Abstract
Tenofovir DF is an antiviral nucleotide with activity against human immunodeficiency virus type 1 (HIV-1). The pharmacokinetics, safety, and activity of oral tenofovir DF in HIV-1-infected adults were evaluated in a randomized, double-blind, placebo-controlled, escalating-dose study of four doses (75, 150, 300, and 600 mg given once daily). Subjects received a single dose of tenofovir DF or a placebo, followed by a 7-day washout period. Thereafter, subjects received their assigned study drug once daily for 28 days. Pharmacokinetic parameters were dose proportional and demonstrated no change with repeated dosing. Reductions in plasma HIV-1 RNA were dose related at tenofovir DF doses of 75 to 300 mg, but there was no increase in virus suppression between the 300- and 600-mg dose cohorts, despite dose-proportional increases in drug exposure. Grade III or IV adverse events were limited to laboratory abnormalities, including elevated creatine phosphokinase and liver function tests, which resolved with or without drug discontinuation and without sequelae. No patients developed detectable sequence changes in the reverse transcriptase gene.
Tenofovir {9-[(R)-2-(phosphonomethoxy)propyl]adenine}, formerly known as PMPA, is a novel nucleotide analog belonging to the class of acyclic nucleoside phosphonates. When administered tenofovir as late as 24 h after intravenous inoculation of simian immunodeficiency virus (SIV), rhesus monkeys were completely protected against acute infection (10, 11). Tenofovir has also demonstrated substantial efficacy in the treatment of chronically infected macaque monkeys (12, 13). When given intravenously to human immunodeficiency virus type 1 (HIV-1)-infected people for 7 consecutive days, tenofovir was well tolerated and demonstrated significant dose-related anti-HIV-1 activity (3). However, tenofovir demonstrated low oral bioavailability in animal studies. Therefore, a prodrug of tenofovir, tenofovir disoproxil fumarate (tenofovir DF) {9-[(R)-2-[[bis[[(isopropoxycarbonyl)oxy]methoxy]phosphinyl]methoxy]propyl]adenine fumarate}; characterized by an advantageous pharmacokinetic profile and therapeutic index was chosen for clinical development (2, 8).
Tenofovir is metabolized intracellularly to its active metabolite, tenofovir diphosphate, which is a competitive inhibitor of HIV-1 reverse transcriptase (RT) that terminates the growing DNA chain. Tenofovir diphosphate, which is active in both resting and activated cells, has a prolonged intracellular half-life that ranges from 12 to 50 h (7). This long intracellular half-life allows infrequent administration. Additionally, tenofovir shows a favorable in vitro resistance profile. Serial passage of an HIV-1 isolate in the presence of increasing concentrations of tenofovir resulted in the emergence of a HIV-1 mutant with a K65R substitution in the RT gene; a recombinant virus expressing HIV-1 RT with the K65R mutation showed an only threefold decrease in tenofovir susceptibility compared to that of the wild-type virus (14). Tenofovir retains activity against a variety of drug-resistant HIV-1 strains in vitro (9, 14). Lamivudine (3TC)-resistant viruses, which carry the M184V mutation, demonstrate approximately twofold increased susceptibility to tenofovir in vitro (5). In vitro testing of tenofovir in combination with other antiretroviral compounds (adefovir, 3TC, zidovudine [AZT], didanosine, stavudine [d4T], saquinavir, ritonavir, nelfinavir, indinavir, abacavir, amprenavir, nevirapine, and delavirdine) demonstrated additive or synergistic activity; no significant antiviral antagonism was observed (6; A. Mulato and J. M. Cherrington, 12th World AIDS Conf., abstr. 41195, 1998).
The current study was designed to evaluate the anti-HIV-1 activity, safety, tolerance, and pharmacokinetics of oral tenofovir DF when administered as a single daily dose for 28 consecutive days.
(The results of this study were presented at the 5th Conference on Retroviruses and Opportunistic Infections, Chicago, Ill., 1 to 5 February 1998 [abstract LB-8] and the 12th World AIDS Conference, Geneva, Switzerland, 28 June to 3 July 1998 [abstracts 12211 and 41218].)
MATERIALS AND METHODS
Study population.
Eligible subjects included men and women with documented HIV-1 infection, HIV-1 RNA levels in plasma of ≥10,000 copies/ml, CD4 cell counts of ≥200 cells/mm3, and Karnofsky performance status scores of ≥80. At the time of screening, subjects were required to have a serum creatinine level of ≤1.5 mg/dl, a calculated creatinine clearance rate of ≥60 ml/min (according to the Cockcroft-Gault formula), a total bilirubin level of ≤1.5 mg/dl, hepatic transaminases less than or equal to three times the upper limit of normal, serum amylase <1.5 times the upper limit of normal, an absolute neutrophil count of ≥1,000 cells/mm3, a platelet count of ≥75,000/mm3, a hemoglobin concentration of ≥8.0 g/dl, and prothrombin and partial thromboplastin times of <1.2 the times upper limit of normal. A negative pregnancy test was required at the time of enrollment for women with childbearing potential. Subjects with a positive serum test for hepatitis B surface antigen were excluded, as were those with active, serious infections. Prior antiretroviral therapy was permitted (other than adefovir dipivoxil); however, concomitant antiretroviral therapy was prohibited from 2 weeks prior to study entry through day 42 of the study. Subjects were excluded if they were receiving ongoing therapy with agents considered to have nephrotoxic potential or to compete for renal elimination via active renal secretion (probenecid).
Study design.
This was a randomized, double-blind, placebo-controlled, dose escalation study of four doses of tenofovir DF (75, 150, 300, and 600 mg). Subjects received a single dose of the study drug in the fasted state and then, after a 7-day washout period, 28 consecutive daily doses of the study drug, each following a meal. Within each cohort, approximately eight subjects were randomly assigned to receive tenofovir DF and two were assigned to receive a placebo. Randomization was performed centrally by a computer-generated random-number program that assigned subject numbers in blocks of five within each dose cohort to active-drug or placebo treatment. Prior to dose escalation to 150 mg of tenofovir DF, five additional subjects (four active and one placebo) were enrolled in the first dosing cohort (75 mg) in order to confirm safety. Subjects were recruited from three centers: The Johns Hopkins University School of Medicine, San Francisco General Hospital, and the University of Washington School of Medicine.
Informed consent was obtained from all subjects. Human experimentation guidelines of the U.S. Department of Health and Human Services and of The Johns Hopkins University School of Medicine, the University of California, San Francisco, and the University of Washington were followed.
The use of a placebo control provided the most rigorous evaluation of the safety, pharmacokinetics, and antiviral profile of a new compound. In addition, for a short-term trial, giving a placebo to HIV-1-positive patients who had never received antiretroviral therapy or who were not responding to or were intolerant of their current regimen was considered acceptable by the participating investigators and institutional review boards. Furthermore, tenofovir data obtained with the SIV model indicated that resistance to tenofovir is slow to develop (13). Although tenofovir DF monotherapy was considered appropriate for this clinical trial design, we are not advocating that monotherapy with any antiretroviral be used in clinical practice.
Study drug.
Each subject received tenofovir DF or identical-appearing, lactose-containing placebo tablets (all of the study medication was supplied by Gilead Sciences, Inc.). Each tenofovir DF tablet contained 75 mg of the active drug. Fasting subjects received a single dose of the study drug at approximately 9 am on day 1 of the study and continued to fast for 2 h after dosing. After a 7-day washout period, the study drug was administered once daily after food for 28 consecutive days (days 8 to 35).
Pharmacokinetic analyses.
Prior to each pharmacokinetic sampling period, subjects were admitted to an inpatient research center at each site. Blood samples were drawn at 0 h (predose) and at 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 h after dosing for determination of serum pharmacokinetic parameters on days 1, 8, and 15 of the study. On study day 1, the drug was administered after an overnight fast; on study days 8 and 15, the drug was given following the consumption of a standardized, high-fat breakfast. Subjects in the 300- and 600-mg cohorts also underwent pharmacokinetic sampling after eating a standardized breakfast on the last day of dosing (day 35); additional samples were obtained at 36 and 48 h. Urine was collected over the intervals 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h after dosing on days of pharmacokinetic sampling, except on day 1, when it was collected for 72 h.
Serum and urine samples were analyzed for tenofovir concentrations by using a validated high-performance liquid chromatographic assay involving precolumn derivatization and fluorescence detection (3). Pharmacokinetic parameters were assessed by application of a nonlinear curve-fitting software package (WinNonlin Standard Edition, version 2.1; Pharsight Corporation, Mountain View, Calif.) using noncompartmental methods. Pharmacokinetic parameters dependent on the drug's terminal half-life were calculated only for patients with drug concentrations in serum greater than the lower limit of quantitation through 12 h following drug administration. Additionally, pharmacokinetic parameters were calculated only if accurate assessment of the area under the concentration-versus-time profile (AUC) and its derived pharmacokinetic parameters was possible. Summary statistics are reported only when greater than 50% of the subjects dosed on a visit contributed data for the given pharmacokinetic metric. The oral bioavailability of tenofovir from tenofovir DF was determined by using historical data from administration of intravenous tenofovir (3).
Safety evaluations.
Clinical assessments were performed, and blood for safety monitoring was obtained regularly during the study. Safety laboratory testing included serum chemistry and electrolyte panels, hematology, coagulation parameters, and urinalyses. Subjects were monitored for 4 weeks (through day 63) after receiving the last dose of the study drug.
Signs, symptoms, and laboratory abnormalities were recorded by using a modified graded toxicity scale based on the AIDS Clinical Trials Group common toxicity grading scale (1). In general, toxicities or abnormalities were classified as follows: mild, grade I; moderate, grade II; severe, grade III; possibly life threatening, grade IV. The treating investigator, without knowledge of treatment assignment, assessed the relationship of an adverse event to the study medication. Treatment assignment remained blinded until receipt of all adverse event reports from the sites and until verification of all data was completed.
Antiviral activity.
Blood samples for HIV-1 RNA levels and T-cell subset analyses (CD4, CD8) were obtained on days 1, 4, 8, 11, 14, 18, 21, 28, and 35. For HIV-1 RNA analysis, plasma was shipped to a central laboratory (Johns Hopkins Research and Reference Laboratory, Baltimore, Md.) and analyzed by using a quantitative RT-PCR assay (Amplicor; Roche Diagnostics Inc.; lower limit of quantification, 400 copies/ml).
Genotypic resistance analyses.
The preparation of the HIV-1 RNA from patient plasma, the generation of the 1.1-kb PCR fragments carrying HIV-1 RT, and analyses of the DNA sequences of these PCR fragments have been previously described (4). Briefly, viral population-based genotypic analyses were performed with RT-PCR fragments carrying HIV-1 RT genes generated from patients' plasma samples taken at baseline and day 35. Nucleotides 1 to 900 (amino acids 1 to 300) of the HIV-1 RT genes generated from the specified plasma samples were manually sequenced by conventional dideoxy sequencing methods (4). From plasmid mixing experiments, mixtures of mutant and wild-type sequences could be detected when present at a frequency of greater than 20%.
Statistical analyses.
Homogeneity of variance among treatment groups at baseline was evaluated by analysis of variance for continuous variables and Pearson's chi-square test for categorical variables. Evaluations of the differences among the four tenofovir DF treatment groups and the pooled placebo group in safety parameters and antiviral activity were done by using the Wilcoxon rank-sum test. For HIV-1 RNA, the baseline value is the geometric mean of the prestudy drug values on days 0 and 1. For CD4 cell counts, the baseline value is the value from day 1. Median values are followed by the interquartile range in parentheses. The safety analysis included each of the 49 subjects who received at least one dose of the study medication. All of the data collected up to 30 days after permanent study medication discontinuation are included.
Rank analysis of variance with Fisher's least-significant-difference tests was performed on pharmacokinetic exposure metrics to assess dose linearity. Signed rank tests were performed to compare pharmacokinetics in the fasted (day 1) state versus the fed (day 8) state for each dose group for assessment of the food effect. Signed rank tests were performed to compare single-dose to steady-state pharmacokinetic values in the fed state (day 8 versus days 15 and 35) to assess changes in tenofovir pharmacokinetics over time. P values of less than or equal to 0.05 were deemed statistically significant. There was no adjustment for multiple comparisons.
RESULTS
Study subjects.
A total of 49 HIV-1-infected subjects (8 females, 41 males) were enrolled in the four cohorts between 6 May 1997 and 18 May 1999 (15 in the 75-mg cohort, 10 in the 150-mg cohort, 11 in the 300-mg cohort, and 13 in the 600-mg cohort). Thirty-eight subjects were assigned to receive the active drug (12 at 75 mg, 8 at 150 mg, 8 at 300 mg, and 10 at 600 mg). All of the 11 other study subjects received a placebo.
The five study groups (pooled placebo and 75, 150, 300, and 600 mg of tenofovir DF) were similar in age and race (Table 1). Although the proportion of subjects who had received prior antiretroviral therapy varied in the four groups, the differences were not significant (Table 1). Most of the antiretroviral agents used prior to study were nucleoside analogues. There were no significant differences in median baseline log10 HIV-1 RNA levels or median baseline CD4 counts among the four groups (Table 1).
TABLE 1.
Characteristics of the study subject population at study entry
| Characteristic | Placebo group | Tenofovir DF dose group
|
Total | |||
|---|---|---|---|---|---|---|
| 75 mg | 150 mg | 300 mg | 600 mg | |||
| No. of subjects | 11 | 12 | 8 | 8 | 10 | 49 |
| Sex (male/female) | 7/4 | 12/0 | 5/3 | 7/1 | 10/0 | 41/8 |
| Age (yrs)a | 37 (33–44) | 38.5 (32.5–42) | 38 (35–40.5) | 41 (33–46) | 42.5 (37–44) | 39 (34–44) |
| No. (%) Caucasian | 4 (50) | 5 (42) | 2 (25) | 5 (63) | 5 (50) | 21 (43) |
| No. (%) African-American | 7 (64) | 6 (50) | 4 (50) | 2 (25) | 4 (40) | 23 (47) |
| No. (%) Hispanic | 0 | 1 (8) | 2 (25) | 1 (13) | 0 (0) | 4 (8) |
| HIV-1 RNA level (log10 copies/ml)a | 4.53 (4.16–4.95) | 4.7 (4.23–5.01) | 4.5 (4.44–5.13) | 4.12 (3.91–4.55) | 4.64 (4.48–4.75) | 4.53 (4.2–4.92) |
| CD4 cell count (cells/mm3)a | 373 (259–444) | 388 (278–435) | 274 (253–466) | 375 (323–564) | 262 (185–289) | 330 (259–444) |
| No. (%) with prior antiretroviral use | 4 (36) | 8 (67) | 7 (88) | 4 (50) | 7 (70) | 30 (61) |
Median (values in parentheses are upper and lower boundaries of interquartile ranges).
Pharmacokinetics.
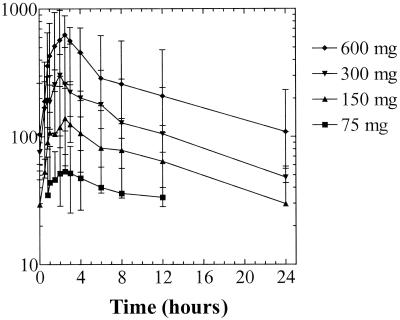
Median steady-state concentration-versus-time curves on day 15 are shown in Fig. 1. Median tenofovir pharmacokinetic parameters obtained on study days 1, 8, 15, and 35 are shown in Table 2. Median peak tenofovir concentrations (Cmax) were proportional to dose. The times required to reach maximum drug concentrations (Tmax) were similar for all doses in the fasted state (0.5 to 1.0 h) and were increased by 0.5 to 2.2 h when the drug was administered with food. In the 75- and 150-mg dose cohorts, many tenofovir concentrations during the pharmacokinetic sampling period were below the limit of quantitation of the bioanalytical assay, resulting in limited calculation of pharmacokinetic parameters (Cmax, Tmax) for these cohorts on some visits.
FIG. 1.
Steady-state concentrations of tenofovir in serum after 7 days of daily oral dosing of HIV-1-infected subjects with tenofovir DF. Data are the median and range for the evaluable (eight or nine) subjects at each dose level.
TABLE 2.
Median tenofovir pharmacokinetic parameters on study days 1, 8, 15, and 35
| Parametera and day | Value achieved with tenofovir DF dose of:
|
|||
|---|---|---|---|---|
| 75 mg | 150 mg | 300 mg | 600 mg | |
| AUC (ng · h/ml) | ||||
| 1 | —b | — | 2,093 | 3,372 |
| 8 | — | — | 3,179 | 4,389c |
| 15 | 717 | 1,613 | 2,937 | 6,073d |
| 35 | NDe | ND | 3,020 | 5,131 |
| Cmax (ng/ml) | ||||
| 1 | 68.6 | 111 | 240 | 618 |
| 8 | 62.9 | 130 | 375 | 573 |
| 15 | 80.8 | 163c | 303 | 633 |
| 35 | ND | ND | 326 | 498 |
| Tmax (h) | ||||
| 1 | 0.80 | 1.00 | 0.80 | 1.00 |
| 8 | 2.00c | 2.00 | 2.00 | 1.50c |
| 15 | 2.00 | 2.50 | 3.00 | 2.50d |
| 35 | ND | ND | 2.30 | 2.00 |
| t1/2 (h) | ||||
| 1 | — | — | 11.9 | 13.0 |
| 8 | — | — | 11.7 | 11.9 |
| 15 | — | 12.0 | 13.7 | 12.1 |
| 35 | ND | ND | 14.4 | 15.4 |
| CL/F (ml/h/kg) | ||||
| 1 | — | — | 910 | 1,083 |
| 8 | — | — | 584 | 671c |
| 15 | 354 | 555 | 521 | 683d |
| 35 | ND | ND | 510 | 641 |
| CLrenal/F (ml/h/kg) | ||||
| 1 | — | — | 203 | — |
| 8 | — | — | 169 | 132 |
| 15 | — | 160 | 161 | 93 |
| 35 | ND | ND | NA | 95 |
| CLcr (ml/h/kg) | ||||
| 1 | 87 | 88 | 88 | 94 |
| 8 | 79 | 91 | 88 | 93 |
| 15 | 85 | 92 | 94 | 82 |
| 35 | ND | ND | 84 | 92 |
The AUC is shown for days 1 and 8 (AUC0–α for day 35); t1/2, half-life of terminal elimination phase; CL/F, apparent oral clearance; CLrenal/F, apparent renal clearance; CLcr, calculated creatinine clearance based on ideal body weight.
—, not reported due to insufficient data.
Fed administration (day 8) was significantly different from fasted administration (day 1) (P<0.05).
Steady-state pharmacokinetics (day 15 or 35) were significantly different from first-dose pharmacokinetics (day 8) (P < 0.05).
ND, not determined on this visit.
Median steady-state pharmacokinetic parameters evaluated on day 15 (and day 35 where applicable) were dose linear across all dose groups. There were no changes in pharmacokinetic parameters over time as assessed by comparison of total drug exposure following the first dose versus at steady state. The total tenofovir exposure over the dosing interval at steady state for the 300- and 600-mg doses was similar to the total tenofovir exposure following the first dose, indicating no unexpected drug accumulation. For the 300- and 600-mg dose cohorts, following the achievement of Cmax, tenofovir concentrations in serum declined in a biphasic manner with terminal half-lives between 12 and 15 h, regardless of the feeding state or pharmacokinetic sampling period. The apparent serum and renal clearances of tenofovir exceeded the calculated creatinine clearance, indicating active tubular secretion of tenofovir by the kidneys.
Oral bioavailability of tenofovir was estimated by using historical data obtained in an evaluation of a 1-mg/kg intravenous dose (3). Oral bioavailability was enhanced by administration with a high-fat meal. The oral bioavailability of the 300- and 600-mg doses of tenofovir DF were estimated to be 25 and 21%, respectively, in the fasted state and 39 and 34%, respectively, in the fed state. The median steady-state levels of tenofovir in serum after administration of eight consecutive doses of 150, 300, and 600 mg of tenofovir DF were 35, 63, and 131%, respectively, of that measured following the administration of a 1-mg/kg intravenous tenofovir dose. Calculated creatinine clearance was not affected by repeated administration of tenofovir DF at any dose level.
Antiviral activity.
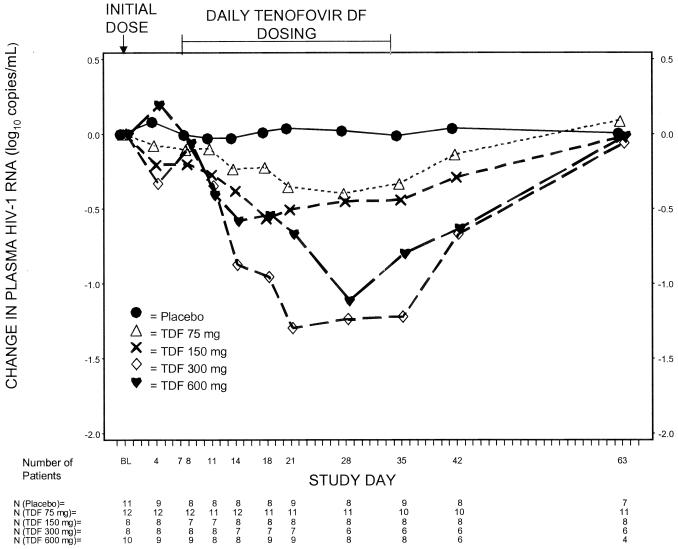
The plasma HIV-1 RNA responses in the subjects in the four tenofovir DF arms and the combined placebo arm are shown in Fig. 2. After administration of a single oral dose of tenofovir DF, median decreases in HIV-1 RNA levels in plasma at day 4 were seen in the 150-mg dose group (−0.20 log10 copies/ml) and in the 300-mg dose group (−0.33 log10 copies/ml). Compared to the placebo group, statistically significant median changes were seen for all tenofovir dose groups at day 35: −0.33 log10 copies/ml (P = 0.003) for the 75-mg dose group, −0.44 log10 copies/ml (P = 0.0002) for the 150-mg dose group, −1.22 log10 copies/ml (P = 0.0004) for the 300-mg dose group, and −0.80 log10 copies/ml (P = 0.0002) for the 600-mg dose group. The median decrease in log10 HIV-1 RNA after 28 days of dosing was greater for subjects in the 300-mg dose group than for those the 150-mg dose group (P = 0.03) and those in the 75-mg dose group (P = 0.0005) but not statistically significantly different for those in the 600-mg dose group.
FIG. 2.
Median changes in HIV-1 RNA in plasma from the baseline among placebo-treated subjects and the three tenofovir DF (TDF)-treated dose groups of patients (as-treated analysis).
The 300-mg dose group contained eight patients, of whom four had had no previous treatment. The median decrease in log10 HIV-1 RNA after 28 days of dosing in the untreated patients was −1.57 log10 copies/ml, while the median decrease in the previously treated patients was −0.97 log10 copies/ml.
Although all of the groups experienced increases in CD4 counts that ranged from 17 to 64 cells/mm3 in the 300- and 600-mg dose group, respectively, none of these changes were significant.
Genotypic analyses.
Baseline and day 35 RT sequences (amino acids 1 to 300) of HIV-1 from the plasma of all tenofovir-treated subjects in the 75-, 150-, 300-, and 600-mg dosing cohorts were analyzed. No subject developed detectable RT sequence changes during the 4-week dosing period. A total of nine subjects had HIV-1 expressing nucleoside-associated RT mutations at baseline (Table 3). The 3TC-associated M184V mutation was identified in the baseline plasma HIV-1 from eight subjects (75 mg, one subject; 150 mg, five subjects; 600 mg, two subjects) treated with tenofovir. By day 35, this mutation was no longer detectable in four of the eight subjects, consistent with previous reports of impaired fitness of M184V-expressing HIV-1. Three subjects expressed 3TC-thymidine analog-associated resistance mutations at baseline, either as mixtures or as full mutants (75 mg, one subject; 600 mg, two subjects; Table 3). These mutations were maintained through day 35 in most subjects; one subject (subject I) demonstrated the loss of an unusual T69A mutation but maintained the M41L and T215Y mutations as mixtures of the mutant and the wild type. Sequence data from patients with detectable mutations have been deposited with GenBank (accession numbers AF375228 to AF375241).
TABLE 3.
Genotypic analysis of RT of tenofovir DF-treated subjects with baseline nucleoside-associated RT mutations
| Tenofovir DF dose (mg) and subject code | Prior antiretroviral therapya | Nucleoside-associated RT mutation(s)b
|
|
|---|---|---|---|
| Day 0 | Day 35 | ||
| 75 | |||
| A | AZT, 3TC, d4T, ddI, IDV | M41L, T215Y | M41L, T215Y |
| B | AZT, 3TC | M184V | M184V |
| 150 | |||
| C | AZT, 3TC | M184V | None |
| D | AZT, 3TC, d4T, IDV | M184V/M | None |
| E | AZT, 3TC, d4T | M184V | M184V |
| F | AZT, 3TC, SQV, IDV | M184V/M | M184V |
| G | AZT, 3TC, d4T, SQV | M184V | M184V |
| 600 | |||
| H | AZT, 3TC, d4T, ddC | M41L/M, M184V/M, T215Y | M41L, T215Y |
| I | AZT, 3TC, d4T, 3TC, ABC, SQV, NFV, RTV | M41L/M, T69A/T, M184V/M, T215Y/T | M41L/M, T215Y/T |
ddI, didanosine; ddC, zalcitabine; ABC, abacavir; IDV, indinavir; SQV, saquinavir, NFV, nelfinavir; RTV, ritonavir.
Nucleoside-associated RT codons are M41, K65, D67, T69, K70, L74, V75, Q151, M184, L210, T215, and K219.
Safety and tolerance.
Forty-nine subjects entered the study; 41 subjects completed the study. Four subjects discontinued the study drug due to laboratory abnormalities according to protocol: 1 in the placebo group, 1 in the 75-mg group, and 2 in the 300-mg group. One additional subject in the placebo group was removed from the study after administration of a single dose due to high amylase and lipase levels at baseline. Other reasons for removal were patient request and noncompliance (two patients in the placebo group and two in the 600-mg dose group).
Adverse events and laboratory abnormalities of severe or life-threatening severity (grade III or IV) judged to be possibly or probably related to the study drug are shown in Table 4. A total of six subjects had grade III or greater elevations in serum creatine kinase (CK) levels (i.e., CK levels greater than four times the upper limit of normal) during the study; five of these subjects received tenofovir, and one subject was in the placebo group (Table 4). In one subject, CK elevation resolved despite continuation of the active study drug (75-mg group). In four of the other five tenofovir-treated subjects with CK elevation, the CK rise was associated with recent exercise. In two of these four subjects, elevations of serum CK recurred upon repetition of similar exercise 2 to 3 weeks after discontinuation of the study drug. In the remaining subject, CK elevation was associated with cocaine use and alcohol intoxication. Only mild symptoms of fatigue were reported at the time of the CK elevations. There were no sequelae, and no dose relationship was apparent.
TABLE 4.
Grade III or IV adverse events (severe or life threatening) possibly or probably related to the study drug
| Group | No. of subjects who received:
|
Total no. of subjects | ||||
|---|---|---|---|---|---|---|
| Placebo | Tenofovir DF dose (mg) of:
|
|||||
| 75 | 150 | 300 | 600 | |||
| All subjects | 11 | 12 | 8 | 8 | 10 | 49 |
| Subjects with an event | 1 | 1 | 0 | 4 | 0 | 6 |
| Subjects with peripheral neuropathy | 1 | 0 | 0 | 1 | 0 | 2 |
| Subjects with CK elevation | 1 | 1 | 0 | 4 | 0 | 6 |
| Subjects with AST and/or ALT elevation | 0 | 1 | 0 | 1 | 0 | 2 |
Three of the five subjects with grade III or IV elevations in CK had concurrent elevations in aspartate aminotransferase (AST) or alanine aminotransferase (ALT) (to greater than five times the upper limit of normal). Two of these episodes were judged possibly or probably related to the study drug. In each, the timing of the rise and fall of AST and ALT was similar to that of serum CK levels, suggestive of a skeletal muscle rather than a hepatic etiology.
Two subjects experienced severe peripheral neuropathy that occurred when they were off the study drug. One subject in the placebo group had a prior history of peripheral neuropathy and was one of the subjects who experienced a grade IV CK elevation. The other subject, who also had a history of peripheral neuropathy, was in the 300-mg tenofovir DF dose group. Because the recurrence was consistent with the subject's “flares” in the past, his physician considered the event to be possibly related to the study drug.
One subject in the 300-mg group had an isolated rise in serum creatinine (1.7 mg/dl) on day 35. Screening and baseline creatinine values were 0.9 and 1.2 mg/dl, respectively. On the following day, the level was 0.9 mg/dl. All subsequent serum creatinine values were normal in this subject, and there was no proteinuria. No subject in the study had grade III or greater (i.e., >3+) proteinuria.
DISCUSSION
Oral tenofovir DF monotherapy resulted in a dose-related effect on the HIV-1 load up to 300 mg, produced predictable and dose-proportional serum tenofovir pharmacokinetics, and was well tolerated when administered once daily over 28 consecutive days to HIV-1-infected subjects who had never before been treated with an antiretroviral drug and to previously treated HIV-1-infected subjects. Although the values were small, responses appeared to be more pronounced in patients never before exposed to antiretroviral therapy.
Previously, clinical evaluation of intravenous tenofovir monotherapy of HIV-1-infected subjects demonstrated a dose-dependent and statistically significant antiviral effect (3), corroborating data derived from studies of monotherapy with tenofovir in SIV-infected macaques (12, 13). In the current study, administration of a single dose of oral tenofovir DF was associated with a statistically significant decrease in HIV-1 RNA levels in plasma compared to that achieved with a placebo. Following 28 days of dosing, administration of tenofovir DF once daily at all of the doses studied resulted in statistically significant decreases in HIV-1 RNA levels in plasma, with the greatest effect achieved with the 300-mg dose. There was no increased effect on changes in HIV-1 RNA levels in plasma from the baseline to day 35 between the 300- and 600-mg dose cohorts (P = 0.28), despite dose-proportional increases in drug exposure (P = 0.02). This may be an indication that the 300-mg dose produces the maximum antiviral effect. Consistent with these data, the maximum antiviral effect seen after administration of seven multiple intravenous doses of tenofovir at 3 mg/kg (AUC from time zero to infinity [AUC0–∞], 16.6 ± 6.05 μg · h/ml) was a median log10 change in HIV-1 RNA levels in plasma from a baseline of −1.1 copies/ml (3).
A maximum tolerated dose was not established in this study. The most frequently reported grade III or more severe adverse event, elevation of CK levels in serum, produced minimal symptoms, was usually associated temporally with exercise, was unrelated to dose, and resolved promptly.
Resistance to tenofovir has been difficult to generate in vitro, but a K65R RT mutation emerges in a fraction of HIV-1 clones after in vitro passage in the presence of tenofovir. A recombinant virus carrying the selected K65R mutation is associated with only a modest three- to fourfold decrease in susceptibility (14). As described in this report, no genotypic changes in the first 300 amino acids of the RT gene were detected following 4 weeks of daily dosing in any of the cohorts. Among the four cohorts, only 9 (24%) of 38 subjects treated with tenofovir DF had detectable nucleoside-associated RT mutations at baseline and, with the exception of the 3TC-associated M184V mutation, these mutations were generally stable through day 35. The small numbers of patients distributed across multiple dosing regimens in this study do not allow conclusions about whether baseline resistance mutations affect the response to tenofovir DF therapy. Additional larger clinical studies of patient populations with baseline resistance are required to address this issue.
In summary, oral tenofovir DF exhibits a pharmacokinetic profile that supports once-daily dosing. Given these encouraging results regarding the antiviral potency and tolerability of the agent, future clinical trials of tenofovir DF will seek to identify a safe and effective dose that can be administered over longer periods of time as a component of combination antiretroviral regimens for the treatment of HIV-1 infection.
ACKNOWLEDGMENTS
We acknowledge the scientific contributions of Julie Cherrington, Stanley C. Gill, Kenneth Cundy, and John Su. We are grateful to Ella Redpath (The Johns Hopkins University), Charles Cooper (Seattle, Wash.), Anna J. Smith, Curtis Head, and Vittorio Marchesin (San Francisco, Calif.) for assistance with this study.
Financial support for this study was provided by Gilead Sciences, Inc., and National Institutes of Health Division of Research Resources General Clinical Research Centers grants M01-RR-00052, M01-RR-00083, and M01-RR-00037 to The Johns Hopkins University School of Medicine, Baltimore, Md. San Francisco General Hospital, San Francisco, Calif., and the University of Washington, Seattle, respectively. James Kahn acknowledges support from the NIH (PM30MH5907).
REFERENCES
- 1.Anonymous. Division of AIDS table for grading severity of adverse experiences. Rockville, Md: National Institute of Allergy and Infectious Diseases; 1996. [Google Scholar]
- 2.Arimilli M, Kim C, Bischofberger N, Dougherty J, Mulato A, Oliyai R, Shaw J P, Cundy K C, Bischofberger N. Synthesis, in vitro biological evaluation and oral bioavailability of prodrugs of the antiretroviral agent 9-[2-(phosphonomethoxy)-propyl]adenine (PMA) Antiviral Chem Chemother. 1997;8:557–564. [Google Scholar]
- 3.Deeks S G, Barditch-Crovo P, Lietman P S, Hwang F, Cundy K C, Rooney J F, Hellmann N S, Safrin S, Kahn J O. The safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob Agents Chemother. 1998;42:2380–2384. doi: 10.1128/aac.42.9.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeks S G, Collier A, Lalezari J, Pavia A, Rodrigue D, Drew W L, Toole J, Jaffe H, Mulato A S, Lamy P D, Li W, Cherrington J M, Hillmann N, Kahn J. The safety and efficacy of adefovir dipivoxil, a novel anti-HIV therapy, in HIV infected adults. J Infect Dis. 1997;176:1517–1523. doi: 10.1086/514150. [DOI] [PubMed] [Google Scholar]
- 5.Miller M D, Anton K E, Mulato A S, Lamy P D, Cherrington J M. Human immunodeficiency virus type 1 expressing the lamivudine-associated M184V mutation in reverse transcriptase shows increased susceptibility to adefovir and decreased replication capacity in vitro. J Infect Dis. 1999;179:92–100. doi: 10.1086/314560. [DOI] [PubMed] [Google Scholar]
- 6.Mulato A S, Cherrington J M. Anti-HIV activity of adefovir (PMEA) and PMPA in combination with antiretroviral compounds: in vitro analyses. Antiviral Res. 1997;36:91–97. doi: 10.1016/s0166-3542(97)00043-0. [DOI] [PubMed] [Google Scholar]
- 7.Robbins B L, Srinivas R V, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother. 1998;42:612–617. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw J-P, Sueoka C M, Oliyai R, Lee W A, Arimilli M, Kim C, Cundy K C. Metabolism and pharmacokinetics of novel oral prodrugs of 9-[(R)-2-(phosphonomethoxy)-propyl]adenine (PMPA) in dogs. Pharm Res. 1997;14:1824–1829. doi: 10.1023/a:1012108719462. [DOI] [PubMed] [Google Scholar]
- 9.Srinivas R V, Fridland A. Antiviral activities of 9-R-2-phosphonomethoxypropyl adenine (PMPA) and bis(isopropyloxymethylcarbonyl)PMPA against various drug-resistant human immunodeficiency virus strains. Antimicrob Agents Chemother. 1998;42:1484–1487. doi: 10.1128/aac.42.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai C-C, Emau P, Follis K E, Beck T W, Benveniste R E, Bishofberger N, Lifson J D, Morton W R. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai C-C, Follis K, Sabo K A, Beck T W, Grant R F, Bischofberger N, Benveniste R E, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl) adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 12.Tsai C-C, Follis K, Beck T W, Sabo A, Bischofberger N, Dailey P I. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy on chronic SIV infection in macaques. AIDS Res Hum Retrovir. 1997;13:707–712. doi: 10.1089/aid.1997.13.707. [DOI] [PubMed] [Google Scholar]
- 13.Van Rompay K K A, Cherrington J M, Marthas M L, Berardi C J, Mulato A S, Spinner A, Tarara R P, Canfield D R, Telm S, Bishofberger N, Pedersen N C. 9-[2-(phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob Agents Chemother. 1996;40:2586–2591. doi: 10.1128/aac.40.11.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainberg M A, Miller M D, Quan Y, Salomon H, Mulato A S, Lamy P D, Margot N A, Anton K E, Cherrington J M. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antiviral Ther. 1999;4:87–94. doi: 10.1177/135965359900400205. [DOI] [PubMed] [Google Scholar]