G1/97 (H9N2) viruses and prevented virus replication in the lungs and brains of infected animals. RWJ-270201 was at least as effective as oseltamivir in in vivo experiments in mice. These results confirmed our previous observations that orally bioavailable oseltamivir is efficacious against H5N1 and H9N2 viruses and that doses of 1.0 and 10 mg/kg/day prevent the death of infected mice (25). Intranasally administered zanamivir was shown to be effective in reducing replication of A/HK/156/97 (H5N1) virus in the lungs of mice and in reducing morbidity and mortality (14). However, zanamivir failed to protect chickens infected with highly virulent viruses of the N1, N2, N3, N7, and N8 subtypes (13, 29).
We assessed the in vivo efficacy of RWJ-270201 against two different influenza viruses. In preventing death and reducing virus titers in the lungs and brains of infected animals, RWJ-270201 was equally effective against A/HK/156/97 (H5N1) virus and A/quail/HK/G1/97 (H9N2) virus at dosages of 1.0 and 10 mg/kg/day. Lower dosages (0.01 and 0.1 mg/kg/day) were more effective against H5N1 virus than against H9N2 virus. Zanamivir and oseltamivir also show disparities in the dosages required to produce significant protection against different influenza strains (14, 25, 30, 37). These differences are closely connected with the balance between the drug dosage, the dose of virus used to inoculate animals, and the unique biological and genetic characteristics of the virus. For example, influenza A/HK/156/97 virus has a 15-amino-acid deletion in the stalk region of the NA (7). Such a deletion is an important factor in host range restriction and tissue tropism (5) and may be implicated in differences between the H5N1 virus and other viruses in virulence and systemic spread in infected hosts. Thus, RWJ-270201 showed high potency against these two highly pathogenic viruses, but additional studies with other viruses with pandemic potential should be conducted to further confirm these results. It was reported recently that RWJ-270201 was inhibitory to influenza A (H1N1), A (H3N2), and B virus infection in mice (38), thus indicating the potential for the oral use of RWJ-270201 in treatment of influenza virus infections in humans.
Our findings provide promising evidence that RWJ-270201 can be used not only for prophylaxis but also for treatment of influenza virus infection. Oral administration of RWJ-270201 to mice 24 h after infection with H5N1 virus resulted in 90% survival. Even when therapy began 48 h after infection, RWJ-270201 protected 50% of animals. Although delayed treatment with oseltamivir yielded higher survival rates than delayed treatment with RWJ-270201 at all time intervals tested, these differences were not statistically significant. Oral therapy with oseltamivir can be delayed until 36 h after exposure to the H5N1 viruses (25) and 48 to 60 h or more after infection with H1N1 virus (37). Our results are consistent with the findings of Sidwell and colleagues (37) that the sensitivity of murine influenza infections to NA inhibitors is dependent on the virus challenge dose. We also suggest that the efficacy of the drug can be dependent on the virus strain used as a challenge because strains can differ in their capacities to spread systemically in the host and in the speed at which they can spread.
This study assessed the potency of the novel oral NA inhibitor RWJ-270201 in the context of pandemic planning, a situation in which antiviral drugs offer enormous advantages, especially when effective vaccines are not available. There are a number of factors that can make an antiviral drug useful in responses to pandemics. These include a broad antiviral spectrum and potency, prophylactic and therapeutic effectiveness, favorable pharmacokinetics, availability to the population at risk, tolerability, and safety (19). We have demonstrated that the novel NA inhibitor RWJ-270201 satisfies the main requirements for usefulness in a pandemic. It is highly efficacious against all nine NA subtypes of avian influenza viruses in vitro, and it provides complete protection against lethal H5N1 and H9N2 influenza virus infection in mice. Although any effective antiviral drug would be useful in the case of a pandemic, orally active NA inhibitors are easily administered and are broadly distributed within the body; antiviral drugs that act at a local site of infection are probably not suitable for treating disseminated virus infections. The long-term stability of the NA inhibitors, which would allow stockpiling of supplies in preparation for a pandemic, has yet to be established. The currently available antiviral agents amantadine and rimantadine remain stable for 25 or more years at ambient temperatures (35). Future studies are necessary to determine whether the efficacious NA inhibitors with their broader range of anti-influenza activity will be able to retain sufficient stability for such stockpiling.
ACKNOWLEDGMENTS
This work was supported by research grants AI29680 and AI95357 from the National Institute of Allergy and Infectious Diseases, by Cancer Center Support (CORE) grant CA-21765, by the American Lebanese Syrian Associated Charities (ALSAC), and by the R. W. Johnson Pharmaceutical Research Institute.
We thank K. Shortridge and M. Peiris for providing the H5N1 and H9N2 influenza viruses, Alice Herren and Laurie Twit for administrative assistance, and Sharon Naron for editorial assistance.
REFERENCES
- 1.Babu Y S, Chand P, Bantia S, Kotian P, Dehghani A, El-Kattan Y, Lin T H, Hutchison T L, Elliott A J, Parker C D, Ananth S L, Horn L L, Laver G W, Montgomery J A. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43:3482–3486. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- 2.Barnett J M, Cadman A, Gor D, Dempsey M, Walters M, Candlin A, Tisdale M, Morley P J, Owens I J, Fenton R J, Lewis A P, Claas E C, Rimmelzwaan G F, De Groot R, Osterhaus A D. Zanamivir susceptibility monitoring and characterization of influenza virus clinical isolates obtained during phase II clinical efficacy studies. Antimicrob Agents Chemother. 2000;44:78–87. doi: 10.1128/aac.44.1.78-87.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belshe R B, Smith H M, Hall C B, Betts R, Hay A J. Genetic basis of resistance to rimantadine emerging during treatment of influenza virus infection. J Virol. 1988;62:1508–1512. doi: 10.1128/jvi.62.5.1508-1512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossart-Whitaker P, Carson M, Babu Y S, Smith C D, Laver W G, Air G M. Three-dimensional structure of influenza A N9 neuraminidase and its complex with inhibitor 2-deoxy-2,3-dehydro-N-acetyl-neuraminic acid. J Mol Biol. 1993;232:1069–1083. doi: 10.1006/jmbi.1993.1461. [DOI] [PubMed] [Google Scholar]
- 5.Castrucci M R, Kawaoka Y. Biologic importance of neuraminidase stalk length in influenza A virus. J Virol. 1993;67:759–764. doi: 10.1128/jvi.67.2.759-764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cauthen A N, Swayne D E, Schultz-Cherry S, Perdue M L, Suarez D L. Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J Virol. 2000;74:6592–6599. doi: 10.1128/jvi.74.14.6592-6599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claas E C, Osterhaus A D, van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 8.Colman P M, Hoyne P A, Lawrence M C. Sequence and structure alignment of paramyxovirus hemagglutinin-neuraminidase with influenza virus neuraminidase. J Virol. 1993;67:2972–2980. doi: 10.1128/jvi.67.6.2972-2980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couch R B, Keitel W A, Cate T R, Quarles J A, Taber L A, Glezen W P. Prevention of influenza virus infection by current influenza virus vaccines. In: Brown L E, Hampson A W, Webster R G, editors. Options for the control of influenza III. Amsterdam, The Netherlands: Elsevier; 1996. pp. 97–106. [Google Scholar]
- 10.De Jong J C, Claas E C, Osterhaus A D M E, Webster R G, Lim W L. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao P, Watanabe S, Ito T, Goto H, Wells K, McGregor M, Cooley A J, Kawaoka Y. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J Virol. 1999;73:3184–3189. doi: 10.1128/jvi.73.4.3184-3189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan Y, Shortridge K F, Krauss S, Webster R G. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc Natl Acad Sci USA. 1999;96:9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gubareva L V, Penn C R, Webster R G. Inhibition of replication of avian influenza viruses by the neuraminidase inhibitor 4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid. Virology. 1995;212:323–330. doi: 10.1006/viro.1995.1489. [DOI] [PubMed] [Google Scholar]
- 14.Gubareva L V, McCullers J A, Bethell R C, Webster R G. Characterization of influenza A/Hong Kong/156/97 (H5N1) virus in a mouse model and protective effect of zanamivir on H5N1 infection in mice. J Infect Dis. 1998;178:1592–1596. doi: 10.1086/314515. [DOI] [PubMed] [Google Scholar]
- 15.Hayden F G, Hay H J. Emergence and transmission of influenza A viruses resistant to amantadine and rimantadine. Curr Top Microbiol Immunol. 1992;176:119–130. doi: 10.1007/978-3-642-77011-1_8. [DOI] [PubMed] [Google Scholar]
- 16.Hayden F G. Amantadine and rimantadine—clinical aspects. In: Richman D D, editor. Antiviral drug resistance. New York, N.Y: John Wiley and Sons, Ltd.; 1996. pp. 59–77. [Google Scholar]
- 17.Hayden F G, Treanor J J, Betts R F, Lobo M, Esinhart J D, Hussey E K. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA. 1996;275:295–299. [PubMed] [Google Scholar]
- 18.Hayden F G, Osterhaus A D, Treanor J J, Fleming D M, Aoki F Y, Nicholson K G, Bohnen A M, Hirst H M, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. GG167 Influenza Study Group. N Engl J Med. 1997;25:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 19.Hayden F G. Antivirals for pandemic influenza. J Infect Dis. 1997;176(Suppl. 1):S56–S61. doi: 10.1086/514177. [DOI] [PubMed] [Google Scholar]
- 20.Hayden F G, Treanor J J, Fritz R S, Lobo M, Betts R F, Miller M, Kinnersley N, Mills R G, Ward P, Straus S E. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann E, Stech J, Leneva I, Krauss S, Scholtissek C, Chin P S, Peiris M, Shortridge K F, Webster R G. Characterization of the influenza A virus gene pool in avian species in southern China: was H6N1 a derivative or a precursor of H5N1? J Virol. 2000;74:6309–6315. doi: 10.1128/jvi.74.14.6309-6315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiser L, Couch R B, Galasso G J, Glezen W P, Webster R G, Wright P F, Hayden F G. First International Symposium on Influenza and Other Respiratory Viruses: summary and overview: Kapalua, Maui, Hawaii, December 4–6. Antivir Res. 1998;42:149–175. doi: 10.1016/S0166-3542(99)00034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim C U, Lew W, Williams M A, Liu H, Zhang L, Swaminathan S, Bischofberger N, Chen M S, Mendel D B, Tai C Y, Laver W G, Stevens R C. Influenza neuraminidase inhibitors possessing novel hydrophobic interactions in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J Am Chem Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- 24.Klenk H-D, Rott R. The molecular biology of influenza virus pathogenicity. Adv Virus Res. 1988;34:247–281. doi: 10.1016/S0065-3527(08)60520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leneva I A, Roberts N, Govorkova E A, Goloubeva O G, Webster R G. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antivir Res. 2000;48:101–115. doi: 10.1016/s0166-3542(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 26.Lew W, Chen X, Kim C U. Discovery and development of GS4104 (oseltamivir): an orally active influenza neuraminidase inhibitor. Curr Med Chem. 2000;7:663–672. doi: 10.2174/0929867003374886. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Eichelberger M C, Compans R W, Air G M. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J Virol. 1995;69:1099–1106. doi: 10.1128/jvi.69.2.1099-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu X, Tumpey T M, Morken T, Zaki S R, Cox N J, Katz J M. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCauley J W, Pullen L A, Forsyth M, Penn C R, Thomas G P. 4-Guanidino-Neu5Ac2en fails to protect chickens from infection with highly pathogenic avian influenza virus. Antivir Res. 1995;27:179–186. doi: 10.1016/0166-3542(95)00005-7. [DOI] [PubMed] [Google Scholar]
- 30.Mendel D B, Tai C Y, Escarpe P A, Li W X, Sidwell R W, Huffman J H, Sweet C, Jakeman K J, Merson J, Lacy S A, Lew W, Williams M A, Zhang L, Chen M S, Bischofberger N, Kim C U. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother. 1998;42:640–646. doi: 10.1128/aac.42.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitnaul L J, Matrosovich M N, Castrucci M R, Tuzikov A B, Bovin N V, Kobasa D, Kawaoka Y. Balanced hemagglutinin and neuraminidase activities are critical for efficient replication of influenza A virus. J Virol. 2000;74:6015–6020. doi: 10.1128/jvi.74.13.6015-6020.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monto A S. Prospects for pandemic influenza control with currently available vaccines and antivirals. J Infect Dis. 1997;176(Suppl. 1):S32–S37. doi: 10.1086/514172. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson K G, Aoki F Y, Osterhaus A D M E, Trottier S, Carewicz O, Mercier C H, Rode A, Kinnersley N, Ward P. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomized controlled trial. Lancet. 2000;355:1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 34.Peiris M, Yuen K Y, Leung C W, Chan K H, Ip P L S, Lai R W M, Orr W K, Shortridge K F. Human infection with influenza H9N2. Lancet. 1999;354:916–917. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 35.Scholtissek C, Webster R G. Long-term stability of the anti-influenza A compounds—amantadine and rimantadine. Antivir Res. 1998;38:213–215. doi: 10.1016/s0166-3542(98)00015-1. [DOI] [PubMed] [Google Scholar]
- 36.Shortridge K F, Zhou N N, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti K G, Norwood M, Senne D, Sims L, Takada A, Webster R G. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 37.Sidwell R W, Huffman J H, Barnard D L, Bailey K W, Wong M N, Morrison A, Syndergaard T, Kim C U. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antivir Res. 1998;37:107–120. doi: 10.1016/s0166-3542(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 38.Sidwell RW, Smee D F, Huffman J H, Barnard D L, Bailey K W, Morrey J D, Babu Y S. In vivo influenza virus-inhibitory effects of the cyclopentane neuraminidase inhibitor RWJ-270201. Antimicrob Agents Chemother. 2001;45:749–757. doi: 10.1128/AAC.45.3.749-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smee D F, Huffman J H, Morrison A C, Barnard D L, Sidwell R W. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activities. Antimicrob Agents Chemother. 2001;45:743–748. doi: 10.1128/AAC.45.3.743-748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suarez D L, Perdue M L, Cox N, Rowe T, Bender C, Huang J, Swayne D E. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T M, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 42.Varghese J N, McKimm-Breschkin J L, Caldwell J B, Kortt A A, Colman P M. The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor. Proteins. 1992;14:327–332. doi: 10.1002/prot.340140302. [DOI] [PubMed] [Google Scholar]
- 43.Venables W N, Ripley B D. Modern applied statistics. 1997. pp. 223–242. , 297–321, 345–350. Springer, New York, N.Y. [Google Scholar]
- 44.von Itzstein M, Wu W-Y, Kok G K, Pegg M S, Dyason J C, Jin B, Van Phan T, Smithe M L, White H F, Oliver S W, Colman P M, Varghese J N, Ryan D M, Woods J M, Bethell R C, Hotham V J, Cameron J M, Penn C R. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]