Abstract
Background and aim
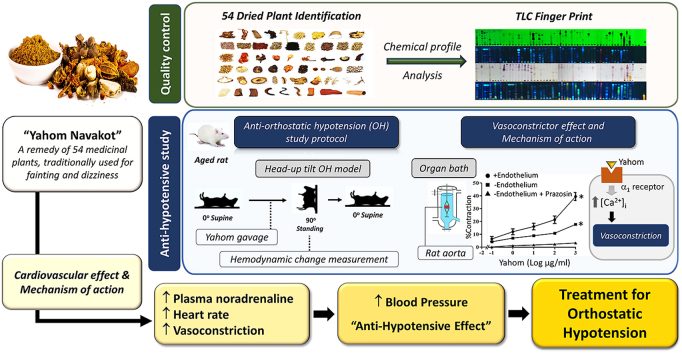
Yahom Navakot (YN), is a Thai traditional medicine, consisting of 54 plants, for treating fainting and dizziness. Thus, YN might relieve orthostatic hypotension (OH) symptoms, but its therapeutic action is unclear. Therefore, this study evaluated YN in OH rats, using a head-up tilt test (HUT).
Experimental procedure
Rats were anesthetized, and OH induced via a 90oHUT, before and after administering vehicle, a YN powder suspension (10, 100 mg/kg), a YN aqueous extract (100 mg/kg), and midodrine (5 mg/kg). The systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MAP), pulse pressure (PP) and heart rate (HR) were determined via the carotid artery. Plasma noradrenaline (NA) was evaluated. YN-induced vasoconstriction of isolated rat aorta rings was determined using organ bath technique.
Results and conclusion
Baseline BP increased with the 100 mg/kg YN powder suspension, the YN aqueous extract or midodrine, while HR decreased, compared with vehicle and control. 90oHUT rapidly reduced SBP, DPB and MAP, but increased HR, for control and vehicle-treated groups, but BP was steady with the 100 mg/kg YN powder suspension, the YN aqueous extract or midodrine. The 90oHUT-increase in HR was most pronounced with the 100 mg/kg YN powder suspension (the traditional formulation). This accords with increased plasma NA. YN also induced vasoconstriction in rat aorta via α1-receptor activation. Thus, the anti-hypotensive action of YN involved a stimulating effect on the heart and blood vessels via sympathetic activation. The results support the traditional use of YN and demonstrated the effectiveness of YN for OH prevention.
Keywords: Yahom Navakot, Orthostatic hypotension, Quality control, Head-up tilt, Blood pressure
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; HR, heart rate; HUT, head-up tilt test; MAP, mean arterial blood pressure; NA, noradrenaline; OH, orthostatic hypotension; PP, pulse pressure; SBP, systolic blood pressure; YN, Yahom Navakot
Graphical abstract
Highlights
-
•
Yahom Navakot is an effective treatment for orthostatic hypotension.
-
•
Yahom Navakot possesses chronotropic effect and vasoconstrictor action.
-
•
Yahom Navakot increases plasma noradrenaline leading to the increase in blood pressure.
1. Introduction
Orthostatic hypotension (OH) is defined as a reduction in systolic blood pressure (SBP) by at least 20 mmHg or diastolic blood pressure (DBP) by at least 10 mmHg within 3 min of attaining an upright position from supine.1 A drop in blood pressure (BP) in OH can cause fainting and dizziness, which is considered to be an acute symptom, and tends to considerably impair the quality of life and may increase the risk of mortality.2 The prevalence of OH increases with age and is 5–23% more prevalent in the elderly.3
Yahom is a traditional Thai recipe, popular among the elderly and commonly used for the treatment of fainting and dizziness. Yahom is a generic name for preparations sold in the markets under different brand names and with different compositions. Most scientific studies on both animals and humans support the traditional use of Yahom. In animal studies, Yahom increased BP after oral administration in rats,4 increased rat regional cerebral blood flow and plantar blood flow5 and stimulated isolated rat atrial and aortic ring contraction.6 A study conducted on healthy human females (aged 20–23 years) reported that the ingestion of 3 g of Yahom increased mean arterial pressure (MAP) and DBP, but had no effect on electrocardiogram (ECG) and heart rate (HR).7 A systematic review on the efficacy and safety of Yahom was summarized from the scientific evidence obtained from investigations in both animal and human studies.8 The findings suggested the following. (i) The commercial Yahom preparations in Thailand are available in many varying compositions, although the exact components of most of them are undisclosed; therefore, quality control of this product is required. (ii) Scientific reports, in particular preclinical studies, on Yahom's action on the cardiovascular system apparently support its use as a treatment for fainting. However, its pharmacological action with respect to specific symptoms, such as the OH, have not been explored. (iii) The toxicity tests on animals have been performed only for Yahom Navakot, but no acute nor chronic toxicity was found.
Yahom Navakot (YN), which consists of 54 medicinal plants and borneol, as listed in the “National List of Essential Medicine” (Table S1), has been approved to be used for prevention of fainting and dizziness.9 An earlier study showed that it increased heart contraction and HR in rats.10 In addition, an aqueous extract of YN increased regional cerebral blood flow, due to the vasodilator effect on cerebral micro blood vessels.5 Oral administration of YN at the doses of 2 and 4 g/kg in mice did not cause any acute toxic signs or lethality.11 Chronic oral toxicity test of YN (10–1,000 mg/kg/day) for 6 months revealed no change on body weight, food consumption, behavior, the general health and clinical chemistry in rats.11 Ingestion of Phikud Navakot (a major ingredient in “Yahom Navakot”) increased MAP and DBP.12 Phikud Navakot contains antioxidant components including phenolics, flavonoids, and anthocyanins,13 volatile oils, and many terpenoids and sesquiterpenoid glycosides.14
These findings supported the use of YN for the treatment of fainting, but its efficacy in the prevention or treatment of OH has yet to be elucidated. Therefore, this study investigated the effects of YN on cardiovascular function in rats, using the head-up tilt test (HUT) to induce OH. In addition, we evaluated the chemical profiles of the 54 constituent herbs in YN, by chromatographic fingerprinting, which offers an efficient way to evaluate the consistency of the YN preparations.
2. Materials and methods
2.1. Drugs and chemicals
The chromatography standards, β-sitosterol and quercetin, were purchased from PhytoLab (Germany). The α1 adrenergic agonist (positive control), midodrine, was purchased from Sigma-Aldrich (USA).
2.2. Quality control of YN preparation
2.2.1. Plant materials
The YN powder preparation and the 54 dried plant materials were provided by the Pra Ajan Fhan Arjaro Hospital, Phanna Nikhom, Sakon Nakhon Province, Thailand. The scientific names and the plant parts used were identified by using macroscopic identification, by comparison with the Thai Herbal Pharmacopoeia,15 and by consultation with a taxonomist of Department of Pharmaceutical Botany, Faculty of Pharmacy, Mahidol University, Thailand (Assist. Prof. Bhanubong Bongcheewin), a taxonomist of Department of Medical Sciences, Medicinal Plant Research Institute, Thailand (Mrs. Pranom Dechwisissakul) and a Thai traditional practitioner of Sireeruckhachati Nature Learning Park (Mr. Ampol Boonpleng). The voucher specimens of all crude drugs were deposited at the Herbarium of Department of Pharmaceutical Botany (PBM), Faculty of Pharmacy, Mahidol University. The detail of crude drugs and voucher numbers are provided in Table S1.
2.2.2. Preparation of plant extracts for quality control
The 54 dried plant materials were powdered and sieved through a no. 60 mesh. Each dried plant and YN preparation were extracted as previously described by Soonthornchareonnon and Ruangwises (2012).16 Briefly, successive extraction using different solvents in the increasing polarity order (hexane, ethanol and water) was carried out in order to yield an extract of the phytochemical compounds in YN with a wide range of different polarities (Fig. S1). Each powdered plant material was extracted separately with hexane (extraction ratio 1:4) by sonication for 60mins, and the residue was removed using Whatman filter paper No.1; the extraction was repeated 3 times, and the supernatants were combined. Then, the residue was sequentially extracted with 95% ethanol and distilled water respectively, as shown in Fig. S1. Finally, all the extracts, i.e., hexane, ethanol and water extracts, were evaporated until dry, and combined (Fig. S1.). The yields of the YN extracts were 29.35% (w/w) of the YN preparation. All dry extracts were kept at 4 °C until used. For phytochemical analysis, the extracts were re-dissolved in methanol: dichloromethane: water (90:5:5) at a concentration of 10 mg/mL.
2.2.3. Phytochemical analysis using thin layer chromatography (TLC) technique
The chromatographic fingerprint analysis represents a comprehensive qualitative approach for the determination of the phytochemical constituents of the raw materials in the YN preparation. Thus, the detailed chemical profile of the plant is critical to ensure the consistency, reliability17 and repeatability of pharmacological effects.18,19 TLC was run on a silica gel 60 GF254 plates (Merck, Darmstadt, Germany), using a mixture of hexane: ethyl acetate (1:1) as a mobile phase system. The running distance was 8 cm and 15 μL of sample solutions were applied. The anisaldehyde-sulphuric acid and the natural product spray reagent were used to detect the terpenes and flavonoids, respectively. The chemical fingerprints were detected under visible light, UV 254 and 366 nm.
2.3. Measurement of hemodynamic changes in postural hypotension rats induced by the head-up tilt test
2.3.1. Preparation of the YN aqueous extract and the YN powder suspension for animal study
The YN aqueous extract was used to compare its efficacy with the YN powder suspension, which represents the traditional use in humans. To obtain the YN aqueous extract, the powder of the YN preparation (500 g) was boiled with distilled water (5 L) for 15min, and then centrifuged at 5,010 g for 20min (Fig. S1). The filtrate was lyophilized (yield 14.9%) and kept in an airtight light-protected container at −20 °C until used.
The test solution for the animal study was freshly prepared by either dissolving the YN aqueous extract or by suspending the YN powder in 1 mL of 35 °C distilled water. The animals were orally administered the YN aqueous extract solution at a dose of 100 mg/kg, and were administered the YN powder suspension at the dose of 10 and 100 mg/kg via an esophageal feeding tube. The YN powder dose used in the present study was extrapolated from the human dose (1–2 g) recommended for the treatment of fainting and dizziness as indicated in the Thai National List of Essential Medicine. A 1–2 g human dose is equivalent to a rat dose of ∼77–154 mg/kg.20 Therefore, 100 mg/kg was chosen. A lower dose of 10 mg/kg was also investigated in order to establish the dose-dependent response. Moreover, based on the previous study, these dosages of YN showed no sign of toxicity.11 The YN aqueous extract was also used in the animal study in order to clarify whether the polar compounds contribute to the pharmacological action of YN or not by comparing its efficacy with YN powder suspension which contains both polar and non-polar compounds.
2.3.2. Experimental animals
The experiments were approved by the Ethics Committee of Naresuan University Animal Care and Use Committee (NUACUC), protocol number NUAE 601020. Male Wistar rats (Nomura Siam International Co. Ltd., Bangkok, Thailand), 72 weeks old and weighing 450–500 g were used. The rats were housed under the environmental conditions at 22 ± 1 °C, 12 h light and dark cycle, fed with a standard rodent diet and tap water in Naresuan University Center for Animal Research (NUCAR) according to the guidelines for care and use of laboratory animals.
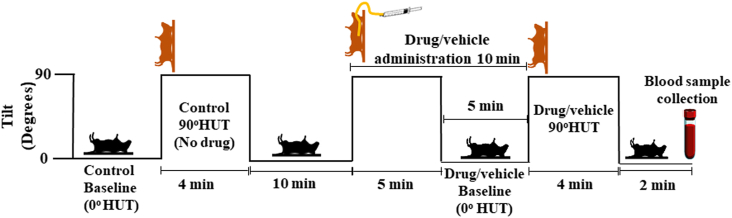
Each rat was anesthetized by intraperitoneal injection (i.p.) with a mixture of ketamine (80 mg/kg) plus xylazine hydrochloride (20 mg/kg). The left common carotid artery was cannulated with a polyethylene tube to monitor the systemic arterial pressure and for final blood sampling. The systemic arterial pressure was monitored by a pressure transducer, and the output was amplified through a bridge amplifier and stored in a computer through a data acquisition and analysis system. An esophageal feeding tube was inserted into the rat for later administration of 5 different treatments, i.e., vehicle (distilled water), 10, 100 mg/kg YN powder suspension, 100 mg/kg YN aqueous extract, or 5 mg/kg midodrine (α1 adrenergic agonist). Then, the rat was placed on an HUT table and supported under the shoulders in the supine position by straps and the experiment was carried out according to the study protocol shown in Fig. 1.
Fig. 1.
Experimental protocol of Yahom Navakot effects on haemodynamic changes in rats with OH using HUT.
2.3.3. Measurement of cardiovascular parameters in HUT-induced OH rats
HUT-induced OH in rats was performed by modifying the protocol described in several previous studies.21,22 Briefly, after the BP and HR stabilized, the control (no vehicle/drug treatment) baseline values were measured and recorded in a supine position (0oHUT) (Fig. 1). OH was induced by raising the table from a horizontal position to a 90oHUT position within approximately 1s and held for 4min. Control measurements of BP, including SBP, DBP, MAP and HR, were performed immediately after the change of position at 1, 2, 3 and 4min without vehicle or drug administration. Then, the rats were returned to the horizontal position within approximately 1s, and rested for another 10min in the supine position, while the baseline measurements of BP and HR were repeated. Then the table was raised to a 90°HUT angle from the horizontal, in order to orally administer the rats with either vehicle, YN powder suspension (10, 100 mg/kg), YN aqueous extract (100 mg/kg) or midodrine (5 mg/kg), and held for 5min before returning to a supine position and rested for 5min. Then, the same 90°HUT as described above was performed and all BP values and HR were measured. Finally, a blood sample was collected in the supine position from a catheter introduced into the carotid artery for plasma noradrenaline (NA) assay.
2.3.4. Plasma noradrenaline assay
Fresh blood was centrifuged at 3,000 rpm for 10min, then the plasma supernatant was stored at −80 °C until assayed. Plasma NA levels were measured using a rat norepinephrine ELISA Kit (MyBiosource, Inc, San Diego, CA, USA).
2.4. Vascular contractile function study
2.4.1. Preparation of YN solution
YN solution was freshly prepared by dissolving the YN powder in warm water, then centrifuged at 3,000 rpm for 10min and the supernatant was collected. The solution was added to the organ bath to give a final concentration of 1-1,000 μg/ml.
2.4.2. Rat isolated aorta preparation
Rats were anesthetized by intraperitoneal injection (i.p.) with a mixture of ketamine (80 mg/kg) plus xylazine hydrochloride (20 mg/kg) after which the aorta was dissected and cleaned of connective tissue and cut into rings (2–3 mm in length). Then, the rings were mounted in an organ bath containing a Krebs solution (mM): NaCl 118, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, NaHCO3 25, glucose 12, pH 7.3), bubbled with 95% O2 and maintained at 37 °C. The vessel segments were allowed to equilibrate for 60min at resting tension of 1 g23 and the Krebs solution was changed every 15min. The viability of the vessels was tested according to their vasoconstriction in response to 80 mM K+ solution. The presence or the absence of the endothelium was assessed by measuring the relaxation response to acetylcholine (10−5 M) in rings pre-contracted by phenylephrine (10−5 M). The endothelium-intact rings were considered to have more than 70% relaxation, whereas the endothelium-denuded rings were considered to have less than 10% relaxation. Changes in tension were obtained via force transducers (CB Sciences Inc., Milford, USA) connected to a Powerlab Data Acquisition System with the Lab Chart software version 7.0 (A.D. Instrument, Castle Hill, Australia).
2.4.3. Experimental protocols for vascular contractile function
A cumulative concentration of YN solution (1-1,000 μg/ml) was added to the organ bath to produce contractile response in either endothelium-intact or endothelium-denuded aortic rings. The same volume of water was added to the control group. The effects were expressed as a percentage of the maximum contraction induced by 80 mM K+ solution. To investigate the involvement of α1-adrenergic receptor, the endothelium-denuded aortic rings were pre-incubated (30min) with prazosin (10−7M),24 an α1-adrenergic receptor blocker, then the cumulative concentration response to YN solution was performed.
2.5. Data analysis
The results were expressed as mean ± SEM. These values were compared using Student's t-test or analysed by analysis of variance (ANOVA) followed by the Tukey–Kramer post hoc test using GraphPad Prism Software Inc. (San Diego, CA, USA), and the differences at P < 0.05 were considered to be statistically significant.
3. Results
3.1. Plant material identification and quality control using the TLC technique
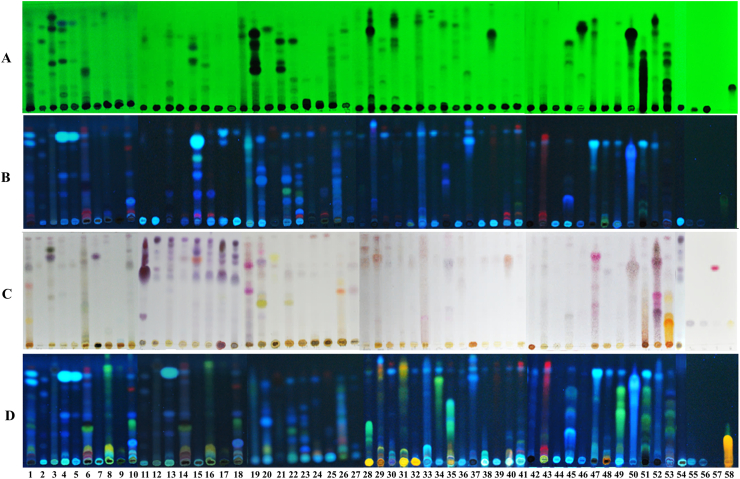
From the 54 plant material samples, 46 of them were successfully identified, and a further 5 of them could be identified at the genus level i.e., Cinnamomum (no. 36 and 41), Ophiopogon (no. 43), Pinus (no. 49) and Santalum (51), whereas no. 5, 9 and 50 were unidentified (Table S1, Fig. S2). Foeniculum vulgare Mill. (no.15) was used in double portion for substitution of Pimminella anisum L. (Thai name: Thian sattabut, Table S1).
The qualitative phytochemical analysis of the YN preparation and its components were performed using the TLC technique (Fig. 2A and B). The TLC fingerprints showed the presence of terpenes (Fig. 2C) and flavonoids (Fig. 2D), after derivatisation with anisaldehyde-sulphuric acid and with the natural product spray reagents, respectively.
Fig. 2.
The phytochemical fingerprints of the Yahom Navakot preparation and its components. TLC normal phase visualized under UV 254 nm (A), 366 nm (B), and post-derivertization with anisaldehyde-sulphuric acid (C) and natural product (D) spray reagent.
3.2. Effect of Yahom Navakot on hemodynamic parameters in HUT-induced OH rats
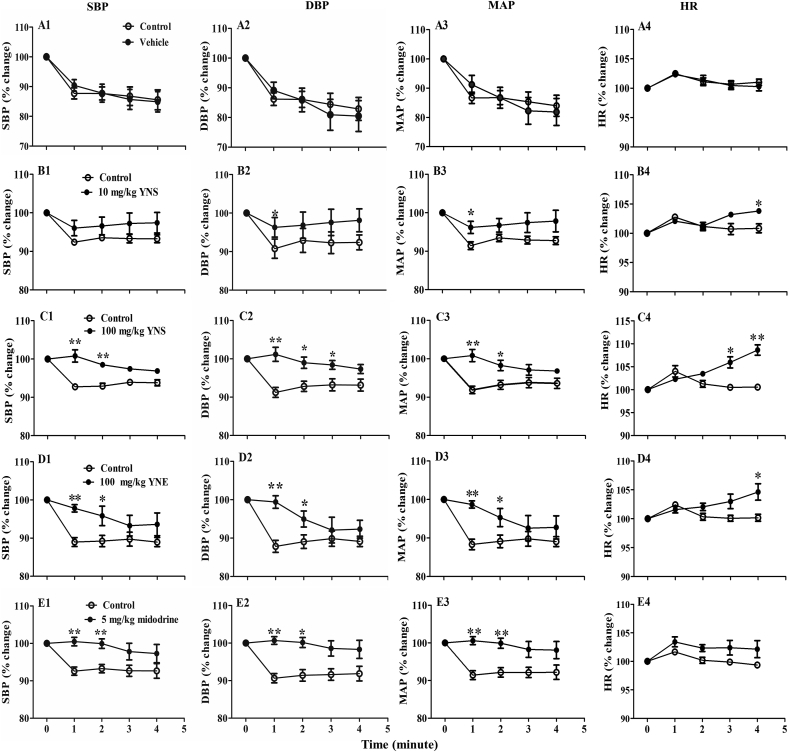
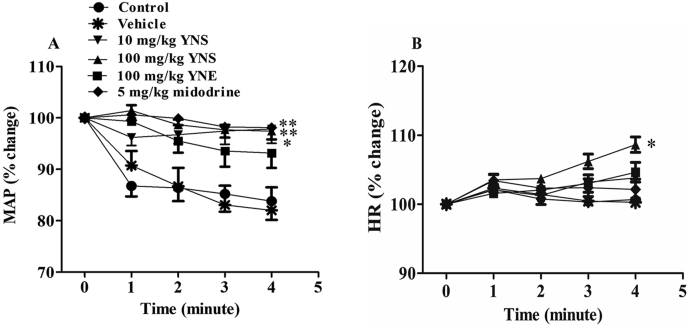
Across all groups, before administering the drugs, the values of the parameter readings (baselines of SBP, DBP, MAP, PP and HR) showed no significant difference when in the supine position (0oHUT). (Table S2). Raising the table from the supine position to a 90oHUT position without any treatment (so called control 90oHUT, Fig. 1) reduced SBP, DBP and MAP in all animal groups (P < 0.01 vs control baseline, Table S2). These BP values rapidly dropped around 10–20 mmHg from the baseline (∼10–15% reduction) within 1min after conducting 90oHUT, then they tended to remain stable throughout the period of 4min (Table S2). In contrast to BP, the HR increased from the baseline (∼2%) within 1min in the 90oHUT control for all groups (P < 0.01 vs control baseline, Table S2). Treatment with vehicle (distilled water) had no effect on the baseline values of all cardiovascular parameters, while 90oHUT in the presence of vehicle reduced SBP, DBP and MAP but increased HR (P < 0.01 vs vehicle baseline, Table S2). The percentage of reduced BP (Fig. 3A1) and increased HR (Fig. 3A4) throughout the 4min of 90oHUT after treatment with vehicle was no different from that of the control 90oHUT.
Fig. 3.
The percentage change of systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MAP) and heart rate (HR) from the baseline (0oHUT), measured at 1, 2, 3, 4min after conducting a 90oHUT in anesthetized rats orally treated with (A1-4) vehicle (distilled water), (B1-4) 10 mg/kg YN powder suspension (YNS), (C1-4) 100 mg/kg YN powder suspension (YNS), (D1-4) 100 mg/kg YN aqueous extract (YNE) and (E1-4) 5 mg/kg midodrine. A control (no vehicle/drug treatment) HUT experiment was performed before each treatment. Values are expressed as mean ± SEM, n = 6; ∗P < 0.05,∗∗P < 0.01, compared with control (paired t-test).
Oral administration of a low dose of YN powder suspension (10 mg/kg) to rats had no effect on the baseline values of SBP, DBP, PP, MAP and HR, when compared with control baseline (Table S2). Performing a 90oHUT in rats treated with the 10 mg/kg YN powder suspension slightly decreased BP from the baseline, but the effect was not significant, although HR was significantly increased (P < 0.05, 0.01 vs drug baseline, Table S2). As for BP, the control showed a greater reduction in DBP and MAP at 1min of 90oHUT, compared to the 10 mg/kg YN powder suspension treatment (P < 0.05, Fig. 3B2 and B3).
Oral administration of a higher dose of YN powder suspension (100 mg/kg) increased baselines of SBP, PP and MAP, but decreased HR, compared to the control baseline (P < 0.05, 0.01, Table S2). There was no change in all BP values compared with the drug baseline throughout the period of the 4min 90oHUT conducted after the 100 mg/kg YN powder suspension treatment (Table S2). Comparing the percentage change of BP from the baseline after 90oHUT between the control and the 100 mg/kg YN powder suspension treatment, it was found that the latter significantly ameliorated the profound drop of BP seen in the control (Fig. 3C1, C2 and C3). Interestingly, the percentage increases of HR observed during 90oHUT in both 10 and 100 mg/kg YN powder suspension treatment groups were significantly greater than in the control 90oHUT (P < 0.05, 0.01, Fig. 3B4 and C4).
In the YN aqueous extract (100 mg/kg) group, there was significant increase in the baseline values of DBP and MAP, but decreased HR, when compared with the control baseline (P < 0.01, Table S2). Performing the 90oHUT after the 100 mg/kg YN aqueous extract administration did not significantly decrease SBP, DBP, PP and MAP, but it increased HR (P < 0.05 vs drug baseline, Table S2). The percentage changes in BP from the baseline after the 90oHUT under the 100 mg/kg YN aqueous extract treatment (Fig. 3D), revealed less reduction in SBP (Fig. 3D1), DBP (Fig. 3D2) and MAP (Fig. 3D3) after 1 and 2 min of the 90oHUT compared to the control (P < 0.05, P < 0.01). Similarly, the 100 mg/kg YN aqueous extract treatment also increased HR during 90oHUT (P < 0.05 vs drug baseline, Table S2) and the percentage change from the baseline of HR was significantly greater than in the control at 4min of 90oHUT (Fig. 3D4).
Treatment with 5 mg/kg midodrine remarkably increased the baselines of SBP, DBP and MAP, but decreased HR, compared to the control baseline (P < 0.01, Table S2). Midodrine did not change the BP response during the 90oHUT for 4min, while HR showed a slight increase at 1min, when compared with the drug baseline (P < 0.05, Table S2). The percentage change from the baseline of BP during the 90oHUT (Fig. 3E), showed greater reduction of SBP (Fig. 3E1), DBP (Fig. 3E2) and MAP (Fig. 3E3) in the control than in the midodrine group (P < 0.05, 0.01), while there was no difference in the percentage change in the HR (Fig. 3E4).
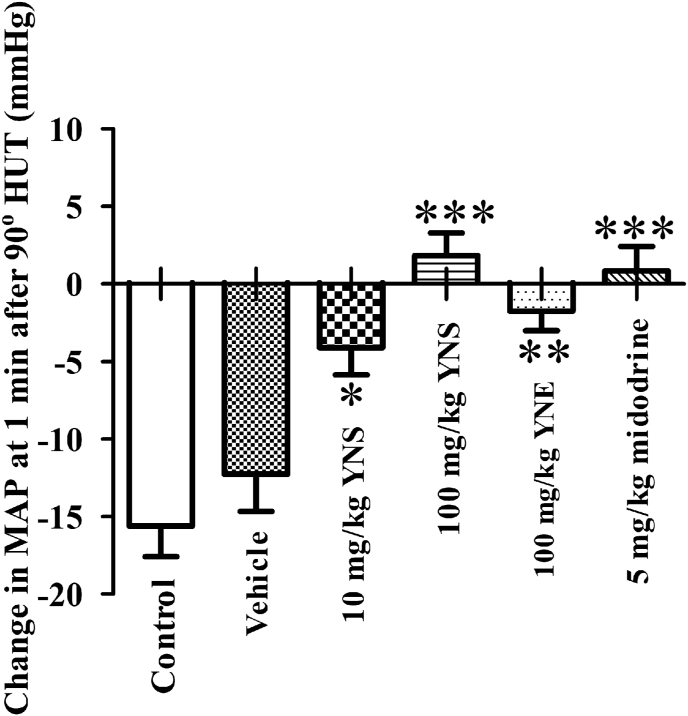
In order to assess the differences in the preventive efficacy against OH of the treatments, the percentage change from the baseline of MAP (Fig. 4A) and HR (Fig. 4B) induced by the 90°HUT was compared in different treatment groups. Clearly, treatment with either the 10 or 100 mg/kg YN powder suspension, or with 5 mg/kg midodrine, ameliorated the reduction of MAP caused by the 90oHUT (P < 0.05, P < 0.01, Fig. 4A), while the 100 mg/kg YN powder suspension significantly accentuated the increase of HR in response to the 90oHUT (P < 0.05, Fig. 4B). Since the 90oHUT induced a rapid drop in MAP at 1min, we compared the effect of different treatments on MAP at 1min after the 90oHUT (Fig. 5). Rapid BP reduction in response to a 90oHUT was significantly prevented by both the YN powder suspension and the YN aqueous extract (P < 0.05, 0.01, 0.001, Fig. 5). Apparently, the 100 mg/kg YN powder suspension was the most effective in prevention against OH (P < 0.001 vs vehicle and control) and its action was dose-dependent (Fig. 5). Midodrine also showed positive results (Fig. 5).
Fig. 4.
Comparison of %change from the baseline of (A) mean arterial blood pressure (MAP) and (B) heart rate (HR) measured at 1, 2, 3, 4min after conducting a 90oHUT in anesthetized rats orally treated with vehicle (distilled water), 10 mg/kg YN powder suspension (YNS), 100 mg/kg YN powder suspension (YNS), 100 mg/kg YN aqueous extract (YNE) and 5 mg/kg midodrine. A control (no vehicle/drug treatment) HUT experiment was performed before the treatment. Values are expressed as mean ± SEM, n = 6; ∗P < 0.05,∗∗P < 0.01 compared with vehicle and control.
Fig. 5.
Changes in mean arterial blood pressure (MAP) at 1min after conducting a 90oHUT of 5 animal groups orally treated with vehicle (distilled water), 10 mg/kg YN powder suspension (YNS), 100 mg/kg YN powder suspension (YNS), 100 mg/kg YN aqueous extract (YNE) and 5 mg/kg midodrine. A control (no vehicle/drug treatment) HUT experiment was performed before the treatment. Values are expressed as mean ± SEM, n = 6; ∗P < 0.05,∗∗P < 0.01,∗∗∗P < 0.001 compared to control.
3.3. Plasma noradrenaline level
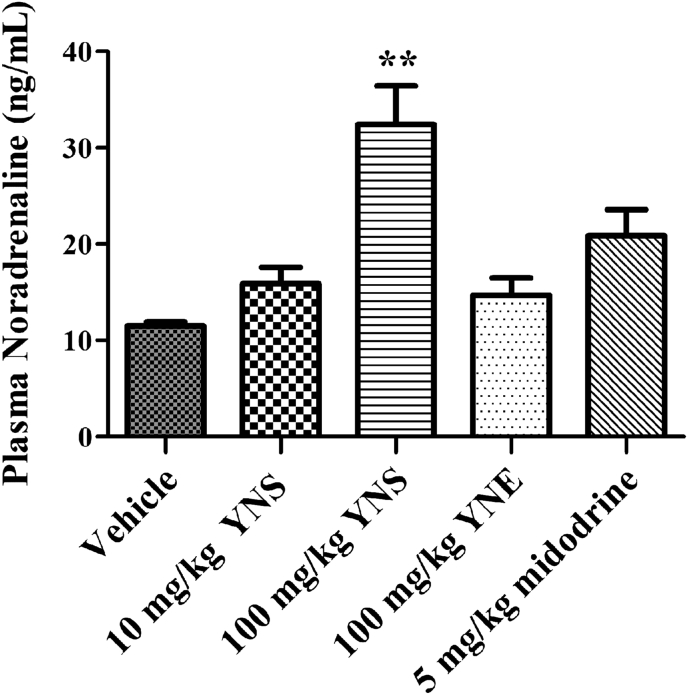
The plasma level of NA significantly increased after administration of the 100 mg/kg YN powder suspension, while it was unchanged after the administration of the 10 mg/kg of YN powder suspension, the 100 mg/kg YN aqueous extract or 5 mg/kg midodrine (Fig. 6).
Fig. 6.
Plasma level of noradrenaline from rats treated with vehicle (distilled water), 10 and 100 mg/kg YN powder suspension (YNS), 100 mg/kg YN aqueous extract (YNE) and 5 mg/kg midodrine. Values are expressed as mean ± SEM, n = 6; ∗∗P < 0.01 compared with vehicle.
3.4. Vasoconstrictor effect of YN
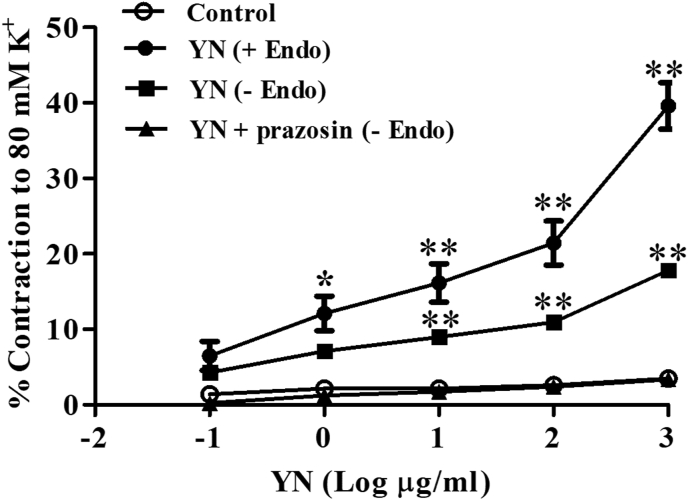
YN powder solution (1-1,000 μg/ml) induced concentration-dependent contractions of rat aorta. The response reached ∼38% of the contraction induced by the 80 mM K+ solution. The endothelial-denuded aortic rings showed less contractile response compared to the endothelial-intact rings (Fig. 7). Preincubation of 10−7M prazosin (α1-adrenergic receptor antagonist) attenuated the vasoconstrictor induced by YN solution (Fig. 7).
Fig. 7.
Concentration-response curve for Yahom Navakot (YN) induced vasoconstriction in endothelium-intact (+Endo), endothelium-denuded (-Endo) and endothelium-denuded (-Endo) pre-incubation with prazosin (10−7 M) rat isolated aorta. Values are expressed as mean ± SEM, n = 6; ∗P < 0.05, ∗∗P < 0.01 compared with control and YN plus prazosin.
4. Discussion
YN is a traditional Thai herbal recipe comprising 54 medicinal plants and borneol. Correct botanical identification, authentication and quality control are an essential measurement for ensuring the safety, efficacy and therapeutic potency of YN. The TLC chemical fingerprint of the YN preparation and each of its components was performed to serve as the reference qualitative data for further research. Three different polarity solvents were used to prepare YN preparation, i.e., hexane, ethanol, and water, to ensure that all the compounds found in YN powder were extracted. The chemical fingerprints showed the presence of terpenes and flavonoids in both the YN preparation and each ingredient. These results are in accordance with previous studies showing that terpenes and flavonoids were found in Phikud Navakot13 and constituent herbs of YN,14 which have previously been showed to possess a cardiovascular effect.25, 26, 27
The present study, conducted in aged rats, demonstrated that the YN powder suspension and the aqueous extract prevented a reduction in BP induced by the 90oHUT. The plasma NA level significantly increased in the rats treated with the YN powder suspension (100 mg/kg), suggesting that its mechanism of action partly involved NA action that caused the increase in HR, myocardial contractility and vascular tone, thus counter-acting the reduced BP and ameliorating OH induced by 90oHUT.
OH is a marked decrease in BP after the upright posture is assumed.1 It occurs in 5% of the middle-aged (45–64 years)28 and up to 20% of elderly29 probably because of a decrease in baroreceptor reflex sensitivity and other forms of autonomic impairment.30 Hence, employing aged rats in the present study to investigate the protective effect of YN against OH would provide a reliable data and can be appropriately extrapolated to human responses. In the animal model, OH can be evoked by HUT,30 a maneuver used to clarify various aspects of cardiovascular compensatory mechanisms in OH and to determine the efficacy of drugs used for preventing or treating OH.31,32 Our HUT-induced OH experiments were conducted in anesthetized rats because this could trigger the cardiovascular responses to acute stress that may influence the sympathetic reactivity involved in the reflex of cardiovascular changes.33
In control rats, BP transiently decreased during 90oHUT, while HR was slightly increased. Such cardiovascular responses were observed in previous HUT experiments on aged rats,34 stressed-induced autonomic imbalance rats35 and rats lacking the baroreceptor reflex.36 During HUT, the gravitational fluid shifts toward the lower part of body, resulting in venous blood pooling, then reduction of venous return and cardiac output (CO), leading to an immediate decrease in BP. Since the baroreceptor reflex plays an important role in BP regulation during HUT,32 if BP does not rapidly return to normal in response to HUT, it is likely that the baroreceptor reflex is not working efficiently. In the present study, the reduction in BP due to 90oHUT was gradually restored back to normal, while HR was slightly increased, thus implying an age-impaired baroreceptor reflex.37 Treatment with vehicle (distilled water) produced the same hemodynamic changes evoked by 90oHUT as observed in the control, suggesting that vehicle did not interfere with the cardiovascular compensatory responses to 90oHUT.
This study used midodrine, a prodrug routinely prescribed for OH,38 as a positive control. Its active metabolite, desglymidodrine, is a selective α1 adrenergic receptor agonist, which produces vasoconstriction and venoconstriction leading to the increases in total peripheral resistance (TPR), venous return and CO, thereby elevating BP without direct stimulating action on the heart.39,40 Our results showed that midodrine increased the baseline BP, while the baseline HR was decreased. The decrease in HR was likely due to a compensatory cardiovascular response to counteract the increased BP, via stimulating the baroreceptor reflex, resulting in the inhibition of the sympathetic activity and in the facilitation of parasympathetic output.41 This is consistent with several previous pharmacological studies showing that midodrine caused a prolonged elevation of BP and a reduction in HR after oral administration in both animal39,42,43 and clinical studies.44 With respect to its vasopressor action, midodrine therefore could effectively attenuate the decrease in BP induced by 90oHUT. Our study demonstrated no fall in BP during 90°HUT in the rats treated with midodrine, thus supporting its effectiveness in OH prevention. Similar results were previously obtained in anesthetized dogs with OH, where midodrine attenuated the decrease in BP during HUT, with increases in CO, cerebral blood flow, vertebral arterial blood flow and femoral arterial blood flow.45 Midodrine also showed a beneficial effect in neurocardiogenic syncope patients by increasing venous return, hence producing less syncope during HUT.46 Another study confirmed that midodrine significantly increased MAP and middle cerebral artery blood flow during HUT in patients with tetraplegia.47
Similar to midodrine, oral administration of either the YN powder suspension (100 mg/kg) or its aqueous extract increased baseline values of MAP, but decreased HR compared with control. These results were in accordance with previous studies in rats showing that Yahom elevated BP.4,12,48 Moreover, increases in the rat microvascular cerebral blood flow5 and local plantar blood flow49 in response to the Yahom aqueous extract were also reported. In humans, ingestion of 3 g of Yahom, increased MAP and DBP.7 Our study demonstrated that either the 100 mg/kg YN powder suspension or the YN aqueous extract attenuated the decreases in SBP, DBP and MAP during 90oHUT, indicating that YN powder suspension and YN aqueous extract counter-acted OH by stabilizing BP. The increase in HR during 90oHUT after treatment with the YN powder suspension and the aqueous extract was more pronounced than in the control and midodrine-treated rats. Thus, it is likely that the anti-hypotensive effect of YN could involve direct stimulating action on the heart, leading to the increases in HR, myocardial contractility, CO, and consequently, elevating BP. Although further experiment is required to clarify YN mechanism of action, a previous investigation showed that in isolated perfused rat heart, YN aqueous extract increased heart contraction and HR, possibly by stimulating the influx of Ca2+ through Ca2+ channels and inhibiting Na+/K+ ATPase, while ß1-adrenergic receptor stimulation may not play a part in YN action because it was not inhibited by propranolol, a ß1-adrenergic receptor antagonist.10 Vasopressor action of YN might also play role in its preventive effect against OH, as a previous report revealed that Yahom extract increased BP by stimulating the aortic ring contractions via activating α1 receptors on the vascular smooth muscle.6 Indeed, the present study also showed vasoconstrictor response to YN solution in rat aorta, which could be blocked by prazosin, an α1-adrenergic receptor antagonist, thus confirming that YN mechanism of action involved α1-adrenoceptor activation.
Since the 90oHUT induced a sudden fall in MAP within 1min, we compared the effect of different treatments on MAP at 1min after the 90oHUT. YN powder suspension showed promising efficacy in preventing OH in a dose-dependent manner and with rapid onset, implying that besides the direct effect on cardiovascular function, its underlying mechanism of action may also involve neuronal modulation such as improving baroreceptor sensitivity and enhancing sympathetic activity. Underpinning this hypothesis, our result revealed that 100 mg/kg YN powder suspension increased the NA plasma level. This implies an increase in sympathetic outflow, leading to the release of catecholamines from the adrenal medulla and the spillover of NA from postganglionic nerve endings into the blood stream.50 An equivalent dose of YN aqueous extract, however, had no effect on the NA level, suggesting that its cardiovascular effect may involve only the direct stimulating action on the heart and blood vessels. This discrepancy could be due to the loss of some volatile oils, which might be the active components, during the extraction process with boiling water. In addition, compared with YN powder suspension, the aqueous extract may contain low non-water-soluble ingredients, which may also contribute to the cardiovascular action of YN.
YN is composed of 54 herbal plants, thus the pharmacological actions of the individual plants could not be easily ascertained to explain the holistic action of YN. Nevertheless, previous studies have revealed cardiovascular actions of some components of YN. For instance, oral administration of nine herbs called “Phikud Navakot”, a major ingredient in YN including the roots of A. dahurica, A. sinensis, and A. lappa; the rhizomes of A. lancea, Ligusticum chuanxiong, and Picrorhiza kurrooa; the roots and rhizomes of Nardostachys jatamansi; the aerial parts of A. annua and the galls of T. chebula, has been shown to increase BP in rats.12 Intravenous injection of A. sinensis increased coronary, cerebral and peripheral blood flow in anesthetized dogs.51 The crude extract of T. crispa and its bioactive components exerted a positive ionotropic effect on isolated left atria of the rat via the adrenergic receptors.52 In contrast, several herbs in YN for example N. sativa,53 T. ammi,54 K. galangal,26 M. elengi27 and Cinnamomum sp.55 possessed anti-hypertensive activity. The vasorelaxant activities in isolated rat aortic rings were also reported in YN components including A. dahurica,56 N. sativa,57 Z. officinale58 and J. Sambac.59 Taken together, the efficacy of YN should be viewed as a whole, as it could result from synergism or antagonism of several ingredients.
4. Conclusions
This is the first study demonstrating that YN powder suspension and its aqueous extract can ameliorate a rapid fall in BP due to the postural change. The YN powder suspension, the native formulation commonly used in humans, was apparently effective for preventing OH in a dose-dependent manner. Thus, the study provides persuasive fundamental therapeutic in vivo data for YN, which supports its traditional use for dizziness and fainting. Nevertheless, further clinical trials designed to evaluate the efficacy and safety of YN in participants or elderly experiencing relevant symptoms or pathologies of OH are still required.
Declaration of competing interest
All authors declare that they have no conflicts of interest to disclose.
Acknowledgements
The authors would like to acknowledge Assist. Prof. Bhanubong Bongcheewin, Mrs. Pranom Dechwisissakul and Mr. Ampol Boonpleng for botanical identification. We are grateful to Mr. Roger A. Williams, a technical writer, for useful discussion and editing the manuscript. This project was financially supported by the National research council of Thailand (grant number 2562/10), the Center of Excellence for Innovation in Chemistry (PERCH-CIC) and the International Research Network (IRN61W0005).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.08.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Freeman R., Wieling W., Axelrod F.B., et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci Basic Clin. 2011;161(1-2):46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Mussi C., Ungar A., Salvioli G., et al. Orthostatic hypotension as cause of syncope in patients older than 65 years admitted to emergency departments for transient loss of consciousness. J Gerontol - Ser A Biol Sci Med Sci. 2009;64(7):801–806. doi: 10.1093/gerona/glp028. [DOI] [PubMed] [Google Scholar]
- 3.Lahrmann H., Cortelli P., Hilz M., Mathias C.J., Struhal W., Tassinari M. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006 doi: 10.1111/j.1468-1331.2006.01512.x. [DOI] [PubMed] [Google Scholar]
- 4.Suvitayavat W., Tunglert S., Thirawarapan S.S., Bunyapraphatsara N. Effects of Ya-hom on blood pressure in rats. J Ethnopharmacol. 2005;97(3):503–508. doi: 10.1016/j.jep.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Jariyapongskul A., Pathumraj S., Niimi H. Effects of Yahom on the regional cerebral blood flow in rat using fluorescence videomicroscopy. Clin Hemorheol Microcirc. 2006;34(1-2):139–144. [PubMed] [Google Scholar]
- 6.Suvitayavat W., Tunlert S., Thirawarapan S., Kitpati C., Bunyapraphatsara N. Actions of Ya-hom, a herbal drug combination, on isolated rat aortic ring and atrial contractions. Phytomedicine. 2005;12(8):561–569. doi: 10.1016/j.phymed.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Suvitayavat W., Praputtam S., Viriyarumpanon A., et al. Cardiovascular effects of Ya-hom in human. Thai J Phytopharm. 2005;12(2) [Google Scholar]
- 8.Chootip K., Chaiyakunapruk N., Soonthornchareonnon N., Scholfield C.N., Fuangchan A. Efficacy and safety of “Yahom” as a traditional Thai herbal therapy: a systematic review. J Ethnopharmacol. 2017;196(November 2016):110–123. doi: 10.1016/j.jep.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 9.National Drug System Development Committee National list of essential drug A.D.2020 (list of herbal medicine products) 2020. http://www.ratchakitcha.soc.go.th/DATA/PDF/2563/E/254/T_0003.PDF Royal Thai Government Gazett Volume 137 Speical (Chapter 254) D (Dated 29 October B.E. 2563) Accessed.
- 10.Ponsane N. Effect of the Yahom extract on cardiac function in isolated perfused rat heart. Chulalongkorn University; ฺBangkok, Thailand: 2004. pp. 59–60. Master of Science Degree Thesis. [Google Scholar]
- 11.Chavalittumrong P., Chivapat S., Attawish A., Soonthornchareonnon N. Chronic toxicity of Yahom Navagoth extract. Thai Tradit Alter Med. 2009;7(2):134–145. (in Thai) [Google Scholar]
- 12.Nusuetrong P., Gerdprasert O., Wetchasit P., Nakchat O., Sotanaphun U. Effect of short-term oral administration of Phikud Navakot in rats. J Med Assoc Thai. 2015;98(Suppl 1):S52–60. [PubMed] [Google Scholar]
- 13.Nalinratana N., Kaewprem W., Tongumpai S., Luechapudiporn R., Sotanaphun U., Meksuriyen D. Synergistic antioxidant action of Phikud Navakot ameliorates hydrogen peroxide-induced stress in human endothelial cells. Integr Med Res. 2014;3(2):74–82. doi: 10.1016/j.imr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burana-osot J., Phattanawasin P., Luangthuwapanit P., Khamwut P., Sotanaphun U. Determination of volatile constituents from crude drugs of Phikud Navakot by gas chromatography-mass spectrometry. Thai Bull Pharm Sci. 2016;11(2):45–60. doi: 10.14456/tbps.2016.11. [DOI] [Google Scholar]
- 15.Department of Medical Sciences M of PH . First edit. Thailand: Bangkok : Department of Medical Sciences Ministry of Public Health; 2019. Thai Herbal Pharmacopoeia 2019. [Google Scholar]
- 16.Soonthornchareonnon N., Ruangwises N. Bangkok. In Thai: Concept Medicus Printing. first ed. 2012. Quality Control of Thai Traditional Medicine. [Google Scholar]
- 17.Köse L.P., Gülçin I., Gören A.C., Namiesnik J., Martinez-Ayala A.L., Gorinstein S. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind Crop Prod. 2015;74:712–721. doi: 10.1016/j.indcrop.2015.05.034. [DOI] [Google Scholar]
- 18.Tohma H., Köksal E., Kılıç Ö., et al. RP-HPLC/MS/MS analysis of the phenolic compounds, antioxidant and antimicrobial activities of salvia L. Species. Antioxidants. 2016;5(4):1–15. doi: 10.3390/antiox5040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bingol Z., Kızıltaş H., Gören A.C., et al. Antidiabetic, anticholinergic and antioxidant activities of aerial parts of shaggy bindweed (Convulvulus betonicifolia Miller subsp.) – profiling of phenolic compounds by LC-HRMS. Heliyon. 2021;7(5) doi: 10.1016/j.heliyon.2021.e06986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair A., Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiyama K., Hora M., Yamagishi R., Kitazawa M. Effects of KMD-3213, a uroselective α1A-adrenoceptor antagonist, on the tilt-induced blood pressure response in normotensive rats. Jpn J Pharmacol. 2002;90(2):131–137. doi: 10.1254/jjp.90.131. [DOI] [PubMed] [Google Scholar]
- 22.Nourian Z., Mow T., Muftic D., et al. Orthostatic hypotensive effect of antipsychotic drugs in Wistar rats by in vivo and in vitro studies of α1-adrenoceptor function. Psychopharmacology (Berlin) 2008;199(1):15–27. doi: 10.1007/s00213-007-1064-9. [DOI] [PubMed] [Google Scholar]
- 23.Wisutthathum S., Kamkaew N., Inchan A., et al. Extract of Aquilaria crassna leaves and mangiferin are vasodilators while showing no cytotoxicity. J Tradit Complement Med. 2019;9(4):237–242. doi: 10.1016/j.jtcme.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paracha T.U., Pobsuk N., Salaloy N., et al. Elucidation of vasodilation response and structure activity relationships of N2,N4-disubstituted quinazoline 2,4-diamines in a rat pulmonary artery model. Molecules. 2019;24(2) doi: 10.3390/molecules24020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Praman S., Mulvany M.J., Allenbach Y., et al. Effects of an n-butanol extract from the stem of Tinospora crispa on blood pressure and heart rate in anesthetized rats. J Ethnopharmacol. 2011;133(2):675–686. doi: 10.1016/j.jep.2010.10.052. [DOI] [PubMed] [Google Scholar]
- 26.Othman R., Ibrahim H., Mohd M.A., Mustafa M.R., Awang K. Bioassay-guided isolation of a vasorelaxant active compound from Kaempferia galanga L. Phytomedicine. 2005;13:61–66. doi: 10.1016/j.phymed.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Dar A., Behbahanian S., Malik A., Jahan N. Hypotensive effect of the methanolic extract of Mimusops elengi in normotensive rats. Phytomedicine. 1999;6(5):373–378. doi: 10.1016/S0944-7113(99)80062-2. [DOI] [PubMed] [Google Scholar]
- 28.Rose K., Eigenbrodt M., Biga R., et al. Orthostatic hypotension predicts mortality in middle-aged adults:the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2006;114(7):630–636. doi: 10.1161/CIRCULATIONAHA.105.598722. [DOI] [PubMed] [Google Scholar]
- 29.Rutan G.H., Hermanson B., Bild D., Steven J., LaBaw F., Tell G.S. Orthostatic hypotension in older adults. The cardiovascular health study. CHS collaborative research group. Hypertension. 1992;19(6):508–519. doi: 10.1161/01.HYP.19.6.508. [DOI] [PubMed] [Google Scholar]
- 30.Freeman R., Abuzinadah A.R., Gibbons C., Jones P., Miglis M.G., Sinn D.I. Orthostatic hypotension: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(11):1294–1309. doi: 10.1016/j.jacc.2018.05.079. [DOI] [PubMed] [Google Scholar]
- 31.Baum T., Vander Vliet G., Glennon J.C., Novak P.J. Antihypertensive and orthostatic responses to drugs in conscious dogs. J Pharmacol Methods. 1981;6(1):21–32. doi: 10.1016/0160-5402(81)90080-2. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura H., Yamasaki M. Changes in blood pressure, blood flow towards the head and heart rate during 90 deg head-up tilting for 30 min in anaesthetized male rats. Exp Physiol. 2018;103(1):31–39. doi: 10.1113/EP086543. [DOI] [PubMed] [Google Scholar]
- 33.Dampney R.A., Coleman M.J., Fontes M.A., et al. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol. 2002;29(4):261–268. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- 34.Ramsey M.W., Behnke B.J., Prisby R.D., Delp M.D. Effects of aging on adipose resistance artery vasoconstriction: possible implications for orthostatic blood pressure regulation. J Appl Physiol. 2007;103(5):1636–1643. doi: 10.1152/japplphysiol.00637.2007. [DOI] [PubMed] [Google Scholar]
- 35.Hata T., Funakami Y., Itoh E. Effects of AF-DX116 and other muscarinic receptor antagonists on orthostatic hypotension in autonomic imbalanced (SART-stressed) rats. J Pharmacol Sci. 2005;97(3):386–392. doi: 10.1254/jphs.FP0040774. [DOI] [PubMed] [Google Scholar]
- 36.Waki H., Katahira K., Yamasaki M., Katsuda S., Shimizu T., Maeda M. Cardiovascular regulation during upright standing behavior in conscious rats. Neurosci Lett. 2009;449(1):10–14. doi: 10.1016/j.neulet.2008.10.087. [DOI] [PubMed] [Google Scholar]
- 37.Borst C., Van Brederode J.F.M., Wieling W. Mechanisms of initial blood pressure response to postural change. Clin Sci. 1984;67(3):321–327. doi: 10.1042/cs0670321. [DOI] [PubMed] [Google Scholar]
- 38.Gilden J.L. In: Primer on the Autonomic Nervous System. third ed. Robertson D., Biaggioni I., Burnstock G., Low P.A., Paton J.F.R., editors. Academic Press; San Diego: 2012. Chapter 130 - midodrine, adrenergic agonists and antagonists; pp. 621–625. third. [Google Scholar]
- 39.Pittner H. The effects of midodrine and alpha-2,5-dimethoxyphenyl-beta-aminoethanol hydrochloride on the rat uterus in situ. Arzneimittelforschung. 1987;37(7):794–796. [PubMed] [Google Scholar]
- 40.Pittner H. Effects of midodrine, ST 1059, methoxamine and glycine on spontaneously beating Guinea-pig atria. Agents Actions. 1976;6(5):584–588. doi: 10.1007/bf01971573. [DOI] [PubMed] [Google Scholar]
- 41.Crystal G.J., Heerdt P.M. Pharmacology and Physiology for Anesthesia. W.B. Saunders; Philadelphia: 2013. Cardiovascular physiology: integrative function; pp. 366–389. [Google Scholar]
- 42.Kolassa N., Schützenberger W.G., Wiener H., Krivanek P. Plasma level of the prodrug midodrine and its active metabolite in comparison with the alpha-mimetic action in dogs. Arch Int Pharmacodyn Ther. 1979;238(1):96–104. [PubMed] [Google Scholar]
- 43.Kurihara J., Takata Y., Suzuki S., Okubo Y., Kato H. Effect of midodrine on chlorpromazine-induced orthostatic hypotension in rabbits: comparison with amezinium, etilefrine and droxidopa. Biol Pharm Bull. 2001;23(12):1445–1449. doi: 10.1248/bpb.23.1445. [DOI] [PubMed] [Google Scholar]
- 44.Grobecker H.F., Kees F. Pharmacokinetic parameters and haemodynamic actions of midodrine in young volunteers. Int Angiol. 1993;12(2):119–124. [PubMed] [Google Scholar]
- 45.Tsuchida K., Yamazaki R., Kaneko K., Aihara H. Effects of midodrine on experimentally-induced postural hypotension in dogs. Arzneimittelforschung. 1986;36(12):1748–1751. [PubMed] [Google Scholar]
- 46.Ward C.R., Gray J.C., Gilroy J.J., Kenny R.A. Midodrine: a role in the management of neurocardiogenic syncope. Heart. 1998;79(1):45–49. doi: 10.1136/hrt.79.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wecht J.M., Rosado-Rivera D., Handrakis J.P., Radulovic M., Bauman W.A. Effects of midodrine hydrochloride on blood pressure and cerebral blood flow during orthostasis in persons with chronic tetraplegia. Arch Phys Med Rehabil. 2010;91(9):1429–1435. doi: 10.1016/j.apmr.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Matangkasombat O. 1974. Interference of Thai Folk Medicine (Ya-Hom) on the Blood Pressure and Cardiac Function in Man and Experimental Animal. [Google Scholar]
- 49.Nernpermpisooth N., Thirawarapan S., Suvitayavat W., Kitpati W., Bunyapraphatsara N. Effects of Ya-hom on cardiovascular functions after long-term oral administration in rats. Pharm Sci Asia. 2015;42(2):55–63. doi: 10.14456/mujps.2015.8. [DOI] [Google Scholar]
- 50.Esler M., Jackman G., Bobik A., et al. Norepinephrine kinetics in essential hypertension. Defective neuronal uptake of norepinephrine in some patients. Hypertension. 1981;3(2):149–156. doi: 10.1161/01.HYP.3.2.149. [DOI] [PubMed] [Google Scholar]
- 51.Chou Y., Huang L., Chen Y., Fan L., Chang L., Tseng K. [The effect of Angelica sinensis on hemodynamics and myocardiac oxygen consumption in dogs (author's transl)] Yao Xue Xue Bao. 1979;14(3):156–160. [PubMed] [Google Scholar]
- 52.Praman S., Mulvany M.J., Williams D.E., Andersen R.J., Jansakul C. Crude extract and purified components isolated from the stems of Tinospora crispa exhibit positive inotropic effects on the isolated left atrium of rats. J Ethnopharmacol. 2013;149(1):123–132. doi: 10.1016/j.jep.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Hebi M., Zeggwagh N., Hajj L., Bouhali B.E., Eddouks M. Cardiovascular effect of Nigella sativa L. Aqueous extract in normal rats. Cardiovasc Haematol Disord - Drug Targets. 2016;16(1):47–55. doi: 10.2174/1871529x16666160729115249. [DOI] [PubMed] [Google Scholar]
- 54.Aftab K., Atta Ur R., Usmanghani K. Blood pressure lowering action of active principle from Trachyspermum ammi (L.) sprague. Phytomedicine. 1995;2:35–40. doi: 10.1016/s0944-7113(11)80046-2. [DOI] [PubMed] [Google Scholar]
- 55.Shen Y., Jia L.N., Honma N., Hosono T., Ariga T., Seki T. Beneficial effects of cinnamon on the metabolic syndrome, inflammation, and pain, and mechanisms underlying these effects-a review. J Tradit Complement Med. 2012;2(1):27–32. doi: 10.1016/S2225-4110(16)30067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plengsuriyakarn T., Viyanant V., Eursitthichai V., et al. Anticancer activities against cholangiocarcinoma, toxicity and pharmacological activities of Thai medicinal plants in animal models. BMC Compl Alternative Med. 2012;12:23. doi: 10.1186/1472-6882-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cherkaoui-Tangi K., Israili Z.H., Lyoussi B. Vasorelaxant effect of essential oil isolated from Nigella sativa L. seeds in rat aorta: proposed mechanism. Pak J Pharm Sci. 2016;29(1):1–8. [PubMed] [Google Scholar]
- 58.Ghayur M.N., Gilani A.H. Ginger lowers blood pressure through blockade of voltage-dependent calcium channels. J Cardiovasc Pharmacol. 2005;45(1):74–80. doi: 10.1097/00005344-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 59.Kunhachan P., Banchonglikitkul C., Kajsongkram T., Khayungarnnawee A., Leelamanit W. Chemical composition, toxicity and vasodilatation effect of the flowers extract of Jasminum sambac (L.) Ait. “g. Duke of Tuscany. Evid base Compl Alternative Med. 2012;2012 doi: 10.1155/2012/471312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.