Abstract
Background and aim: Echinodorus macrophyllus (Kunth.) Micheli is popularly used for acute and chronic inflammatory conditions. The anti-inflammatory activity was previously demonstrated for its flavonoid-enriched fractions. The aim of this work assessed the antinociceptive properties of both aqueous extract and its fractions. Experimental procedure: The antinociceptive activity was determined by acetic acid-induced writhing, formalin test, tail immersion test, hot-plate test, xylene-induced ear edema methods, and the evaluation of its mechanism was performed in the writhing model. The aqueous extract of Echinodorus macrophyllus (AEEm) was fractionated, yielding Fr20, and Fr40. Fr40 composition was determined by HPLC-DAD-ESI-MS. Results and conclusion: Fr20 (all doses) and Fr40 (100 mg/kg) reduced the nociception in the tail-flick model. Both fractions increased the percentage of maximum possible effect with 25 mg/kg, in the hot-plate assay, at 60 min, while AEEm reduced pain only with 50 and 100 mg/kg. There was a reduction in xylene-edema index, with Fr40 (25 mg/kg), AEEm (50 mg/kg) and Fr20 (50 mg/kg). All doses of AEEm, Fr20, and Fr40 reduced both phases of the formalin model. In the abdominal contortion model, Fr40 presented the highest activity, reducing 96% of contortions and its antinociceptive mechanism was evaluated. The results indicated the involvement of NO and adrenergic activation pathways. The main components of Fr40 are swertisin, swertiajaponin, isoorientin 7,3′-dimethyl ether, swertisin-O-rhamnoside, isoorientin, isovitexin, isovitexin-Orhamnoside, and isovitexin-7-O-glucoside. The aqueous extract of E. macrophyllus leaves and its fractions exhibited significant analgesic effect, mediated through both peripheral and central mechanisms being considered a potentially antinociceptive drug.
Keywords: Medicinal plants, Chemical, Thermal and neurogenic assays, Adrenergic pathway, NO pathway, Flavonoid derivatives
Abbreviations: AEEm, aqueous extract of E. macrophyllus; Fr20, fraction isolated from EAEm on Sephadex LH-20 with 20% ethanol; Fr40, fraction isolated from EAEm on Sephadex LH-20 with 40% ethanol; MPE, percentage of the maximum possible effect; NO, nitric oxide; ODQ, 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one; GMPc, cyclic guanosine monophosphate; 7-NI, 7-nitroindazole; DAD-UV diode array detector, ultraviolet; Q-TOF, quadrupole time-of-flight; HPLC, high-performance liquid chromatography; ESI-MS, electrospray ionization mass spectrometer; HPTLC, high-performance thin-layer chromatography; NSAIDs, nonsteroidal anti-inflammatory drugs; NP/PEG, natural products reagent/polyethylene glycol.
Graphical abstract
Highlights of the findings and novelties
-
•
AEEm/fractions exhibited chemical, thermal and neurogenic antinociceptive actions.
-
•
Fr40 fraction presents high antinociceptive activity in all nociceptive models.
-
•
The Fr40 action on the adrenergic and NO pathways was demonstrated.
-
•
Swertisin, wertiajaponin, isoorientin, isovitexin were the main components of Fr40.
1. Introduction
Natural products, such as medicinal herbs and supplements, are used by about 80% of the population with attention to health, their effectiveness has been demonstrated in only a few of them and is poorly monitored.1 In Brazil, despite the growth of the pharmaceutical industry in the second half of the 20th century, modifying traditional Brazilian medicine,2 there is still an urgent need to collect, document, and save economic botanical resources. Echinodorus macrophyllus (Kunth) Micheli (Alismataceae) is vulgarly known as chapéu-de-couro and is listed in the Brazilian Pharmacopoeia.3 The leaves are popularly prepared as an infusion and used as a diuretic and to treat inflammatory conditions.4 The phytochemistry of this species found in the literature shows the presence of polyphenols, flavonoids, diterpenes, and sesquiterpenes.5, 6, 7
The aqueous extract of E. macrophyllus (AEEm) has shown the T cell immune response suppression in mice.8 Anti-inflammatory effects were observed for AEEm, its flavonoid-enriched fraction (in vitro and in vivo),9 and the ethanolic extract (acute and subchronic action).10 No mutagenic, genotoxic, or cytotoxic effects were evidencied after six weeks of continuous oral treatment of mice with AEEm11,12 and apoptosis was not observed.13
Few scientific data are supporting the antinociceptive action of this plant. We have previously demonstrated this activity for the essential14 and the hexanic extract of E. macrophyllus.15
In this work, the correlation of phenolic compounds and flavonoids with the analgesic effect was evaluated based on the compounds’ presence in the chemical composition of this plant. In addition, the antinociceptive effect of AEEm and its fractions was assessed using different experimental models of pain in mice. Finally, for their most active fraction (Fr40), its possible mechanism of action was also investigated.
2. Materials and methods
2.1. Plant material
The Echinodorus macrophyllus, collected in Nova Friburgo, Rio de Janeiro, was obtained commercially from a Medicinal Plant distributor (Alcantara - Rio de Janeiro) and maintained at 5 °C. A voucher specimen was identified and deposited at the Herbarium Bradeannum UERJ, Rio de Janeiro, Brazil (deposit number HB84807).
2.2. E. macrophyllus extract and fractions
Leaves were ground and dried before infusion (100 g/2 l), protected from light. After reaching room temperature, filtering, and lyophilization, the AEEm (yielding 12.5%) was fractioned (7 g/5 ml ultrapure water), as previously related on Sephadex LH-20,9,14 yielding fractions Fr20 (92.0%) and Fr40 (7.9%). The solvents of fractions were submitted to evaporation in a rotary evaporator under reduced pressure, lyophilized, and stored at −15 °C. The samples were reconstituted in water or sterile physiological saline, for oral (p.o.) or intraperitoneal (i.p.) treatment, respectively.
2.3. In vivo assays
Male Swiss Webster (SW) or DBA/1 J mice of 3–4 months, weighing 25–35 g, were obtained from the State University of Rio de Janeiro or Vital Brazil Institute. Mice were housed in a climate-controlled room at constant temperature (23 ± 2 °C), under a 12 h light/dark period, and free access to food and water. One hour before the noxious stimulus, food and water were removed, and SW mice (n = 5/group) were pretreated by gavage (p.o.) with different doses of the AEEm, or its fractions, or with the vehicle (water, control group). For the neurogenic inflammation, DBA/1 J mice groups (n = 5) were treated by intraperitoneal route (i.p.) with different doses of AEEm, its fractions, or physiological saline (control group).
In the acetic acid-induced writhing model,16,17 the number of writhes was counted between 5 and 15 min after i.p. injection of 0.6% acetic acid (10 ml/kg), and the antinociceptive activity was calculated by comparison with the control group. As a positive control, mice were treated with dipyrone (50 mg/kg).
In the formalin test,18 the hyperalgesic responses were measured between 0 and 5 min (neurogenic phase) and 15–25 min (inflammatory phase) after sub-plantar injection of 20 μl formalin in the right hind paw. Control antinociceptive groups were treated with morphine (10 mg/kg, s.c.) or dipyrone (50 mg/kg, p.o.) 30 min before the challenge.
The tail immersion test was performed by submersion of the mice's tail in the water (55 ± 1 °C) and determining its removal time (latency) with a cut-off of 10 s to minimize tissue damage.19 Mice with latency values between 1.5 and 3.5 s were selected for the assays, and data were expressed as the tail immersion latency time. A positive control group was treated with morphine 10 mg/kg b.w. (i.p.) 45 min before the test.
As previously described,20 the hot-plate assay was carried out by placing mice individually on a hot-plate maintained at a constant temperature of 55 ± 0.5 °C (Insight Equipamentos, Ribeirão Preto, SP, Brazil) within an area confined by a transparent removable acrylic cylinder. Mice with mean basal values around 6–8 s were selected for the tests, and this response was determined at 30, 60, and 120 min post-challenge with a maximum cut-off of 30 s to prevent tissue damage to mouse's paw. A positive control group received morphine 10 mg/kg b.w. (i.p.) 30 min before the test. The hot-plate latencies were converted to a percentage of the maximum possible effect: %MPE= ((post-treatment latency-baseline latency)/(cut-off time-baseline latency))x100.
The neurogenic inflammation21 was induced by the topical application of 30 μl xylene on the right ear's internal/external surfaces in mice sedated (s.c.) with phenobarbital (10 mg/kg). A positive control group received indomethacin 10 mg/kg (i.p.) 1 h before the test. After 30 min, the animals were euthanized, both ears were sampled with a punch (6 mm) and immediately weighed. The increase in the weight of the right ear punch compared to the left ear (control) indicated the inflammatory response.
2.4. Evaluation of antinociceptive mechanism of fr40
The Fr40 mechanism was evaluated in SW mice groups (n = 5/group) treated before the acetic-acid 0.6% injection (i.p.) with the following groups:
Opiate action: 1) Control group - treated (p.o.) with the vehicle of Fr40 dilution (water); 2) Morphine 5 mg/kg, (i.p.) 30 min before; 3) Naloxone (non-selective opioid receptor antagonist) 5 mg/kg subcutaneously (s.c.) in the back of the head 45 min before; 4) Morphine 5 mg/kg, and naloxone 5 mg/kg, 30 min and 15 min before respectively; 5) Fr40 25 mg/kg (p.o.), 1 h before; 6) Fr40 25 mg/kg (p.o.), 1 h before and naloxone 15 min before the injection of the irritant.
Adrenergic mechanism: 1) Control group received 100 μl water by gavage; 2) Yohimbine 1 mg/kg (α2 antagonist) treated (s.c.) on the dorsum, 1 h before; 3) Clonidine 30 μg/kg (α2 adrenergic agonist) treated (i.p.), 30 min before; 4) Yohimbine 1 mg/kg and clonidine 30 μg/kg, treated 30 min and 15 min before, respectively; 5) Fr40 25 mg/kg (p.o.), 1 h before; 6) Fr40 25 mg/kg (p.o.) and with yohimbine 1 mg/kg treated 1 h and 15 min before, respectively.
Involvement of the NO-GMPc (nitric oxide-cyclic guanosine monophosphate) pathway: 1) Control group treated with 100 μl water by gavage; 2) ODQ 2.5 mg/kg ((1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one) treated by intramuscular (i.m.) injection, 1 h before; 3) 7-nitroindazole 3 m/kg (7-NI) treated by i.m. injection, 1 h before; 4) Fr40 25 mg/kg (v.o.), treated 1 h before; 5) Fr40 25 mg/kg (v.o.) and ODQ 2.5 mg/kg (i.m.) treated 1 h and 25 min before; 6) One group was treated with Fr40 25 mg/kg (v.o.), 1 h before, and with 7-NI 3 mg/kg (i.m.), respectively.
2.5. Phytochemical analysis
The analysis was performed by high-performance liquid chromatography (HPLC Prominence, LC-20A pumps, diode array detector DAD-UV SPD-M20A, Shimadzu, Shimadzu Corporation, Brazil) coupled to an Electrospray Ionization Mass Spectrometer (ESI-MS) with Accurate-Mass Quadrupole Time-of-Flight (Q-TOF) from Bruker Corporation, MA, USA. Samples were injected onto a Shim-pack HR-ODS column (150 × 2.1 mm, 3 μm, Shimadzu Corporation, Brazil). Gradient elution was performed with 0.1% acetic acid/water (solvent A) and methanol (solvent B) at a constant flow rate of 0.2 ml/min. The elution gradient was 5–20% B over 30 min, 20% B isocratic 30–40 min, 20–95% B for 12 min, and re-equilibration of the column with 5% B until 64 min, using a flow rate of 200 μl/min. Spectra were recorded in negative and positive ionization mode between m/z 50 and 1200. Phenolic acids and flavonoids were also detected on silica gel 60 on HPTLC (High-performance thin-layer chromatography) plate 3 × 7 cm using ethyl acetate: dichloromethane: acetic acid: water (10:2.5:2:1, v/v) as the mobile phase. After drying, the plates were sprayed with the natural products reagent/polyethylene glycol (NP/PEG). The chromatograms were observed at 365 nm, and fluorescence bands were recorded and photographed.
2.6. Statistical analysis
The results are shown as mean values ± S.D. Data statistical analysis was done by one-way ANOVA, followed by Dunnett's or Tukey's post-hoc tests, with a significant level of p ≤ 0.05, using the program GraphPad Prism®.
3. Results
3.1. Antinociceptive activity of AEEm and its fractions
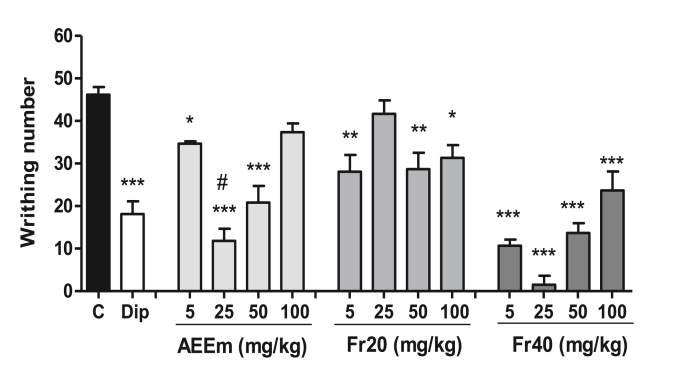
The oral treatment with AEEm reduced the number of writhes by 26% (5 mg/kg), 75% (25 mg/kg) and 55% (50 mg/kg) versus control group (46.68 ± 5.77 writhes). The Fr20 at 5 mg/kg, 50 mg/kg and 100 mg/kg doses decreased the writhing by 39%, 38% and 32%, respectively. The Fr40 produced a reduction of writhes at all doses, being of 77% (5 mg/kg), 96% (25 mg/kg), 70% (50 mg/kg) and 48% (100 mg/kg), and dipyrone (50 mg/kg) inhibited 51% of contortions (Fig. 1).
Fig. 1.
Effect of the treatment with AEEm, Fr20, and Fr40 on the acetic acid-induced writhing test. SW male mice (n = 5/group) were orally treated 60 min before 0.6% acetic acid intraperitoneal injection with different doses of the AEEm, Fr20 or Fr40. Control groups were treated with the vehicle (C) or dipyrone 50 mg/kg (Dip). Data represent the mean ± SD of three experiments. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 vs. control group (ANOVA followed by Dunnet's test) and #p < 0.05 relative to dipyrone (ANOVA followed by Tukey's test).
3.2. AEEm and its fractions exhibited antinociceptive activity in the formalin test
AEEm, Fr20, and Fr40 have reduced at all doses the licking time of both phases of the formalin test (Table 1). AEEm exhibited maximum inhibition of 70.9% at the neurogenic phase (50 mg/kg) and 74.6% in the inflammatory one (25 mg/kg). Fr20 reduced 92.3% of the licking time in the first phase (100 mg/kg) and 99.9% in the second phase (50 mg/kg) being more active than dipyrone.
Table 1.
Effects of AEEm, Fr20 and Fr40 on the formalin-induced nociception.
| aGroups | Dose |
1st Phase |
2nd Phase |
||
|---|---|---|---|---|---|
| mg/kg | bLicking time (s) |
cInhibition % |
bLicking time (s) |
cInhibition % |
|
| Control | - | 99.2 ± 5.2 | – | 169.9 ± 6.8 | – |
| Morphine | 10 mg | 20.4 ± 2.1#∗ | 79.4 | 20.9 ± 4.5#∗ | 87.7 |
| Dipyrone | 50 mg | 61.0 ± 7.6# | 38.5 | 33.2 ± 7.2# | 80.4 |
| AEEm | 25 mg | 41.8 ± 5.9#∗ | 57.9 | 43.1 ± 8.3# | 74.6 |
| 50 mg | 28.8 ± 4.9#∗ | 70.9 | 85.6 ± 8.1# | 49.6 | |
| 100 mg | 32.3 ± 5.0#∗ | 67.4 | 78.4 ± 7.7# | 53.8 | |
| Fr20 | 25 mg | 27.5 ± 8.8#∗ | 72.4 | 14.7 ± 5.1#∗ | 91.3 |
| 50 mg | 17.7 ± 11.6#∗ | 82.1 | 0.2 ± 0.4#∗§ | 99.9 | |
| 100 mg | 7.6 ± 6.8#∗§ | 92.3 | 1.7 ± 3.5#∗§ | 99.0 | |
| Fr40 | 25 mg | 18.1 ± 5.8#∗b | 81.7 | 60.7 ± 12.3# | 64.3 |
| 50 mg | 59.3 ± 11.8# | 40.2 | 45.7 ± 17.7# | 73.1 | |
| 100 mg | 27.5 ± 4.4#∗b | 72.3 | 33.2 ± 12.9#a | 80.4 | |
#p < 0.001 vs. control (Dunnett's test); ∗p < 0.05 vs. dipyrone (Tukey's test); §p < 0.05 vs. morphine (Tukey's test); ap < 0.001 vs. Fr40 25 mg (Tukey's test); bp < 0.001 vs. Fr40 50 mg (Tukey's test).
SW mice (n = 5/group) were treated with the vehicle (control group) or different doses of AEEm, Fr20 or Fr40 (p.o.), 60 min before formalin injection. Drug control groups were treated with dipyrone (p.o.) or morphine (s.c.) 30 min before formalin injection.
Mean of licking time ± S.D. of three independent experiment, between 0 and 5 min (1st phase) and 15–25 min (2nd phase) after formalin injection.
Inhibition in relation to control group.
Fr40 showed an inhibitory effect on U inverted in the first phase, being the nociceptive response with 50 mg/kg significantly different from the other doses (p < 0.001, Tukey's test). Fr40 showed a dose-response effect at the inflammatory phase with a significant difference between the lower and higher doses, with the highest inhibition of 80.4% (100 mg/kg). Morphine (10 mg/kg, s.c.) showed a high reduction of licking time in both neurogenic (79.4%) and inflammatory phases (87.7%). Dipyrone (50 mg/kg, p.o.) decreased the response time significantly in the first phase (38.5%), but mainly in the inflammatory period (80.4%).
3.3. Thermal antinociceptive potential
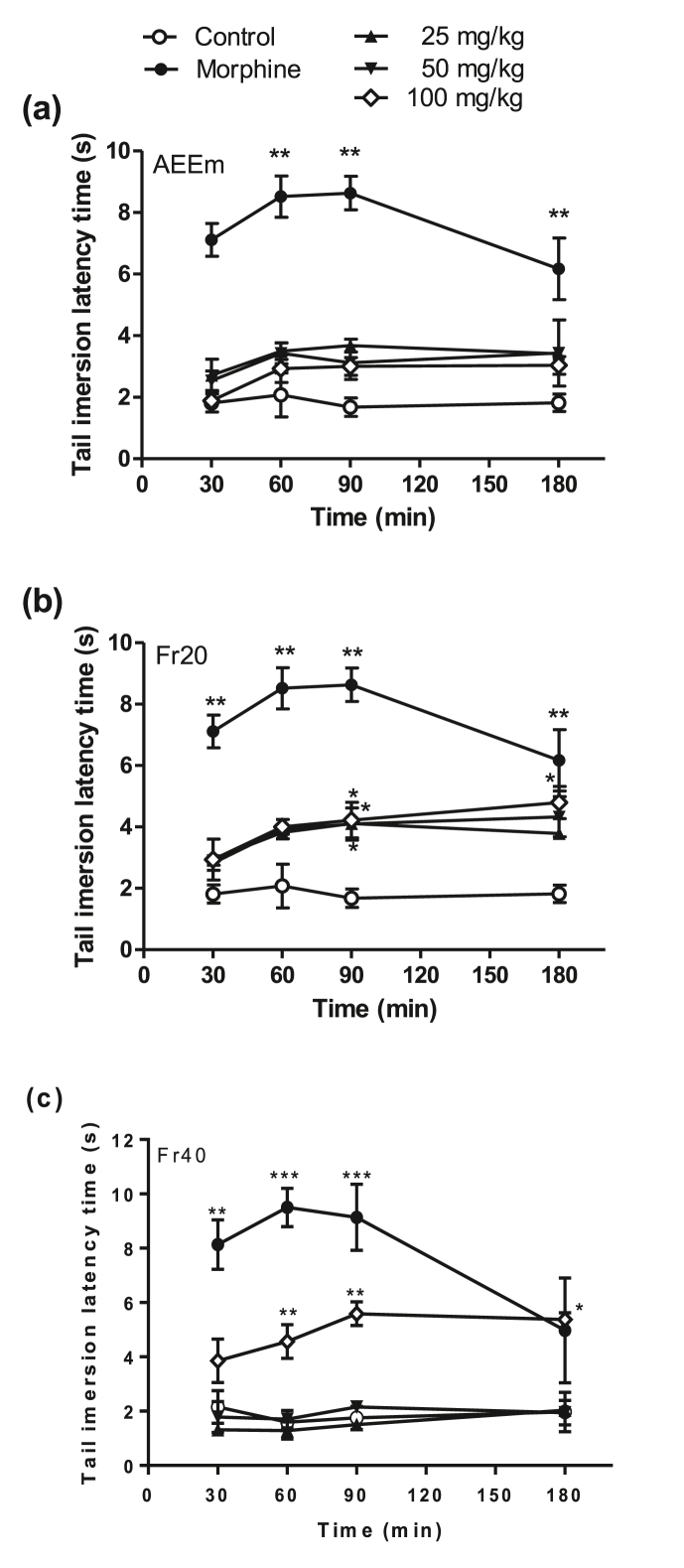
No significant results were observed after treatment with AEEm in the tail immersion test (Fig. 2a). Otherwise, the oral treatment with all doses of Fr20 fractions (Fig. 2b) produced a significant reduction of a painful sensation at 90 min challenge (latency time 2.5x high), compared to the control group. Fr40 (Fig. 2c) increased the latency time only in the higher dose (100 mg/kg), at 60–180 min. Morphine exhibited significant antinociceptive response at all times analyzed (30–180 min), increasing the response time by up to 6.5 times in 90 min.
Fig. 2.
Effect of treatment with AEEm and its fractions in the tail immersion model. SW male groups (n = 5/group) were orally treated with the vehicle (C), different doses (p.o.) of AEEm (a), Fr20 (b) and Fr40 (c), or morphine (10 mg/kg, i.p.) 60 min before the tail immersion at 50 °C. Data represent the mean ± SD of latency time per group of three experiments. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 vs. control group (ANOVA followed by Dunnett's test).
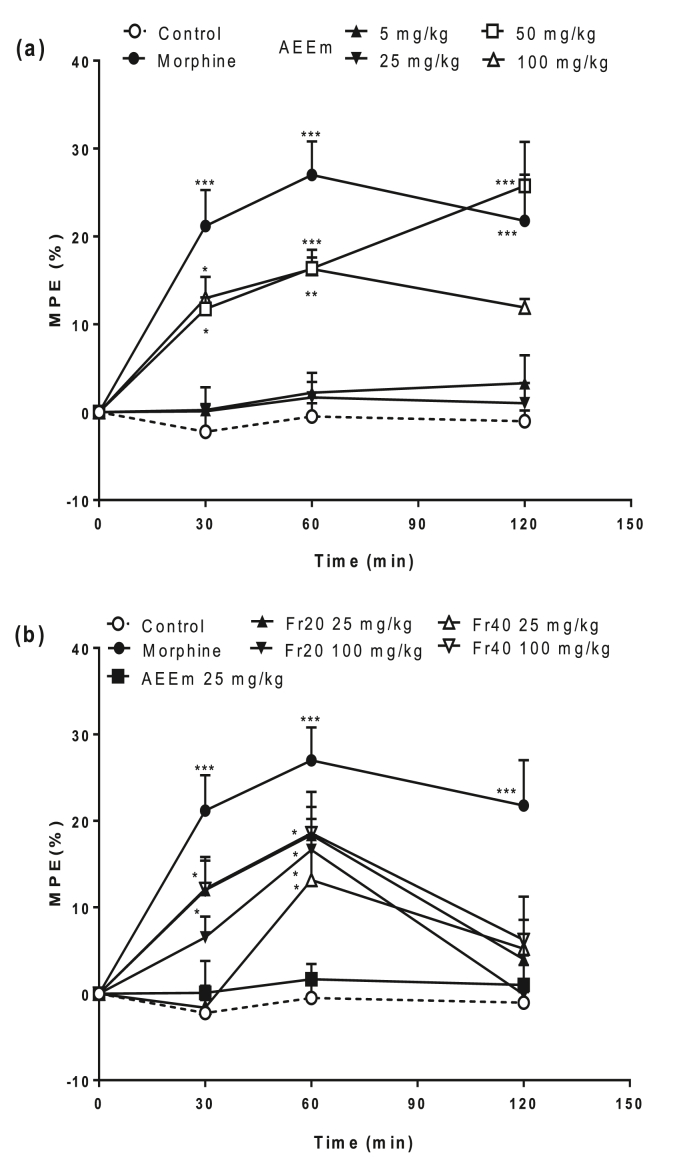
AEEm presented thermal antinociceptive activity in the hot plate model in the higher doses (50 and 100 mg/kg) when evaluated at 30 and 60 min (Fig. 3a). Although no response was observed in the mice group treated with AEEm at 25 mg/kg, the oral treatment with its fractions Fr20 and Fr40 (Fig. 3b) showed a significant increase of the percentage of the maximum possible effect (MPE%), both at 30 and 60 min of a challenge. Morphine exhibited significant antinociceptive responses at all times (30–120 min).
Fig. 3.
Analgesic effects induced by treatment with AEEm (a) and its fractions (b) assessed by the hot plate test. SW male groups (5/group) were orally treated with the vehicle (C), different doses of AEEm, Fr20, and Fr40 (p.o.) or morphine (10 mg/kg, i.p.) 60 min before the challenge. Data represent the mean ± SD of maximum possible effect (MPE) percentage of three experiments. ∗p < 0.05 ∗∗p < 0.01 and ∗∗∗p < 0.001 vs. control group (ANOVA followed by Dunnett's test).
3.4. The neurogenic anti-inflammatory potential in the xylene-induced ear edema
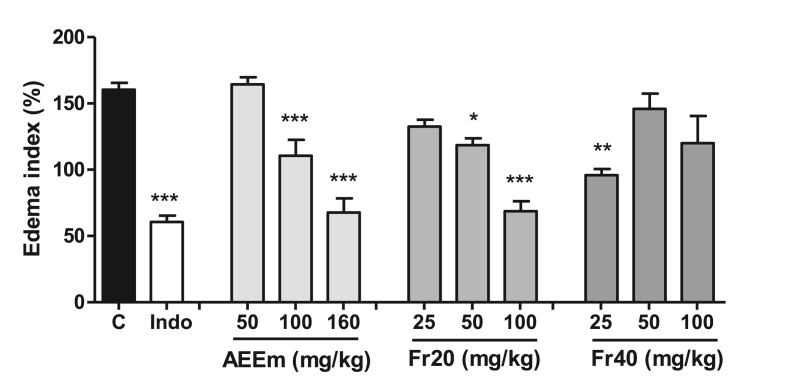
The treatment with AEEm or Fr20 (Fig. 4) produced a dose-response effect in this model (p < 0.01, Tukey's test), with a maximum reduction of the edema index of 62.6% and 57.1%, respectively. The treatment with Fr40 (Fig. 4) lowered the edema index only at 25 mg/kg (40.1%).
Fig. 4.
Effect of treatment with AEEm, Fr20, and Fr40 on xylene-induced ear edema in mice. Different doses (p.o.) of AEEm, Fr20 and Fr40, or indomethacin 10 mg/kg (Indo, i.p.) were administered to DBA/1 J mice (n = 5/group) 60 min before topical application of xylene. The increase in xylene-induced weight was assessed by the difference between the weight of the right treated ear section and the untreated left ear section. Data represent the mean ± SD of three experiments. ∗p < 0.05 ∗∗p < 0.01 and ∗∗∗p < 0.001 vs. control group (ANOVA followed by Dunnett's test).
3.5. Evaluation of antinociceptive mechanism of Fr40
The Fr40 presented antinociceptive activity in all the evaluated models and higher activity than Fr20 in the writhing test and was chosen to study its antinociceptive mechanism in this model (Table 2).
Table 2.
Assessment of antinociceptive mechanism of Fr40 in the writhing model.
| Groups | Writhing number | % inhibition |
|---|---|---|
| Control | 50.67 ± 2.08 | – |
| Naloxone | 46.25 ± 1.71 | 8.72 |
| Morphine | 1.20 ± 2.17∗∗∗ | 97.63 |
| Naloxone + morphine | 20.50 ± 4.93∗∗∗# | 59.45 |
| Fr40 | 12.60 ± 5.94∗∗∗ | 75.13 |
| Naloxone + Fr40 | 13.67 ± 4.51∗∗∗ | 73.02 |
| Yohimbine | 47.25 ± 3.77 | 6.75 |
| Clonidine | 0.20 ± 0.45∗∗∗ | 99.60 |
| Yohimbine + clonidine | 40.80 ± 6.65## | 19.47 |
| Fr40 | 12.60 ± 5.94∗∗∗ | 75.13 |
| Yohimbine + Fr40 | 35.50 ± 9.81∗§ | 29.93 |
| ODQ | 41.00 ± 6.08 | 19.08 |
| Fr40 | 12.60 ± 5.94∗∗∗ | 75.13 |
| ODQ + Fr40 | 4.50 ± 2.89∗∗∗& | 91.12 |
| 7-NI | 41.60 ± 7.09 | 17.90 |
| Fr40 | 12.60 ± 5.94∗∗∗ | 75.13 |
| 7-NI + Fr40 | 37.50 ± 6.35§ | 25.99 |
SW male mice treated (n = 5–6/group) before the i.p. injection of HAC 0.6% with: Fr40 25 mg/kg (v.o.); naloxone (5 mg/kg s.c.); morphine (5 mg/kg i. p.); yohimbine (1 mg/kg s.c.); clonidine (30 μg/kg, i. p.); ODQ 2.5 mg/kg i.m. (1H-[1,2,4] oxadiazolo[4,3-a] quinoxalin-1-one); 7-NI 3 mg/kg i.m. (7-nitroindazole). The results represent mean ± SD of contortions of two experiments measured between 5 and 15 min after i.p. injection of HAC 0.6%. The % inhibition was calculated in relation to the control. ANOVA followed Tukey's test. ∗p < 0.05 and ∗∗∗p < 0.001 vs. control; #p < 0.01 vs. morphine; ##p < 0.001 vs. clonidine; §p < 0.001 vs. Fr40; and &p < 0.001 vs. ODQ.
The opiate mechanism was evaluated using, as an agonist control morphine and as an antagonist, naloxone. Naloxone did not interfere with the abdominal contortions when compared to the control group (p > 0.05) and was able to revert by 61%, the antinociceptive effect of morphine (p < 0.001). Fr40 has reduced the number of contortions vs. the control group by 75.13%, which was not reversed by naloxone.
The adrenergic mechanism was evaluated employing yohimbine as the antagonist and clonidine as the agonist. Yohimbine did not interfere with the abdominal constrictions versus the control group (p > 0.05) and has reverted by 80.4% the effect of clonidine (p < 0.001). Fr40 reduced the number of contortions by 75.13%, and its effect was reversed by 60.2% by yohimbine.
The involvement of the activation of guanylate cyclase and the generation of GMPc in the antinociceptive response of Fr40 was evaluated employing ODQ. This inhibitor of the guanylate cyclase enzyme has not interfered in the writhing number versus the control group (p > 0.05). Fr40 reduced by 75.13% the number of contortions concerning the control group, but ODQ did not reverse this effect.
The contribution of NO in the antinociceptive effect of Fr40 was evaluated using 7-NI, a neuronal NO synthase antagonist. The 7-NI did not interfere with the abdominal contortions compared to the control group (p > 0.05). However, the Fr40 reduced by 75.13% the number of contortions, and its effect was partially reversed (65.4%) by 7-NI.
3.6. Phytochemical analysis
The AEEm and its fractions were submitted to HPTLC sprayed with NP/PEG. The extract and its fractions exhibited bands with red, orange/yellow, green, and blue fluorescence, suggesting flavonoids, flavonoids glycosides, and phenolic acids. Fr40 presented mainly yellow and orange fluorescent bands, besides blue and green ones. Fr20 gave predominantly blue fluorescent bands (Appendice 1). The analysis by HPLC-ESI-MS-Q-TOF (Appendice 2) and comparison with literature data22 showed that Fr40 exhibited as main components swertisin (37.4%), and swertiajaponin (35.79%), besides isoorientin 7,3′-dimethyl ether (9.29%), swertisin-O-rhamnoside (7.86%), isoorientin (5.42%), isovitexin (2.07%), isovitexin-O-rhamnoside (1.21%) and isovitexin-7-O-glucoside (1.02%), as shown in Table 3.
Table 3.
Composition of Fr40 determined by HPLC-ESI-MS.
| Peak | RT (min) | % | λmax (nm) | MS1 (−) (m/z) |
MS (m/z) | Propose struture |
|---|---|---|---|---|---|---|
| 1 | 24.8 | 1.02 | 269, 335 | 593.1495 | 593,150(100), 217.004(60) | Isovitexin-7-O-glucoside |
| 2 | 26.1 | 5.41 | 241, 342 | 447.0932 | 447.093(100), 217.004(16) | Isoorientin |
| 3 | 26.9 | 35.79 | 243, 347 | 461.1094 | 461.109(100) | Swertiajaponin |
| 4 | 30.2 | 1.21 | 269, 335 | 577.1546 | 577.155(100), 461.108(39), 217.004(49) | Isovitexin-O-rhamnoside |
| 5 | 30.6 | 2.07 | 240, 335 | 431.0979 | 431.098(100), 217.004(30) | Isovitexin |
| 6 | 31.2 | 7.86 | 271, 336 | 591.1709 | 591.171(100), 445.114(8), 217.004(4) | Swertisin-O-rhamnoside |
| 7 | 31.5 | 37.34 | 270, 336 | 445.1144 | 445.114(100) | Swertisin |
| 8 | 33.1 | 9.29 | 270, 345 | 475.1244 | 475.124(100), 217.004(4) | Isoorientin 7,3′-dimethylether |
RT retention time (min); peak of identified compounds.
4. Discussion
Animal models of nociception (pain) have been crucial in our understanding of acute and chronic pain. Pain is a complex phenomenon that usually differs depending on the affected tissue and the mechanism of injury. In this work, AEEm and its fractions were assayed by thermal, inflammatory, and neurogenic nociceptive methods.
Acetic acid-induced abdominal constriction23 promotes the release of mediators involved in neurogenic and peripheral pain, inducing visceral pain by stimulating of the sensorial primary afferent nerve and C fibers. In addition, there is an involvement of different mediators, including acid-sensing ion channels and prostaglandin pathways, stimulating the nociceptive neurons sensitive to nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids.24,25 Thus, AEEm and its fractions can act by antinociceptive mechanisms similar to non-narcotics and narcotic drugs, possibly blocking the receptor or releasing of endogenous compounds that excite pain nerve endings. Fr40 shows the highest inhibition levels of pain, reducing the constrictions by 96% with half a dipyrone dose.
Samples were also evaluated in the formalin test, which produces distinct biphasic nociception represented by a neurogenic (0–5 min, intense pain) and an inflammatory (15–30 min, moderate pain) phase.21 The earlier stage seems to be caused mainly by the activation of C-fibers after peripheral stimulation (direct stimulation of nociceptors). The late phase is originated from peripheral mechanisms. There is a release of inflammatory mediators by activating N-methyl-d-aspartate receptors and changes in the dorsal horn of the spinal cord. Activation of central sensitized neurons occurs due to peripheral inflammation, and the ongoing activity of primary afferents and direct activation of TRPA1. This cation channel plays an essential role in inflammatory pain.26, 27, 28 Both phases are inhibited by central analgesic drugs (narcotics), while the later period is suppressed mainly by peripherally acting drugs (steroids, NSAIDs).29 The inhibition of the neurogenic phase by Fr40 and Fr20 (25 mg/kg) was higher than dipyrone (50 mg/kg) and as potent as morphine (10 mg/kg). AEEm also presented more significant analgesic action than dipyrone in the early phase. These findings may be the result of its direct effects on nociceptors. In the inflammatory phase, Fr20 (all doses) and Fr40 (50 or 100 mg/kg) showed a higher effect than dipyrone, resulting from the reduction of synthesis or release of prostaglandins, or both, or other inflammation mediators.
The tail immersion test consists of applying a heat stimulus and recording the latency to remove the tail. The tail-flick reflex results from the activation of cutaneous nociceptors, conduction within the central nervous system (central delay), ventral horn, and tail muscles activation.30 The increased latency time after the oral administration with Fr20 at all doses, at 90 min, and with Fr40 in the higher doses (60–90 min), suggests a thermal antinociceptive activity. These results could be bonded to the inhibition of agents that activate the release of the endogenous peptide by periaqueductal gray matter, which is carried to the spinal cord to inhibit the pain muscle transmission within the dorsal horn.31
The hot-plate test involves recording the latency for either withdrawing the paw from the hot-plate or licking the paw. Fr20 and Fr40 have exhibited higher antinociceptive responses than AEEm in this assay, prolonging the nocifensive withdrawal reflex response from the heat source. This response could draw in from modulation of the medullary or central pain level since this test has meditated for both or from the direct inhibitory activity on nerve endings or transmission pathways.31 Thus, they may be acting either at the peripheral or the central level, or both.32
Fr40, Fr20, and AEEm were active in the neurogenic phase of the formalin test. So, its effect was evaluated in the xylene-induced ear edema, a neurogenic inflammatory assay. This essay analyses the antinociceptive properties of topical anti-inflammatory steroids and nonsteroidal antiphlogistic agents, especially those inhibiting phospholipase A2.33
The xylene application causes vasodilatation, increases vascular permeability, and plasma extravasations, leading to ear swelling.34 This inflammation process is initiated by mediators such as serotonin, acetylcholine, histamine, bradykinin, and prostaglandins, which release neuropeptides like substance P, which activate its receptors, causing neurogenic inflammation.35 Fr40 exhibited a significant effect in this model, at half a dose of dipyrone, suggesting a possible inhibition of neuro-mediators action or release.
The centrally acting protective effects were corroborated by the first phase of formalin-induced pain and immersion test results. This immersion test indicates acute pain and central mechanisms of spinal nociceptive reflexes. In addition, previous work showed that Fr20 has higher anti-inflammatory activity than the AEEm, with substantial decrease in NO and LTB4 levels, vasodilation, and neutrophil migration.9 These results suggest that although the mechanisms remain somewhat unknown, the analgesic effects of AEEm and Fr20 can also be due to the modulation of inflammatory mediators.
The Fr40 fraction showed antinociceptive potential at all experimental models assayed in this work, exhibited lower complexity by HPTLC, and higher antinociceptive activity than dipyrone (p < 0.05) in the acetic acid-induced writhing. Therefore, it was chosen for phytochemical analysis and the study of the mechanism of action in the acetic acid-induced writhing model at the most effective dose (25 mg/kg).
The opioid system modulates the pain perception by interaction with its receptors (μ, δ, κ) on the cell membrane, coupled to G-protein. It is expressed in the central nervous system, and peripherals sensory neurons. This system participates primarily in the analgesia but also acts in the innate and acquired immune responses.36 Opioid agonists, like morphine, activate intracellular signaling leading to a reduction in the excitability of neurons, partially blocking the transmission of the painful stimulus.37 The opiate mechanism was evaluated (Table 2) using as controls morphine (opioid agonist) and naloxone (antagonist of the non-selective opioid receptor). The effect of Fr40 was not reversed by naloxone, suggesting that this fraction does not act by the opioid system.
Clonidine is an α-2 agonist drug of the pre-synaptic α-2 adrenergic receptors found in the central and peripheral nervous system (brain, spinal cord, and dorsal root ganglia), and the activation of these receptors reduces the local release of catecholamines, decreasing the pain and allodynia.38, 39, 40 Yohimbine, a selective α2-antagonist, has reversed 60.2% of Fr40 antinociception (Table 2), suggesting an adrenergic mechanism of this fraction. It was demonstrated that yohimbine eliminated the clonidine analgesic effect partially, as observed in our work, and that it acts blocking Na + channels and vanilloid VR1 receptors.41
The involvement of the activation of NO-sensitive guanylate cyclase and GMPc generation was evaluated (Table 2) by employing the potent and selective inhibitor ODQ. The Fr40 reduced by 75.13% the number of contortions, and the ODQ did not reverse its effect.
NO modulates the synaptic transmission both in the central nervous system and in the peripheral system.42,43 In nerve endings, it is produced by the neuronal NO synthase. It may diffuse to the extracellular medium by activating guanylate cyclase, inducing the formation of GMPc. Thus, depending on the experimental conditions, NO can produce pronociceptive or antinociceptive effects.44 The Fr40 effect was partially reversed by the neuronal NOs inhibitor 7-NI, which competes with l-arginine, substrate by binding to the NOs enzyme, suggesting the involvement of this pathway antinociceptive action (Table 2).
Fr40 showed by phytochemical screening the flavonoids swertiajaponin, swertisin, isoorientin, isovitexin, and its derivatives. These compounds were also identified from the Echinodorus grandiflorus leaves.45 Isovitexin and isoorientin were found in all the samples of Echinodorus scaber and E. grandiflorus.46
Isoorientin decreased the NO and tumor necrosis factor-alfa production by RAW 264.7 cells.47 Swertisin and 2-O-rhamnosyl-swertisin were effective in inhibiting the hypernociceptive response induced by carrageenan.48 The inhibition of the mechanical sensitization caused by complete Freund's adjuvant or Prostaglandin E2 by the 2″-O-rhamnosyl-swertisin, isolated from A. moluccana,48 was due to its activity on the peripheral and central pathways of pain.49 Isovitexin (apigenin-6-C-glucoside), generally purified together with vitexin, its isomer, exhibit several pharmacological properties, including antioxidant, anti-inflammatory, anti-hyperalgesic, and neuroprotective ones.50 So, the Fr40 antinociceptive activity may be due to the pharmacological properties of its flavonoid compounds.
5. Conclusion
This work demonstrated, the antinociceptive effects of Echinodorus macrophyllus leaves infusion, as it is popularly used. Its fractionation resulted in the Fr40 fraction with higher antinociceptive activity than traditional analgesic drugs. Besides, the results may suggest central, peripheral, or both antinociceptive responses for AEEm, Fr20, and Fr40, providing relevant scientific evidence that supports the traditional use of this plant due to its analgesic properties. Fr40, a flavonoid-rich fraction, did not act via the opioid pathway, and by activation of guanylate cyclase. Otherwise, the involvement of adrenergic activation and NO pathways was demonstrated.
Ethics approval and consent to participate
All experiments were in agreement with guidelines for ethical standards of investigation of experimental procedures in animals. The Committee for Ethics in Animal Research (CEA-IBRAG committee/protocol 07/2013, 07/2017, 013/2018) approved this study, which was performed by norms of the National Council for Animal Experimentation Control (CONCEA).
Authors’ contributions
DCF and MGPC conceived, designed the research, and wrote the manuscript; BPM, GPS, and SVMS performed experimental analgesic models; ENF and CRMG performed fractionation; LSMV made phytochemistry analysis; KCCS and MGPC assisted the research work. The authors declare that they approved the manuscript.
Funding
This study was supported by FAPERJ (E−26/110.541/2014), CNPq (Doctoral Scholarship), and State University of Rio de Janeiro.
Declaration of competing interest
The authors declare that they have no conflicts of interest to disclose.
Acknowledgments
We thank the personnel of LIA-BPPN for their technical assistance and the Vital Brazil Institute for the supply of SW mice.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.07.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandao M.G.L., Grael C.F.F., Fagg C.W. In: Biological Diversity and Sustainable Resources Use. Grillo O., Venora G., editors. InTech; Rijeka, Croatia: 2011. European naturalists and medicinal plants of Brazil; pp. 101–120. [DOI] [Google Scholar]
- 3.ANVISA . fifth ed. ANVISA; Brasília, Brazil: 2010. Brazilian Pharmacopoeia. Brazilian Pharmacopeia Convention. [Google Scholar]
- 4.de La Cruz M.G., Carlini, Caniato . Plantas medicinais de Mato Grosso - A Farmacopeia Popular dos Raizeiros. Cuiabá, MT, Brazil; 2008. Plantas utilizadas por raizeiros na medicina popular em Cuiabá, Mato Grosso; pp. 63–128. [Google Scholar]
- 5.Kobayashi J., Sekiguchi M., Shigemori H., Ohsaki A. Echinophyllins A and B, novel nitrogen-containing clerodane diterpenoids from Echinodorus macrophyllus. Tetrahedron Lett. 2000;41:2939–2943. doi: 10.1016/S0040-4039(00)00314-2. [DOI] [PubMed] [Google Scholar]
- 6.Shigemori H., Shimamoto S., Sekiguchi M., et al. New cembrane diterpenoids with an eight-membered lactone ring from the leaves of Echinodorus macrophyllus. J Nat Prod. 2002;65:82–84. doi: 10.1021/np0104119. [DOI] [PubMed] [Google Scholar]
- 7.Silva T.M., Miranda R.R.S., Ferraz V.P., Pereira M.T., Siqueira E.P., Alcântara A.F.C. Changes in the essential oil composition of leaves of Echinodrus macrophyllus exposed to γ-radiation. Rev Bras Farmacog. 2013;23:600–607. doi: 10.1590/S0102-695X2013005000049. [DOI] [Google Scholar]
- 8.Pinto A.C., Rego G.C., Siqueira A.M., et al. Immunosuppressive effects of Echinodorus macrophyllus aqueous extract. J Ethnopharmacol. 2007;111:435–439. doi: 10.1016/j.jep.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Silva G.P., Fernandes D.C., Vigliano M.V., et al. Flavonoid-enriched fraction from Echinodorus macrophyllus aqueous extract exhibits high in-vitro and in-vivo anti-inflammatory activity. J Pharm Pharmacol. 2016;68:1584–1596. doi: 10.1111/jphp.12620. [DOI] [PubMed] [Google Scholar]
- 10.Tannus-Rangel E., Santos S.R., Lima J.C.S., et al. Topical and systemic anti-inflammatory effects of Echinodorus macrophyllus (Kunth.) Micheli (Alismataceae) J Med Food. 2010;13:1161–1166. doi: 10.1089/jmf.2009.0247. [DOI] [PubMed] [Google Scholar]
- 11.Lopes C.L., Albano F., Laranja G.A.T., et al. Toxicological evaluation by in vitro and in vivo assays of an aqueous extract prepared from Echinodorus macrophyllus leaves. Toxicol Lett. 2000;116:189–198. doi: 10.1016/S0378-4274(00)00220-4. [DOI] [PubMed] [Google Scholar]
- 12.Vidal L.S., Alves A.M., Kuster R.M., Lage C., Leitão A.C. Genotoxicity and mutagenicity of Echinodorus macrophyllus (chapéu-de-couro) extracts. Genet Mol Biol. 2010;33:549–557. doi: 10.1590/S1415-47572010005000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaz M.S.M., Vaz da Silva M.S., Oliveira R.J., et al. Evaluation of the toxicokinetics and apoptotic potential of ethanol extract from Echinodorus macrophyllus leaves in vivo. Regul Toxicol Pharmacol. 2016;82:32–38. doi: 10.1016/j.yrtph.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes D.C., Velozo L.S.M., Alves R.A., et al. Antinociceptive activity of essential oil from Echinodorus macrophyllus (Kunth.) Micheli (Alismataceae) Rev Fitos. 2012;7:245–251. [Google Scholar]
- 15.Fernandes D.C., Martins B.P., Medeiros D.L.F., et al. Antinociceptive and anti-inflammatory activities of the hexanic extract of Echinodorus macrophyllus (Kunth.) Micheli in mice. Bras J Health Biom Sci. 2019;18:25–32. [Google Scholar]
- 16.Koster R., Anderson M., de Beer E.J. Acetic acid for analgesic screening. Fed Proc. 1959;18:412. [Google Scholar]
- 17.Loro J.F., Del Rio I., Pérez-Santana L. Preliminary studies of analgesic and anti-inflammatory properties of Opuntia dillenii aqueous extract. J Ethnopharmacol. 1999;67:213–218. doi: 10.1016/s0378-8741(99)00027-6. [DOI] [PubMed] [Google Scholar]
- 18.Shibata M., Ohkubo T., Takahashi H., Inok R. Modified formalin test: characteristic biphasic pain response. Pain. 1989;38:347–352. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- 19.Sewell R.D.E., Spencer P.S.J. Antinociceptive activity of narcotic agonist and partial agonist analgesics and other agents in the tail-immersion test in mice and rats. Neuropharmacology. 1976;15:683–688. doi: 10.1016/0028-3908(76)90037-x. [DOI] [PubMed] [Google Scholar]
- 20.Tita B., Abdel-Haq H., Vitalone A., Mazzanti G., Saso L. Analgesic properties of Epilobium angustifolium, evaluated by the hot plate test and the writhing test. Farmaco. 2011;56:341–343. doi: 10.1016/s0014-827x(01)01046-1. [DOI] [PubMed] [Google Scholar]
- 21.Young J.M., de Young J.M. In: Pharmacological Methods in the Control of Inflammation. Spector J., Back N., editors. 1989. Cutaneous models of inflammation for the evaluation of topical and systemic pharmacological agents; pp. 215–231. New York. [Google Scholar]
- 22.Prando T.B.L., Barboza L.N., Araújo V.O., et al. Involvement of bradykinin B2 and muscarinic receptors in the prolonged diuretic and antihypertensive properties of Echinodorus grandiflorus (Cham. & Schltdl.) Micheli. Phytomedicine. 2016;23:1249–1258. doi: 10.1016/j.phymed.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Collier H.O.J., Dinneen L.C., Johnson C.A., Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawynok J. Topical and peripherally acting analgesics. Pharmacol Rev. 2003;55:1–20. doi: 10.1124/pr.55.1.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Gregory N., Harris A.L., Robinson C.R., Dopugherty P.M., Fuchs P.N., Sluka K.A. An overview of animal models of pain: disease models and outcome measures. J Pain. 2013;14:1255–1269. doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunskaar S., Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 27.Coderre T.J., Abbott F.V., Sawynok J. In: Encyclopedia of Pain. Gebhart G.F., Schmidt R.F., editors. Springer; Berlin, Heidelberg: 2013. Formalin test; pp. 1303–1308. [Google Scholar]
- 28.Gong N., Huang Q., Chen Y., Xu M., Ma S., Wang Y. Pain assessment using the rat and mouse formalin tests. Bio-protocol. 2014;4:e1288. doi: 10.21769/BioProtoc.1288. [DOI] [Google Scholar]
- 29.Voilley N. Acid-sensing ion channels (ASICs): new targets for the an algesic effects of Non-Steroid Anti-Inflammatory Drugs (NSAIDs) Curr Drug Targets - Inflamm Allergy. 2004;3:71–79. doi: 10.2174/1568010043483980. [DOI] [PubMed] [Google Scholar]
- 30.Danneman P.J., Kiritsy-Roy J.A., Morrow T.J., Casey K.L. Central delay of the laser-activated rat tail-flick reflex. Pain. 1994;58:39–44. doi: 10.1016/0304-3959(94)90183-X. [DOI] [PubMed] [Google Scholar]
- 31.Shilo Y., Pascoe P.J. In: Pain Management in Veterinary Practice. Egger C.M., Love T., Doherty T., editors. John Wiley & Sons, Inc.; 2014. Anatomy, physiology, and Pathophysiology of pain; pp. 9–27. [Google Scholar]
- 32.Bomba F.D.T., Wandji B.A., Piegang B.N., et al. Antinociceptive properties of the aqueous and methanol extracts of the stem bark of Petersianthus macrocarpus (P. Beauv.) Liben (Lecythidaceae) in mice. J Ethnopharmacol. 2015;174:66–73. doi: 10.1016/j.jep.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Akindele A.J., Adeyemi O.O. Antiinflamatory activity of the aqueous leaf extract of Byrsocarpus coccineus. Fitoterapia. 2007;78:25–28. doi: 10.1016/j.fitote.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Richardson J.D., Vasko M.R. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Therapeut. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 35.Banki E., Hajna Z., Kemeny A., et al. The selective PAC1 receptor agonist maxadilan inhibits neurogenic vasodilation and edema formation in the mouse skin. Neuropharmacology. 2014;85:538–547. doi: 10.1016/j.neuropharm.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Liang X., Liu R., Chen C., Ji F., Li T. Opioid system modulates the immune function: a review. Transl Perioper & Pain Med. 2016;1:5–13. [PMC free article] [PubMed] [Google Scholar]
- 37.Bodnar R.J. Endogenous opiates and behavior: 2016. Peptides. 2018;101:167–212. doi: 10.1016/j.peptides.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Heyneman C.A., Lawless-Liday C., Wall G.C. Oral versus topical NSAIDs in rheumatic diseases: a comparison. Drugs. 2000;60:555–574. doi: 10.2165/00003495-200060030-00004. [DOI] [PubMed] [Google Scholar]
- 39.Mason N.J., Artis D., Hunter C.A. New lessons from old pathogens: what parasitic infections have taught us about the role of nuclear factor-κB in the regulation of immunity. Immunol Rev. 2004;201:48–56. doi: 10.1111/j.0105-2896.2004.00189.x. [DOI] [PubMed] [Google Scholar]
- 40.Gilsbach R., Hein L. Are the pharmacology and physiology of alpha(2) adrenoceptors determined by alpha(2)-heteroreceptors and autoreceptors respectively? Br J Pharmacol. 2012;165:90–102. doi: 10.1111/j.1476-5381.2011.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dessaint J., Yu W., Krause J.E., Yue L. Yohimbine inhibits firing activities of rat dorsal root ganglion neurons by blocking Na+ channels and vanilloid VR1 receptors. Eur J Pharmacol. 2004;485:11–20. doi: 10.1016/j.ejphar.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 42.Salter M., Strijbos F.P.J.L.M., Neale S., Duffy C., Follenfant R.L., Garthwaites J. The nitric oxide-cyclic GMP pathway is required for nociceptive signaling at specific loci within the somatosensory pathway. Neuroscience. 1996;73:649–655. doi: 10.1016/j.ejphar.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 43.Jain N.K., Kulkarni S.K., NAME L.- A nitric oxide synthase inhibitor, modulates cholinergic antinociception. Methods Find Exp Clin Pharmacol. 1999;21:161–165. doi: 10.1358/mf.1999.21.3.534824. [DOI] [PubMed] [Google Scholar]
- 44.Sakurada T., Manome Y., Tan-No K., et al. Involvement of nitric oxide in spinally mediated capsaicin and glutamate-induced behavioural responses in the mouse. Neurochem Int. 1996;29:271–278. doi: 10.1016/0197-0186(96)00004-6. [DOI] [PubMed] [Google Scholar]
- 45.Schnitzler M., Petereit F., Nahrsted A. Trans-Aconitic acid, glucosyl flavones and hydroxycinnamoyl tartaric acids from the leaves of Echinodorus grandiflorus ssp. aureus, a Brazilian medicinal plant. Rev Bras Farmacogn. 2007;17:149–154. doi: 10.1590/S0102-695X2007000200002. [DOI] [Google Scholar]
- 46.Strada C.L., Lima K.C., da Silva V.C., et al. Isovitexin as marker and bioactive compound in the antinociceptive activity of the Brazilian crude drug extracts of Echinodorus scaber and E. grandiflorus. Rev Bras Farmacogn. 2017;27:619–626. doi: 10.1016/j.bjp.2017.05.011. [DOI] [Google Scholar]
- 47.Luyen B.T.T., Tai B.H., Thao N.P., Cha J.Y., Lee Y.M., Kim Y.H. A new phenolic component from Triticum aestivum Sprouts and its effects on LPS-stimulated production of nitric oxide and TNF-α in RAW 264.7 cells. Phytother Res. 2014;28:1064–1070. doi: 10.1002/ptr.5097. [DOI] [PubMed] [Google Scholar]
- 48.Quintão N.L.M., Meyre-Silva C., Silva G.F., et al. Aleurites moluccana (L.) Willd. leaves: mechanical antinociceptive properties of a standardized dried extract and its chemical markers. Evid Based Complement Alternat Med. 2011;1–10 doi: 10.1155/2011/179890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quintão N.M.L., Antonialli C.S., Silva G.F., et al. Aleurites moluccana and its main active ingredient, the flavonoid 2’’-O-rhamnosylswertisin, have promising antinociceptive effects in experimental models of hypersensitivity in mice. Pharmacol Biochem Behav. 2012;102:302–311. doi: 10.1016/j.pbb.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 50.He M., Min J.W., Kong W.L., He X.H., Li J.X., Peng B.W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia. 2016;115:74–85. doi: 10.1016/j.fitote.2016.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.