Abstract
Human dental pulp stem cells (HDPSCs) have great potential to be used in regenerative medicine. To use these stem cells effectively for this purpose, they should be grown in a 3D cell culture that mimics their natural niches instead of a 2D conventional cell culture. The aim of this study was to grow the HDPSCs in the 3D cell culture created by Transglutaminase-crosslinked collagen hydrogels (Col-Tgel) in two different strengths to find a suitable 3D cell culture environment for these stem cells. Two stiffness of the 3D Col-Tgel were used to grow the HDPSCs: soft and medium matrix with strength of 0.9–1.5 kPa and 14–20 kPa, respectively. HDPSCs express markers similar to MSCs, therefore seven such markers were analyzed in the HDPSCs during their growth in the 2D and in the 3D soft and medium Col-Tgel. The CD105 and CD90 markers were significantly (p < 0.05) downregulated in HDPSCs cultured in both 3D cell culture conditions compared with HDPSCs in 2D cell culture. Furthermore, CD34 marker, a negative marker, expressed by a few cells in HDPSCs culture was upregulated (p < 0.05) in HDPSCs cultured in medium 3D Col-Tgel, indicating cells that expressing the marker grow better in medium 3D Col-Tgel. The apoptosis results revealed that HDPSCs in medium 3D Col-Tgel had the least number of live cells and a significantly (p < 0.05) higher early apoptosis rate compared to HDPSCs in 2D and 3D Col-Tgel medium. MTT analysis also showed a significant difference among the three cell culture conditions. We conclude that HDPSCs cultured on 3D soft Col-Tgel showed better proliferation than cells cultured in 3D medium gel. These results demonstrate that the ideal environment to grow HDPSCs in 3D is the soft Col-Tgel not medium Col-Tgel.
Keywords: Human dental pulp stem cells, 2D cell culture, 3D cell culture, Col-Tgel
1. Introduction
Human dental pulp stem cells (HDPSCs) are adult stem cells with great therapeutic potential in regenerative medicine because of their broader differentiation capabilities. Dental pulp is soft tissues which comprises of neural fibers, blood vessels as well as connective tissue; the HDPSCs are located at the center of the tooth (Lan et al., 2019). Other dental tissues such as exfoliated deciduous teeth (SHED), periodontal ligament (PDLSCs), apical papilla (SCAP) and dental follicle progenitor cells (DFPCs) are the sources of the stem cells (Gronthos et al., 2000, Miura et al., 2003, Seo et al., 2004). HDPSCs are known to be heterogenous in nature having different subtypes; one subtype of the stem cells originates from migrating neural crest cells, while another subtypes originates from mesodermal and ectodermal lineage (Lan et al., 2019). These stem cells have wide differentiation potential and have been differentiated into a variety of cell types such as osteoblasts, chondrocytes, adipocytes, neurons, myocytes, liver cells, epithelial cells of cornea, skin cells, dentin forming odontoblasts and Beta cells (Noda et al., 2019, Potdar and Jethmalani, 2015, Yu et al., 2010, Yu et al., 2007, Almushayt et al., 2006, d'Aquino et al., 2007, Kadar et al., 2009, Paino et al., 2010, Luke et al., 2020).
These HDPSCs have similar morphology as that of Mesenchymal Stem Cells (MSCs). They have fibroblasts like morphology as well as an adherence tendency to plastic surfaces (Pisciotta et al., 2015). Pisciotta et al. showed that HDPSCs and MSCs express similar markers. HDPSCs are shown to be positive for CD90, CD105 and CD73 markers and negative for hematopoietic markerCD34 (Noda et al., 2019, Gopinath et al., 2020).
The source of HDPSCs is mainly from third molars (wisdom teeth) which makes them easily accessible as yearly more than 10 million third molars are extracted in the United States (Friedman et al., 2007). In addition to their ease of extraction, HDPSCs use does not create any ethical issue which make the HDPSCs a very good choice with promising future potential.
Conventionally HDPSCs are grown in 2D cell culture in vitro. However, these stem cells have 3D niches in the pulp. Hence, concentrated efforts should be done to grow stem cells in 3D cell culture to completely mimic their natural in vivo environment. The 3D cell culture will display the inherent characteristics of these cells and may be more therapeutically effective. The dental pulp consists of HDPSCs, neurons, odontoblasts, endothelial cells, immune cells, growth factors (GFs) and the extracellular matrix (ECM), a mineralized form of collagen matrix makes up the dentin which encapsules the pulp; all of these components create its microenvironment (Monteiro & Yelick, 2019), whereas the flat glass/plastic surface in 2D culture provides an environment completely alien to the cells. Recent research shows that 2D culture provides unnatural conditions and physiologically compromised cells due to the absence of matrix-like environment; this led to failure rates in drug testing as cells in 2D culture were not true representatives of cells in vivo (Dutta & Dutta, 2018). It is now known that 3D environment allows the cells to acquire their natural characteristics (Bettinger et al., 2009). Hence, to evaluate the innate potential of these stem cells, a suitable 3D cell culturing needs to be invented.
In this study the 3D cell culture created by Col-Tgel was tested to grow the HDPSCs under different strengths of the gel. The gel is a tailorable collagen-based hydrogel that forms a solid substrate after cross-linking. It is a 3D cell culture matrix that provides a collagen backbone for cell attachment and can be used for stem cells, tumor cells, and primary cells (https://www.101bio.com/P720_3D_cell_culture_gel.php Accessed August 12, 2021). Furthermore, it is operated at room temperature and contains cell essential nutrients for cell survival; cells can be embedded in the matrix or plated on top, and the transparency and biocompatibility of the gel allows direct observation under the light microscope. Previous studies used the Col-Tgel in different experimentations; a study performed by Kuwahara et al. demonstrated that using Col-Tgel for cell delivery of viable cells in the wound sites of mice could successfully be conducted (Kuwahara et al., 2010). A study by Fang et al. modulated the 3D gel of the tumor cells to mimic the 3D in vivo environment of the cancer cells. Col-Tgel was cross linked to produce heterogeneous 3D environments resembling in vivo tumor environment on many aspects such as nutrition diffusion, hypoxia environment, pH gradients and various mechanical strengths indicating the flexibility of the hydrogel (Fang et al., 2014).
The purpose of this study was to find a suitable 3D cell culture for the growth of HDPSCs. Col-Tgel is available in different strengths; it can be made as soft gel with strength of 0.9–1.5 kPa and medium gel with strength of 14–20 kPa. The growth of HDPSCs was assessed in the soft and medium gels in addition to their growth in 2D conventional cell culture environment. This study proposes a suitable 3D cell culture for the growth of HDPSCs.
2. Materials and methods
2.1. Extraction of third molar and isolation HDPSCs
Normal human third molar teeth were collected from three individuals (age 15–25) at the University of Sharjah hospital under approved guidelines. The ethical committee at the University of Sharjah approved our study with approval number: REC-20–01-28–02.
Informed written consents were obtained from every donor for use of dental pulp tissue. The HDPSCs were extracted as described previously (Ac et al., 2020). Briefly, pulp tissue was gently separated after tooth surface was cleaned and placed in Phosphate Buffered Saline (PBS) (Sigma-Aldrich, USA) with pen/strep (Sigma-Aldrich, USA). Tissue was cut into small pieces, followed by digestion with collagenase/dispase (Roche Diagnostics GmbH, Germany), after digestion the debris were removed by centrifugation and single-cell suspensions were obtained by passing cells through a 70-μm cell strainer (Corning, USA).
Single cell suspension was incubated with α-MEM (Sigma-Aldrich, USA) supplemented with 20% fetal bovine serum (Sigma-Aldrich, USA), 1% L-Glutamine (Sigma-Aldrich, USA), and 1% penicillin streptomycin (Sigma-Aldrich, USA) antibiotics at 37 °C in humidified chamber with 5% CO2. These cells were initially grown in 2D flasks (T25 and T75) to assess proliferation and efficiency of growth (Fig. 1).
Fig. 1.
Schematic diagram showing the outline of the study. Extraction of third molar was performed and the pulp was removed asectipcally. Pulp tissue was digested by collagenase and seeded to grow human dental pulp stem cells. The HDPSCs were passaged in T75 flasks for further use in the 3D culturing and for stocking cells for future use. HDPSCs were seeded into 24 well plates for 2D culture. HDPSCs were extracted from T75 flasks and mixed with components from Col-Tgel in soft and medium strengths and seeded in drop formations in 24 suspension cell culture plates. After growth of 4 days, HDPSCs were extracted from all conditions: 2D, 3D soft gel and 3D medium gel and various analysises were performed.
2.2. Conventional 2Dimentional cell culture
Human dental pulp stem cells were taken from the previously growing passages and 60 thousand cells per well were seeded into 24 well plates for 4 days as control, 1 mL of α-MEM (Sigma-Aldrich, USA) media was added to each well on day1, sufficient for 4-day period after which cells were harvested (Fig. 1). All the experiments were performed three times in triplicates.
2.3. 3D Col-Tgel model
3D Collagen gel (101 Bio USA) was prepared by pouring a mixture of Col-Tgel component A and component B (20:1 v/v) onto non-sticky culture dishes and incubating at 37 °C for 45 min. The gel had two stiffness used in this experiment: soft matrix with strength of 0.9–1.5 kPa and medium matrix with 14–20 kPa. According to the manufacture instruction, the ratio of cells to gel was kept (2:1); 60 thousand cells were counted for plating per well, and the two components were mixed. A droplet of 20 mL cell-gel mixture was casted on each well of a 24 well suspension cell culture plate and formed a semi-domed shape (Fig. 1). α-MEM media (Sigma-Aldrich, USA) was added to each well to submerge the droplet to provide cells with the essential nutrients for 4-day period after which the cells were extracted. All these experiments were performed three times in triplicates.
2.4. Flow cytometry analysis
2.4.1. Apoptosis assay
Cells were harvested, washed with PBS, suspended in Annexin-V binding buffer, staining was performed with FITC-Annexin-V and PI Detection kit (BD Pharmigen, USA) according to the manufacturer’s instructions. After staining, cells were acquired in BD FACS-ARIA III (BD-Bioscience, Franklin Lakes, NJ, USA) flow cytometer using FACSDiva software (Fig. 1). Compensation was performed by running single color controls and analysis was performed using FlowJo software (BD-Bioscience, Franklin Lakes, NJ, USA).
2.4.2. Stem cell markers characterization
Cells were fixed with Cytofix/CytoPerm kit (BD Biosciences, USA) as per manufacturer’s instructions. Surface markers staining was performed using CD106-PE, CD271-PE, CD34-APC, CD105-APC, CD90-FITC, CD73-FITC and CD146-PE from Invitrogen as per manufacturer’s instructions. Markers expressions were characterized by acquiring samples in BD FACS-ARIA III (BD-Bioscience, Franklin Lakes, NJ, USA) using FACSDiva software. Compensation was performed by running either single color controls or BD CompBeads and analysis was performed using FlowJo software (BD-Bioscience, Franklin Lakes, NJ, USA).
2.5. Cell proliferation analysis
Using a 96 well plate, 5 × 103 cells were plated per well and Promega CellTiter 96® AQueous One Solution (Promega, USA) was added, the plate was incubated for 3–4 h in a growth chamber and absorbance was read at 490 nm using Crocodile Workstation/software (Titertek Berthold, Germany).
2.6. Statistical analysis
The data are presented as Mean ± SD. The data were analyzed using one-way analysis of variance (ANOVA) followed by Posthoc Tukey analysis. The statistical significance was accepted at p < 0.05.
3. Results
3.1. Morphological characteristics of the HDPSCs at the three culturing conditions
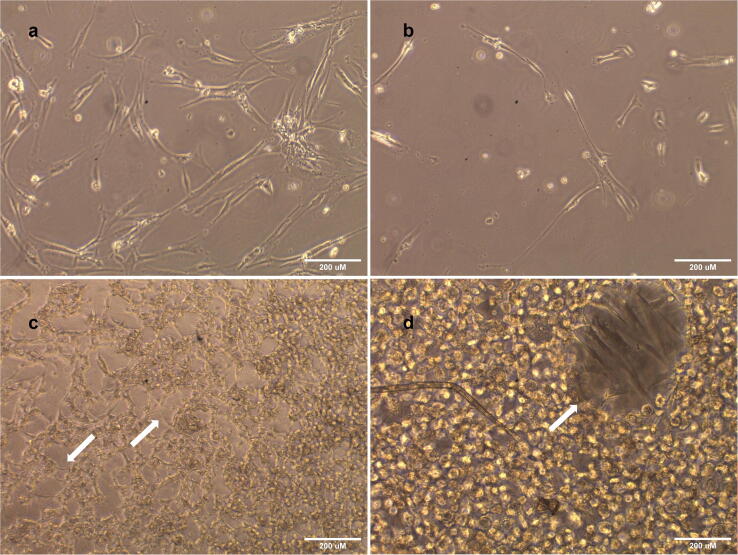
Light microscopic images of 10x and 20x magnifications showed a clear difference between 2D cell morphology and 3D medium (Fig. 2). The cells grown in 2D, and 3D soft gel showed standard fibroblast-like morphologies, while the cells grown in 3D medium gel showed a more circular morphology, different from their fibroblastic-like morphology.
Fig. 2.
Light Microscopic images of HDPSCs from day1 till day4 of proliferation in the three conditions: 2D culture, 3D soft Col-Tgel and 3D medium Col-Tgel. HDPSCs in 2D culture at 10X magnification and 3D culture images at 20X magnification. HDPSCs in 2D and 3D soft gel showing fibroblasts like morphology while HDPSCs in 3D medium gel showing a more circular morphology. The white arrows point towards the morphology in each condition.
HDPSCs adopted the same morphology after extraction from 3D soft gel and 3D medium gel when they were re-seeded in 2D culture (Fig. 3a & b), indicating the 3D medium gel is responsible for the changes in the stem cells morphology,. HDPSCs on 3D soft gel showed the same fibroblasts -like morphology, once the drop was moved and flattened slightly; the cells showed a clear morphology as that of 2D culture, proving that 3D soft gel with strength 0.9–1.5 kPa is optimum for the HDPSCs growth. These cells in 3D soft gel were viewed from above showing layers of cells in the gel (Fig. 3c). 3D soft gel observed under the microscope showing layers within the hydrogel, cells were seen to express fibroblast -like morphology in the layers within the gel, observed at different magnification (Fig. 3d).
Fig. 3.
Light Microscopic Images of HDPSCs; (a) Cells express morphology similar to morphology gown in 2D once extracted from 3D soft Col-Tgel and seeded again in 2D environment (b) Cells express morphology similar to cells grown in 2D morphology after extraction from 3D medium Col-Tgel and seeded again in 2D culture (c) 3D soft gel under light microscope at a magnification of 10× with arrows pointed towards and showing layers of cells, revealing HDPSCs to express similar morphology to cells in 2D enviroment (d) Higher magnification 20× of 3D soft gel with arrows pointing towards the HDPSCs.
3.2. Apoptosis
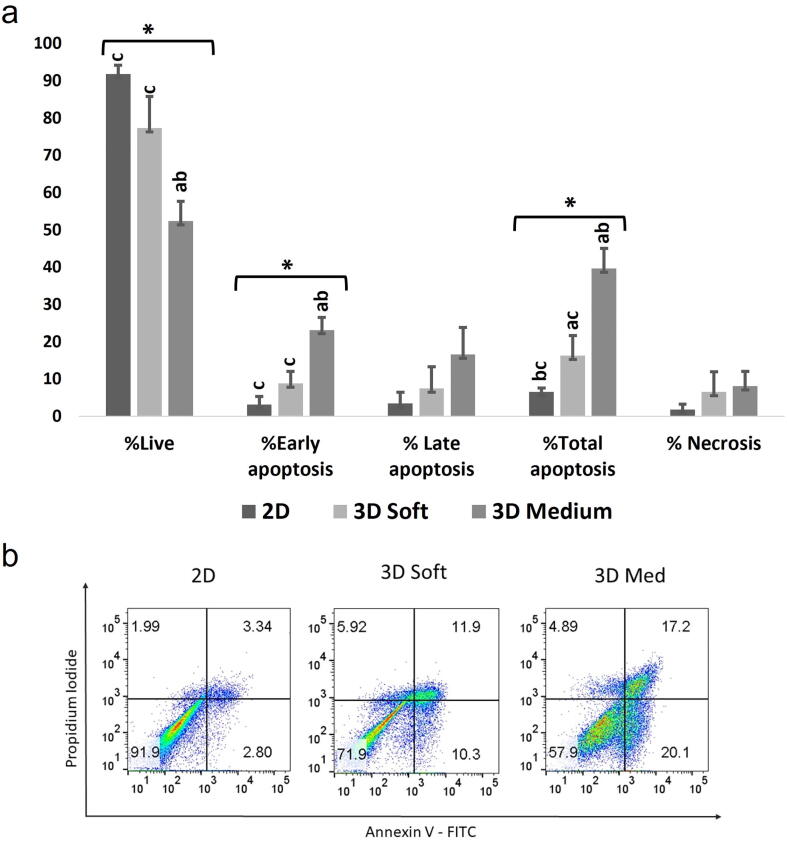
As shown in Fig. 4a the results show that cellular viability is highest in 2D cell culture condition. As the collagen strength increases the rate of apoptosis increases in HDPSCs. There is a significant difference in early apoptosis between 2D and 3D medium gels and between 3D soft and 3D medium gels. The total apoptosis also showed significance between 2D and 3D soft gels; between 2D and 3D medium and between 3D soft and 3D medium. The percentage of live cells are 91.73%, 77.30% and 52.33% for 2D, 3D soft and 3D medium gel respectively; there is also significant variation in the live cells between 2D and 3D medium gels and between 3D soft and medium gels (Fig. 4a). The flow cytometry data on early apoptosis, total apoptosis and live cells were obtained using Annexin and PI (Fig. 4b).
Fig. 4.
The effect of the three cell culture conditions, 2D, 3D soft gel and 3D medium gels, on HDPSCs growth; (a) HDPSCs were stained with Annexin/PI and analyzed using flow cytometry. The graph shows percentages of live, early/late apoptotic, total apoptosis and necrotic cells in all conditions denoted by different shades, *shows statistically significant difference for ANOVA. a: significant variation (p < 0.05) with 2D; b: significant variation (p < 0.05) with 3D soft, c: significant variation (p < 0.05) with 2D medium. (b) Representative Flow Cytometry results for Annexin-V /PI on cells extracted from 2D, 3D soft gel and 3D Medium gel conditions.
3.3. Stem cell markers characterization
Marker study showed positive mesenchymal markers CD105, CD90 and CD73 were expressed by the cells in 2D and negative marker such as hematopoietic marker CD34 was expressed by few cells. The CD90 and CD105 markers were expressed by HDPSCs in both 3D conditions (soft and medium) albeit their expressions were significantly lower in cells in both 3D gels (soft and medium) than in cells in 2D (Fig. 5). In contrast, CD34 expression was increased significantly in cells in 3D medium gel compared with cells in 3D soft and 2D gels, which indicate that specific subpopulation with CD34 might increase and have favorable environment in 3D medium condition (Fig. 5). The mean values for these three markers and also mean value of other markers were normalized. For example, the mean values/percentages for CD34 expressions were 3.47, 6.33 and 17.13 in cells in 2D, 3D soft gel and 3D medium gel, respectively. The 2D values have been normalized to 100% and the other values were also normalized compared with 2D values. Hence, the CD34 values in the three different cell culture conditions were normalized from the above mean percentage to 100 %, 183 % and 494% for 2D, 3D soft gel and 3D medium gel, respectively (Fig. 5). The expression of other markers CD106, CD146 and CD271 was not statistically significantly expressed in all three conditions (Fig. 5 & Fig. 6). Furthermore, from Fig. 6 it can be observed that not all HDPSCs express CD146 and CD 271.
Fig. 5.
The effect of the three cell culture conditions (2D, 3D soft gel and 3D medium gel) on the expression of 7 key marks by HDPSCs. HDPSCs were stained with CD antibodies and analyzed using flow cytometry. The graph shows normalized mean expression percentages of cell surface markers: CD106, CD90, CD105, CD146, CD73, CD34 and CD271 in all conditions denoted by different shades, *shows statistically significant difference (p < 0.05) for ANOVA. There is significant difference in CD90 and CD105 between 2D and 3D soft gel as well as 2D and 3D medium gel. There is also a significant difference in CD34 between 2D and 3D medium gel as well as 3D soft gel and 3D medium gel. a: significant variation (p < 0.05) with 2D; b: significant variation (p < 0.05) with 3D soft, c: significant variation (p < 0.05) with 2D medium.
Fig. 6.
Graphical representation of flow cytometry results of cell surface markers under unstained (US), 2D, 3D soft gel and 3D medium gel conditions. (a) CD106 (b) CD90 (c) CD105 (d) CD146 (e) CD73 (f) CD34 (g) CD271.
3.4. Cell viability analysis
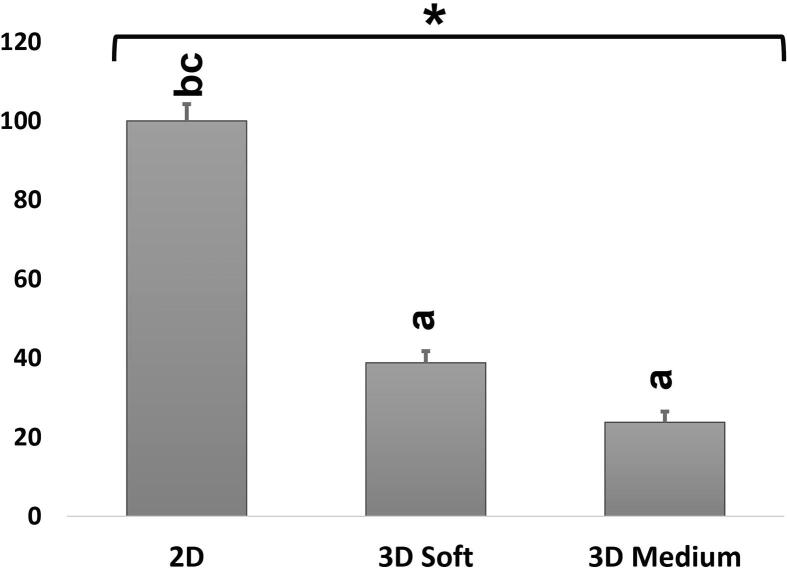
The mean values of replicates of cell viability for all conditions 2D, 3D soft gel and 3D medium gel were 1.25, 0.49 and 0.30 respectively, which were further normalized for graphical representation. Cell viability data shows 2D condition was the most suitable environment for HDPSCs proliferation, followed by 3D soft gel and lastly 3D medium gel condition, there was a significant difference in the data when comparing the three conditions: 2D with 3D soft gel and medium gel (Fig. 7).
Fig. 7.
The effect of the three conditions on HDPSCs viability. The graph shows normalized percent means in all three conditions. *shows statistically significant difference (p < 0.05) for ANOVA. a: significant variation (p < 0.05) with 2D; b: significant variation (p < 0.05) with 3D soft, c: significant variation (p < 0.05) with 2D medium.
4. Discussion
HDPSCs are increasingly being characterized and evaluated as a viable stem cell source for the application of effective regenerative therapies for clinical use (Alraies et al., 2020). These therapeutic applications are based on accessibility of HDPSCs from the permanent dentition. Furthermore, the stem cells have self-renewal and multilineage differentiation capabilities (Potdar and Jethmalani, 2015, Luke et al., 2020). Consequently, studies in animal model have shown that DPSCs are beneficial to repair tissues and organs (Lan et al., 2019).
There is lack of information about the growth of cells in 3D environments as well as their comparison with 2D environment. Cells normally grow in a multi-interactive environment with the extracellular matrix playing a crucial role in vivo.
Scaffolds and matrices are widely used in cell culturing. These scaffolds guide the 3D growth of cells. They are able to provide 3D environment to cells by mimicking the microenvironment of tissues. Scaffolds should permit transportation of oxygen, nutrition, and removal of waste products; they should also be biodegradable (Ashri et al., 2015).
In this study, we used Col-Tgel with different stiffness to grow HDPSCs; this hydrogel mimics the physiochemical properties of the ECM (Dutta & Dutta, 2018). The transparency of Col-Tgel allowed us to view the cells in real-time under the light microscope. The microscopic images of 10x and 20x magnification revealed different morphologies of cells grown in 2D vs 3D environment in soft and medium stiffness (Fig. 2). The cells in both 2D and 3D soft Col-Tgel cultures showed elongated fibroblast-like morphology. However, cells in 3D medium gel showed a circular morphology with no change in the empty spaces in the gel, unlike the other two gel types which could be the result of very slow or no growth in the 3D Col-Tgel medium (Fig. 2). The elastic modulus of dental pulp tissue is 5.5 kPa (Datko Williams et al., 2018) which means the HDPSCs are sensitive to the pressure and at the medium pressure of 14–20 kPa the HDPSCs cannot proliferate well. This led us to believe that the cells in 3D medium Col-Tgel gel were not doubling and could have been either in G0 phase or going through apoptosis or necrosis. A study using breast cancer MDA-MB-231, prostate cancer cells (C4–2B), and colon cancer cells (HCT116) showed difference in the morphologies of the cells in the 2D and 3D cell culture similar to our study; in 3D matrix, cells formed microspheroids while in 2D culture the cells were elongated (Fang et al., 2014). Another study reporting the culturing of adipose derived stromal cells (ADSCs) on Col-Tgel of soft, medium, and stiff strengths revealed that cells embedded in the Soft Col-Tgel have the morphology of mesh-like and exhibited extended actin filaments. In contrast, cells grew in the medium and stiff Col-Tgels displayed dot-like morphology (Tan et al., 2015), the reported morphological changes are similar to our cell culturing in the soft and medium of Col-Tgels. Fig. 2 shows that cells growing in 2D surface have standard fibroblast like elongation similar to cells growing in 3D soft Col-Tgel gel which have shorter fibroblast like extension elongation. Similarly, the study using ADSCs originally formed a monolayer and displayed an elongated morphology in the 2D setting in contrast to less elongated morphology by cells in the 3D environment (Tan et al., 2015).
Furthermore, after extraction of cells from both soft and medium Col-Tgel, some cells were collected and regrown on 2D culture plates to observe if the cells could get back same characteristics of 2D culture. The cells retained their morphology and showed the same doubling time as the control cells on the 2D culture (Fig. 3).
According to manufactures of the Col-Tgel, the 3D soft gel would work optimum for the growth of the stem cells, although no research was previously performed specifically on the growth of HDPSCs. Our study indicated that apoptosis was induced in HDPSCs in 3D medium gel. This indicates the strength of 14 to 20 kPa is not good for the growth of HDPSCs. In this strength the cells were unable to proliferate as the porosity was lower. Consequently, increased strength is not the most efficient environment for HDPSCs (Fig. 4a). When comparing total apoptosis (late and early) this also showed that cells in 2D are experiencing least apoptosis while cells in 3D soft gel is experiencing significantly more apoptosis than 2D, while HDPSCs in 3D medium gel displayed most apoptosis (Fig. 4a). This further proves that the environment created by soft Col-Tgel is better suited to the HDPSCs compared to the 3D medium Col-Tgel environment. The genotype and phenotype of HDPSCs is also dependent on the interaction with its surrounding ECM, and endogenous ECM remodeling has crucial role in cells assembly and differentiation. One study grew mouse C2C12 myoblast cells in three different rigidities 3% (soft), 6% (medium), and 9% (stiff) of Col-Tgel; It was observed, similar to our study, the number of cells in the 3% TG-Gel increased at a more rapid rate than those cells in the 6% and 9% Col-Tgel (Tan et al., 2014). Another study used adipose derived stromal cells and showed similar trends as our study; the study grew ADSCs (Adipose derived stem cells) on all three stiffness, soft, medium, and stiff. The results revealed that increase in gel stiffness led to reduction in the cell proliferation and viability (Tan et al., 2015). The study concluded that the elasticity of the Med Col-Tgel (14–20 kPa), mimics striated muscle elasticity (∼8–17 kPa) and this environment was found to be the optimal microenvironment for ADSCs (Tan et al., 2015). The ADSCs and HDSCs are both mesenchymal cells and it is surprising that ADSCs, unlike our HDPSCs, are able to proliferate in the 3D medium Col-Tgel.
MTT assay indicated that proliferation was highest in 2D cell culture again indicating 2D environment is the most suitable to HDPSCs, followed by the environment of 3D soft gel (Fig. 7). Despite the rapid growth in 2D cell culture, 3D cell culture is better as it mimics the in vivo environment. A study performed by Tan et al. using C2C12 cells also performed MTT assay to assess mitochondrial activity and, similarly to our results, location of most viable cells was observed around the periphery of 3% TG-Gel (which was equivalent to soft gel in our study) in comparison to the 6% and 9% which were the medium and stuff gel respectively (Tan et al., 2014). Proliferation was also measured in a study using multiple cancer cell lines in Col-Tgel and the results mirrored our findings with cells in soft gel displayed highest proliferation and the proliferation decreased with an increase in gel stiffness (Fang et al., 2016).
Markers study has now become a very integral part of most stem cells research as it is a crucial tool for identifying subpopulations that exist within heterogeneous cell populations. In this study we studied seven positive markers (CD105, CD90, CD73, CD146, CD106 and CD271) and a negative surface marker CD34 for mesenchymal/dental pulp stem cells to assess their expressions in 3D and 2D cell culture environments. As HDPSCs express similar markers to that of MSCs, positive expressions of CD105 and CD90 and negative expression of CD34 were obtained (Alraies et al., 2020, Gopinath et al., 2020). In our study, 2D cultured HDPSCs also showed positive expressions for CD90 and CD105 and negative expression for CD34. Cells cultured in 3D soft Col-Tgel showed similar trends as that of 2D with a positive expression of CD90 and CD105. However, their expression was significantly down regulated as stiffness increased. In contrast, the CD 34 expression was upregulated in 3D medium Col-Tgel suggesting the hematopoietic like cells favour the 3D medium Col-Tgel environment (Fig. 5). Vishwanath et al. and Gronthos et al. also showed the profile of HDPSCs showed a negative expression with hematopoietic marker CD34 (Vishwanath et al., 2013, Gronthos et al., 2002). This study also mirrors the results of a previous study with HDPSCs profiles showing a positive expression of CD90 and CD105 (Atari et al., 2012, Noda et al., 2019, Gopinath et al., 2020). CD105 is an Endoglin (ENG) which is a component of the Transforming Growth Factor β Receptor Complex which has high affinity to TGFβ1 and TGFβ3. The upregulation of CD34 in 3D medium gel indicates a subpopulation of HDPSCs with CD34 may be favorably growing in 3D medium gel or the higher strength may differentiate HDPSCs into hematopoietic lineage. Differences in strength might be a contributing factor in stem cells differentiation. One study indicates that strength does have effect on the differentiation of stem cells (Tan et al., 2014), and therefore we can conclude that the 3D medium Col-Tgel might be favoring to a subpopulation of cells expressing CD34. The expression of other cell surface markers such as lymphocyte differentiation marker CD73 and endothelial cell marker CD106 showed no significant difference, similar to the study performed by Vishwanath et al. and Atari et al. (Vishwanath et al., 2013, Atari et al., 2012).
5. Conclusion
This study revealed that 3D soft Col-T-gel is better than medium Col-Tgel for the 3D growth of the HDPSCs. The HDPSCs exhibited best proliferation in 2D culture followed by 3D soft Col-Tgel and lastly 3D medium Col-Tgel gel culture. The medium gel Col-T-gel induces apoptosis in HDSCs. Consequently, the expressions of specific markers such as CD34 are differentially regulated, and it proves that medium Col-T-gel is not suited for the 3D cell culturing of HDPSCs. The soft Col-T-gel is suited for the 3D culturing of the HDPSCs. However, the effects of the soft Col-Tgel gel on the differential regulation of CD90 and CD105 markers warrant further investigation. Further study using a mixture of gels to create a strength between soft and medium gel can also be tested to see the proliferation and differentiation trends of HDPSCs. The stiff Col-Tgel will not be a suitable model hydrogel as it will unlikely support stem cell growth; the stiff gel will have most strength and will further enhance the apoptosis of HDPSCs. Furthermore, as we have observed that strengths of Col-Tgel affect apoptosis, markers and other proteins might also affect the differentiation. Hence, the transcriptomic, and proteomics analysis of these HDPSCs at different strengths of the Col-Tgel should be performed to unravel the molecular processes that are governed during these 3D cultures.
Funding
This study was funded by College of Graduate Studies at University of Sharjah, UAE, for thesis support for master study and by targeted grant for research group by University of Sharjah with grant number: 1802145073.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
This study was funded by targeted grant (1802145073) for research group at the University of Sharjah and by master thesis support grant by College of Graduate study at the University of Sharjah.
We created the Fig. 1 using the biorender website (www.biorender.com).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Sakina Eqbal Hussain Tayabally, Email: U18104628@sharjah.ac.ae.
Amir Ali Khan, Email: amkhan@sharjah.ac.ae.
References
- Ac S.A., Islam M.S., Samsudin A.R. Investigation of the effect of a time delay on the characteristics and survival of dental pulp stem cells from extracted teeth. Arch. Oral Biol. 2020;119 doi: 10.1016/j.archoralbio.2020.104896. [DOI] [PubMed] [Google Scholar]
- Almushayt A., Narayanan K., Zaki A.E., George A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 2006;13(7):611–620. doi: 10.1038/sj.gt.3302687. [DOI] [PubMed] [Google Scholar]
- Alraies A., Waddington R.J., Sloan A.J., Moseley R. Evaluation of dental pulp stem cell heterogeneity and behaviour in 3D type I collagen gels. Biomed Res. Int. 2020;2020:1–12. doi: 10.1155/2020/3034727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashri N.Y., Ajlan S.A., Aldahmash A.M. Dental pulp stem cells: biology and use for periodontal tissue engineering. Saudi Med. J. 2015;36(12):1391–1399. doi: 10.15537/smj.2015.12.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atari M., Gil-Recio C., Fabregat M., García-Fernández D., Barajas M., Carrasco M.A., Giner L. Dental pulp of the third molar: a new source of pluripotent-like stem cells. J. Cell Sci. 2012;125(14):3343–3356. doi: 10.1242/jcs.096537. [DOI] [PubMed] [Google Scholar]
- Bettinger C., Langer R., Borenstein J. Engineering substrate topography at the micro-and nanoscale to control cell function. Angew. Chem. Int. Ed. 2009;48(30):5406–5415. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Aquino R., Graziano A., Sampaolesi M., Laino G., Pirozzi G., De Rosa A., Papaccio G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14(6):1162–1171. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- Datko Williams L., Farley A., Cupelli M., Alapati S., Kennedy M.S., Dean D. Effects of substrate stiffness on dental pulp stromal cells in culture. J. Biomed. Mater. Res. Part A. 2018;106(7):1789–1797. doi: 10.1002/jbm.a.36382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R.C., Dutta A.K. CRC Press; 2018. 3D Cell Culture: Fundamentals and Applications in Tissue Engineering and Regenerative Medicine. [Google Scholar]
- Fang J.Y., Tan S.J., Wu Y.C., Yang Z., Hoang B.X., Han B. From competency to dormancy: a 3D model to study cancer cells and drug responsiveness. J. Transl. Med. 2016;14(1):1–13. doi: 10.1186/s12967-016-0798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J.Y., Tan S.-J., Yang Z., Tayag C., Han B.o., Burns J.S. Tumor bioengineering using a transglutaminase crosslinked hydrogel. PLoS ONE. 2014;9(8):e105616. doi: 10.1371/journal.pone.0105616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J.W. The prophylactic extraction of third molars: a public health hazard. Am. J. Public Health. 2007;97(9):1554–1559. doi: 10.2105/AJPH.2006.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath V.K., Soumya S., Jayakumar M.N. Osteogenic and odontogenic differentiation potential of dental pulp stem cells isolated from inflamed dental pulp tissues (I-DPSCs) by two different methods. Acta Odontol. Scand. 2020;78(4):281–289. doi: 10.1080/00016357.2019.1702716. [DOI] [PubMed] [Google Scholar]
- Gronthos S., Brahim J., Li W., Fisher L.W., Cherman N., Boyde A., DenBesten P., Robey P.G., Shi S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002;81(8):531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.101bio.com/P720_3D_cell_culture_gel.php Accessed August 12, 2021.
- Kadar K., Kiraly M., Porcsalmy B., Molnar B., Racz G.Z., Blazsek J., Varga G. Differentiation potential of stem cells from human dental origin-promise for tissue engineering. J. Physiol. Pharmacol. 2009;60(Suppl 7):167–175. [PubMed] [Google Scholar]
- Kuwahara K., Yang Z., Slack G.C., Nimni M.E., Han B.o. Cell delivery using an injectable and adhesive transglutaminase–gelatin gel. Tissue Eng. Part C: Methods. 2010;16(4):609–618. doi: 10.1089/ten.TEC.2009.0406. [DOI] [PubMed] [Google Scholar]
- Lan X., Sun Z., Chu C., Boltze J., Li S. Dental pulp stem cells: an attractive alternative for cell therapy in ischemic stroke. Front. Neurol. 2019;10:824. doi: 10.3389/fneur.2019.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke A.M., Patnaik R., Kuriadom S., Abu-Fanas S., Mathew S., Shetty K.P. Human dental pulp stem cells differentiation to neural cells, osteocytes and adipocytes-An in vitro study. Heliyon. 2020;6(1):e03054. doi: 10.1016/j.heliyon.2019.e03054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M., Gronthos S., Zhao M., Lu B., Fisher L.W., Robey P.G., Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro N., Yelick P.C. Principles of Regenerative Medicine. Academic Press; 2019. Dental tissue engineering; pp. 907–921. [Google Scholar]
- Noda S., Kawashima N., Yamamoto M., Hashimoto K., Nara K., Sekiya I., Okiji T. Effect of cell culture density on dental pulp-derived mesenchymal stem cells with reference to osteogenic differentiation. Sci. Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-41741-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paino, F., Ricci, G., De Rosa, A., D'Aquino, R., Laino, L., Pirozzi, G., Papaccio, G., 2010. Ecto-mesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. [DOI] [PubMed]
- Pisciotta A., Carnevale G., Meloni S., Riccio M., De Biasi S., Gibellini L., Ferrari A., Bruzzesi G., De Pol A. Human dental pulp stem cells (hDPSCs): isolation, enrichment and comparative differentiation of two sub-populations. BMC Dev. Biol. 2015;15(1) doi: 10.1186/s12861-015-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potdar P.D., Jethmalani Y.D. Human dental pulp stem cells: applications in future regenerative medicine. World J. Stem Cells. 2015;7(5):839. doi: 10.4252/wjsc.v7.i5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo B.-M., Miura M., Gronthos S., Mark Bartold P., Batouli S., Brahim J., Young M., Gehron Robey P., Wang C.Y., Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. The Lancet. 2004;364(9429):149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Tan S.J., Fang J.Y., Wu Y., Yang Z., Liang G., Han B. Muscle tissue engineering and regeneration through epigenetic reprogramming and scaffold manipulation. Sci. Rep. 2015;5(1):1–13. doi: 10.1038/srep16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ShihJye, Fang J.Y., Yang Z., Nimni M.E., Han B.o. The synergetic effect of hydrogel stiffness and growth factor on osteogenic differentiation. Biomaterials. 2014;35(20):5294–5306. doi: 10.1016/j.biomaterials.2014.02.040. [DOI] [PubMed] [Google Scholar]
- Vishwanath V.R., Nadig R.R., Nadig R., Prasanna J.S., Karthik J., Pai V.S. Differentiation of isolated and characterized human dental pulp stem cells and stem cells from human exfoliated deciduous teeth: an in vitro study. J. Conserv. Dent.: JCD. 2013;16(5):423. doi: 10.4103/0972-0707.117509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., He H., Tang C., Zhang G., Li Y., Wang R., Shi J., Jin Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010;11(1) doi: 10.1186/1471-2121-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Wang Y., Deng Z., Tang L., Li Y., Shi J., Jin Y. Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol. Cell. 2007;99(8):465–474. doi: 10.1042/BC20070013. [DOI] [PubMed] [Google Scholar]