Abstract
Osteonecrosis of the femoral head (ONFH) is a recalcitrant ischemic disorder, which could be classified into two major categories: traumatic and nontraumatic. Regardless of different risk factors, it has been testified that ONFH results from primitive vascular problems, leading to temporary or permanent loss of blood supply to bone tissue. Histopathological and microarchitectural alterations ensues, which is a gradual evolutionary process involving bone marrow and osteocyte necrosis, progressive destruction of subchondral bone, unsuccessful reparative process, and eventual articular collapse and degenerative arthritis. Based on the imaging features of ONFH, different classification systems have been developed to evaluate the severity and prognosis of the disease, which is pivotal for implementation of treatment strategy, especially the joint-preserving surgery. However, patients classified with the same severity stage, especially in the peri-collapse stage, sometimes responded differently after similar joint-preserving surgery. The unusual phenomenon may be attributed to the limitation of the current imaging classification systems, which might underestimate the disease severity, especially when referring to the early stages. In this review, we briefly summarize the etiology and pathogenesis of ONFH. The imaging features and staging classification systems of ONFH are also described. More importantly, we focus on histopathological and microstructural alterations of the femoral head, and provide an overview of their essential contribution to ONFH progression. Given the observation of discordance between imaging characteristics and histopathological alterations, a substantial amount of research on the relationship between imaging and histopathological features is required to further modify and revise the current wide-accepted classification systems.
Keywords: Osteonecrosis of the femoral head, Pathogenesis, Histopathology, Microarchitecture, Imaging, Staging
1. Introduction
Osteonecrosis of the femoral head (ONFH) is a recalcitrant and common disease, which is triggered by disruption of blood supply in the femoral head with various etiologies [1]. ONFH has an extremely high disability rate, affecting the quality of living of patients, which occurs frequently in young and middle-aged population [2]. ONFH brings about necrosis of the peri-articular bone and cartilage degeneration in the femoral head, ultimately leading to joint collapse [3].
Although ONFH has been attracting extensive research interest in last decades, there is no wide-accepted consensus to the exact etiology and pathogenesis [4]. Regardless of different risk factors and underlying diseases, it has been testified that ONFH results from primitive vascular problems, leading to temporary or permanent loss of blood supply to the bone tissue [5]. Histopathological and microarchitectural alterations ensues, which is a gradual process involving bone marrow and osteocyte necrosis, progressive destruction of the subchondral bone, unsuccessful reparative process around the necrotic zone, and eventual articular collapse and degenerative arthritis of the joint [6,7]. Based on the imaging features of ONFH, different classification systems have been developed to evaluate the stage of severity and prognosis of the disease, which is pivotal for implementation of the treatment strategy, especially the joint-preserving surgery [8,9]. However, patients classified with the same severity stage responded differently after similar joint-preserving surgery [10]. The unusual phenomenon may be attributed to the limitation and defect of the current imaging classification systems, and inconsistency between histopathology and radiological stage has been observed [11].
In this review, we briefly summarize the etiology and pathogenesis of ONFH. The imaging features and staging classification systems of ONFH were also described. More importantly, we focus on histopathological and microstructural alterations of the femoral head in ONFH, and provide an overview of their essential contribution to the progression of ONFH.
2. The etiology and pathogenesis of ONFH
ONFH can be divided into two major categories: traumatic and nontraumatic. The main etiological factors for traumatic ONFH include femoral head and neck fracture, acetabular fracture, hip dislocation, and severe hip sprain or contusion (no fracture, with intraarticular haematoma) [[12], [13], [14], [15], [16], [17]].
Nontraumatic ONFH is not only closely associated with glucocorticoid (GC) and alcohol use, but is also related with blood dyscrasia, metabolic and coagulation disorders [4,18]. GC administration is the most common cause of secondary osteoporosis, and the main cause of nontraumatic osteonecrosis. In patients receiving long-term therapy, GC-induced fractures were found in 30 %–50 % of these patients and osteonecrosis in 9 %–40 % [19,20]. Non-traumatic ONFH is found to be correlated with low bone mineral density, evidenced by a study showing that fractural stages of ONFH were associated with a 5-fold risk of osteoporosis [21].
There are five theories about the pathogenesis of GC-induced ONFH: 1) The lipid metabolism disorder theory; 2) The decreased osteogenesis potential of bone marrow mesenchymal stem cells theory; 3) Insufficient blood supply theory; 4) The inflammation and cell apoptosis theory; 5) The gene polymorphism and non-coding RNA theory [22]. The study have shown the relevance between ONFH and dose and treatment duration of GC, although it is debatable [23]. The comparative study of Kameda et al. presented that the progression of ONFH relied on the response to a high dose of GC therapy and a decrease in the bone mineral density value in the 1st year [24]. Saito et al. pointed out a noteworthy dose–response relationship between the development of ONFH and the entire dose of GC administered in the first 2 weeks after renal transplantation [25]. Platelet activation is involved in development of GC-induced ONFH and the effect may be secondary to endothelial cell damage induced by GC [26]. Wada et al. discovered that warfarin reduced the occurrence of ONFH in spontaneously hypertensive rats [27]. Motomura et al. demonstrated that the combined use of warfarin and probucol facilitates to prevent GC-induced ONFH in rabbits [28]. Kang et al. also noticed that combination treatment with enoxaparin and lovastatin lowered the incidence of GC-induced ONFH in the rabbit [29]. Therefore, coagulation abnormalities may play a vital role in GC-induced ONFH.
Underlying diseases have been widely considered to play as an important risk factor in the pathogenesis and progression of osteonecrosis [23]. Systemic lupus erythematosus (SLE) is reported to be positively correlated with ONFH as an independent risk factor [30]. Shigemura et al. led a prospective study and revealed a higher rate of GC-induced ONFH in the SLE group than the non-SLE group [31]. Apostolopoulos and Morand provided evidence for the destructive effects of GC in SLE and proposed therapeutic options that would downgrade reliance on GC [32]. Additional study displayed that an elevated triglyceride level was a crucial risk factor for silent ONFH in patients with SLE [33].
There has been a consensus that osteonecrosis results from primitive vascular problems, including vessel infarction, stenosing arteritis, arterio-sclerotic disease, extraosseous arterial involvement or extraosseous venous abnormality, or hypercoagulability and hypo-fibrinolysis. The inherited hypofibrinolysis has been considered to be a risk factor of idiopathic ONFH [5]. Jones et al. observed intraosseous fibrin thromboses after induction of experimental fat emboli and speculated that fat emboli could initiate intravascular coagulation [34]. Sun et al. investigated the etiology of post-severe acute respiratory syndrome (SARS) ONFH and revealed that Plasminogen activator inhibitor-1 (PAI-1) was a sensitive blood symbol for screening high-risk susceptible populations [35]. After early damage of endothelial cells activated by GCs or additional factors, a hypercoagulable state is formed. This is followed by vascular problems (thrombosis, poor blood flow, and ischemia), and this conversely brings about endothelial cell damage, which may be cyclic [26,36,37]. Endothelial cell apoptosis consequently promotes thrombus formation and osteonecrosis by two major mechanisms. First, apoptotic bodies can indirectly cause coagulopathic changes by endothelial dysfunction. Second, apoptotic endothelial cells can accelerate adhesion of platelets to endothelial cells and activate platelets, ultimately leading to thrombus formation [26]. Interruption or a reduction in blood flow along capillaries could also play an etiological part in endothelial cell apoptosis [38,39]. Diminished resistance of the affected bone is supposed to determine secondary vascular impairment at the capillary level either through compression from comparatively inelastic fat cells or through the rupture of small intra-trabecular vessels [40]. Osteonecrosis involves more frequently the convex articular surfaces, which may be owe to the smaller diameter of the terminal vessels of this region and the lack of collateral vascularization [18].
3. Histopathological and microstructural alterations of ONFH
ONFH is an evolutionary course involving marrow necrosis and osteocytic death in the femoral head [6,7], reparative process around the necrotic zone, and collapse of the articular surface and following degenerative arthritis of the hip [41]. According to the severity and stage of the disease, the site involved and the factors stimulating its pathogenesis and progression, the morbid anatomy and histopathology of osteonecrosis present a different appearance [18]. Despite different risk factors and postulated associated mechanisms, it is generally used to be thought that pathologic features of ONFH are homologous, regardless of the etiology [42,43]. The histopathologic features of necrosis and repair were reported to be similar in GC-induced and non-GC-induced ONFH, despite a more rapid evolution of pathologic alterations in the GC-induced ONFH [43].
The organizational structure of the femoral head incorporates articular cartilage, subchondral bone, and deep cancellous bone. Owing to its special bone structure characteristics, the femoral head can endure greater compressive stress without collapse within the normal physiological limit [44]. There is evidence that anterior portion of the bone that most often suffers the greater overall collapse, and regional differences exist in the quantity or quality of femoral head bone or in stress distribution in the femoral head [45]. L. Wang et al. also showed that bone resorption triggered further weakening of the subchondral bone and resulted eventually in progressive collapse of the subchondral trabeculae. Consequently, they hypothesized that osteonecrosis is chronologically correlated with these pathological changes in subchondral bone microstructure in rabbits [46].
According to the research by Catto et al. a four-stage system (Stage I, II, III and IV) has been proposed to illustrate the pathological and microarchitectural alterations in ONFH, which has been extensively recognized [47,48]. In the last 60 years, many studies have been conducted to clarify pathological changes in each stage, despite little progress in the prognosis of ONFH. The exact alterations of microarchitecture and pathology of the involved regions in the femoral head require further investigation.
3.1. Stage I
In this stage of ONFH, the femoral head typically reveals no gross or macroscopic microarchitectural manifestations and is limited to microscopic alterations [49]. The early phase of osteonecrosis is cell death, interruption of cell enzymes, and loss of cell metabolic activity [49]. However, the cells that make up bone differ in their ability to resist ischemic injury. Hematopoietic cells are most sensitive and die within 6–12 h [50]. Correspondingly, the earliest signs indicative of bone ischemia have been observed in the marrow spaces, where there is loss of nuclear staining of marrow cells [18]. Bone cells (osteoclasts, osteoblasts, and osteocytes) may survive from 12 to 48 h [50,51]. Nonetheless, chondrocytes are normally adapted to relatively low oxygen tension and do not become devitalized, with the exception of cells in the calcified cartilage, seen as absence of chondrocytes [49].
The gross and histologic changes in the cartilage without radiographic evidence of degeneration were described by several authors. In stage I, standard radiology is of limited value, and only abnormal alterations in soft tissues (such as plasmostasis and adipocyte alteration) are observable on magnetic resonance imaging (MRI) [52,53]. MRI does not always detect accurate labral injury or chondral damage, and arthroscopy remains the gold standard for detection and staging of chondral lesions [54,55]. Ruch et al. presented poor correlation between radiographic and arthroscopic assessment of femoral head cartilage in patients with early post-collapse osteonecrosis, as arthroscopic evaluation revealed osteochondral lesions that had not been noted on radiograph or MR [56,57]. Mukisi-Mukaza et al. declared that there is no correspondence between histopathology and radiological stage in patients with ONFH [11].
In the early stage of ONFH, marrow edema is detected in subchondral bone and deep cancellous bone, indicative of declined blood supply in this region [58]. Owing to obstructions of the intraosseous venous system, particularly in the transitional zone at the border of the sequester, a significant feature in the pathogenesis of ONFH is obstruction of the venous outflow [26]. Bone marrow mesenchymal stem cells (BMSCs) residing are multipotent cells that have the ability to differentiate into an extensive array of specialized cells such as bone, cartilage, adipose, and muscle [59]. The capacity for multipotent differentiation of BMSCs is dependent upon the local milieu where the cell is placed and the various cytokines and growth factors with which it comes into contact [59]. BMSCs offer a source of osteoblasts to the lesion involved, and these cells may play an important part in osteogenesis and repair the necrotic defect [[60], [61], [62]]. It has been discovered that low-dose allogeneic BMSCs could promote bone regeneration in osteonecrotic lesions of the femoral head [63]. Not only do BMSCs offer the precursor cells for repair, but they also secrete growth factors and pro-angiogenic factors such as BMP-2 and VEGF stimulating the local repair processes in ONFH [64,65].
Skeletal system homeostasis is constantly preserved through the collaborative actions of bone cells, involving bone resorption by osteoclasts and bone formation by osteoblasts, whereas osteocytes act as mechanosensors and orchestrators of bone remodeling process. This process is under the regulation of local and systemic factors [66]. Osteoclasts are multinucleated, giant cells of hematopoietic origin, which are formed by the fusion of mononuclear pre-osteoclasts derived from myeloid cells. Fusion-mediated giant cell formation is crucial for osteoclast maturation, and bone resorption is incompetent without it [67,68]. Osteoclasts have positive and negative regulatory effects on osteoblast functions [67], and the formation, differentiation, and maturation of osteoclasts are also synchronized by various solubility factors released by osteoblasts [69]. There is evidence that modifications of bone remodeling activity and weakening of bone structure with formation of microfractures are implicated in the pathogenesis and development of ONFH [18]. Wang et al. speculated that the altered osteoblast and osteoclast activity bring about a reduction in macroscopic mechanical strength [70]. Interestingly, evaluation of transiliac bone-biopsy specimens from patients with aseptic osteonecrosis and normal kidney function disclosed a marked reduction in osteoblastic appositional rate and in bone-formation rate at the cell and tissue level, signifying that non-apparent metabolic disturbances are present in patients with aseptic necrosis [40].
Non-surgical treatment, including reduced weight-bearing, pharmacotherapy and physiotherapy, could be selected for ONFH patients in Stage I. Alternatively, core decompression is currently the most common operative procedure in the early stages of ONFH, showing significantly higher success rate than that of nonsurgical management [71].
3.2. Stage II
The gross appearance of the femoral head does not change considerably in stage II (Fig. 1A) [72]. Due to the progression of reparative processes, the infarcted subarticular necrotic area appears better demarcated on section (Fig. 1), and a peripheral rim of sclerosis is visible radiologically [18].
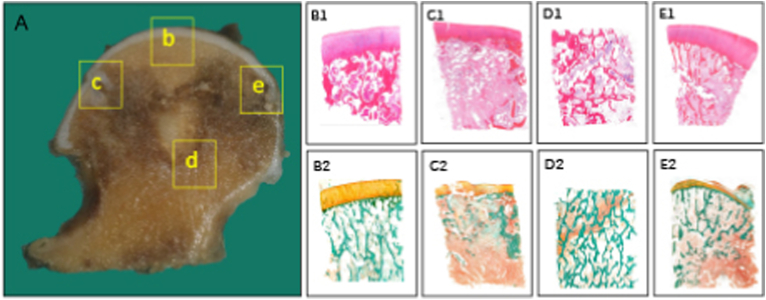
Figure 1.
Histopathological alterations of osteonecrotic femoral heads in Stage II (A) Coronally sectioned gross specimen. The articular surface is not altered considerably as in stage I (b, B1, B2) Necrotic area: There is an accumulation of bone marrow cell debris and bone trabeculae demonstrating empty lacunae, occasionally containing some pycnotic nuclei of osteocytes (c, C1, C2) Lateral transitional zone: Increased vascularity and marrow reaction with inflammatory fibrovascular infiltration is observed, leading to the eventual formation of subchondral cyst (d, D1, D2) Inferior transitional zone: The proliferating capillaries and fibroblasts extend into the necrotic area, and viable new bone tissue is regenerated by osteoblasts, resulting in the sclerotic rim (e, E1, E2) Medial transitional zone: New blood vessels propagate into the peripheral region of the necrotic lesion, and dead bone trabeculae are removed by osteoclasts. Stain: Haematoxylin and Eosin (B1-E1), Goldner's Trichrome (B2-E2), Magnification: × 10.0.
The articular surface is unaltered as in stage I. In a baboon animal model, interruption of contact between articular cartilage and vascularized subchondral bone brought about cartilage degeneration, which takes three years [73]. The interface between the subchondral bone and calcified cartilage contains numerous vascular canals [74]. Imhof et al. illustrated the dense subchondral vasculature in close proximity to the cartilage and the micro-channels that penetrate the subchondral bone and permit communication between the bone and cartilage [75]. Experiments in joints obtained immediately after death demonstrated that, the tidemark and calcified cartilage are permeable to low-molecular-weight solutes [76]. There are likely to be both vascular and other means, including via the osteocyte lacuna-canalicular network of bone, for signaling molecules to traverse between the bone and cartilage [77]. The zone of calcified cartilage forms an important interface between cartilage and bone for transmitting force, attaching cartilage to bone, and limiting diffusion from bone to the deeper layers of cartilage [78]. The stiffness and hardness of calcified cartilage was typically lower than subchondral bone for the same mineral content [79]. No collagen fibers are successive between the calcified cartilage and the cortical endplate. Consequently, the osteochondral junction is considered as an area of weakness [80]. In contrast, the tidemark which separates the calcified cartilage from the articular cartilage, is traversed by collagen fibrils, leading to a quite strong connection between these two regions. It has been hypothesized that alterations of the calcified cartilage may compromise its mechanical function as a connective and cushioning tissue, leading to degeneration of the overlying articular cartilage [74,78,79].
The tissue adjacent to the necrotic area reveals increased vascularity and marrow reaction with increased inflammatory fibrovascular infiltration (Fig. 1) [81]. The proliferating capillaries and fibroblasts extend into the necrotic area, and dead bone trabeculae are removed by osteoclasts and substituted by viable new ones, laid down by osteoblasts, resulting in the sclerotic rim [18,82]. Utsunomiya et al. speculated that the concentration of stress may increase in association with the increased osteoblastic activity at the lateral boundary of the femoral head in early-stage osteonecrosis [83]. The study by Karasuyama et al. demonstrated that both shear stress and shear strain tend to be concentrated on thickened bone trabeculae at the boundary along with the progression of sclerotic changes, whereas increased osteoclastic activity is not observed unless collapse has occurred [84]. New blood vessels frequently can only propagate into the peripheral region of the necrotic lesion, and new bone tissue emerges surrounding the necrotic region and develops a sclerotic band over time, which further inhibits blood vessels from growing into the center of the necrotic area [85]. Consequently, this area cannot be successfully repaired in most cases and eventually subchondral fracture occurs, which leads to the collapse of the femoral head in late stage. The peak stress from sclerosis rim may be a main factor inducing the formation of cystic lesion in ONFH via an osteoarthritis-like mechanism [86]. Additionally, sclerotic changes are reported to elicit subchondral fractures at the lateral boundary [83].
Combination treatment regimens are commonly employed in the treatment of ONFH patients in Stage II, including non-surgical management and joint salvaging procedures. The most commonly used procedures are core decompression and vascularized bone-grafting [87]. Nonetheless, a more comprehensive consideration of risk factors, pathologic and imaging stages, and symptoms should be conducted in the decision of therapeutic interventions for early-stage ONFH.
3.3. Stage III
There is an obvious alteration of the appearance of the femoral head, due to the subchondral fractures and subsequent collapse of the necrotic bone at this stage (Fig. 2) [18]. As ONFH develops, subchondral fractures appear, and most fractures are initially found between necrotic and sclerotic areas which are caused by abnormal pressure distribution and osteoclast absorption [83,88,89]. Motomura et al. found that the size of the necrotic lesion seemed to contribute to the distribution of subchondral fractures [90]. The most important factor in the prognosis of osteonecrosis is the estimated percentage of compromised load surface [91]. Ma J et al. hypothesized that discordance between bone structure and function of the femoral head may be involved in the progression of ONFH and more attention should be paid to the prevention and treatment of such discordance [92].
1) Subchondral Fracture
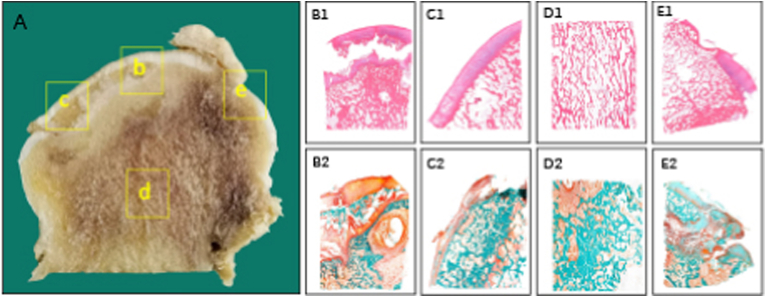
Figure 2.
Histopathological alterations of osteonecrotic femoral heads in stage III (A) Coronally sectioned gross specimen. Subchondral collapse is observed just beneath the osteochondral junction, which is verified by the histological section (b, B1, B2) Necrotic area: Subchondral trabecular fracture is located just beneath the subchondral bone plate level (c, C1, C2) Lateral transitional zone: The initial fracture of the subchondral plate, which is mostly located in the region between necrotic and sclerotic bone tissue, is considered as a starting point to further trigger the collapse of the femoral head (d, D1, D2) Inferior transitional zone: The reactive interface undergoes progressive remodeling at the junction with regenerated bone tissue observed, and vascular-rich granulation tissue is detected in the marrow space (e, E1, E2) Medial transitional zone: When the medial boundary of the necrotic lesion is located lateral to the fovea of the femoral head, revascularization and active bone remodeling occurs in the region. Stain: Haematoxylin and Eosin (B1-E1), Goldner's Trichrome (B2-E2), Magnification: × 10.0.
Structural modification of the overlying cartilage seemed morphologically sound, even if subchondral fractures had been observed in some patients [56]. Subchondral fracture consists of the fracture of the subchondral bone plate and that of the subchondral trabeculae underneath. As for the fracture in the subchondral trabeculae, it is characterized by a linear or slightly serpiginous thin trace of low signal intensity on all sequences paralleling the articular surface in MRI [93]. The nanoindentation modulus of the subchondral trabeculae is enhanced in the early stage of ischemic osteonecrosis of the immature femoral head and makes it more susceptible to microcrack formation [94]. The specific trabecular fracture is usually located just below the subchondral bone plate level, or less frequently within the deeper necrotic cancellous area or at its periphery on the frontal section [18]. The initial fracture of the subchondral plate, which is mostly located in the region between necrotic and sclerotic bone tissue, is considered as a starting point to further trigger the collapse of the femoral head [89].
A previous report showed that the depth of the low-intensity band on the T1-weighted image is a valuable implement for the differentiation of subchondral insufficiency fracture of the femoral head from ONFH in accordance with the histopathological diagnoses [95]. However, it is reported that the crescent sign is not satisfactory to distinguish subchondral insufficiency fracture from ONFH in cases with collapsed femoral heads, as alterations in bone matrix are triggered only during the regeneration period [96].
In the late stage of collapse, fractures of the necrotic bone at the sclerotic boundary were detected and a low-density tissue that appeared to be fibrous granulation was situated along the necrotic bone side of the sclerotic boundary [97]. Persistent hip pain in ONFH patients is usually considered to be due to subchondral fracture and subsequent femoral head collapse in post-collapse stage, which was not detected in the early stages of ONFH [98,99]. Meier et al. detected a strong correlation between subchondral fracture and bone marrow edema in the post-collapse stage of ONFH [100]. Bone marrow edema of the femoral head represents a secondary sign of subchondral fracture, indicating ARCO Stage III in patients with ONFH [100]. It is rational to assume that bone marrow edema in ONFH might be an inflammatory change to mechanical stress of subchondral fractures or to tissue ischemia around the necrotic area [[101], [102], [103]]. Any lesions vulnerable to collapse ought to be handled to recuperate fundamental strength of the femoral head and halt or even slow the progression of this disease [9].
2) Collapse of the femoral head and related mechanism
In the early-collapse stage of ONFH, initial fracture cracks proceeded between separated bone resorption regions at the anterosuperior portion of the femoral head [97]. Recurrent slight damage could bring about subchondral microfractures, which allows the synovial fluid from the joint cavity to infiltrate into the subchondral bone underneath, lifting the intraosseous pressure. The increased intraosseous pressure affects the local microcirculation and triggers a vicious circle which ultimately results in subchondral cyst formation and articular collapse. Cystic lesion in ONFH plays a significant part in exacerbating the development of femoral head collapse [86]. The osteonecrosis complicated by cystic lesions is more likely to cause microfracture, collapse, and crescent sign which specify structural instability of the femoral head [86]. The absence of immobilization, as well as constant weight bearing could eventually result in further subchondral collapse and cartilage damage [80].
Zhang et al. proposed a new definition of the peri-collapse stage of ONFH, which refers to a continuous period in the development of ONFH from the occurrence of subchondral fracture to early collapse (<2 mm) [9]. The peri-collapse stage is a conception that is first recommended in an attempt to assess a continuous period before and after head collapse as a whole [104]. Theruvath et al. described 14 patients with subchondral fracture, and 12 patients experienced collapse with a mean follow-up of 2.6 years [101]. The peri-collapse stage with distinct clinical and imaging characteristics provides a last good opportunity for the application of joint-preserving techniques [105]. It is necessary to separate the peri-collapse stage as an independent state in evaluating the natural progression of ONFH and selecting an appropriate treatment algorithm.
The collapse of femoral head strongly influences the staging and prognosis of ONFH [106]. In regard to its pathogenesis, Bullough and DiCarlo proposed the following three potential causes: 1) the cumulative effect of microfractures induced by fatigue within the necrotic zone; 2) weakness of the trabeculae in the reparative front as a result of osteoclastic activity; 3) focal concentration of stress at the junction between the thickened sclerotic trabeculae of the reparative zone and the necrotic trabeculae [90].
Bergmann et al. reported that the greatest force is applied to the anterior-superior aspect of the femoral head [107]. It was reported that the anterior hemisphere might be subject to less total load but experiences higher peak load, as it continually osculates between being covered and uncovered by the acetabulum [[108], [109], [110]]. Christian J et al. suggested these osculation-induced peak loads account for the lower bone quality in the anterior hemisphere and the increased susceptibility for it to collapse in ONFH [45]. As such, reinforcement of the femoral head in ONFH should focus on the anterior hemisphere.
Utsunomiya et al. showed that when it was at the early-stage of ONFH, both equivalent stress and shear stress were concentrated at the lateral boundary of the necrotic lesion, where sclerotic changes were observed [83]. As echoing the mechanical findings in the early-stage, collapse has been found to consistently involve subchondral fractures at the lateral boundary in the late-stage of ONFH [56,111]. Radiography may also demonstrate early indications of articular collapse in ONFH, particularly involving the anterolateral and anterior femoral head, in which both frontal and frog-leg lateral projections should be obtained [81]. However, Hengsberge et al. found that the micromechanical properties of bone tissue do not directly affect the overall macro-mechanical properties of bone tissue [112]. Therefore, the collapse of the ONFH is unlikely to be caused by reduced micromechanical properties of bone trabeculae in the weight-bearing region, which could explain that the micromechanical properties of bone trabeculae in collapsed femoral heads with ONFH did not differ significantly between the necrotic zone and the healthy zone in the study by Wang et al. [70]. GC-induced ONFH was characterized by multiple ‘osteolytic bone destruction’, while the alcohol-induced ONFH was manifested by some kinds of ‘coagulative destruction’, exhibiting different features of osteonecrosis [113]. Interestingly, Brown et al. suggested that the degree of structural degradation of the cancellous bone within the main infarct body might be more important than by the degree of structural degradation within the subchondral plate in the onset of collapse [114]. They postulated the trabeculae subjacent to the subchondral plate in the weightbearing region initiates the collapse as a result of its poor quality.
Despite the relative normal structural appearance of the femoral head in the early stage, osteoblast and osteoclast activities had already changed in different regions of the femoral head with osteonecrosis. Specifically, osteoclast activity increased in subchondral bone and the necrotic region while osteoblast activity increased in the sclerotic region [72]. Some authors have conducted experiments using resected femoral heads with late-stage ONFH to investigate the mechanism of collapse. It has been speculated that the altered osteoblast and osteoclast activity leads to a reduction in macroscopic mechanical strength and eventual collapse of the femoral head [70]. Accordingly, it has been proposed that ONFH could be intervened during the early stage, in which osteoblast activity could be promoted and osteoclast activity inhibited as early as possible to prevent collapse of the affected femoral head.
3) Reparative procedure and pathological alterations in and about the reactive interface
The reactive interface undergoes progressive remodeling at the junction with the necrotic area [49]. Enchondral ossification is the physiological way of bone formation when fracture ends are well vascularized [96], which could also be observed in the reactive interface. Phemister et al. coined the term “creeping substitution” to indicate the slow replacement of aseptic dead bone [115]. The procedure of osteoblast accumulation of appositional bone, often upon dead trabeculae, and osteoclastic resorption of devitalized bone, occurs in and about the reactive interface [49]. In terms of the bone remodeling related to the necrotic zone, Fan et al. successfully established an emu collapse model of ONFH, in which the extensive trabecular structure was rebuilt and the original trabeculae was absorbed [116].
Alterations in the remodeling of bone tissue may contribute to the progression of osteonecrosis in several ways, for instance by inducing a healing defect of microfractures and thus facilitating subchondral fractures ultimately leading to femoral head collapse [40]. Subchondral fractures frequently start at the bone resorption area around the reparative interface zone in histological sections of collapsed femoral heads [117]. Focal necrosis of the repair tissue around the area of fracture-collapse may ensue and it should not be interpreted as recurrent osteonecrosis, since it is likely not correlated to a recurrent ischemia but rather to the fracture itself [118]. Histologically, the reparative interface zone is found to be an admixture of fragmented bony trabeculae and cartilage with reparative tissues, including reactive woven bone, metaplastic cartilage, vascularized fibrous and granulation tissue, which is the appearance of unstable fracture elsewhere in the skeleton [18]. Despite the reparative process and reactive woven trabeculae in the area, the subchondral fracture failed to heal. Interference with revascularization occurs repeatedly in the repair process of the necrotic femoral head [119].
Joint-preserving procedures including vascularized bone-grafting and osteotomy, are highly recommended for ONFH patients in the early phase of Stage III, which is usually referred as peri-collapse stage. As for patients in the late phase of Stage III with more severely collapsed femoral head, joint-preserving procedures are still worthy of trying in youngsters, while joint arthroplasty is indicated for older people (>55 years old) [87].
3.4. Stage IV
Due to the progressive detachment of osteochondral fragments from the infracted area, the shape of the femoral head is severely deformed at this stage (Fig. 3) [18]. The degenerated cartilage on the femoral head side caused by depression results in cartilage damage on the acetabular side, and ONFH progresses to secondary osteoarthritis (OA) [120]. Osteochondral debris could be detected in the capsular and synovial tissues [18]. When osteoarthritic changes are fully developed, it may not be possible to recognize the initial cause as that of subarticular avascular necrosis [18]. Chondrocytes close to the necrotic lesion preserve the integrity of their morphology, but when it progresses towards the superficial layer of cartilage, chondrocytes vary in the shape and structure. Cellularity of bone tissue diminishes as the disease is in a more advanced stage [56].
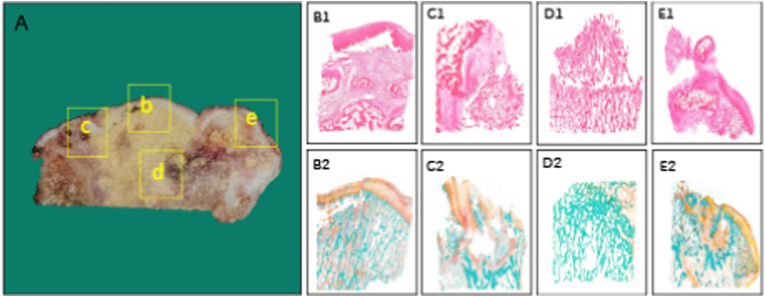
Figure 3.
Histopathological alterations of osteonecrotic femoral heads in stage IV (A) Coronally sectioned gross specimen. The shape of the femoral head is severely deformed at this stage (b, B1, B2) Necrotic area: Chondrocytes close to the necrotic lesion preserve the morphology integrity, but when it progresses towards the superficial layer of cartilage, chondrocytes vary in the shape and structure. Weight-bearing zone of the femoral head is replaced by fibrous tissue, secondary collapse ensues (c, C1, C2) Lateral transitional zone: The osteochondral structure is obviously damaged and shows a chaotic, irregular morphology (d, D1, D2) Inferior transitional zone: Histomorphological characteristics of this region in stage IV is similar to that in stage III (e, E1, E2) Medial transitional zone: Despite the reparative process and reactive woven trabeculae in the area, substitution of necrotic bone with viable new bone tissue fail, exhibiting apparently disarranged structure. Stain: Haematoxylin and Eosin (B1-E1), Goldner's Trichrome (B2-E2), Magnification: × 10.0.
In the late phase of ONFH, arterial endothelial hyperplasia occurs, the arterial diameter decreases, and the loss of arterial structure is further aggravated, leading to complete arterial occlusion, which indicates OA of the hip joint [121]. This ischemic state is manifested by pathologic and histological changes similar to those that occur in the mid phase of blood supply changes. New vascular penetration occupies the subchondral bone and collapse ensues when weight-bearing zone of the femoral head is replaced by fibrous tissue, and flatness of the head can be easily understood as a secondary episode to collapse [98]. Serious hip function loss or pain often occurs in this stage, and joint replacement should be selected [122].
4. Imaging features of osteonecrosis
4.1. Radiograph
Imaging evaluation of ONFH should begin with radiography (Figure 4, Figure 5, Figure 6, Figure 7), as it is the least expensive and most widely available method of radiologic assessment [81]. Radiography is insensitive for early changes of ONFH, and a normal image obtained by X-ray does not necessarily translate in the absence of the disease [123]. Between the debut of the injury and the first suggestive images of ONHF on radiography, a period of up to five years can pass [123]. However, the imaging features are often characteristic and may obviate the need for additional radiologic evaluation [81]. Radiography manifestations are typically osteosclerosis, cystic change, and a “crescent sign” in earlier stages. Early changes of articular collapse typically occur at the junction of the serpentine sclerotic rim and the articular surface, where stress is maximally exerted [81]. After collapse, there is a loss of sphericity of the femoral head and degenerative arthritis in the late stages. X-ray images obtained using plane radiography can be differentiated from those seen in other disorders: transitory osteoporosis, osteomyelitis, fractures, epiphyseal dysplasia, osteosarcoma, various cancers, bone metastases [123].
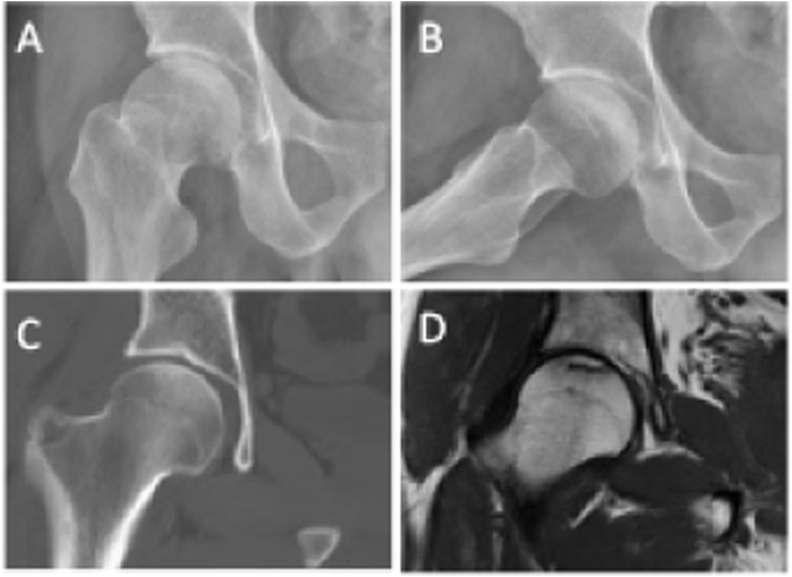
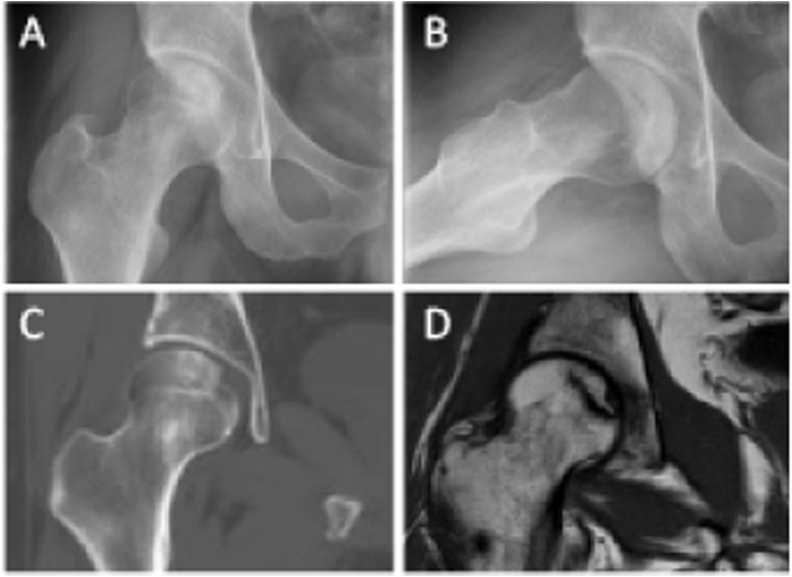
Figure 4.
ON (ARCO Stage I) of the right femoral head in a 38-year-old male patient (A) Frontal radiograph (B) Frog-leg lateral radiograph (C) Coronal CT image (D) Coronal T1-weighted MR image.
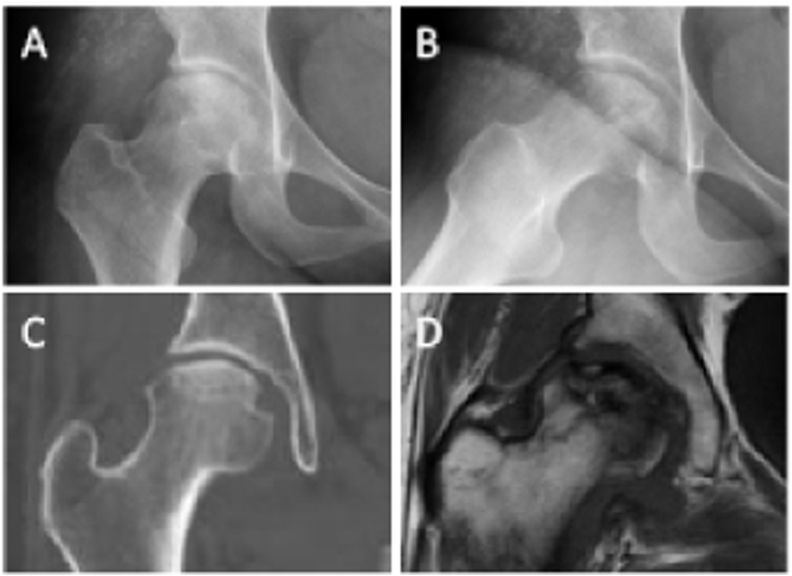
Figure 5.
ON (ARCO Stage II) of the right femoral head in a 30-year-old male patient (A) Frontal radiograph (B) Frog-leg lateral radiograph (C) Coronal CT image (D) Coronal T1-weighted MR image.
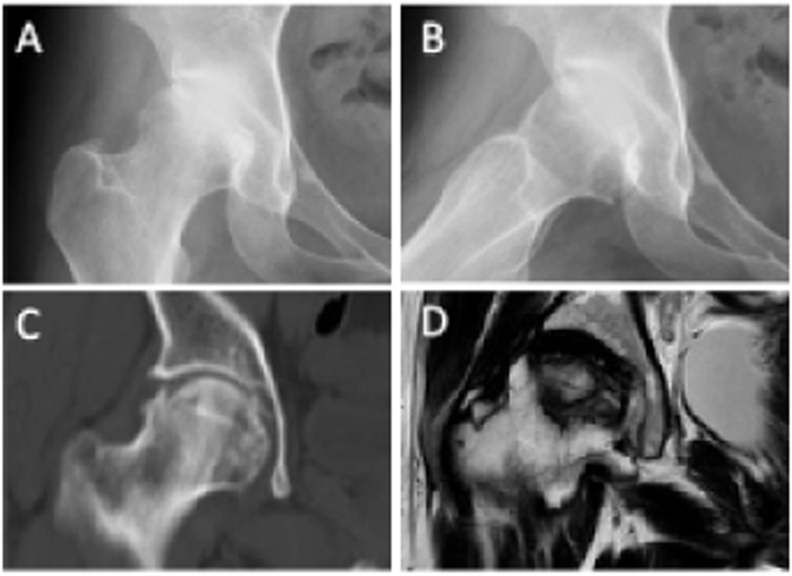
Figure 6.
ON (ARCO Stage III) of the right femoral head in a 24-year-old female patient (A) Frontal radiograph (B) Frog-leg lateral radiograph (C) Coronal CT image (D) Coronal T1-weighted MR image.
Figure 7.
ON (ARCO Stage IV) of the right femoral head in a 30-year-old male patient (A) Frontal radiograph (B) Frog-leg lateral radiograph (C) Coronal CT image (D) Coronal T1-weighted MR image.
4.2. Radionuclide imaging
The relative insensitivity of radiography for early changes of ONFH has led to use of additional imaging modalities including nuclear medicine [81]. The addition of single-photon-emission computed tomography (SPECT) may improve the accuracy of radionuclide imaging for diagnosis of ONFH and increase the sensitivity [[124], [125], [126]]. SPECT/CT with 99mTc-methylene diphosphonate showed that osteoblastic activity gradually increased around the necrotic lesion in early-stage ONFH [127]. Bone scintigraphy with 99mTc-methylene diphosphonate, shows high sensitivity for early detection since the radionuclide activity reflects osteoblastic activity and blood flow which are absent in ONFH [128,129]. For symptomatic disease, the method is able to provide positive findings in 2–3 days after the onset of symptoms (“cold within hot”) and later “hot lesion” reflecting revascularization [130]. However, for the asymptomatic ONFH, there are not many available data to suggest the diagnostic usefulness of the method [130]. In a study by Ryu and colleagues, SPECT was determined to be more sensitive than MRI (100 % vs 66 %) in detecting early osteonecrosis following renal transplantation [125]. Positron emission tomography may detect signs of ONFH earlier than MRI and SPECT, and can predict progression of ONFH [131]. In addition, nuclear medicine imaging is also applied in post-operative period to detect graft viability or infective complications [132].
4.3. CT
CT is a very useful assessment tool in later stages of ONFH, for determining the extent of the lesions such as sclerosis and other events occurring in the state of repair [123]. CT provides a detailed analysis of the morphological aspects (Figure 4, Figure 5, Figure 6, Figure 7) [123]. CT usually reveals zones of osteosclerosis surrounding the necrotic bone and repaired bone or shows subchondral bone fracture [[133], [134], [135]]. CT assessment of early ONFH may reveal alteration of the normal “asterisk” that is formed by condensation of the compressive and tensile trabeculae [81]. However, CT is not advocated for early detection of ONFH and is less sensitive than scintigraphy or MRI [136]. Later stages of ONFH are well depicted with CT, and similar to radiography, show a serpentine or undulating sclerotic margin [81]. CT provides more information for identifying possible subchondral fractures than does MRI and serves as the most sensitive tool for grading the peri-collapse lesion stage [105]. CT is superior to MRI in identifying subchondral fracture and can help diagnose and predict the prognosis of ONFH [137]. CT is not a routinely performed technique, but is useful to rule out the presence of a subchondral fracture when MRI is doubtful or contraindicated [132]. Lesions which are relevant to indication for hip arthroplasty, tend to be underestimated on conventional MRI compared to high-resolution quantitative computed tomography (HR-QCT), suggesting the importance of CT imaging in ONFH [138]. Nonetheless, multidetector CT, with high resolution, has not been extensively studied in evaluation of ONFH [81]. Multidetector CT is useful for detecting articular collapse location and extent in epiphyseal osteonecrosis and was superior to both radiography and MRI in several studies [139,140]. This information is particularly important in surgical planning for rotational arthroplasty [141].
4.4. MRI
MRI, a non-invasive imaging technique, is generally regarded as the most sensitive and specific image modality for identification of ONFH [130,[142], [143], [144], [145], [146], [147]]. MR could detect the repair process before conventional radiographs and thus would be useful for evaluating the necrotic area and detecting these rare cases of extended osteonecrosis (Figure 4, Figure 5, Figure 6, Figure 7) [148,149]. Monitoring of high-risk patients with periodic hip MR would help diagnose necrosis in its early stage. It can detect early abnormal lesions in asymptomatic patients, long before they can be viewed on plain radiography, thus facilitating the initiation of treatment, providing a better response to initial therapy and a favorable prognosis [123]. MRI findings of osteonecrosis have been reported to be present as early as 1 week subsequent to inducted vascular injury in an animal study by Brody et al. [150]. There are only rare reports of osteonecrosis with a normal MRI examination [151]. A previous case report indicated that osteonecrosis could possibly occur within 3 weeks after initiation of high-dose corticosteroid therapy [152]. A long-term prospective study showed that serial MRI evaluation might be useful in following up the progression of small asymptomatic lesions of ONFH in patients with SARS [153]. Hiroyuki Hatanaka et al. demonstrated that symptomatic pre-collapse ONFH diagnosed based on plain radiographic findings could be distinguished from asymptomatic pre-collapse ONFH by the presence of bone marrow edema on MRI and thus bone marrow edema may be a sign of occult fracture [154].
MRI also helps quantify the area and extent of ONFH in different planes and could also be used to guide treatments as a validated technique in following up patients. The most common MRI pattern seen in osteonecrosis is an area of yellow marrow surrounded by a low-signal-intensity rim with all pulse sequences [81]. The double-line sign may also partially result from chemical-shift misregistration artifact [146,155]. Two MRI features that increase the risk of development of ONFH are a thick epiphyseal scar and early conversion to yellow marrow [156]. The MRI images correlate closely with the histological changes occurring in the affected hip, and they could describe in details the size of the lesions, enabling accurate staging of the disease, which is used to decide a later therapeutic algorithm [140,157,158]. Mitchell and colleagues described that the MR signal intensity in the area of osteonecrosis may also show intrinsic characteristics other than adipose tissue in a minority of patients, which includes hemorrhage (high signal intensity on T1-and T2-weighted images), cystic areas (low signal intensity on T1-weighted images and high signal intensity on T2-weighted images), and fibrous tissue (low signal intensity with all pulse sequences) [128]. But Murphey et al. suggested that this variability is much more common in epiphyseal areas of osteonecrosis and does not have prognostic significance as originally suggested. But they suggested that this more nonspecific MRI pattern is unusual and is often associated in patients with diffuse marrow disease or red marrow reconversion (sickle cell anemia, Gaucher disease, and chronic renal failure patients treated with erythropoietin) [81]. Additional variations in the MRI appearance of osteonecrosis are also described, including nonspecific diffuse marrow signal abnormality (decreased signal intensity on T1-weighted images and variable signal intensity on T2weighted images) [146,159,160].
The use of contrast-enhanced MRI in adult osteonecrosis is typically not necessary for diagnosis or assessment in the vast majority of cases [81]. However, in animal studies, Nadal and colleagues showed that dynamic contrast enhanced MRI was most sensitive to detect osteonecrosis in early surgically-induced femoral head disease [161]. The typical appearance of osteonecrosis on post-contrast MRI is lack of enhancement of the devitalized tissue [81]. There is often a peripheral rim of enhancement corresponding to the zone of creeping substitution granulation tissue [81]. Dynamic contrast-enhanced studies reveal increased peak enhancement and delayed time to peak enhancement [162,163]. More variable patterns of enhancement, perhaps corresponding to mixtures of ischemia and fibrosis, have been reported by Li and Hauzeur [164,165]. There are results which indicate that contrast-enhanced MRI may be useful for noninvasive evaluation of femoral head perfusion after fracture of the femoral neck [166]. Dynamic MRI is a reliable tool to evaluate vascularity of femoral heads and reduces the uncertainty of outcome of treatment of intracapsular femoral neck fracture (ICFNF), which can be a useful tool to formulate a treatment algorithm in management of ICFNF [47,167]. Foci of osteonecrosis as a result of femoral neck fractures are common and have been reported in up to 75 % of cases at histologic evaluation [47]. MRI with a diffusion sequence, T2 mapping, and apparent diffusion coefficient (ADC) mapping has also been advocated more recently to evaluate epiphyseal osteonecrosis, although these techniques remain investigational [[168], [169], [170]].
5. Clinical staging systems of ONFH
Patients with suspected ONFH would have one or more of the following criteria: (1) throbbing, deep groin pain, and one or more associated risk factors; and (2) a previous ONFH in another joint [171]. ONFH classification systems are excellent tools based on imaging data that are widely used to stratify the severity and prognosis, and guide the treatment strategy [8]. There are various staging systems which have been developed for assessment of adult ONFH. These include the Marcus, Ficat and Arlet, Steinberg, Association Research Circulation Osseous (ARCO) classifications, and the China–Japan Friendship Hospital (CJFH) classification system.
In 1973, Marcus et al. classified ONFH into six stages according to the radiographic findings of the hip [172]. Its main feature is simple, but due to the limitations of imaging techniques at that time, the areas and ranges of necrosis could not be accurately evaluated and staged. Therefore, this classification is no longer used. In 1977, Ficat et al. developed a classification system for ONFH, in which four stages were classified according to clinical symptoms, radiograph, MRI, and isotope bone scan [172,173]. Although this classification system is more detailed and accurate than the Marcus classification system, the areas and ranges of necrosis have not been included either, which are quite important for the selection of assessment of disease progression and treatment chosen [173,174]. In 1995, Steinberg proposed a new classification system [175]. This system is the first to incorporate the size of lesion measurements as part of a complete system [176]. This classification system provides a more detailed staging method, which is much more helpful for the selection of clinical treatment and improvement of the prognosis. However, the assessment methods of this classification system are too tedious, and there is no reproducibility for the determination of stage III, and the distinction between stage V and stage VI is also obscure. The classification system of Association Research Circulation Osseous (ARCO) is more detailed than the Steinberg classification system and takes into account the necrotic areas and extent [172]. The ARCO system originates from the Steinberg classification, and several amendments have been made over the years [87]. This system does not provide a method to evaluate either pre-radiographic lesions or lesions in which the joint line and the acetabulum are involved. The location of osteonecrotic lesion is detected and the relative information is added to each stage in ARCO system as a supplement, but its specific value is uncertain [177,178]. The ARCO classification system is more favorable to the guide of clinical treatment and the assessment of the progress and prognosis of ONFH [179]. However, its disadvantage is that the evaluation criteria of this classification are too detailed and complex to be widely used in clinical practice. The Japanese Orthopaedic Association (JOA) system originated from the Ficat and Arlet system with the location and size of the lesion added to its classification. However, this system only evaluates Ficat Stages II and III and not Stages I and IV [180,181].
In 2013, Professor Li Zirong proposed a new classification system based on the three-pillar structure of the femoral head—China-Japan Friendship Hospital (CJFH) classification, also known as Li's classification [182]. According to the midcoronal section on MRI (stage I and II) and CT (stage II and III), the coronal section of the femoral head was divided into three pillars by two parallel lines to femoral neck axis: lateral (30 %), central (40 %), and medial (30 %) pillars. The pathogenesis of ONFH was identified with its location on these pillars. Preservation of the lateral pillar is the keystone for forestalling the collapse of the femoral head [183].
Our team analyzed the characteristics of the Ficat classification system and proposed improvements based on the pathological and micro-imaging findings. Subchondral bone is composed of the dome-like subchondral plate and subchondral cancellous bone, which is closely related to the occurrence and development of many joint diseases, such as femoral head necrosis and osteoarthritis [184]. Subchondral fracture impairs the immobilization function of subchondral bone to articular cartilage and initiates head collapse [185,186]. Focal resorption of the broken subchondral plate and secondary compaction of necrotic cancellous bone result in the formation of a cavum below the subchondral plate, which appears as a crescent sign or radiolucent line on X-ray radiographs and joint effusion and closely correlates with the occurrence of bone marrow edema and hip pain [159,187,188].
Currently, for the treatment of ONFH, there is a lack of consensus regarding diagnostic methods, evaluation systems, and indications of various treatment options [8]. Therefore, we should incorporate all these together to find a preferable treatment option for ONFH.
6. Conclusion
Regardless of etiologies, ONFH results from primitive vascular problems, including obstruction of intraosseous microcirculation, extraosseous arterial involvement or extraosseous venous abnormality. In the aspect of histopathology and micromorphology, ONFH is an evolutionary course involving subchondral bone infarction, progressive destruction of the osteochondral complex, reparative process around the necrotic region, and collapse of the femoral head and following degenerative arthritis. Accordingly, a four-stage system has been proposed to demonstrate the pathological and microarchitectural alterations in ONFH, and every specific stage exhibits distinguished characteristics, despite poor correlation with the existing relative radiological stage.
Many classification systems have been developed to assess the severity of the disease, which is based on the imaging features. These ONFH classification systems have been widely applied in clinic, despite constant suspicion about their efficacy in the guide of joint-preserving strategy. The considerable failure rate brings about the suspicion about the validity and reliability of the imaging-oriented grading systems, which might underestimate the severity of the early stages of ONFH. A substantial amount of research on the relationship between imaging and histopathological features is required to further modify and revise the current wide-accepted classification systems.
Authors’ contributions
All authors listed have read and approved all versions of the manuscript YWC and GYL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy. Critical revision of the article for important intellectual content: YWC, YM, KXL, FX, GYL, CQZ. All authors approved the final version to be submitted for publication.
Declaration of competing interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (grant no. 81702181) and the Projects of International Coopera-tion and Exchanges of National Natural Science Foundation of China of China (grant no. 81820108020).
Contributor Information
Yiwei Chen, Email: chenyiwei@sjtu.edu.cn.
Yu Miao, Email: miaoyuno1@163.com.
Kexin Liu, Email: 2890299383@qq.com.
Feng Xue, Email: drxfeng@163.com.
Bin Zhu, Email: zhubin@njmu.edu.cn.
Changqing Zhang, Email: zhangcq@sjtu.edu.cn.
Guangyi Li, Email: guangyi.li@shsmu.edu.cn.
References
- 1.Mont M.A., Salem H.S., Piuzzi N.S., Goodman S.B., Jones L.C. Nontraumatic osteonecrosis of the femoral head: where do we stand today?: a 5-year update. J Bone Joint Surg Am. 2020;102(12):1084–1099. doi: 10.2106/JBJS.19.01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieberman J.R., Engstrom S.M., Meneghini R.M., SooHoo N.F. Which factors influence preservation of the osteonecrotic femoral head? Clin Orthop Relat Res. 2012;470(2):525–534. doi: 10.1007/s11999-011-2050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardozo J.B., Andrade D.M., Santiago M.B. The use of bisphosphonate in the treatment of avascular necrosis: a systematic review. Clin Rheumatol. 2008;27(6):685–688. doi: 10.1007/s10067-008-0861-9. [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Rosenblum A., Cui Q. Osteonecrosis of the femoral head. Orthop Clin N Am. 2019;50(2):139–149. doi: 10.1016/j.ocl.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Gagala J., Buraczynska M., Mazurkiewicz T., Ksiazek A. Prevalence of genetic risk factors related with thrombophilia and hypofibrinolysis in patients with osteonecrosis of the femoral head in Poland. BMC Muscoskel Disord. 2013;14 doi: 10.1186/1471-2474-14-264. 264–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arlet J. Nontraumatic avascular necrosis of the femoral head. Past, present, and future. Clin Orthop Relat Res. 1992;277:12–21. [PubMed] [Google Scholar]
- 7.Mont M.A., Hungerford D.S. Non-traumatic avascular necrosis of the femoral head. J Bone Jt Surg Am Vol. 1995;77(3):459–474. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Mont M.A., Marulanda G.A., Jones L.C., Saleh K.J., Gordon N., Hungerford D.S., et al. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Jt Surg Am Vol. 2006;88(Suppl 3):16–26. doi: 10.2106/JBJS.F.00457. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q.Y., Li Z.R., Gao F.Q., Sun W. Pericollapse stage of osteonecrosis of the femoral head: a last chance for joint preservation. Chin Med J. 2018;131(21):2589–2598. doi: 10.4103/0366-6999.244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoji T., Yamasaki T., Ota Y., Saka H., Yasunaga Y., Adachi N. Intra-articular pathology affects outcomes after joint preserving surgery for osteonecrosis of the femoral head. Int Orthop. 2020;44(7):1295–1303. doi: 10.1007/s00264-020-04550-9. [DOI] [PubMed] [Google Scholar]
- 11.Mukisi-Mukaza M., Gomez-Brouchet A., Donkerwolcke M., Hinsenkamp M., Burny F. Histopathology of aseptic necrosis of the femoral head in sickle cell disease. Int Orthop. 2011;35(8):1145–1150. doi: 10.1007/s00264-010-1121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panteli M., Rodham P., Giannoudis P.V. Biomechanical rationale for implant choices in femoral neck fracture fixation in the non-elderly. Injury. 2015;46(3):445–452. doi: 10.1016/j.injury.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Thompson G.H., Lea E.S., Chin K., Liu R.W., Son-Hing J.P., Gilmore A. Closed bone graft epiphysiodesis for avascular necrosis of the capital femoral epiphysis. Clin Orthop Relat Res. 2013;471(7):2199–2205. doi: 10.1007/s11999-013-2819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehlinger M., Moser T., Adam P., Bierry G., Gangi A., de Mathelin M., et al. Early prediction of femoral head avascular necrosis following neck fracture. Orthop Traumatol Surg Res. 2011;97(1):79–88. doi: 10.1016/j.otsr.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 15.de Palma L., Santucci A., Verdenelli A., Bugatti M.G., Meco L., Marinelli M. Outcome of unstable isolated fractures of the posterior acetabular wall associated with hip dislocation. Eur J Orthop Surg Traumatol. 2014;24(3):341–346. doi: 10.1007/s00590-013-1200-7. [DOI] [PubMed] [Google Scholar]
- 16.Tannast M., Pleus F., Bonel H., Galloway H., Siebenrock K.A., Anderson S.E. Magnetic resonance imaging in traumatic posterior hip dislocation. J Orthop Trauma. 2010;24(12):723–731. doi: 10.1097/BOT.0b013e3181d76918. [DOI] [PubMed] [Google Scholar]
- 17.Bartoníček J., Vávra J., Bartoška R., Havránek P. Operative treatment of avascular necrosis of the femoral head after proximal femur fractures in adolescents. Int Orthop. 2012;36(1):149–157. doi: 10.1007/s00264-011-1272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fondi Cristina. Franchi. A. Definition of bone necrosis by the pathologist. Clinical Cases in Mineral and Bone Metabolism. 2007;4(1):21–26. [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein R.S. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365(1):62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein R.S. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41(2):183–190. doi: 10.1007/s12020-011-9580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gangji V., Soyfoo M.S., Heuschling A., Afzali V., Moreno-Reyes R., Rasschaert J., et al. Non traumatic osteonecrosis of the femoral head is associated with low bone mass. Bone. 2018;107:88–92. doi: 10.1016/j.bone.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Wang A., Ren M., Wang J. The pathogenesis of steroid-induced osteonecrosis of the femoral head: a systematic review of the literature. Gene. 2018;671:103–109. doi: 10.1016/j.gene.2018.05.091. [DOI] [PubMed] [Google Scholar]
- 23.Liu L.-H., Zhang Q.-Y., Sun W., Li Z.-R., Gao F.-Q. Corticosteroid-induced osteonecrosis of the femoral head: detection, diagnosis, and treatment in earlier stages. Chin Med J. 2017;130(21):2601–2607. doi: 10.4103/0366-6999.217094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kameda H., Amano K., Nagasawa H., Ogawa H., Sekiguchi N., Takei H., et al. Notable difference between the development of vertebral fracture and osteonecrosis of the femoral head in patients treated with high-dose glucocorticoids for systemic rheumatic diseases. Intern Med. 2009;48(22):1931–1938. doi: 10.2169/internalmedicine.48.2414. [DOI] [PubMed] [Google Scholar]
- 25.Saito M., Ueshima K., Fujioka M., Ishida M., Goto T., Arai Y., et al. Corticosteroid administration within 2 weeks after renal transplantation affects the incidence of femoral head osteonecrosis. Acta Orthop. 2014;85(3):266–270. doi: 10.3109/17453674.2014.916490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boss J.H., Misselevich I. Osteonecrosis of the femoral head of laboratory animals: the lessons learned from a comparative study of osteonecrosis in man and experimental animals. Vet Pathol. 2003;40(4):345–354. doi: 10.1354/vp.40-4-345. [DOI] [PubMed] [Google Scholar]
- 27.Wada M., Kumagai K., Murata M., S-Yamashita Y., Shindo H. Warfarin reduces the incidence of osteonecrosis of the femoral head in spontaneously hypertensive rats. J Orthop Sci. 2004;9(6):585–590. doi: 10.1007/s00776-004-0829-9. [DOI] [PubMed] [Google Scholar]
- 28.Motomura G., Yamamoto T., Miyanishi K., Jingushi S., Iwamoto Y. Combined effects of an anticoagulant and a lipid-lowering agent on the prevention of steroid-induced osteonecrosis in rabbits. Arthritis Rheum. 2004;50(10):3387–3391. doi: 10.1002/art.20517. [DOI] [PubMed] [Google Scholar]
- 29.Kang P., Gao H., Pei F., Shen B., Yang J., Zhou Z. Effects of an anticoagulant and a lipid-lowering agent on the prevention of steroid-induced osteonecrosis in rabbits. Int J Exp Pathol. 2010;91(3):235–243. doi: 10.1111/j.1365-2613.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adikari M., Gunawardane A., Illangantilaka S., Atukorale H., Rubasinghe J. A case of systemic lupus erythematosus presenting as bilateral avascular necrosis of femur. BMC Res Notes. 2016;9 doi: 10.1186/s13104-016-2198-9. 392–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shigemura T., Nakamura J., Kishida S., Harada Y., Ohtori S., Kamikawa K., et al. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study. Rheumatology. 2011;50(11):2023–2028. doi: 10.1093/rheumatology/ker277. [DOI] [PubMed] [Google Scholar]
- 32.Apostolopoulos D., Morand E.F. It hasn't gone away: the problem of glucocorticoid use in lupus remains. Rheumatology. 2017;56(suppl_1):i114–i122. doi: 10.1093/rheumatology/kew406. [DOI] [PubMed] [Google Scholar]
- 33.Kuroda T., Tanabe N., Wakamatsu A., Takai C., Sato H., Nakatsue T., et al. High triglyceride is a risk factor for silent osteonecrosis of the femoral head in systemic lupus erythematosus. Clin Rheumatol. 2015;34(12):2071–2077. doi: 10.1007/s10067-015-3075-y. [DOI] [PubMed] [Google Scholar]
- 34.Jones J.P., Jr. Fat embolism, intravascular coagulation, and osteonecrosis. Clin Orthop Relat Res. 1993;292:294–308. [PubMed] [Google Scholar]
- 35.Sun W., Li Z., Shi Z., Wang B., Gao F., Yang Y., et al. Relationship between post-SARS osteonecrosis and PAI-1 4G/5G gene polymorphisms. Eur J Orthop Surg Traumatol. 2014;24(4):525–529. doi: 10.1007/s00590-013-1223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redlich M., Maly A., Aframian D., Shabat S., Ezov N., Levin-Harrus T., et al. Histopathologic changes in dental and oral soft tissues in 2-butoxyethanol-induced hemolysis and thrombosis in rats∗. J Oral Pathol Med. 2004;33(7):424–429. doi: 10.1111/j.1600-0714.2004.00224.x. [DOI] [PubMed] [Google Scholar]
- 37.Jones J.P., Jr., Ramirez S., Doty S.B. The pathophysiologic role of fat in dysbaric osteonecrosis. Clin Orthop Relat Res. 1993;296:256–264. [PubMed] [Google Scholar]
- 38.Shabat S., Nyska A., Long P.H., Goelman G., Abramovitch R., Ezov N., et al. Osteonecrosis in a chemically induced rat model of human hemolytic disorders associated with thrombosis–a new model for avascular necrosis of bone. Calcif Tissue Int. 2004;74(3):220–228. doi: 10.1007/s00223-003-0068-7. [DOI] [PubMed] [Google Scholar]
- 39.Langille B.L., Bendeck M.P., Keeley F.W. Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol. 1989;256(4 Pt 2):H931–H939. doi: 10.1152/ajpheart.1989.256.4.H931. [DOI] [PubMed] [Google Scholar]
- 40.Arlot M.E., Bonjean M., Chavassieux P.M., Meunier P.J. Bone histology in adults with aseptic necrosis. Histomorphometric evaluation of iliac biopsies in seventy-seven patients. J Bone Jt Surg Am Vol. 1983;65(9):1319–1327. [PubMed] [Google Scholar]
- 41.Jones L.C., Hungerford D.S. Osteonecrosis: etiology, diagnosis, and treatment. Curr Opin Rheumatol. 2004;16(4):443–449. doi: 10.1097/01.moo.0000127829.34643.fd. [DOI] [PubMed] [Google Scholar]
- 42.Kenzora J.E., Glimcher M.J. Pathogenesis of idiopathic osteonecrosis: the ubiquitous crescent sign. Orthop Clin N Am. 1985;16(4):681–696. [PubMed] [Google Scholar]
- 43.Chernetsky S.G., Mont M.A., LaPorte D.M., Jones L.C., Hungerford D.S., McCarthy E.F. Pathologic features in steroid and nonsteroid associated osteonecrosis. Clin Orthop Relat Res. 1999;368:149–161. [PubMed] [Google Scholar]
- 44.Qiu S.J., Dai K.R. The arch structure of trabeculae in normal femoral head and its biomechanical significance. Chin Med J. 1992;105(3):237–240. [PubMed] [Google Scholar]
- 45.Zaino C.J., Leali A., Fetto J.F. Regional variations of bone quantity and quality impact femoral head collapse. Clin Orthop Relat Res. 2010;468(1):276–282. doi: 10.1007/s11999-009-1041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L., Zhang L., Pan H., Peng S., Zhao X., Lu W.W. Abnormal subchondral bone microstructure following steroid administration is involved in the early pathogenesis of steroid-induced osteonecrosis. Osteoporos Int. 2016;27(1):153–159. doi: 10.1007/s00198-015-3225-8. [DOI] [PubMed] [Google Scholar]
- 47.Catto M. A histological study of avascular necrosis of the femoral head after transcervical fracture. J Bone Joint Surg Br. 1965;47(4):749–776. [PubMed] [Google Scholar]
- 48.Catto M. The histological appearances of late segmental collapse of the femoral head after transcervical fracture. J Bone Joint Surg Br. 1965;47(4):777–791. [PubMed] [Google Scholar]
- 49.Inoue A., Ono K. A histological study of idiopathic avascular necrosis of the head of the femur. J Bone Joint Surg Br. 1979;61(2):138–143. doi: 10.1302/0301-620X.61B2.438261. [DOI] [PubMed] [Google Scholar]
- 50.Imhof H., Breitenseher M., Trattnig S., Kramer J., Hofmann S., Plenk H., et al. Imaging of avascular necrosis of bone. Eur Radiol. 1997;7(2):180–186. doi: 10.1007/s003300050131. [DOI] [PubMed] [Google Scholar]
- 51.Utting J.C., Robins S.P., Brandao-Burch A., Orriss I.R., Behar J., Arnett T.R. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res. 2006;312(10):1693–1702. doi: 10.1016/j.yexcr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Hauzeur J.P., Pasteels J.L., Schoutens A., Hinsenkamp M., Appelboom T., Chochrad I., et al. The diagnostic value of magnetic resonance imaging in non-traumatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 1989;71(5):641–649. [PubMed] [Google Scholar]
- 53.Hernigou P., Voisin M.C., Marichez M., Despres E., Goutallier D. [Comparison of nuclear magnetic resonance and histology in necrosis of the femoral head] Rev Rhum Mal Osteoartic. 1989;56(11):741–744. [fre] [PubMed] [Google Scholar]
- 54.Zlatkin M.B., Pevsner D., Sanders T.G., Hancock C.R., Ceballos C.E., Herrera M.F. Acetabular labral tears and cartilage lesions of the hip: indirect MR arthrographic correlation with arthroscopy–a preliminary study. AJR Am J Roentgenol. 2010;194(3):709–714. doi: 10.2214/AJR.07.3669. [DOI] [PubMed] [Google Scholar]
- 55.Ho C.P., Ommen N.D., Bhatia S., Saroki A.J., Goljan P., Briggs K.K., et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808–1813. doi: 10.1016/j.arthro.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Traistaru M.R., Kamal D., Kamal K.C., Rogoveanu O.C., Popescu M., Bondari S., et al. Imaging and histopathological aspects in aseptic osteonecrosis of the femoral head. Rom J Morphol Embryol. 2015;56(4):1447–1453. [PubMed] [Google Scholar]
- 57.Ruch D.S., Sekiya J., Dickson Schaefer W., Koman L.A., Pope T.L., Poehling G.G. The role of hip arthroscopy in the evaluation of avascular necrosis. Orthopedics. 2001;24(4):339–343. doi: 10.3928/0147-7447-20010401-15. [DOI] [PubMed] [Google Scholar]
- 58.Schwarz Lausten G. Non-traumatic necrosis of the femoral head. Int Orthop. 1999;23(4):193–197. doi: 10.1007/s002640050348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang C., Wang Y., Meng H.Y., Yuan X.L., Xu X.L., Wang A.Y., et al. Application of bone marrow mesenchymal stem cells to the treatment of osteonecrosis of the femoral head. Int J Clin Exp Med. 2015;8(3):3127–3135. [PMC free article] [PubMed] [Google Scholar]
- 60.Hernigou P., Trousselier M., Roubineau F., Bouthors C., Chevallier N., Rouard H., et al. Stem cell therapy for the treatment of hip osteonecrosis: a 30-year review of progress. Clin Orthop Surg. 2016;8(1):1–8. doi: 10.4055/cios.2016.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu S., Zhang L., Jin H., Shan L., Zhou L., Xiao L., et al. Autologous stem cells combined core decompression for treatment of avascular necrosis of the femoral head: a systematic meta-analysis. BioMed Res Int. 2017;2017:6136205. doi: 10.1155/2017/6136205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan Z., Hang D., Guo C., Chen Z. Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head. J Orthop Res. 2009;27(4):442–446. doi: 10.1002/jor.20759. [DOI] [PubMed] [Google Scholar]
- 63.López Fernández A., Barro V., Ortiz-Hernández M., Manzanares-Céspedes M.C., Vivas D., Vives J., et al. Effect of allogeneic cell-based tissue engineered treatments in a sheep osteonecrosis model. 17th–18th ed. Tissue Eng Part A. 2020;26:993–1004. doi: 10.1089/ten.TEA.2019.0339. [eng] [DOI] [PubMed] [Google Scholar]
- 64.Song H., Tao L., Wang F., Wang W., Wei Y., Shen W., et al. Effect of bone mesenchymal stem cells transplantation on the micro-environment of early osteonecrosis of the femoral head. Int J Clin Exp Pathol. 2015;8(11):14528–14534. [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan H.F., Zhang J., Guo C.A., Yan Z.Q. Clinical outcomes of osteonecrosis of the femoral head after autologous bone marrow stem cell implantation: a meta-analysis of seven case-control studies. Clinics. 2016;71(2):110–113. doi: 10.6061/clinics/2016(02)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan C.K., Chen C.C., Luppen C.A., Kim J.B., DeBoer A.T., Wei K., et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457(7228):490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee S.-H., Rho J., Jeong D., Sul J.-Y., Kim T., Kim N., et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med. 2006;12(12):1403–1409. doi: 10.1038/nm1514. [DOI] [PubMed] [Google Scholar]
- 68.Ben A.A. Scheven, J.W. Visser, P.J. Nijweide. In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population. Nature. 1986 May 1-7;321(6065):79–81. doi: 10.1038/321079a0. [DOI] [PubMed] [Google Scholar]
- 69.Kubota K., Sakikawa C., Katsumata M., Nakamura T., Wakabayashi K. Platelet-derived growth factor BB secreted from osteoclasts acts as an osteoblastogenesis inhibitory factor. J Bone Miner Res. 2002;17(2):257–265. doi: 10.1359/jbmr.2002.17.2.257. [DOI] [PubMed] [Google Scholar]
- 70.Wang C., Wang X., Xu X-l, Yuan X-l, Gou W-l, Wang A-y, et al. Bone microstructure and regional distribution of osteoblast and osteoclast activity in the osteonecrotic femoral head. PloS One. 2014;9(5) doi: 10.1371/journal.pone.0096361. e96361–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao D.W., Hu Y.C. Chinese experts' consensus on the diagnosis and treatment of osteonecrosis of the femoral head in adults. Orthop Surg. 2012;4(3):125–130. doi: 10.1111/j.1757-7861.2012.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang C., Meng H., Wang Y., Zhao B., Zhao C., Sun W., et al. Analysis of early stage osteonecrosis of the human femoral head and the mechanism of femoral head collapse. Int J Biol Sci. 2018;14(2):156–164. doi: 10.7150/ijbs.18334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malinin T., Ouellette E.A. Articular cartilage nutrition is mediated by subchondral bone– a long-term autograft study in baboons. Osteoarthritis Cartilage. 2000;8:483–491. doi: 10.1053/joca.1999.0324. [DOI] [PubMed] [Google Scholar]
- 74.Clark J.M., Huber J.D. The structure of the human subchondral plate. J Bone Joint Surg Br. 1990;72(5):866–873. doi: 10.1302/0301-620X.72B5.2211774. [DOI] [PubMed] [Google Scholar]
- 75.Imhof H., Sulzbacher I., Grampp S., Czerny C., Youssefzadeh S., Kainberger F. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest Radiol. 2000;35(10):581–588. doi: 10.1097/00004424-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Arkill K.P., Winlove C.P. Solute transport in the deep and calcified zones of articular cartilage. Osteoarthritis Cartilage. 2008;16(6):708–714. doi: 10.1016/j.joca.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Findlay D.M., Kuliwaba J.S. Bone-cartilage crosstalk: a conversation for understanding osteoarthritis. Bone Res. 2016;4:16028. doi: 10.1038/boneres.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oegema T.R., Jr., Carpenter R.J., Hofmeister F., Thompson R.C., Jr. The interaction of the zone of calcified cartilage and subchondral bone in osteoarthritis. Microsc Res Tech. 1997;37(4):324–332. doi: 10.1002/(SICI)1097-0029(19970515)37:4<324::AID-JEMT7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 79.Gupta H.S., Schratter S., Tesch W., Roschger P., Berzlanovich A., Schoeberl T., et al. Two different correlations between nanoindentation modulus and mineral content in the bone-cartilage interface. J Struct Biol. 2005;149(2):138–148. doi: 10.1016/j.jsb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 80.Madry H., van Dijk C.N., Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):419–433. doi: 10.1007/s00167-010-1054-z. [DOI] [PubMed] [Google Scholar]
- 81.Murphey M.D., Foreman K.L., Klassen-Fischer M.K., Fox M.G., Chung E.M., Kransdorf M.J. From the radiologic pathology archives imaging of osteonecrosis: radiologic-pathologic correlation. Radiographics. 2014;34(4):1003–1028. doi: 10.1148/rg.344140019. [DOI] [PubMed] [Google Scholar]
- 82.Bullough P.G., DiCarlo E.F. Subchondral avascular necrosis: a common cause of arthritis. Ann Rheum Dis. 1990;49(6):412–420. doi: 10.1136/ard.49.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Utsunomiya T., Motomura G., Ikemura S., Kubo Y., Sonoda K., Hatanaka H., et al. Effects of sclerotic changes on stress concentration in early-stage osteonecrosis: a patient-specific, 3D finite element analysis. J Orthop Res. 2018;36(12):3169–3177. doi: 10.1002/jor.24124. [DOI] [PubMed] [Google Scholar]
- 84.Karasuyama K., Yamamoto T., Motomura G., Sonoda K., Kubo Y., Iwamoto Y. The role of sclerotic changes in the starting mechanisms of collapse: a histomorphometric and FEM study on the femoral head of osteonecrosis. Bone. 2015;81:644–648. doi: 10.1016/j.bone.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y., Yin J., Ding H., Zhang C., Gao Y.S. Vitamin K2 ameliorates damage of blood vessels by glucocorticoid: a potential mechanism for its protective effects in glucocorticoid-induced osteonecrosis of the femoral head in a rat model. Int J Biol Sci. 2016;12(7):776–785. doi: 10.7150/ijbs.15248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao F., Han J., He Z., Li Z. Radiological analysis of cystic lesion in osteonecrosis of the femoral head. Int Orthop. 2018;42(7):1615–1621. doi: 10.1007/s00264-018-3958-z. [DOI] [PubMed] [Google Scholar]
- 87.Zhao D., Zhang F., Wang B., Liu B., Li L., Kim S.Y., et al. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version) J Orthop Translat. 2020;21:100–110. doi: 10.1016/j.jot.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Castañeda S., Roman-Blas J.A., Largo R., Herrero-Beaumont G. Subchondral bone as a key target for osteoarthritis treatment. Biochem Pharmacol. 2012;83(3):315–323. doi: 10.1016/j.bcp.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 89.Karasuyama K., Yamamoto T., Motomura G., Sonoda K., Kubo Y., Iwamoto Y. The role of sclerotic changes in the starting mechanisms of collapse: a histomorphometric and FEM study on the femoral head of osteonecrosis. Bone. 2015;81:644–648. doi: 10.1016/j.bone.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 90.Motomura G., Yamamoto T., Yamaguchi R., Ikemura S., Yn T., Mawatari Y.I. Morphological analysis of collapsed regions in osteonecrosis of the femoral head. J Bone Joint Surg [Br] 2011;93-B doi: 10.1302/0301-620X.93B225476. [DOI] [PubMed] [Google Scholar]
- 91.Lafforgue P., Dahan E., Chagnaud C., Schiano A., Kasbarian M., Acquaviva P.C. Early-stage avascular necrosis of the femoral head: MR imaging for prognosis in 31 cases with at least 2 years of follow-up. Radiology. 1993;187(1):199–204. doi: 10.1148/radiology.187.1.8451413. [DOI] [PubMed] [Google Scholar]
- 92.Ma J.-X., He W.-W., Zhao J., Kuang M.-J., Bai H.-H., Sun L., et al. Bone microarchitecture and biomechanics of the necrotic femoral head. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-13643-2. 13345–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takatori Y., Kokubo T., Ninomiya S., Nakamura S., Morimoto S., Kusaba I. Avascular necrosis of the femoral head. Natural history and magnetic resonance imaging. J Bone Joint Surg Br. 1993;75(2):217–221. doi: 10.1302/0301-620X.75B2.8444940. [DOI] [PubMed] [Google Scholar]
- 94.Aruwajoye O.O., Patel M.K., Allen M.R., Burr D.B., Aswath P.B., Kim H.K. Microcrack density and nanomechanical properties in the subchondral region of the immature piglet femoral head following ischemic osteonecrosis. Bone. 2013;52(2):632–639. doi: 10.1016/j.bone.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 95.Ikemura S., Mawatari T., Matsui G., Iguchi T., Mitsuyasu H. The depth of the low-intensity band on the T1-weighted MR image is useful for distinguishing subchondral insufficiency fracture from osteonecrosis of the collapsed femoral head. Arch Orthop Trauma Surg. 2018;138(8):1053–1058. doi: 10.1007/s00402-018-2948-3. [DOI] [PubMed] [Google Scholar]
- 96.Ikemura S., Yamamoto T., Motomura G., Nakashima Y., Mawatari T., Iwamoto Y. MRI evaluation of collapsed femoral heads in patients 60 years old or older: differentiation of subchondral insufficiency fracture from osteonecrosis of the femoral head. AJR Am J Roentgenol. 2010;195(1):W63–W68. doi: 10.2214/AJR.09.3271. [DOI] [PubMed] [Google Scholar]
- 97.Hamada H., Takao M., Sakai T., Sugano N. Subchondral fracture begins from the bone resorption area in osteonecrosis of the femoral head: a micro-computerised tomography study. Int Orthop. 2018;42(7):1479–1484. doi: 10.1007/s00264-018-3879-x. [DOI] [PubMed] [Google Scholar]
- 98.Ohzono K., Saito M., Takaoka K., Ono K., Saito S., Nishina T., et al. Natural history of nontraumatic avascular necrosis of the femoral head. J Bone Joint Surg Br. 1991;73(1):68–72. doi: 10.1302/0301-620X.73B1.1991778. [DOI] [PubMed] [Google Scholar]
- 99.Kim Y.M., Oh H.C., Kim H.J. The pattern of bone marrow oedema on MRI in osteonecrosis of the femoral head. J Bone Joint Surg Br. 2000;82(6):837–841. doi: 10.1302/0301-620x.82b6.10740. [DOI] [PubMed] [Google Scholar]
- 100.Meier R., Kraus T.M., Schaeffeler C., Torka S., Schlitter A.M., Specht K., et al. Bone marrow oedema on MR imaging indicates ARCO stage 3 disease in patients with AVN of the femoral head. Eur Radiol. 2014;24(9):2271–2278. doi: 10.1007/s00330-014-3216-8. [DOI] [PubMed] [Google Scholar]
- 101.Theruvath A.J., Sukerkar P.A., Bao S., Rosenberg J., Luna-Fineman S., Kharbanda S., et al. Bone marrow oedema predicts bone collapse in paediatric and adolescent leukaemia patients with corticosteroid-induced osteonecrosis. Eur Radiol. 2018;28(1):410–417. doi: 10.1007/s00330-017-4961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koo K.H., Ahn I.O., Kim R., Song H.R., Jeong S.T., Na J.B., et al. Bone marrow edema and associated pain in early stage osteonecrosis of the femoral head: prospective study with serial MR images. Radiology. 1999;213(3):715–722. doi: 10.1148/radiology.213.3.r99dc06715. [DOI] [PubMed] [Google Scholar]
- 103.Patel S. Primary bone marrow oedema syndromes. Rheumatology. 2014;53(5):785–792. doi: 10.1093/rheumatology/ket324. [DOI] [PubMed] [Google Scholar]
- 104.Liu Y., Zhou C., Chen L., Sun Y., He W. [A summary of hip-preservation surgery based on peri-collapse stage of osteonecrosis of femoral head] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31(8):1010–1015. doi: 10.7507/1002-1892.201611084. [chi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Q.-Y., Li Z.-R., Gao F.-Q., Sun W. Pericollapse stage of osteonecrosis of the femoral head: a last chance for joint preservation. Chin Med J. 2018;131(21):2589–2598. doi: 10.4103/0366-6999.244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takashima K., Sakai T., Hamada H., Takao M., Sugano N. Which classification system is most useful for classifying osteonecrosis of the femoral head? Clin Orthop Relat Res. 2018;476(6):1240–1249. doi: 10.1007/s11999.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bergmann G., Graichen F., Rohlmann A. Hip joint loading during walking and running, measured in two patients. J Biomech. 1993;26(8):969–990. doi: 10.1016/0021-9290(93)90058-m. [DOI] [PubMed] [Google Scholar]
- 108.Hodge W.A., Carlson K.L., Fijan R.S., Burgess R.G., Riley P.O., Harris W.H., et al. Contact pressures from an instrumented hip endoprosthesis. J Bone Jt Surg Am Vol. 1989;71(9):1378–1386. [PubMed] [Google Scholar]
- 109.Krebs D.E., Elbaum L., Riley P.O., Hodge W.A., Mann R.W. Exercise and gait effects on in vivo hip contact pressures. Phys Ther. 1991;71(4):301–309. doi: 10.1093/ptj/71.4.301. [DOI] [PubMed] [Google Scholar]
- 110.Yoshida H., Faust A., Wilckens J., Kitagawa M., Fetto J., Chao E.Y.S. Three-dimensional dynamic hip contact area and pressure distribution during activities of daily living. J Biomech. 2006;39(11):1996–2004. doi: 10.1016/j.jbiomech.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 111.Motomura G., Yamamoto T., Yamaguchi R., Ikemura S., Nakashima Y., Mawatari T., et al. Morphological analysis of collapsed regions in osteonecrosis of the femoral head. J Bone Joint Surg Br. 2011;93(2):184–187. doi: 10.1302/0301-620X.93B225476. [DOI] [PubMed] [Google Scholar]
- 112.Hengsberger S., Ammann P., Legros B., Rizzoli R., Zysset P. Intrinsic bone tissue properties in adult rat vertebrae: modulation by dietary protein. Bone. 2005;36(1):134–141. doi: 10.1016/j.bone.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 113.Wei Q., Yang F., Chen X., He M., Chen Z., Zhang Q., et al. Microarchitecture features and pathology of necrotic region in patients with steroid-induced and alcohol-induced osteonecrosis of femoral head. Chin J Reparative Reconstr Surg. 2018;32(7):866–872. doi: 10.7507/1002-1892.201711130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown T.D., Baker K.J., Brand R.A. Structural consequences of subchondral bone involvement in segmental osteonecrosis of the femoral head. Journal of orthopaedic research. official publication of the Orthopaedic Research Society. 1992;10(1):79–87. doi: 10.1002/jor.1100100110. [DOI] [PubMed] [Google Scholar]
- 115.Phemister D.B. Repair of bone in the presence of aseptic necrosis resulting from fractures, transplantations, and vascular obstruction. 1930. J Bone Jt Surg Am Vol. 2005;87(3):672. doi: 10.2106/00004623-200503000-00029. [DOI] [PubMed] [Google Scholar]
- 116.Fan M., Peng J., Wang A., Zhang L., Liu B., Ren Z., et al. Emu model of full-range femoral head osteonecrosis induced focally by an alternating freezing and heating insult. J Int Med Res. 2011;39(1):187–198. doi: 10.1177/147323001103900120. [DOI] [PubMed] [Google Scholar]
- 117.Li W., Sakai T., Nishii T., Nakamura N., Takao M., Yoshikawa H., et al. Distribution of TRAP-positive cells and expression of HIF-1alpha, VEGF, and FGF-2 in the reparative reaction in patients with osteonecrosis of the femoral head. J Orthop Res. 2009;27(5):694–700. doi: 10.1002/jor.20802. [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto T., DiCarlo E.F., Bullough P.G. The prevalence and clinicopathological appearance of extension of osteonecrosis in the femoral head. J Bone Joint Surg Br. 1999;81(2):328–332. [PubMed] [Google Scholar]
- 119.Atsumi T., Kuroki Y., Yamano K. A microangiographic study of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1989;246:186–194. [PubMed] [Google Scholar]
- 120.Kato S., Yamada H., Terada N., Masuda K., Lenz M.E., Morita M., et al. Joint biomarkers in idiopathic femoral head osteonecrosis: comparison with hip osteoarthritis. J Rheumatol. 2005;32(8):1518–1523. [PubMed] [Google Scholar]
- 121.Zhao D., Zhang F., Wang B., Liu B., Li L., Kim S.-Y., et al. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version) 21st ed. J Orthop Translat. 2020;6:100–110. doi: 10.1016/j.jot.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bedard N.A., Callaghan J.J., Liu S.S., Greiner J.J., Klaassen A.L., Johnston R.C. Cementless THA for the treatment of osteonecrosis at 10-year follow-up: have we improved compared to cemented THA? J Arthroplasty. 2013;28(7):1192–1199. doi: 10.1016/j.arth.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 123.Trăistaru M.R., Kamal D., Kamal K.C., Rogoveanu O.C., Popescu M., Bondari S., et al. Imaging and histopathological aspects in aseptic osteonecrosis of the femoral head. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2015;56(4):1447–1453. [PubMed] [Google Scholar]
- 124.Luk W.H., Au-Yeung A.W.S., Yang M.K.W. Diagnostic value of SPECT versus SPECT/CT in femoral avascular necrosis: preliminary results. Nucl Med Commun. 2010;31(11):958–961. doi: 10.1097/MNM.0b013e32833e7732. [DOI] [PubMed] [Google Scholar]
- 125.Ryu J.-S., Kim J.S., Moon D.H., Kim S.M., Shin M.J., Chang J.S., et al. Bone SPECT is more sensitive than MRI in the detection of early osteonecrosis of the femoral head after renal transplantation. J Nucl Med. 2002;43(8):1006–1011. [PubMed] [Google Scholar]
- 126.Motomura G., Yamamoto T., Karasuyama K., Iwamoto Y. Bone SPECT/CT of femoral head subchondral insufficiency fracture. Clin Nucl Med. 2015;40(9):752–754. doi: 10.1097/RLU.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 127.Motomura G., Yamamoto T., Abe K., Nakashima Y., Ohishi M., Hamai S., et al. Scintigraphic assessments of the reparative process in osteonecrosis of the femoral head using SPECT/CT with 99mTc hydroxymethylene diphosphonate. Nucl Med Commun. 2014;35(10):1047–1051. doi: 10.1097/MNM.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 128.Mitchell D.G., Rao V.M., Dalinka M.K., Spritzer C.E., Alavi A., Steinberg M.E., et al. Femoral head avascular necrosis: correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology. 1987;162(3):709–715. doi: 10.1148/radiology.162.3.3809484. [DOI] [PubMed] [Google Scholar]
- 129.Beltran J., Herman L.J., Burk J.M., Zuelzer W.A., Clark R.N., Lucas J.G., et al. Femoral head avascular necrosis: MR imaging with clinical-pathologic and radionuclide correlation. Radiology. 1988;166(1 Pt 1):215–220. doi: 10.1148/radiology.166.1.3336682. [DOI] [PubMed] [Google Scholar]
- 130.Malizos K.N., Karantanas A.H., Varitimidis S.E., Dailiana Z.H., Bargiotas K., Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol. 2007;63(1):16–28. doi: 10.1016/j.ejrad.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 131.Dasa V., Adbel-Nabi H., Anders M.J., Mihalko W.M. F-18 fluoride positron emission tomography of the hip for osteonecrosis. Clin Orthop Relat Res. 2008;466(5):1081–1086. doi: 10.1007/s11999-008-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Manenti G., Altobelli S., Pugliese L., Tarantino U. The role of imaging in diagnosis and management of femoral head avascular necrosis. Clin Cases Miner Bone Metab. 2015;12(Suppl 1):31–38. doi: 10.11138/ccmbm/2015.12.3s.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dihlmann W. CT analysis of the upper end of the femur: the asterisk sign and ischaemic bone necrosis of the femoral head. Skeletal Radiol. 1982;8(4):251–258. doi: 10.1007/BF02219619. [DOI] [PubMed] [Google Scholar]
- 134.Mitchell D.G., Kressel H.Y., Arger P.H., Dalinka M., Spritzer C.E., Steinberg M.E. Avascular necrosis of the femoral head: morphologic assessment by MR imaging, with CT correlation. Radiology. 1986;161(3):739–742. doi: 10.1148/radiology.161.3.3786725. [DOI] [PubMed] [Google Scholar]
- 135.Conway W.F., Totty W.G., McEnery K.W. CT and MR imaging of the hip. Radiology. 1996;198(2):297–307. doi: 10.1148/radiology.198.2.8596820. [DOI] [PubMed] [Google Scholar]
- 136.Hauzeur J.P., Pasteels J.L., Orloff S. Bilateral non-traumatic aseptic osteonecrosis in the femoral head. An experimental study of incidence. J Bone Jt Surg Am Vol. 1987;69(8):1221–1225. [PubMed] [Google Scholar]
- 137.Hu L.B., Huang Z.G., Wei H.Y., Wang W., Ren A., Xu Y.Y. Osteonecrosis of the femoral head: using CT, MRI and gross specimen to characterize the location, shape and size of the lesion. Br J Radiol. 2015;88(1046):20140508. doi: 10.1259/bjr.20140508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kolb A.R., Patsch J.M., Vogl W.D., Benca E., Stelzeneder D., Windhager R., et al. The role of the subchondral layer in osteonecrosis of the femoral head: analysis based on HR-QCT in comparison to MRI findings. Acta Radiol. 2019;60(4):501–508. doi: 10.1177/0284185118786070. [DOI] [PubMed] [Google Scholar]
- 139.Stevens K., Tao C., Lee S.-U., Salem N., Vandevenne J., Cheng C., et al. Subchondral fractures in osteonecrosis of the femoral head: comparison of radiography, CT, and MR imaging. AJR Am J Roentgenol. 2003;180(2):363–368. doi: 10.2214/ajr.180.2.1800363. [DOI] [PubMed] [Google Scholar]
- 140.Yeh L.-R., Chen C.K.H., Huang Y.-L., Pan H.-B., Yang C.-F. Diagnostic performance of MR imaging in the assessment of subchondral fractures in avascular necrosis of the femoral head. Skeletal Radiol. 2009;38(6):559–564. doi: 10.1007/s00256-009-0659-0. [DOI] [PubMed] [Google Scholar]
- 141.Beckmann J., Matsuura M., Grässel S., Köck F., Grifka J., Tingart M. A muCT analysis of the femoral bone stock in osteonecrosis of the femoral head compared to osteoarthrosis. Arch Orthop Trauma Surg. 2009;129(4):501–505. doi: 10.1007/s00402-008-0666-y. [DOI] [PubMed] [Google Scholar]
- 142.Piyakunmala K., Sangkomkamhang T., Chareonchonvanitch K. Is magnetic resonance imaging necessary for normal plain radiography evaluation of contralateral non-traumatic asymptomatic femoral head in high osteonecrosis risk patient. J Med Assoc Thai. 2009;92(6):S147–S151. [PubMed] [Google Scholar]
- 143.Glickstein M.F., Burk D.L., Jr., Schiebler M.L., Cohen E.K., Dalinka M.K., Steinberg M.E., et al. Avascular necrosis versus other diseases of the hip: sensitivity of MR imaging. Radiology. 1988;169(1):213–215. doi: 10.1148/radiology.169.1.3420260. [DOI] [PubMed] [Google Scholar]
- 144.Kamata N., Oshitani N., Sogawa M., Yamagami H., Watanabe K., Fujiwara Y., et al. Usefulness of magnetic resonance imaging for detection of asymptomatic osteonecrosis of the femoral head in patients with inflammatory bowel disease on long-term corticosteroid treatment. Scand J Gastroenterol. 2008;43(3):308–313. doi: 10.1080/00365520701676773. [DOI] [PubMed] [Google Scholar]
- 145.Sen R.K., Tripathy S.K., Aggarwal S., Marwaha N., Sharma R.R., Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplasty. 2012;27(5):679–686. doi: 10.1016/j.arth.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 146.Vande Berg B.E., Malghem J.J., Labaisse M.A., Noel H.M., Maldague B.E. MR imaging of avascular necrosis and transient marrow edema of the femoral head. Radiographics. 1993;13(3):501–520. doi: 10.1148/radiographics.13.3.8316660. [DOI] [PubMed] [Google Scholar]
- 147.Karantanas A.H., Drakonaki E.E. The role of MR imaging in avascular necrosis of the femoral head. Semin Muscoskel Radiol. 2011;15(3):281–300. doi: 10.1055/s-0031-1278427. [DOI] [PubMed] [Google Scholar]
- 148.Koo K.H., Kim R. Quantifying the extent of osteonecrosis of the femoral head. A new method using MRI. J Bone Joint Surg Br. 1995;77(6):875–880. [PubMed] [Google Scholar]
- 149.Shimizu K., Moriya H., Akita T., Sakamoto M., Suguro T. Prediction of collapse with magnetic resonance imaging of avascular necrosis of the femoral head. J Bone Jt Surg Am Vol. 1994;76(2):215–223. doi: 10.2106/00004623-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 150.Brody A.S., Strong M., Babikian G., Sweet D.E., Seidel F.G., Kuhn J.P. John Caffey Award paper. Avascular necrosis: early MR imaging and histologic findings in a canine model. AJR Am J Roentgenol. 1991;157(2):341–345. doi: 10.2214/ajr.157.2.1853819. [DOI] [PubMed] [Google Scholar]
- 151.Koo K.H., Kim R., Cho S.H., Song H.R., Lee G., Ko G.H. Angiography, scintigraphy, intraosseous pressure, and histologic findings in high-risk osteonecrotic femoral heads with negative magnetic resonance images. Clin Orthop Relat Res. 1994;308:127–138. [PubMed] [Google Scholar]
- 152.Karol S.E., Yang W., Van Driest S.L., Chang T.Y., Kaste S., Bowton E., et al. Genetics of glucocorticoid-associated osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2015;126(15):1770–1776. doi: 10.1182/blood-2015-05-643601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao F-c, Li Z-r, Zhang N-f, Wang B-l, Sun W., Cheng L-m, et al. Lesion size changes in osteonecrosis of the femoral head: a long-term prospective study using MRI. Int Orthop. 2010;34(6):799–804. doi: 10.1007/s00264-009-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hatanaka H., Motomura G., Ikemura S., Kubo Y., Utsunomiya T., Baba S., et al. Differences in magnetic resonance findings between symptomatic and asymptomatic pre-collapse osteonecrosis of the femoral head. Eur J Radiol. 2019;112:1–6. doi: 10.1016/j.ejrad.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 155.Duda S.H., Laniado M., Schick F., Claussen C.D. The double-line sign of osteonecrosis: evaluation on chemical shift MR images. Eur J Radiol. 1993;16(3):233–238. doi: 10.1016/0720-048x(93)90081-w. [DOI] [PubMed] [Google Scholar]
- 156.Jiang C.C., Shih T.T. Epiphyseal scar of the femoral head: risk factor of osteonecrosis. Radiology. 1994;191(2):409–412. doi: 10.1148/radiology.191.2.8153314. [DOI] [PubMed] [Google Scholar]
- 157.Yamaguchi R., Yamamoto T., Motomura G., Ikemura S., Iwamoto Y. MRI-detected double low-intensity bands in osteonecrosis of the femoral head. J Orthop Sci. 2011;16(4):471–475. doi: 10.1007/s00776-011-0059-x. [DOI] [PubMed] [Google Scholar]
- 158.Takao M., Nishii T., Sakai T., Yoshikawa H., Sugano N. Repair in osteonecrosis of the femoral head: MR imaging features at long-term follow-up. Clin Rheumatol. 2010;29(8):841–848. doi: 10.1007/s10067-010-1404-8. [DOI] [PubMed] [Google Scholar]
- 159.Iida S., Harada Y., Shimizu K., Sakamoto M., Ikenoue S., Akita T., et al. Correlation between bone marrow edema and collapse of the femoral head in steroid-induced osteonecrosis. AJR American journal of roentgenology. 2000;174(3):735–743. doi: 10.2214/ajr.174.3.1740735. [DOI] [PubMed] [Google Scholar]
- 160.Kim Y.M., Oh H.C., Kim H.J. The pattern of bone marrow oedema on MRI in osteonecrosis of the femoral head. J Bone Joint Surg Br. 2000;82(6):837–841. doi: 10.1302/0301-620x.82b6.10740. [DOI] [PubMed] [Google Scholar]
- 161.Nadel S.N., Debatin J.F., Richardson W.J., Hedlund L.W., Senft C., Rizk W.S., et al. Detection of acute avascular necrosis of the femoral head in dogs: dynamic contrast-enhanced MR imaging vs spin-echo and STIR sequences. AJR Am J Roentgenol. 1992;159(6):1255–1261. doi: 10.2214/ajr.159.6.1442396. [DOI] [PubMed] [Google Scholar]
- 162.Chan W.P., Liu Y.-J., Huang G.-S., Lin M.-F., Huang S., Chang Y.-C., et al. Relationship of idiopathic osteonecrosis of the femoral head to perfusion changes in the proximal femur by dynamic contrast-enhanced MRI. AJR Am J Roentgenol. 2011;196(3):637–643. doi: 10.2214/AJR.10.4322. [DOI] [PubMed] [Google Scholar]
- 163.Lee Y.-K., Yoo J.J., Koo K.-H., Yoon K.S., Min B.W., Kim H.J. Collapsed subchondral fatigue fracture of the femoral head. Orthop Clin N Am. 2009;40(2):259–265. doi: 10.1016/j.ocl.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 164.Li K.C., Hiette P. Contrast-enhanced fat saturation magnetic resonance imaging for studying the pathophysiology of osteonecrosis of the hips. Skeletal Radiol. 1992;21(6):375–379. doi: 10.1007/BF00241816. [DOI] [PubMed] [Google Scholar]
- 165.Hauzeur J.P., Sintzoff S., Jr., Appelboom T., De Maertelaer V., Bentin J., Pasteels J.L. Relationship between magnetic resonance imaging and histologic findings by bone biopsy in nontraumatic osteonecrosis of the femoral head. J Rheumatol. 1992;19(3):385–392. [PubMed] [Google Scholar]
- 166.Lang P., Mauz M., Schörner W., Schwetlick G., Henkes H., Berthezene Y., et al. Acute fracture of the femoral neck: assessment of femoral head perfusion with gadopentetate dimeglumine-enhanced MR imaging. AJR Am J Roentgenol. 1993;160(2):335–341. doi: 10.2214/ajr.160.2.8424346. [DOI] [PubMed] [Google Scholar]
- 167.Kaushik A., Sankaran B., Varghese M. Prognostic value of dynamic MRI in assessing post-traumatic femoral head vascularity. Skeletal Radiol. 2009;38(6):565–569. doi: 10.1007/s00256-009-0667-0. [DOI] [PubMed] [Google Scholar]
- 168.Öner A.Y., Aggunlu L., Akpek S., Celik A., Le Roux P., Tali T., et al. Staging of hip avascular necrosis: is there a need for DWI? Acta Radiol. 2011;52(1):111–114. doi: 10.1258/ar.2010.100231. [DOI] [PubMed] [Google Scholar]
- 169.Yamamoto S., Watanabe A., Nakamura J., Ohtori S., Harada Y., Kishida S., et al. Quantitative T2 mapping of femoral head cartilage in systemic lupus erythematosus patients with noncollapsed osteonecrosis of the femoral head associated with corticosteroid therapy. J Magn Reson Imag. 2011;34(5):1151–1158. doi: 10.1002/jmri.22685. [DOI] [PubMed] [Google Scholar]
- 170.Mackenzie J.D., Hernandez A., Pena A., Ruppert K., Khrichenko D., Gonzalez L., et al. Magnetic resonance imaging in children with sickle cell disease–detecting alterations in the apparent diffusion coefficient in hips with avascular necrosis. Pediatr Radiol. 2012;42(6):706–713. doi: 10.1007/s00247-011-2327-5. [DOI] [PubMed] [Google Scholar]
- 171.Marker D.R., Seyler T.M., McGrath M.S., Delanois R.E., Ulrich S.D., Mont M.A. Treatment of early stage osteonecrosis of the femoral head. J Bone Jt Surg Am Vol. 2008;90(4):175–187. doi: 10.2106/JBJS.H.00671. [DOI] [PubMed] [Google Scholar]
- 172.Marcus N.D., Enneking W.F., Massam R.A. The silent hip in idiopathic aseptic necrosis. treatment by bone-grafting. J Bone Joint Surg Am. 1973;55(7):1351–1366. [PubMed] [Google Scholar]
- 173.Ficat R.P. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985;67(1):3–9. doi: 10.1302/0301-620X.67B1.3155745. [DOI] [PubMed] [Google Scholar]
- 174.Jawad M.U., Haleem A.A., Scully S.P. In brief: Ficat classification: avascular necrosis of the femoral head. Clin Orthop Relat Res. 2012;470(9):2636–2639. doi: 10.1007/s11999-012-2416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Steinberg M.E., Hayken G.D., Steinberg D.R. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77(1):34–41. [PubMed] [Google Scholar]
- 176.Steinberg M.E., Bands R.E., Parry S., Hoffman E., Chan T., Hartman K.M. Does lesion size affect the outcome in avascular necrosis? Clinical orthopaedics and related research. 1999;367:262–271. [PubMed] [Google Scholar]
- 177.Ito H., Matsuno T., Omizu N., Aoki Y., Minami A. Mid-term prognosis of non-traumatic osteonecrosis of the femoral head. J Bone Joint Surg Br. 2003;85(6):796–801. [PubMed] [Google Scholar]
- 178.Nishii T., Sugano N., Ohzono K., Sakai T., Haraguchi K., Yoshikawa H. Progression and cessation of collapse in osteonecrosis of the femoral head. Clin Orthop Relat Res. 2002;400:149–157. doi: 10.1097/00003086-200207000-00019. [DOI] [PubMed] [Google Scholar]
- 179.Bohndorf K., Roth A. [Imaging and classification of avascular femoral head necrosis] Orthopä. 2018;47(9):729–734. doi: 10.1007/s00132-018-3615-7. [ger] [DOI] [PubMed] [Google Scholar]
- 180.Iwamoto Y. The role of the JOA in the globalization of orthopedics. J Orthop Sci. 2009;14(2) doi: 10.1007/s00776-009-1319-x. 131–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ohzono K., Saito M., Sugano N., Takaoka K., Ono K. The fate of nontraumatic avascular necrosis of the femoral head. A radiologic classification to formulate prognosis. Clin Orthop Relat Res. 1992;277:73–78. [PubMed] [Google Scholar]
- 182.Zi-rong L., Z-h Liu, Sun W., Shi Z., Wang B-l Zhao F-c, et al. The classification of osteonecrosis of the femoral head based on the three pillars structure: China Japan Friendship Hospital (CJFH) classification. Chin J Orthop. 2012;32:515–520. [Google Scholar]
- 183.Sun W., Li Z.R., Wang B.L., Liu B.L., Zhang Q.D., Guo W.S. Relationship between preservation of the lateral pillar and collapse of the femoral head in patients with osteonecrosis. Orthopedics. 2014;37(1):e24–e28. doi: 10.3928/01477447-20131219-12. [DOI] [PubMed] [Google Scholar]
- 184.Li G. Subchondral bone as a key target for osteoarthritis treatment. Biochem Pharmacol. Arthritis Research & Therapy. 2013;15:223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hamada H., Takao M., Sakai T., Sugano N. Subchondral fracture begins from the bone resorption area in osteonecrosis of the femoral head: a micro-computerised tomography study. Int Orthop. 2018;42(7):1479–1484. doi: 10.1007/s00264-018-3879-x. [DOI] [PubMed] [Google Scholar]
- 186.Board T.N. Subchondral insufficiency fracture of the femoral head and acetabulum: indications for total hip arthroplasty. J Bone Jt Surg Am Vol. 2003;85(3) doi: 10.2106/00004623-200303000-00036. 572–72. [DOI] [PubMed] [Google Scholar]
- 187.Koo K.H., Ahn I.O., Kim R., Song H.R., Jeong S.T., Na J.B., et al. Bone marrow edema and associated pain in early stage osteonecrosis of the femoral head: prospective study with serial MR images. Radiology. 1999;213(3):715–722. doi: 10.1148/radiology.213.3.r99dc06715. [DOI] [PubMed] [Google Scholar]
- 188.Lee G.C., Khoury V., Steinberg D., Kim W., Dalinka M., Steinberg M. How do radiologists evaluate osteonecrosis? Skeletal Radiol. 2014;43(5):607–614. doi: 10.1007/s00256-013-1803-4. [DOI] [PubMed] [Google Scholar]