Abstract
Objective
Temporomandibular joint (TMJ) osteoarthritis (OA) is a type of TMJ disorders with clinical symptoms of pain, movement limitation, cartilage degeneration and joint dysfunction. This review article is aiming to summarize recent findings on signaling pathways involved in TMJ OA development and progression.
Methods
Most recent findings in TMJ OA studies have been reviewed and cited.
Results
TMJ OA is caused by inflammation, abnormal mechanical loading and genetic abnormalities. The molecular mechanisms related to TMJ OA have been determined using different genetic mouse models. Recent studies demonstrated that several signaling pathways are involved in TMJ OA pathology, including Wnt/β-catenin, TGF-β and BMP, Indian Hedgehog, FGF, NF-κB, and Notch pathways, which are summarized in this review article. Alterations of these signaling pathways lead to the pathological changes in TMJ tissues, affecting cartilage matrix degradation, catabolic metabolism and chondrocyte apoptosis.
Conclusion
Multiple signaling pathways were involved in the pathological process of TMJ OA. New therapeutic strategies, such as stem cell application, gene editing and other techniques may be utilized for TMJ OA treatment.
The translational potential of this article
TMJ OA is a most important subtype of TMJ disorders and may lead to substantial joint pain, dysfunction, dental malocclusion, and reduced health-related quality of life. This review article summarized current findings of signaling pathways involved in TMJ OA, including Wnt/β-catenin, TGF-β and BMP, Indian Hedgehog, FGF, NF-κB, and Notch pathways, to better understand the pathological mechanisms of TMJ OA and define the molecular targets for TMJ OA treatment.
Keywords: Temporomandibular joint, Osteoarthritis, Cartilage degradation, Mechanical loading, Molecular signaling
1. Temporomandibular joint osteoarthritis: definition and prevalence
Osteoarthritis (OA) is the most common degenerative disease in the temporomandibular joint (TMJ) causing severe pain and dysfunction [1]. TMJ is composed of the mandibular condyle, articular disc, and the glenoid fossa of the temporal bone, which is responsible for the most complicated movement in the body. As a synovial joint, TMJ is developed from three separate mesenchymal condensations representing the glenoid fossa of the temporal bone, the condylar process of the mandibular ramus, and the articular disc [2]. Compared with other synovial joints, TMJ showed better healing potential and interstitial growth of cartilage, because the articular surface of the TMJ mandibular condyle is fibrocartilage, which contains both type I and type II collagen, compared to articular hyaline cartilage, which only contains type II collagen [3]. Another unique feature of TMJ is that the cartilage of the mandible condyle is a secondary cartilage compared to the articular cartilage in other joints, which is a primary cartilage [4]. More specifically, cartilage of TMJ is developed in association with specific bones formed by intra-membranous ossification beginning with undifferentiated cells comprising mesenchymal tissue covering the prenatal or postnatal condyle. While the primary cartilage is associated with endochondral ossification, where the cartilage precedes the bone formation beginning in the cartilage cells within the central layer of an epiphyseal plate [5]. TMJ has rotational and translational movement. Mandibular motions lead to the static and dynamic loading to the TMJ [6]. Pain and dysfunction are the main symptoms of TMJ OA. Radiographic changes of the condyle and articular eminence, such as erosive resorption, sclerosis, attrition, osteophyte formation, and cyst-like changes, are the key clinical feature for the diagnosis of TMJ OA [1,7,8]. At least one sign of TMJ disorders, including pain, limited mandibular motion, or TMJ sounds, has been reported in 40–75% adults in US [9]. It was reported 67% of patients with hand OA had cone-beam computed tomography (CBCT) -defined TMJ OA [10]. A population-based study from a German cohort, in which patients were analyzed by the magnetic resonance imaging (MRI) technique, showed TMJ OA is common in older people (about 70%) [11]. The frequency of TMJ disorders also increased with age. The occurrence of TMJ OA below 30 years of age was 14.56%. In contrast, patients from 30 to 80 years of age the TMJ OA occurrence was 28–32% [7,8]. Additionally, TMJ OA has marked sexual dimorphism. Several retrospective studies showed that the frequency of TMJ OA was almost twofold higher in women than in men [8].
2. TMJ OA: pathogenesis
The pathogenesis of TMJ OA is different from OA in the knee or hip and is highly complicated, including inflammation, mechanical overload, and cartilage degradation and etc.
2.1. Inflammation
Although TMJ OA has been described as non-inflammatory arthritic condition resulting from degenerative changes of the joint, multiple inflammatory cytokines could play an important role in TMJ OA pathogenesis. It has been well established that inflammation dysregulates the catabolism of the cartilage matrix leading to the deterioration of chondrocyte function [12]. CBCT images clearly showed osseous changes which often occur in response to TMJ inflammation [13]. It has been reported that mRNA levels of IL-1β, IL-2, IL-12, IL-17, IL-18, TNF-α, TNF-β, and IFN-γ were significantly higher in the TMJ synovial fluid from patients with TMJ OA than that of normal subjects [14]. Among them, IL-12 was the predominant cytokine expressed in TMJ tissues in patients with TMJ OA [14]. Expression of IL-1β and TNF-α has been reported to be associated with cartilage degradation and suppression of the synthesis of cartilage matrix in TMJ OA [15]. IL-17, IL-1β and TNF-α expressed by synovial cells induce the RANKL expression in synovial fibroblasts and osteoblasts, promoting osteoclast formation and bone resorption [16]. Toll-like receptor 4 (TLR4), which mediates the innate immune reaction, aggravates the damage of cartilage and subchondral bone in discectomy-induced TMJ OA through activation of MyD88/NF-κB pathway in mice [17]. In addition, inflammatory cytokines are also in close correlation with pain in TMJ OA. It was reported that TNF-α is related to sensory neuron hyperexcitability and can directly stimulate nociceptors [18].
2.2. Mechanical loading
Single most important etiological factor for degenerative TMJ disease is the alteration of mechanical loading that surpasses the adaptive capacity of the joint. Multiple animal models suggest adverse joint loading without any surgical manipulation of the joint tissues can induce joint inflammation in vivo [19]. The TMJ, which is a bi-condylar joint, is formed by the articulation of the mandible and the temporal bone of the cranium. Between the condyle and the articular fossa there is a disc which is made of fibrocartilage that acts as a cushion to absorb stress and allows the condyle to move easily when the mouth opens and closes [20]. Although the relationship between disc displacement and TMJ OA remains to be further elucidated, it has been reported that disc displacement causes OA [21]. In contrast, a recent study also suggested that there is no significant relationship with osseous changes between disc displacement and TMJ OA [22].
Many studies focus on mechanical sensing during pathological processes of TMJ OA and knockdown of high mobility group protein B2 (HMGB2) attenuated the sensitivity of chondrocytes in response to pressure loading, suggesting an important role of HMGB2 in the TMJ pathogenesis [23]. The connexin (Cx) 43 hemichannel located in the primary cilium is responsible for mediating exchanges of small molecules, such as ATP, Ca2+, and prostaglandin E2 (PGE2) between TMJ chondrocytes and matrix under mechanical stimulation [24]. PGE2 acts as a hormone in mediating cartilage metabolism and OA associated pain.
Abnormal subchondral bone remodeling also affects mechanical balance of TMJ. It has been reported during the early stages of TMJ OA, biglycan and fibromodulin double KO (bgn−/−; fmod−/−) mice showed increased bone resorption and decrease type I collagen expression in subchondral bone of TMJ [25]. An in vivo longitudinal studies demonstrated that new bone, measured by micro-CT, was formed after predominantly resorptive activity in the subchondral bone of TMJ condyles in the initial phases of experimental disordered occlusion [26].
2.3. Cartilage degradation
As resident cell of cartilage, chondrocytes are the key mediators for maintaining the cartilage matrix homeostasis. Compromising of chondrocyte function and survival would disturb the cartilage homeostasis and accelerate OA proceeding. Recent study showed that a novel circRNA, namely circGCN1L1, increases chondrocyte apoptosis by targeting miR-330–3p and TNF-α in TMJ OA [27]. Inhibition of the expression of circGCN1L1 in a rat TMJ OA model could ameliorate TMJ OA [27]. Programmed cell death protein 4 (PDCD4) was initially identified in apoptosis-inducing mice and regarded as a tumor suppressor and was reported to suppress autophagy and enhance apoptosis in the cartilage of sodium mono iodoacetate (MIA)-induced TMJ OA and in IL-1β-treated chondrocytes in rats [28]. The interaction between subchondral bone and cartilage is like shoe and feet, which is important for the maintenance of articular integrity and physiology. It was reported that endoplasmic reticulum stress is significantly activated by mechanical stress-induced mandibular cartilage thinning induced by mechanical stress and may be a novel mechanism of chondrocyte apoptosis induced by mechanical stimulation [29]. Extracorporeal shockwave therapy improved structure and bone quality of subchondral bone and alleviation of inflammation and chondrocyte apoptosis in the MIA-induced TMJ OA [30].
About two-thirds of the dry weight of adult articular cartilage is collagen. It was well established that fibrochondrocytes participate in the inflammation induced matrix degradation by production and activation of matrix metalloproteinases (MMPs) [31,32]. Fibrochondrocytes expressed mRNAs for MMP2, 3, 7, 8, 9, 11, 13, 14, 16, 17, and 19 as well as TIMP1, 2, and 3. Among them, MMP3, 7, 8, 9, 13, 16, 17, and 19 were significantly upregulated after IL-β stimulation in rat primary fibrocartilage which was harvested from TMJ discs [32]. The catabolic genes MMP3, MMP13, and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5) were elevated in the condylar head of MIA-induced TMJ OA in rats [31]. Inhibition of SDF-1-CXCR4 signaling alleviates the severity of TMJ OA by decreasing MMP3, MMP9, and p-ERK expression [33]. Treatment with simvastatin could decrease MMP3 and MMP13 expression in TMJ OA mice induced by high fat diet and excessive compressive mechanical loading [34]. The suppression of expression of estrogen-related receptor γ (ERR-γ) by ERR-γ siRNAs decreases the levels of the MMP-3/9/13 expression and reduces the extent of cartilage destruction [35].
3. Molecular signaling during TMJ OA development
3.1. Wnt/β-catenin signaling
Wnt/β-catenin signaling pathway is a conserved cellular communication system that has been studied for decades in stem cell self-renewal and cell proliferation and differentiation during embryonic development and adult tissue homeostasis. It regulates the pathogenesis of OA and other types of arthritis [36,37]. Wnt/β-catenin pathway triggers the signaling at the cell surface where Wnt glycoprotein interacting with receptors Frizzled and low-density lipoprotein receptor-related proteins 5 or 6 (LRP5/6) to form a complex which stabilizes intracellular β-catenin and makes it translocate to the nucleus. Wnt targeted proteins can then be expressed when β-catenin protein binds to transcription factors in the nucleus [38].
It has been reported that progressive TMJ defects, joint space narrowing and OA-like defects in TMJ can be found in β-catenin conditional activation mice, β-catenin(ex3)Agc1ER [39]. Specifically, expression of enzymes such as MMP13, Adamts4, and Adamts5, which caused cartilage degradation, was significantly increased; while the expression of chondrocyte hypertrophy-related protein, Col-X, was also upregulated in β-catenin(ex3)Agc1ER mice [39]. Moreover, decreased cell proliferation and increased cell apoptosis were observed in the condylar cartilage of these mice [40]. All this information implicates that β-catenin plays a critical role in TMJ pathogenesis and Wnt/β-catenin signaling can be a potential therapeutic target for the treatment of TMJ OA [41]. Another study showed that Wnt5a/Ror2 signaling in bone marrow stromal cells (BMSCs) of TMJ subchondral bone was enhanced in unilateral anterior crossbites (UAC)-treated rats, which promoted the osteoclast formation and the TMJ subchondral bone loss [42]. Inhibitors of Wnt signaling pathway, such as StAx-35 R and SAH-Bcl9, were used in the treatment of OA by inhibition of β-catenin transcriptional activity [43,44].
3.2. TGF-β and BMP signaling
Transforming growth factor β (TGF-β)/bone morphogenic protein (BMP) signaling has been extensively studied in the bone formation and plays versatile roles throughout life [45]. TGF-β superfamily includes over forty members, such as TGF-βs, bone morphogenetic proteins (BMPs), and activin. They are embedded in the bone matrix and regulate bone remodeling, or modulate bone or cartilage formation [46]. TGF-β signal pathway first transmits signals through the formation and successive activation of a heteromeric complex of type II and type I serine/threonine kinase receptors followed by the phosphorylation of specific Smad proteins, R-Smads, and then intracellular signaling is initiated [47]. The phosphorylated R-Smads could heterodimerize with co-Smad, Smad4, thereby translocate to nucleus and activate the transcription of target genes [48].
One ex vivo study showed the decreased TGF-β3 and Smad3 when transfected with miR-140–5p mimics in the primary mandibular condylar chondrocytes (MCCs) treated with IL-1β, which induced OA-like changes in TMJ tissues [49]. Indeed, Smad3 plays an important role in the transmission of signals from TGF-β receptor. For instance, OA was induced when Smad3 was specifically deleted in chondrocytes and progressive cartilage degradation of subchondral bone of mandibular condylar was observed in Smad3−/− mice [50]. It has been shown that in the TGF-β/Smad3 signaling pathway, a bioactive lipid, sphingosine 1-phosphate (S1P) is generated to serve as an intracellular mediator or an extracellular ligand for distinct receptors, resulting in inflammation, cell migration, and angiogenesis. The crosstalk between TGF-β/Smad3 and S1P/S1P3 and Smad3/S1P3 signaling in chondrocytes may be responsible for the development of TMJ OA [50,51]. Furthermore, it has also been reported that overexpression of TGF-β1 induced abnormal subchondral bone remodeling leading to degradation of mandibular condylar cartilage and TMJ OA progression in mice [52]. Meanwhile, TGF-β2 deletion may also alleviate trend of TMJ OA. When tissue-specific TGFβR2 deletion in mature chondrocytes was achieved by generating Agc1-CreER;Tgfbr2fl/fl mice, cartilage degradation observed in mechanical stress-induced TMJ OA (caused by partial discectomy) was delayed [53]. The conflict findings observed in Smad3 and Tgfbr2 KO mice may reflect the stage-specific or cell type-specific effects of these molecules. Other studies showed that BMP-2 aggrevated the TMJ OA in Bgn−/−; Fmod−/− double KO mice compared with the WT mice [54]. Moreover, BMP-2 was demonstrated to be involved in the pathogenesis or the repair process in the patients with TMJ internal derangement [55].
3.3. Indian Hedgehog signaling
Indian hedgehog (Ihh) is a signaling molecule in Hh family and plays a critical role in regulation of skeletal development [56]. It is mainly expressed in pre-hypertrophic and hypertrophic chondrocytes during endochondral ossification. It regulates many processes during cartilage development, including expression of parathyroid hormone-related protein (PTHrP) in periarticular tissue [57]. Ihh signaling starts from the binding of Ihh to Patched 1 (Ptch1) which causes the displacement of Ptch1 from primary cilium. Then Smoothened (Smo) is phosphorylated and the glioma-associated oncogene (Gli) proteins, including Gli1, Gli2, and Gli3, are activated. Without Hh ligands binding to Ptch1, Ptch1 localized at the base of the primary cilia and the activation of Smo is prevented [58]. Under this condition, Hh target genes can be repressed by C-terminal truncated Gli2 and Gli3 formed through proteolytic cleavage, while Gli1 is not phosphorylated and cleaved and functions as an activator in the Hh signaling [59].
Ihh may promote TMJ OA development which was implicated in the study that enhanced Ihh signaling promotes the terminal differentiation of deep zone chondrocytes in TMJ osteoarthritic cartilage stimulated by unilateral anterior cross-bite (UAC); while the OA-like lesions and UAC-promoted chondrocytes terminal differentiation were rescued by the deletion of Smo in mice [60]. Another study showed that Ihh promoted OA development by regulating the genes related to cartilage degeneration, while OA can be attenuated via the inhibition of Ihh [61]. Ihh, Smo, and Gli1 were activated in the TMJ OA induced by bite-raising, indicating that Ihh signaling may promote TMJ OA by stimulating the chondrocyte hypertrophy, and activation of Ihh signaling was observed in adjuvant-induced TMJ OA in rats [62]. In the cultured glenoid fossa cells, chondrocyte differentiation and maturation can be prevented by the hedgehog inhibitor [63]. The chondrocyte terminal differentiation in TMJ OA can be prevented by the inhibition of Ihh signaling in Col2-CreER; Pth1rfl/fl; Smofl/fl mice [60].
3.4. FGF signaling
Fibroblast growth factor (FGF) signaling pathway is essential in the regulation of skeletal development, especially in the maintenance of articular cartilage. There are 22 ligands of FGF family members which exert various functions mainly through binding to 4 distinct FGF receptors (FGFRs) [64]. The typical FGF/FGFRs signaling initiated from the binding of FGFs to the extracellular domain of FGFRs, the target proteins are then recruited and phosphorylated at the cytoplasmic tail of FGFRs followed by the activation of various downstream signaling events. Many signaling pathways, such as phosphoinositide 3-kinase/Akt, phospholipase C, mitogen-activated protein kinase (MAPK), and signal transducers and activators of transcription (STAT) 1/p21 pathway, are involved in the FGF downstream signaling activities [65]. In addition, MEK/ERK, as an important downstream signaling molecule of FGFR1, connects FGF signaling pathway with the progression of OA [66].
Studies showed that FGF signaling may be enhanced in TMJ OA and this was revealed by the report that deletion of Fgfr1 in TMJ chondrocytes delayed TMJ OA progression in specific OA models [67]. Indeed, results of immunohistochemical staining showed that the expression of several protein markers, such as MMP13, ADAMTS5, and Col-X, decreased while the aggrecan expression level increased in the Fgfr1 deficiency mice. Furthermore, deletion of Fgfr1 in TMJ chondrocytes ameliorated the TMJ OA progression partially by promoting autophagic activity [67]. FGFR3 is also critical in skeletal development, especially in the cartilage tissues [68]. When Fgfr3 was conditionally deleted in chondrocytes at the adult stage, the TMJ OA-like changes were observed in these KO mice. Moreover, the expression of Ihh and Runx2 was increased in TMJ tissues of Fgfr3 cKO mice and the inhibition of Ihh significantly decreased the expression of Runx2, Col10, Mmp13 and Adamts5, alleviating OA-like defects in the TMJ of Fgfr3 cKO mice, indicating that FGFR3/Ihh signaling is essential in maintenance of the TMJ articular cartilage intact at the adult stage [69]. When a FGFR1 inhibitor, G141, was used to treat primary chondrocytes derived from the condylar cartilage and stimulated by IL-1β, expression of TMJ OA markers, such as Runx2, Mmp13, and Adamts5, was decreased [67].
3.5. NF-κB signaling
NF-κB is considered as an important mediator in immunity, inflammatory process, stress responses, cell proliferation and cell death. NF-κB family comprises five proteins, RelA, RelB, c-Rel, NF-κB1, and NF-κB2, which are responsible for the formation of active complexes, interaction with NF-κB inhibitors, nuclear translocation and DNA binding to regulate the expression of NF-κB-target genes [70]. Classical NF-κB signaling pathway is mediated by tumor necrosis factor receptor (TNF-R), Toll-like receptor (TL-R) or T-cell receptor (TC-R) with activation of IKK-α/IKK-β/IKK-γ-NEMO complex. NF-κB dimers bound to inhibitory NF-κB (I-κB) proteins in the cytoplasm as an inactive form. When cells are stimulated by mechanical and chemical signals, I-κBs are phosphorylated by I-κB kinases and degradated by ubiquitin-proteasome system, NF-κB heterodimers are then free to translocate into the nucleus to activate expression of target genes [71].
It has been reported that NF-κB signaling pathway is essential in the pathogenesis of TMJ OA. NF-κB signaling can be induced by periostin, a matricellular protein, then the MMPs and inflammatory cytokines can be upregulated NF-κB signaling activation to accelerate the OA pathogenesis [72]. Genistein played a protective role in condylar cartilage of OA by downregulating expression of NF-κB in rats [73]; while inflammation of TMJ chondrocytes can be ameliorated by Yohimbine through suppression of NF-κB signaling pathway [74]. Celecoxib may protect excessive mechanical stress-induced TMJ OA partially through inhibition of NF-κB signaling and suppression of MMP production [75]. NF-κB can also be suppressed in experimental TMJ OA model when the mice were treated with rebamipide [75]. The ADAMTS5 expression is induced by activation of the NF-κB pathway by periostin, leading to proteoglycan and collagen degradation and aggravation of TMJ OA [76]. It has been shown that expression of TLR4 and NF-κB was elevated in the synovium in TMJ OA patients and in discectomy-induced TMJ OA in mice. TLR4 was also elevated in the damaged cartilage and subchondral bone through activation of MyD88/NF-κB [17]. It has been shown that the cartilage degeneration of TMJ OA can be inhibited by blocking the periostin–NF–κB-ADAMTS5 pathway [76].
3.6. Notch signaling
The Notch receptor is a single-pass transmembrane receptor located at the cell surface and is critical in cell differentiation and apoptosis. The Notch signaling pathway is highly conserved and is composed of several molecules such as Notch ligands, Notch receptors, transcriptional effector and target genes [77]. It is initiated by Notch ligands binding to the Notch receptors, and then Notch receptors are cleaved, the intracellular domain of Notch receptors then translocate to the nucleus and downstream target genes are activated [78]. Notch signaling pathway plays a dual role in the cartilage maintenance, which regulates the molecules involved in the cartilage formation and degradation [79]. Recent studies showed that altering Notch signaling may lead to TMJ OA and it is known that Notch signaling is important in angiogenesis of condylar cartilage and disc which is essential in the development of TMJ OA [80].
Several studies implicated that Notch signaling may be activated in TMJ OA. For instance, high Notch1 activity was found in osteoarthritis cartilage, especially in the most damaged areas of the OA, compared with healthy cartilage [81]. Degradative proteinases and inflammatory cytokines, such as MMP13, IL-1β, and IL-6, are induced by Notch activation in OA which contribute to the cartilage loss [82]. Notch ligands and receptors are also upregulated in the mouse model of TMJ OA. Notch was highly expressed in the TMJ OA with OA-like lesions in mandibular condyles in rats [83]. When Notch signaling was blocked by DAPT, an inhibitor of Notch, the progression of cartilage damage was delayed, implicating that the inhibition of Notch signaling pathway can partially delay the progression of TMJ OA [84] (see Fig. 1).
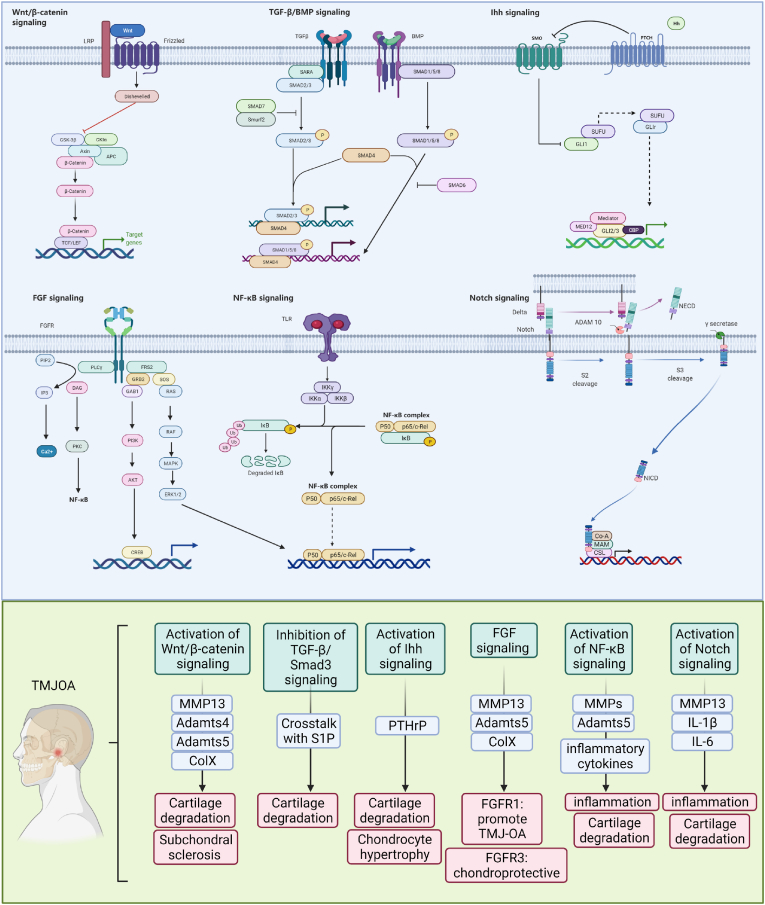
Figure 1.
Schematic diagram of multiple signaling pathways in TMJ OA chondrocytes. Alterations of Wnt/β-catenin, TGF-β, Ihh, FGF, NF-κB, and Notch signaling pathways lead to increased expression of matrix degrading proteases, including MMPs and Adamts4/5, promoting cartilage degradation. The activation of Wnt/β-catenin signaling also affect subchondral bone of TMJ. The activation of Ihh pathway affects chondrocyte differentiation and maturation through PTHrP. The activation of NF-κB, and Notch pathways induces inflammation and accelerates TMJ OA development.
4. Perspective
Because it was difficult to acquire TMJ tissue samples from patients, many animal models were developed for TMJ OA studies. Partial perforations of the discs, in which one-third of the disc in the anterior and lateral regions of the joint was resected, were performed on rabbits for inducing TMJ OA [85]. It has been reported that TMJ OA model can be successfully generated in mice and rats using an elastic rubber band to create disordered occlusion, which causes OA-like lesions in the mandibular condyle [29]. Compressive loading of mechanical stress onto the TMJ can be applied to mice and rats to induce TMJ OA [26,52]. Additionally, injection of MIA, a chondrocyte metabolism inhibitor, into TMJ can also induce TMJ OA phenotype in rats and rabbits [28,29]. Despite these success, the current animal models of TMJ OA are still insufficient in mimicking complex clinical conditions. Novel or improved animal models of TMJ OA may be developed in future studies. Primary TMJ cells have been used for in vitro studies [86], but it is difficult to obtain fibrocatilage samples from patients’ TMJ tissues. Generation of a human immortalized cell line of fibrocatilage chondrocyte may benefit future TMJ OA studies. Besides, application of new techniques, such as organoid, single cell sequencing, 3D printing, could accelerate TMJ OA research.
5. Conclusion
Multiple signaling pathways were involved in the pathological process of TMJ OA mainly by directly or indirectly affecting cartilage matrix degradation, chondrocyte metabolism and chondrocyte apoptosis.New therapeutic strategies of TMJ OA such as engineered cartilage, stem cell applicaton, gene editing and regenerative techniques may be applied in TMJ OA studies.
Funding
This research was supported by the grant from National Natural Science Foundation of China (NSFC) 82030067 to D.C.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
All persons who have made substantial contributions to the work reported in the manuscript (e.g., technical help, writing and editing assistance, general support), but who do not meet the criteria for authorship, are named in the Acknowledgements and have given us their written permission to be named. If we have not included an Acknowledgements, then that indicates that we have not received substantial contributions from non-authors.
References
- 1.Kalladka M., Quek S., Heir G., Eliav E., Mupparapu M., Viswanath A. Temporomandibular joint osteoarthritis: diagnosis and long-term conservative management: a topic review. J Indian Prosthodont Soc. 2014;14(1):6–15. doi: 10.1007/s13191-013-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scrivani S.J., Keith D.A., Kaban L.B. Temporomandibular disorders. N Engl J Med. 2008;359(25):2693–2705. doi: 10.1056/NEJMra0802472. [DOI] [PubMed] [Google Scholar]
- 3.Wadhwa S., Kapila S. TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Educ. 2008;72(8):930–947. [PMC free article] [PubMed] [Google Scholar]
- 4.Mizoguchi I., Nakamura M., Takahashi I., Kagayama M., Mitani H. A comparison of the immunohistochemical localization of type I and type II collagens in craniofacial cartilages of the rat. Acta Anat. 1992;144(1):59–64. doi: 10.1159/000147286. [DOI] [PubMed] [Google Scholar]
- 5.Breeland G., Sinkler M.A., Menezes R.G. StatPearls. Treasure. 2021. Embryology, bone ossification. Island (FL) [Google Scholar]
- 6.Palla S., Gallo L.M., Gossi D. Dynamic stereometry of the temporomandibular joint. Orthod Craniofac Res. 2003;6(Suppl 1):37–47. doi: 10.1034/j.1600-0544.2003.233.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang X.D., Zhang J.N., Gan Y.H., Zhou Y.H. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res. 2015;94(5):666–673. doi: 10.1177/0022034515574770. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y.P., Zhang Z.Y., Wu Y.T., Zhang W.L., Ma X.C. Investigation of the clinical and radiographic features of osteoarthrosis of the temporomandibular joints in adolescents and young adults. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(2):e27–34. doi: 10.1016/j.tripleo.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 9.Alzarea B.K. Temporomandibular disorders (TMD) in edentulous patients: a review and proposed classification (dr. Bader's classification) J Clin Diagn Res. 2015;9(4):ZE06–9. doi: 10.7860/JCDR/2015/13535.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrahamsson A.K., Kristensen M., Arvidsson L.Z., Kvien T.K., Larheim T.A., Haugen I.K. Frequency of temporomandibular joint osteoarthritis and related symptoms in a hand osteoarthritis cohort. Osteoarthritis Cartilage. 2017;25(5):654–657. doi: 10.1016/j.joca.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Schmitter M., Essig M., Seneadza V., Balke Z., Schroder J., Rammelsberg P. Prevalence of clinical and radiographic signs of osteoarthrosis of the temporomandibular joint in an older persons community. Dentomaxillofacial Radiol. 2010;39(4):231–234. doi: 10.1259/dmfr/16270943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldring M.B., Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larheim T.A., Abrahamsson A.K., Kristensen M., Arvidsson L.Z. Temporomandibular joint diagnostics using CBCT. Dentomaxillofacial Radiol. 2015;44(1):20140235. doi: 10.1259/dmfr.20140235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernal R., Velasquez E., Gamonal J., Garcia-Sanz J.A., Silva A., Sanz M. Expression of proinflammatory cytokines in osteoarthritis of the temporomandibular joint. Arch Oral Biol. 2008;53(10):910–915. doi: 10.1016/j.archoralbio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Wang X.D., Cui S.J., Liu Y., Luo Q., Du R.J., Kou X.X., et al. Deterioration of mechanical properties of discs in chronically inflamed TMJ. J Dent Res. 2014;93(11):1170–1176. doi: 10.1177/0022034514552825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wehmeyer C., Pap T., Buckley C.D., Naylor A.J. The role of stromal cells in inflammatory bone loss. Clin Exp Immunol. 2017;189(1):1–11. doi: 10.1111/cei.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X., Cai H.X., Cao P.Y., Feng Y., Jiang H.H., Liu L., et al. TLR4 contributes to the damage of cartilage and subchondral bone in discectomy-induced TMJOA mice. J Cell Mol Med. 2020;24(19):11489–11499. doi: 10.1111/jcmm.15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaible H.G. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther. 2014;16(5):470. doi: 10.1186/s13075-014-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujisawa T., Kuboki T., Kasai T., Sonoyama W., Kojima S., Uehara J., et al. A repetitive, steady mouth opening induced an osteoarthritis-like lesion in the rabbit temporomandibular joint. J Dent Res. 2003;82(9):731–735. doi: 10.1177/154405910308200914. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka E., Koolstra J.H. Biomechanics of the temporomandibular joint. J Dent Res. 2008;87(11):989–991. doi: 10.1177/154405910808701101. [DOI] [PubMed] [Google Scholar]
- 21.Das S.K. TMJ osteoarthritis and early diagnosis. J Oral Biol Craniofac Res. 2013;3(3):109–110. doi: 10.1016/j.jobcr.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song H., Lee J.Y., Huh K.H., Park J.W. Long-term changes of temporomandibular joint osteoarthritis on computed tomography. Sci Rep. 2020;10(1):6731. doi: 10.1038/s41598-020-63493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y., Lu H., Deng L., Lin C.H., Pennington Klein K., Wu M. HMGB2 is associated with pressure loading in chondrocytes of temporomandibular joint: in vitro and in vivo study. Cytokine. 2020;126:154875. doi: 10.1016/j.cyto.2019.154875. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J., Zhang H.Y., Zhang M., Qiu Z.Y., Wu Y.P., Callaway D.A., et al. Connexin43 hemichannels mediate small molecule exchange between chondrocytes and matrix in biomechanically-stimulated temporomandibular joint cartilage. Osteoarthritis Cartilage. 2014;22(6):822–830. doi: 10.1016/j.joca.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Embree M., Ono M., Kilts T., Walker D., Langguth J., Mao J., et al. Role of subchondral bone during early-stage experimental TMJ osteoarthritis. J Dent Res. 2011;90(11):1331–1338. doi: 10.1177/0022034511421930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J., Jiao K., Zhang M., Zhou T., Liu X.D., Yu S.B., et al. Occlusal effects on longitudinal bone alterations of the temporomandibular joint. J Dent Res. 2013;92(3):253–259. doi: 10.1177/0022034512473482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H., Hu Y., Wang C., Zhang X., He D. CircGCN1L1 promotes synoviocyte proliferation and chondrocyte apoptosis by targeting miR-330-3p and TNF-alpha in TMJ osteoarthritis. Cell Death Dis. 2020;11(4):284. doi: 10.1038/s41419-020-2447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L., Feng Y.P., Hu S.Y., Li H.M., Li Y.Y., Ke J., et al. PDCD4 suppresses autophagy and promotes apoptosis via Akt in chondrocytes of temporomandibular joint osteoarthritis. Oral Dis. 2021;27(3):547–558. [English] [Google Scholar]
- 29.Li H., Zhang X.Y., Wu T.J., Cheng W., Liu X., Jiang T.T., et al. Endoplasmic reticulum stress regulates rat mandibular cartilage thinning under compressive mechanical stress. J Biol Chem. 2013;288(25):18172–18183. doi: 10.1074/jbc.M112.407296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y.H., Bang J.I., Son H.J., Kim Y., Kim J.H., Bae H., et al. Protective effects of extracorporeal shockwave on rat chondrocytes and temporomandibular joint osteoarthritis; preclinical evaluation with in vivo(99m)Tc-HDP SPECT and ex vivo micro-CT. Osteoarthritis Cartilage. 2019;27(11):1692–1701. doi: 10.1016/j.joca.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Liao L., Zhang S., Zhou G.Q., Ye L., Huang J., Zhao L., et al. Deletion of Runx2 in condylar chondrocytes disrupts TMJ tissue homeostasis. J Cell Physiol. 2019;234(4):3436–3444. doi: 10.1002/jcp.26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deschner J., Rath-Deschner B., Agarwal S. Regulation of matrix metalloproteinase expression by dynamic tensile strain in rat fibrochondrocytes. Osteoarthritis Cartilage. 2006;14(3):264–272. doi: 10.1016/j.joca.2005.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X.Y., Chen Y., Tang X.J., Jiang L.H., Ji P. AMD3100 attenuates matrix metalloprotease-3 and -9 expressions and prevents cartilage degradation in a monosodium iodo-acetate-induced rat model of temporomandibular osteoarthritis. J Oral Maxillofac Surg. 2016;74(5):927 e1–27 e13. doi: 10.1016/j.joms.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Du J., Jiang Q., Mei L., Yang R., Wen J., Lin S., et al. Effect of high fat diet and excessive compressive mechanical force on pathologic changes of temporomandibular joint. Sci Rep. 2020;10(1):17457. doi: 10.1038/s41598-020-74326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao H., Liu S., Ma C., Ma S., Chen G., Yuan L., et al. Estrogen-related receptor gamma induces angiogenesis and extracellular matrix degradation of temporomandibular joint osteoarthritis in rats. Front Pharmacol. 2019;10:1290. doi: 10.3389/fphar.2019.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu M., Tang D., Wu Q., Hao S., Chen M., Xie C., et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24(1):12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie W., Zhou L., Li S., Hui T., Chen D. Wnt/beta-catenin signaling plays a key role in the development of spondyloarthritis. Ann N Y Acad Sci. 2016;1364:25–31. doi: 10.1111/nyas.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhanot P., Brink M., Samos C.H., Hsieh J.C., Wang Y., Macke J.P., et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382(6588):225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 39.Hui T., Zhou Y., Wang T., Li J., Zhang S., Liao L., et al. Activation of beta-catenin signaling in aggrecan-expressing cells in temporomandibular joint causes osteoarthritis-like defects. Int J Oral Sci. 2018;10(2):13. doi: 10.1038/s41368-018-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y., Shu B., Xie R., Huang J., Zheng L., Zhou X., et al. Deletion of Axin1 in condylar chondrocytes leads to osteoarthritis-like phenotype in temporomandibular joint via activation of beta-catenin and FGF signaling. J Cell Physiol. 2019;234(2):1720–1729. doi: 10.1002/jcp.27043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M., Li S., Xie W., Shen J., Im H.J., Holz J.D., et al. Activation of beta-catenin signalling leads to temporomandibular joint defects. Eur Cell Mater. 2014;28:223–235. doi: 10.22203/ecm.v028a15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang T., Zhang J., Cao Y., Zhang M., Jing L., Jiao K., et al. Wnt5a/Ror2 mediates temporomandibular joint subchondral bone remodeling. J Dent Res. 2015;94(6):803–812. doi: 10.1177/0022034515576051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takada K., Zhu D., Bird G.H., Sukhdeo K., Zhao J.J., Mani M., et al. Targeted disruption of the BCL9/beta-catenin complex inhibits oncogenic Wnt signaling. Sci Transl Med. 2012;4(148):148ra17. doi: 10.1126/scitranslmed.3003808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grossmann T.N., Yeh J.T., Bowman B.R., Chu Q., Moellering R.E., Verdine G.L. Inhibition of oncogenic Wnt signaling through direct targeting of beta-catenin. Proc Natl Acad Sci U S A. 2012;109(44):17942–17947. doi: 10.1073/pnas.1208396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katagiri T., Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8(3):147–159. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- 46.Tang Y., Wu X., Lei W., Pang L., Wan C., Shi Z., et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15(7):757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner D.O., Sieber C., Bhushan R., Borgermann J.H., Graf D., Knaus P. BMPs: from bone to body morphogenetic proteins. Sci Signal. 2010;3(107):mr1. doi: 10.1126/scisignal.3107mr1. [DOI] [PubMed] [Google Scholar]
- 48.Yi J.J., Barnes A.P., Hand R., Polleux F., Ehlers M.D. TGF-beta signaling specifies axons during brain development. Cell. 2010;142(1):144–157. doi: 10.1016/j.cell.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W., Zhao S., Yang H., Zhang C., Kang Q., Deng J., et al. Potential novel prediction of TMJ-OA: MiR-140-5p regulates inflammation through smad/TGF-beta signaling. Front Pharmacol. 2019;10:15. doi: 10.3389/fphar.2019.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mori H., Izawa T., Tanaka E. Smad3 deficiency leads to mandibular condyle degradation via the sphingosine 1-phosphate (S1P)/S1P3 signaling axis. Am J Pathol. 2015;185(10):2742–2756. doi: 10.1016/j.ajpath.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 51.Alvarez S.E., Milstien S., Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metabol. 2007;18(8):300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Jiao K., Zhang M., Niu L., Yu S., Zhen G., Xian L., et al. Overexpressed TGF-beta in subchondral bone leads to mandibular condyle degradation. J Dent Res. 2014;93(2):140–147. doi: 10.1177/0022034513513034. [DOI] [PubMed] [Google Scholar]
- 53.Fang J., Xiao L., Chen R., Zhao Z. Conditional removal of the canonical TGF-beta1 signaling delays condylar cartilage degeneration induced by a partial discectomy in mice. PloS One. 2017;12(5) doi: 10.1371/journal.pone.0177826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shirakura M., Kram V., Robinson J., Sikka S., Kilts T.M., Wadhwa S., et al. Extracellular matrix mediates BMP-2 in a model of temporomandibular joint osteoarthritis. Cells Tissues Organs. 2017;204(2):84–92. doi: 10.1159/000464102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki T., Bessho K., Segami N., Nojima T., Iizuka T. Bone morphogenetic protein-2 in temporomandibular joints with internal derangement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(6):670–673. doi: 10.1016/s1079-2104(99)70007-7. [DOI] [PubMed] [Google Scholar]
- 56.Alman B.A. The role of hedgehog signalling in skeletal health and disease. Nat Rev Rheumatol. 2015;11(9):552–560. doi: 10.1038/nrrheum.2015.84. [DOI] [PubMed] [Google Scholar]
- 57.Lanske B., Karaplis A.C., Lee K., Luz A., Vortkamp A., Pirro A., et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273(5275):663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 58.Nachury M.V., Mick D.U. Establishing and regulating the composition of cilia for signal transduction. Nat Rev Mol Cell Biol. 2019;20(7):389–405. doi: 10.1038/s41580-019-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong S.Y., Reiter J.F. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang H., Zhang M., Liu Q., Zhang H., Zhang J., Lu L., et al. Inhibition of Ihh reverses temporomandibular joint osteoarthritis via a PTH1R signaling dependent mechanism. Int J Mol Sci. 2019;20(15) doi: 10.3390/ijms20153797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckland J. Osteoarthritis: blocking hedgehog signaling might have therapeutic potential in OA. Nat Rev Rheumatol. 2010;6(2):61. doi: 10.1038/nrrheum.2009.270. [DOI] [PubMed] [Google Scholar]
- 62.Xu T., Xu G., Gu Z., Wu H. Hedgehog signal expression in articular cartilage of rat temporomandibular joint and association with adjuvant-induced osteoarthritis. J Oral Pathol Med. 2017;46(4):284–291. doi: 10.1111/jop.12497. [DOI] [PubMed] [Google Scholar]
- 63.Bechtold T.E., Saunders C., Decker R.S., Um H.B., Cottingham N., Salhab I., et al. Osteophyte formation and matrix mineralization in a TMJ osteoarthritis mouse model are associated with ectopic hedgehog signaling. Matrix Biol. 2016;52-54:339–354. doi: 10.1016/j.matbio.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ornitz D.M., Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su N., Du X., Chen L. FGF signaling: its role in bone development and human skeleton diseases. Front Biosci. 2008;13:2842–2865. doi: 10.2741/2890. [DOI] [PubMed] [Google Scholar]
- 66.Martin G., Bogdanowicz P., Domagala F., Ficheux H., Pujol J.P. Rhein inhibits interleukin-1 beta-induced activation of MEK/ERK pathway and DNA binding of NF-kappa B and AP-1 in chondrocytes cultured in hypoxia: a potential mechanism for its disease-modifying effect in osteoarthritis. Inflammation. 2003;27(4):233–246. doi: 10.1023/a:1025040631514. [DOI] [PubMed] [Google Scholar]
- 67.Wang Z., Huang J., Zhou S., Luo F., Tan Q., Sun X., et al. Loss of Fgfr1 in chondrocytes inhibits osteoarthritis by promoting autophagic activity in temporomandibular joint. J Biol Chem. 2018;293(23):8761–8774. doi: 10.1074/jbc.RA118.002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foldynova-Trantirkova S., Wilcox W.R., Krejci P. Sixteen years and counting: the current understanding of fibroblast growth factor receptor 3 (FGFR3) signaling in skeletal dysplasias. Hum Mutat. 2012;33(1):29–41. doi: 10.1002/humu.21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou S., Xie Y., Li W., Huang J., Wang Z., Tang J., et al. Conditional deletion of Fgfr3 in chondrocytes leads to osteoarthritis-like defects in temporomandibular joint of adult mice. Sci Rep. 2016;6:24039. doi: 10.1038/srep24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q., Lenardo M.J., Baltimore D. 30 Years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168(1–2):37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ge X.P., Gan Y.H., Zhang C.G., Zhou C.Y., Ma K.T., Meng J.H., et al. Requirement of the NF-kappaB pathway for induction of Wnt-5A by interleukin-1beta in condylar chondrocytes of the temporomandibular joint: functional crosstalk between the Wnt-5A and NF-kappaB signaling pathways. Osteoarthritis Cartilage. 2011;19(1):111–117. doi: 10.1016/j.joca.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 72.Chijimatsu R., Kunugiza Y., Taniyama Y., Nakamura N., Tomita T., Yoshikawa H. Expression and pathological effects of periostin in human osteoarthritis cartilage. BMC Muscoskel Disord. 2015;16:215. doi: 10.1186/s12891-015-0682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan J., Ding W., Wu N., Jiang S., Li W. Protective effect of genistein on condylar cartilage through downregulating NF-kappaB expression in experimentally created osteoarthritis rats. BioMed Res Int. 2019;2019:2629791. doi: 10.1155/2019/2629791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ou F., Huang Y., Sun J., Su K., He Y., Zeng R., et al. Yohimbine ameliorates temporomandibular joint chondrocyte inflammation with suppression of NF-kappaB pathway. Inflammation. 2021;44(1):80–90. doi: 10.1007/s10753-020-01310-0. [DOI] [PubMed] [Google Scholar]
- 75.Su S.C., Tanimoto K., Tanne Y., Kunimatsu R., Hirose N., Mitsuyoshi T., et al. Celecoxib exerts protective effects on extracellular matrix metabolism of mandibular condylar chondrocytes under excessive mechanical stress. Osteoarthritis Cartilage. 2014;22(6):845–851. doi: 10.1016/j.joca.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 76.Fan B., Liu X., Chen X., Xu W., Zhao H., Yang C., et al. Periostin mediates condylar resorption via the NF-kappaB-ADAMTS5 pathway. Inflammation. 2020;43(2):455–465. doi: 10.1007/s10753-019-01129-4. [DOI] [PubMed] [Google Scholar]
- 77.D'Souza B., Meloty-Kapella L., Weinmaster G. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kopan R., Ilagan M.X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saito T., Tanaka S. Molecular mechanisms underlying osteoarthritis development: notch and NF-kappaB. Arthritis Res Ther. 2017;19(1):94. doi: 10.1186/s13075-017-1296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Q.Y., Dai J., Kuang B., Zhang J., Yu S.B., Duan Y.Z., et al. Osteochondral angiogenesis in rat mandibular condyles with osteoarthritis-like changes. Arch Oral Biol. 2012;57(6):620–629. doi: 10.1016/j.archoralbio.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 81.Mahjoub M., Sassi N., Driss M., Laadhar L., Allouche M., Hamdoun M., et al. Expression patterns of Notch receptors and their ligands in human osteoarthritic and healthy articular cartilage. Tissue Cell. 2012;44(3):182–194. doi: 10.1016/j.tice.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 82.Yoon K., Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8(6):709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y., Zhao B., Zhu Y., Zhao H., Ma C. HIF-1-VEGF-Notch mediates angiogenesis in temporomandibular joint osteoarthritis. Am J Transl Res. 2019;11(5):2969–2982. [PMC free article] [PubMed] [Google Scholar]
- 84.Luo X., Jiang Y., Bi R., Jiang N., Zhu S. Inhibition of notch signaling pathway temporally postpones the cartilage degradation progress of temporomandibular joint arthritis in mice. J Cranio-Maxillo-Fac Surg. 2018;46(7):1132–1138. doi: 10.1016/j.jcms.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 85.Liu X.W., Hu J., Man C., Zhang B., Ma Y.Q., Zhu S.S. Insulin-like growth factor-1 suspended in hyaluronan improves cartilage and subchondral cancellous bone repair in osteoarthritis of temporomandibular joint. Int J Oral Maxillofac Surg. 2011;40(2):184–190. doi: 10.1016/j.ijom.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 86.Wang L., Lazebnik M., Detamore M.S. Hyaline cartilage cells outperform mandibular condylar cartilage cells in a TMJ fibrocartilage tissue engineering application. Osteoarthritis Cartilage. 2009;17(3):346–353. doi: 10.1016/j.joca.2008.07.004. [DOI] [PubMed] [Google Scholar]