Abstract
Background and aim
Substantial evidence suggests the effectiveness of plant-based medicine in stress-related diseases. Kamikihito (KKT), a Japanese traditional herbal medicine (Kampo), has been used for anemia, insomnia, and anxiety. Recent studies revealed its ameliorating effect on cognitive and memory dysfunction in several animal models. We, therefore, determined whether daily supplementation of KKT has an antidepressant-like effect on the stress-induced behavioral and neurological changes in rats.
Experimental procedure
The effect of KKT against the stress-induced changes in anxiety- and depressive-like behaviors and hippocampal neurogenesis were determined using a rat model of chronic restraint stress (CRS). KKT was orally administered daily at 300 or 1000 mg/kg during 21 consecutive days of CRS (6 h/day). The effect of CRS and KKT on physiological parameters, including body weight gain, food/water consumptions, plasma corticosterone (CORT) levels, and percentage of adrenal gland weight to body weight, were firstly measured. Anxiety- and depressive-like behaviors in rats were assessed in the open field test (OFT), sucrose preference test (SPT), and forced swimming test (FST). Hippocampal neurogenesis was determined by immunohistochemistry.
Results and conclusion
CRS for 21 days caused a significant decrease in body weight gain and increase in plasma CORT levels and percentage of adrenal gland weight to body weight, which were rescued by KKT treatment. KKT also suppressed the CRS-induced anxiety- and depressive-like behaviors and impairment of hippocampal neurogenesis. These results suggest that daily treatment of KKT has a protective effect against physiological, neurological, and behavioral changes in a rat model of depression.
Keywords: Kamikihito (加味帰脾湯), Antidepressant-like effect, Major depressive disorder, Chronic restraint stress, Hippocampal neurogenesis
Abbreviations: BDNF, brain-derived neurotrophic factor; CORT, corticosterone; CRS, chronic restraint stress; DCX, doublecortin; DG, dentate gyrus; DNA, methyltransferase; FST, forced swimming test; HPA, hypothalamus-pituitary-adrenal; KKT, Kamikihito; MAO, monoamine oxidase; MDD, major depressive disorder; NSPCs, neural progenitor/stem cells; OFT, open field test; ROS, reactive oxygen species; SPT, sucrose preference test
Graphical abstract
Highlights
-
•
Kamikihito (KKT) showed beneficial effects in a rat model of depression.
-
•
KKT prevented the chronic restraint stress (CRS)-induced physiological changes.
-
•
KKT ameliorated anxiety- and anhedonic-like behaviors caused by CRS.
-
•
Reduced hippocampal neurogenesis induced by CRS was rescued by KKT treatment.
1. Introduction
Major depressive disorder (MDD) is a debilitating psychiatric illness with a lifetime prevalence of 10 %–20 %.1 Environmental factors, such as stressful or traumatic events, are considered to influence the susceptibility to MDD. Recent evidence suggests that impairment in the complex systems, such as monoamine neurotransmission, the hypothalamus-pituitary-adrenal (HPA) axis (glucocorticoids), and neurotrophic factors are involved in the incidence of MDD. Because of the limited availability and the variable conditions of human samples, animal models of depression have been developed.2 Among these animal models, chronic restraint stress (CRS) is known to be a well-established stress paradigm that leads to anxiety- and depressive-like behaviors in rodents.3, 4, 5
The hippocampus which is known for its important roles in learning and memory, cognitive function, mood regulation, and stress response6 attracts considerable attention from clinicians and researchers as the tissue seems to be highly correlated with stress and the resultant depressive symptoms. Morphological change in the hippocampus is frequently reported in patients with MDD.7 Importantly, the reduced volume in the hippocampus can be reproduced in the rat model of depression induced by CRS.8
A growing body of evidence in the basic research shows that the number of newly generated neurons (neurogenesis) from neural progenitor/stem cells (NSPCs) in the dentate gyrus (DG), subregion of the hippocampus, is negatively correlated with anxiety and depressive behaviors.9,10 Antidepressant treatment can ameliorate both the stress-induced behavioral changes and the suppression of neurogenesis in the DG of the hippocampus.11 It is, therefore, considered that hippocampal neurogenesis is an indicator of the depressive state and could be a key target of the medical treatment for MDD patients.6
Depression and anxiety are commonly treated by pharmacological therapy. Although these drugs appear to have a beneficial effect in some severe cases, others complaint that the drugs are not effective for all cases and incur diverse adverse effects.12 On the other hand, a number of studies have recently demonstrated the effectiveness of complementary and alternative medicine, such as herbal medicine, in the treatment of patients with anxiety and depression.12, 13, 14
Kamikihito (KKT), one of the Japanese traditional herbal medicine (Kampo), composed of fourteen medicinal plants, has been used to treat anemia, insomnia, and mental anxiety. It has been reported to be effective for cognitive and memory dysfunction and behavioral abnormalities induced by beta-amyloid,15 toxic chemicals,16 and aging,17 suggesting that KKT could act on and exert beneficial effects in the central nervous system (CNS).
We, therefore, investigated the effect of KKT in an animal model of depression. We exposed rats to daily restraint stress after administration of KTT at two different doses and determined whether KKT has protective effects on the CRS-induced changes in anxiety- and depressive-like behaviors and hippocampal neurogenesis.
2. Materials and methods
2.1. Animals
Wistar rats (male, 7 weeks) were purchased from Nippon Bio-Supp. Center (Tokyo, Japan). Rats were housed two to three per cage in 12:12 h light/dark cycle (lights on at 7 a.m.) at a temperature of 25 ± 1 °C, humidity 40–50 %, and free access to food (CLEA Japan, CE-2, Tokyo, Japan) and water for 10 days before testing.
All animal experiments were approved by the Committee of Animal Care and Welfare of our University and performed in accordance with the guidelines established with the Committee of Animal Care and Welfare of our University. Each experiment was repeated two times and all efforts were made to minimize animal suffering and to reduce the number of animals used in this study.
2.2. Drug administration
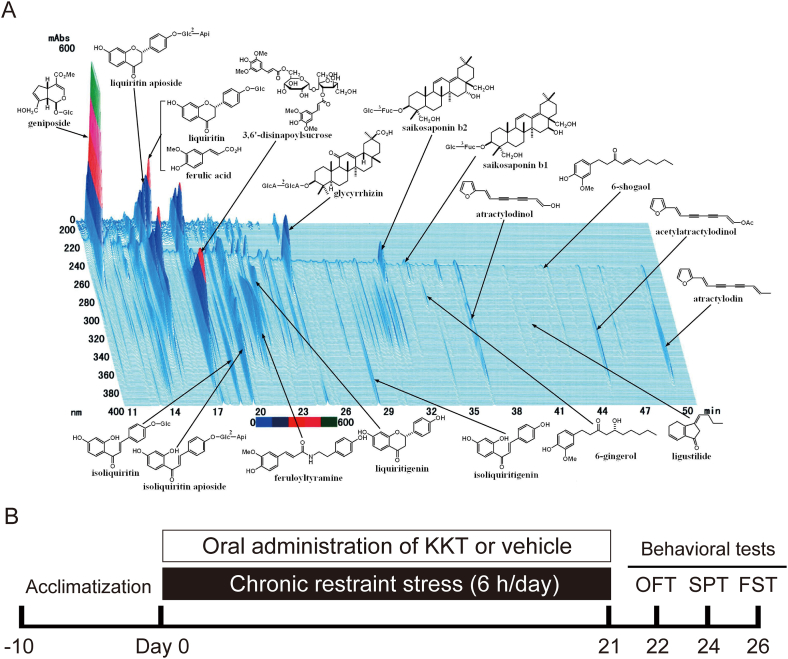
The dry powdered extract of KKT was supplied by Tsumura & Co. (#TJ-137, Tokyo, Japan). Dried powder of 5.0 g KKT contains extracts from the following herbs. 3.0 g Astragali Radix (黄耆; huáng qí), 3.0 g Bupleuri Radix (柴胡; chái hú), 3.0 g Zizyphi Semen (酸棗仁; suān zǎo rén), 3.0 g Atractylodis Rhizoma (蒼朮; cāng zhú), 3.0 g Ginseng Radix (人參; Rén Shēn), 3.0 g Poria (茯苓; fú líng), 3.0 g Longanae Arillus (龍眼肉; lóng yǎn ròu), 2.0 g Polygalae Radix (遠志; yuǎn zhì), 2.0 g Gardeniae Fructus (山梔子; shān zhī zǐ), 2.0 g Zizyphi Fructus (大棗; dà zǎo), 2.0 g Angelicae Radix (当帰; dāng guī), 1.0 g Glycyrrhizae Radix (甘草; gān cǎo), 1.0 g Zingiberis Rhizoma (生姜; shēng jiāng), 1.0 g Saussureae Radix (木香; mù xiāng). These herbs were mixed and boiled with purified water at 95.1 °C for 1 h. The macerate was filtered and dried by removing water under reduced pressure. The three-dimensional high-performance liquid chromatography (3D-HPLC) profile chart of KKT was provided by Tsumura & Co. (Fig. 1A). The amounts of some bioactive compounds contained in KKT were confirmed as follows; 27–81 mg geniposide, 6–18 mg glycyrrhizinic acid, 0.8–3.2 mg saikosaponin b2 in 5 g of KKT.15
Fig. 1.
(A) Constituents of the KKT extract identified by 3D-HPLC (provided by Tsumura & Co). (B) The schematic experimental design for treatments and behavioral tests. KKT or vehicle was administrated to the rats before subjected to daily 6-h CRS for 21 days. OFT: open field test; SPT: sucrose preference test; FST: forced swimming test.
Based on our primary experiment (data not published) and previous reports15, 16, 17 showing beneficial effects of KKT in rodents by using 200–2000 mg/kg/day, KKT was freshly dissolved in distilled water and orally administered to the rats 30 min before the start of CRS at 300 or 1000 mg/kg/day. Vehicle treated rats were received a constant volume of distilled water (10 ml/kg) at the same time.
2.3. Experimental design
Rats were randomly assigned to one of the four experimental groups: control group (CON, without restraint stress; n = 5), chronic restraint stress (CRS; n = 5), CRS + KKT 300 mg/kg/day (CRS + KKT300; n = 5), and CRS + KKT 1000 mg/kg/day (CRS + KKT1,000; n = 5).
2.4. Restraint stress
Rats were restrained for 6 h/day (09:00–15:00 h) in an acrylic made immobilization box (W × L × H = 5 × 15 × 5.5 cm) for 21 consecutive days. The cages were adjusted to prevent any movements of the animal. The CON rats were kept in their home cages without access to both food and water during the 6-h CRS. The body weight and 24 h consumptions of food and water were measured daily. The schematic design of the experiment is shown in Fig. 1B.
2.5. Behavioral tests
2.5.1. Open field test (OFT)
The OFT was performed using a 90 × 90 cm square field with 40 cm-high walls. Each rat was placed in the center of the field and tested for 5 min. Total distance, the number of entries into the central zone (45 × 45 cm, the central zone), distance traveled, and time spent in it and in the remaining area near the walls (the peripheral zone) were recorded. The data analysis was performed automatically using SMART video tracking software (version 3.0.05, Panlab, Spain).
2.5.2. Sucrose preference test (SPT)
Individually housed rats were trained to drink water from two bottles for 2 days. The bottles and food were removed 12 h before the test. The changes in the weight of the two bottles containing tap water or 2 % sucrose solution were measured 24 h after putting back the bottles. The two bottles were placed randomly and the position was changed after 12 h. Sucrose preference was calculated by the following formula: (the weight of consumed sucrose solution)/[the weight of consumed (sucrose solution + drinking water)] × 100.
2.5.3. Forced swimming test (FST)
The FST was conducted for 5 min by placing rats in individual acrylic cylinders (50 cm height × 20 cm diameter) filled with water (24 °C) 33 cm deep. All rats experienced a training session for 15 min on the day before the test. The time spent climbing, swimming, and being immobile were determined. The data was acquired and analyzed by the SMART software.
2.5.4. Plasma concentration of corticosterone
Blood samples were collected on the final day of the experiment. The samples were spun at 3000 g for 10 min at 4 °C to separate the plasma. The Plasma samples were stored at −80 °C until assay. Corticosterone (CORT) levels were determined by Enzyme-linked immunosorbent assay (ELISA) kit (Enzo Life Science, NY, USA), according to the manufacturer's instructions.
2.6. Immunohistochemistry
Rats were deeply anesthetized 16 h after the FST with intraperitoneal administration of a combination of three anesthetics medetomidinehydrochloride, 0.3 mg/kg (Domitol; Nippon Zenyaku Kogyo Co.,Ltd., Fukushima, Japan); midazolam, 4.0 mg/kg (Sandoz; Sandoz K.K. Tokyo, Japan) and butorphanol, 5.0 mg/kg (Vetorphale; Meiji Seika Pharma Co., Ltd., Japan), and intracardially perfused with cold phosphate buffered saline (PBS) at pH 7.4, followed by 4 % paraformaldehyde in 0.1 M PBS. After perfusion, brains were removed and further fixed in the same PFA solution overnight. The brains were equilibrated in 30 % sucrose. The tissues were subsequently embedded and frozen in the Tissue-Tek OCT compound (Tissue-Tek, Sakura Finetek, Tokyo Japan) and stored at −80 °C until sectioning. After cutting the tissue into 30 μm thickness using a cryostat (CM1860; Leica Biosystems, Germany), immunostaining was performed on 6 series of free-floating hippocampal sections.
Sections were rinsed three times in PBS, subsequently incubated with blocking solution (10 % goat serum with 0.3 % Triton X-100 in PBS). The sections were then incubated with following primary antibodies for 48 h at 4 °C; anti-doublecortin (DCX) antibody (1:200, #ab18723, Abcam, MA, USA) or anti-Ki67 antibody (1:100, #ab15580, Abcam) and anti-Neu N (1:500, #MAB377, Millipore, CA, USA). Alexa-Fluor 488 (1:200, Invitrogen, CA, USA) and Alexa-Fluor 546 (1:200, Invitrogen) were used as secondary antibodies (1 h at room temperature). Sections were subsequently mounted on slides and coverslipped with mounting medium containing DAPI (Santa Cruz Biotechnology, CA, USA).
Immunofluorescent images were obtained with Olympus FV1000D confocal microscope (Olympus, Tokyo, Japan). DCX- and Ki67-positive cells were counted along the DG of the six hippocampal sections.
2.7. Statistical analysis
All data are presented as means and standard deviation (SD). The statistical significance was determined by one-way ANOVA followed by Tukey's HSD post-hoc test, using SPSS ver. 25 (IBM Japan, Tokyo, Japan). The relationship between the CORT levels and percentage of adrenal gland/body weight was assessed by the Pearson's correlation coefficient test. In all cases, a value of p < 0.05 was considered statistically significant.
3. Results
3.1. Negative effect of CRS on body weight gain and an ameliorating action of KKT
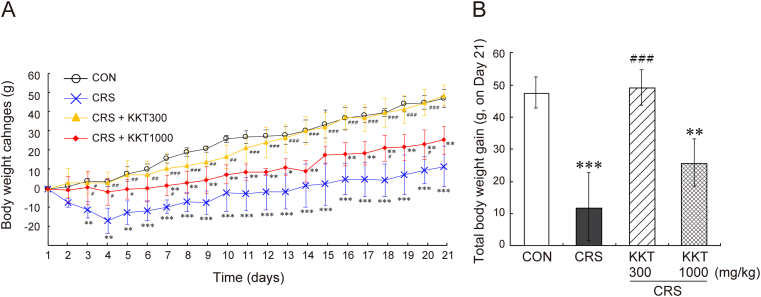
The negative effect of CRS on body weight gain has appeared from day 3 (Fig. 2A). The reduced weight gain continued until the end of the experiment (Fig. 2A and B). KKT administration at the dose of 1000 mg/kg/day significantly rescued this decline. KKT (300 mg/kg/day) almost completely blocked the loss of body weight gain caused by CRS (Day 21: F(3,19) = 23.6, p < 0.001) (Fig. 2A and B).
Fig. 2.
KKT rescued the reduced body weight gain caused by CRS. (A) Body weight changes of rats compared to the body weight of Day 0. (B) Total body weight gain on the Day 21. CRS + KKT300: rats administered 300 mg/kg KKT daily with CRS, CRS + KKT1000: rats administered 1000 mg/kg KKT daily with CRS. Values are the mean ± SD, ∗∗p < 0.01, ∗∗∗p < 0.001 vs CON; #p < 0.05, ##p < 0.01, ###p < 0.001 vs CRS group.
The daily food intake was reduced by CRS from day 2 to day 15, which seems not to be restored by KKT administration (Fig. 3A). The two doses of KKT showed an undistinguishable food intake during the period of CRS. Both CRS and KKT had no strong effect on the daily water intake (Fig. 3B).
Fig. 3.
Effects of CRS and KKT on (A) daily food intake and (B) daily water intake. Values are the mean ± SD, ∗p < 0.05, ∗∗p < 0.01 vs CON group.
3.2. KKT inhibited the CRS-induced increase in plasma corticosterone levels and adrenal hypertrophy
Daily 6-h CRS for 21 days significantly increased plasma CORT levels (F(3,20) = 12.3, p < 0.001) and the adrenal gland/body weight ratio (F(3,20) = 10.6, p < 0.001) (Table 1). KKT administration inhibited the CRS-induced increase of these parameters in both doses (300 and 1000 mg/kg/day) (Table 1). A significant positive correlation between the plasma CORT levels and the adrenal gland/body weight ratio was revealed by the Pearson's correlation coefficient (r = 0.628, p < 0.01).
Table 1.
Changes in plasma CORT levels and percentage of the adrenal gland weight to the body weight after CRS and KKT treatment. n = 5, data represent mean ± SD. ∗∗∗p < 0.001 vs CON; #p < 0.05,##p < 0.01,###p < 0.001 vs CRS group (Tukey's HSD post-hoc test).
| plasma corticosterone (ng/ml) | adrenal grand/body weight (%) | |
|---|---|---|
| CON | 70.5 ± 44.8 | 0.0168 ± 0.00108 |
| CRS | 201 ± 50.2 ∗∗∗ | 0.0226 ± 0.00146 ∗∗∗ |
| CRS + KKT300 | 62.8 ± 21.8 ### | 0.0186 ± 0.00167 ## |
| CRS + KKT1000 | 105 ± 18.6 ## | 0.0190 ± 0.00198 # |
3.3. The CRS-induced anxiety- and depressive-like behaviors were restored by KKT
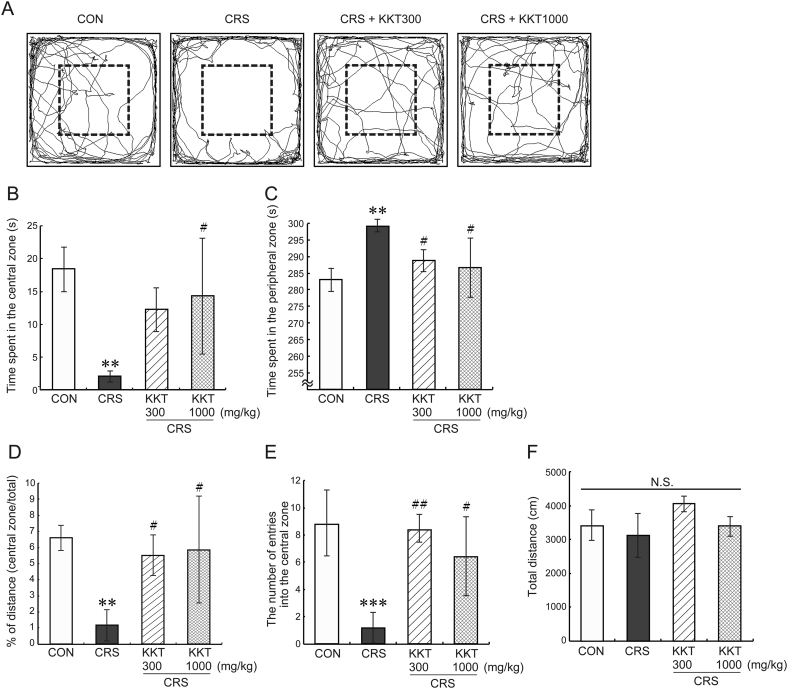
We determined whether KKT ameliorates the anxiety- and depressive-like behaviors induced by CRS. CRS for 21 days clearly developed anxiety-like behavior in the OFT (Fig. 4). The time spent in the central and peripheral zone was significantly decreased (F(3,19) = 7.33, p < 0.01) (Fig. 4A and B) and increased (F(3,19) = 7.42, p < 0.01) (Fig. 4A and C) respectively, compared to the CON group. KKT (1000 mg/kg/day) administration restored these values (Fig. 4B and C). KKT (300 mg/kg/day) significantly blocked to decrease the time spent in the peripheral zone (Fig. 4C). This dose of KKT did not show a significant effect in the CRS-reduced time spent in the central zone although it showed a tendency to improve that (p = 0.057 vs CRS) (Fig. 4C). The values of percentage of distance traveled in the central zone (F(3,19) = 6.78, p < 0.01) (Fig. 4D) and the number of entries into the central zone (F(3,19) = 11.9, p < 0.001) (Fig. 4E) were decreased in the CRS group, which were clearly recovered by both doses of KKT treatment. There was no statistical significance in the total distance traveled during the test among four groups (F (3,19) = 3.192, p > 0.05) (Fig. 4F).
Fig. 4.
Effects of KKT against CRS-induced anxiety-like behavior observed in the open field test. (A) Representative trajectories of rats' movement during the 5-min test period. The central zone is shown by a square with dot line in each field. Time spent in (B) the central zone and (C) the peripheral zone. (D) Percentage of distance traveled in the central zone. (E) The number of entries into the central zone. (F) Total distance traveled in the field during the test. Values are the mean ± SD, ∗∗p < 0.01, ∗∗∗p < 0.001 vs CON; #p < 0.05, ##p < 0.01 vs CRS group. N.S., not significant.
To evaluate anhedonic behavior and behavioral despair in rats, the SPT and FST were employed (Fig. 5). Rats exposed to CRS showed a significant reduction in the percentage of sucrose consumption (F(3,19) = 16.6, p < 0.001) (Fig. 5A), suggesting that 21-day CRS induced anhedonia in rats. Both doses of KKT inhibited developing anhedonia-like behavior in rats with CRS (Fig. 5A). The rats of the CRS group also exhibited behavioral despair as significantly increased immobility time (Immobility: F (3,19) = 15.9, p < 0.001) and decreased climbing time (Climbing: F(3,19) = 7.93, p < 0.01) (Fig. 5B) in the FST. These changes in depressive-like behaviors were suppressed close to the CON group by daily administration of KKT at both doses (Fig. 5B). No significant difference was observed in swimming time among all groups (Fig. 5B). These data suggest that KKT in the dose of 300–1000 mg/kg/day has an anti-depressant-like effect on the CRS-induced anxiety- and depressive-like behaviors.
Fig. 5.
Effects of KKT on CRS-induced depressive-like behaviors. Depressive-like behaviors were assessed by (A) the sucrose preference test (anhedonia) and (B) the forced swimming test. Values are the mean ± SD, ∗∗p < 0.01, ∗∗∗p < 0.001 vs CON; #p < 0.05, ##p < 0.01, ###p < 0.001 vs CRS group. N.S., not significant.
3.4. KKT ameliorated the impaired hippocampal neurogenesis caused by CRS
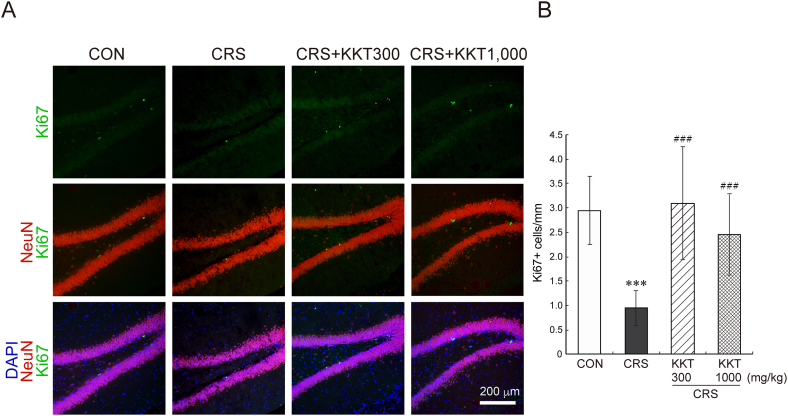
We next investigated the effect of CRS and KKT treatment in hippocampal neurogenesis. Immunohistochemical analysis showed that CRS for 21 days remarkably reduced the number of proliferating cells and immature neurons in the DG (Fig. 6, Fig. 7). The reduction in the number of Ki67-positive proliferating cells in the DG was almost completely blocked by KKT (F(3,70) = 19.2, p < 0.001) (Fig. 6A and B). Similarly, the number of DCX-positive immature newborn neurons was dramatically reduced by CRS, which was recovered by KKT administration (F(3,74) = 21.8, p < 0.001) (Fig. 7A and B). No significant difference was observed between the two groups of CRS + KKT. These data clearly showed the negative effect of CRS on the proliferation of NSPCs and survival/maturation of newly generated neurons, and the protective effect of KKT against CRS.
Fig. 6.
KKT restored the reduced NSPCs proliferation in the DG caused by CRS. (A) Proliferating cells in the DG (Ki67-positive cells, green) and neurons (NeuN-positive cells, Red). Nuclei were visualized by DAPI (Blue) (B) Quantitative data of the number of Ki67-positive cells/mm in the DG. The hippocampal sections were obtained from 3 to 4 rats in each group. The number of sections determined in this experiment were: CON, 15; CRS, 16; CRS + KKT300, 21; CRS + KKT1000, 19. Values are the mean ± SD, ∗∗∗p < 0.001 vs CON; ###p < 0.001 vs CRS group. White bar = 200 μm.
Fig. 7.
KKT suppressed the CRS-induced decline in the number of immature neurons. (A) immature newborn neurons in the DG were visualized as DCX-positive cells (Green). Nuclei were visualized by DAPI (Blue) (B) Quantitative analysis of the number of DCX-positive cells/mm in the DG. The hippocampal sections were obtained from 3 to 4 rats in each group. The number of sections determined in this experiment were: CON, 19; CRS, 18; CRS + KKT300, 19; CRS + KKT1000, 22. Values are the mean ± SD, ∗∗∗p < 0.001 vs CON; ###p < 0.001 vs CRS group. White bar = 200 μm.
4. Discussion
This study was undertaken to determine whether oral KKT treatment can attenuate the behavioral changes and neuronal damages induced by CRS for 21 days since it has been used as an anti-anxiety medication in traditional medicine. To the best of our knowledge, this is the first report revealing the antidepressant-like effect of KKT through restoring hippocampal neurogenesis under depressive conditions.
The CRS paradigm used in this study was a strong stressor that changed physiological parameters, such as reduction in body weight gain, hypercorticosteronemia, and hypertrophy of the adrenal gland, in line with the previous reports using similar stress paradigms.3,18 Although less body weight gain was observed in the CRS + KKT1000 group compared to the CON and CRS + KKT300 group, other CRS-induced physiological changes were prevented by simultaneous treatment with KKT at both doses. The tendency of losing body weight observed on the first four days of the stress paradigm has occurred parallelly with the reduction of food intake. However, while food intake in the CRS rats gradually caught up with the CON levels, it was not accompanied by the recovery of the body weight gain. One possible reason is the sustained elevation of CORT levels in the CRS rats. Kim et al. reported that the changes in plasma CORT levels in mice following restraint stress were negatively correlated with the changes in body weight.19 Glucocorticoids are able to promote the catabolism of muscle and adipose tissues, which leads to a reduction in body weight gain.
We also confirmed the CRS-induced anxiety- and depressive-like behaviors similar to the previous studies.4,5 In the OFT, the CRS rats tended to stay close to the walls of the arena, which reflects the fear to explore the central zone and new environment. This anxious behavior was completely suppressed by the KKT treatment. Similarly, the anxiolytic effect of repeated KKT administration was demonstrated in the elevated plus maze (EPM) test.15 Furthermore, extract of Zingiberis Rhizoma (生姜; shēng jiāng),20 a component of KKT, and rutin, a major flavonoid component of Zizyphi Fructus (大棗; dà zǎo)21 independently showed an anxiolytic effect in the EPM test. Therefore, it is possible that the anxiolytic effect of KKT may act through multiple neuropathways rather than a single neurotransmitter system.
Despite some studies reported reduced locomotor activity after chronic stress,4,14 our results supported other reports showing no remarkable change in locomotor activity.22,23 This discrepancy could attribute to the use of different strains of animals, chronic stress models, and arena dimensions.
The decrease in sucrose preference and the increase in immobility time in the FST in our experiment demonstrated that a rat model of depression was successfully established by our CRS procedure. It was reported that depressed patients and suicide victims24 also showed enlarged adrenal gland (hypertrophy), which probably due to the impaired negative feedback function of the HPA axis that leads to enhancement of CORT production. Thus, it is plausible that KKT exerted antidepressant-like effects via the functions of some therapeutic compounds that directly target the HPA axis. It was also reported that geniposide, a major compound in Gardeniae fructus (山梔子; shān zhī zǐ), functions as monoamine oxidase (MAO)-B inhibitor.25 Moreover, treatment with geniposide exerts antidepressant-like effects in CRS-induced depressive mice via glucagon-like peptide-1 receptor (GLP-1R)/AKT signaling pathway.26 In this regard, the presence of geniposide as a bioactive compound in KKT may at least partly explain the antidepressant-like effect demonstrated in the present experiment although the detailed molecular mechanisms through which KKT prevents CRS-induced depressive behavior need to be explored.
The hippocampus is a key brain region for memory, cognition, mood, and emotion while it is highly sensitive to glucocorticoid hormones. The DG region contains a high density of glucocorticoid receptors and it is one of the two regions in the adult mammalian brain that can give rise to new neurons. Chronic administration of glucocorticoids and the chronic stress-induced elevated glucocorticoids levels have been reported to affect cell proliferation, neuronal differentiation as well as cell survival, which in turn induced anxiety- and depressive-like behaviors in rodents.3,7 Although some studies suggest that the suppression of hippocampal neurogenesis may not be essential to develop depressive symptoms and the restoration of HPA axis feedback function is sufficient to maintain the therapeutic effect, other reports clearly showed that enhanced neurogenesis is necessary and sufficient for the effects of antidepressants in the rodent models of depression.10,11
In accordance with the above observations, our findings confirmed that CRS markedly suppressed cell proliferation (Ki67 positive cells) coupled with neurogenesis (DCX positive cells) in the hippocampus, suggesting a possible relationship between the reduced neurogenesis and their anxiety- and depressive-like behaviors. It is well established that a neurotrophin BDNF (brain-derived neurotrophic factor) positively regulates neurogenesis, differentiation, survival, and plasticity of NSPCs and new-born neurons in the adult brain and its expression is negatively regulated by stressful conditions.4,5,18 Ye et al. reported that genipin which is generated by removing a glucose from geniposide contained in KKT could increase BDNF expression in the hippocampus by inhibiting the DNA methyltransferase 1 (DNMT1) in mice.27 Since KKT treatment suppressed the CRS-induced depressive behaviors and rescued cell proliferation and neurogenesis, it is possible that geniposide can be one of the active compounds in the antidepressant-like activity of KKT. We, however, have to keep in mind that KKT is a mixture of fourteen plants, including Ginseng Radix (人參; Rén Shēn), Polygalae Radix (遠志; yuǎn zhì), Astragali Radix (黄耆; huáng qí), and Gardeniae Fructus (山梔子; shān zhī zǐ), which have been reported to contain several bioactive compounds such as flavonoids, terpenoids, and saponins with strong antioxidant, anti-inflammatory, and neuroprotective effects.28, 29, 30, 31, 32 The exposure to prolonged stress or high levels of glucocorticoids can increase ROS (reactive oxygen species) in the hippocampus, which causes a severe reduction in neuronal production.33 As KKT contains a large number of antioxidant molecules that can modulate the intracellular signaling cascades and lipid kinases. Therefore, KKT might have protected NSPCs and new-born neurons from the ROS-dependent adverse effects on neurogenesis.
The precise mechanisms by which KKT exerted the anti-anxiety and antidepressant-like effects and improved hippocampal neurogenesis are not completely known and determining the functions of all compounds in KKT are not easy. It is important, however, to consider that the synergistic and additive effects of multiple compounds targeting different targets are necessary for herbal/kampo medicine. The absence of a positive control group was a limitation of the present study. Future studies, therefore, could focus on clarifying the molecular mechanisms of the anti-anxiety and anti-depressive effects of KKT, comparing to the action of positive controls whose mechanisms are well established.
5. Conclusion
In conclusion, the present investigation gives evidence to support the use of KKT as a medication for anxiety and depression. It also demonstrated that KKT treatment can protect neurogenesis in the hippocampus under stressful conditions.
Ethics approval
This study was approved by the Ethics Committees of our University (certificate NO. 09082).
Consent for publication
All authors critically revised the manuscript and approved the final version before submission.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Kessler R.C., Bromet E.J. The epidemiology of depression across cultures. Annu Rev Publ Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q., Timberlake M.A., II, Prall K., Dwivedi Y. The recent progress in animal models of depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;77:99–109. doi: 10.1016/j.pnpbp.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenbrock H., Koros E., Bloching A., Podhorna J., Borsini F. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res. 2005;1040:55–63. doi: 10.1016/j.brainres.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 4.Naert G., Ixart G., Maurice T., Tapia-Arancibia L., Givalois L. Brain-derived neurotrophic factor and hypothalamic-pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. Mol Cell Neurosci. 2011;46:55–66. doi: 10.1016/j.mcn.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Liang S., Wang T., Hu X., et al. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 6.DeCarolis N.A., Eisch A.J. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010;58:884–893. doi: 10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacQueen G., Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatr. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 8.Schoenfeld T.J., McCausland H.C., Morris H.D., Padmanaban V., Cameron H.A. Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biol Psychiatr. 2017;82:914–923. doi: 10.1016/j.biopsych.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anacker C., Zunszain P.A., Cattaneo A., et al. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatr. 2011;16:738–750. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill A.S., Sahay A., Hen R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology. 2015;40:2368–2378. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santarelli L., Saxe M., Gross C., et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 12.Liu L., Liu C., Wang Y., Wang P., Li Y., Li B. Herbal medicine for anxiety, depression and insomnia. Curr Neuropharmacol. 2015;13:481–493. doi: 10.2174/1570159X1304150831122734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unützer J., Klap R., Sturm R., et al. Mental disorders and the use of alternative medicine: results from a national survey. Am J Psychiatr. 2000;157:1851–1857. doi: 10.1176/appi.ajp.157.11.1851. [DOI] [PubMed] [Google Scholar]
- 14.Wang D., An S.C., Zhang X. Prevention of chronic stress-induced depression-like behavior by inducible nitric oxide inhibitor. Neurosci Lett. 2008;433:59–64. doi: 10.1016/j.neulet.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Oizumi H., Miyazaki S., Tabuchi M., Endo T., Omiya Y., Mizoguchi K. Kamikihito enhances cognitive functions and reward-related behaviors of aged c57bl/6j mice in an automated behavioral assay system. Front Pharmacol. 2020;11:1037. doi: 10.3389/fphar.2020.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tohda C., Nakada R., Urano T., Okonogi A., Kuboyama T. Kamikihi-to (KKT) rescues axonal and synaptic degeneration associated with memory impairment in a mouse model of Alzheimer's disease, 5XFAD. Int J Neurosci. 2011;121:641–648. doi: 10.3109/00207454.2011.602809. [DOI] [PubMed] [Google Scholar]
- 17.Araki R., Nishida S., Hiraki Y., Li F., Matsumoto K., Yabe T. Kamikihito ameliorates lipopolysaccharide-induced sickness behavior via attenuating neural activation, but not inflammation, in the hypothalamic paraventricular nucleus and central nucleus of the amygdala in mice. Biol Pharm Bull. 2016;39:289–294. doi: 10.1248/bpb.b15-00707. [DOI] [PubMed] [Google Scholar]
- 18.Chigr F., Rachidi F., Segura S., et al. Neurogenesis inhibition in the dorsal vagal complex by chronic immobilization stress in the adult rat. Neuroscience. 2009;158:524–536. doi: 10.1016/j.neuroscience.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Kim J.G., Jung H.S., Kim K.J., Min S.S., Yoon B.J. Basal blood corticosterone level is correlated with susceptibility to chronic restraint stress in mice. Neurosci Lett. 2013;555:137–142. doi: 10.1016/j.neulet.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 20.Vishwakarma S.L., Pal S.C., Kasture V.S., Kasture S.B. Anxiolytic and antiemetic activity of Zingiber officinale. Phytother Res. 2002;16:621–626. doi: 10.1002/ptr.948. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Leon A., González-Trujano M.E., Fernández-Guasti A. The anxiolytic-like effect of rutin in rats involves GABAA receptors in the basolateral amygdala. Behav Pharmacol. 2017;28:303–312. doi: 10.1097/FBP.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 22.Dhamija I., Parle M., Kumar S. Antidepressant and anxiolytic effects of Garcinia indica fruit rind via monoaminergic pathway. 3 Biotech. 2017;7:131. doi: 10.1007/s13205-017-0766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan J.N.M., Lee J.C.D., Lee S.S., et al. Interaction effect of social isolation and high dose corticosteroid on neurogenesis and emotional behavior. Front Behav Neurosci. 2017;11:18. doi: 10.3389/fnbeh.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marini S., Vellante F., Matarazzo I., et al. Inflammatory markers and suicidal attempts in depressed patients: a review. Int J Immunopathol Pharmacol. 2016;29:583–594. doi: 10.1177/0394632015623793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J., Duan P., Cui Y., Li Q., Shi Y. Geniposide alleviates depression-like behavior via enhancing BDNF expression in hippocampus of streptozotocin-evoked mice. Metab Brain Dis. 2016;31:1113–1122. doi: 10.1007/s11011-016-9856-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y., Li H., Fang F., et al. Geniposide improves repeated restraint stress-induced depression-like behavior in mice by ameliorating neuronal apoptosis via regulating GLP-1R/AKT signaling pathway. Neurosci Lett. 2018;676:19–26. doi: 10.1016/j.neulet.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Ye D., Zhang L., Fan W., Zhang X., Dong E. Genipin normalizes depression-like behavior induced by prenatal stress through inhibiting DNMT1. Epigenetics. 2018;13:310–317. doi: 10.1080/15592294.2018.1450033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demir I., Kiymaz N., Gudu B.O., et al. Study of the neuroprotective effect of ginseng on superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) levels in experimental diffuse head trauma. Acta Neurochir. 2013;155:913–922. doi: 10.1007/s00701-013-1672-6. [DOI] [PubMed] [Google Scholar]
- 29.Lv S., Ding Y., Zhao H., Liu S., Zhang J., Wang J. Therapeutic potential and effective components of the Chinese herb Gardeniae fructus in the treatment of senile disease. Aging Dis. 2018;9:1153. doi: 10.14336/AD.2018.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni Y., Li L., Zhang W., et al. Discovery and LC-MS characterization of new crocins in Gardeniae fructus and their neuroprotective potential. J Agric Food Chem. 2017;65:2936–2946. doi: 10.1021/acs.jafc.6b03866. [DOI] [PubMed] [Google Scholar]
- 31.Kuboyama T., Hirotsu K., Arai T., Yamasaki H., Tohda C. Polygalae Radix extract prevents axonal degeneration and memory deficits in a transgenic mouse model of Alzheimer's disease. Front Pharmacol. 2017;8:805. doi: 10.3389/fphar.2017.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong A.G., Duan R., Wang H.Y., et al. Evaluation of the pharmaceutical properties and value of Astragali Radix. Medicine. 2018;5:46. doi: 10.3390/medicines5020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan T.F., Gu S., Shan C., Marchado S., Arias-Carrión O. Oxidative stress and adult neurogenesis. Stem Cell Rev Rep. 2015;11:706–709. doi: 10.1007/s12015-015-9603-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.