Abstract
Rationale & Objective:
Most circulating biomarkers of chronic kidney disease (CKD) progression focus on factors reflecting glomerular filtration. Few biomarkers capture non-glomerular pathways of kidney injury or damage, which may be particularly informative in populations at high risk for CKD progression such as individuals with diabetes.
Study Design:
Cohort study.
Setting & Participants:
594 participants (mean age 70, 53% women) of the Reason for Geographic and Racial Differences in Stroke study who had diabetes and an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73m2 at baseline.
Exposures:
Plasma biomarkers of inflammation/fibrosis (TNFR-1 and 2, suPAR, MCP-1, YKL-40) and tubular injury (KIM-1) measured at the baseline visit.
Outcomes:
Incident kidney failure needing replacement therapy (KFRT).
Analytical approach:
Cox proportional hazards regression and Lasso regression adjusted for established risk factors for kidney function decline, baseline eGFR and urine albumin-to-creatinine ratio (ACR).
Results:
A total of 98 KFRT events were observed over a mean 6.2 (standard deviation 3.5) years of follow-up. Plasma biomarkers were modestly associated with baseline eGFR (correlation coefficients ranging from −0.08 to −0.65) and ACR (0.14 to 0.56). In individual biomarker models adjusted for eGFR, ACR, and established risk factors, hazard ratios for incident KFRT per two-fold higher biomarker concentrations were 1.52 (95%CI: 1.25,1.84) for plasma KIM-1; 1.54 (95%CI: 1.08,2.21) for TNFR-1; 1.91 (95%CI: 1.16,3.14) for TNFR-2; and 1.39 (95%CI: 1.05,1.84) for YKL-40. In Lasso regression models accounting for biomarkers in parallel, plasma KIM-1 and TNFR1 remained associated with incident KFRT.
Limitations:
Single biomarker measurement, lack of follow-up eGFR measurements
Conclusion:
Individual plasma markers of inflammation/fibrosis (TNFR-1, TNFR-1, YKL-40) and tubular injury (KIM-1) were associated with risk of incident KFRT in adults with diabetes and an eGFR <60 ml/min/1.73m2 after adjustment for established risk factors.
Keywords: end-stage kidney disease, end-stage renal disease, biomarkers, dialysis, diabetes, chronic kidney disease, renal failure
Plain language summary:
Diabetes commonly damages kidney tubules by causing fibrosis and inflammation. However, little is known whether biomarkers of kidney tubule injury, fibrosis and inflammation are associated with risk of developing kidney failure requiring dialysis. We examined whether blood biomarkers of inflammation, fibrosis and tubular injury were associated with the development of kidney failure in individuals with diabetes and chronic kidney disease. We found that higher concentrations of several of these biomarkers were associated with higher risk of developing kidney failure, particularly kidney injury marker 1 (KIM-1) and tumor necrosis factor receptor 1 (TNFR1). These data provide important information on factors that cause kidney failure in those with diabetes.
INTRODUCTION
Diabetes is the leading cause of chronic kidney disease (CKD) globally and a strong risk factor for progression of CKD to kidney failure needing replacement therapy (KFRT).1,2 Rates of CKD progression in individuals with diabetes can be heterogeneous, with some demonstrating rapid declines in estimated glomerular filtration rate (eGFR) whereas others experience a more indolent course.3,4 Identifying biomarkers associated with development of KFRT in those with diabetes and CKD is of interest for several reasons. The clinical care of these patients—such as timing of referral for kidney transplant evaluation—is highly sensitive to their risk for progressive kidney function decline. Such information could also be used to more efficiently identify individuals who are ideal candidates for recruitment into clinical trials. Additionally, determining which biomarkers most strongly associate with KFRT can provide novel insights into pathophysiology of progression of kidney disease in the setting of diabetes.
Circulating CKD biomarkers currently used in clinical practice focus on creatinine- or cystatin C-based eGFR, both of which largely reflect aspects of glomerular filtration. Non-glomerular pathways of injury or structural damage are only partially captured by eGFR. Inflammation and fibrosis contribute to CKD progression. Therefore, biomarkers related to these pathways may provide novel insights into CKD pathophysiology.5–11 Circulating markers of tubular health also provide important pathophysiologic and prognostic information above and beyond eGFR.12,13 Few studies have examined markers of inflammation, fibrosis, and tubular injury in parallel to compare their relative strengths as predictors in individuals at high risk for CKD progression, such as those with diabetes and an eGFR < 60 ml/min/1.73m2. Accordingly, the major goals of this study were to examine the association of circulating biomarkers of inflammation/fibrosis—soluble tumor necrosis factor receptors 1 and 2 (TNFR1 and TNFR2), chitinase 3-like 1 (YKL-40), monocyte chemotactic protein-1 (MCP-1) and soluble urokinase-type plasminogen activator receptor (suPAR)—and tubular injury (kidney injury marker 1, KIM-1) with risk of KFRT in individuals with diabetes and an eGFR <60 ml/min/1.73m2 who participated in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study.
METHODS
The REGARDS study is a population-based, longitudinal cohort study designed to examine underlying causes for racial and regional differences in stroke rates in the United States. Details of the study design have been published elsewhere.14 Briefly, the study was designed to provide approximately equal representation of men and women, and oversampled individuals who were black as well as individuals living in the southeastern United States. Trained interviewers conducted computer-assisted telephone interviews to obtain information including participants’ socio-demographics, cardiovascular risk factors, and use of anti-hypertensive, anti-glycemic, and cholesterol-lowering medication. Following this call, trained personnel conducted an in-home study visit that included an electrocardiographic recording, blood pressure, height and weight measurements, inventory of medications and collection of blood and urine samples. Overall, 30,239 individuals were enrolled between January 2003 and October 2007 (42% black, 55% women). The REGARDS study protocol was approved by the Institutional Review Boards at the participating centers and all participants provided written informed consent.
Primary Exposures
The primary exposures were plasma concentrations of TNFR-1, TNFR-2, YKL-40, MCP-1, suPAR and KIM-1 sampled at participants’ baseline visit. Biomarkers were measured in plasma samples stored at −80°C after a single freeze-thaw using a multiplex assay on the Meso Scale Discovery platform (Meso Scale Discovery, Gaithersburg, MD). Overall, intra- and inter-assay coefficients of variation were 3.6–7.9% and 7.8–10.1%, respectively. Each biomarker was measured in duplicate for each sample, and results were averaged to improve precision. All assays were performed blinded to clinical outcomes.
Outcome of Interest
Incident KFRT was ascertained from linkage to the United States Renal Data System, which collects information on persons initiating dialysis or receiving kidney transplants.15 The current analysis included KFRT cases identified through June 3, 2014.
Covariates of Interest
Age, sex, race, smoking history, and educational attainment were determined by self-report. Body mass index (BMI) was determined using height and weight obtained at the baseline in-home visit. Blood pressure was defined as the average of two measures taken on seated participants after a 5-minute rest. Use of medications for hypertension was obtained by self-report. History of coronary heart disease (CHD) was defined as having any of the following: evidence of myocardial infarction on the baseline electrocardiograph, self-report of a prior history of a cardiac procedure (coronary artery bypass surgery or percutaneous coronary intervention), or self-reported history of myocardial infarction. History of stroke was determined by self-report. Estimated GFR was determined from isotope dilution mass spectrometry-traceable serum creatinine concentration measurements using the CKD Epidemiology Collaboration equation.16 Urine albumin concentration was measured by the BNII ProSpec nephelometer (Siemens AG) and urine creatinine concentration was measured by the rate Jaffé method (Roche/Hitachi, Basel, Switzerland). The urine albumin-to-creatinine ratio (ACR) was expressed as mg/g.
Derivation of Study Population
We restricted the analysis to participants with diabetes and an eGFR <60 ml/min/1.73m2 who did not have prevalent KFRT at the baseline visit (n=1,092). Diabetes was defined as self-reported use of insulin or oral hypoglycemic agents, fasting blood glucose concentration of 126 mg/dL or higher, or a non-fasting blood glucose concentration of 200 mg/dL or higher. We originally intended to use a case-cohort study design to provide an unbiased estimate of the relative hazard of KFRT without requiring measurement of biomarkers in all participants of interest.17 Cases included participants who developed incident KFRT (n=174). The subcohort sample was randomly selected from the 1,092 participants eligible for analysis (n=600), without regard to biomarker status or KFRT outcome. A total of 99 incident KFRT cases were included in the subcohort sample, leaving 75 incident KFRT cases that arose outside the subcohort sample. Six participants (one of whom was a case) were excluded as they did not have an adequate volume of stored plasma for biomarker measurement.
Following the measurement of biomarkers in case-cohort samples, it was noted that all cases arising outside the subcohort were inadvertently run on a single set of plates assayed in duplicate on the same day. Inspection of Levey-Jennings plots from quality control samples included on each plate revealed that, although measured values fell within the quality control limits established by Westgard rules, the mean concentrations of multiple biomarkers on this single set of plates were substantially lower than mean concentrations in plates run on other days (consisting almost entirely of subcohort samples in which cases and controls were more evenly distributed). This introduced systematic bias in the measurement of biomarkers in cases arising outside the subcohort. Because of this uncorrectable bias, we focused all analyses on the randomly-sampled subcohort alone, excluding cases measured outside the subcohort, which still provided an unbiased study of REGARDS participants, albeit with slightly less statistical power than originally intentioned.
Statistical Analysis
Descriptive statistics were used to compare participant characteristics within study cohort across quartiles of each plasma biomarker. Pearson partial correlation controlling for age and sex were used to examine associations of plasma biomarkers with each other and with eGFR and ACR. After confirming the assumption of proportionality of hazards, Cox regression models were used to estimate the hazard ratio of incident KFRT as a function of each biomarker, separately. Model 1 adjusted for age, sex, race, and education. Model 2 was further adjusted for BMI, systolic blood pressure, use of hypertension medications, number of hypertension medications, use of renin-angiotensin-aldosterone system (RAAS) inhibitors, smoking status, history of CHD, history of stroke and high-sensitivity C-reactive protein (hsCRP). Model 3 adjusted additionally for baseline eGFR and ACR. In all models, plasma biomarkers were analyzed in quartiles, with the lowest quartile serving as the referent group and on a continuous scale after log base 2 transformation (interpreted as per two-fold higher of each biomarker). Since our predictors are highly correlated, we used a data-driven regularization method to select the best set of biomarkers. The least absolute shrinkage and selection operator (Lasso) is a good method for automatic variable selection.18 This regression method penalizes the absolute size of the regression coefficients. A Lasso penalty with leave-one-out cross-validation was used to estimate the penalty parameters and results were reported using the penalty that gave the best cross-validated fit. The biomarkers selected from the Lasso were then entered into Cox proportional hazards regression models. We used the implementation in the R glmnet package. We examined for effect modification by race and sex by testing the statistical significance of a multiplicative interaction term in fully-adjusted models for each biomarker separately. To determine whether addition of biomarkers improved 2- and 5-year prediction of KFRT, we calculated point estimates for Harrell’s c-index for models including the Kidney Failure Risk Equation (KFRE) without and with each biomarker. In addition, Wald test statistics were used to assess the relative importance of biomarkers for predicting KFRT. In this method, the percentage contribution of each variable (variable-specific Wald test) to the global Wald χ2 score is calculated, with a higher score (i.e., greater percentage contribution in global Wald χ2 score) indicating that a variable is a stronger predictor than those with lower scores. A two-tailed P value < 0.05 was considered statistically significant for all analyses including interaction terms. Analyses were performed using IBM SPSS version 26 (Armonk, NY: IBM Corp).
RESULTS
Study Population
The 594 participants were comprised of 47% men and 53% black individuals, with a baseline mean age of 70 years and mean eGFR of 44 ml/min/1.73m2. Other baseline characteristics of the study sample are depicted overall and by quartiles of plasma KIM-1 in Table 1. Baseline characteristics across quartiles of other biomarkers largely aligned with KIM-1 and are depicted in Supplemental Tables 1–5. In general, participants in higher quartiles of KIM-1 were younger, more likely to be black, were more likely to be current smokers and have a history of CHD and stroke, had higher systolic and diastolic blood pressure, were more likely to be taking anti-hypertensive medications, and had lower eGFR and higher ACR. As compared to participants who did not develop KFRT, those who did were on average older, more likely to be black, men, current smokers and have a history of hypertension, had higher blood pressure, and had lower eGFR and higher ACR at baseline (Supplemental Table 6).
Table 1.
Baseline characteristics of study participants by quartile of KIM-1
| Variable | All Participants | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|---|
|
| |||||
| N | 594 | 149 | 148 | 148 | 149 |
| Range (pg/ml) | < 322 | 323 – 522 | 523 – 954 | > 954 | |
| Age, years | 70 (9) | 72 (8) | 71 (10) | 70 (8) | 68 (8) |
| Men | 278 (47) | 61(41) | 78 (53) | 71 (48) | 68 (46) |
| Black Education |
316 (53) | 74 (50) | 64 (43) | 79 (53) | 99 (66) |
| < high school | 133 (22) | 31(21) | 38 (26) | 31 (21) | 33 (22) |
| high school graduate | 161 (27) | 30 (20) | 39 (26) | 44 (30) | 48 (32) |
| some college | 148 (25) | 44 (30) | 35 (24) | 32 (22) | 37 (25) |
| ≥ college graduate | 151 (26) | 43 (29) | 36 (24) | 41 (28) | 31 (21) |
| Income | |||||
| < $20k | 173 (29) | 42 (28) | 48 (32) | 40 (27) | 43 (29) |
| $20k-$34k | 169 (29) | 39 (26) | 41 (28) | 38 (26) | 51 (34) |
| $35k-$74k | 138 (23) | 35 (24) | 39 (26) | 34 (23) | 30 (20) |
| ≥ $75k | 41 (7) | <11 (8)* | 12 (8) | 12 (8) | <11 (8)* |
| refuse to answer | 73 (12) | 23 (15) | < 11 (8)* | 24 (16) | 18 (12) |
| BMI Smoking status |
31.9 (6.6) | 32.3 (6.3) | 31.6 (6.6) | 31.5 (6.8) | 32.1 (6.8) |
| Never | 259 (44) | 67 (45) | 67 (45) | 59 (40) | 66 (44) |
| Former | 278 (47) | 68 (46) | 71 (48) | 76 (51) | 63 (42) |
| Current | 57 (10) | 14 (9) | <11* | 13 (9) | 20 (13) |
| Hypertension | 516 (87) | 128 (86) | 127 (86) | 128 (87) | 133 (90) |
| Coronary heart Disease | 239 (42) | 53 (36) | 58(41) | 66 (46) | 62 (43) |
| Stroke | 95 (16) | 14 (10) | 22 (15) | 31 (21) | 28 (19) |
| Systolic BP, mmHg | 132 (19) | 131 (18) | 127 (17) | 131 (18) | 141 (21) |
| Diastolic BP, mmHg | 74 (11) | 73 (10) | 71 (10) | 73 (11) | 78 (11) |
| Hypertension medication | 494 (87) | 123 (86) | 120 (85) | 123 (85) | 128 (91) |
| RAAS inhibitor | 444 (75) | 116 (78) | 108 (73) | 114 (77) | 106 (71) |
| Number of hypertension meds | 2.5 (1.2) | 2.4 (1.2) | 2.5 (1.2) | 2.6 (1.1) | 2.5 (1.2) |
| Insulin use | 175 (30) | 38 (26) | 40 (27) | 40 (27) | 57 (38) |
| Metformin use | 127 (21) | 34 (23) | 26 (18) | 31 (21) | 36 (24) |
| hsCRP, mg/L | 3.1 [1.3, 7.4] | 2.7 [0.9, 6.1] | 3.1 [1.3, 7.4] | 3.2 [1.5, 6.5] | 3.6 [1.7, 7.9] |
| eGFR, ml/min/1.73m2 | 44 (12) | 49 (9) | 45 (11) | 44 (12) | 38 (13) |
| urine albumin (mg/L) | 32 [11, 212] | 14 [7, 30] | 30 [11, 100] | 26 [11, 156] | 502 [73, 1460] |
| urine creatinine (mg/dL) | 101 [71, 143] | 115 [79, 156] | 113 [72, 146] | 93 [66, 138] | 93 [67, 130] |
| Urine ACR, mg/g | 32 [9, 224] | 12 [6, 31] | 27 [9, 87] | 27 [12, 126] | 459 [64, 1765] |
Abbreviations: Q, quartile, BMI, body mass index; BP, blood pressure; RAAS, renin-angiotensin-aldosterone inhibitor; eGFR, estimated glomerular filtration rate; ACR, albumin to creatinine ratio; hsCRP, high-sensitivity C-reactive Protein Data are depicted as means (standard deviation), N (%), or medians [interquartile range]
Values for cells with ten or fewer participants are suppressed as per USRDS policy
Table 2 depicts age- and sex-adjusted Spearman correlations between individual plasma biomarkers and eGFR and ACR. Each plasma biomarker was inversely associated with eGFR (correlation coefficients ranging from −0.08 to −0.65) and positively associated with ACR (correlation coefficients ranging from 0.14 to 0.56). Plasma TNFR-1 had the highest correlation with eGFR (ρ= 0.65) and plasma KIM-1 had the highest correlation with ACR (ρ= 0.56). In general, biomarkers were modestly correlated with each other with the exception of TNFR-1, TNFR-2 and suPAR, which were strongly intercorrelated (correlation coefficients ranging from 0.76 to 0.83).
Table 2.
Age- and sex-adjusted Pearson partial correlations of baseline plasma biomarkers (KIM-1, TNFR-1, TNFR-2, MCP-1, suPAR, YKL-40), urine albumin to creatinine ratio, and estimated glomerular filtration rate in study participants
| KIM-1 | TNFR-1 | TNFR-2 | MCP-1 | suPAR | YKL-40 | BMP-7 | UACR | eGFR | |
|---|---|---|---|---|---|---|---|---|---|
| KIM-1 | 1.0 | 0.39** | 0.41** | 0.15** | 0.37** | 0.33** | 0.23** | 0.56** | −0.32** |
| TNFR-1 | 1.0 | 0.83** | 0.14** | 0.79** | 0.40** | 0.18** | 0.52** | −0.65** | |
| TNFR-2 | 1.0 | 0.15** | 0.76** | 0.39** | 0.08 | 0.52** | −0.60** | ||
| MCP-1 | 1.0 | 0.17** | −0.01 | −0.01 | 0.14** | −0.08 | |||
| suPAR | 1.0 | 0.37** | 0.23** | 0.45** | −0.60** | ||||
| YKL-40 | 1.0 | 0.20** | 0.26** | −0.23** | |||||
| BMP-7 | 1.0 | 0.15** | −0.09* | ||||||
| UACR | 1.0 | −0.43** | |||||||
| eGFR | 1.0 |
Abbreviations: TNFR-1 and TNFR-2, soluble tumor necrosis factor receptors 1 and 2; YKL-40, chitinase 3-like 1; MCP-1, monocyte chemotactic protein-1; suPAR, soluble urokinase-type plasminogen activator receptor; KIM-1, kidney injury marker 1.
Correlation is significant at the 0.01 level (2-tailed)
Association of plasma biomarkers with incident KFRT
During the mean (standard deviation) follow-up of 6.2 (3.5) years, 98 participants developed incident KFRT. Multivariable-adjusted associations of plasma biomarkers with incident KFRT are shown in Table 3. Incidence rates of KFRT increased with increasing quartiles of each biomarker. For KIM-1, TNFR-1, and TNFR-2, KFRT rates rose almost 20-fold across quartiles, from approximately 0.5 events of incident KFRT per year in the lowest quartile to 9 in the highest quartile. In analyses adjusted for age, sex, race and education, higher concentrations of each biomarker were associated with greater risk of incident KFRT. Hazard ratios [HR] per two-fold higher concentration of biomarker were—KIM-1: 2.01, 95% confidence interval [CI] 1.73,2.33; TNFR-1: 2.87, 95% CI 2.34,3.52; TNFR-2: 5.64, 95% CI 3.98,8.00; MCP-1: 1.36, 95%CI 1.09,1.70; suPAR: 3.23, 95% CI 2.49,4.19; YKL-40: 1.86, 95% CI 1.47,2.36. The associations were modestly attenuated after further adjustment for BMI, systolic blood pressure, use of anti-hypertensive medications, current smoking, and a history of CHD and stroke. After further adjustment for eGFR and ACR, associations were attenuated further but remained statistically significant for all biomarkers except MCP-1 and suPAR (Figure 1). None of the associations were modified by race or sex (Pinteraction>0.05 for all).
Table 3.
Association of baseline plasma biomarkers (KIM-1, TNFR-1, TNFR-2, MCP-1, suPAR, YKL-40) with incident KFRT in random subcohort of 594 REGARDS participants with diabetes and eGFR<60 at baseline
| N | cases | IR (%/yr) | Unadjusted HR (95% CI) |
Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 3 HR (95% CI) |
|||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| KIM-1 Continuous | |||||||||
| per two-fold higher | 594 | 98 | 2.67 | 2.24 (1.95, 2.58) | 2.01 (1.73, 2.33) | 1.92 (1.64, 2.25) | 1.51 (1.24, 1.83) | ||
| Quartiles | |||||||||
| Range (pg/ml) | Median | ||||||||
| Q1 | < 322 | 232 | 149 | <11* | 0.55 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 | 322 – 522 | 400 | 148 | 11 | 1.14 | 2.07 (0.76, 5.59) | 1.82 (0.67, 4.98) | 1.98 (0.72, 5.44) | 0.84 (0.29, 2.46) |
| Q3 | 523 – 954 | 682 | 148 | 18 | 2.12 | 3.85 (1.53, 9.71) | 3.33 (1.32, 8.43) | 3.13 (1.23, 7.98) | 1.40 (0.52, 3.76) |
| Q4 | > 954 | 1611 | 149 | 63 | 8.3 | 15.21 (6.57, 35.19) | 11.20 (4.80, 26.14) | 9.94 (4.22, 23.42) | 2.78 (1.09, 7.09) |
| TNFR-1 Continuous | |||||||||
| per two-fold higher | 594 | 98 | 2.67 | 3.02 (2.52, 3.62) | 2.87 (2.34, 3.52) | 2.71 (2.17, 3.39) | 1.44 (1.03, 2.15) | ||
| Quartiles | |||||||||
| Range (pg/ml) | Median | ||||||||
| Q1 | < 1598 | 1270 | 149 | <11* | 0.57 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 | 1598 – 2252 | 1918 | 148 | 10 | 1.02 | 1.90 (0.69, 5.23) | 2.00 (0.72, 5.50) | 2.00 (0.72, 5.53) | 1.18 (0.42, 3.33) |
| Q3 | 2253 – 3509 | 2813 | 149 | 23 | 2.51 | 4.72 (1.92, 11.61) | 6.15 (2.48, 15.28) | 6.39 (2.56, 15.96) | 2.38 (0.91,6.21) |
| Q4 | > 3509 | 4826 | 148 | 59 | 9.01 | 17.52 (7.52, 40.81) | 18.50 (7.87, 43.46) | 17.88 (7.41, 43.11) | 4.33 (1.61, 11.68) |
| TNFR-2 Continuous | |||||||||
| per two-fold higher | 594 | 98 | 2.67 | 6.81 (4.77, 9.72) | 5.64 (3.98, 8.00) | 5.33 (3.69, 7.69) | 1.80 (1.06, 3.03) | ||
| Quartiles | |||||||||
| Range (pg/ml) | Median | ||||||||
| Q1 | < 20563 | 16857 | 149 | <11* | 0.34 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 | 20563 – 26647 | 23595 | 148 | 12 | 1.24 | 3.67 (1.18, 11.37) | 3.88 (1.24, 12.08) | 4.28 (1.36, 13.47) | 3.52 (1.11, 11.14) |
| Q3 | 26648 – 36960 | 30662 | 149 | 27 | 3.12 | 9.25 (3.24, 26.47) | 10.57 (3.66, 30.52) | 11.40 (3.91, 33.30) | 5.08 (1.70, 15.21) |
| Q4 | > 36960 | 44976 | 148 | 55 | 8.33 | 25.49 (9.21, 70.58) | 26.27 (9.44, 73.07) | 26.71 (9.37, 76.17) | 5.97 (1.88, 18.91) |
| MCP-1 Continuous | |||||||||
| per two-fold higher | 594 | 98 | 2.67 | 1.23 (0.97, 1.55) | 1.36 (1.09, 1.70) | 1.37 (1.09, 1.73) | 1.19 (0.92, 1.55) | ||
| Quartiles | |||||||||
| Range (pg/ml) | Median | ||||||||
| Q1 | < 98 | 79 | 149 | 18 | 1.89 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 | 98 – 127 | 111 | 148 | 25 | 2.71 | 1.43 (0.78, 2.62) | 1.83 (0.99, 3.37) | 2.01 (1.08, 3.76) | 1.89 (0.97, 3.67) |
| Q3 | 128 – 162 | 142 | 148 | 21 | 2.19 | 1.16 (0.62, 2.18) | 1.50 (0.80, 2.83) | 1.58 (0.82, 3.02) | 1.28 (0.62, 2.65) |
| Q4 | > 162 | 197 | 149 | 34 | 4.07 | 2.15 (1.21, 3.80) | 2.75 (1.54, 4.90) | 3.00 (1.64, 5.50) | 2.49 (1.30, 4.79) |
| suPAR Continuous | |||||||||
| per two-fold higher | 594 | 98 | 2.67 | 3.38 (2.68, 4.27) | 3.23 (2.49, 4.19) | 3.39 (2.52, 4.56) | 1.42 (0.89, 2.28) | ||
| Quartiles | |||||||||
| Range (pg/ml) | Median | ||||||||
| Q1 | < 5455 | 4794 | 149 | <11* | 0.61 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 | 5455 – 7112 | 6318 | 148 | 16 | 0.92 | 2.47 (1.02, 6.00) | 2.80 (1.15, 6.86) | 2.57 (1.05, 6.31) | 1.81 (0.72, 4.50) |
| Q3 | 7113 – 9416 | 8131 | 149 | 17 | 1.28 | 3.46 (1.43, 8.37) | 4.11 (1.68, 10.04) | 3.78 (1.53, 9.39) | 2.48 (0.98, 6.28) |
| Q4 | > 9416 | 11991 | 148 | 58 | 7.13 | 15.93 (7.23, 35.10) | 14.98 (6.76, 33.18) | 14.15 (6.24, 32.07) | 4.17 (1.69, 10.31) |
| YKL-40 Continuous | |||||||||
| per two-fold higher | 594 | 98 | 2.67 | 1.72 (1.36, 2.18) | 1.86 (1.47, 2.36) | 1.80 (1.40, 2.31) | 1.30 (1.00, 1.73) | ||
| Quartiles | |||||||||
| Range (pg/ml) | Median | ||||||||
| Q1 | < 67338 | 46048 | 149 | <11* | 0.92 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 | 67338 – 114901 | 89580 | 148 | 23 | 2.6 | 2.81 (1.33, 5.99) | 3.32 (1.57, 7.02) | 2.92 (1.37, 6.27) | 1.79 (0.82, 3.91) |
| Q3 | 114902 – 198632 | 150559 | 149 | 33 | 6.61 | 3.89 (1.92, 7.91) | 5.13 (2.51, 10.64) | 4.47 (2.14, 9.37) | 1.63 (0.75, 3.56) |
| Q4 | >198632 | 239541 | 148 | 32 | 4.07 | 4.46 (2.19, 9.09) | 5.34 (2.59, 11.02) | 4.77 (2.26, 10.05) | 2.18 (1.00, 4.76) |
Abbreviations: KFRT, end-stage kidney disease; TNFR1 and TNFR2, soluble tumor necrosis factor receptors 1 and 2; YKL-40, chitinase 3-like 1; MCP-1, monocyte chemotactic protein-1; suPAR, soluble urokinase-type plasminogen activator receptor; KIM-1, kidney injury marker 1
Model 1 adjusted for age, race, sex, education
Model 2 adjusted for variables in Model 1 plus body mass index, systolic blood pressure, use of hypertension medications, use of renin-angiotensin-aldosterone system inhibitors, number of hypertension medications, smoking status, coronary heart disease, stroke, and high-sensitivity C-reactive protein
Model 3 adjusted for variables in Model 2 plus urine albumin to creatinine ratio and estimated glomerular filtration rate.
Values for cells with ten or fewer participants are suppressed as per USRDS policy
Figure 1.
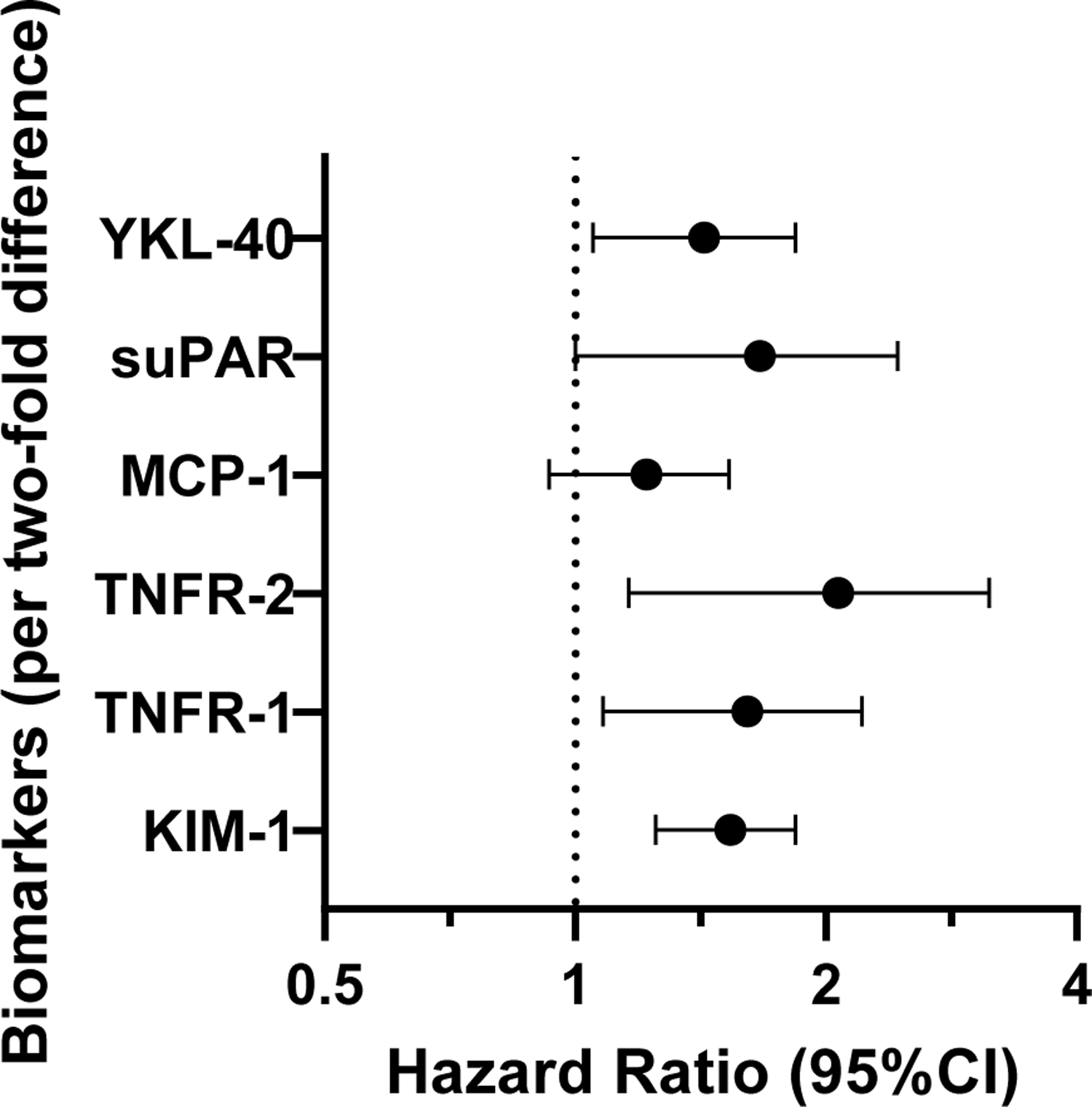
Hazard ratio (95% confidence interval) of incident kidney failure needing replacement therapy as a function of each biomarker in models adjusted for age, race, sex, education, body mass index, systolic blood pressure, use of anti-hypertensive mediations, smoking status, coronary heart disease, stroke, urine albumin to creatinine ratio, and estimated glomerular filtration rate. All biomarkers were analyzed on a continuous scale (per two-fold higher concentration).
When biomarkers were modeled in quartiles, the results were consistent, with the highest quartile associated with greater risk of incident KFRT as compared to the lowest quartile in fully adjusted models—KIM-1: HR 2.51, 95%CI 0.99,6.35; TNFR-1: HR 4.67, 95%CI 1.78,12.23; TNFR-2: HR 4.48, 95%CI 1.36,14.78; MCP-1: HR 2.22, 95%CI 1.18,4.16; suPAR: HR 3.33, 95%CI 1.27,8.73; YKL-40: HR 1.87, 95%CI 0.86,4.09. When examining biomarkers in parallel to identify the best set of markers using Lasso regression, KIM-1 (HR per two-fold higher concentration 1.50, 95%CI 1.24,1.83) and TNFR-1 (HR 1.48, 95%CI 1.03,2.13) remained associated with risk of incident KFRT in fully adjusted models (Supplemental Table 7). Addition of KIM-1 or TNFR-1 to a model including the KFRE score did not significantly improve 2- or 5-year prediction of KFRT, either when added separately or together (Table 4). In fully-adjusted models including all biomarkers, eGFR and ACR, KIM-1 was the strongest predictor of KFRT (Supplemental Figure).
Table 4.
Change in c-statistic for predicting 2- and 5-year risk of kidney failure needing replacement therapy when adding kidney injury marker 1 (KIM-1 ), tumor necrosis factor receptor 1 (TNFR-1 ), individually and together, to a model including the kidney failure risk equation score.
| 2-year risk of KFRT (N Events = 22) | 5-year risk of KFRT (N Events = 69) | |||
|---|---|---|---|---|
|
| ||||
| c-statistic | P | c-statistic | P | |
| KFRE (4 variable) | 0.832 | base model | 0.859 | base model |
| KFRE + KIM-1 | 0.852 | 0.48 | 0.853 | 0.76 |
| KFRE + TNFR-1 | 0.844 | 0.46 | 0.836 | 0.22 |
| KFRE + KIM1 + TNFR-1 | 0.861 | 0.28 | 0.868 | 0.61 |
Abbreviations: KFRE, kidney failure risk equation; KFRT, kidney failure needing replacement therapy
P-value compares c-statistics to base model
DISCUSSION
In this study of older adults with diabetes and CKD, plasma markers of inflammation/fibrosis (TNFR-1 and 2, and YKL-40) and tubular injury (KIM-1) were associated with higher risk of KFRT independent of established risk factors including baseline eGFR and ACR.
Prior studies have shown that higher concentrations of TNFR-1 and TNFR-2 are associated with greater risk of kidney function decline in individuals with diabetes and CKD.8,9,11 The results of the current study are in accordance with these prior studies, and extend those findings to a population of community-dwelling adults that is more racially heterogeneous (53% of participants were black in our study) and with a lower eGFR at baseline on average. Both TNFR-1 and 2 are markers of the TNF pathway, which is upregulated in individuals with diabetes and kidney disease. The TNF pathway is implicated in the endothelial injury and glomerular damage observed in diabetic nephropathy.19,20 The consistency of the associations between TNFR-1 and 2 with kidney function decline in multiple distinct cohorts of individuals with diabetes and CKD from across a broad range of kidney function underscores the central role of inflammation in the pathogenesis of kidney disease in individuals with diabetes.
Few studies examined the association of plasma YKL-40 and kidney function decline in individuals with diabetes and CKD and the results have been inconsistent. In a recent study of participants of the Chronic Renal Insufficiency Cohort (CRIC) study with diabetes and an eGFR <60 ml/min/1.73m2 at the baseline visit, higher plasma YKL-40 was associated with greater risk of kidney disease progression (defined as incident KFRT or ≥40% decline in eGFR during follow-up) independently of traditional risk factors and baseline kidney function.11 Moreover, the magnitude of association observed in the aforementioned study was nearly identical to that observed in the current study (HR per two-fold higher concentration 1.35, 95% CI: 1.16,1.57). In contrast, a study of individuals with diabetes attending an outpatient clinic in the Netherlands did not find a statistically significant association of plasma YKL-40 with kidney function decline when accounting for established risk factors.21 Importantly, participants in the latter study were not strictly selected for an eGFR < 60 ml/min/1.73m2 as was the case for the current study and the aforementioned CRIC study, suggesting that plasma YKL-40 may be a more useful risk factor for kidney function decline in individuals with more advanced stages of diabetes-related kidney disease.
Our finding that higher circulating KIM-1 concentrations were independently associated with greater risk of incident KFRT is consistent with multiple prior studies of individuals with diabetes and kidney disease.12,22–26 The added novelty of the current results derives from our finding that KIM-1 was one of two biomarkers (along with TNFR-1) that remained significantly associated with incident KFRT in a fully-adjusted Cox regression model after application of Lasso regression. This is in line with the aforementioned CRIC study, which reported that KIM-1 was a part of a panel of biomarkers that remained associated with diabetic kidney disease progression after backward stepwise regression (along with TNFR-2 and YKL-40).11 The consistency of plasma KIM-1 as an independent biomarker of kidney function decline across these two studies, and the finding that KIM-1 was the strongest individual predictor of KFRT in the current study (including eGFR and ACR), underscores the importance of tubular injury in the progression of kidney disease in those with diabetes and advanced CKD, and may inform future efforts to identify a heuristic set of biomarkers for kidney disease progression in individuals with advanced diabetic kidney disease. It is also noteworthy that urine KIM-1 is already used for detecting kidney injury in pre-clinical drug development and has been qualified by the Food and Drug Administration as part of a composite panel for use in healthy volunteers to detect drug toxicity, and so it may have a shorter path towards being approved for identifying participants at high risk for CKD progression and early monitoring of responses to drugs used to slow CKD progression.
Strengths of our study include the use of a large, community-dwelling, geographically- and racially-diverse population; the linkage with USRDS which identifies nearly all incident KFRT cases; and the measurement of all plasma biomarkers in the same central laboratory with detailed quality control measures. Our study has limitations as well. Because of the inadvertent assaying of samples on one plate from virtually all cases that arose outside of the subcohort, we reverted to a cohort study rather than the initially planned case-cohort design. While this reduced our study power, we were still able to detect significant associations between several plasma biomarkers and incident KFRT. We did not have follow-up measures of eGFR in all participants and so were unable to determine whether the plasma biomarkers associated with eGFR decline in addition to the development of KFRT. In addition, we only had single measurements of biomarkers at the baseline visit which did not allow us to examine the association of changes in biomarker concentrations over time with incident KFRT. Finally, we did not have measurements of diabetes control, such as hemoglobin A1c.
In summary, plasma markers of inflammation/fibrosis (TNFR-1 and 2, YKL-40) and tubular injury (KIM-1) were independently associated with greater risk of incident KFRT in community-dwelling adults with diabetes and an eGFR < 60 ml/min/1.73m2. Further, in this setting, plasma KIM-1 and TNFR-1 appeared to be most strongly associated with risk of KFRT after accounting for other biomarkers and kidney function.
Supplementary Material
Figure S1. Percentage contribution in global Wald χ2 score for individual biomarkers.
Table S1. Baseline characteristics of study participants in the random subcohort overall and by quartile of TNFR-1.
Table S2. Baseline characteristics of study participants in the random subcohort overall and by quartile of TNFR-2.
Table S3. Baseline characteristics of study participants in the random subcohort overall and by quartile of MCP-1.
Table S4. Baseline characteristics of study participants in the random subcohort overall and by quartile of sUPAR.
Table S5. Baseline characteristics of study participants in the random subcohort overall and by quartile of YKL-40.
Table S6: Baseline characteristics of study participants by development of kidney failure needing replacement therapy.
Table S7. Strength of association of 6 plasma biomarkers with incident KFRT determined by LASSO regression.
ACKNOWLEDGEMENTS:
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
SUPPORT: This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service.. This work was also supported by award U01DK102730 from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Gutierrez was supported by award K24DK116180. Dr. Schrauben was supported by award K23DK118198-01A1. Drs. Schrauben and Feldman also receive support from the Chronic Kidney Disease Biomarker Consortium (U01-DK103225). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. The opinions expressed in this paper do not necessarily reflect those of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, the Department of Health and Humans Services or the government of the United States. The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government
FINANCIAL DISCLOSURE: Dr. Gutierrez discloses that he has received honoraria and grant support from Akebia and Amgen; honoraria from AstraZeneca, Reata, and Ardelyx; grant support from GSK; and serves on a data monitoring committee for QED. Dr. Shlipak discloses that he has received consulting income from Cricket Health and Intercept Pharmaceuticals. Dr. Ix discloses that he is principal investigator of an investigator initiated research grant supported by Baxter International, serves as a member of a data safety monitoring board for Sanifit Therapeutics, is a member of the scientific advisory board for Alpha Young, and has served on advisory boards for AstraZeneca and Ardelyx. Dr. Coca is a member of the scientific advisory board of Renalytix AI and owns equity in the same; and has received consulting fees from Renalytix AI, CHF Solutions, Relypsa (now Vifor), Bayer, Boehringer-Ingelheim, ProKidney, and Takeda Pharmaceuticals in the past three years. Dr. Parikh is a member of the advisory board of and owns equity in RenalytixAI. He also serves as a consultant for Genfit and Novartis. Dr. Bonventre is co-inventor on KIM-1 patents assigned to MassGeneralBrigham. He also has equity and is a consultant for Renalytix.
Footnotes
PEER REVIEW: Received March 30, 2021. Evaluated by 3 external peer reviewers, with editorial input from a Statistics/Methods Editor and an Acting Editor-in-Chief (Editorial Board Member Paul J. Phelan, MB BCh, MD). Accepted in revised form September 7, 2021. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Koye DN, Shaw JE, Reid CM, Atkins RC, Reutens AT, Magliano DJ. Incidence of chronic kidney disease among people with diabetes: a systematic review of observational studies. Diabet Med. 2017;34(7):887–901. [DOI] [PubMed] [Google Scholar]
- 2.Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017;389(10075):1238–1252. [DOI] [PubMed] [Google Scholar]
- 3.Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving HH. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004;66(4):1596–1605. [DOI] [PubMed] [Google Scholar]
- 4.Skupien J, Smiles AM, Valo E, et al. Variations in Risk of End-Stage Renal Disease and Risk of Mortality in an International Study of Patients With Type 1 Diabetes and Advanced Nephropathy. Diabetes Care. 2019;42(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colhoun HM, Marcovecchio ML. Biomarkers of diabetic kidney disease. Diabetologia. 2018;61(5):996–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayek SS, Sever S, Ko YA, et al. Soluble Urokinase Receptor and Chronic Kidney Disease. N Engl J Med. 2015;373(20):1916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ix JH, Katz R, Bansal N, et al. Urine Fibrosis Markers and Risk of Allograft Failure in Kidney Transplant Recipients: A Case-Cohort Ancillary Study of the FAVORIT Trial. Am J Kidney Dis. 2017;69(3):410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23(3):507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavkov ME, Nelson RG, Knowler WC, Cheng Y, Krolewski AS, Niewczas MA. Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int. 2015;87(4):812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt IM, Hall IE, Kale S, et al. Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol. 2013;24(2):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrauben SJ, Shou H, Zhang X, et al. Association of Multiple Plasma Biomarker Concentrations with Progression of Prevalent Diabetic Kidney Disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabbisetti VS, Waikar SS, Antoine DJ, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25(10):2177–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waikar SS, Sabbisetti V, Ärnlöv J, et al. Relationship of proximal tubular injury to chronic kidney disease as assessed by urinary kidney injury molecule-1 in five cohort studies. Nephrol Dial Transplant. 2016;31(9):1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Renal Data System. 2015 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: 2015. [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165–1172. [DOI] [PubMed] [Google Scholar]
- 18.Tibshirani R Regression Shrinkage and Selection via the Lasso. J R Stat Soc Ser B. 1994;58:267–288. [Google Scholar]
- 19.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19(3):433–442. [DOI] [PubMed] [Google Scholar]
- 20.Pichler R, Afkarian M, Dieter BP, Tuttle KR. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol Renal Physiol. 2017;312(4):F716–f731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pena MJ, Heinzel A, Heinze G, et al. A panel of novel biomarkers representing different disease pathways improves prediction of renal function decline in type 2 diabetes. PLoS One. 2015;10(5):e0120995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bob F, Schiller A, Timar R, et al. Rapid decline of kidney function in diabetic kidney disease is associated with high soluble Klotho levels. Nefrologia. 2019;39(3):250–257. [DOI] [PubMed] [Google Scholar]
- 23.Coca SG, Nadkarni GN, Huang Y, et al. Plasma Biomarkers and Kidney Function Decline in Early and Established Diabetic Kidney Disease. J Am Soc Nephrol. 2017;28(9):2786–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombo M, Looker HC, Farran B, et al. Serum kidney injury molecule 1 and β(2)-microglobulin perform as well as larger biomarker panels for prediction of rapid decline in renal function in type 2 diabetes. Diabetologia. 2019;62(1):156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowak N, Skupien J, Niewczas MA, et al. Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int. 2016;89(2):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowak N, Skupien J, Smiles AM, et al. Markers of early progressive renal decline in type 2 diabetes suggest different implications for etiological studies and prognostic tests development. Kidney Int. 2018;93(5):1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Percentage contribution in global Wald χ2 score for individual biomarkers.
Table S1. Baseline characteristics of study participants in the random subcohort overall and by quartile of TNFR-1.
Table S2. Baseline characteristics of study participants in the random subcohort overall and by quartile of TNFR-2.
Table S3. Baseline characteristics of study participants in the random subcohort overall and by quartile of MCP-1.
Table S4. Baseline characteristics of study participants in the random subcohort overall and by quartile of sUPAR.
Table S5. Baseline characteristics of study participants in the random subcohort overall and by quartile of YKL-40.
Table S6: Baseline characteristics of study participants by development of kidney failure needing replacement therapy.
Table S7. Strength of association of 6 plasma biomarkers with incident KFRT determined by LASSO regression.


