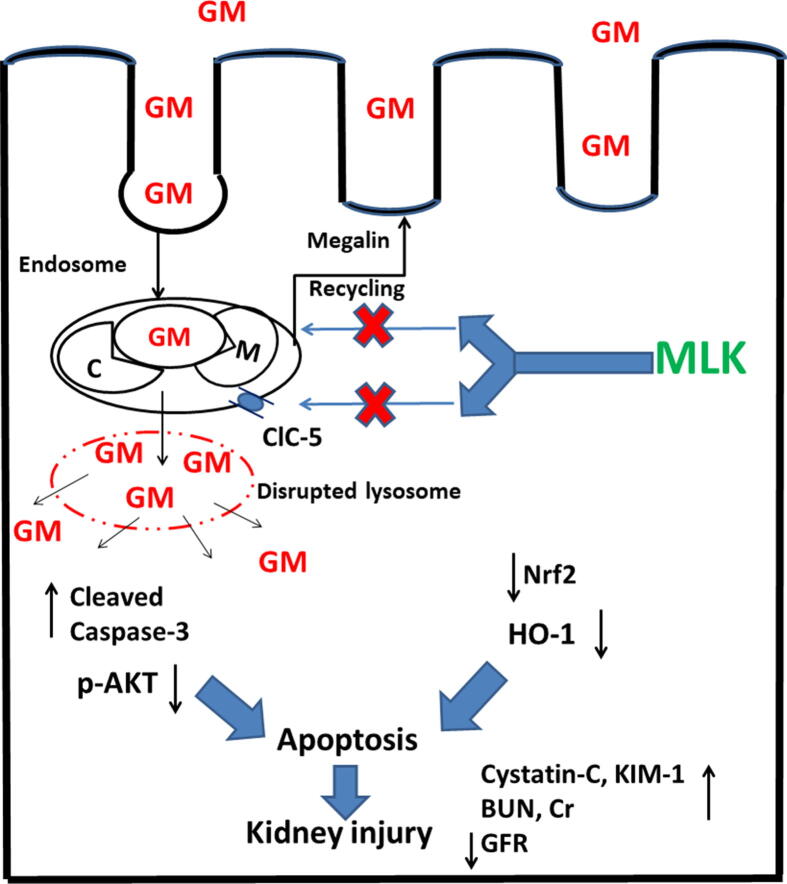
Graphical abstract
Keywords: Gentamicin endocytosis, Megalin, Chloride channel-5, Montelukast, Apoptosis
Abbreviations: BUN, Blood urea nitrogen; ClC-5, Chloride channel-5; Cr, Creatinine; FITC-BSA, Fluorescein isothiocyanate conjugate-bovine serum albumin; GM, Gentamicin; GFR, Glomerular filtration rate; HO-1, Heme oxygenase-1; H & E, Hematoxylin and eosin; KIM-1, Kidney injury molecule-1; MLK, Montelukast; Nrf2, Nuclear factor erythroid 2-related factor 2; PI3K, Phosphoinositide 3-kinase; Bcl-2, B-cell lymphoma 2; Bad, Bcl-2 associated agonist of cell death; Bcl-xL, B-cell lymphoma-extra large
Highlights
-
•
GM administration upregulated megalin and ClC-5 protein expressions.
-
•
Concurrent treatment with MLK downregulated ClC-5 protein expression.
-
•
Interference with ClC-5 by MLK reduced megalin expression.
-
•
MLK reduced renal uptake of FITC-BSA indicating impaired endocytic function.
-
•
MLK regulated p-AKT1/cleaved caspase-3 apoptotic pathway and kidney functions.
Abstract
Megalin receptor-mediated endocytosis participates a crucial role in gentamicin (GM) uptake, accumulation, and toxicity. In this study, we investigated the potential effects of montelukast (MLK) on megalin expression/endocytic function against GM nephrotoxicity. Male Wistar rats were administered GM (120 mg/kg; i.p.) daily in divided doses along 4 hr; 30 mg/kg/hr; for 7 days. MLK (30 mg/kg/day) was orally administered 7 days before and then concurrently with GM. The protein expressions of megalin and chloride channel-5 (ClC-5); one of the essential regulators of megalin endocytic function; were determined by Western blotting. Besides, the endocytic function of megalin was evaluated by the uptake of bovine serum albumin labeled with fluorescein isothiocyanate (FITC-BSA) into proximal tubular epithelial cells. Moreover, kidney function biomarkers (Cr, BUN, GFR, KIM-1, cystatin-C) and apoptosis markers (p-AKT1, cleaved caspase-3) were estimated. Co-treatment with MLK downregulated ClC-5 expression leading to reduced recycling of megalin to the plasma membrane, reduced expression, and so impaired endocytic function that was evidenced by reduced uptake of FITC-BSA in proximal tubular epithelial cells. The protein expression of the apoptotic executioner cleaved caspase-3 was significantly reduced, while that of the antiapoptotic p-AKT1 was elevated. These results were confirmed by the improvement of kidney functions and histological findings. Our data suggest that MLK could interfere with megalin expression/endocytic function that could be attributed to downregulation of ClC-5 protein expression. That eventually reduces renal cell apoptosis and improves kidney functions after GM administration without affecting the antibacterial activity of GM. Therefore, reduced expression of ClC-5 and interference with megalin expression/endocytic function by MLK could be an effective strategy against GM nephrotoxicity.
1. Introduction
Aminoglycosides are widely used for the treatment of various infections caused by Gram-negative bacteria, in spite of new antibiotic generations (Krause et al., 2016). Gentamicin (GM) is one of the most commonly used aminoglycoside antibiotics because of its broad spectrum and rapid bactericidal activity, relatively low resistance rate, and low cost (Nagai and Takano, 2014, Krause et al., 2016). However, its clinical use is reduced due to toxic effects on different tissues (Ali et al., 2020, Hu and Ma, 2021, Mousavinasab et al., 2021). Kidney functions should be closely monitored in patients receiving GM because of the high risk of acute renal failure (Bell et al., 2014). Despite the generation of antibiotics with less side effects, the synergistic effect of aminoglycosides with different antibiotics, as well as the development of multidrug resistance have led to reconsider treatment with aminoglycosides a better choice (Wargo and Edwards, 2014, Krause et al., 2016).
A key factor in GM nephrotoxicity is its accumulation in proximal tubule epithelial cells. The entry and accumulation of GM depend on a transport system because of its hydrophilic properties that hinder penetration across renal cell membrane. Therefore, a specific transport system for proteins and cations in the proximal tubules is responsible for GM transportation via endocytosis (Nagai and Takano, 2014, Randjelovic et al., 2017).
The macromolecular complex of endocytosis in the proximal tubules consists of megalin, cubilin, amnionless, disabled-2 (Dab2), and chloride channel-5 (ClC-5) (Trimarchi et al., 2020). GM uptake directly correlates to expression/functionality of megalin. Knockout of megalin, greatly reduced GM uptake, indicating that endocytosis is the major pathway for GM accumulation in the kidney and consequently its nephrotoxic effect (Schmitz et al., 2002, Mahadevappa et al., 2014). After endocytosis, GM is transferred to lysosomes for degradation. At certain concentration, lysosomal membrane disruption occurs leading to GM release into the cytosol and eventually renal cell apoptosis (Quiros et al., 2011, McWilliam et al., 2017, Randjelovic et al., 2017).
The 2Cl-/H+ exchanger ClC-5 is an important regulator of megalin endocytosis, where its deficiency leads to defective endocytosis and reduced megalin expression by impairing its recycling to the cell membrane (Christensen et al., 2003, De et al., 2014). Therefore, interference with ClC-5 expression with subsequently affected megalin receptor could be an effective strategy to protect against the nephrotoxic effect of GM.
Montelukast sodium (MLK) is an orally active blocker of cysteinyl leukotriene receptor 1 (CysLTR1) in the bronchial smooth muscles, so it is used to alleviate the symptoms of chronic asthma and allergic rhinitis (Nayak, 2004, Yokomizo et al., 2018). Several studies have reported the nephroprotective effect of MLK against different nephrotoxicants and gamma radiation through its antioxidant, anti-inflammatory, and antiapoptotic properties (Otunctemur et al., 2013, Gad et al., 2017, Köse et al., 2019, Hormati et al., 2020). Interestingly, it has been demonstrated that leukotriene receptor blockade downregulates Cl- conductance in hepatocytes (Meng et al., 1997). Therefore, we hypothesized that MLK could downregulate renal ClC-5 expression which has a pivotal role in megalin endocytosis. Accordingly, interference with ClC-5 could reduce the expression and endocytic function of renal megalin, and thereby afford nephroprotective effect against GM.
2. Materials and methods
2.1. Animals
Wistar male rats (180 - 200 g) were purchased from Nahda Animal Facility, Nahda University, Beni-Suef, Egypt. Rats were maintained two weeks for adaptation and kept under controlled conditions of room temperature (23 ± 2 °C) and 12/12 hr dark-light cycles. Rats were allowed free access to standard diet and water. The procedures performed on animals were in accordance with the National Institutes of Health guide for care and use of laboratory animals. In addition, the experimental procedures have been approved by the Institutional Animal Care and Use Committee, Beni-Suef University (IACUC, 019–81).
2.2. Drugs, chemicals, kits, and antibodies
Montelukast sodium was obtained from Merck & Co. Inc. (USA). Garamycin® ampoules (Schering-Plough) containing 80 mg/2 ml of GM sulphate were used in this experiment. Fluorescein isothiocyanateconjugated bovine serum albumin (FITC-BSA) was purchased from Sigma-Aldrich (USA, CAT# A9771) and dissolved in 10 mM Tris, pH 7. ELISA kits including, rat kidney injury molecule-1(KIM-1) (CAT# 18654) and rat cystatin-C (CAT# 18659) were purchased from Glory Science Co. (China). Creatinine (Cr) and blood urea nitrogen (BUN) colorimetric kits were purchased from Bio-Med (Egypt), while those of albumin and calcium were purchased from SPINREACT (Spain). The primary antibodies of mouse monoclonal megalin antibody (CAT# H-10, sc-515772), anti-p-AKT1 (CAT# 104A282, sc-52940), and anti-β-actin (CAT# ACTBD11B7, sc-81178) were purchased from Santa Cruz Biotechnology, while polyclonal anti-ClC-5 antibody (CAT# C1116) was purchased from Sigma-Aldrich (USA). The polyclonal anti-cleaved caspase-3 (CAT# YPA2210), anti-HO-1 (CAT# YPA1919), and anti-Nrf2 (CAT# YPA1621) antibodies were purchased from Biospes (China).
2.3. Experimental design
At the beginning, we carried out a pilot study to investigate the suitable dose of GM to induce renal glomerular and tubular dysfunction. Thirty rats were divided into five groups, each of six rats. Rats of the first group (control) were administered saline intraperitoneally. For the second group, rats were administered GM (100 mg/kg; i.p.) once daily for 1 week, while those of the third group were administered GM (100 mg/kg; i.p.) divided along 4 hr; 25 mg/kg/hr; daily for 1 week. Rats the fourth group were administered GM (120 mg/kg; i.p.) once daily for 1 week, while those of the fifth group were administered GM (120 mg/kg; i.p.) divided along 4 hr; 30 mg/kg/hr; daily for 1 week. The first dose was administered at the same time every day. After 24 hr of the last GM dose, blood and kidney tissue samples were collected for estimation of serum Cr and BUN levels, as well as histopathological investigation.
Afterwards, we carried out the main investigation to evaluate the potential effects of MLK against GM nephrotoxicity. Twenty-four animals were divided into four groups each consisting of six rats. The first group (control) received distillated water orally. The second group received MLK (30 mg/kg/day, p.o.) for 14 consecutive days (İçer et al., 2016). The third group was injected with GM at a dose of 120 mg/kg/day i.p. divided along 4 hr; 30 mg/kg/hr; daily for 1 week. The fourth group received both MLK and GM, where MLK was administered for 7 days before GM injection and then concurrently with GM for another 1 week. Treatment regimens were chosen according to previous studies and confirmed by the pilot study.
On the 14th day after the last dose of GM, each rat was placed in a metabolic cage for 24 hr to collect urine. The collected urine samples were centrifuged at 2805 × g for 10 min, and then kept at −20 °C for determination of Cr and cystatin-C levels. On the 15th day, blood samples and kidney tissue samples were collected. Serum was separated by centrifugation at 5610 × g and then stored at −20 °C until analysis. One of the isolated kidneys was used to prepare 20% w/v homogenate using ice-cold 0.1 M phosphate buffer saline (pH = 7.4). The other kidney was either preserved in RIPA lysis buffer containing protease inhibitor cocktail and then kept at −20 °C till Western blot analysis or fixed in 10% formol saline for histopathological assessment.
2.4. Assessment of kidney functions
The levels of Cr and BUN were determined in serum and urine samples for assessment of acute kidney injury according to the instructions of each kit manufacturer. Besides, KIM-1 was determined in kidney samples, while cystatin-C was estimated in both serum and urine samples by ELISA kits. The glomerular filtration rate (GFR) was estimated according to the previously reported formula: (Cr in urine × urine flow)/Cr in serum, where the urine flow (ml/min) was calculated from urine volume of 24 hr/1440 (Abdelrahman 2018).
2.5. Estimation of the endocytic function of megalin receptor
On the 14th day, FITC-BSA (20 mg/kg) (Abd El-Lateef et al., 2019) was injected intraperitoneally one hr after the last dose of GM. Kidneys were collected after 20 min and then preserved in 10% neutral buffered formalin solution, processed, sectioned and stained with alcian blue dye (Carleton 1980). Images of tissue sections were taken using fluorescence microscope fitted with DS-Fi15-Meg Color C digital camera and the blue fluorescence intensity was evaluated using ImageJ software version 1.46.
In addition, the urinary excretion of the megalin ligands albumin and calcium was estimated as another indicator to the effect of MLK on megalin endocytic function. The levels of albumin and calcium in urine samples were measured according to kit manufacturer’s instructions.
2.6. Determination of megalin, ClC-5, p-AKT, cleaved caspase-3, Nrf2, and HO-1 protein expressions by Western blotting
Kidney samples (50 mg) were homogenized in RIPA lysis buffer with protease inhibitor cocktail (Biospes, China) at 4 °C for 30 min, then the protein concentration of each sample was determined using Biuret method (Wang et al., 1996).
The transcription factor Nrf2 was estimated in the nuclear fraction that was extracted as follows: kidney tissue (100 mg) was rinsed twice in PBS then re-suspended in 1 ml of the lysis buffer containing DTT (0.1 M) and protease inhibitors. The tissue was homogenized until more than 90% of the cells were broken and the nuclei were visualized under the microscope. The disrupted cells were centrifuged for 20 min at 10,000–11,000 × g. The resultant supernatant is the cytoplasmic fraction. The nuclei pellets were re-suspended in ∼ 140 µl of extraction buffer (20 mM HEPES pH 7.9, with 1.5 mM MgCl2, 0.42 M NaCl, 0.2 mM EDTA, 25% (v/v) Glycerol) containing DTT and protease inhibitor. Finally, homogenization for short period can be done to facilitate nuclear extraction followed by shaking gently for 30 min, centrifugation for 5 min at 10,000 – 12,000 × g, then separation and storage of the supernatant at –20 °C.
After protein extraction, samples were loaded on 10–12% SDS-polyacrylamide gel for electrophoresis then transferred to PVDF membrane (Millipore, MERCK, Germany) using semidry transfer method (Towbin et al., 1979). The membrane was blocked with 5% non-fat milk in TBST buffer for 1 h, then incubated at 4 °C overnight with the primary antibody specific for the target protein. That was followed by incubation with alkaline phosphatase-conjugated anti-goat secondary antibody. Protein bands were detected by BCIP/NBT colorimetric detection kit (Biospes, China) and quantified using densitometric analysis software (ImageJ, USA), with relative quantification to beta actin.
2.7. Histological investigation
The kidney samples were fixed in 10% formol-saline solution for 24 hr then dehydrated using serial dilutions of alcohol. Afterwards, specimens were embedded in paraffin wax in hot air oven for 24 h at 56 °C. Paraffin blocks (5 μm) were transversely sectioned using sledge microtome. Each section was stained with Hematoxylin and Eosin (H&E) as illustrated previously (Bancroft and Gamble 2008), followed by scoring of the lesions as previously demonstrated (Abd El-Lateef et al., 2019).
2.8. Antibacterial activity assay
The agar disc diffusion method was utilized to test the effect of MLK on the antibacterial activity of GM. Briefly, Escherichia coli was cultured in Mueller-Hinton broth and adjusted to a concentration equivalent to 0.5 McFarland Standard onto Mueller-Hinton agar plates. The 1st well was filled with GM (10 mg), the 2nd with MLK (10 mg), and the 3rd with mixture of MLK (10 mg) and GM (10 mg). The plate was incubated overnight at 37 °C followed by examination of the bacterial growth and zone of inhibition.
2.9. Statistical analysis
All the results were expressed as means ± standard deviation (SD). All statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test using Graph Pad Prism 8, (GraphPad Software Inc., USA). Dunnett’s post-hoc test was used in the preliminary study to compare different doses of GM to the control group. The results were deemed statistically significant at p < 0.05.
3. Results
3.1. Effect of MLK on endocytosis pathway
3.1.1. Effect of MLK on ClC-5 and megalin protein expressions
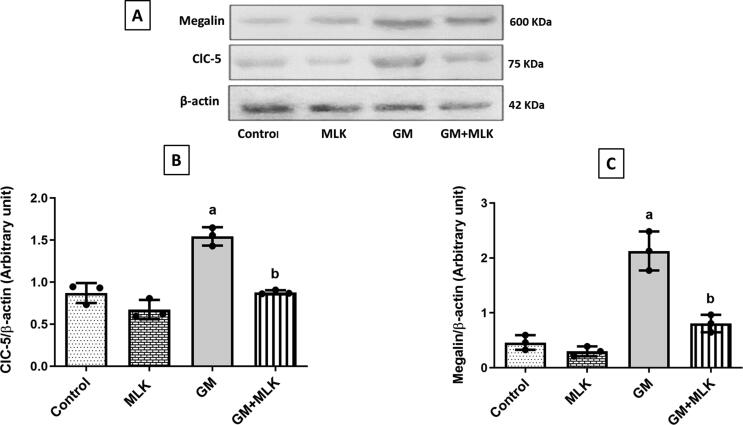
Gentamicin group showed a significant increase in the protein expressions of ClC-5 and megalin by 77.61% and 363%, respectively compared to the control group. Concurrent treatment with MLK significantly decreased these protein expressions by 43.13% and 62.15%, respectively compared to GM group (Fig. 1).
Fig. 1.
Effect of MLK on GM endocytic proteins. (A) Western blots for ClC-5 and megalin. (B, C) Graphical presentations for the changes in protein expression of ClC-5 and megalin, respectively. The protein expressions of ClC-5 and megalin were increased in GM-treated rats. Concurrent treatment with MLK significantly reduced their expressions. Dots represent individual values, while bars represent mean ± SD (n = 3). Comparisons were made using one-way ANOVA followed by Tukey’s post-hoc test. a Significantly different from control group at p < 0.05, b Significantly different from GM group at p < 0.05.
3.1.2. Effect of MLK on the endocytic function of megalin receptor
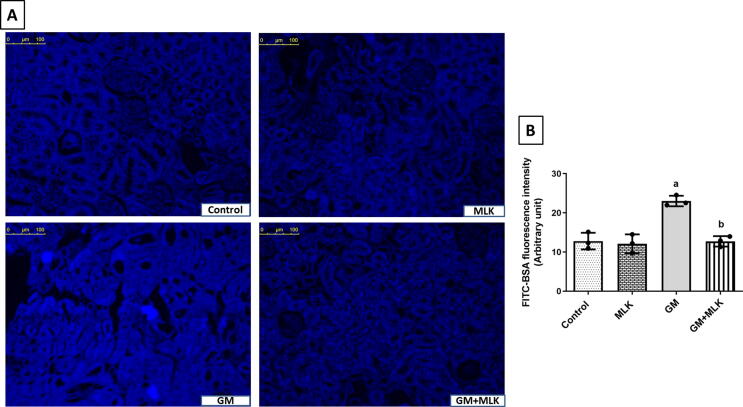
To prove the effect of MLK on the endocytic function of megalin receptor in the proximal tubular epithelial cells, we analyzed the fluorescence of FITC-BSA where its uptake is mediated through megalin receptor. We detected a statistically significant increase in the fluorescence intensity in GM group by 79.91% compared to the control group. Co-treatment with MLK reduced the fluorescence intensity of FITC-BSA by 44.72% as compared to GM-treated group (Fig. 2).
Fig. 2.
Effect of MLK on megalin endocytic function in GM-treated rats. (A) Representative images demonstrating FITC-BSA uptake in renal cells with increased blue fluorescence intensity in GM-treated group, while the fluorescence intensity was reduced in GM + MLK group. (B) Graphical presentation of the changes in blue fluorescence intensity in different groups. Dots represent individual values, while bars represent mean ± SD (n = 3). Comparisons were made using one-way ANOVA followed by Tukey’s post-hoc test. a Significantly different from control group at p < 0.05, b Significantly different from GM group at p < 0.05.
3.1.3. Effect of MLK on urinary excretion of some megalin ligands
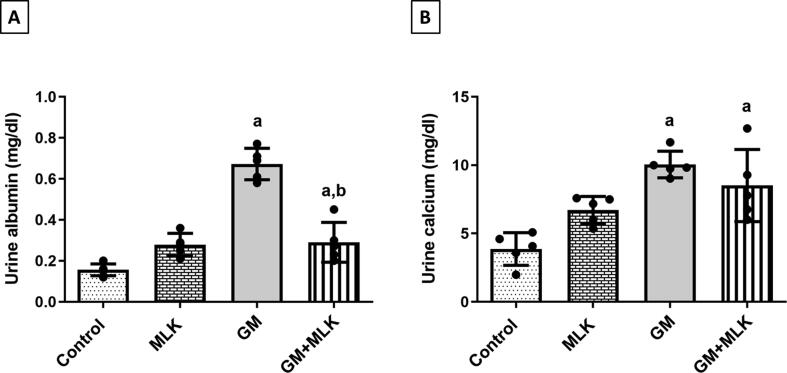
We assessed the effect of treatment with GM alone and concurrently with MLK on the urinary excretion of some megalin ligands (albumin and calcium). We found that GM significantly increased albumin and calcium levels by 3.31- and 1.60-fold, while co-treatment with MLK showed significantly decreased albumin level by 56.85% and a slight non-significant decrease in calcium level when compared to GM-treated group (Fig. 3).
Fig. 3.
Effect of MLK on urinary excretion of some megalin ligands in GM-treated rats. (A) Albumin, and (B) Calcium. The levels of albumin and calcium were significantly increased in urine samples of GM-treated rats. Concurrent treatment with MLK significantly ameliorated albumin levels, while those of calcium were still elevated. Dots represent individual values, while bars represent mean ± SD (n = 5). Comparisons were made using one-way ANOVA followed by Tukey’s post-hoc test. a Significantly different from control group at p < 0.05, b Significantly different from GM group at p < 0.05.
3.2. Effect of MLK on GM-induced apoptosis.
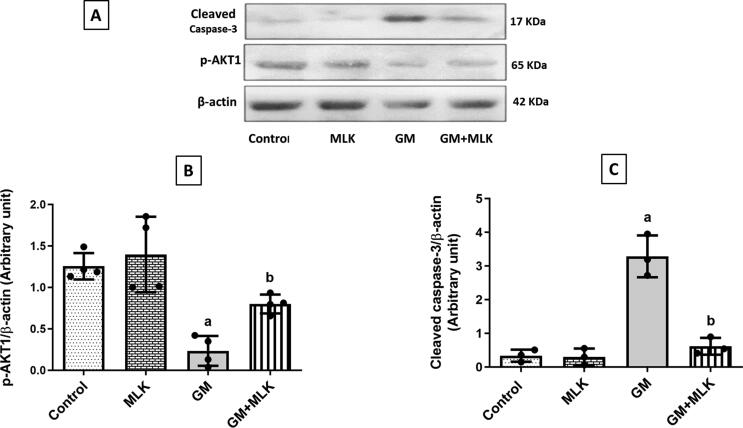
Gentamicin administration induced apoptosis which was evident by increased expression of cleaved caspase-3 by 8.81-fold and decreased expression of the antiapoptotic p-AKT1 by 81.31% when compared to the control group. Our results elucidated the antiapoptotic effect of MLK through a significantly reduced cleaved caspase-3 expression by 81.26% and increased p-AKT1 expression by 2.41-fold as compared to GM-treated group (Fig. 4).
Fig. 4.
Effect of MLK on the apoptotic pathway p-AKT1/cleaved caspase-3 in GM-treated rats. (A) Western blots for p-AKT1 and cleaved caspase-3. (B, C) Graphical presentations for the changes in protein expression of p-AKT1 and cleaved caspase-3, respectively. Administration of MLK with GM significantly attenuated the apoptotic effect of GM through enhancing p-AKT1 protein expression, while reducing that of cleaved caspase-3. Dots represent individual values, while bars represent mean ± SD (n = 3–4). Comparisons were made using one-way ANOVA followed by Tukey’s post-hoc test. a Significantly different from control group at p < 0.05, b Significantly different from GM group at p < 0.05.
3.3. Effect of MLK on antioxidant defense in GM-treated rats.
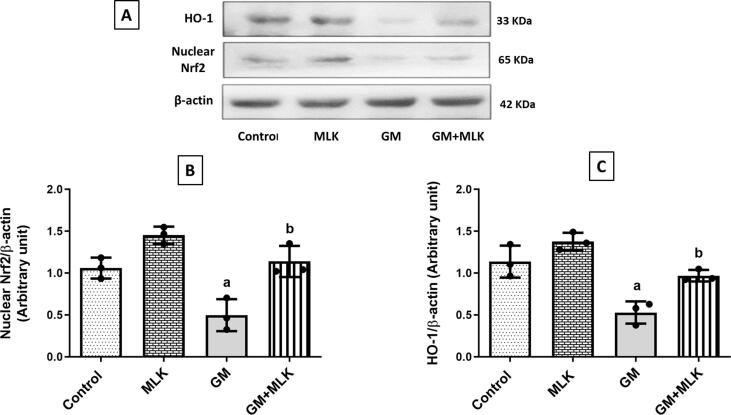
Results of the current study revealed that GM administration reduced renal antioxidant defense, as evidenced by downregulation of the nuclear fraction of the transcription factor Nrf2 and the antioxidant enzyme HO-1 expressions by about 53.10% and 53.52%, respectively when compared to the control group. MLK administration to GM-treated rats enhanced the antioxidant defense in kidney tissue through increased expressions of nuclear Nrf2 and HO-1 by 1.29- and 0.83-fold, respectively as compared to GM group (Fig. 5).
Fig. 5.
Effect of MLK on the antioxidant pathway Nrf2/HO-1 in GM-treated rats. (A) Western blots for nuclear Nrf2 and HO-1. (B, C) Graphical presentations for the changes in protein expression of nuclear Nrf2 and HO-1, respectively. Administration of GM 120 mg/kg/day for 7 days significantly reduced the expression of the transcription factor Nrf2 and consequently the antioxidant enzyme HO-1, while co-treatment with MLK restored these protein expressions to the normal level. Dots represent individual values, while bars represent mean ± SD (n = 3). Comparisons were made using one-way ANOVA followed by Tukey’s post-hoc test. a Significantly different from control group at p < 0.05, b Significantly different from GM group at p < 0.05.
3.4. Effect on kidney function biomarkers
3.4.1. Effect of single and divided dosing of two different doses of GM on Cr and BUN
Regarding Cr level, there was no significant difference between control group, GM (100 mg/kg) single-dose group, GM (120 mg/kg) single-dose group, and GM (100 mg/kg) divided-dose group. However, a significant increase in Cr level was shown between control and GM (120 mg/kg) divided-dose group by 4.54-fold.
Meanwhile, BUN levels for GM (100 mg/kg) single-dose group, GM (120 mg/kg) single-dose group, and GM (100 mg/kg) divided-dose group were significantly increased by 1.23–, 1.64-, and 1.20-fold, respectively. GM (120 mg/kg) divided dosing significantly increased BUN by 2.68-fold when compared to control group (Table 1).
Table 1.
Changes in Cr and BUN after single and divided dosing per day of GM for 1 week.
| Groups | Cr | BUN |
|---|---|---|
| Control | 0.56 ± 0.08 | 34.82 ± 2.01 |
| GM (100 mg/kg) single-dose | 0.53 ± 0.02 | 77.71 ± 17.80a |
| GM (100 mg/kg) divided-dose | 0.71 ± 0.24 | 76.53 ± 19.08a |
| GM (120 mg/kg) single-dose | 0.65 ± 0.04 | 92.05 ± 13.90a |
| GM (120 mg/kg) divided-dose | 3.10 ± 0.34a | 128.00 ± 1.23a |
Divided dosing of GM (120 mg/kg) significantly elevated both serum Cr and BUN levels compared to control, while other dosing systems significantly elevated BUN levels only. Data are expressed as mean ± SD (n = 6). Comparisons were made using one-way ANOVA followed by Dunnett’s post-hoc test. a Significantly different from control group at p < 0.05.
3.4.2. Effect of MLK on traditional kidney function biomarkers in GM-treated rats
In addition to significantly increasing Cr and BUN levels, divided dosing of GM 120 mg/kg/day for 7 days produced a significant decrease in GFR by 88.89%, when compared to control group (Table 2).
Table 2.
Effect of MLK on traditional kidney function biomarkers in GM-treated rats.
| Groups | Cr (mg/dl) | BUN (mg/dl) | GFR (ml/min) |
|---|---|---|---|
| Control | 0.50 ± 0.04 | 34.82 ± 2.01 | 0.45 ± 0.09 |
| MLK | 0.46 ± 0.03 | 34.66 ± 2.07 | 0.44 ± 0.10 |
| GM | 3.10 ± 0.34a | 128.00 ± 1.23a | 0.05 ± 0.01a |
| GM + MLK | 0.83 ± 0.12a,b | 71.74 ± 16.34a,b | 0.30 ± 0.04a,b |
Serum Cr and BUN levels were significantly increased, while GFR was decreased in GM group. Concurrent treatment with MLK significantly reduced Cr and BUN levels, in addition to improved GFR compared to GM group. Data are expressed as mean ± SD (n = 6). Comparisons were made using one-way ANOVA followed by Tukey’s post-hoc test. a Significantly different from control group at p < 0.05, b Significantly different from GM group at p < 0.05.
Treatment with MLK concurrently with GM resulted in a reduction in Cr, BUN by 73.23%, 43.95%, along with increased GFR by 5-fold compared to GM-treated group (Table 2).
3.4.3. Effect of MLK treatment on sensitive kidney function biomarkers
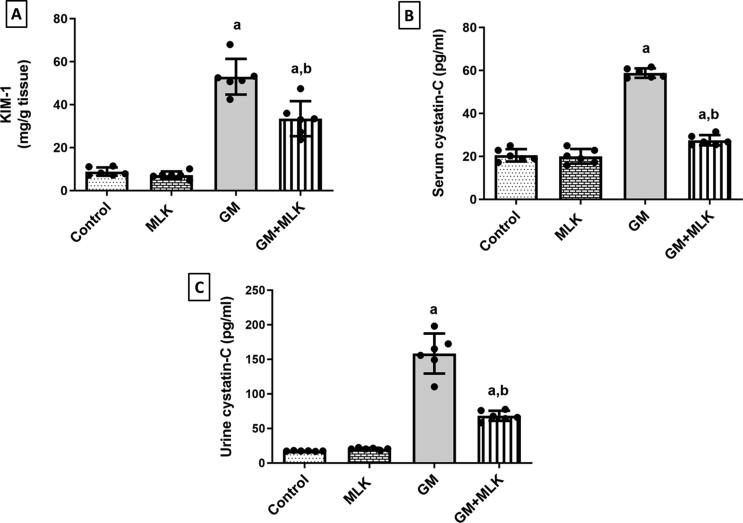
Divided dosing of GM 120 mg/kg/day for 7 days produced a significant increase in KIM-1, serum cystatin-C, and urine cystatin-C by 5.01-, 1.86-, and 8.09-fold, respectively compared to control rats. Co-treatment with MLK resulted in reduction of KIM-1, serum cystatin-C, and urine cystatin-C by 37%, 53.22%, and 56.91%, respectively compared to GM group (Fig. 6).
Fig. 6.
Effect of MLK on sensitive and specific kidney function biomarkers in GM-treated rats. (A) KIM-1, (B) serum cystatin C, and (C) urine cystatin C levels. Tubular damage was indicated by increased levels of those sensitive and specific kidney function biomarkers in GM-treated rats. Concurrent treatment with MLK significantly ameliorated these elevations. Dots represent individual values, while bars represent mean ± SD (n = 6). Comparisons were made using one-way ANOVA followed by Tukey’s post-hoc test. a Significantly different from control group at p < 0.05, b Significantly different from GM group at p < 0.05.
3.5. Effect of MLK on histopathological changes
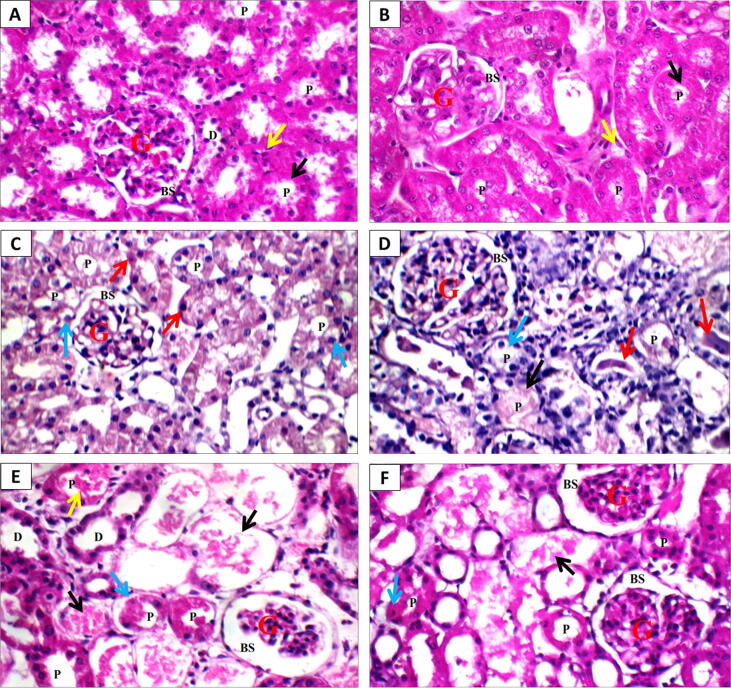
Photomicrographs of kidney sections from control and MLK groups showed normal renal architecture (capsule, glomeruli and Bowman’s space, proximal tubules with brush borders, renal medulla and collecting tubules with epithelial lining and interstitium) (Fig. 7A & B). Kidneys from rats administered single-dose of GM (100 and 120 mg/kg/day) showed normal glomeruli and Bowman’s spaces, scattered necrotic proximal tubules with intra-tubular hyaline casts (Fig. 7 C & D). However, in divided-dose GM (120 mg/kg/day) group, the kidney showed irregular renal capsule, distorted hypercellular glomeruli with obliterated Bowman’s spaces, marked tubular necrosis, proximal tubules with apoptotic epithelial lining, and renal medulla showed collecting tubules with intra-tubular hyaline casts. While sections from MLK treated group showed marked improvement in kidney architecture including, renal capsule, glomeruli with Bowman’s spaces, mild tubular necrosis, proximal tubules with mild apoptotic epithelial lining, and renal medulla with epithelial lining of collecting tubules showing few scattered intra-tubular hyaline casts (Fig. 7E & F). The scoring of histopathological changes is presented in Table 3.
Fig. 7.
Effect of MLK on histopathological alterations in kidney sections stained with H&E (x400) from GM-treated rats. A & B) Control and MLK groups, respectively showing normal glomeruli (G) with normal Bowman’s spaces (BS), normal proximal tubules (P) with preserved brush borders (black arrow), normal distal tubules (D), and normal interstitium (yellow arrow), C) GM single-dose (100 mg/kg) group showing normal glomerulus (G) and Bowman’s space (BS), scattered proximal tubules (P) with edematous (blue arrows) apoptotic epithelial lining (red arrows). D) GM single-dose (120 mg/kg) group showing normal glomerulus (G) and Bowman’s space (BS), scattered completely necrotic proximal tubules (black arrow) and others with complete loss of brush borders (blue arrow), and intra-tubular hyaline casts (red arrows). E) GM divided-dose (120 mg/kg) group showing atrophied glomerulus (G) with wide Bowman’s space (BS), marked tubular necrosis (black arrow), and scattered viable proximal tubules (p) with apoptotic epithelial lining (blue arrow) and intra-tubular hyaline casts (yellow arrow). F) GM + MLK group showing normal glomeruli (G) with normal Bowman’s spaces (BS), mild tubular necrosis (black arrow), and proximal tubules (P) with mild apoptotic epithelial lining (blue arrow).
Table 3.
Histopathological scoring.
| Groups | Glomeruli | Boman’s spaces | Tubules |
Interstitium | Medulla | ||
|---|---|---|---|---|---|---|---|
| lining | Brush border | lumen | |||||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MLK | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GM | ++ | + | ++ | ++ | ++ | ++ | ++ |
| GM + MLK | 0 | 0 | + | + | 0 | 0 | 0 |
Glomeruli: 0: Normal +: Edematous/congested ++: Small-sized/atrophied
Bowman’s spaces (BS): 0: Normal +: Widened/dilated ++: Narrow/obliterated
Tubules:
■ Lining: 0: Normal +: Mild apoptosis ++: Moderate/marked necrosis
■ Brush border: 0: Preserved +: Partial loss ++: Complete loss
■ Lumen: 0: Free +: Intra-tubular debris ++: Intra-tubular casts
Interstitium: 0: Normal +: Mild inflammatory infiltrate ++: Moderate/marked inflammatory infiltrate
Medulla: 0: Normal +: Few hyaline casts ++: Intra-tubular hyaline casts
3.6. Effect of MLK on the antibacterial activity of GM
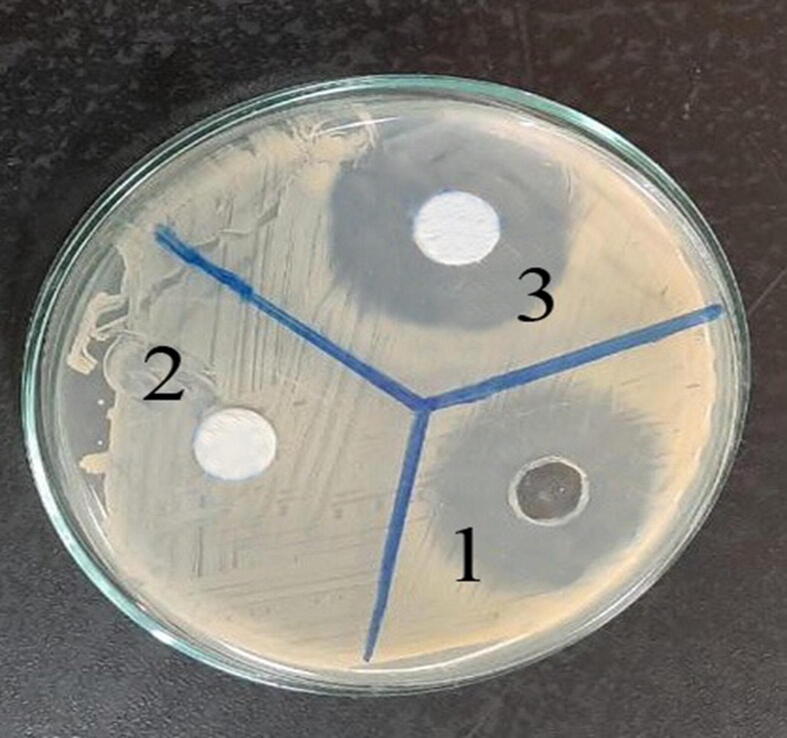
Finally, we found that MLK did not inhibit the antibacterial activity of GM using agar diffusion method. The well containing 10 mg MLK showed no growth-inhibition zone against Escherichia coli (zero). The well containing GM + MLK revealed an adequate inhibitory zone (34 mm) that was similar to the inhibitory zone of GM in the absence of MLK (34 mm). These findings demonstrate that MLK has no synergistic or inhibitory effect on the bacteriostatic action of GM (Fig. 8).
Fig. 8.
Effect of MLK on the antimicrobial activity of GM by Agar diffusion method. Drugs were placed on Mueller-Hinton agar plates that had been incubated with Escherichia coli. The contents of the numbered wells were as follows: 1: GM (10 mg), 2: MLK (10 mg), and 3: GM (10 mg) + MLK (10 mg). Well 3 showed the same inhibitory zone as well 1, and well 2 showed no inhibitory zone, demonstrating that MLK has no synergistic or inhibitory effect on the bacteriostatic action of GM.
4. Discussion
In this study, we focused on the main pathophysiological mechanism responsible for GM nephrotoxicity. The uptake of GM through endocytosis leads to its accumulation in lysosomes of the proximal tubules resulting in lysosomal membrane rupture and consequently tubular cell death (Quiros et al., 2011, Randjelovic et al., 2017).
In the proximal tubular epithelial cells, megalin receptor is considered the gait for GM uptake and accumulation, where megalin expression elevates in GM-treated rats (Dagil et al., 2013, Jado et al., 2020). In accordance, our study elucidated an increase in megalin and ClC-5 expressions in GM-treated group.
After participation in ligand endocytosis, megalin recycles to the plasma membrane. Accumulated evidence has attributed reduced megalin expression and impaired recycling to the deficiency or dysfunction of ClC-5 (Christensen et al., 2003, Novarino et al., 2010, Hryciw et al., 2012). ClC-5 is a Cl-/H+ transporter primarily expressed in the kidney to participate mainly in endosome acidification which is essential for uptake and trafficking in the proximal tubules. The acidification of endosomes facilitates dissociation between megalin and its ligand followed by megalin recycling to the brush border membrane (Hara-Chikuma et al., 2005, Wellhauser et al., 2010).
Our results clearly demonstrated reduced megalin expression upon 14 days administration of MLK to GM-treated rats. This effect could be attributed to downregulation of ClC-5 expression. The effect of MLK on renal ClC-5 is in accordance with the previously reported effect of leukotriene receptor blockade on Cl- conductance in hepatocytes, where different subtypes of ClC are expressed in the liver (Meng et al., 1997, Li and Weinman, 2002).
Interestingly, albumin uptake into renal proximal tubular cells is mediated through megalin/cubilin complex (Ren et al., 2020), and so its administration one hr after the last dose of GM may reflect GM uptake into renal cells. FITC-BSA injected in GM-treated group produced an increase in the fluorescence intensity due to increased megalin expression as previously illustrated (Bryniarski et al., 2018). These results give an indication to increased GM concentration in the renal cortex compared to the control group. Contrarily, the decrease in fluorescence intensity of FITC-BSA in GM + MLK group could be attributed to downregulation of megalin receptor. In parallel, de Barros Peruchetti et al., (2018) have reported that the decrease in megalin expression induced by high glucose leads to decreased albumin endocytosis. Therefore, decreased albumin endocytosis in GM + MLK group could reflect reduced megalin expression and consequently GM endocytosis into renal cells.
Furthermore, urine analysis revealed an increase in urinary excretion of calcium, which is a megalin ligand (Christensen and Nielsen 2006), in GM group in spite of the overexpression of megalin receptor. That could be attributed to the interference of GM with cations for the uptake by megalin/cubilin complex in renal tubular cells, and thus urinary calcium excretion increases (Randjelovic et al., 2017). Regarding urinary calcium level in GM + MLK group, our results demonstrated slight non-significant decrease in calcium level that may be due to impaired endocytosis by MLK. The increased albuminuria that was observed in GM-treated group could be attributed to glomerular injury induced by GM that permits leakage of albumin in the glomerular filtrate which passes to the urine when the proximal tubular reabsorptive capacity is defective or saturated (Udupa and Prakash, 2019, Aziz et al., 2020). On the other hand, the protective effect of MLK against GM-induced glomerular dysfunction may explain the significant decrease in albuminuria in GM + MLK-treated group, in spite of defective endocytosis.
After endocytosis, cytosolic GM released from lysosomes triggers mitochondrial apoptotic pathway that leads to release of cytochrome c in the cytosol, in addition to reduced ATP stores and formation of reactive oxygen species (ROS) (Randjelovic et al., 2017). Released cytochrome c facilitates the conversion of caspase-3 into its active form (cleaved caspase-3) which cleaves functional proteins to induce apoptosis of cells, so it is called apoptosis executioner (Quiros et al., 2011, Hsu et al., 2014). Besides, the formed ROS activate caspase-3 by reducing mitochondrial membrane potential leading to translocation of cytochrome c to the cytosol (Cao et al., 2021). Apoptotic mitochondrial pathway is under control of the antiapoptotic protein Bcl-2 (Opferman and Kothari 2018). AKT signaling activates the antiapoptotic Bcl-2 and Bcl-xL, while retards the proapoptotic Bax and the executioner cleaved caspase-3 (Bao et al., 2017, He et al., 2020). In parallel to previous studies, our results revealed that the protein expression of cleaved caspase-3 was significantly increased, while that of p-AKT1 was decreased indicating the induction of apoptosis by GM (Kandemir et al., 2015, Kaplan et al., 2017, Kucharava et al., 2019).
On the other hand, administration of MLK with GM displayed antiapoptotic effect via enhanced p-AKT1 and reduced cleaved caspase-3 expressions, which is in accordance with previous studies on MLK (Hashim et al., 2018, Zovko et al., 2018). Similarly, zafirlukast which is another leukotriene receptor blocker has been reported to enhance the expression of p-AKT (Song et al., 2020).
The activation of AKT1 results in phosphorylation of both Thr308 and Ser473, while the antibody used in our study is more specific for estimation of p-AKT1 (Ser473). Several studies have reported the antiapoptotic effect AKT and that its deficiency leads to apoptosis (Chen et al., 2020, Qiu et al., 2020, Xu et al., 2021). Upon AKT phosphorylation by PI3K, the activated AKT phosphorylates the Bcl-2 associated agonist of cell death (Bad) and inhibits its proapoptotic effect. However, dephosphorylated Bad interacts with Bcl-2 or Bcl-xL on mitochondrial membrane to antagonize their antiapoptotic effects, resulting in cytochrome c release and eventually apoptosis by activation of caspases (Liu et al., 2020, Xu et al., 2021). Therefore, the activation of AKT signaling guards against apoptosis by preventing the deleterious effects of Bad.
The transcriptional factor Nrf2 plays a vital role in protection against oxidative stress by nuclear translocation and enhancing the transcription of antioxidant enzymes such as HO-1, thus reducing kidney injury (Shelton et al., 2013, Lu et al., 2019). Nrf2/HO-1 signaling is an important cellular protective mechanism against increased ROS and subsequently apoptosis (Chen and Shaikh, 2009, Wan et al., 2019, Zhu et al., 2019).
After GM treatment, nuclear Nrf2 and HO-1 protein expressions were significantly decreased, that has also been elucidated previously (Subramanian et al., 2015). Meanwhile, treatment with GM + MLK enhanced Nrf2 and HO-1 protein expressions which is in accordance with previous studies (Jiang et al., 2017, Jung et al., 2020). Furthermore, the effect of MLK on activation of Nrf2/HO-1 signaling could also be correlated to reduced megalin expression according to the study of Reisman et al., (2012).
Concerning kidney functions, we observed from our preliminary investigation that i.p. administration of 100 mg/kg and 120 mg/kg GM once/day for 1 week have no effect on glomerular system. That was elucidated through the histopathological investigation that showed normal glomeruli, in addition to serum Cr level that didn’t significantly change after GM administration. These results match the findings of Sun et al. who have reported that GM nephrotoxicity results in tubular necrosis (Sun et al., 2018), but glomerular toxicity occurs as a result of exposure to a large dose of GM (Stojiljkovic et al., 2008, Randjelovic et al., 2017).
Although 100 mg/kg and 120 mg/kg GM are considered large doses, no effect on glomerular system was observed. That may be attributed to GM kinetics, where it has been previously demonstrated that uptake of GM from the inner ear tissues and renal cortex occurs rapidly resulting in early saturation. That was confirmed by failure to detect stable concentration after single i.m. injection of 10 or 100 mg/kg GM compared to 3 hr constant infusion of 15 μg/min (Huy et al., 1986). Similarly, Giuliano et al., (1986) have attributed the nonlinear correlation between GM concentration in renal cortex and the stable serum concentration after 6 hr continuous infusion to uptake saturation. Consequently, it has been elucidated that injection of GM at a single high dose reduces its nephrotoxic effect compared to continuous infusion (Kim et al., 2016). In parallel, we found that divided dosing of GM (120 mg/kg) along 4 hr significantly induced deterioration in kidney functions.
From results of our principle experiment, GM (120 mg/kg) divided dosing led to full prone nephrotoxicity that was evident by elevation of glomerular and tubular damage biomarkers, as well as the histopathological alterations.
Creatinine and BUN, which are subjected to glomerular filtration, are considered traditional kidney function biomarkers. Besides, Cr is used as an index for determination of GFR (Udupa and Prakash 2019). Tubular damage is indicated by increased KIM-1 which is a transmembrane glycoprotein of the proximal renal tubules. Marked upregulation of KIM-1 occurs upon acute and also chronic kidney injury, so its estimation can sensitively detect proximal tubular damage (Luft 2021). Cystatin-C, which is a cysteine protease inhibitor present in all cells, is subjected to glomerular filtration and proximal tubular reabsorption/catabolism (Luft 2021). Therefore, serum and urinary cystatin-C levels can be used to evaluate glomerular filtration and renal tubular damage. Interestingly, it has been reported that KIM-1 and cystatin-C are more sensitive and accurate compared to traditional biomarkers for early detection of GM nephrotoxicity (Udupa and Prakash 2019).
Our results demonstrated that co-treatment with MLK reduced KIM-1, serum and urinary cystatin-C levels in GM-treated rats. Parallel to our results, a previous study has demonstrated that exposure to low levels of fluoride could attenuate the nephrotoxicity of GM by reducing megalin expression and also urinary KIM-1 and cystatin-C excretion (Cárdenas-González et al., 2016).
Unexpectedly, administration of MLK to normal animals did not show significant elevation of urinary cystatin-C. It is worthy to mention that urine level of cystatin-C is very low in normal conditions (Helmersson-Karlqvist et al., 2016). Therefore, inhibition of megalin-mediated uptake of cystatin-C by MLK may not produce significant elevation of urinary cystatin-C level, particularly if serum cystatin-C level is low. In GM + MLK group, the significant reduction of serum and urinary cystatin-C levels could be attributed to improved renal glomerular and tubular functions. On the other hand, Jensen et al., (2017) have demonstrated that cystatin-C binds with high affinity to megalin/cubilin complex and that defective cystatin-C uptake in megalin deficient mice leads to elevated urinary cystatin-C excretion (Cárdenas-González et al., 2016).
Previous reports have demonstrated the ability of MLK to normalize levels of Cr, BUN, as well as GFR after exposure to nephrotoxic agents (Gad et al., 2017, Köse et al., 2019). In agreement, our results showed significant improvement of glomerular filtration and reduction of tubular injury biomarkers in GM + MLK group, that was also confirmed by the histopathological findings. That could be attributed to reduced GM uptake due to impaired megalin endocytic function by MLK, and hence improved kidney functions.
5. Conclusion
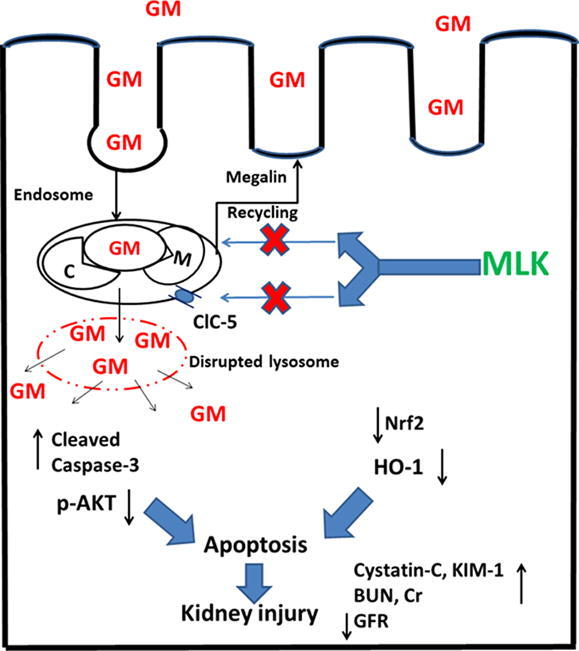
Gentamicin nephrotoxicity could be attenuated by MLK through interference with the expression/endocytic function of megalin receptor, which is responsible for the uptake and accumulation of GM in the proximal tubular cells. Reduced expression/endocytic function of megalin by MLK could be attributed to downregulation of ClC-5, which is one of the key regulators of megalin endocytic function. That eventually reduces renal cell apoptosis and improves kidney functions after GM administration without affecting the antibacterial activity of GM (Fig. 9).
Fig. 9.
Overview of the molecular protective mechanisms of MLK against GM nephrotoxicity. Endocytosis of GM in proximal tubular epithelial cells is mediated through megalin (M)/cubilin (C) complex. The endocytic function of megalin receptor is regulated by ClC-5 through acidification of endosomes to facilitate dissociation between megalin and its ligand, then megalin recycles to the brush border membrane. MLK downregulates ClC-5 expression resulting in impaired endocytic function, megalin recycling, and so its expression on the cell membrane. Therefore, MLK could reduce GM uptake into renal cells and so reduce apoptosis and improve kidney functions.
6. Future perspectives
The current study sheds light on a novel and promising nephroprotective strategy against GM nephrotoxicity through downregulation of ClC-5 and subsequent interference with megalin expression/endocytic function by MLK. Such strategy may be applied in the future against other nephrotoxic agents that depend on megalin-mediated endocytosis in their uptake into renal cells. Clinical trials are also required to prove such effects in human.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors are so grateful to Prof. Sayed Abdel-Raheem, Histopathology Department, Faculty of Medicine, Al-Azhar University, Cairo, for his efforts in the histopathological investigation.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Lateef S.M., El-Sayed E.-S., Mansour A.M., et al. The protective role of estrogen and its receptors in gentamicin-induced acute kidney injury in rats. Life Sci. 2019;239:117082. doi: 10.1016/j.lfs.2019.117082. [DOI] [PubMed] [Google Scholar]
- Abdelrahman R.S. Protective effect of apocynin against gentamicin-induced nephrotoxicity in rats. Hum. Exp. Toxicol. 2018;37(1):27–37. doi: 10.1177/0960327116689716. [DOI] [PubMed] [Google Scholar]
- Ali F.E.M., Hassanein E.H.M., Bakr A.G., et al. Ursodeoxycholic acid abrogates gentamicin-induced hepatotoxicity in rats: Role of NF-κB-p65/TNF-α, Bax/Bcl-xl/Caspase-3, and eNOS/iNOS pathways. Life Sci. 2020;254:117760. doi: 10.1016/j.lfs.2020.117760. [DOI] [PubMed] [Google Scholar]
- Aziz N.M., Elbassuoni E.A., Kamel M.Y., et al. Hydrogen sulfide renal protective effects: possible link between hydrogen sulfide and endogenous carbon monoxide in a rat model of renal injury. Cell Stress Chaperones. 2020;25(2):211–221. doi: 10.1007/s12192-019-01055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft J.D., Gamble M. Theory and practice of histological techniques. 6th ed. Elsevier health sciences; USA: 2008. [Google Scholar]
- Bao R.K., Zheng S.F., Wang X.Y. Selenium protects against cadmium-induced kidney apoptosis in chickens by activating the PI3K/AKT/Bcl-2 signaling pathway. Environ. Sci. Pollut. Res. 2017;24(25):20342–20353. doi: 10.1007/s11356-017-9422-6. [DOI] [PubMed] [Google Scholar]
- Bell S., Davey P., Nathwani D., et al. Risk of AKI with gentamicin as surgical prophylaxis. J. Am. Soc. Nephrol. 2014;25(11):2625–2632. doi: 10.1681/ASN.2014010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryniarski M.A., Yee B.M., Jaffri I., et al. Increased megalin expression in early type 2 diabetes: role of insulin-signaling pathways. Am. J. Physiol. Renal. Physiol. 2018;315(5):F1191–F1207. doi: 10.1152/ajprenal.00210.2018. [DOI] [PubMed] [Google Scholar]
- Cao Y., Xu J., Cui D., et al. Protective effect of carnosine on hydrogen peroxide–induced oxidative stress in human kidney tubular epithelial cells. Biochem. Biophys. Res. Commun. 2021;534:576–582. doi: 10.1016/j.bbrc.2020.11.037. [DOI] [PubMed] [Google Scholar]
- Cárdenas-González M., Jacobo Estrada T., Rodríguez-Muñoz R., et al. Sub-chronic exposure to fluoride impacts the response to a subsequent nephrotoxic treatment with gentamicin. J. Appl. Toxicol. 2016;36(2):309–319. doi: 10.1002/jat.3186. [DOI] [PubMed] [Google Scholar]
- Carleton H.M., Drury R.A.B., Wallington E.A. Oxford University Press; USA: 1980. Carleton’s Histological technique. [Google Scholar]
- Chen B., Yang B.o., Zhu J., et al. Hsp90 relieves heat stress-induced damage in mouse kidneys: Involvement of antiapoptotic PKM2-AKT and autophagic HIF-1α signaling. Int. J. Mol. Sci. 2020;21(5):1646. doi: 10.3390/ijms21051646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Shaikh Z.A. Activation of Nrf2 by cadmium and its role in protection against cadmium-induced apoptosis in rat kidney cells. Toxicol. Appl. Pharmacol. 2009;241(1):81–89. doi: 10.1016/j.taap.2009.07.038. [DOI] [PubMed] [Google Scholar]
- Christensen E., Nielsen R. Role of megalin and cubilin in renal physiology and pathophysiology. Rev. Physiol. Biochem. Pharmacol. 2006;158:1–22. doi: 10.1007/112_0604. [DOI] [PubMed] [Google Scholar]
- Christensen E.I., Devuyst O., Dom G., et al. Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc. Natl. Acad. Sci. 2003;100(14):8472–8477. doi: 10.1073/pnas.1432873100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagil R., O'Shea C., Nykjær A., et al. Gentamicin binds to the megalin receptor as a competitive inhibitor using the common ligand binding motif of complement type repeats: insight from the nmr structure of the 10th complement type repeat domain alone and in complex with gentamicin. J. Biol. Chem. 2013;288(6):4424–4435. doi: 10.1074/jbc.M112.434159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Barros Peruchetti D., Silva-Aguiar R.P., Siqueira G.M., et al. High glucose reduces megalin-mediated albumin endocytosis in renal proximal tubule cells through protein kinase B O-GlcNAcylation. J. Biol. Chem. 2018;293(29):11388–11400. doi: 10.1074/jbc.RA117.001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S., Kuwahara S., Saito A. The endocytic receptor megalin and its associated proteins in proximal tubule epithelial cells. Membranes. 2014;4(3):333–355. doi: 10.3390/membranes4030333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad A.M., El-Raouf O.M.A., El-Sayeh B.M., Fawzy H.M., Abdallah D.M., et al. Renoprotective effects of montelukast in an experimental model of cisplatin nephrotoxicity in rats. J. Biochem. Mol. Toxicol. 2017;31(12):1–6. doi: 10.1002/jbt.21979. [DOI] [PubMed] [Google Scholar]
- Giuliano R.A., Verpooten G.A., Verbist L., et al. In vivo uptake kinetics of aminoglycosides in the kidney cortex of rats. J. Pharmacol. Exp. Ther. 1986;236:470–475. [PubMed] [Google Scholar]
- Hara-Chikuma M., Wang Y., Guggino S.E., Guggino W.B., Verkman A.S. Impaired acidification in early endosomes of ClC-5 deficient proximal tubule. Biochem. Biophys. Res. Commun. 2005;329(3):941–946. doi: 10.1016/j.bbrc.2005.02.060. [DOI] [PubMed] [Google Scholar]
- Hashim A., Helmy M., Mouneir S. Cysteinyl leukotrienes predominantly mediate cisplatin-induced acute renal damage in male rats. J. Physiol. Pharmacol. 2018;69:779–787. doi: 10.26402/jpp.2018.5.12. [DOI] [PubMed] [Google Scholar]
- He M., Li Y., Wang L., Guo B., Mei W., Zhu B., Zhang J., Ding Y., Meng B., Zhang L., Xiang L., Dong J., Liu M., Xiang L., Xiang G. MYDGF attenuates podocyte injury and proteinuria by activating Akt/BAD signal pathway in mice with diabetic kidney disease. Diabetologia. 2020;63(9):1916–1931. doi: 10.1007/s00125-020-05197-2. [DOI] [PubMed] [Google Scholar]
- Helmersson-Karlqvist J., Ärnlöv J., Carlsson A.C., Lind L., Larsson A. Urinary KIM-1, but not urinary cystatin C, should be corrected for urinary creatinine. Clin. Biochem. 2016;49(15):1164–1166. doi: 10.1016/j.clinbiochem.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Hormati A., Ahmadpour S., Afkhami Ardekani M., Khodadust F., Refahi S. Radioprotective effects of montelukast, a selective leukotriene CysLT1 receptor antagonist, against nephrotoxicity induced by gamma radiation in mice. J. Biochem. Mol. Toxicol. 2020;34(6) doi: 10.1002/jbt.v34.610.1002/jbt.22479. [DOI] [PubMed] [Google Scholar]
- Hryciw D.H., Jenkin K.A., Simcocks A.C., Grinfeld E., McAinch A.J., Poronnik P. The interaction between megalin and ClC-5 is scaffolded by the Na+–H+ exchanger regulatory factor 2 (NHERF2) in proximal tubule cells. Int. J. Biochem. Cell Biol. 2012;44(5):815–823. doi: 10.1016/j.biocel.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Hsu Y.-H., Chen T.-H., Wu M.-Y., Lin Y.-F., Chen W.-L., Cheng T.-H., Chen C.-H. Protective effects of Zhibai Dihuang Wan on renal tubular cells affected with gentamicin-induced apoptosis. J. Ethnopharmacol. 2014;151(1):635–642. doi: 10.1016/j.jep.2013.11.031. [DOI] [PubMed] [Google Scholar]
- Hu Y., Ma X. Icariin treatment protects against gentamicin-induced ototoxicity via activation of the AMPK-SIRT3 pathway. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.620741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Ba Huy P., Bernard P., Schacht J. Kinetics of gentamicin uptake and release in the rat. Comparison of inner ear tissues and fluids with other organs. J. Clin. Invest. 1986;77(5):1492–1500. doi: 10.1172/JCI112463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İçer M., Zengin Y., Gunduz E., Dursun R., Durgun H.M., Turkcu G., Yuksel H., Üstündağ M., Guloglu C. Is montelukast as effective as N-acetylcysteine in hepatic injury due to acetaminophen intoxication in rats? Exp. Toxicol. Pathol. 2016;68(1):55–59. doi: 10.1016/j.etp.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Jado J.C., Humanes B., González-Nicolás M.Á., Camaño S., Lara J.M., López B., Cercenado E., García-Bordas J., Tejedor A., Lázaro A. Nephroprotective Effect of Cilastatin against Gentamicin-Induced Renal Injury In Vitro and In Vivo without Altering Its Bactericidal Efficiency. Antioxidants. 2020;9(9):821. doi: 10.3390/antiox9090821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen D., Kierulf-Lassen C., Kristensen M.L.V., Nørregaard R., Weyer K., Nielsen R., Christensen E.I., Birn H., Armando I. Megalin dependent urinary cystatin C excretion in ischemic kidney injury in rats. PLoS ONE. 2017;12(6):e0178796. doi: 10.1371/journal.pone.0178796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Tan W., Chen X., et al. Montelukast Inhibits the Inflammation of Human Bronchial Epithelial Cells Infected by Respiratory Syncytial Virus. J. Sun Yat-sen Univ. (Med. Sci.). 2017;38:676–684. [Google Scholar]
- Jung T.-Y., Lee A.Y., Song J.-H., Lee M.Y., Lim J.-O., Lee S.-J., Ko J.-W., Shin N.-R., Kim J.-C., Shin I.-S., Kim J.-S. Scrophularia koraiensis nakai attenuates allergic airway inflammation via suppression of NF-κB and enhancement of Nrf2/HO-1 signaling. Antioxidants. 2020;9(2):99. doi: 10.3390/antiox9020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandemir F.M., Ozkaraca M., Yildirim B.A., Hanedan B., Kirbas A., Kilic K., Aktas E., Benzer F. Rutin attenuates gentamicin-induced renal damage by reducing oxidative stress, inflammation, apoptosis, and autophagy in rats. Ren. Fail. 2015;37(3):518–525. doi: 10.3109/0886022X.2015.1006100. [DOI] [PubMed] [Google Scholar]
- Kaplan H.M., Şingirik E., Erdoğan K.E., Doran F. Protective effect of alpha-linolenic acid on gentamicin-induced ototoxicity in mice. Somatosens. Mot. Res. 2017;34(3):145–150. doi: 10.1080/08990220.2017.1356283. [DOI] [PubMed] [Google Scholar]
- Kim S., LesherPerez S.C., Kim B.C.C., Yamanishi C., Labuz J.M., Leung B., Takayama S. Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication. 2016;8(1):015021. doi: 10.1088/1758-5090/8/1/015021. [DOI] [PubMed] [Google Scholar]
- Köse E., Oğuz F., Vardi N., et al. Therapeutic and protective effects of montelukast against doxorubicin-induced acute kidney damage in rats. Iran J. Basic Med. Sci. 2019;22:407–411. doi: 10.22038/ijbms.2019.33493.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K.M., Serio A.W., Kane T.R., Connolly L.E. Aminoglycosides: an overview. Cold Spring Harb Perspect Med. 2016;6(6):a027029. doi: 10.1101/cshperspect.a027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharava K., Sekulic-Jablanovic M., Horvath L., Bodmer D., Petkovic V. Pasireotide protects mammalian cochlear hair cells from gentamicin ototoxicity by activating the PI3K–Akt pathway. Cell Death Dis. 2019;10(2) doi: 10.1038/s41419-019-1386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Weinman S.A. Chloride channels and hepatocellular function: prospects for molecular identification. Annu. Rev. Physiol. 2002;64(1):609–633. doi: 10.1146/annurev.physiol.64.090501.145429. [DOI] [PubMed] [Google Scholar]
- Liu C., Chen K., Wang H., Zhang Y.e., Duan X., Xue Y., He H., Huang Y.u., Chen Z., Ren H., Wang H., Zeng C. Gastrin attenuates renal ischemia/reperfusion injury by a PI3K/Akt/Bad-mediated anti-apoptosis signaling. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.540479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Wang P., Qiao Y., Jiang C., Ge Y., Flickinger B., Malhotra D.K., Dworkin L.D., Liu Z., Gong R. GSK3β-mediated Keap1-independent regulation of Nrf2 antioxidant response: A molecular rheostat of acute kidney injury to chronic kidney disease transition. Redox Biol. 2019;26:101275. doi: 10.1016/j.redox.2019.101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft F.C. Biomarkers and predicting acute kidney injury. Acta Physiol. 2021;231(1) doi: 10.1111/apha.2021.231.issue-110.1111/apha.13479. [DOI] [PubMed] [Google Scholar]
- Mahadevappa R., Nielsen R., Christensen E.I., Birn H. Megalin in acute kidney injury: foe and friend. Am. J. Physiol. Renal. Physiol. 2014;306(2):F147–F154. doi: 10.1152/ajprenal.00378.2013. [DOI] [PubMed] [Google Scholar]
- McWilliam S.J., Antoine D.J., Smyth R.L., Pirmohamed M. Aminoglycoside-induced nephrotoxicity in children. Pediatr. Nephrol. 2017;32(11):2015–2025. doi: 10.1007/s00467-016-3533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.J., Carruth M.W., Weinman S.A. Leukotriene D4 activates a chloride conductance in hepatocytes from lipopolysaccharide-treated rats. J. Clin. Invest. 1997;99(12):2915–2922. doi: 10.1172/JCI119486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavinasab S.R., Akhoundi-Meybodi Z., Mahmoudi L., Karimzadeh I. A randomized double-blinded placebo-controlled clinical trial on protective effects of pentoxifylline on gentamicin nephrotoxicity in infectious patients. Clin. Exp. Nephrol. 2021;25(8):844–853. doi: 10.1007/s10157-021-02032-9. [DOI] [PubMed] [Google Scholar]
- Nagai J., Takano M. Entry of aminoglycosides into renal tubular epithelial cells via endocytosis-dependent and endocytosis-independent pathways. Biochem. Pharmacol. 2014;90(4):331–337. doi: 10.1016/j.bcp.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Nayak A. A review of montelukast in the treatment of asthma and allergic rhinitis. Expert Opin. Pharmacother. 2004;5(3):679–686. doi: 10.1517/14656566.5.3.679. [DOI] [PubMed] [Google Scholar]
- Novarino G., Weinert S., Rickheit G., Jentsch T.J. Endosomal chloride-proton exchange rather than chloride conductance is crucial for renal endocytosis. Science. 2010;328(5984):1398–1401. doi: 10.1126/science.1188070. [DOI] [PubMed] [Google Scholar]
- Opferman J.T., Kothari A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018;25(1):37–45. doi: 10.1038/cdd.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otunctemur A., Ozbek E., Cekmen M., Cakir S.S., Dursun M., Polat E.C., Somay A., Ozbay N. Protective effect of montelukast which is cysteinyl-leukotriene receptor antagonist on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Ren. Fail. 2013;35(3):403–410. doi: 10.3109/0886022X.2012.761040. [DOI] [PubMed] [Google Scholar]
- Qiu M., Liu J., Su Y., Guo R., Zhao B., Liu J. Diosmetin induces apoptosis by downregulating AKT phosphorylation via P53 activation in human renal carcinoma ACHN cells. Protein Pept. Lett. 2020;27(10):1022–1028. doi: 10.2174/0929866527666200330172646. [DOI] [PubMed] [Google Scholar]
- Quiros Y., Vicente-Vicente L., Morales A.I., Lopez-Novoa J.M., Lopez-Hernandez F.J. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol. Sci. 2011;119(2):245–256. doi: 10.1093/toxsci/kfq267. [DOI] [PubMed] [Google Scholar]
- Randjelovic P., Veljkovic S., Stojiljkovic N., et al. Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. EXCLI J. 2017;16:388–399. doi: 10.17179/excli2017-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman S.A., Chertow G.M., Hebbar S., Vaziri N.D., Ward K.W., Meyer C.J. Bardoxolone methyl decreases megalin and activates nrf2 in the kidney. J. Am. Soc. Nephrol. 2012;23(10):1663–1673. doi: 10.1681/ASN.2012050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q., Weyer K., Rbaibi Y., Long K.R., Tan R.J., Nielsen R., Christensen E.I., Baty C.J., Kashlan O.B., Weisz O.A. Distinct functions of megalin and cubilin receptors in recovery of normal and nephrotic levels of filtered albumin. Am. J. Physiol. Renal. Physiol. 2020;318(5):F1284–F1294. doi: 10.1152/ajprenal.00030.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C., Hilpert J., Jacobsen C., Boensch C., Christensen E.I., Luft F.C., Willnow T.E. Megalin deficiency offers protection from renal aminoglycoside accumulation. J. Biol. Chem. 2002;277(1):618–622. doi: 10.1074/jbc.M109959200. [DOI] [PubMed] [Google Scholar]
- Shelton L.M., Kevin Park B., Copple I.M. Role of Nrf2 in protection against acute kidney injury. Kidney Int. 2013;84(6):1090–1095. doi: 10.1038/ki.2013.248. [DOI] [PubMed] [Google Scholar]
- Song Q., Hu Z., Xie X., Cai H. Zafirlukast prevented ox-LDL-induced formation of foam cells. Toxicol. Appl. Pharmacol. 2020;409 doi: 10.1016/j.taap.2020.115295. [DOI] [PubMed] [Google Scholar]
- Stojiljkovic N., Mihailovic D., Veljkovic S., Stoiljkovic M., Jovanovic I. Glomerular basement membrane alterations induced by gentamicin administration in rats. Exp. Toxicol. Pathol. 2008;60(1):69–75. doi: 10.1016/j.etp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Subramanian P., Anandan R., Jayapalan J.J., Hashim O.H. Hesperidin protects gentamicin-induced nephrotoxicity via Nrf2/HO-1 signaling and inhibits inflammation mediated by NF-κB in rats. J. Funct. Foods. 2015;13:89–99. [Google Scholar]
- Sun H., Yang H., Ruan H., Li W., He X., Wang L., Liu F., Zhang J. The protective effect of Sika deer antler protein on gentamicin-induced nephrotoxicity in vitro and in vivo. Cell. Physiol. Biochem. 2018;50(3):841–850. doi: 10.1159/000494471. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi H., Ceol M., Gianesello L., Priante G., Iotti A., Del Prete D. Downregulation of megalin, cubilin, ClC-5 and podocin in Fabry nephropathy: potential implications in the decreased effectiveness of enzyme replacement therapy. J. Nephrol. 2021;34(4):1307–1314. doi: 10.1007/s40620-020-00835-9. [DOI] [PubMed] [Google Scholar]
- Udupa V., Prakash V. Gentamicin induced acute renal damage and its evaluation using urinary biomarkers in rats. Toxicol. Rep. 2019;6:91–99. doi: 10.1016/j.toxrep.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan T., Wang Z., Luo Y., et al. FA-97, a New Synthetic Caffeic Acid Phenethyl Ester Derivative, Protects against Oxidative Stress-Mediated Neuronal Cell Apoptosis and Scopolamine-Induced Cognitive Impairment by Activating Nrf2/HO-1 Signaling. Oxid. Med. Cell. Longevity. 2019;2019 doi: 10.1155/2019/8239642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.F., Abouzied M.M., Smith D.M. Proteins as potential endpoint temperature indicators for ground beef patties. J. Food Sci. 1996;61(1):5–7. [Google Scholar]
- Wargo K.A., Edwards J.D. Aminoglycoside-Induced Nephrotoxicity. J. Pharm. Pract. 2014;27(6):573–577. doi: 10.1177/0897190014546836. [DOI] [PubMed] [Google Scholar]
- Wellhauser L., D’Antonio C., Bear C.E. ClC transporters: discoveries and challenges in defining the mechanisms underlying function and regulation of ClC-5. Pflügers Arch. 2010;460(2):543–557. doi: 10.1007/s00424-009-0769-5. [DOI] [PubMed] [Google Scholar]
- Xu F., Wu M., Lu X., Zhang H., Shi L., Xi Y., Zhou H., Wang J., Miao L., Gong D.-W., Cui W. Effect of Fc-Elabela-21 on renal ischemia/reperfusion injury in mice: mediation of anti-apoptotic effect via Akt phosphorylation. Peptides. 2021;147:170682. doi: 10.1016/j.peptides.2021.170682. [DOI] [PubMed] [Google Scholar]
- Xu Y., Li D., Wu J., Zhang M., Shao X., Xu L., Tang L., Zhu M., Ni Z., Zhang M., Mou S. Farnesoid X receptor promotes renal ischaemia-reperfusion injury by inducing tubular epithelial cell apoptosis. Cell Prolif. 2021;54(4) doi: 10.1111/cpr.v54.410.1111/cpr.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo T., Nakamura M., Shimizu T. Leukotriene receptors as potential therapeutic targets. J. Clin. Invest. 2018;128:2691–2701. doi: 10.1172/JCI97946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Zhao Y., Hou W., Guo L. MiR-153 regulates cardiomyocyte apoptosis by targeting Nrf2/HO-1 signaling. Chromosome Res. 2019;27(3):167–178. doi: 10.1007/s10577-019-09608-y. [DOI] [PubMed] [Google Scholar]
- Zovko A., Yektaei-Karin E., Salamon D., Nilsson A., Wallvik J., Stenke L. Montelukast, a cysteinyl leukotriene receptor antagonist, inhibits the growth of chronic myeloid leukemia cells through apoptosis. Oncol. Rep. 2018;40(2):902–908. doi: 10.3892/or.2018.6465. [DOI] [PubMed] [Google Scholar]