Abstract
The antiviral efficacy of orally administered adefovir dipivoxil was evaluated in an 18-week study (12 weeks of treatment and 6 weeks of recovery) conducted with woodchucks chronically infected with woodchuck hepatitis virus (WHV). Adefovir dipivoxil is a prodrug of adefovir designed to enhance its oral bioavailability. Following administration of 15 mg of adefovir dipivoxil per kg of body weight in four WHV-infected animals, the mean maximum concentration of adefovir in serum was 0.462 μg/ml, with an elimination half-life of 10.2 h, and the oral bioavailability of adefovir was estimated to be 22.9% (±11.2%). To study antiviral efficacy, the animals were divided into three groups. There were six animals each in a high-dose group (15 mg/kg/day) and a low-dose group (5 mg/kg/day). A vehicle control group consisted of five animals because WHV DNA was detectable only by PCR at the time of the study in one of the original six animals. Efficacy was evaluated by determining the levels of WHV DNA in serum. The geometric mean WHV DNA level for the high-dose group diminished by >40-fold (>1.6 log10) after 2 weeks of treatment and >300-fold (>2.5 log10) at 12 weeks. There was a >10-fold reduction in five of six low-dose animals by 2 weeks, but levels were unchanged in one animal. By 12 weeks of treatment there was a >45-fold (>1.6 log10) reduction of WHV DNA levels, and serum WHV DNA levels were below the limit of quantification in three of six animals. Viral DNA levels returned to pretreatment levels during the 6-week recovery period. There were no clinically significant changes in body weight, hematology, or serum chemistry values, including bicarbonate or lactate, in any of the treated animals. No histologic evidence of liver injury was apparent in the biopsies. Under the conditions of this study, adefovir dipivoxil was an effective antihepadnaviral agent.
Chronic hepatitis B virus (HBV) infection is a serious threat to the health and well-being of more than 350 million people, approximately 5% of the world's population, because of the associated risk of significant liver disease, including cirrhosis and hepatocellular carcinoma (15, 29). There are few treatment options available for people who are currently infected and therefore would not benefit from the effective vaccine that is available.
Current treatment options include two major groups, modifiers of the immune response and inhibitors of viral replication. Alfa interferon is approved for clinical use, but more than two-thirds of patients treated with alfa interferon do not respond (14). Broad application of interferon therapy is limited by a variety of interferon-associated side effects, a treatment regimen that involves repeated parenteral injection, and restriction to patients with compensated liver disease.
Effective antiviral chemotherapeutic agents, most often nucleoside analogues, that target hepadnaviral replication are being developed in order to provide suitable treatment for infected individuals (17, 18, 21, 28). Testing drugs in development is facilitated by the availability of suitable animal models with natural hepadnaviral infections. In vitro and in vivo studies in ducks infected with duck HBV and woodchucks infected with woodchuck hepatitis virus (WHV) have provided valuable information on the efficacy and toxicity of novel antiviral drugs (5, 6, 18, 19, 24, 31). In vivo testing is advantageous because pharmacokinetics and toxicity can be assessed in addition to antiviral efficacy. Woodchucks are a particularly suitable model for anti-HBV studies because the natural history of liver disease during chronic infection is similar to that in humans (26). The sensitivities of WHV and HBV reverse transcriptase to nucleoside analogues are quite similar (12). In addition, the woodchuck model also displayed the lethal hepatotoxicity observed with the 2′-fluorinated nucleoside analog fialuridine (31).
Adefovir (9-[2-phosphonylmethoxyethyl]adenine) is an acyclic nucleotide analogue of AMP that has been shown to have broad-spectrum activity against a variety of viruses in vitro (1) and in vivo (2). Adefovir dipivoxil, the bis(pivaloyloxy-methyl) ester of adefovir, is an oral prodrug of adefovir. It has been effective in animal models and in clinical trials against human immunodeficiency virus (3, 11) and HBV (13). In this report we present results of an 18-week study of adefovir dipivoxil treatment in chronically WHV-infected woodchucks to assess its pharmacokinetics, safety, and antiviral efficacy in reducing serum WHV DNA levels.
MATERIALS AND METHODS
Pharmacokinetics.
For pharmacokinetic analysis, four (two male and two female) WHV carrier woodchucks (HC307, HC297, HC298, and HC302) were sedated with 1 ml of acepromazine maleate (10 mg/ml) intramuscularly and orally dosed with adefovir dipivoxil (15 mg/kg) in grape juice. Blood was collected at 0, 0.25, 0.5, 1, 2, 4, 8, and 24 h postadministration. Each animal was given subcutaneous fluids to maintain fluid balance during the study period. Blood was collected in heparinized tubes and placed on ice. Plasma was separated by centrifugation within 30 min and frozen at −70°C until analysis. Plasma was analyzed by reverse-phase ion-pair high-pressure liquid chromatography with fluorescence derivatization (27). Pharmacokinetic analysis was performed using noncompartmental methods.
Drug preparation and dosing.
Adefovir dipivoxil was supplied in tablet form by Gilead Sciences (Foster City, Calif.). The tablets were ground into a fine powder, and the weighed drug was mixed into a suspension with 3 ml of diluted grape juice concentrate. Animal doses were prepared daily.
Animals were dosed daily. High-dose animals received 15 mg of adefovir dipivoxil/kg of body weight, low-dose animals received 5 mg of adefovir dipivoxil/kg, and controls received diluted grape juice. Each animal was dosed orally using a feeding tube. Animals were observed to determine that there was complete consumption of the dose.
Animals.
All animals were acclimated and housed in the Laboratory Animal Resources Facility of the College of Veterinary Medicine at North Carolina State University (NCSU). The animals were allowed access to water ad libitum and were fed Agway (Richmond, Ind.) rabbit pellets (5p26) and Agway monkey chow (5049) ad libitum. A photoperiod of 12 h of light and 12 h of dark was maintained. All procedures were performed as described in the currently approved Institutional Animal Care and Use Committee protocol.
Twelve wild-caught naturally infected WHV carrier woodchucks were purchased from Northeastern Wildlife (S. Plymouth, N.Y.). Six WHV carrier animals from the NCSU woodchuck colony were also used. Four of these animals were wild caught and two were captive bred. The woodchucks were divided into three groups of six, but one control animal was removed from the study because its WHV DNA level was below the limit of detection by the hybridization assay. All animals appeared normal by physical examination and complete blood counts. Five animals had minor serum chemistry abnormalities. One animal (HC212) had a mildly elevated blood urea nitrogen (BUN) level of 43 mg/dl and was assigned to the control group. Serum gamma glutamyltranspeptidase levels, a measure of the presence of liver neoplasia, were less than 10 IU/liter in all of the high-dose animals and control animals. In the low-dose group, four animals (HC305, HC199, HC247, and HC197) had gamma glutamyltranspeptidase levels that ranged from 14 to 41 IU/liter, indicative of early hepatocellular neoplasia. None of the animals exhibited physical signs or hematological abnormalities indicating any debility from tumor development during the course of the study.
Liver biopsies.
Wedge liver biopsy samples were taken from all high-dose animals and three control animals by laparotomy several days before the drug trial started and on the last day of the recovery period. The surgery was performed in the Central Procedures Laboratory of the College of Veterinary Medicine at NCSU. Animals were presedated with 0.2 ml of Innovar-Vet (fentanyl, 0.4 mg/ml; droperidol, 20 mg/ml; Pittman-Moore, Mundelein, Ill.) and anesthesia was maintained by isoflurane inhalation. A total dose of oxymorphone (0.02 mg/kg intramuscularly; Mallinckrodt, Mundelein, Ill.) was administered for control of postoperative pain. Each animal was given subcutaneous fluids to maintain fluid balance during the surgery and recovery periods. The liver sample was placed in 10% neutral buffered formalin for histological examination.
Histopathology.
Fixed liver was processed routinely into paraffin. Embedded liver was sectioned at a 6-μm thickness and stained with hematoxylin and eosin. Liver injury was evaluated on a subjective scale that has been described previously (22). Inflammation was the major determinant, with other factors such as necrosis, vacuolization, biliary hyperplasia, and variation in nuclear size influencing the degree of injury.
Blood samples.
Animals were sedated with 0.2 ml of ketamine (100 mg/ml; Fort Dodge Laboratories, Fort Dodge, Iowa) and 0.5 ml of acepromazine maleate (10 mg/ml) intramuscularly for blood collection. Blood samples were collected 6 weeks prior to the study (2 weeks prior to the study for the three control animals and the three low-dose animals transferred from the colony). Blood samples were also collected at day 0 and weeks 2, 4, 12, and 18. Serum chemistry was determined by an automatic analyzer (Monarch, Lexington, Mass.) maintained by the Clinical Pathology Laboratory of the College of Veterinary Medicine at NCSU. A panel of enzyme and biochemical parameters was run in order to monitor organ function and to screen for toxic effects of the test compound. The liver-related enzymes sorbitol dehydrogenase and alanine aminotransferase were monitored. Venous blood samples for analysis of bicarbonate levels were collected into heparin-containing tubes, placed immediately on ice, and analyzed on a blood gas instrument (Gempremier Plus; Instrument Laboratory, Lexington, Mass.). Samples for blood lactate levels were collected in sodium fluoride-containing tubes and placed on wet ice immediately. Samples were then centrifuged and plasma was removed within 15 min of collection. Also, whole blood was collected into EDTA-containing tubes and submitted for complete blood counts. Complete blood counts were performed on an automatic analyzer (Serono Baker, Allentown, Pa.), in the Clinical Pathology Laboratory of the College of Veterinary Medicine at NCSU.
Analysis of variance with Dunnett's comparisons was performed to test for significant differences between adefovir dipivoxil-treated groups and controls for each parameter at each time point. When the data were found to deviate from normality assumptions, the ranks of the data rather than the values themselves were analyzed. P values less than or equal to 0.05 were considered statistically significant.
Slot blot analysis of serum DNA.
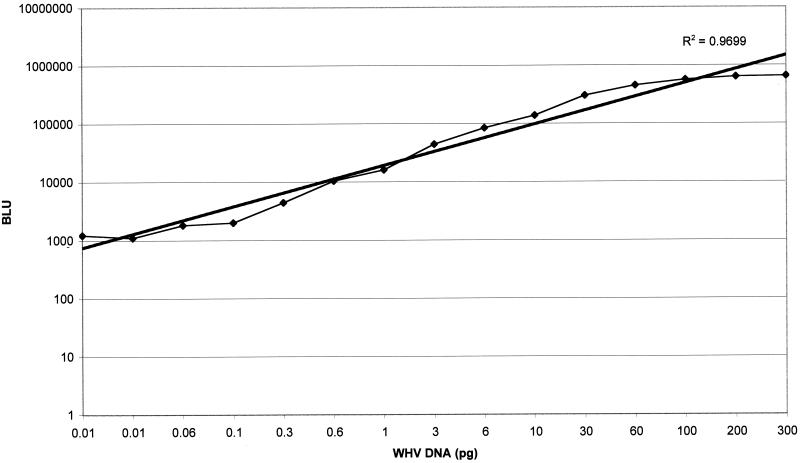
For analysis of WHV DNA, whole blood was collected into glass tubes without anticoagulant and allowed to clot at room temperature. Serum was collected and frozen at −70°C until analysis. WHV DNA was extracted from 200 μl of serum and eluted into 200 μl of Tris buffer (10 mM, pH 8.5) using a QIAamp blood kit (Qiagen, Valencia, Calif.). The probe was an EcoRI-digested WHV2 genome (3.32 kb) labeled with digoxigenin-II-dUTP (DIG High Prime DNA labeling detection starter kit II; Boehringer Mannheim, Indianapolis, Ind.). The WHV DNA standard used as a control was whole WHV2 genomic DNA linearized with EcoRI. Ten microliters of extracted DNA solution was denatured with 30 μl of denaturing buffer at 65°C for 40 min and was loaded on each slot. Denatured DNA was fixed to a nylon filter (Nytran; Schleicher & Schuell, Keene, N.H.) by a UV cross-linking method. The filter and probe were incubated together, and after washing, the membranes were immediately scanned using the Lumi-Imager (Boehringer Mannheim) to generate a numerical value for the strength of the hybridization signal. To establish the linear range for the analysis, a standard curve relating the unit of measurement (Boehringer light units) versus DNA values ranging from 0.01 to 300 pg of WHV DNA was generated (Fig. 1). The analysis was linear over the range of input WHV DNA with an R2 of 0.97. The lowest level of detection of the assay was determined to be 0.06 pg of WHV DNA by review of background versus DNA standard dilutions. The estimated sensitivity of this assay was 6 pg of WHV DNA/ml, or 2 × 106 genome equivalents/ml.
FIG. 1.
Regression analysis of the relationship of WHV DNA levels to detection units. BLU, Boehringer light units.
For analysis, all samples were run in triplicate and the geometric mean and standard deviation were established. Extracted negative and positive sera and several dilutions of standard DNA (0.01, 0.03, 0.06, and 0.1 ng of DNA) were included on each membrane. The standard curve was established using the analysis program provided by Boehringer Mannheim. The prestudy and time zero values were used to get a baseline geometric mean for each animal. The log fold reduction at each time point was calculated as the baseline value minus the value at that time point. During the course of treatment, the serum WHV DNA level for each animal was compared to the pretreatment level to determine the fold reduction of viral DNA levels. The geometric mean of the log fold reduction values was calculated for each group at each time point. Geometric mean fold reduction (or increase) was calculated as 10log fold reduction where log fold reduction is >0 and −1 · (10−log fold reduction) where log fold reduction is <0.
A mean reduction for each group was determined for each time point as the mean of the individual animal values. Analysis of variance was performed to compare the log fold reduction values among the groups. Pairwise contrasts were also performed to test for differences between controls and each of the adefovir dipivoxil groups. Any P value less than or equal to 0.05 was reported as significant.
RESULTS
Pharmacokinetics.
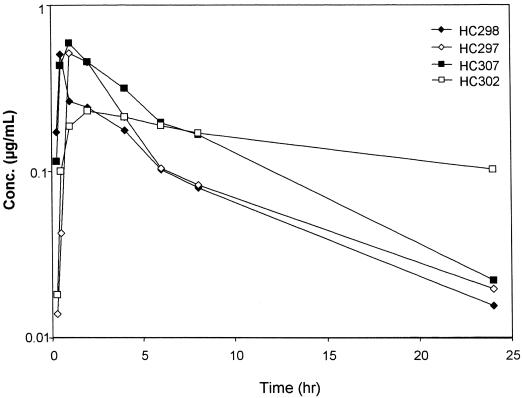
Following oral administration of adefovir dipivoxil, the mean maximum concentration of adefovir in serum was 0.462 μg/ml, with a mean apparent half-life of 10.2 h (ranging from 5.6 to 20.9 h) (Fig. 2). The oral bioavailability of adefovir in woodchucks was estimated to be 22.9% (±11.2%). In the absence of any evidence for species-specific differences in drug metabolism, an area under the concentration-time curve for intravenous administration of 17.6 μg · h/ml was obtained using allometric scaling of plasma clearance of adefovir versus body weight.
FIG. 2.
Plasma concentration-versus-time profiles for adefovir in individual animals following oral administration of 15 mg of adefovir dipivoxil/kg to WHV-infected woodchucks.
Serum WHV DNA.
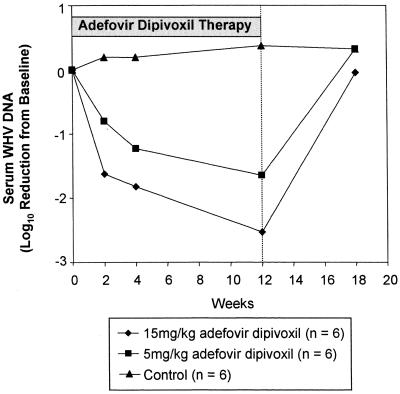
Results from the analysis of serum WHV DNA for all groups are summarized in Fig. 3. The fold reductions are shown in Table 1. There was a marked (>1.6 log10) diminution in serum WHV DNA levels by the second week of treatment compared to the average pretreatment levels in all high-dose animals. At 2 weeks, a >10-fold reduction occurred in five of six low-dose animals, but no reduction was seen in one of the animals. Viral levels were reduced progressively during the 12-week course of therapy in all animals. In the high-dose group, the WHV DNA levels were reduced >65-fold (>1.8 log10) at 4 weeks and >300-fold (>2.5 log10) after 12 weeks of treatment. Two animals (HC307 and HC299) had reductions to the lower limits of detection (0.06 pg of WHV DNA). In the low-dose woodchucks, WHV DNA levels were reduced by >6-fold at 2 weeks and >45-fold (>1.6 log10) after 12 weeks of treatment. Three animals (HC199, HC247, and HC197) had reductions to the lower limit of detection. WHV viral DNA levels returned to pretreatment levels within 6 weeks after drug administration stopped. In the low-dose group, the serum WHV DNA level in one animal (HC305) did not appear to be reduced at 2 weeks. Review of this sample suggested that there had been evaporative loss during storage that may have concentrated the viral DNA, producing an erroneous elevation in DNA level. There was a minimal 2.4-fold (0.38 log10) rise in serum WHV DNA levels in the control animals during the course of treatment, but this was within the expected range of normal variation.
FIG. 3.
Anti-WHV activity of adefovir dipivoxil administered orally to WHV-infected woodchucks for 12 weeks.
TABLE 1.
WHV DNA reduction versus baseline
| Adefovir dipivoxil dosage (mg/kg/day) and animala | Baseline DNA | Fold reduction in DNAb
|
|||
|---|---|---|---|---|---|
| Wk 2 | Wk 4 | Wk 12 | Wk 18 | ||
| 15 mg/kg/day | |||||
| HC297 | 83.3 | 5.1 | 416.7 | 39.7 | −2.7 |
| HC298 | 75.5 | 15.4 | 8.5 | 228.7 | −2.0 |
| HC299 | 135.7 | 393.2 | 33.4 | >2,228.1 | 1.2 |
| HC302 | 165.3 | 118.1 | 4.2 | 570.0 | 1.5 |
| HC303 | 101.1 | 16.6 | 51.0 | 40.4 | 2.6 |
| HC307 | 195.6 | 93.1 | >3,259.5 | >3,259.5 | 2.0 |
| Mean | 118.3 | 42.1cd | >66.0c | >339.0c | 1.1 |
| SE | 18.4 | 27.8 | >67.0 | >264.2 | 0.3 |
| 5 mg/kg/day | |||||
| HC197 | 15.1 | 7.2 | 50.2 | 251.0 | −1.8 |
| HC199 | 34.0 | 17.0 | 20.0 | >566.0 | −3.0 |
| HC247 | 22.8 | 67.2 | >380.6 | >380.6 | −6.5 |
| HC304 | 41.8 | 5.0 | 4.3 | 43.1 | −1.4 |
| HC305 | 56.7 | −1.7 | 2.0 | 2.1 | −1.9 |
| HC312 | 141.2 | 2.5 | 7.1 | 1.7 | 1.0 |
| Mean | 39.7 | 6.3e | >16.9e | >45.0c | −2.1 |
| SE | 12.6 | 4.1 | >13.1 | >48.0 | 0.1 |
| Control | |||||
| HC212 | 11.7 | −1.6 | −2.1 | −9.4 | −9.3 |
| HC268 | 22.4 | −2.1 | −2.1 | −2.5 | −3.8 |
| HC271 | 8.6 | −3.0 | −4.2 | −2.1 | −1.1 |
| HC296 | 34.6 | −1.1 | −1.5 | −1.6 | −1.6 |
| HC309 | 44.8 | 1.0 | 2.2 | −1.1 | 1.6 |
| Mean | 20.3 | −1.6 | −1.6 | −2.4 | −2.1 |
| SE | 6.4 | 0.1 | 0.2 | 0.2 | 0.2 |
Means are geometric means; SE, geometric standard error estimate.
Analysis of variance with pairwise contrasts was performed to compare log fold reductions among groups. Groups were significantly different from the control if P was ≤0.05. Negative numbers indicate an increase.
Significantly different from control, P ≤ 0.01.
Significantly different from 5 mg/kg/day, 0.01 < P ≤ 0.05.
Significantly different from control, 0.01 < P ≤ 0.05.
Hematology and clinical chemistry.
There were no clinically relevant changes in the hematology or clinical chemistry of any the animals based on treatment. Statistical analysis revealed no consistent evidence of or trends in the alteration of organ function or bone marrow activity detected based on treatment. The blood lactate levels and bicarbonate levels were similar in treated and control animals.
One animal in the control group (HC212) had a moderate elevation in serum BUN levels (approximately 40 mg/dl) throughout the study and creatinine levels that increased slightly during the course of the study. There were no statistically significant differences in BUN levels between groups. Creatinine levels were lower in the high-dose animals in week 18, but the difference was not clinically relevant.
Physical observations.
All animals appeared to be in general good health and were bright, alert, and responsive during the study. Several high-dose (15 mg/kg) animals (HC298, HC299, and HC307) and two low-dose (5 mg/kg) animals (HC197 and HC247) developed focal cheilitis during the course of treatment. HC298 and HC197 developed lesions within 3 to 5 weeks, and HC299, HC307, and HC247 developed lesions within 8 to 9 weeks. The cheilitis was characterized by erythematous, erosive lesions on the lateral aspect of the mouth. Affected animals salivated excessively. The lesions resolved within several days once drug administration was discontinued. Animal body weights were not affected by drug treatment.
Liver histopathology.
Hematoxylin-and-eosin-stained liver biopsy samples from all six high-dose animals and three control animals (HC212, HC296, and HC306) collected at day 0 and week 18 were examined (Table 2). The initial liver samples of the high-dose (15-mg/kg/day) woodchucks were characterized by mild to moderate lymphoplasmacytic infiltrates of the portal areas and multiple small inflammatory foci within the parenchyma consistent with chronic WHV infection. Other features of the livers (i.e., vacuolization and necrosis) were within normal limits for chronically WHV-infected woodchucks. One high-dose (15-mg/kg) woodchuck (HC303) had evidence of infection with Taenia crassiceps characterized by cysticerci that were free in the peritoneal cavity and occasionally embedded in the hepatic parenchyma.
TABLE 2.
Liver biopsy histology for high-dose and control woodchucks prior to and 6 weeks following drug treatment
| Animal | Dose (mg/kg/day) | Injury score
|
|
|---|---|---|---|
| Pretreatment | Posttreatment | ||
| HC302 | 15 | 1 | 1 |
| HC298 | 15 | 2 | 2 |
| HC307 | 15 | 2 | 1 |
| HC297 | 15 | 1 | 1 |
| HC299 | 15 | 2 | 1 |
| HC303 | 15 | 2 | 1a |
| HC296 | Control | 1 | 1 |
| HC212 | Control | 3 | 1 |
This animal was infected with T. crassiceps cysticerci, and liver was evaluated at sites that were not apparently affected by the parasite.
The posttreatment biopsy samples were similar to the pretreatment samples except that the intensity of hepatic inflammation was diminished slightly in four of the high-dose animals (HC298, HC299, HC303, and HC307). There was no histologic evidence of toxic hepatic injury to the livers of any of the animals. One of the high-dose animals (HC303) had a moderate increase in macrovesicular and microvesicular vacuolization of hepatocytes that was attributed to a marked infection with cestode (T. crassiceps) cysticerci that filled the abdominal cavity and involved the liver parenchyma at the time of the second liver biopsy.
One of the control woodchucks (HC296) had mild inflammation at the first biopsy, and there was moderate to intense inflammation in a second animal (HC212). The posttreatment biopsy results for HC296 were the same as the initial biopsy results. There was a moderate reduction in inflammation in the biopsy sample from HC212. No other significant histologic changes were noted.
DISCUSSION
This study has demonstrated the potent dose-related antihepadnaviral efficacy of oral adefovir dipivoxil in chronically WHV-infected woodchucks. Serum WHV DNA levels were reduced by more than 2.5 logs in woodchucks that received 15 mg of adefovir dipivoxil/kg daily and more than 1.6 logs in those treated with 5 mg of adefovir dipivoxil/kg daily in the course of 12 weeks of treatment. Diminution of serum WHV levels was evident within 14 days, at the first sample period. Viral levels remained suppressed throughout the course of treatment and rebounded to pretreatment levels once drug exposure was discontinued. These findings are in accord with studies in WHV-infected primary hepatocytes, duck HBV-infected ducks, HBV-expressing cell lines, and HBV-infected humans (5, 9,13, 24, 32). Although direct comparisons are not possible because of different dosage regimens and methods of analysis, the antiviral activity of adefovir in chronically WHV-infected woodchucks appears to be similar to activities of other antihepadnaviral nucleoside analogs, such as emtricitabine, adenine-5′-arabinoside monophosphate (Ara-AMP), famciclovir, and zidovudine (6, 18, 22).
Although adefovir has a potent and selective activity against hepadnavirus and retrovirus replication (3, 10, 32) it is not absorbed well from the digestive tract because of limited intestinal permeativity of the phosphonate group, which is charged at physiologic pH (25). The prodrug, adefovir dipivoxil, is better absorbed than adefovir in several animal species, including humans, nonhuman primates, dogs, and rats (3, 7, 8, 27). The oral bioavailability of adefovir dipivoxil in woodchucks was quite similar to that in humans and the other species despite the differences in the anatomy of the digestive tracts. As a result, a single daily oral dose was sufficient to reduce serum WHV DNA levels and to maintain the reduction during the course of therapy.
The antiviral efficacy of nucleoside analogues is usually evident within 1 or 2 weeks of treatment in vivo; however, toxic effects can develop when treatment is extended beyond several weeks. Long-term treatment with Ara-AMP can lead to neuropathy, and long-term ribavirin can lead to disturbed erythropoietic activity (20, 30). WHV-infected woodchucks treated with fialuridine had a marked reduction in serum WHV DNA within 1 to 2 weeks of treatment. Toxicity was not evident in treated woodchucks until they had been exposed for approximately 8 weeks, after which time lethal liver damage ensued (31). Fialuridine-treated patients have also experienced severe toxic effects (23). In order to assess the risk of delayed toxicity in this study, adefovir dipivoxil was administered to woodchucks for 12 weeks and animals were monitored for an additional 6 weeks after treatment. Analysis of a broad series of serum biochemical analytes, targeting liver and renal function in particular, showed no evidence of clinically significant toxicity in treated woodchucks. In addition to standard serum biochemistry, serum bicarbonate and lactate levels were measured in all the woodchucks because the levels of these analytes have been abnormal in patients with fialuridine-induced hepatic mitochondrial damage (4). In clinical studies with high doses of adefovir dipivoxil (60 and 120 mg daily) for the treatment of HIV infection, the most frequent adverse events were a mild nephrotoxicity characterized by elevated serum creatinine and/or hypophosphatemia that was reversible upon dose discontinuation. In ongoing clinical studies for the treatment of HBV infection, a much lower daily dose of 10 mg is being tested and serum creatinine and phosphate levels are being monitored closely. In the woodchuck study, there were no clinically relevant or statistically significant changes in serum creatinine or serum phosphate at week 12 compared to those in control animals, suggesting that there is no evidence of nephrotoxicity in woodchucks treated with these relatively high doses. There was no evidence of clinically significant abnormalities in the hematological data from the adefovir dipivoxil-treated woodchucks. Importantly, serum bicarbonate remained within normal limits, thus showing no evidence of metabolic acidosis. Also, there was no evidence of the marked treatment-related microvesicular and macrovesicular vacuolization seen in fialuridine-treated woodchucks or humans in the livers of high-dose (15 mg of adefovir dipivoxil/kg daily) woodchucks after the recovery period (16, 31).
The pathogenesis of the cheilitis is unclear. Irritation of the lips appeared to be related to exposure to the adefovir, since it occurred only in the animals that were treated and resolved quickly when exposure was discontinued. Physical trauma seems unlikely since the animals accepted the drug in grape juice without reluctance. Although it is possible that there was a direct irritant effect of the drug to the mucous membranes, this seems unlikely in view of the fact that there were no lesions in the oral cavity of any of the animals. Possibly there was more salivation in response to the drug that led to persistent moisture and a secondary bacterial dermatitis at the commissural region of the lips that led to the cheilitis.
In summary, adefovir dipivoxil was shown to be an effective antihepadnaviral agent in chronically WHV-infected feral woodchucks. Serum WHV DNA levels were markedly suppressed in a dose-related fashion during 12 weeks of treatment. The oral bioavailability of the prodrug, adefovir dipivoxil, was sufficient to permit an efficacious single daily oral administration regime. There was no evidence of toxicity during the 12-week treatment period or during the 6-week follow-up period. Continued development of adefovir dipivoxil for the treatment of chronic hepatitis B in patients is warranted.
ACKNOWLEDGMENTS
We appreciate the careful review of the manuscript by Frank Richardson and Mick Hitchcock.
Funds for the support of this study were provided by Gilead Sciences.
REFERENCES
- 1.Balzarini J, Naesens L, Herdewijn P, Rosenberg I, Holy A, Pauwels R, Baba M, Johns D G, De Clercq E. Marked in vivo antiretrovirus activity of 9-(2-phosponylmethoxyethyl) adenine, a selective anti-human immunodeficiency virus agent. Proc Natl Acad Sci USA. 1991;86:332–336. doi: 10.1073/pnas.86.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini J, Naesens L, Slachmuylders J, Niphuis H, Rosenberg I, Holy A, Schellekens H, De Clercq E. 9-(2-Phosphonylmethoxyethyl) adenine (PMEA) effectively inhibits retrovirus replication in vitro and simian immunodeficiency virus infection in rhesus monkeys. AIDS. 1991;5:21–28. doi: 10.1097/00002030-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Barditch-Crovo P, Toole J H C W, Cundy K C, Ebeling D, Jaffe H S, Lietman P S. Anti-human immunodeficiency virus (HIV) activity, safety, and pharmacokinetics of adefovir dipivoxil (9-[2-(bis-pivaloyloxymethyl)-phosphonylmethoxyethyl] adenine) in HIV-infected patients. J Infect Dis. 1997;176:406–413. doi: 10.1086/514057. [DOI] [PubMed] [Google Scholar]
- 4.Colacino J M. Mechanisms for the anti-hepatitis B virus activity and mitochondrial toxicity of fialuridine. Antivir Res. 1996;29:125–139. doi: 10.1016/0166-3542(95)00836-5. [DOI] [PubMed] [Google Scholar]
- 5.Colledge D, Civitico G, Locarnini S, Shaw T. In vitro antihepadnaviral activities of combinations of penciclovir, lamivudine, and adefovir. Antimicrob Agents Chemother. 2000;44:551–560. doi: 10.1128/aac.44.3.551-560.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullen J M, Smith S L, Davis M G, Dunn S E, Botteron C, Cecchi A, Linsey D, Linzey D, Frick L, Paff M, Goulding A, Biron K. In vivo antiviral activity and pharmacokinetics of (−)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in woodchuck hepatitis virus-infected woodchucks. Antimicrob Agents Chemother. 1997;41:2076–2082. doi: 10.1128/aac.41.10.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cundy K C, Fishback J A, Shaw J P, Lee M L, Soike K F, Visor G C, Lee W A. Oral bioavailability of the antiretroviral agent 9–2(phosphonylmethoxyethyl) adenine (PMEA) from three formulations of the prodrug bis(pivaloyloxymethyl) PMEA in fasted male cynomolgus monkeys. Pharm Res. 1994;11:839–843. doi: 10.1023/a:1018925723889. [DOI] [PubMed] [Google Scholar]
- 8.Cundy K C, Sue I L, Visor G C, Marshburn J, Nakamura C, Lee W A, Shaw J P. Oral formulations of adefovir dipivoxil: in vitro dissolution and in vivo bioavailability in dogs. J Pharm Sci. 1997;86:1334–1338. doi: 10.1021/js970264s. [DOI] [PubMed] [Google Scholar]
- 9.Dandri M, Burda M R, Will H, Petersen J. Increased hepatocyte turnover and inhibition of woodchuck hepatitis B virus replication by adefovir in vitro do not lead to reduction of the closed circular DNA. Hepatology. 2000;32:139–146. doi: 10.1053/jhep.2000.8701. [DOI] [PubMed] [Google Scholar]
- 10.De Clercq E. Broad-spectrum anti-DNA virus and anti-retrovirus activity of phosphonylmethoxyalkylpurines and pyrimidines. Biochem Pharmacol. 1991;42:963–972. doi: 10.1016/0006-2952(91)90276-b. [DOI] [PubMed] [Google Scholar]
- 11.Deeks S G, Collier A, Lalezari J, Pavia A, Rodrigue D, Drew W L, Toole J, Jaffe H S, Mulato A S, Lamy P D, Li W, Cherrington J M, Hellmann N, Kahn J. The safety and efficacy of adefovir dipivoxil, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 1997;176:1517–1523. doi: 10.1086/514150. [DOI] [PubMed] [Google Scholar]
- 12.Fourel I, Hantz O, Cova L, Allaudeen H S, Trepo C. Main properties of duck hepatitis B virus DNA polymerase: comparison with the human and woodchuck hepatitis B virus DNA polymerases. Antivir Res. 1987;8:189–199. doi: 10.1016/0166-3542(87)90073-8. [DOI] [PubMed] [Google Scholar]
- 13.Gilson R J, Chopra K B, Newell A M, Murray-Lyon I M, Nelson M R, Rice S J, Tedder R S, Toole J, Jaffe H S, Weller I V. A placebo-controlled phase I/II study of adefovir dipivoxil in patients with chronic hepatitis B virus infection. J Viral Hepatitis. 1999;6:387–395. doi: 10.1046/j.1365-2893.1999.00182.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoofnagle J H. Therapy of viral hepatitis. Digestion. 1998;59:563–578. doi: 10.1159/000007532. [DOI] [PubMed] [Google Scholar]
- 15.Kane M. Global status of HBV immunization. Lancet. 1996;348:696. doi: 10.1016/S0140-6736(05)65598-5. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner D E, Gaffey M J, Sallie R, Tsokos M, Nichols L, McKenzie R, Strauss S E, Hoofnagle J H. Histopathologic changes associated with fialuridine hepatotoxicity. Mod Pathol. 1997;10:192–199. [PubMed] [Google Scholar]
- 17.Korba B A, Xie H, Wright K N, Hornbuckle W E, Gerin J L, Tennant B C, Hostetler K Y. Liver-targeted antiviral nucleosides: enhanced antiviral activity of phosphatidyl-dideoxyguanosine versus dideoxyguanosine in woodchuck hepatitis virus infection in vivo. Hepatology. 1996;23:958–963. doi: 10.1002/hep.510230503. [DOI] [PubMed] [Google Scholar]
- 18.Korba B E, Cote P, Hornbuckle W, Tennant B C, Gerin J L. Treatment of chronic woodchuck hepatitis virus infection in the eastern woodchuck (Marmota monax) with nucleoside analogues is predictive of therapy for chronic hepatitis B virus in man. Hepatology. 2000;31:1165–1175. doi: 10.1053/he.2000.5982. [DOI] [PubMed] [Google Scholar]
- 19.Korba B E, Schinazi R F, Cote P, Tennant B C, Gerin J L. Effect of oral administration of emtricitabine on woodchuck hepatitis virus replication in chronically infected woodchucks. Antimicrob Agents Chemother. 2000;44:1757–1760. doi: 10.1128/aac.44.6.1757-1760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lok A S, Wilson L A, Thomas H C. Neurotoxicity associated with adenine arabinoside monophosphate in the treatment of chronic hepatitis B virus infection. J Antimicrob Chemother. 1984;14:93–99. doi: 10.1093/jac/14.1.93. [DOI] [PubMed] [Google Scholar]
- 21.Marion P L. Development of antiviral therapy for chronic infection with hepatitis B virus. Curr Top Microbiol Immunol. 1991;168:167–183. doi: 10.1007/978-3-642-76015-0_8. [DOI] [PubMed] [Google Scholar]
- 22.Mason W S, Cullen J M, Moraleda G, Saputelli J, Aldrich C E, Miller D S, Tennant B C, Frick L, Averett D, Condreay L D, Jilbert A R. Lamivudine therapy of WHV-infected woodchucks. Virology. 1998;245:18–32. doi: 10.1006/viro.1998.9150. [DOI] [PubMed] [Google Scholar]
- 23.McKensie R, Fried M W, Sallie R, Conjeevaram H, DiBisceglie A M, Park Y, Savarese B, Kleiner D, Tsokos M, Luciano C, Pruett T, Stotka J L, Straus S E, Hoofnagle J H. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N Engl J Med. 1995;333:1099–1105. doi: 10.1056/NEJM199510263331702. [DOI] [PubMed] [Google Scholar]
- 24.Nicoll A J, Colledge D L, Toole J J, Angus P W, Smallwood R A, Locarnini S A. Inhibition of duck hepatitis virus replication by 9-(2-phosphonylmethoxymethyl)adenine, an acyclic phosphonate nucleoside analogue. Antimicrob Agents Chemother. 1998;42:3130–3135. doi: 10.1128/aac.42.12.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palu G, Stefanelli S, Rassu M, Parolin C, Balzarini J, De Clercq E. Cellular uptake of phosphonylmethoxyalkylpurine derivatives. Antivir Res. 1991;16:115–119. doi: 10.1016/0166-3542(91)90063-w. [DOI] [PubMed] [Google Scholar]
- 26.Roggendorf M, Tolle T K. The woodchuck: an animal model for hepatitis B virus infection in man. Intervirology. 1995;38:100–112. doi: 10.1159/000150418. [DOI] [PubMed] [Google Scholar]
- 27.Shaw J P, Louie M S, Krishnamurthy V V, Arimilli M N, Jones J J, Bidgood A M, Lee W A, Cundy K C. Pharmacokinetics and metabolism of selected prodrugs of PMEA in rats. Drug Metab Dispos. 1997;25:362–366. [PubMed] [Google Scholar]
- 28.Shaw T, Locarnini S. Hepatic purine and pyrimidine metabolism: implications for antiviral chemotherapy of viral hepatitis. Liver. 1995;15:169–184. doi: 10.1111/j.1600-0676.1995.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 29.Sherker A H, Marion P L. Hepadnaviruses and hepatocellular carcinoma. Annu Rev Microbiol. 1991;45:475–507. doi: 10.1146/annurev.mi.45.100191.002355. [DOI] [PubMed] [Google Scholar]
- 30.Tappero G, Ballare M, Farina M, Negro F. Severe anemia following combined alpha-interferon/ribavirin therapy of chronic hepatitis C. J Hepatol. 1998;29:1033–1034. doi: 10.1016/s0168-8278(98)80138-4. [DOI] [PubMed] [Google Scholar]
- 31.Tennant B C, Baldwin B H, Graham L A, Ascenzi M A, Hornbuckle W E, Rowland P H, Tochkov I A, Yeager A E, Erb H N, Colacino J M, Lopez C, Engelhardt J A, Bowsher R R, Richardson F C, Lewis W, Cote P J, Korba B E, Gerin J L. Antiviral activity and toxicity of fialuridine in the woodchuck model of hepatitis B virus infection. Hepatology. 1998;28:179–191. doi: 10.1002/hep.510280124. [DOI] [PubMed] [Google Scholar]
- 32.Yokuta T, Mochizuki S, Konno K, Mori S, Shigeta S, De Clercq E. Inhibitory effects of selected antiviral compounds on human hepatitis B virus DNA synthesis. Antimicrob Agents Chemother. 1991;35:394–397. doi: 10.1128/aac.35.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]