Abstract
Objective
Literature examining the relationship between elevated blood pressure and osteoarthritis (OA) has yielded conflicting results. This study aimed to systematically review the relationship between hypertension and OA in both load-bearing and non-load-bearing joints.
Methods
A systematic literature search was performed on Embase, Emcare, MEDLINE and Ovid Nursing Database. The associations between hypertension and OA development in knees, hips and hands were analysed by calculating the odds ratio (OR).
Results
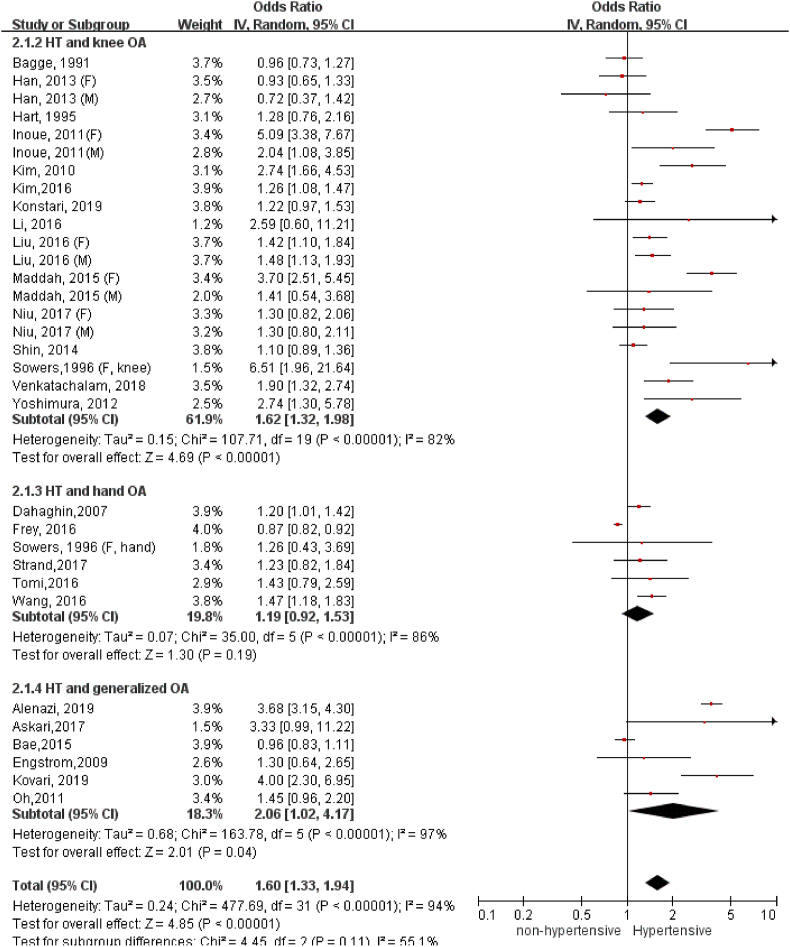
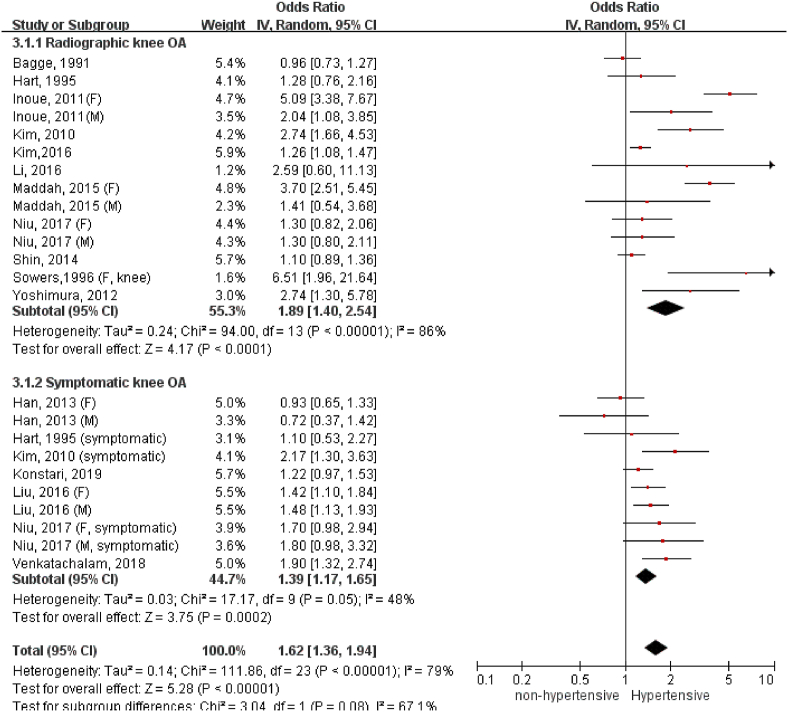
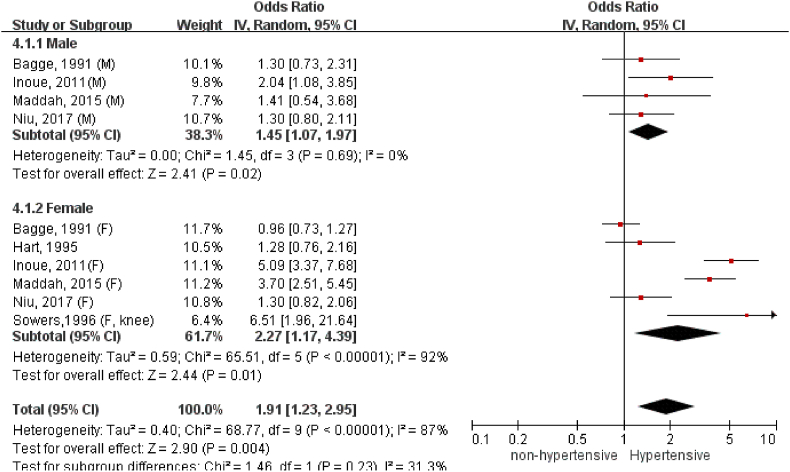
A total of 26 studies with 97,960 participants were included. The overall odds of having OA significantly increased in the people with hypertension compared to the normotensive ones (OR = 1.60, 95%CI = 1.33, 1.94). The association of hypertension with OA was detected in knee (OR = 1.62, 95%CI = 1.32, 1.98), not in hand (OR = 1.19, 95%CI = 0.92, 1.53). Moreover, there existed a stronger association of hypertension with radiographic knee OA (OR = 1.89, 95%CI = 1.40, 2.54) than symptomatic knee OA (OR = 1.39, 95%CI = 1.17, 1.65). The association between hypertension and radiographic knee OA remained statistically significant for the studies that adjusted for body mass index (BMI) (OR = 1.42, 95%CI = 1.13, 1.78), and was particularly strong in women (OR = 2.27, 95%CI = 1.17, 4.39).
Conclusion
A BMI-independent association between hypertension and radiographic knee OA existed with potential sex variation, which warrants further investigations into the underlying genetic, hormonal and environmental factors.
The translational potential of this article: Blood pressure has been reported to link with OA for years ago, however, its contribution to OA is still unclear and conflicted in different reports. This review indicated an intimate relationship between hypertension and structural damages of knee OA, rather than simply chronic joint pain, especially in women. This finding not only provides stronger support for further investigations into the causal risk factor, i.e. hypertension, of OA from tissue level to molecular level, but also putting forward a novel thinking in OA pathogenesis and its therapy strategies.
Orthopedic translation
This study further strengthen the association between hypertension and radiographic knee OA. It points in a vascular aetiology hypothesis of OA. It might open up a new avenue for repositioning anti-hypertensive medications for osteoarthritis treatment.
Keywords: Hypertension, Metabolic syndrome, Osteoarthritis
1. Introduction
Osteoarthritis (OA) is a prevalent and costly debilitating condition that affects 240 million people worldwide. OA is a whole-joint disorder, not only involving articular cartilage degradation but also subchondral bone disturbance, synovitis, etc. OA afflicts both load-bearing (knee and hip) and non-load-bearing joints (hand). It is a leading cause of chronic pain, functional disability and poor quality of life in older adults. Currently, there is no cure for OA. The patients with OA have to wait for surgical replacement of damaged joint at the later stage of disease. Moreover, the patients with OA are at a higher risk of cardiovascular events and all-cause mortality than those without OA [[1], [2], [3]].
Elevated blood pressure, which is the frequently encountered comorbidity in patients with OA, is a key modifiable risk factor in cardiovascular events [4]. There has been a growing interest in the relationship between hypertension and OA. Yet conflicting data have been reported [[5], [6], [7]]. High systolic blood pressure (SBP) was associated with incident radiographic knee OA from the data of Osteoarthritis Initiative [6]. However, such association diminished after adjustment for body mass index (BMI) [5]. Recently, a novel causal association between low SBP and clinical diagnosis of OA was demonstrated via Mendelian Randomization in UK Biobank [7]. In short, the relationship between blood pressure and OA remains controversial.
Here, we conducted a systematic review and meta-analysis to further explore the relationship between hypertension and OA in both load-bearing (knee) and non-load-bearing joints (hand). Moreover, we examined the associations of hypertension with radiographic and symptomatic OA, respectively. Lastly, we examined how their relationship may differ by the potential confounding factors such as sex and BMI adjustment.
2. Materials and methods
2.1. Search strategy
Systematic literature search and meta-analyses were done according to the PRISMA (Preferred Reporting Items Systematic Reviews and Meta-Analysis) guidelines [8]. Four bibliographic databases were used, namely Embase, Emcare, MEDLINE and Ovid Nursing Database from inception to December 2020 using the key words as follows, “osteoarthritis”, “metabolic”, “hypertension” and “blood pressure”. The literature search was then extended with a wide manual search and reviewing of the reference lists of selected publications and relevant articles suggested by search engines. Only studies published in English were considered. The search strategy has been registered in PROSPERO (CRD42021227828).
2.2. Selection criteria
Two independent investigators conducted the initial screening on all related literatures. Studies that met the following criteria were included in this systematic review: 1) human studies and 2) observational studies including cohort, case–control and cross-sectional study design. If no agreement for the study selection was reached, the third investigator decided if an inclusion was appropriate.
2.3. Data extraction and quality assessment
The following data was extracted by two investigators from the selected studies: name of first author, year, country, study setting, sample size, age, % of female, study design, staging of OA, localizations of OA, definition of hypertension, odds ratios (ORs) and 95% confidence intervals (CIs). Hypertension was the exposure factor, and the occurrence of OA was the outcome. Prevalence of OA among hypertensive and non-hypertensive population, and prevalence of HT among OA and non-OA population were also extracted from included studies. Case-control studies were not included for prevalence calculation due to a lower representativeness to the whole population when compared with cross-sectional and cohort studies.
2.4. Exposure and outcomes
The exposures and outcomes of the present review included hypertension and OA. We included studies that defined hypertension as an exposure and provided specific diagnostic criteria of essential hypertension, either using pre-defined cut-off values of SBP/DBP or the use of anti-hypertension medications in medical record [9]. With OA as the outcome, we stratified it into radiographic OA, which is defined as Kellgren–Lawrence (K-L) grade ≥2, and/or symptomatic OA with persistent chronic joint pain for 3 months. Studies that were focusing on the severe OA cases with K-L grade 4 or arthroplasty were recorded as radiographic OA.
2.5. Statistical analysis
In this meta-analysis, 95% confidence intervals (CIs) were considered as the effect size for all studies. The heterogeneity among the studies was estimated by Cochran Q test and I2 statistics, with an I2 between 50% and 90% possibly representing substantial heterogeneity [10]. Due to the inter-study variations, we built random effects models using the inversed variance approach to pool the estimates from individual studies, and then we summarized the results by forest plots. Odds ratios were calculated as the effect estimate to express the association between hypertension and different sites of OA. Subgroup analysis was conducted to demonstrate how the association between hypertension and OA differ by the anatomic sites (knee, hand or generalized [OA affecting multiple joints]) [11], types (symptomatic or radiographic), sex (male or female) and adjustment for BMI in regression analyses (yes or no). Statistical significance was indicated when p values < 0.05. To detect potential publication bias, Egger's test was conducted for outcome(s) that included at least 10 studies [12]. As recommended by Cochrane Handbook, when there are fewer studies the power of the tests is too low to distinguish chance from real bias [10]. If significant publication bias was detected, a trim-a-fill technique was performed to examine whether studies should be imputed to make the results more symmetrical, i.e., less publication bias [13]. Sensitivity analyses were performed by omitting one study at a time and then calculating a pooled estimate for the remaining studies. We aimed to evaluate whether the results were affected markedly by a single study. Most analyses were performed using Review Manager 5.3, while publication bias was examined by STATA (Stata Statistical Software: Release 16. College Station, TX, USA).
3. Result
3.1. Study characteristics
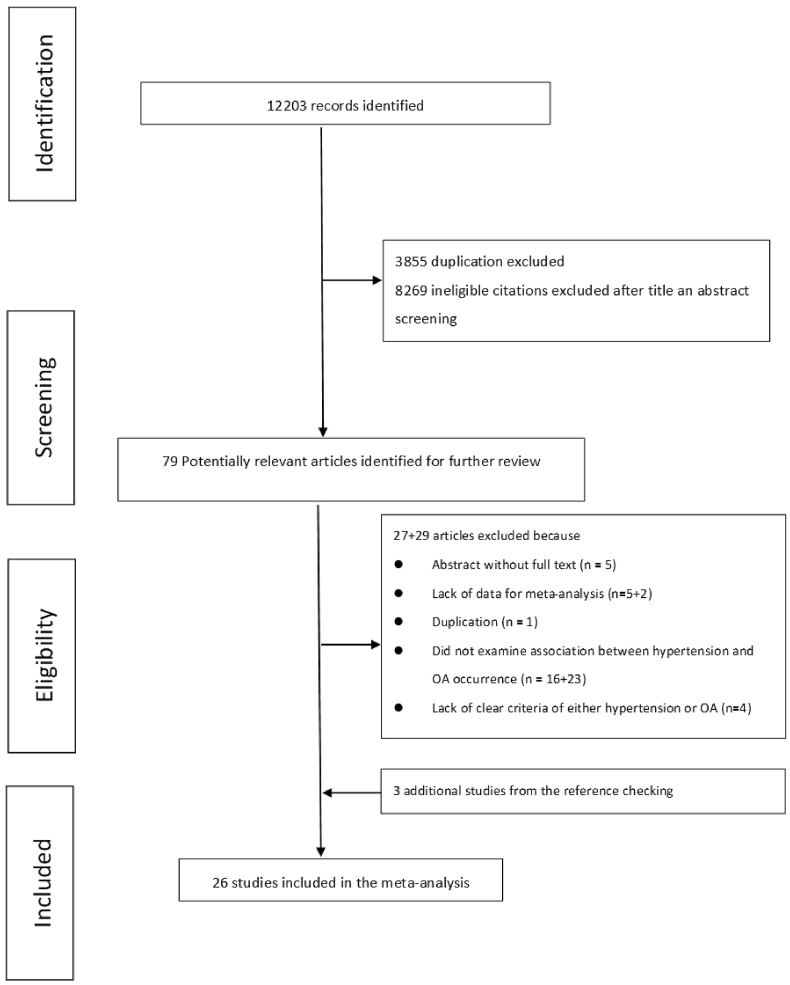
A total of 12,203 studies were included in the primary electronic screening (Fig. 1). After screening the titles, abstracts and full-texts, 26 studies with 97,960 participants were included in this review (Table 1). There were 15 cross-sectional studies [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]], 5 case–control studies [[29], [30], [31], [32], [33]] and 6 cohort studies [5,[34], [35], [36], [37], [38]]. The percentage of female participants in each study ranged from 12.0% to 100%.
Figure 1.
Flow chart of the searching and screening process.
Table 1.
Characteristics of 26 included studies.
| Author, year | Country | Study design | No. of subjects | Range of/average age | Gender (female %) | BMI as confounder | OA definition | HTN definition |
|---|---|---|---|---|---|---|---|---|
| Knee OA | ||||||||
| Konstari, 2019 | Finland | Cohort | 6274 | ≥30 | 50.1% | Yes | Symptomatic | SBP ≥130 mmHg or DBP ≥85 mmHg or antihypertensive medication |
| Venkatachalam, 2018 | India | CS | 1986 | ≥18 | 63.5% | No | Symptomatic | SBP >140 mmHg and/or DBP >90 mmHg |
| Niu, 2017 | USA | Cohort | 991 | 54.2 | 55.1% | Yes | Radiographic/symptomatic | ≥130/85 mmHg or use of antihypertensive medications |
| Kim, 2016 | Korea | CS | 9514 | ≥50 | 57.3% | Yes | Radiographic | Prehypertension: 120–139/80-89 mmHg Hypertension: ≥140/90 mmHg or use of antihypertensive medications |
| Li, 2016 | China | CC | 151 | 50–80 | 81.5% | No | Radiographic | Prehypertension: SBP 120–139 or DBP 80-89 Stage 1 hypertension: SBP 140–159 or DBP 90-99 Stage 2 hypertension: SBP ≥160 or DBP ≥100 |
| Liu, 2016 | China | CS | 3428 | ≥40 | 51.5% | Yes | Symptomatic | ≥140/90 mmHg or use of antihypertensive medications |
| Maddah, 2015 | Iran | CS | 625 | 54.64 | 80.0% | No | Radiographic | ≥130/85 mmHg or use of antihypertensive medications |
| Shin, 2014 | Korea | CS | 2363 | ≥50 | 57.4% | Yes | Radiographic | ≥130/85 mmHg or use of antihypertensive medications |
| Han, 2013 | Korea | CC | 2234 | 40–91 | 54.3% | No | Symptomatic | ≥130/85 mmHg or use of antihypertensive medications |
| Yoshimura, 2012 | Japan | Cohort | 1384 | 63.9 | 66.3% | Yes | Radiographic | ≥130/85 mmHg |
| Inoue, 2011 | Japan | CS | 795 | 30–86 | 62.9% | No | Radiographic | ≥130/85 mmHg |
| Kim, 2010 | Korea | CS | 504 | ≥50 | 54.4% | Yes | Radiographic/symptomatic | ≥140/90 mmHg |
| Hart, 1995 | UK | CS | 979 | 45–64 | 100% | Yes | Radiographic/symptomatic | use of antihypertensive medications |
| Bagge, 1991 | Sweden | CS | 340 | ≥79 | 60.3% | Yes | Radiographic | DBP ≥110 mmHg or use of antihypertensive medications |
| Hand OA | ||||||||
| Strand, 2017 | USA | Cohort | 1089 | 59.2 | 51.8% | Yes | Radiographic | ≥130/85 mmHg or on medication |
| Frey, 2016 | USA | CC | 27000 | 30–90 | 74.2% | No | Medical record | Medical record |
| Wang, 2016 | China | CS | 6218 | ≥40 | 53.1% | No | Radiographic/symptomatic | Clinical diagnosis or use of antihypertensive medications |
| Tomi, 2016 | France | CS | 301 | 53.4 | 12.0% | No | Radiographic | ≥140/90 mmHg |
| Dahaghin, 2007 | Netherland | CS | 3585 | ≥55 | 58.2% | Yes | Radiographic | ≥160/100 mmHg or use of antihypertensive medications |
| Generalized OA | ||||||||
| Alenazi, 2019 (unspecified) | Unspecified | CS | 3855 | 66.43 | 60.9 | Yes | Symptomatic | Medical record |
| Kovari, 2019 (hand and knee) | Hungarian | CC | 400 | 65.47 | 100% | No | Symptomatic | Medical record |
| Askari, 2017 (knee and/or hip) | Iran | CC | 393 | 54.6 | 81.9% | Yes | Radiographic | ≥130/85 mmHg |
| Oh, 2011 (shoulder OA) | Korea | Cohort | 679 | 71.8 | 58.3% | No | Radiographic | Medical record |
| Engstrom, 2009 (knee and/or hip) | Sweden | Cohort | 5171 | 57.5 | 58.5% | Yes | Radiographic | ≥130/85 mmHg or use of antihypertensive medications |
| Sowers, 1996 (knee and/or hand) | USA | CS | 573 | 24–45 | 100% | Yes | Radiographic | SBP ≥ 124 mmHg or self-reported |
| Bae, 2015 (unspecified) | Korea | CS | 17128 | ≥20 | 57.6% | Yes | Symptomatic | Prehypertension: 120–139/80-89 mmHg Hypertension: ≥140/90 mmHg or use of antihypertensive medications |
Abbreviations: HTN: hypertension; OA: osteoarthritis; BMI: body mass index; CS: cross-sectional study; CC: case–control study; DBP: diastolic blood pressure; SBP: systolic blood pressure.
3.2. Prevalence of OA among hypertensive and non-hypertensive population
After excluding 5 studies in case–control nature and 9 studies lacking any relevant data for prevalence calculation, there were 12 studies with 52,830 participants for the subsequent analysis on the prevalence of OA among the hypertensive and normotensive population, and the prevalence of HT among the OA and non-OA population. The prevalence of OA in knee and hand was as high as 26.3% (7 studies) and 15.7% (2 studies) among hypertensive patients in contrast to the normotensive ones (knee: 25.1%; hand: 8.6%). The prevalence of generalized OA was also higher among the people with hypertension (3 studies, 38.2% versus 13.4%).
3.3. Prevalence of hypertension among population with or without OA
On the other hand, the prevalence of hypertension was also higher in the people with knee OA than those without knee OA (7 studies, 42.6 versus 20.1%). Similar differences were also found that hand OA (2 studies, 26.2% versus 7.4%) and generalized OA (3 studies, 37.9% versus 13.5%).
3.4. A significant association between hypertension and knee OA
The OR of OA development in the stage of established hypertension (Fig. 2) was as high as 1.60 (95%C.I. = 1.33, 1.94). When stratifying the results by the different anatomic sites of OA, the association of hypertension with knee OA (OR = 1.62, 95%C.I. = 1.32, 1.98) was apparently higher compared to either hand OA (OR = 1.19, 95% C.I. = 0.92, 1.53). The association of hypertension with generalized OA was marginally significant (OR: 2.06, 95%C.I. = 1.02, 4.17). Egger's test was performed to detect publication bias of the association between hypertension and knee OA because it included >10 studies. Although the test for publication bias has been significant (Z = 2.09, p = 0.0367), trim-and-fill analysis showed that no study needs to be imputed to make the results more symmetrical.
Figure 2.
Association of hypertension with OA in varied anatomic locations.
3.5. A closer association between hypertension and radiographic knee OA
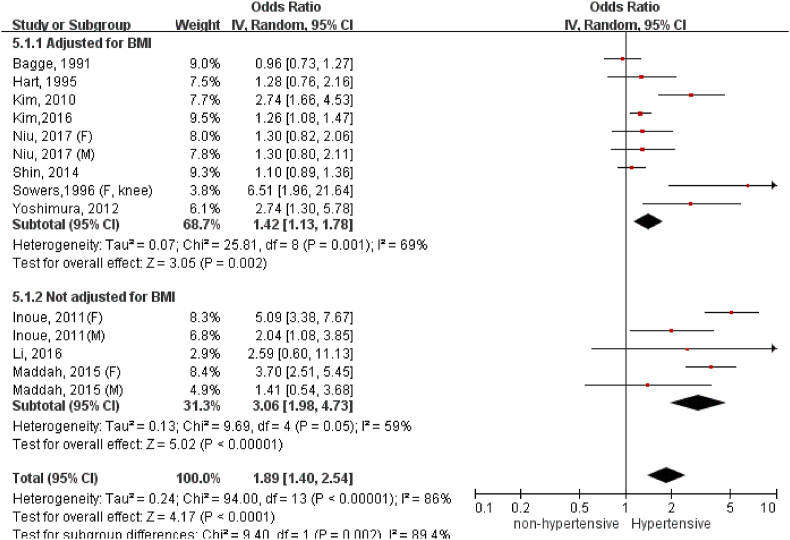
We have conducted subgroup analysis to compare association between hypertension and radiographic or symptomatic knee OA (Fig. 3). Hypertension appeared to have stronger association with radiographic (OR: 1.89, 95%C.I. = 1.40, 2.54) than symptomatic knee OA (OR: 1.39, 95%C.I. = 1.17, 1.65), but the subgroup difference was statistically insignificant (p = 0.08).
Figure 3.
Association of hypertension with radiographic and symptomatic knee OA.
We have conducted more subgroup analyses according to sex and BMI adjustment. We only included analyses on hypertension and radiographic knee OA since it contained the largest number of studies for this comparison. Four studies have provided male-specific association and six studies reported female-specific association for meta-analysis (Fig. 4). The association appeared to be stronger among females (OR: 2.27, 95%C.I. = 1.17, 4.39) than males (OR: 1.45, 95%C.I. = 1.07, 1.97) despite no statistical significance in subgroup difference (p = 0.23). As shown in Fig. 5, the link between hypertension and radiographic knee OA became weaker yet remained statistically significant for studies with BMI adjustment in regression analysis (OR: 1.42, 95%C.I. = 1.13, 1.78). Of note, without adjusting BMI as the confounding factor, dramatically magnified the odds of hypertension-associated OA (OR: 3.06, 95%C.I. = 1.98, 4.73).
Figure 4.
Association between hypertension and radiographic knee OA in different gender.
Figure 5.
Association between hypertension and radiographic knee OA after BMI adjustment.
3.6. Heterogeneity and sensitivity analysis
The inter-study heterogeneity in most analyses was high (I2>50%). Two exceptions were the sub-group analysis on the relationship between hypertension and symptomatic knee OA (I2 = 48%, 7 studies), and the relationship between hypertension and radiographic knee OA in male subjects (I2 = 0%, 4 studies). When excluding one study at a time, only excluding Frey et al.‘s study would substantially change the heterogeneity among the studies on hypertension and hand OA (I2 = 0%), and the magnitude of their association (OR:1.29, 95% C.I. = 1.14, 1.46) [32].
4. Discussion
This meta-analysis has advanced current understanding of the relationship between hypertension and OA. To the best of our knowledge, this study revealed the associations of the anatomic location and type of OA with its vascular comorbidity, i.e. hypertension. First, hypertension conferred 62% increased odds for knee OA yet only 19% increment for hand OA. A significant increase of hypertension-associated OA in the load-bearing joint (knee) instead of in the non-load-bearing joint (hand) suggested the interplay between local mechanics and systemic vascular pathology in the pathogenesis of OA. Second, hypertension contributed 89% increased odds for radiographic knee OA but only 39% increase for symptomatic knee OA. Our data indicated an intimate relationship between hypertension and structural damages of knee OA, rather than chronic joint pain only. Third, we attempted to address the confounding factors in observational studies associations between hypertension and OA such as BMI, which have not been examined in previous meta-analyses [39]. After BMI adjustment, the strength of association between hypertension and radiographic knee OA dropped from 3.06 to 1.42 but remained statistically significant. In addition to BMI, we demonstrated how sex might confound the relationship between high blood pressure and radiographic knee OA. Hypertension-associated radiographic knee OA was more likely to occur in women (127% increased odds in women Vs. 45% in men).
The aetiology of knee OA is multifactorial. However, the common risk factors such as ageing and obesity cannot fully explain the doubled prevalence of knee OA since the mid-19th century [40]. A growing body of epidemiological evidence from observational studies showed a close association between metabolic syndrome (MetS) and OA [41]. MetS is a cluster of at least three out of five of following conditions: central obesity, hypertension, elevated blood glucose, high cholesterol and low high-density lipoprotein levels. Given the fact of hypertension as a key component of MetS, we modified our searching strategy by adding “metabolic” into key words with more original research articles being included. Compared with the previous meta-analysis [39], we identified additional studies that enabled us to detect a closer association of high blood pressure with radiographic knee OA than symptomatic knee OA [25,27,31,38]. In addition, one implication from our study is to highlight knee OA as one of key common comorbidities of hypertension. According to the 2020 International Society of Hypertension Global Hypertension Practice Guidelines [42], the common comorbidities and complications for hypertension are cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, acquired immunodeficiency syndrome, diabetes, lipid disorders, metabolic syndrome, inflammatory rheumatic diseases, and psychiatric diseases. Despite numerous studies that demonstrated significant associations between hypertension and knee OA, knee OA has not been considered as a comorbidity for hypertension management until now.
We are cautious that there remain a few limitations in our meta-analysis, which are the confounding effects and inverse causation. First, this meta-analysis has not exhaustively addressed all the confounders in the association between hypertension and OA although we have stratified the major ones such as sex and BMI adjustment. The other key confounders such as smoking, trauma, menopausal status, and the history of medications such as anti-hypertensive drugs, steroid or non-steroidal anti-inflammatory drugs were not reported as common in these observational studies, among which the use of three or more anti-hypertensive medications was associated with a lower risk of radiographic knee OA [6]. More importantly, this meta-analysis has not confirmed the causal relationship between hypertension and knee OA because most included studies were cross-sectional in nature. It warrants more prospective cohort investigations. In addition, we have detected publication bias for the association between hypertension and knee OA. However, trim-and-fill analysis showed that no study was in need to make the results more symmetrical, therefore the issue of publication bias does not appear to affect the validity of findings substantially.
Recent developments in analytic techniques such as Mendelian randomization (MR) have provided a powerful control for confounding and inverse causation [43]. It uses genetic variants as instrumental variables to infer whether a risk factor causally affects a health outcome. By deploying this big data analytics tool, Funck-Brentano and colleagues reported an inverse causal association of genetically-determined blood pressure with the risk of clinical diagnosis of (symptomatic) knee/hip OA and surgical replacements in UK Biobank [7]. Data from MR study as well as our meta-analysis does suggest a vascular aetiology of OA. However, the observation via MR points in an opposite direction to our meta-analysis that elevated blood pressure is highly likely a consequence, rather a cause of symptomatic knee OA. As reported in our meta-analysis, there did exist a potential ethnical variation in the associations between hypertension and knee OA. The observation on a race-dependent association of angiotensin converting enzyme gene polymorphism with knee OA as well as hypertension might help explain this phenomenon [[44], [45], [46], [47]]. In light of the discrepancy between Funck et al.‘s and our studies [7], the significance of this meta-analysis is to raise a meaningful argument. It prompts the need to investigate the interrelationship between hypertension and chronic pain sensation in knee OA in different races.
Blood flow has been recently shown to control bone vascular function and osteogenesis [48]. Bone perfusion abnormalities, characterized by a reduction in both arterial inflow and venous outflow, have been well documented in human knee OA [49]. Reduced blood flow can lead to subchondral bone ischemia and apoptosis of osteocytes, which would initiate osteoclasts-mediated bone resorption [50]. Without proper treatment, it would further develop into cystic lesions, which compromise the mechanical support for overlying cartilage, interfere gas and metabolites exchange in the bone-cartilage functional unit, and ultimately contribute to the initiation and development of OA [51]. As documented in a swine model, systemic blood pressure positively correlates with intra-osseous pressure in the long bone [52]. Under the situation of high blood pressure, high intra-osseous pressure would lead to both arterial and venous occlusion. In view of this, both low and high blood pressure can cause a reduction of bone blood flow in OA. Therefore, our observation in this meta-analysis remained valid that hypertension is detrimental instead of protective in the development of OA. Furthermore, altered bone blood pressure in response to systemic blood pressure might not necessarily associate with knee pain [53,54]. It might partially explain our observation on the closer association of hypertension with radiographic knee OA than symptomatic knee OA.
The causal association between blood pressure and OA makes a strong case to revisit the role of the vascular system in joint disease. Whereas the concept of OA as a whole joint disorder has gained much popularity in the last decade, the role of the vascular system and particularly angiogenesis has not been explored in depth. Importantly, experimental studies in postnatal long bones have conclusively demonstrated that a drug-induced reduction of blood flow leads to loss of mineralized bone and treatment with bisphosphonates enhanced blood flow and vessel growth in bone [48]. However, most findings have been obtained in metaphyseal and diaphysial bone under non-inflammatory and non-degenerative conditions. Furthermore, three-dimensional visualization and analysis of the vascular system in subchondral bone of mice and humans has until recently remained technically challenging. Microangiography of osteoarthritic subchondral bone tissue in mice revealed an increment in vascular volume and number of blood vessels in established disease [51]. Optical clearing of bone tissues enabled advanced light and fluorescent microscopy of murine and human bone tissues and led to the identification of a previously unknown blood vessel type in cortical bone [55]. Further exploiting these techniques in human clinical specimens and experimental OA models is warranted to unravel insight into the vascular mechanisms underpinning development or progression of disease. Furthermore, through identifying the common pathways in managing blood pressure and bone properties, researchers may identify antihypertensive medications that may prevent the onset of OA, or vice versa.
To conclude, this meta-analysis shows a BMI-independent association between hypertension and radiographic knee OA with potential sex variations. This finding warrants further investigations into the underlying genetic, hormonal and environmental mechanism through large-scale prospective cohorts and laboratory studies.
Authors’ contributions
Kenneth Lo: Study design, analysis and interpretation of the results, critical revision of the manuscript. Manting Au: Study design, data acquisition and interpretation, drafting of the manuscript. Junguo Ni: Data interpretation, critical revision of the manuscript. Chunyi Wen: Study design, critical revision of the manuscript, project funding. All authors have read and approved the final submitted manuscript.
Funding
This work received the support from Research Grants Council of Hong Kong Early Career Scheme (PolyU 251008/18M), PROCORE-France/Hong Kong Joint Research Scheme (F-PolyU504/18), Health and Medical Research Fund Scheme (01150087, 15161391, 16172691), the project of strategic importance from Hong Kong Polytechnic University.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgement
The authors would like to acknowledge the contribution of Aoyang Pu, Adrian Weber, Jeroen GEURTS in literature search and scientific discussion to this manuscript.
References
- 1.Kendzerska T., Juni P., King L.K., Croxford R., Stanaitis I., Hawker G.A. The longitudinal relationship between hand, hip and knee osteoarthritis and cardiovascular events: a population-based cohort study. Osteoarthritis Cartilage. 2017;25(11):1771–1780. doi: 10.1016/j.joca.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Haugen I.K., Ramachandran V.S., Misra D., Neogi T., Niu J., Yang T., et al. Hand osteoarthritis in relation to mortality and incidence of cardiovascular disease: data from the Framingham heart study. Ann Rheum Dis. 2015;74(1):74–81. doi: 10.1136/annrheumdis-2013-203789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nuesch E., Dieppe P., Reichenbach S., Williams S., Iff S., Juni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ (Clinical research ed) 2011;342:d1165. doi: 10.1136/bmj.d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh G., Miller J.D., Lee F.H., Pettitt D., Russell M.W. Prevalence of cardiovascular disease risk factors among US adults with self-reported osteoarthritis: data from the Third National Health and Nutrition Examination Survey. Am J Manag Care. 2002;8(15 Suppl):S383–S391. [PubMed] [Google Scholar]
- 5.Niu J., Clancy M., Aliabadi P., Vasan R., Felson D.T. Metabolic syndrome, its components, and knee osteoarthritis: the framingham osteoarthritis study. Arthritis Rheum. 2017;69(6):1194–1203. doi: 10.1002/art.40087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo G.H., McAlindon T.E., Katz J.N., Driban J.B., Price L.L., Eaton C.B., et al. Systolic and pulse pressure associate with incident knee osteoarthritis: data from the Osteoarthritis Initiative. Clin Rheumatol. 2017;36(9):2121–2128. doi: 10.1007/s10067-017-3656-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funck-Brentano T., Nethander M., Moverare-Skrtic S., Richette P., Ohlsson C. Causal factors for knee, hip and hand osteoarthritis: a Mendelian randomization study in the UK Biobank. Arthritis Rheum. 2019;71(10):1634–1641. doi: 10.1002/art.40928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Jr., Collins K.J., Dennison Himmelfarb C., et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical Practice guidelines. J Am Coll Cardiol 2018. 2017;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) Cochrane; 2011. https://www.training.cochrane.org/handbook Available from. [Google Scholar]
- 11.Nelson A.E., Smith M.W., Golightly Y.M., Jordan J.M. Generalized osteoarthritis": a systematic review. Semin Arthritis Rheum. 2014;43(6):713–720. doi: 10.1016/j.semarthrit.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin L., Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74(3):785–794. doi: 10.1111/biom.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [English] [DOI] [PubMed] [Google Scholar]
- 14.Kim H.S., Shin J.S., Lee J., Lee Y.J., Kim M.R., Bae Y.H., et al. Association between knee osteoarthritis, cardiovascular risk factors, and the framingham risk score in south Koreans: a cross-sectional study. PloS One. 2016;11(10) doi: 10.1371/journal.pone.0165325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Zhang H., Liang N., Fan W., Li J., Huang Z., et al. Prevalence and associated factors of knee osteoarthritis in a rural Chinese adult population: an epidemiological survey. BMC Publ Health. 2016;16:94. doi: 10.1186/s12889-016-2782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maddah S., Mahdizadeh J. Association of metabolic syndrome and its components with knee osteoarthritis. Acta Med Iran. 2015;53(12):743–748. [PubMed] [Google Scholar]
- 17.Shin D. Association between metabolic syndrome, radiographic knee osteoarthritis, and intensity of knee pain: results of a national survey. J Clin Endocrinol Metab. 2014;99(9):3177–3183. doi: 10.1210/jc.2014-1043. [DOI] [PubMed] [Google Scholar]
- 18.Inoue R., Ishibashi Y., Tsuda E., Yamamoto Y., Matsuzaka M., Takahashi I., et al. Medical problems and risk factors of metabolic syndrome among radiographic knee osteoarthritis patients in the Japanese general population. J Orthop Sci. 2011;16(6):704–709. doi: 10.1007/s00776-011-0157-9. [DOI] [PubMed] [Google Scholar]
- 19.Kim I., Kim H.A., Seo Y.I., Song Y.W., Jeong J.Y., Kim D.H. The prevalence of knee osteoarthritis in elderly community residents in Korea. J Kor Med Sci. 2010;25(2):293–298. doi: 10.3346/jkms.2010.25.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart D.J., Doyle D.V., Spector T.D. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol. 1995;22(6):1118–1123. [PubMed] [Google Scholar]
- 21.Bagge E., Bjelle A., Eden S., Svanborg A. Factors associated with radiographic osteoarthritis: results from the population study 70-year-old people in Goteborg. J Rheumatol. 1991;18(8):1218–1222. [PubMed] [Google Scholar]
- 22.Wang F., Shi L., Xue Q.Y. Association of metabolic factors with symptomatic hand osteoarthritis in the Chinese han population aged 40 Years and above. Chin Med J. 2016;129(19):2301–2307. doi: 10.4103/0366-6999.190660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomi A.L., Sellam J., Lacombe K., Fellahi S., Sebire M., Rey-Jouvin C., et al. Increased prevalence and severity of radiographic hand osteoarthritis in patients with HIV-1 infection associated with metabolic syndrome: data from the cross-sectional METAFIB-OA study. Ann Rheum Dis. 2016;75(12):2101–2107. doi: 10.1136/annrheumdis-2016-209262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahaghin S., Bierma-Zeinstra S.M., Koes B.W., Hazes J.M., Pols H.A. Do metabolic factors add to the effect of overweight on hand osteoarthritis? The Rotterdam Study. Ann Rheum Dis. 2007;66(7):916–920. doi: 10.1136/ard.2005.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sowers M.F., Hochberg M., Crabbe J.P., Muhich A., Crutchfield M., Updike S. Association of bone mineral density and sex hormone levels with osteoarthritis of the hand and knee in premenopausal women. Am J Epidemiol. 1996;143(1):38–47. doi: 10.1093/oxfordjournals.aje.a008655. [DOI] [PubMed] [Google Scholar]
- 26.Bae Y.H., Shin J.S., Lee J., Kim M.R., Park K.B., Cho J.H., et al. Association between hypertension and the prevalence of low back pain and osteoarthritis in Koreans: a cross-sectional study. PloS One. 2015;10(9) doi: 10.1371/journal.pone.0138790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venkatachalam J., Natesan M., Eswaran M., Johnson A.K.S., Bharath V., Singh Z. Prevalence of osteoarthritis of knee joint among adult population in a rural area of Kanchipuram District, Tamil Nadu. Indian J Publ Health. 2018;62(2):117–122. doi: 10.4103/ijph.IJPH_344_16. [DOI] [PubMed] [Google Scholar]
- 28.Alenazi A.M., Alothman S., Alshehri M.M., Rucker J., Waitman L.R., Wick J., et al. The prevalence of type 2 diabetes and associated risk factors with generalized osteoarthritis: a retrospective study using ICD codes for clinical data repository system. Clin Rheumatol. 2019;38(12):3539–3547. doi: 10.1007/s10067-019-04712-0. [DOI] [PubMed] [Google Scholar]
- 29.Askari A., Ehrampoush E., Homayounfar R., Arasteh P., Naghizadeh M.M., Yarahmadi M., et al. Relationship between metabolic syndrome and osteoarthritis: the fasa osteoarthritis study. Diabetes Metab Syndr. 2017;11(Suppl 2):S827–S832. doi: 10.1016/j.dsx.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Li H., George D.M., Jaarsma R.L., Mao X. Metabolic syndrome and components exacerbate osteoarthritis symptoms of pain, depression and reduced knee function. Ann Transl Med. 2016;4(7):133. doi: 10.21037/atm.2016.03.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han C.D., Yang I.H., Lee W.S., Park Y.J., Park K.K. Correlation between metabolic syndrome and knee osteoarthritis: data from the Korean national health and nutrition examination survey (KNHANES) BMC Publ Health. 2013;13:603. doi: 10.1186/1471-2458-13-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frey N., Hugle T., Jick S.S., Meier C.R., Spoendlin J. Type II diabetes mellitus and incident osteoarthritis of the hand: a population-based case-control analysis. Osteoarthritis Cartilage. 2016;24(9):1535–1540. doi: 10.1016/j.joca.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Kovari E., Kaposi A., Bekes G., Kiss Z., Kurucz R., Mandl P., et al. Comorbidity clusters in generalized osteoarthritis among female patients: a cross-sectional study. Semin Arthritis Rheum. 2020;50(2):183–191. doi: 10.1016/j.semarthrit.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimura N., Muraki S., Oka H., Tanaka S., Kawaguchi H., Nakamura K., et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage. 2012;20(11):1217–1226. doi: 10.1016/j.joca.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Strand M.P., Neogi T., Niu J., Felson D.T., Haugen I.K. Association between metabolic syndrome and radiographic hand osteoarthritis: data from a community-based longitudinal cohort study. Arthritis Care Res. 2018;70(3):469–474. doi: 10.1002/acr.23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh J.H., Chung S.W., Oh C.H., Kim S.H., Park S.J., Kim K.W., et al. The prevalence of shoulder osteoarthritis in the elderly Korean population: association with risk factors and function. J Shoulder Elbow Surg. 2011;20(5):756–763. doi: 10.1016/j.jse.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Engstrom G., Gerhardsson de Verdier M., Rollof J., Nilsson P.M., Lohmander L.S. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage. 2009;17(2):168–173. doi: 10.1016/j.joca.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Konstari S., Saaksjarvi K., Heliovaara M., Rissanen H., Knekt P., Arokoski J.P.A., et al. Associations of metabolic syndrome and its components with the risk of incident knee osteoarthritis leading to hospitalization: a 32-year follow-up study. Cartilage. 2019 doi: 10.1177/1947603519894731. 1947603519894731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y.M., Wang J., Liu X.G. Association between hypertension and risk of knee osteoarthritis: a meta-analysis of observational studies. Medicine (Baltim) 2017;96(32) doi: 10.1097/MD.0000000000007584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace I.J., Worthington S., Felson D.T., Jurmain R.D., Wren K.T., Maijanen H., et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci U S A. 2017;114(35):9332–9336. doi: 10.1073/pnas.1703856114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhuo Q., Yang W., Chen J., Wang Y. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol. 2012;8(12):729–737. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 42.Unger T., Borghi C., Charchar F., Khan N.A., Poulter N.R., Prabhakaran D., et al. International society of hypertension global hypertension Practice guidelines. Hypertension 2020. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 43.Smith G.D., Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33(1):30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]
- 44.Poornima S., Subramanyam K., Khan I.A., Hasan Q. The insertion and deletion (I28005D) polymorphism of the angiotensin I converting enzyme gene is a risk factor for osteoarthritis in an Asian Indian population. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2015;16(4):1281–1287. doi: 10.1177/1470320314547403. [DOI] [PubMed] [Google Scholar]
- 45.Hong S.J., Yang H.I., Yoo M.C., In C.S., Yim S.V., Jin S.Y., et al. Angiotensin converting enzyme gene polymorphism in Korean patients with primary knee osteoarthritis. Exp Mol Med. 2003;35(3):189–195. doi: 10.1038/emm.2003.26. [DOI] [PubMed] [Google Scholar]
- 46.Lin C., Chen H.C., Fang W.H., Wang C.C., Peng Y.J., Lee H.S., et al. Angiotensin-converting enzyme insertion/deletion polymorphism and susceptibility to osteoarthritis of the knee: a case-control study and meta-analysis. PloS One. 2016;11(9) doi: 10.1371/journal.pone.0161754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shehab D.K., Al-Jarallah K.F., Alawadhi A.M., Al-Herz A., Nahar I., Haider M.Z. Prevalence of angiotensin-converting enzyme gene insertion-deletion polymorphism in patients with primary knee osteoarthritis. Clin Exp Rheumatol. 2008;26(2):305–310. [PubMed] [Google Scholar]
- 48.Ramasamy S.K., Kusumbe A.P., Schiller M., Zeuschner D., Bixel M.G., Milia C., et al. Blood flow controls bone vascular function and osteogenesis. Nat Commun. 2016;7:13601. doi: 10.1038/ncomms13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aaron R.K., Racine J.R., Voisinet A., Evangelista P., Dyke J.P. Subchondral bone circulation in osteoarthritis of the human knee. Osteoarthritis Cartilage. 2018;26(7):940–944. doi: 10.1016/j.joca.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Chan P.M.B., Wen C., Yang W.C., Yan C., Chiu K. Is subchondral bone cyst formation in non-load-bearing region of osteoarthritic knee a vascular problem? Med Hypotheses. 2017;109(11):80–83. doi: 10.1016/j.mehy.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 51.Zhen G., Wen C., Jia X., Li Y., Crane J.L., Mears S.C., et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704–712. doi: 10.1038/nm.3143. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Lorenzo R.A., Ward J.A., Jordan B.S., Hanson C.E. Relationships of intraosseous and systemic pressure waveforms in a Swine model. Acad Emerg Med. 2014;21(8):899–904. doi: 10.1111/acem.12432. [DOI] [PubMed] [Google Scholar]
- 53.Arnoldi C.C., Lemperg K., Linderholm H. Intraosseous hypertension and pain in the knee. J Bone Joint Surg Br. 1975;57(3):360–363. [PubMed] [Google Scholar]
- 54.Seah S., Wheaton D., Li L., Dyke J.P., Talmo C., Harvey W.F., et al. The relationship of tibial bone perfusion to pain in knee osteoarthritis. Osteoarthritis Cartilage. 2012 Dec;20(12):1527–1533. doi: 10.1016/j.joca.2012.08.025. [Eng] [DOI] [PubMed] [Google Scholar]
- 55.Grüneboom A., Hawwari I., Weidner D., Culemann S., Müller S., Henneberg S., et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat Met. 2019;1(2):236–250. doi: 10.1038/s42255-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]