Abstract
Resistance to penicillin and multiple antimicrobial agents among Streptococcus pneumoniae strains is becoming an increasing problem worldwide and in Asia. To determine the prevalence of carriage of S. pneumoniae isolates not susceptible to penicillin in young children, we obtained nasopharyngeal swab specimens from 1,978 children (ages, 2 to 6 years) attending 79 day care centers or kindergartens. Three hundred eighty-three strains of S. pneumoniae were isolated from these children. Fifty-eight percent of these isolates had reduced susceptibility to penicillin, 123 (32.1%) were intermediate, and 100 (26.1%) were resistant. A very high penicillin MIC (4 μg/ml) was found in 3.3% of the isolates. The isolates also demonstrated high rates of resistance to other antimicrobial agents (51.2% to cefaclor, 50.2% to cefuroxime, 42.8% to cefotaxime, 80.7% to trimethoprim-sulfamethoxazole, 77% to erythromycin, 60% to clindamycin, and 33.7% to chloramphenicol). No isolate was resistant to fluoroquinolone. Multidrug resistance (not susceptible to the β-lactams and three or more other classes) was found in 39.4% of the isolates. Risk factors for the carriage of S. pneumoniae not susceptible to penicillin were multiple physician visits in the preceding 3 months and use of antimicrobial agents by the individual or by household members in the preceding 3 months. In the logistic regression analysis, only the use of antimicrobial agents in the preceding 3 months was an independent risk factor (P = 0.004; odds ratio, 2; 95% confidence interval, 1.2 to 3.2). This study demonstrated the high prevalence of antibiotic-resistant S. pneumoniae in healthy young children in the community in Hong Kong.
Streptococcus pneumoniae is an important cause of bacteremia, bacterial meningitis, otitis media, and pneumonia in children worldwide. The issue of antibiotic resistance has been an increasing concern in the last decade around the world (2, 4). In Hong Kong, the rate of resistance to penicillin was 5% in 1991; this rate increased to 28.9 and 69.1% in 1993 to 1995 and in 1998, respectively (11, 12). Among the penicillin-resistant strains, 70 to 90% of them are currently resistant to erythromycin, cotrimoxazole, tetracycline, and chloramphenicol.
Nasopharyngeal carriage of S. pneumoniae can serve as an indicator of the prevalence of resistant strains in the community and has been used to assess the antibiotic resistance of S. pneumoniae in different populations (9, 14, 20). In Hong Kong, no large-scale data on S. pneumoniae colonization in young children are available. The purpose of this study was to obtain data on the susceptibility and the rate of prevalence of carriage of S. pneumoniae isolates not susceptible to penicillin (PNSSP isolates) among the pediatric population throughout Hong Kong. The approach was a cross-sectional survey of nasopharyngeal carriage among children between 2 and 6 years of age attending day care centers or kindergartens.
MATERIALS AND METHODS
Study design.
Children between 2 and 6 years of age who attend day care centers or kindergartens in Hong Kong were recruited. We planned to sample about 2,000 children. We estimated that between 10 and 25% of children would carry S. pneumoniae and that around 50% of those carrying S. pneumoniae would have isolates with reduced susceptibility to penicillin. We estimated that a sample size of 2,000 children would yield 200 to 500 children carrying S. pneumoniae and 100 to 250 isolates with reduced penicillin susceptibility. Hong Kong is divided into 18 school districts, and the sample size of each district was calculated according to the number of day care center and kindergarten places in each district (Table 1). Ages of children who attend day care centers and kindergartens range between 2 and 6 years and 3 and 6 years, respectively. Normally, all children attend 5 days per week for 7 to 9 h a day in day care centers and for 3 to 4 h a day in kindergartens. The average class size was about 30. Out of the total of 159,113 day care center and kindergarten places, day care centers accounted for 25% while kindergartens accounted for 75%. This ratio was used to calculate the number of children to be recruited from day care centers and kindergartens from each district to make up the study population of 2,000. District 18 is a conglomerate of several sparsely populated small outlying islands in Hong Kong, and since the number of children to be recruited was small, the quota was distributed over other areas nearby.
TABLE 1.
Distribution of children recruited for study by district and day care center and kindergarten places in Hong Kong
| District | Day care centers
|
Kindergartens
|
||
|---|---|---|---|---|
| No. of places (n = 38,767) (%) | No. of children planned (actually recruited) | No. of places (n = 120,346) (%) | No. of children planned (actually recruited) | |
| 1 | 1,130 (2.9) | 15 (15) | 3,031 (2.5) | 38 (42) |
| 2 | 2,154 (5.5) | 28 (30) | 5,041 (4.2) | 63 (62) |
| 3 | 3,961 (10) | 50 (52) | 11,046 (9.2) | 138 (131) |
| 4 | 1,086 (2.8) | 14 (13) | 3,212 (2.7) | 41 (40) |
| 5 | 1,456 (3.7) | 19 (19) | 4,142 (3.4) | 53 (52) |
| 6 | 4,145 (10.7) | 54 (54) | 10,370 (8.6) | 130 (131) |
| 7 | 2,259 (5.8) | 29 (29) | 6,722 (5.6) | 84 (89) |
| 8 | 2,337 (6.0) | 31 (31) | 7,255 (6.0) | 92 (90) |
| 9 | 3,109 (8.0) | 41 (41) | 9,415 (7.8) | 117 (121) |
| 10 | 1,452 (3.8) | 20 (21) | 4,285 (3.6) | 54 (24) |
| 11 | 2,810 (7.3) | 28 (26) | 5,877 (4.9) | 75 (97) |
| 12 | 3,320 (8.6) | 44 (45) | 11,845 (9.8) | 147 (137) |
| 13 | 1,594 (4.1) | 22 (20) | 9,607 (8.0) | 120 (121) |
| 14 | 1,486 (3.8) | 21 (20) | 5,540 (4.6) | 69 (62) |
| 15 | 1,711 (4.4) | 23 (23) | 6,319 (5.2) | 78 (94) |
| 16 | 3,680 (9.5) | 48 (49) | 11,030 (9.2) | 138 (137) |
| 17 | 740 (1.9) | 11 (15) | 4,880 (4.1) | 62 (68) |
| 18 | 338 (0.9) | 4 (0) | 747 (0.6) | 10 (0) |
In the recruitment phase, letters explaining the study were sent randomly to the day care centers and kindergartens of each school district asking for participation. In each school, a random sample of parents were initially approached by the teachers. Those that consented to participate were then sent a letter explaining the study, a consent form, and a questionnaire regarding potential risk factors for infection with nonsusceptible S. pneumoniae. The participation rate of parents who were approached by the teachers was about 90%. On average, 25 children (standard deviation, 11 children) from each institute were recruited. At a prearranged date, a trained study nurse went to each school to collect the completed questionnaire and nasopharyngeal swab samples from the children whose parents had consented to participate. A calcium alginate-tipped swab on a flexible aluminum wire (TRANSWAB per nasal; Medical Wire and Equipment Co. Ltd., Corsham, Wilts, England) was used. The specimens were brought back to the laboratory within 4 h for processing.
Definitions.
Recent use of antibiotics was defined as consumption of any antibiotic in the 3 months prior to the date of surveillance. Overcrowding was defined as a living space of ≤5.5 m2/person in accordance with the guideline of the Hong Kong Housing Authority. Nonsusceptible organisms included those that were both intermediate and resistant. Multidrug resistance was defined as nonsusceptibility to three or more classes in addition to β-lactams.
Bacterial isolation and identification.
For selective isolation of S. pneumoniae, swabs were inoculated onto 5% horse blood agar supplemented with gentamicin (5 μg/ml) and incubated in 5% CO2 for 16 to 24 h. All isolates were identified using colony morphology, Gram stain, optochin susceptibility, and bile solubility.
Antimicrobial agents and susceptibility testing.
E-test strips of penicillin, amoxicillin (as amoxicillin-clavulanate, 2:1), erythromycin, clindamycin, cefaclor, cefuroxime, cefpodoxime, cefotaxime, quinupristin-dalfopristin, linezolid, ciprofloxacin, and levofloxacin were purchased from AB Biodisk (Solna, Sweden). E-test MICs were determined in accordance with the manufacturer's instructions (11). Test inocula were prepared from pneumococcal colonies grown on sheep blood agar that had been incubated for 20 to 24 h in 5% CO2. Colonies were suspended in 0.9% saline to obtain a suspension equivalent to the turbidity of a 0.5 McFarland standard. From this suspension, E-tests were performed on Mueller-Hinton agar with 5% sheep blood (BBL, Becton Dickinson Microbiology Systems, Cockeysville, Md.). The plates were incubated at 35°C in 5% CO2 for 20 to 24 h. MICs falling between two marks on the E-test strip were rounded up to the next higher twofold dilution, as recommended in the instructions. The disk diffusion method was used for trimethoprim-sulfamethoxazole and chloramphenicol. Quality control strains (S. pneumoniae ATCC 49619, Staphylococcus aureus ATCC 29213, and Escherichia coli ATCC 25922) were included with each run. Interpretation of results was performed by using published breakpoints of the National Committee for Clinical Laboratory Standards (NCCLS) (22). For ciprofloxacin, the assumed breakpoints were as follows: ≤2 μg/ml, sensitive; 4 μg/ml, intermediate; ≥8 μg/ml, resistant.
Statistical analysis.
Potential risk factors for carriage of PNSSP were identified by univariate analysis. The χ2 test was used for categorical variables. Continuous variables were tested by Student's t test or the Mann-Whitney U test. Variables that were significant in the univariate analysis and those that could increase the risk of PNSSP carriage from a clinical point of view were further tested by logistic regression using the forward-conditional method (25). The following parameters were entered for analysis in the logistic regression models: age, gender, location of day care center or kindergarten, number of young siblings with age <12 years, hospitalization, recent use of antibiotic by subject, recent use of antibiotic by household member, and overcrowding. A P value of <0.05 was considered to be statistically significant. A statistical package (SPSS 10.0; SPSS Hong Kong Ltd., Hong Kong) was used for all analysis.
RESULTS
Demographics.
Between December 1999 and June 2000, nasopharyngeal swab samples were taken from a total of 2,001 children between 2 and 6 years of age from 79 day care centers and kindergartens in Hong Kong. Twenty-three children were found to be older than 72 months and were excluded. The median age (interquantile range) for the remaining 1,978 children was 5.3 years (4.3 to 5.3 years); the mean age was 5 years. About one-half of the children were male (52.7%). The questionnaire was returned for all participants. Sixty-three percent of surveyed children had siblings 12 years old or below; 51.4% had one sibling, 9.7% had two siblings, 1.5% had three siblings, and 0.4% had four siblings. The living environment was overcrowded for 14% of the children in this study. At the time of surveillance, 5.2% (103 of 1,978) of all children were reported to be taking antimicrobial agents. In the 3 months prior to the study, 77.6% (1,535 of 1,978) of them had visited their family doctor and 3.2% had been hospitalized. Of these 1,535 children, there were a total of 1,931 doctor visits in the 3-month period prior to the collection of the nasal sample, with 468 children having three or more visits and 139 having five or more visits. Fifty percent (883 of 1,747) of the children and 34.7% (616 of 1,777) of their household members reported recent use of antimicrobial agents. In 13.6% (241 of 1,777) of the households, two or more household members had taken antimicrobial agents recently.
S. pneumoniae carriage and antimicrobial susceptibility.
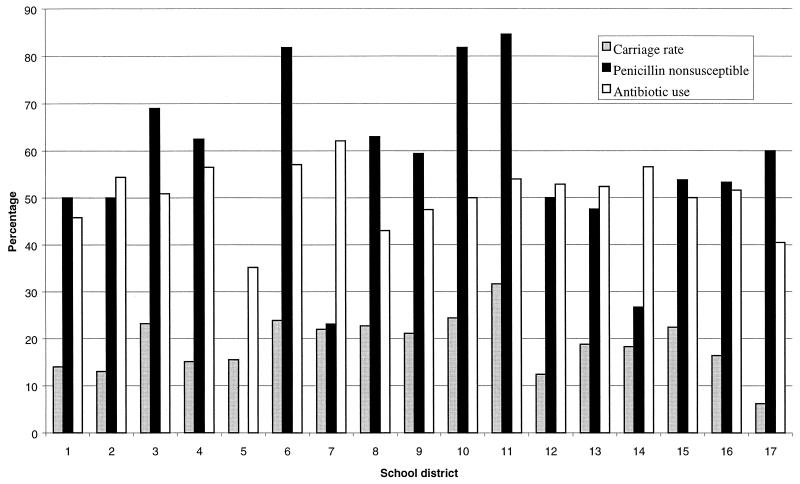
Overall, the carriage rate of S. pneumoniae was 19.4% (383 of 1,978). Carriage was more common at a younger age: 28.8% (17 of 59) for children 2 to 3 years old, 32.6% (95 of 291) for children 4 years old, 20.1% (97 of 483) for children 5 years old, and 15.2% (174 of 1,145) for children 6 years old (P < 0.001; Mann-Whitney U test). Carriage rates according to regional location of the day care centers and kindergartens are shown in Fig. 1. Variations in the carriage rate were found in the different regions, highest (31.7%) in region 11 and lowest (6.2%) in region 17 (P = 0.001). With the exception of region 5, between 23.1 and 84.6% of the S. pneumoniae isolates were not susceptible to penicillin (P < 0.001). Overall, 58.2% (223 of 383) of the isolates were not susceptible to penicillin, including 29.5% (123 of 383) intermediate and 26.1% (100 of 383) resistant. The susceptibilities of the 383 pneumococcus isolates to 12 antimicrobial agents are summarized in Table 2. A very high MIC of penicillin (4 μg/ml) was found in 3.3% (13 of 383) of the strains. Larger percentages of isolates with reduced susceptibility to penicillin were resistant to trimethoprim-sulfamethoxazole and chloramphenicol, 98.6 (220 of 223) and 45.3% (101 of 223), respectively, than of penicillin-susceptible strains 55.6 (89 of 160) and 17.5% (28 of 160) (P < 0.001). Overall, 39.4% (151 of 383) of isolates were multiply resistant.
FIG. 1.
Frequencies of S. pneumoniae, PNSSP carriage, and antibiotic use for the 3 months prior to sampling in children attending day care centers or kindergartens by school district.
TABLE 2.
Susceptibilities of 383 isolates of S. pneumoniae stratified by penicillin susceptibilitya
| Drug | Penicillin susceptibilityb | n | MIC (μg/ml)c
|
% Susceptible | % Intermediate | % Resistant | ||
|---|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | ||||||
| Penicillin | All | 383 | 0.016–4 | 0.25 | 2 | 41.8 | 32.1 | 26.1 |
| S | 160 | 0.016–0.064 | 0.032 | 0.064 | 100.0 | 0.0 | 0.0 | |
| I | 123 | 0.125–1 | 1 | 1 | 0.0 | 100.0 | 0.0 | |
| R | 100 | 2–4 | 2 | 4 | 0.0 | 0.0 | 100.0 | |
| Amoxicillin | All | 0.016–4 | 0.25 | 2 | 99.0/53.3 | 1.0/23.5 | 0.0/23.2 | |
| S | 0.016–0.125 | 0.032 | 0.032 | 100.0/100.0 | 0.0/0.0 | 0.0/0.0 | ||
| I | 0.032–4 | 1 | 2 | 99.2/35.8 | 0.8/51.2 | 0.0/13.0 | ||
| R | 1–4 | 2 | 2 | 97.0/0.0 | 3.0/27.0 | 0.0/73.0 | ||
| Cefaclor | All | 0.25–≥256 | 4 | 64 | 47.8 | 1.3 | 50.9 | |
| S | 0.25–1 | 0.5 | 0.5 | 99.4 | 0.0 | 0.6 | ||
| I | 0.25–≥256 | 16 | 64 | 19.5 | 4.1 | 76.4 | ||
| R | 16–≥256 | 64 | 128 | 0.0 | 0.0 | 100.0 | ||
| Cefuroxime | All | 0.016–16 | 1 | 4 | 49.9 | 1.6 | 48.6 | |
| S | 0.016–0.25 | 0.032 | 0.125 | 100.0 | 0.0 | 0.0 | ||
| I | 0.016–4 | 2 | 4 | 25.2 | 4.9 | 69.9 | ||
| R | 2–16 | 4 | 4 | 0.0 | 0.0 | 100.0 | ||
| Cefpodoxime | All | 0.016–16 | 0.5 | 4 | 50.1 | 0.8 | 49.1 | |
| S | 0.016–0.5 | 0.032 | 0.064 | 100.0 | 0.0 | 0.0 | ||
| I | 0.032–4 | 2 | 4 | 26.0 | 2.4 | 71.5 | ||
| R | 2–16 | 4 | 4 | 0.0 | 0.0 | 100.0 | ||
| Cefotaxime | All | 0.016–2 | 0.5 | 2 | 56.9 | 30.5 | 12.5 | |
| S | 0.016–0.25 | 0.032 | 0.064 | 100.0 | 0.0 | 0.0 | ||
| I | 0.016–2 | 1 | 1 | 47.2 | 52.0 | 0.8 | ||
| R | 1–2 | 1 | 2 | 0.0 | 53.0 | 47.0 | ||
| Erythromycin | All | 0.032–≥256 | 16 | ≥256 | 23.0 | 0.0 | 77.0 | |
| S | 0.032–≥256 | 16 | ≥256 | 41.2 | 0.0 | 58.8 | ||
| I | 0.125–≥256 | ≥256 | ≥256 | 17.1 | 0.0 | 82.9 | ||
| R | 0.25–≥256 | 16 | ≥256 | 1.0 | 0.0 | 99.0 | ||
| Clindamycin | All | 0.125–≥256 | 0.5 | ≥256 | 39.9 | 15.9 | 44.1 | |
| S | 0.125–≥256 | 0.5 | ≥256 | 46.3 | 17.5 | 36.3 | ||
| I | 0.125–≥256 | ≥256 | ≥256 | 28.5 | 8.1 | 63.4 | ||
| R | 0.25–≥256 | 0.5 | ≥256 | 44.0 | 23.0 | 33.0 | ||
| Quinupristin-dalfopristin | All | 0.25–2 | 0.5 | 0.5 | 98.2 | 1.8 | 0.0 | |
| S | 0.25–2 | 0.5 | 0.5 | 98.8 | 1.2 | 0.0 | ||
| I | 0.25–2 | 0.5 | 1 | 98.4 | 1.6 | 0.0 | ||
| R | 0.25–2 | 0.5 | 0.5 | 97.0 | 3.0 | 0.0 | ||
| Linezolid | All | 1–4 | 2 | 2 | NAd | NA | NA | |
| S | 1–4 | 2 | 2 | NA | NA | NA | ||
| I | 1–2 | 2 | 2 | NA | NA | NA | ||
| R | 1–4 | 2 | 2 | NA | NA | NA | ||
| Ciprofloxacin | All | 0.5–2 | 1 | 1 | 100.0 | 0.0 | 0.0 | |
| S | 0.5–2 | 1 | 2 | 100.0 | 0.0 | 0.0 | ||
| I | 0.5–2 | 1 | 2 | 100.0 | 0.0 | 0.0 | ||
| R | 0.5–2 | 1 | 1 | 100.0 | 0.0 | 0.0 | ||
| Levofloxacin | All | 0.5–2 | 1 | 1 | 100.0 | 0.0 | 0.0 | |
| S | 0.5–2 | 1 | 1 | 100.0 | 0.0 | 0.0 | ||
| I | 0.5–2 | 1 | 1 | 100.0 | 0.0 | 0.0 | ||
| R | 0.5–1 | 1 | 1 | 100.0 | 0.0 | 0.0 | ||
Results were interpreted according to NCCLS M7-A5 (22). For amoxicillin, interpretation was according to a previous recommendation, with interpretation according to NCCLS M100-S9 also shown (to the right of the shill) for comparison (21). The assumed breakpoints for ciprofloxacin were as follows: ≥2 μg/ml, sensitive; 4 μg/ml, intermediate; ≥8 μg/ml, resistant.
S, susceptible; I, intermediate; R, resistant.
50% and 90%, MICs at which 50 and 90% of isolates are inhibited, respectively.
NA, no NCCLS breakpoints available.
Risk factors for carriage of PNSSP.
The result of univariate analysis is shown in Table 3. Three parameters were statistically significant. These were multiple physician visits and recent use of antimicrobial agents by the subject and by household members. Use of antibiotics in the preceding 3 months was significantly correlated (P < 0.001) with physician visit, multiple physician visits, and use of antibiotics by family members. In the logistic regression analysis, only use of antibiotics in the preceding 3 months was independently associated with isolation of PNSSP (P = 0.004; odds ratio, 2; 95% confidence interval, 1.2 to 3.2).
TABLE 3.
Risk factors of nasopharyngeal carriage of PNSSP
| Parameter | Pa | ORb | 95% confidence interval (OR) |
|---|---|---|---|
| Use of antibiotic in preceding 3 mo | 0.003 | 1.9 | 1.2–3 |
| Current use of antimicrobial agents | 0.3 | 1.6 | 0.6–4.0 |
| Hospitalization | 0.9 | 0.9 | 0.3–2.5 |
| Gender | 0.9 | 1 | 0.7–1.5 |
| Young age (36 mo) | 0.1 | 2.6 | 0.8–8.1 |
| Overcrowding | 0.8 | 0.9 | 0.5–1.6 |
| Use of antimicrobial agents among household members in the preceding 3 mo | 0.006 | 2.0 | 1.2–3.2 |
| Having siblings 12 yr old or below | 0.9 | 1 | 0.6–1.5 |
| Had physician visit past 3 mo | 0.07 | 1.6 | 1–2.6 |
| Multiple physician visits (≥3) | 0.001 | 2.5 | 1.5–4.3 |
By univariate analysis. A P value of <0.05 was considered to be significant.
OR, odds ratio.
DISCUSSION
This is a cross-sectional study and the first large-scale study in Hong Kong to examine the epidemiology of S. pneumoniae carriage and the antibiotic resistance pattern of this organism. There has long been the suspicion that the antibiotic resistance pattern of S. pneumoniae in Hong Kong is similar to those seen in nearby Asian countries and in the West. Pediatric data have been scarce. One earlier study showed reduced penicillin susceptibility in 40% of 45 pediatric isolates (17). Another study of 621 Hong Kong children between 2 months and 5 years of age reported an S. pneumoniae carriage rate of 10% (28). Testing of susceptibility to penicillin was not performed, but the authors reported no ampicillin resistance.
The S. pneumoniae carriage of 19.4% in this study lies somewhere between the low carriage rate of 3.5% and the high rate of 77% reported in different areas in the world (3, 6, 10, 15, 24, 25). In general, the highest rates were reported in studies that involved predominately children less than 2 years of age. Our relatively low rates reflect the older age of children studied. However, this was double the carriage rate reported in a smaller study of Hong Kong children (mostly 2 to 5 years to old) attending one maternal and child health center (28). This carriage rate closely approximates that reported from nearby Taiwan (21%) (6). Carriage of S. pneumoniae has been correlated with the emergence of clinical disease, and the antibiotic susceptibility pattern of isolates carried in the nasopharynx reflects the susceptibility pattern of invasive strains.
Despite early and recent reports of increasing prevalence of multidrug-resistant S. pneumoniae in the United States, Asian countries have some of the highest frequencies of PNSSP: 80% in Korea, 71% in Taiwan, 65% in Japan, 58% in Thailand, and 53% in Vietnam (6, 24, 26, 30). Previous data on invasive strains of S. pneumoniae in Hong Kong indicated that 69% were PNSSP (MIC, >0.06 μg/ml) (11). The present study on S. pneumoniae isolated from healthy children in the community showed a rate of penicillin nonsusceptibility of 58.2%, similar to those in neighboring areas in Asia and reflective of invasive isolates from hospitalized patients. This is in contrary to a study of 502 children between 3 months and 5 years of age with upper respiratory tract infections (URI), who presented to a general outpatient clinic at Beijing, People's Republic of China (31). The nasopharyngeal carriage rate of pneumococcus was 37.8%, but only 9% of the isolates were not susceptible to penicillin. Resistance to erythromycin and trimethoprim-sulfamethoxazole, however, was high, at 76.8 and 74.8%, respectively. The marked difference in rates of PNSSP between Hong Kong and Beijing is intriguing. We speculate that this might be related in part to differences in the pattern of antibiotic usage. In Hong Kong and Beijing, as in other industrial areas (23, 29), antibiotics are commonly prescribed to children with URI and hence the high rates. The β-lactams, such as amoxicillin and cefaclor, are most commonly used in Hong Kong, while in Beijing (31) erythromycin is the most popular.
Trimethoprim-sulfamethoxazole and erythromycin represent two classes of antimicrobial agents commonly prescribed by physicians as first-line drugs. Macrolide resistance in S. pneumoniae varies in different geographic areas. It was reported to be low (≤10%) in Latin America, Canada, and Russia but high in the Asia Pacific region (>30%) and 92% in Taiwan (6, 8, 13, 27). Almost 80% of the S. pneumoniae isolates in our study were resistant to erythromycin, and, among penicillin-resistant strains, the resistance was over 90%. Since the mechanisms of action are identical for all members of the macrolide family, resistance to erythromycin also signifies resistance to all the newer members, rendering them all inappropriate choices as first-line antibiotics for treating community-acquired infection when S. pneumoniae is suspected. Trimethoprim-sulfamethoxazole resistance is common in S. pneumoniae in different parts of the world, both in penicillin-susceptible and nonsusceptible strains. The overall incidence of resistance ranges from 20 to over 70% (3, 16, 27). Here we report a high (over 80%) incidence of trimethoprim-sulfamethoxazole resistance in all S. pneumoniae isolates and over 99% in PNSSP. In addition, resistance to clindamycin is high, especially among isolates with reduced penicillin susceptibility. Using either clindamycin, a macrolide, or trimethoprim-sulfamethoxazole as an alternative treatment of PNSSP should not be recommended in areas such as Hong Kong with a high prevalence of resistance unless specific susceptibility is known. In view of the high prevalence of PNSSP, these drugs should not be used as first-line therapy in Hong Kong when S. pneumoniae is suspected.
Pneumococcal resistance to ciprofloxacin, levofloxacin, and trovafloxacin has been documented in 12.1, 5.5, and 2.2% of pneumococcal strains isolated from hospitalized patients in Hong Kong (11). With the documentation of quinolone-resistant pneumococcus in Hong Kong there was concern that these resistant strains could be passed from the adult carriers to children and then became disseminated in day care centers and kindergartens. Quinolone resistance was not documented in the present study. However, there needs to be continual monitoring of quinolone resistance among pneumococcal isolates from children in areas with significant quinolone resistance such as Hong Kong.
As in many other studies, prior exposure to antimicrobial agents was a risk factor for the carriage of PNSSP (3, 15, 16, 19, 25). Children in our study seem to be brought to medical attention frequently. These children are representative of children in this age group, who are expected to experience 5 to 10 colds per year. The frequent doctor consultation, however, reflects the attitudes of parents, as well as the easy access to health care in Hong Kong. What is of concern is that one-half of these consultations resulted in antibiotic usage in this group of children, in whom viral infection should account for most cases of febrile illness. Chan reported in a survey of >500 Hong Kong parents that almost all parents thought that URI in children would not resolve without seeing a doctor. Fifty-four percent thought that bacteria were the cause of URI, and 37% of parents requested antimicrobial agents for their children (5). These misconceptions were similar to some reports in the United States (7, 18). Education of both parents and physicians on the judicious use of antimicrobial agents in children as well as adults is needed to tackle this problem of antibiotic misuse and overuse.
As in previous studies, a young age is a risk factor for PNSSP carriage (15, 19, 25). Surprisingly, although crowding has been implicated, it was not a risk factor in our study, but the definition of crowding used (<5.5 m2/person) is very harsh. Recent hospitalization is also not a factor. There had been suggestion that the highly resistant strains of S. pneumoniae identified in invasive diseases in hospitalized patients were a result of antibiotic use in hospitals and were not reflective of the situation in the community. This study shows that the problem in the hospital only reflects the situation in the community.
With the emergence of multidrug-resistant S. pneumoniae worldwide, the development of effective vaccine for young children is of utmost importance. The licensing and effectiveness of the heptavalent pneumococcal conjugate vaccine have resulted in the recommendation by the American Academy of Pediatrics for universal vaccination in children 23 months old and younger (1). Further analysis of the serotypes of the isolates will provide information on the applicability of the licensed conjugate pneumococcal vaccine in Hong Kong.
ACKNOWLEDGMENTS
This work was supported by the Health Services Research Committee/Health Care and Promotion Fund (no. 921030).
We thank C. Y. Loo for swabbing all the children single-handedly and Wilfred H. S. Wong for technical support.
REFERENCES
- 1.American Academy of Pediatrics. Recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics. 2000;106:362–366. doi: 10.1542/peds.106.2.362. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Arnold K E, Leggiadro R J, Breiman R F, Lipman H B, Schwartz B, Appleton M A, Cleveland K O, Szeto H C, Hill B C, Tenover F C, Elliott J A, Facklam R R. Risk factors for carriage of drug-resistant Streptococcus pneumoniae among children in Memphis, Tennessee. J Pediatr. 1996;128:757–764. doi: 10.1016/s0022-3476(96)70326-8. [DOI] [PubMed] [Google Scholar]
- 4.Breiman R F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 5.Chan C S. What do patients expect from consultations for upper respiratory tract infections? Fam Pract. 1996;13:229–235. doi: 10.1093/fampra/13.3.229. [DOI] [PubMed] [Google Scholar]
- 6.Chiou C C, Liu Y C, Huang T S, Hwang W K, Wang J H, Lin H H, Yen M Y, Hsieh K S. Extremely high prevalence of nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae among children in Kaohsiung, Taiwan. J Clin Microbiol. 1998;36:1933–1937. doi: 10.1128/jcm.36.7.1933-1937.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collett C A, Pappas D E, Evans B A, Hayden G F. Parental knowledge about common respiratory infections and antibiotic therapy in children. South Med J. 1999;92:971–976. doi: 10.1097/00007611-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Critchley I A, Thornsberry C, Piazza G, Jones M, Hickey M L, Barth A L, Mendes C, Rossi F F, Sader H S, Teixeira L M, Sahm D F. Antimicrobial susceptibility of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis collected from five centers in Brazil, 1997–98. Clin Microbiol Infect. 2000;6:178–184. doi: 10.1046/j.1469-0691.2000.00063.x. [DOI] [PubMed] [Google Scholar]
- 9.Fairchok M P, Ashton W S, Fischer G W. Carriage of penicillin-resistant pneumococci in a military population in Washington, DC: risk factors and correlation with clinical isolates. Clin Infect Dis. 1996;22:966–972. doi: 10.1093/clinids/22.6.966. [DOI] [PubMed] [Google Scholar]
- 10.Givon L N, Dagan R, Fraser D, Yagupsky P, Porat N. Marked differences in pneumococcal carriage and resistance patterns between day care centers located within a small area. Clin Infect Dis. 1999;29:1274–1280. doi: 10.1086/313465. [DOI] [PubMed] [Google Scholar]
- 11.Ho P L, Que T L, Tsang D N, Ng T K, Chow K H, Seto W H. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob Agents Chemother. 1999;43:1310–1313. doi: 10.1128/aac.43.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho P L, Yuen K Y, Yam W C, Wong S S Y, Luk W K. Changing patterns of susceptibilities of blood, urinary and respiratory pathogens in Hong Kong. J Hosp Infect. 1995;31:305–317. doi: 10.1016/0195-6701(95)90209-0. [DOI] [PubMed] [Google Scholar]
- 13.Hoban D J, Doern G V, Fluit A C, Roussel-Delvallez M, Jones R N. Worldwide prevalence of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the SENTRY antimicrobial surveillance program, 1997–1999. Clin Infect Dis. 2001;32(Suppl. 2):S81–S93. doi: 10.1086/320181. [DOI] [PubMed] [Google Scholar]
- 14.Kellner J D, Ford-Jones E L. Streptococcus pneumoniae carriage in children attending 59 Canadian child care centers. Toronto Child Care Centre Study Group. Arch Pediatr Adolesc Med. 1999;153:495–502. doi: 10.1001/archpedi.153.5.495. [DOI] [PubMed] [Google Scholar]
- 15.Kellner J D, McGeer A, Cetron M S, Low D E, Butler J C, Matlow A, Talbot J, Ford-Jones E L. The use of Streptococcus pneumoniae nasopharyngeal isolates from healthy children to predict features of invasive disease. Pediatr Infect Dis J. 1998;17:279–286. doi: 10.1097/00006454-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Lopez B, Cima M D, Vazquez F, Fenoll A, Gutierrez J, Fidalgo C, Caicoya M, Mendez F J. Epidemiological study of Streptococcus pneumoniae carriers in healthy primary-school children. Eur J Clin Microbiol Infect Dis. 1999;18:771–776. doi: 10.1007/s100960050399. [DOI] [PubMed] [Google Scholar]
- 17.Luey K Y, Kam K M. Vaccine coverage of Streptococcus pneumoniae in Hong Kong with attention to the multiple-antibiotic-resistant strains. Vaccine. 1996;14:1573–1580. doi: 10.1016/s0264-410x(96)00156-9. [DOI] [PubMed] [Google Scholar]
- 18.Mainous A G, Hueston W J, Clark J R. Antibiotics and upper respiratory infection: do some folks think there is a cure for the common cold. J Fam Pract. 1996;42:357–361. [PubMed] [Google Scholar]
- 19.Melander E, Molstad S, Persson K, Hansson H B, Soderstrom M, Ekdahl K. Previous antibiotic consumption and other risk factors for carriage of penicillin-resistant Streptococcus pneumoniae in children. Eur J Clin Microbiol Infect Dis. 1998;17:834–838. doi: 10.1007/s100960050202. [DOI] [PubMed] [Google Scholar]
- 20.Musher D M, Groover J E, Reichler M R, Riedo F X, Schwartz B, Watson D A, Baughn R E, Breiman R F. Emergence of antibody to capsular polysaccharides of Streptococcus pneumoniae during outbreaks of pneumonia: association with nasopharyngeal colonization. Clin Infect Dis. 1997;24:441–446. doi: 10.1093/clinids/24.3.441. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing, 9th informational supplement. M100–S9. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7–A5. 5th ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 23.Nyquist A C, Gonzales R, Steiner J F, Sande M A. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA. 1998;279:875–877. doi: 10.1001/jama.279.11.875. [DOI] [PubMed] [Google Scholar]
- 24.Parry C M, Diep T S, Wain J, Hoa N T, Gainsborough M, Nga D, Davies C, Phu N H, Hien T T, White N J, Farrar J J. Nasal carriage in Vietnamese children of Streptococcus pneumoniae resistant to multiple antimicrobial agents. Antimicrob Agents Chemother. 2000;44:484–488. doi: 10.1128/aac.44.3.484-488.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Principi N, Marchisio P, Schito G C, Mannelli S. Risk factors for carriage of respiratory pathogens in the nasopharynx of healthy children. Ascanius Project Collaborative Group. Pediatr. Infect Dis J. 1999;18:517–523. doi: 10.1097/00006454-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Song J H, Lee N Y, Ichiyama S, Yoshida R, Hirakata Y, Fu W, Chongthaleong A, Aswapokee N, Chiu C H, Lalitha M K, Thomas K, Perera J, Yee T T, Jamal F, Warsa U C, Vinh B X, Jacobs M R, Appelbaum P C, Pai C H. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Clin Infect Dis. 1999;28:1206–1211. doi: 10.1086/514783. [DOI] [PubMed] [Google Scholar]
- 27.Stratchounski L S, Kretchikova O I, Kozlov R S, Reshedko G K, Stetsiouk O U, Tarasova G D, Blochin B M, Egorova O A, Boyko L M. Antimicrobial resistance of Streptococcus pneumoniae isolated from healthy children in day-care centers: results of a multicenter study in Russia. Pediatr Infect Dis J. 2000;19:196–200. doi: 10.1097/00006454-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Sung R Y, Ling J M, Fung S M, Oppenheimer S J, Crook D W, Lau J T, Cheng A F. Carriage of Haemophilus influenzae and Streptococcus pneumoniae in healthy Chinese and Vietnamese children in Hong Kong. Acta Paediatr. 1995;84:1262–1267. doi: 10.1111/j.1651-2227.1995.tb13545.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang E E, Einarson T R, Kellner J D, Conly J M. Antibiotic prescribing for Canadian preschool children: evidence of overprescribing for viral respiratory infections. Clin Infect Dis. 1999;29:155–160. doi: 10.1086/520145. [DOI] [PubMed] [Google Scholar]
- 30.Whitney C G, Farley M M, Hadler J, Harrison L H, Lexau C, Reingold A, Lefkowitz L, Cieslak P R, Cetron M, Zell E R, Jorgensen J H, Schuchat A, Facklam R R. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343:1917–1924. doi: 10.1056/NEJM200012283432603. [DOI] [PubMed] [Google Scholar]
- 31.Yu S J, Wang J F, Li J, Li Y, Wang S L, Whitney C G, Levince O S, Dowell S F, Yang Y H. Resistance, serotypes and hidden resistant clones of Streptococcus pneumoniae among children in Beijing. Chin J Pediatr. 2000;38:424–427. [Google Scholar]