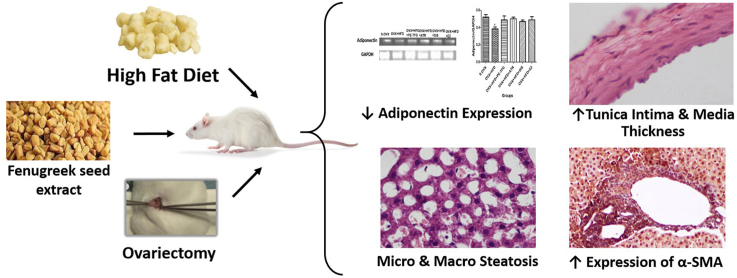
Abstract
Background and aim
Trigonella foenum-graecum L. seeds (TFG) are used as spices in Indian cuisine. In Indian traditional medicine, TFG is used to treat diabetes, dyslipidemia, obesity, arthritis, cancer, digestive disorders, and postmenopausal conditions. Pathophysiology of postmenopausal diseases involves low-grade systemic inflammation. The purpose of this study is to investigate the prophylactic effect of petroleum ether fraction of TFG-extract (PE-TFG) on inflammatory markers, and histopathological changes in ovariectomized rats (OVX-rats) fed with a high-fat diet (HFD).
Experimental procedure
OVX female Sprague Dawley rats were used for the study. Three weeks after ovariectomy, rats were randomized in different groups and administered PE-TFG, atorvastatin, diosgenin, 17β-estradiol for 12 weeks along with HFD. The sham-operated rats (S.OVX) were fed with a standard pellet diet. At the end of 12-weeks, rats were sacrificed, and blood samples were used to estimate lipid profile, glucose, hepatic markers, TNF-α, and leptin. Liver, kidney, and common carotid artery were isolated for testing oxidative stress markers, mRNA expression of adiponectin, PPAR-γ, and histopathological changes.
Results
Administration of PE-TFG significantly decreased (P < 0.05) total cholesterol, LDL, hepatic markers, leptin, TNF-α and improved mRNA expression of adiponectin and PPAR-γ in HFD-fed OVX-rats. Further, micro and macro hepatic steatosis, inflammation, glomerular hypertrophy, degenerated tubules in kidney, increased tunica intima, and media thickness of common carotid artery and the pathological changes were not significant upon PE-TFG administration compared to S.OVX-rats.
Conclusion
PE-TFG protects cellular inflammation and metabolic alternations in HFD-fed OVX-rats and thus can be explored further in postmenopausal diseases as a prophylactic agent.
Keywords: Adiponectin, PPAR- γ, TNF-α, Leptin, α-smooth muscle actin
Abbreviations: TFG, Trigonella foenum graecum; PE, Petroleum ether fraction; OVX, Ovariectamised; HFD, High-fat diet; CVD, Cardiovascular disease; PPAR-γ, Peroxisome proliferator-activated receptor-gamma; TNF-, tumor necrosis factor-alpha; BMI, Body mass index; CMC, Carboxymethylcellulose; TC, Total Cholesterol; TG, triglycerides; LDL, low-density lipoprotein; HDL, High-density lipoprotein; DIS, Diosgenis; ATR, Atorvastatin; E2, 17β-estradiol; AST, Aspartate transaminase; ALT, Alanine transaminase; GSH, Glutathione; TBARS, thiobarbituric acid reactive substances; H&E stain, Hematoxylin and Eosin stain; α-SMA, α -smooth muscle actin; CCA, Common carotid artery; CRI, Cardiac risk index; AI, Atherrogenc index
Graphical abstract
Highlights
-
•
Ovariectomy and high-fat diet increased inflammatory markers and lipids in faemale Wistar rats.
-
•
Dilated sinusoids, necrosis around central vein, lipid droplets in hepatocytes were seen in liver.
-
•
Marked glomerular hypertrophy in kidney, increased thickness of tunica intima and media.
-
•
PE-TFG attenuated leptin, TNF-α adiponectin, PPAR-γ in ovariectomized high-fat-fed rats.
-
•
PE-TFG normalized the liver changes, reduced glomerular hypertrophy.
1. Introduction
Menopause is a physiological condition with a complete cessation of menstruation caused by estrogen deficiency. Women experience many psychological changes like depression, dementia, anxiety, irritability, and sleep disturbances. Blood pressure variations, obesity, hyperlipidemia, and cardiovascular disease (CVD) are common postmenopausal diseases.1 Changes in estrogen and progesterone levels, genetic disposition, poor lifestyle patterns, and depression at mid-age contribute to menopausal symptoms.2 Obesity is one of the risk factors for severe menopausal symptoms. The most important factors leading to obesity are consumption of a high-fat diet (HFD), sedentary lifestyle, and lack of physical activity. Women with central abdominal obesity have high vasomotor scores, nervousness, dissatisfaction in personal life, depression, memory loss, joint pains, and sleeping disorders.3 In comparison to pre-menopausal women, postmenopausal women have a statistically higher rate of overweight/obesity (51.2% vs 41.5%) and abdominal obesity (52% vs 43%).4 The menopausal transition is associated with an increased risk of CVD and increased tunica intima and media thickness.5 Inflammation during menopause is linked to dyslipidemia and is the underlying cause of the metabolic syndrome and etiopathogenesis of atherosclerosis. TNF-α and lipopolysaccharide are inflammatory signals that promote leptin and leptin receptor expression.4 Adiponectin possessing anti-inflammatory and anti-atherogenic properties. Decreased levels of adiponectin are associated with an increased risk for metabolic disorders. PPAR-γ improves the expression of some genes encoding proteins involved in lipid and glucose metabolism and possesses anti-atherogenic and anti-inflammatory actions in endothelial cells.6 Menopausal obesity with increased expression of adiponectin and PPAR-γ can prevent insulin resistance.7 In menopause, the standard treatment is hormone replacement therapy, but it has serious drawbacks, including an increased risk of breast and endometrial cancers.8 Herbal medicines are recommended nowadays because they act on multiple targets and modulate and treat menopausal symptoms holistically. Phytoestrogens are naturally occurring plant-derived compounds that mimic estrogen and improve the quality of life of women.
Trigonella foenum-graecum (TFG) seeds are essential Indian spices that are used commonly in foods. TFG, usually known as fenugreek, belongs to the family Fabaceae. It is widely distributed throughout the world and mainly cultivated in Africa, Asia, and Mediterranean countries. TFG has various medicinal properties, including anti-diabetes, anti-hyperlipidemic, analgesic, anticancer activities, and antioxidant activity.9 TFG seeds contain phytoestrogen, which functions as estrogen mimics and aid in the prevention of hyperlipidemia.10 In our previous study, PE-TFG seed extract demonstrated anti-hyperlipidemic and anti-inflammatory activity in an ovariectomy model.11 Limited studies reporting the hypolipidemic effect of petroleum ether fraction of TFG seed extract on HFD and ovariectomy-induced obesity in rat models at the molecular and histopathological level. Consequently, the present study was focused on investigating the prophylactic effect of petroleum ether fraction of Trigonella foenum-graecum L. seed extract on inflammatory markers and histopathological changes in ovariectomized rats fed with HFD.
2. Materials and methods
2.1. Animals for experiment
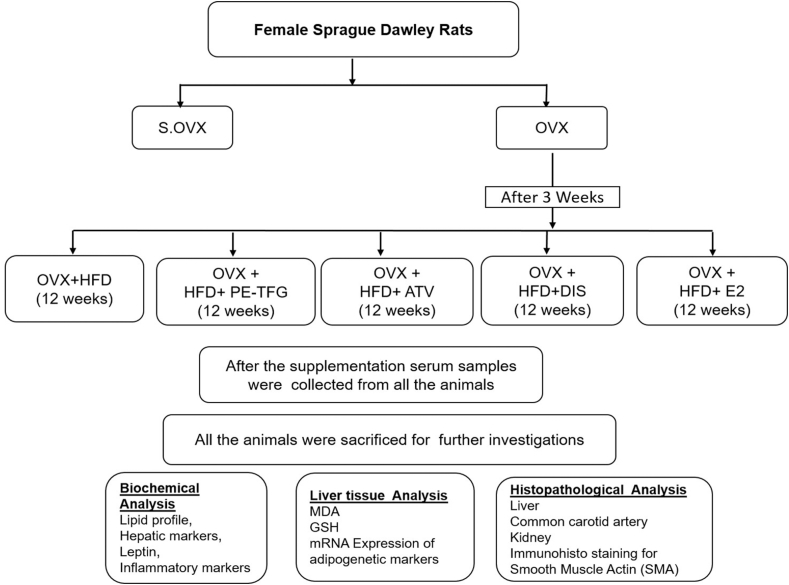
Ethical committee approval (IAEC/KMC/16/2016) was obtained before starting the experiment. The rats used in this study were healthy female Sprague Dawley rats weighing 150–200 g and aged 2–3 months. Animals have been maintained according to the guidelines of the “committee for the purpose of control and supervision of experiments on animals” guidelines, Government of India. Sham ovariectomy rats were fed with a normal pellet diet and water ad libitum.
2.2. Induction of menopause and study design
Thirty-six rats were randomly grouped as Sham ovariectomy (S.OVX) and OVX. The OVX rats were sedated by intraperitoneal (i.p) injection of a mixture of ketamine (50 mg/kg b.w) and xylazine (5 mg/kg b.w). The rats were prepared for lower abdominal surgery after examining the withdrawal, blinking reflexes, and bilateral ovariectomy was performed.12 The above surgical procedure was carried out in sham-operated animals without removing ovaries. After three weeks of ovariectomy (OVX), the rats were assigned randomly into Ovariectomy + High-fat diet (OVX + HFD), Ovariectomy + HFD + Petroleum ether fraction of TFG (OVX + HFD + PE-TFG), Ovariectomy + HFD + Atorvastatin (OVX + HFD + ATR), Ovariectomy + HFD + Diosgenin (OVX + HFD + DIS), Ovariectomy + HFD + 17β-estradiol (OVX + HFD + E2) groups and treated with HFD along with PE-TFG seed extract, ATR, DIS, and E2 respectively for 12 weeks (see the supplementary material for details: Fig. S1).
2.3. Preparation of test materials
PE-TFG was formulated in 0.5% Carboxymethylcellulose (CMC) and given orally at 50 mg/kg/day. Diosgenin: (Sigma-Aldrich) was formulated in 0.5% CMC and given orally at 50 mg/kg/day. Atorvastatin was formulated in 0.5% CMC and given orally at 10 mg/kg/day. 17β-estradiol: (E8515-5 G, Sigma-Aldrich) was formulated in sesame oil and injected subcutaneously at 100 μg/kg/day. Preparation of PE-TFG and HP-TLC investigations were as per our earlier reports.13
Composition of high-fat diet (5 kg): The composition of the high-fat diet was based on the previous literature.14 Lard - 2 kg, Normal pellet powder - 1½ kg, Casein - ½ kg, Sucrose - ½ kg, Cholesterol – 250 g, Vitamin - 100 ml and Choline bitartrate 50 g.
2.4. Methodology
After 12 weeks of supplementation, a fasting sample (2 ml blood) was collected from rats through puncturing retro-orbital plexus under anaesthesia, and serum was collected. The liver, kidney, and common carotid artery (CCA) were immediately separated for further analysis after all the rats were sacrificed. The liver tissue was used for adiponectin, PPAR- γ expression of mRNA, estimation of antioxidant and oxidative stress markers. 10% formalin was used to fix a small piece of kidney, liver, and CCA for histopathological examination.
2.5. Body mass index (BMI) and biochemical parameters
BMI was calculated by body weight (g)/Body length cm.2 Lipid profile, glucose, AST, and ALT were assessed by kit method (Aspen Ltd. New Delhi, India). Low-density lipoprotein (LDL) was calculated by the Friedewald formula. The cardiac risk index (CRI = TC/HDL) and atherogenic index (AI = log (TG/HDL) were calculated.10 Glutathione (GSH) and thiobarbituric acid reactive substances (TBARS) were evaluated by standard methods.11 The serum leptin (RayBio Leptin ELISA kit) and TNF- (ELR-TNFa-CL, RayBio®) were estimated according to the manufacturer's protocol.
2.6. Expression of adiponectin and PPAR-γ
The PureLink RNA isolation, Superscript III First-strand synthesis kit (Invitrogen, USA) was used to obtain RNA from liver tissue and cDNA, respectively. The cDNA template was amplified for GAPDH, PPAR-γ, and adiponectin genes. PeqSTAR Thermal cycler (PeqLab, Germany) was used to carry out PCR. Ethidium bromide-stained agarose gel was used to detect PCR products. The sequence of the primers are as follows: GAPDH Forward –CTAGAGACAGCCGCATC; GAPDH Reverse- GGGTAGAGTCATACTGGAAC: Adiponectin Forward-AATCCTGCCCAGTCATGAAG; Adiponectin Reverse- CATCTCCTGGGTCACCCTTA: PPAR-γ Forward- CATGACCAGGGAGTTCCTCAA. PPAR-γ Reverse- GCAAACTCAAACTTAGGCTCCATAA. The number of replicates for each group was six. Image J software was used to obtain the intensity of the bands and represented as a graph.
2.7. Histopathological analysis
Formalin-fixed liver tissue, kidney, and CCA were processed, stained with hematoxylin and eosin (H&E).15 Further, immunohistochemistry was performed to see the expression of α -smooth muscle actin in the liver tissue was as per the standard protocol.16
2.8. Statistical analysis
One-way ANOVA with Bonferroni's post-hoc test was used to analyze the data by Graph Pad Prism Version 5.0. Results were expressed as mean ± SEM. P ≤ 0.05 was considered significant.
3. Results
3.1. Body mass index (BMI)
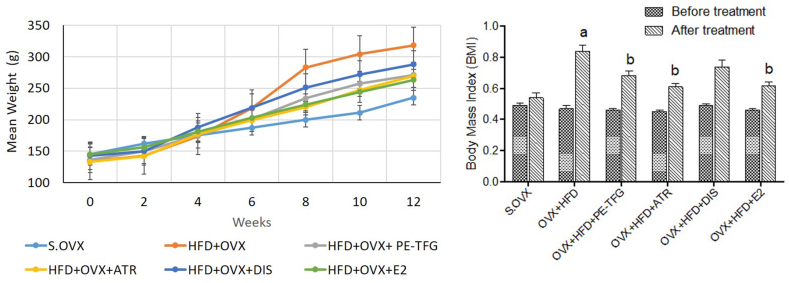
The BW of the animals was measured for 12 weeks at weekly intervals. There was no evident change in the mean BW of the different groups at zero weeks viz., S.OVX (145 ± 3.4), OVX + HFD (134 ± 4), OVX + HFD + PE-TFG (136 ± 2.1), OVX + HFD + ATR (133 ± 2.2), OVX + HFD + DIS (143 ± 4), and OVX + HFD + E2 (145 ± 3). Body weight was elevated significantly at the end of the 12th week (P < 0.05) in the OVX + HFD group (318 ± 3.2) compared to the S.OVX group (235 ± 3.8). The BW of the treated groups decreased significantly (P < 0.05; OVX + HFD + PE-TFG: 271 ± 3.3; OVX + HFD + ATR: 269 ± 4.1; OVX + HFD + DIS: 288 ± 3.2 and OVX + HFD + E2: 263 ± 3) compared to the OVX + HFD group.
There was no substantial variation in BMI between the groups at the starting of the experiment. However, a significant change was observed (P < 0.05) in the BMI of the OVX + HFD group (0.83 ± 0.03) compared to S.OVX (0.51 ± 0.03) after 12 weeks. Subsequently, treatment with PE-TFG (0.68 ± 0.02), ATR (0.62 ± 0.02), DIS (0.73 ± 0.03), and E2 (0.63 ± 0.02), the BMI level was significantly reduced compared to the OVX + HFD group (see Supplementary material: Fig. S2).
3.2. Results of biochemical tests
3.2.1. Lipid profile and glucose levels
The serum lipid profile (TC, TG, and LDL) levels were significantly elevated (P = 0.01) in OVX + HFD compared to S.OVX group. However, after the supplementation with test materials, lipid profile levels were reduced (P = 0.05) in OVX + HFD group. Serum HDL decreased significantly (P = 0.02) in OVX + HFD group compared to S.OVX group. Serum HDL levels were increased though it was not significant compared to OVX + HFD group, after the administration of test materials. The percentage efficacy of the test material (PE-TFG) for TC (65%), TG (62%) and LDL (45%) compared to S.OVX. The atherogenic index (AI) and cardiac risk index (CRI) increased in OVX + HFD group compared to S.OVX group. These values significantly decreased (P = 0.04) after the administration of test materials. Glucose level was increased (P = 0.03) in OVX + HFD group compared to S.OVX. Following the supplementation of test materials, this level was reversed (see Table 1).
Table 1.
Effects of PE-TFG on lipid profile and glucose in ovariectomized high-fat-fed rats.
| Parameters | S.OVX | OVX + HFD | OVX + HFD + PE-TFG | OVX + HFD + ATR | OVX + HFD + DIS | OVX + HFD + E2 |
|---|---|---|---|---|---|---|
| Total Cholesterol (TC, mg/dl) | 62.4 ± 3.3 | 109 ± 4.5a | 82.7 ± 4.3 b | 77.6 ± 4.1b | 74 ± 3.2 b | 67.8 ± 3.6 b |
| Triglycerides (mg/dl) | 62.7 ± 3.5 | 125 ± 3.6a | 83 ± 3.9b | 71 ± 3 b | 68.3 ± 1.7 b | 78.3 ± 2.3 b |
| LDL (mg/dl) | 26 ± 4.1 | 70.5 ± 4.1a | 48.2 ± 4.5b | 43 ± 4.6 b | 40 ± 3.4 b | 32.3 ± 3.8 b |
| HDL (mg/dl) | 24 ± 1.6 | 14 ± 0.7a | 18 ± 0.9 | 19 ± 0.7 | 19 ± 0.7 | 18.2 ± 0.5 |
| Atherogenic index (AI) | 0.39 ± 0.04 | 0.91 ± 0.02a | 0.62 ± 0.03b | 0.53 ± 0.02 b | 0.63 ± 0.01 b | 0.58 ± 0.02 b |
| Cardiac risk index (CRI) | 2.6 ± 0.23 | 7.8 ± 0.35a | 4.5 ± 0.32b | 3.9 ± 0.27 b | 4 ± 0.1 b | 3.4 ± 0.2 b |
| Glucose (mg/dl) | 67 ± 3 | 122 ± 2.1a | 98 ± 1.9b | 115 ± 1.1 | 97 ± 1.3 b | 105 ± 1.6 b |
The values are expressed in mean ± SEM. aP <0.05: S.OVX vs. OVX + HFD and treated groups; bP < 0.05: OVX + HFD vs. OVX + HFD treated groups (One-way ANOVA, Bonferroni's test); S.OVX: Sham ovariectomy, OVX: Ovariectomy, HFD: High-fat diet, PE-TFG: Petroleum ether fraction of Trigonella foenum-graecum, ATR: Atorvastatin, DIS: Diosgenin, E2: 17β- Estradiol.
3.2.2. Serum AST and ALT levels
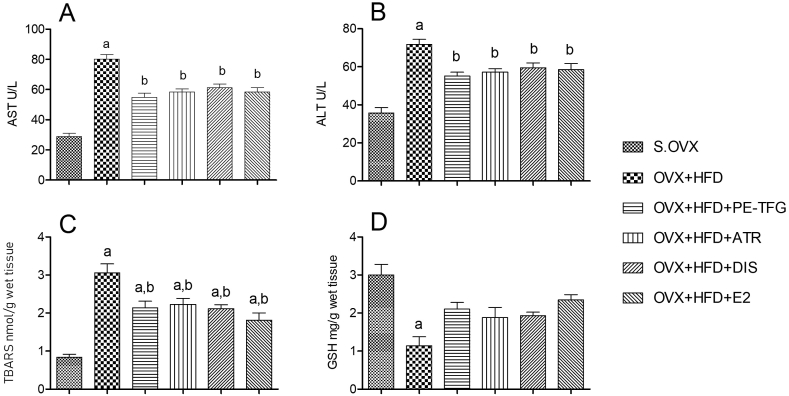
AST and ALT levels were significantly increased (P = 0.01) in OVX + HFD group compared to S.OVX group. Supplementation with test materials to OVX + HFD rats significantly reduced (P = 0.05) the hepatic makers compared to OVX + HFD group. The percentage efficacy of the PE-TFG for AST (49%) and ALT (36%) compared to S.OVX (Fig. 1A and B).
Fig. 1.
Serum and liver parameters in prophylactic treatments to the ovariectomized high-fat-fed rat model. A: Serum AST; B: Serum ALT; C: Liver TBARS; D: Liver GSH; aP <0.05: S.OVX vs. OVX + HFD and treated groups; bP < 0.05: OVX + HFD vs. OVX + HFD treated groups; AST: Alanine transaminase; AST: Aspartate transaminase; TBARS: Thiobarbituric acid reactive substances; GSH: Reduced glutathione; S.OVX: Sham ovariectomy, OVX: Ovariectomy, HFD: High-fat diet, PE-TFG: Petroleum ether fraction of Trigonella foenum-graecum, ATR: Atorvastatin, DIS: Diosgenin, E2: 17β- Estradiol.
3.2.3. TBARS and reduced glutathione (GSH) levels in the liver lysate
TBARS are the potent marker of lipid peroxidation. The TBARS were significantly (P = 0.001) elevated, and GSH was reduced in OVX + HFD rats compared to S.OVX. Supplementation with test materials reduced TBARS (P = 0.03), and markedly elevated GSH compared to OVX + HFD group. The percentage efficacy of the PE-TFG for TBARS (56%) compared to S.OVX (Fig. 1C and D).
3.2.4. Tumor necrosis factor-α (TNF-α) and leptin in serum
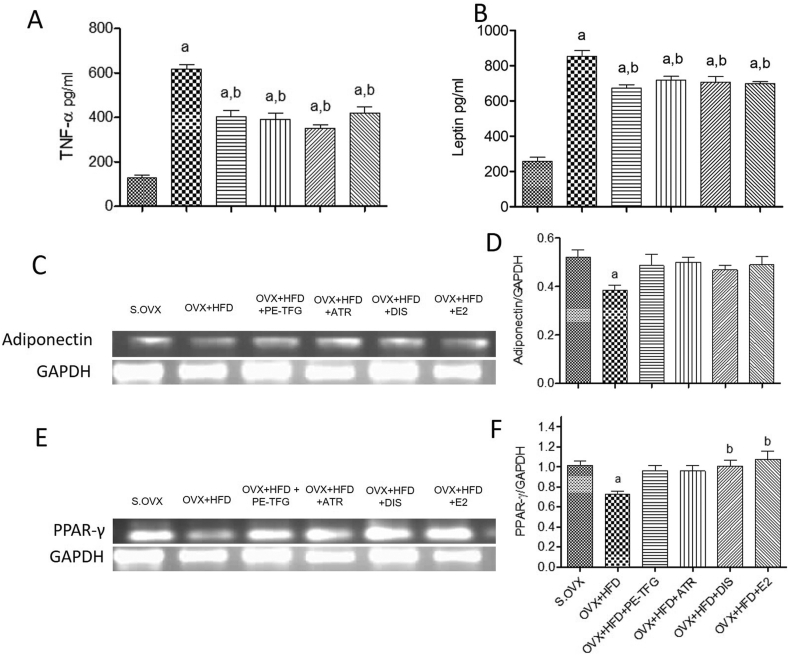
Serum TNF-α and leptin were elevated significantly (P = 0.001) in OVX + HFD group compared to S.OVX group. Serum TNF-α level leptin levels significantly decreased after supplementation with PE-TFG extract and ATR The percentage efficacy of the PE-TFG for TNF-α (48%) and leptin (59%) compared to S.OVX (Fig. 2A and B).
Fig. 2.
Blood and liver inflammatory markers in prophylactic treatments to the ovariectomized high-fat-fed rat model. A: Blood TNF-α; B: Blood leptin; C: Adiponectin, and GAPDH mRNA expression; D: Quantification of Adiponectin expression; E: PPAR-γ and GAPDH mRNA expression; F: Quantification of PPAR-γ expression; aP <0.05: S.OVX vs. OVX + HFD and treated groups; bP < 0.05. OVX + HFD vs. OVX + HFD treated groups; TNF-α: Tumor necrosis factor alfa; PPAR-γ: Peroxisome proliferator-activated receptor-gamma; S.OVX: Sham ovariectomy, OVX: Ovariectomy, HFD: High-fat diet, PE-TFG: Petroleum ether fraction of Trigonella foenum-graecum, ATR: Atorvastatin, DIS: Diosgenin, E2: 17β- Estradiol.
3.2.5. Adiponectin and PPAR-γ mRNA expression in liver tissue
In OVX + HFD group, adiponectin and PPAR-γ expression were significantly lower (P = 0.04) than the S.OVX group (Fig. 2C and D). Supplementation with PE-TFG improved the adiponectin and PPAR- γ mRNA expression (Fig. 2E and F).
3.3. Histopathological analysis
3.3.1. Histology of liver
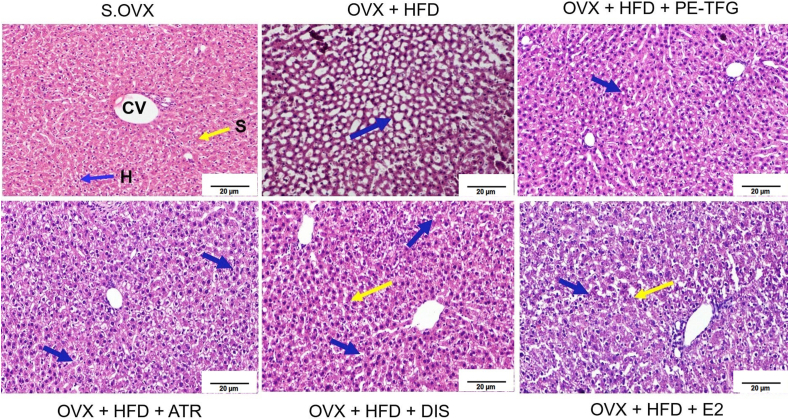
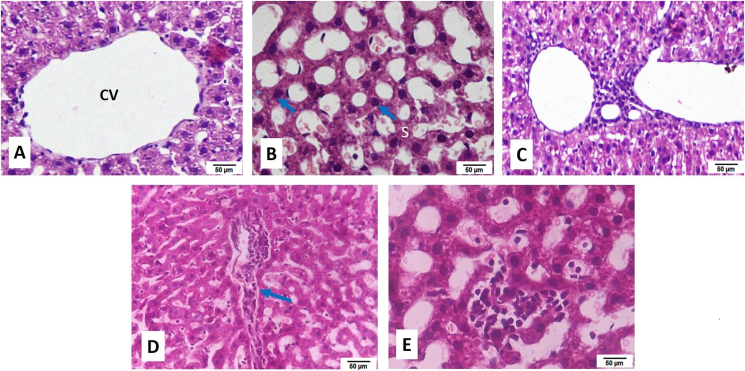
OVX + HFD group showed dilated sinusoids and massive macro steatosis. Micro steatosis and mild inflammation were observed in OVX + HFD + PE-TFG treated group. Dilation of sinusoids and micro steatosis was observed in atorvastatin (OVX + HFD + ATR), diosgenin (OVX + HFD + DIS), and estradiol (OVX + HFD + E2) treated groups (Fig. 3).
Fig. 3.
Liver histopathology of ovariectomized high-fat-fed rats after prophylactic treatments. H&E staining; objective lens, X40; S.OVX group demonstrated usual liver morphology, with definite hepatocytes (H), central vein (CV), sinusoidal spaces (S), and well-preserved cytoplasm with conspicuous nuclei. Blue Arrow-Hepatocytes (H), Yellow arrow- Sinusoids (S). S.OVX: Sham ovariectomy, OVX: Ovariectomy, HFD: High-fat diet, PE-TFG: Petroleum ether fraction of Trigonella foenum-graecum, ATR: Atorvastatin, DIS: Diosgenin, E2: 17β- Estradiol.
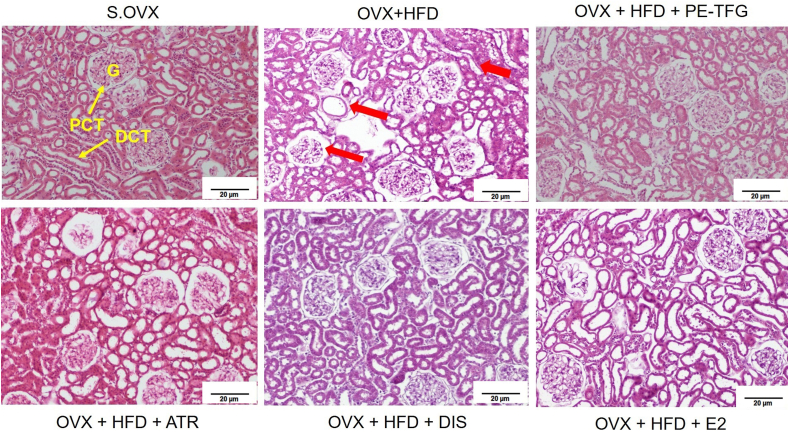
3.3.2. Histology of kidney
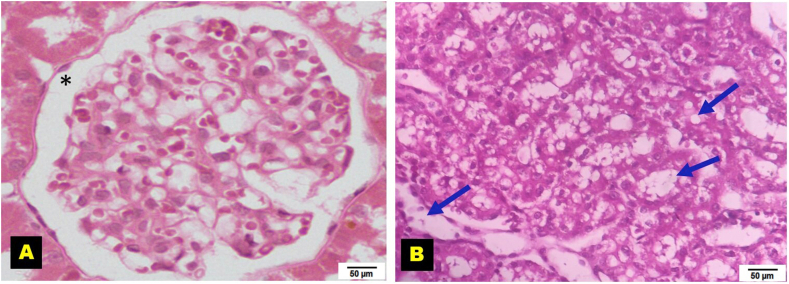
The S.OVX group had a normal medullary structure, while the OVX + HFD group had glomerular hypertrophy, severe inflammation, and degenerated tubules. The decreased inflammation and regeneration of the cells was observed in treated groups (Fig. 4; for detailed aspects, see supplementary figures Figs. S3 and S4).
Fig. 4.
Kidney histopathology of ovariectomized high-fat-fed rats after prophylactic treatments. H&E staining; objective lens, ×40; G: Glomerulus, DCT: Distal convoluted tubule, PCT: Proximal convoluted tubule. S.OVX: Sham ovariectomy, OVX: Ovariectomy, HFD: High-fat diet, PE-TFG: Petroleum ether fraction of Trigonella foenum-graecum, ATR: Atorvastatin, DIS: Diosgenin, E2: 17β- Estradiol.
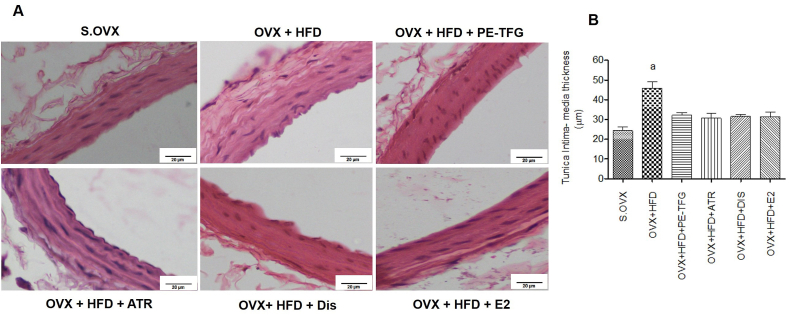
3.3.3. Histology of common carotid artery
OVX + HFD group had increased (P = 0.05) the thickness of tunica intima and media (45.9 ± 3.2) compared to S.OVX group (24.3 ± 2.1). After the treatment, the thickness was reduced in PE-TFG (36.1 ± 1.9), ATR (33.3 ± 1.7), DIS (37 ± 3.2), and E2 (35.3 ± 1.7) compared to OVX + HFD group (Fig. 5).
Fig. 5.
Histopathology Common Carotid Artery of ovariectomized high-fat-fed rats after prophylactic treatments. A: H&E staining of CCA in a prophylactic model of OVX group fed with HFD (objective lens, ×40). B: Thickness of tunica intima and media of the CCA in a prophylactic model of OVX group fed with HFD. aP<0.05: S.OVX vs. OVX + HFD; CCA: Common carotid artery; S.OVX: Sham ovariectomy, OVX: Ovariectomy, HFD: High-fat diet, PE-TFG: Petroleum ether fraction of Trigonella foenum-graecum, ATR: Atorvastatin, DIS: Diosgenin, E2: 17β- Estradiol.
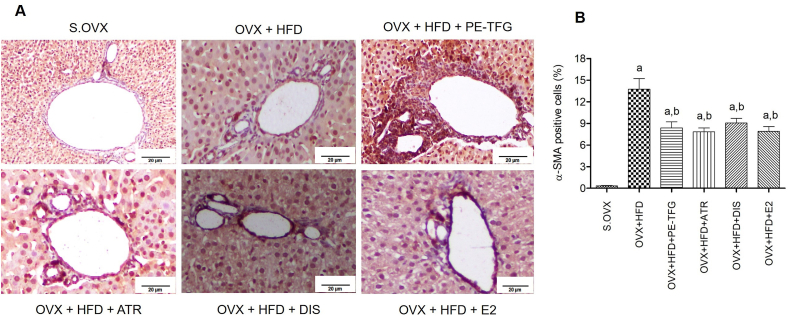
3.4. Immunohistochemistry of the liver sections
In the OVX + HFD group, the percentage area of the fibrotic field was enhanced (P = 0.05) compared to the S.OVX group. However, the same was found to be significantly reversed after the treatment with test materials (Fig. 6).
Fig. 6.
A: Immunohistochemistry of α-smooth muscle actin (α-SMA) of liver sections after treatment in the prophylactic rat model. The objective lens, X40; Percentage of α- SMA liver sections in the prophylactic model of OVX rats fed with HFD; aP<0.05: Treatment groups Vs S.OVX; b P<0.05: HFD+OVX vs. OVX+HFD+treated groups. S.OVX, Sham ovariectomy, OVX: Ovariectomy, HFD: High-fat diet, PE-TFG: Petroleum ether-Trigonella foenum-graecum, ATR: Atorvastatin, DIS: Diosgenin, E2: 17β- Estradiol.
4. Discussion
The bilateral ovariectomized rat model is a commonly used animal model to represent human menopausal features like ovarian hormone cessation, obesity, osteoporosis, metabolic changes, oxidative damage and immune dysfunction.10 We have used ovariectomized rats fed with HFD to see the inflammatory stress and to assess the modulations by TFG seed extract. In comparison to S.OVX rats, the BMI of OVX + HFD rats had significantly increased. Obesity is induced by consumption of high-fat alone and, in tandem with ovariectomy, the ovariectomy-induced changes were exacerbated. In our study, it was observed that OVX + HFD rats were vulnerable to weight gain and increased fat mass relative to S.OVX. This effect may be due to the loss of ovarian hormones, and HFD induced obesity favoured the production of pro-inflammatory cytokines. In support of our results, feeding HFD for 8-weeks did not change body weight significantly. However, a combination of HFD diet and OVX increased body weight and improved retroperitoneal and mesenteric tissue weight.17 Similar to our findings, the hyperlipidemic diet-fed ovariectomized rats for four weeks induced hyperlipidemia, increased body weight, and lipid content in carcasa.18 The Oxphos-CR genes, PGC1-responsive genes involved in fatty acid metabolism and energy expenditure, are downregulated in adipose tissue by ovariectomy.19 A 12-week HFD administration impaired the body composition and augmented visceral adiposity. However, co-administering a standardized TFG seed extract based on low molecular weight galactomannans considerably inhibited the HFD-induced mice body weight.20 Contrary to these results, mice fed with HFD without TFG showed high body weight, and incorporating whole TFG seed in food (2% w/w) did not impact body weight composition and caloric intake of HFD-mice.21 The therapeutic effect of diosgenin on obesity was previously reported. OVX induced weight gain and uterine wet weight loss was reversed after the treatment with diosgenin at different doses for 12 weeks.22
In comparison to S.OVX rats, OVX + HFD rats had significantly increased TC, TG, and LDL levels. Though, the serum lipid levels were reversed after treated with PE-TFG extract and DIS. These findings are supported by a previous study, OVX animals fed with high dietary cholesterol showed cholesterol accumulation in the liver, reducing the gene expression of key VLDL synthesis markers leading to a decline in the elimination of cholesterol from the liver. Vijayakumar et al., 2010, investigated lowered SREBP-1 (sterol regulatory element-binding protein-1) and higher LDL receptors after treatment with aqueous extract of TFG in rats fed with HFD and observed that TFG had a potential effect on dyslipidemia and inhibited the lipid accumulation.23 These results support the use of TFG in a postmenopausal condition other than its proven effects on dyslipidemia, partly decrease in fat accumulation. Treatment with TFG seed extract (200 mg/kg) reduced TC and TG due to the increased activity of lecithin cholesterol acyltransferase (LCAT-favoring the inclusion of more cholesterol in HDL and uptake by the liver) by 47%, lipoprotein lipase (20.8%), triglyceride lipase (34%), and improved the elimination of bile acids.24 The estrogenic activity of TFG was also reported in a recent study which is attributed to the presence of phytoestrogens.25 Lipid levels are lowered by TFG seed extract due to its saponin content, the fiber mannose, and galactose, which are the main ingredients of the gum.26 Other natural phytoestrogen White Kwao Krua supplementation (100 mg/kg/day) reduced TC, LDL cholesterol, increased HDL, and alleviated vascular damage in HFD fed rabbits.27
Cardiac Risk Index (CRI) is perceived to be a reliable predictor for evaluating the risk of coronary heart disease (CHD). Furthermore, dyslipidemia and hypertension are well-known risk factors of CHD. Variations in the level of any lipid profile cause the individuals more susceptible to atherosclerotic complications. There is a positive association between AI and TC, LDL, triglyceride, and a substantial negative correlation between AI and HDL. An increase in AI was observed in a previous study with an increase in TC, triglyceride, and LDL. Aqueous extract of TFG is a potential applicant in the management of cardiac risk indexes.28 In our study, TFG seed extract reduced the lipid levels due to its phytoestrogen action and high fiber content. OVX-rats fed with HFD showed increased serum glucose levels. In contrast to our findings, ovariectomy followed by HFD/STZ (streptozotocin) had no impact on fasting blood sugar levels but increased insulin resistance. The loss of ovarian hormones involved in insulin resistance independent of their energy status. Insulin sensitivity is also associated with changes to adipocytokine secretion and low-chronic inflammation in white adipose tissue (WAT). Ovarian hormones contribute to the maintenance of insulin sensitivity and preventing low-grade inflammation of WAT. Hence, animal experiments on ovariectomy in rats have decreased glucose absorption and insulin sensitivity.29 TFG controls blood sugar levels mainly by inhibiting carbohydrate digestion and absorption and enhancing the peripheral activity of insulin. The existence of soluble glucomannan fiber in TFG seeds, which reduces the absorption of sugars from the intestine. Trigonelline and fenugrecin are well-known hypoglycemic alkaloids, and the amino acid 4-hydroxy isoleucine acts on the β-cells of the pancreas to produce insulin.24 In a study TC, TG and LDL were significantly decreased after 4-weeks of intervention in the green tea taking postmenopausal women group compared with control.30 A study demonstrated decreased glucose levels in diosgenin pretreated control rats and in STZ induced diabetic rats. Mean blood glucose levels in rats treated with different doses of diosgenin are significantly decreased.31
An increased level of liver enzymes (ALT, AST) indicates liver dysfunction. Inflammatory hepatocellular disorders can increase the transaminase level considerably.32 According to studies, nonalcoholic fatty liver disease developed in OVX rats fed with HFD (three weeks) had significantly higher AST and ALT levels.29 TFG seeds contain biologically active flavonoids such as vitexin, naringenin, tricin, tricin-7-O-beta-d-glucopyranoside, and cortsin, which can protect against liver damage initiated by oxidative stress. Saponin (DIS) isolated from TFG was found to lower serum ALT and eliminate lipid zones in the liver tissue of rats fed HFD.33
Elevated TBARS in OVX and OVX rats fed with HFD were observed in the present study. Declined estrogen levels in menopause are correlated with increased oxidative stress. Two weeks post-OVX, rats fed with HFD for 8-weeks showed 27% higher TBARS levels and 25% greater in the sham-HFD group than in the respective control groups. GSH in OVX-HFD rats was 27% lower and 29% lesser in sham-HFD rats compared to healthy controls.34 TFG administration reduced lipid peroxidation might also be attributed to its estrogenic components, including saponins (diosgenin), trigonelline, and flavonoids.
In our study, increased TNF-α was observed in OVX rats fed with HFD. Excess formation of cytokines may be affected by the fat and bone marrow. TNF-α contributes to vascular dysfunction and decreases the NO synthase expression in endothelial cells. Earlier experiments observed higher TNF-α levels in postmenopausal women compared to pre-menopausal women. Intraperitoneal administration of estradiol (10 μg/kg) one day after ovariectomy and intervention for 42 days substantially reduced the levels of IL-1, IL-6, and TNF- α in the OVX group.10 TFG extract significantly reduced serum pro-inflammatory cytokines (IL-1,6 and TNF-α).35 NF-κB and glucocorticoid receptors (GRs) regulate the secretion of these cytokines. Diosgenin increased the expression and activation of GRs because both glucocorticoids and diosgenin have a common steroid structure. Activated GRs suppress the expression of NF-κB by binding directly to cAMP response element-binding protein, which results in reduced pro-inflammatory cytokine secretion.36
Leptin levels are elevated in OVX + HFD compared to S.OVX rats. Previously, OVX mice treated with HFD resulted in significant weight gain and leptin insensitivity. However, after one month of supplementation with E2, mice were responsive to leptin's anorectic effect.37 Estrogen can modulate the activity of leptin by modifying the LEPR expression in growth plate chondrocytes via the estrogen receptors and triggering the eRK1/2 signaling pathways.38 The deposition of fat and fat cells is sex-dependent and influences leptin secretion. Recent experiments have confirmed that the plasma concentration of leptin in obese female rats was two times higher than in obese male rats.39 In a previous study, treatment with AqE-TFG in reduced leptin levels resulted in substantial loss of adipocytes in obese rats induced by HFD.28 Low molecular weight water-soluble galactomannan-based TFG seed extract in brown adipose tissue, improved HFD-induced obesity in C57BL/6 mice by inhibiting up-regulated mRNA levels of leptin and TRIP-Br2.20
PE-TFG seed extract enhanced expression of adiponectin in OVX + HFD rats. Adiponectin mRNA in rats treated with E2 and HFD were greater than in rats treated with HFD, and a comparable pattern was observed in rats treated with E2 compared to the control group.40 Adiponectin can influence steroid gene expression and steroidogenesis, and the effect depends on the hormone levels. A study supports these findings, HFD fed mice treated with TFG (2 g/kg/day) increased adiponectin expression in subcutaneous inguinal adipose tissue but had no effect in visceral epididymal adipose tissue.21 Diosgenin supplementation reversed the downregulation of mRNA expressions of PPAR-γ, adiponectin, and arginase-1.41 Obesity is associated with decreased PPAR-γ activity. The positive effects of TFG on obesity and hyperlipidemia are due to the insulin-like effect, increased adiponectin, and PPAR-γ expression. Supporting our findings, two weeks of TFG extract supplementation substantially improved mRNA PPAR expression in high fructose diet (eight weeks), feeding rats compared to the control group.42 HFD fed mouse treated with Chinese yam sanyaku and diosgenin showed moderately up-regulated lipoprotein lipase mRNA levels and PPAR-γ.43
Lipid metabolism is affected by estrogen deficiency and increases COX-2-dependent visceral fat storage.44 In another study, the OVX group, fed with high-fat and fructose diet-induced nonalcoholic steatohepatitis (NASH), significantly in both non-OVX and OVX-rats.45 In another study, HFD (2 weeks) ∕ Streptozotocin (120 mg ∕kg) intraperitoneally induced type II diabetes showed improved adiposity, mild and focal microvascular steatosis. The liver showed standard architecture with no inflammation, steatosis, and necrosis with the supplementation of TFG seed extract (100 mg/kg/BW). These results indicate that TFG possesses good antioxidant activity.46 Contrary to our results, a study observed that TFG had minimal or no influence on hepatic steatosis.21
ROS-mediated inflammatory cytokine production takes place in postmenopausal women with a hormone deficiency, which leads to changes in renal architecture. TFG provides defense against morphological alterations in diabetic rats of the kidneys by elevating antioxidant activity and preventing the aggregation of oxidized DNA.47 The pro-inflammatory cytokine levels are decreased by the sapogenins (diosgenin), polyphenols, 4-hydroxy-isoleucine, alkaloids, and trigonelline present in TFG seed extract.24
High cholesterol diets damage the endothelial lining of the major arteries and the heart, resulting in hypertension and, ultimately, atherosclerosis in both perimenopausal and postmenopausal women.48 The key pathways implicated in diminished vascular response in OVX-models are correlated with reduced NO bioactivity and attenuation of endothelial-derived hyperpolarizing factors. Increased superoxide anion levels and protein expression of NADPH subunits, as gp91phox and p22phox, were accompanied by decreased endothelial function in OVX rats.49 Ovariectomy along with HFD changed the TI/TM ratio, and these findings may be indicators of early atherosclerosis. It is found that diosgenin prevents the vascular cell adhesion molecule (VCAM-1), intracellular adhesion molecule (ICAM-1), proteins expression associated with atherosclerosis, prevents the generation of intracellular ROS by TNF-α and NF-κB and IκB kinase activation as well as degradation of IκBα and nuclear translocation of NF-κB. TFG and diosgenin have antioxidant properties, as well as the ability to inhibit H2O2-induced apoptosis in human endothelial cells.46
α-SMA is a specific marker for smooth muscle cell differentiation. In our study, lack of estrogen and HFD developed nonalcoholic liver steatosis. E2 treatment significantly reduced AST, ALT, hyaluronic acid (HA), collagen type IV (C-IV) in serum, hepatic collagen was suppressed, and hepatic stellate cells were significantly decreased for α-SMA in ovariectomized female rats with hepatic fibrosis caused by carbon tetrachloride. CCl4 toxicity-induced rat liver showed vacuolization of cytoplasm, lymphocyte infiltration in the central vein, fibrosis, and calcification. The fibrosis was reversed after 7-weeks of therapy with 10% TFG seed extract.50 This beneficial impact may be attributed to the presence of flavonoids, polyphenols, and polysaccharides, which are effective in preventing lipid peroxidation, hepatic stellate cell activation by disrupting signal transduction, and protein cell activation.51 Genistin (structurally similar to 17β-estradiol) and exercise ameliorated liver fibrosis and suppressed the IL-13 expression in OVX rats fed with a high-fat, high fructose diet.52
Abendinzade et al., 2015, reported hexanic (50 and 150 mg/dL) and ethanolic extract (50 and 150 mg/dL) of TFG, estradiol decreases (P < 0.05) IL-1, IL-6 and TNF-α in the ovariectomized rats.10 Whereas, in the current study, a single dose of PE-TFG (50 mg/kg) seed extract was used. In OVX + HFD rats, it was observed significantly increased glucose, cholesterol, triglyceride, LDL, TBARS, hepatic markers, leptin and TNF- α and decreased HDL, GSH levels. PE-TFG treatment significantly decreased (P < 0.05) total cholesterol, LDL, hepatic markers, leptin, and TNF-α. Increased mRNA expression of adiponectin and PPAR-γ in OVX rats fed with HFD. Further, micro and macro hepatic steatosis, inflammation, glomerular hypertrophy, degenerated tubules in the kidney, increased tunica intima and media thickness of the common carotid artery were observed. These pathological changes were not seen in rats supplemented with PE-TFG. Thus the current study explains the role of TFG in the mitigation of inflammation induced by menopause.
5. Limitations of the study
The present study observed the beneficial effects of supplementation of PE-TFG seed extract in ovariectomy followed by high-fat diet model. The study focused only on the effect of PE-TFG seed extract on biochemical and cellular inflammatory modifications. Further investigations directed towards identifying the multiple signaling pathways involved in the protective effects of the PE-TFG seed extract may help us better understand the molecular mechanisms. Another limitation in our study was we have used only a single dose of TFG seed extract. Further studies to optimize the dose of TGF extract may provide insight into its dose-related biological activity.
6. Conclusion
The present study results indicate that ovariectomy with high-fat diet significantly increases inflammatory markers, oxidative stress, lipid accumulation in the liver, increase of tunica intima and media thickness of common carotid artery, reduced mRNA expression of adiponectin and PPAR-γ. These adverse inflammatory effects were reversed by the supplementation of PE-TFG seed extract. The protective effect of TFG in ovariectomy followed by HFD is attributed to the presence of diosgenin that mimics the estrogenic activity along with other phenols and flavonoids present in the extract. Further studies are essential to appreciate the phytoestrogen effect of TFG in postmenopausal inflammatory diseases.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Approval from the Institutional Animal Ethical Committee of Manipal Academy of Higher Education, Manipal, was obtained before the study (IAEC/KMC/16/2016).
Declaration of competing interest
The authors declare that there is no conflict of interest in publishing the data generated in this work.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.07.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
References
- 1.Augoulea A., Moros M., Lykeridou A., Kaparos G., Lyberi R., Panoulis K. Psychosomatic and vasomotor symptom changes during transition to menopause. Prz Menopauzalny. 2019;18(2):110–115. doi: 10.5114/pm.2019.86835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Namazi M., Sadeghi R., Moghadam Z.B. Social determinants of health in menopause: an integrative review. Int J Womens Health. 2019;11:637–647. doi: 10.2147/IJWH.S228594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis S.R., Castelo-Branco C., Chedraui P., et al. Writing group of the international menopause society for world menopause day 2012. Understanding weight gain at menopause. Climacteric. 2012;15(5):419–429. doi: 10.3109/13697137.2012.707385. [DOI] [PubMed] [Google Scholar]
- 4.Huang W.-Y., Chang C.-C., Chen D.-R., Kor C.-T., Chen T.-Y., Wu H.-M. Circulating leptin and adiponectin are associated with insulin resistance in healthy postmenopausal women with hot flashes. PloS One. 2017;12(4) doi: 10.1371/journal.pone.0176430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ieamtairat P., Soontrapa S., Kaewrudee S., Promsorn J., Takong W., Somboonporn W. Difference in carotid intima-media thickness between pre and postmenopausal women. Menopause. 2019;26(1):39–44. doi: 10.1097/GME.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 6.Tyagi S., Gupta P., Saini A.S., Kaushal C., Sharma S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. "J Adv Pharm Technol Research"" (JAPTR)". 2011;2(4):236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn S.V., Jung D.-H., Yadav D., Kim J.-Y., Koh S.-B. Relative contribution of obesity and menopause to the association between serum adiponectin and incident metabolic syndrome. Menopause. 2018;25(2):154–159. doi: 10.1097/GME.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 8.Deli T., Orosz M., Jakab A. Hormone replacement therapy in cancer survivors – review of the literature. Pathol Oncol Res. 2020;26(1):63–78. doi: 10.1007/s12253-018-00569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zameer S., Najmi A.K., Vohora D., Akhtar M. A review on therapeutic potentials of Trigonella foenum graecum (fenugreek) and its chemical constituents in neurological disorders: complementary roles to its hypolipidemic, hypoglycemic, and antioxidant potential. Nutr Neurosci. 2018;21(8):539–545. doi: 10.1080/1028415X.2017.1327200. [DOI] [PubMed] [Google Scholar]
- 10.Abedinzade M., Nasri S., Omodi M.J., Ghasemi E., Ghorbani A. Efficacy of Trigonella foenum-graecum seed extract in reducing metabolic and inflammatory alterations associated with menopause. Iran Red Crescent Med J. 2015;17(11) doi: 10.5812/ircmj.26685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagamma T., Konuri A., Nayak C.D., Kamath S.U., Udupa P.E.G., Nayak Y. Dose-dependent effects of fenugreek seed extract on the biochemical and haematological parameters in high-fat diet-fed rats. J Taibah Univ Med Sci. 2019;14(4):383–389. doi: 10.1016/j.jtumed.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anjaneyulu K., Rai K.S., Rajesh T., Nagamma T., Bhat K.M.R. Therapeutic efficacy of fenugreek extract or/and choline with docosahexaenoic acid in attenuating learning and memory deficits in ovariectomized rats. J Krishna Inst Med Sci Univ. 2018;7(2):10–20. [Google Scholar]
- 13.Nagamma T., Konuri A., Maheshwari R., Udupa E.G., Nayak Y. Efficacy of trigonella foenum-graecum seed extract on ovariectomy-induced hyperlipidemia, oxidative stress, and histopathological changes in rats. Phcog Mag. 2019;15:S274–s279. doi: 10.4103/pm.pm_4_19. [DOI] [Google Scholar]
- 14.Buettner R., Schölmerich J., Bollheimer L.C. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15(4):798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 15.Xu S.-P., Mao X.-Y., Cheng X., Chen B. Ameliorating effects of casein glycomacropeptide on obesity induced by high-fat diet in male Sprague-Dawley rats. Food Chem Toxicol. 2013;56:1–7. doi: 10.1016/j.fct.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Nagamma T., Konuri A., Bhat K.M.R., Maheshwari R., Udupa P., Nayak Y. Modulation of inflammatory markers by petroleum ether fraction of Trigonella foenum-graecum L. seed extract in ovariectomized rats. J Food Biochem. 2021;45(4) doi: 10.1111/jfbc.13690. [DOI] [PubMed] [Google Scholar]
- 17.Gorres B.K., Bomhoff G.L., Gupte A.A., Geiger P.C. Altered estrogen receptor expression in skeletal muscle and adipose tissue of female rats fed a high-fat diet. J Appl Physiol. 2011;110(4):1046–1053. doi: 10.1152/japplphysiol.00541.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neto N.I.P., Rodrigues M.E.S., Hachul A.C.L., et al. A hyperlipidic diet combined with short-term ovariectomy increases adiposity and hyperleptinemia and decreases cytokine content in mesenteric adipose tissue. Mediat Inflamm. 2015:923248. doi: 10.1155/2015/923248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camporez J.P.G., Jornayvaz F.R., Lee H.-Y., et al. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology. 2013;154(3):1021–1028. doi: 10.1210/en.2012-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandhare A.D., Bandyopadhyay D., Thakurdesai P.A. Low molecular weight galactomannans-based standardized fenugreek seed extract ameliorates high-fat diet-induced obesity in mice via modulation of FASn, IL-6, leptin, and TRIP-Br2. RSC Adv. 2018;8(57):32401–32416. doi: 10.1039/c8ra05204b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knott E.J., Richard A.J., Mynatt R.L., Ribnicky D., Stephens J.M., Bruce-Keller A. Fenugreek supplementation during high-fat feeding improves specific markers of metabolic health. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-12846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z., Song C., Fu X., et al. High-dose diosgenin reduces bone loss in ovariectomized rats via attenuation of the RANKL/OPG ratio. Int J Mol Sci. 2014;15(9):17130–17147. doi: 10.3390/ijms150917130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijayakumar M.V., Pandey V., Mishra G.C., Bhat M.K. Hypolipidemic effect of fenugreek seeds is mediated through inhibition of fat accumulation and upregulation of LDL receptor. Obesity. 2010;18(4):667–674. doi: 10.1038/oby.2009.337. [DOI] [PubMed] [Google Scholar]
- 24.Geberemeskel G.A., Debebe Y.G., Nguse N.A. Antidiabetic effect of fenugreek seed powder solution (trigonella foenum-graecum L.) on hyperlipidemia in diabetic patients. J Diabetes Res. 2019;2019 doi: 10.1155/2019/8507453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brogi H., Elbachir H., El Amrani N., Amsaguine S., Radallah D. Fenugreek seeds estrogenic activity in ovariectomized female rats. Curr Issues Pharm Med Sci. 2019;32(3):138–145. [Google Scholar]
- 26.Sundaram G., Ramakrishnan T., Parthasarathy H., Raja M., Raj S. Fenugreek, diabetes, and periodontal disease: a cross-link of sorts! J Indian Soc Periodontol. 2018;22(2):122–126. doi: 10.4103/jisp.jisp_322_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratanachamnong P., Phivthong-Ngam L., Namchaiw P. Daily White kwao krua dietary supplement alleviates LDL oxidative susceptibility, plasma LDL level and improves vasculature in a hypercholesterolemia rabbit model. J Tradit Complement Med. 2020;10(5):496–503. doi: 10.1016/j.jtcme.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar P., Bhandari U., Jamadagni S. Fenugreek seed extract inhibit fat accumulation and ameliorates dyslipidemia in high fat diet-induced obese rats. BioMed Res Int. 2014;2014 doi: 10.1155/2014/606021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pósa A., Szabó R., Kupai K., et al. Exercise training and calorie restriction influence the metabolic parameters in ovariectomized female rats. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/787063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tadayon M., Movahedi S., Abedi P., Syahpoosh A. Impact of green tea extract on serum lipid of postmenopausal women: a randomized controlled trial. J Tradit Complement Med. 2018;8(3):391–395. doi: 10.1016/j.jtcme.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato K., Fujita S., Iemitsu M. Acute administration of diosgenin or dioscorea improves hyperglycemia with increases muscular steroidogenesis in STZ-induced type 1 diabetic rats. J Steroid Biochem Mol Biol. 2014;143:152–159. doi: 10.1016/j.jsbmb.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Chang B.Y., Kim D.S., Kim S.Y. Improvement in menopause-associated hepatic lipid metabolic disorders by herbal formula HPC03 on ovariectomized rats. Evid base Compl Alternative Med. 2020:2020. doi: 10.1155/2020/1409376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z., Lei Y.-L., Wang W.-P., et al. Effects of saponin from trigonella foenum-graecum seeds on dyslipidemia. Iran J Med Sci. 2017;42(6):577–585. [PMC free article] [PubMed] [Google Scholar]
- 34.Vuković R., Blažetić S., Oršolić I., et al. Impact of ovariectomy, high fat diet, and lifestyle modifications on oxidative/antioxidative status in the rat liver. Croat Med J. 2014;55(3):218–227. doi: 10.3325/cmj.2014.55.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steels E., Steele M.L., Harold M., Coulson S. Efficacy of a proprietary trigonella foenum-graecum L. De-husked seed extract in reducing menopausal symptoms in otherwise healthy women: a double-blind, randomized, placebo-controlled study. Phyther Res. 2017;31(9):1316–1322. doi: 10.1002/ptr.5856. [DOI] [PubMed] [Google Scholar]
- 36.Junchao Y., Zhen W., Yuan W., et al. Anti-trachea inflammatory effects of diosgenin from Dioscorea nipponica through interactions with glucocorticoid receptor α. J Int Med Res. 2017;45(1):101–113. doi: 10.1177/0300060516676724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Litwak S.A., Wilson J.L., Chen W., et al. Estradiol prevents fat accumulation and overcomes leptin resistance in female high-fat diet mice. Endocrinology. 2014;155(11):4447–4460. doi: 10.1210/en.2014-1342. [DOI] [PubMed] [Google Scholar]
- 38.Yin C., Kang L., Lai C., et al. Effects of 17β-estradiol on leptin signaling in anterior pituitary of ovariectomized rats. Exp Anim. Published online. 2016:16–87. doi: 10.1538/expanim.16-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozcan S., Ulker N., Bulmus O., Yardimci A., Ozcan M., Canpolat S. The modulatory effects of irisin on asprosin, leptin, glucose levels and lipid profile in healthy and obese male and female rats. Arch Physiol Biochem. Published online. 2020 doi: 10.1080/13813455.2020.1722706. [DOI] [PubMed] [Google Scholar]
- 40.Shang C.G., Liu Z.H., Wang X.H., Feng Z.H., Zhang Y. Effect of high-fat diet-induced disorders on rat with endometrial hyperplasia and adiponectin system in circulation and uterus. Chin Med J. 2017;130(15):1831–1837. doi: 10.4103/0366-6999.211551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y., Xu X., Zhang Y., et al. Diosgenin regulates adipokine expression in perivascular adipose tissue and ameliorates endothelial dysfunction via regulation of AMPK. J Steroid Biochem Mol Biol. 2016;155(Pt A):155–165. doi: 10.1016/j.jsbmb.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Mohammadi A., Gholamhosseinian A., Fallah H. Trigonella foenum-graecum water extract improves insulin sensitivity and stimulates PPAR and γ gene expression in high fructose-fed insulin-resistant rats. Adv Biomed Res. 2016;5(1):54. doi: 10.4103/2277-9175.178799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashidume T., Sasaki K., Hirata J., et al. Effects of sanyaku and its constituent diosgenin on the fasted and postprandial hypertriacylglycerolemia in high-fat-diet-fed KK- ay mice. J Agric Food Chem. 2018;66(38):9968–9975. doi: 10.1021/acs.jafc.8b03040. [DOI] [PubMed] [Google Scholar]
- 44.Kamada Y., Kiso S., Yoshida Y., et al. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am J Physiol Gastrointest Liver Physiol. 2011;301(6) doi: 10.1152/ajpgi.00211.2011. [DOI] [PubMed] [Google Scholar]
- 45.Pummoung S., Werawatganon D., Klaikeaw N., Siriviriyakul P. Genistein-attenuated hepatic steatosis and inflammation in nonalcoholic steatohepatitis with bilateral ovariectomized rats. Phcog Mag. 2018;14(55):S20–S24. doi: 10.4103/pm.pm_603_17. [DOI] [Google Scholar]
- 46.Al-Dabbagh B., Elhaty I.A., Al Hrout A., et al. Antioxidant and anticancer activities of Trigonella foenum-graecum, Cassia acutifolia and Rhazya stricta. BMC Compl Alternative Med. 2018;18(1):1–12. doi: 10.1186/s12906-018-2285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue W., Lei J., Li X., Zhang R. Trigonella foenum graecum seed extract protects kidney function and morphology in diabetic rats via its antioxidant activity. Nutr Res. 2011;31(7):555–562. doi: 10.1016/j.nutres.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Costello B.T., Sprung K., Coulter S.A. The rise and fall of estrogen therapy: is testosterone for “manopause” next? Tex Heart Inst J. 2017;44(5):338–340. doi: 10.14503/THIJ-17-6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caliman I.F., Lamas A.Z., Dalpiaz P.L.M., et al. Endothelial relaxation mechanisms and oxidative stress are restored by atorvastatin therapy in ovariectomized rats. PloS One. 2013;8(11) doi: 10.1371/journal.pone.0080892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu R., Nikolajczyk B.S. Tissue immune cells fuel obesity-associated inflammation in adipose tissue and beyond. Front Immunol. 2019;10:1587. doi: 10.3389/fimmu.2019.01587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mbarki S., Alimi H., Bouzenna H., Elfeki A., Hfaiedh N. Phytochemical study and protective effect of Trigonella foenum graecum (Fenugreek seeds) against carbon tetrachloride-induced toxicity in liver and kidney of male rat. Biomed Pharmacother. 2017;88:19–26. doi: 10.1016/j.biopha.2016.12.078. [DOI] [PubMed] [Google Scholar]
- 52.Witayavanitkul N., Werawatganon D., Chayanupatkul M., Klaikeaw N., Siriviriyakul P. Genistein and exercise treatment reduced NASH related HDAC3, IL-13 and MMP-12 expressions in ovariectomized rats fed with high fat high fructose diet. J Tradit Complement Med. 2021 doi: 10.1016/j.jtcme.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.