Graphical abstract
Keywords: Interleukin 1β (IL1- β), Malondialdehyde (MDA), Total antioxidant capacity (TAC), Parascaris equorum, Pro-inflammatory cytokines (TNF-α)
Abstract
This study aimed to assess the effects of Parascaris equorum (P. equorum) in infected donkeys through evaluation the oxidative stress and different gene parameters in infected tissues. Fifty donkeys were examined in Giza Zoo abattoir from the period of January to March 2021. Blood and sera samples were collected from each examined donkey. P. equorum were subjected for identification through scanning electron microscope study and the infected tissues were subjected into gene expression analysis using two genes; interleukin 1β (IL1- β); and pro-inflammatory cytokines (TNF-α) with assessment of the antioxidant and free radicals released from the animals during the infection. Eighteen donkeys were positive for P. equorum adult or larvae by postmortem examination of the intestine and abdomen with prevalence rate of 36 %. The examined infected donkeys with P. equorum showed significantly higher of Total antioxidant capacity (TAC) levels and the serum malondialdehyde (MDA) 2.45 ± 0.53 than that in non-infected control donkeys. The levels of AST enzyme were 278.54 ± 0.45 while ALT enzyme was 14.97 ± 0.87 which was significantly higher than that of control negative donkeys. The infected donkeys with P. equorum showed significantly upregulation of the TNF-α and IL-1β which classify according to number of collected worms. The P. equorum infected donkeys exerted at least 100 eggs of parasite in feces. The fecal egg count was marked decreased after treatment with moxidectin. Moxidectin is considered a novel active ingredient that has a marvelous result with high persistency and protection for long time, in addition to, broad spectrum activity and low or no resistance. We recommend the periodical deworming with different molecules as more economic and lifesaving over a single treatment every 12 months parallel with parasitic testing.
1. Introduction
Donkeys (Equus asinus) are one of early-domesticated equines, they are easy to rear, which representing a cheap mean for human transportation; about 40 million donkeys distributed world-wide and 13 million in Africa, while around 3 million working donkeys approximately in Egypt (Hilali et al., 2015, Attia et al., 2018, Attia and Mahdy, 2021) They are accustomed to low quality forages or grass and spread widely in Africa and rural areas (Ahmed et al., 2011). unfortunately, donkeys subjected to bad management, work for long hours under stress, malnutrition and fed with low quality grass and forages which considered as source for their infection with a variety of parasites (Parsani et al., 2013, Jajere et al., 2016, Mahdy and Attia, 2021). In developing countries, one of the most prominent factors limiting the health and performance of donkeys is gastrointestinal parasites, where it considered as the most serious disease problem for horses and donkeys (Pereira and Vianna, 2006). Donkeys infected with a wide range of internal parasites as roundworms (families: Ascaridae, Trichostronglidae, Oxyuridae and Stronglidae) and tapeworm (family: Anoplocephalidae) which induce severe gastrointestinal damages that appears in shape of diarrhea, inappetence, weight loss, poor performance, general weakness, colic, and may end with death in case of heavy infestation subsequent to intestinal impaction, obstruction or perforation (Pereira and Vianna, 2006, Getahun and Tihune, 2017, Attia et al., 2018). Parascaris equorum is one of gut helminths that represent a serious problem for equines, it has a direct life cycle takes about three months in which the adult round worm lives in the small intestine lies millions of rebellious eggs that passed daily in equine feces to the environment and develop into infective stage within 10–14 days that ingested by a new host and hatch in its intestine, larvae liberate and migrate through liver, lungs and may attack other tissues for 2–4 weeks, then return to small intestine and mature (Urqhart et al., 1988, Rose and Hodgson, 1993, Getachew et al., 2008). Parascaris equorum infection revealed inflammatory lesions mainly in small intestine, gut ischemia, severe colic, intestinal obstruction, intestinal rupture, toxemia, and death. Also, respiratory manifestation (nasal discharge, intermittent cough, and dyspnea); liver lesions and nervous manifestations may by occasionally observed due to larval migration (Rose and Hodgson, 1993, Getachew et al., 2008). Many diagnostic tools were used for parasitic detection as fecal examination, ELISA, and PCR. Enzyme Linked Immunosorbent Assay (ELISA) is a quantitative, highly sensitive technique customized for the detection of target analyze found in biological samples as serum, and plasma, where ELISA be used for detection and accurate quantification of substances such as cytokines, hematological factors, hormones, peptides, and immunoglobulins (Shalaby et al., 2008). Anthelmintic resistance specially against cyathostomine (small strongyle) and other nematodes has been extended to many active ingredients as benzimidazoles or pyrantels (50% of populations) and occasional piperazine (Drudge et al., 1983, Kaplan, 2004, Kaplan et al., 2004, Brazik et al., 2006). Similarly, P. equorum has been found to be resistant to fenbendazole, Piperazine and macrocyclic lactones, particularly ivermectin, which has been observed in numerous countries (Peregrine et al., 2014) and Egypt (Ali et al., 2015). On the other hand, moxidectin alone or in combination with praziquantel found to be effective treatment against internal worms in horses (Cobb and Boeckh, 2009). As a result, there is a need to continue monitoring anthelmintic resistance in all equine populations, as well as to promote methods that limit anthelmintic use (Nielsen et al., 2014). Procedures exerted for P. equorum control were mainly depends on the managemental and biosecurity measures side-by-side with the rotational deworming programs to cover the most parasites threaten the equestrians. So, future studies should focus on new control strategies and medicaments selection to overcome this resistance (Reinemeyer, 2009). Therefore, this study aimed to investigate the immunological effects of P. equorum in donkeys’ tissues through analysis of different immunological genes as interleukin 1β and tumor necrosis factor α; as well as assessment of different oxidative stress to evaluate the antioxidant capacity released through P. equorum infection with treatment trial to evaluate the efficacy of moxidectin in the treatment of such infection
2. Materials and methods
2.1. Animal and sampling
Fifty donkeys were examined in Giza Zoo abattoir from the period of January to March 2021. Blood was collected from each examined donkey in sterilized tubes with and without EDTA. The samples were collected and preserved as recorded in Attia et al. (2020).
The abdomen of each donkey was opened, and the intestine was removed and opened to examine the presence of P. equorum. As well as; collection of fecal samples from each examined donkey. The labeled fecal samples and the Parascaris nematodes as well as the blood and sera were collected and examined in the Faculty of Veterinary Medicine, Cairo University for further analysis.
2.2. Fecal samples
Examination of fecal samples were done to exclude other internal parasitic infection where eggs or oocyst were traced using direct fecal smear and floatation technique to exclude their presence (Soulsby, 1986). Therefore, the only used donkeys’ sera as reference was that free from all parasites except presence of P. equorum either eggs or adults.
2.3. Blood and sera samples
Ten ml of blood samples from each animal were collected during slaughtering from jugular vein; on plain tube which used for estimation of different biochemical analysis as aspartate amino transferase (AST) and alanine amino transferase (ALT) following the specific test kits instructions (spectrum-diagnostics, Egypt). Also, thin blood film from each animal whole blood with EDTA was done and stained with Giemsa stain for detecting any blood parasites (Zaki et al. 2021).
2.3.1. Identification of P. equorum Adult and Eggs
All the collected nematodes from intestine and its eggs from feces were examined in the parasitology laboratory of the Faculty of Veterinary Medicine, Cairo University for larval identification. The identification of the collected nematodes was done following Soulsby, 1986, Attia et al., 2018.
2.3.2. Ultrastructure Identification of The Collected P. equorum using Scanning Electron Microscopy (SEM) study (JOEL)
Adult P. equorum were washed several times using 0.9% saline (Attia and Salem, 2021). The collected worms were fixed in 2.5% Glutaraldehyde following to Abdelsalam et al., (2020); then the worms were removed from Glutaraldehyde and dehydrated using ethanol series (50%; 70%; 90%; 100%); then the fixed Nematoda was dried in CO2 critical point drier (Autosamdri-815, Germany). The adult was glued over stubs (as anterior end and posterior end) and then; coated with 20 nm gold (Abu-Elala et al., 2018) in a sputter coater (Spi-Module sputter Coater, UK). All the specimens were photographed with a scanning electron microscope (JSM 5200, Electron prob); Microanalyzer Jeol, Japan; at Faculty of Agriculture, Cairo University, as described by Salem and Attia, (2021).
2.4. Assessment of the oxidative stress markers
Oxidative stress markers were studied in sera samples as malondialdehyde (MDA); Total antioxidant capacity (TAC) according to Aytekin and Unubol Aypak, 2011, Aktas et al., 2017, Salem et al., 2018, Attia et al., 2019.
2.5. Evaluation of pro-inflammatory cytokines (TNF-α) and interleukin 1β
Infected intestine with the parasites were aseptically dissected and then preserved in freezer in −20 °C. Samples from 5 uninfected donkeys were free from any parasites and have no gross lesion used as negative controls (Attia et al., 2020).
2.5.1. RNA Isolation
Isolation of mRNA by total RNA kit (Ambion, Applied Biosystems), from 200 mg of infected intestine with P. equorum. Homogenization of the intestinal tissues were applied in Lysing Matrix D tubes (MP Biomedicals) using a FastPrep-24 homogenizer (MP Biomedicals, 2 cycles of 30 s at 6 m/s). Nanodrop (Thermo Scientific) were assess the mRNA purity and quantity. A 500 ng of mRNA were made with DNaseI amplification grade (Invitrogen) following the manufacturer's instructions. The reverse transcription of treated RNA was performed by High-Capacity cDNA Archive Kit (Applied Biosystems) (Attia et al., 2020, Younis et al., 2020).
2.5.2. Quantitive Real-Time PCR protocol (qRT-PCR)
PCR primer sets were designed according to presence in Gene Bank specific for donkeys (Table 1). The reference gene used was the β-actin and used for sample normalization. Real-time PCR run protocol were followed according to Attia and Mahdy, (2021).
Table 1.
The sequences of the forward and reverse primer used in the quantitative real-time PCR.
| Genes | sequence | Accession number | References |
|---|---|---|---|
| IL-1β | F: AAAACAGTGAGGGAGAAATT | XM_014852743 | Huang et al., 2015 |
| R: AGAAACTTCTTCTTGGGTAG | |||
| TNF- α | F: ATGTTTCAGTCACATTTCAG | XM_014831267 | Huang et al., 2015 |
| R: CCTACCGGTT CCCATCTCAA | |||
| β-Actin | F: CAGCAAGCAGGAGTACGATGAG | AF035774 | Swiderski et al. 1999 |
| R: TGTGTGGTGTGTGGTTGTTTTG |
2.6. Treatment trials
Ten naturally infected donkeys with P. equorum in a private collecting station were subdivided into two groups; 1st group was treated with moxidectin (Equest®, Zoetis), at a dose of 0.4 mg/kg oral paste with interval of 3 months and 2nd group kept untreated. The efficacy of the treatment was estimated by fecal egg count reduction (FECR) test. Individual freshly voided fecal samples were collected weekly for one month using veterinary gloves. All fecal samples were kept in labeled plastic pages and transported on ice box rapidly to Parasitology Department, Faculty of Veterinary Medicine, Cairo University for further investigations. Fecal samples were stored under refrigeration and examined within 24 h. Fecal egg counts were estimated using modified McMaster technique (Raynaud, 1970) using saturated solution of sodium chloride (specific gravity 1.20).
2.7. Statistical analysis
Data were expressed as means ± standard errors and the data were statistically analyzed using independent t-test of ANOVA test using SPSS Inc., Chicago, IL, USA; Version 18.0 software. P-value was considered significant when it less than 0.05.
3. Results
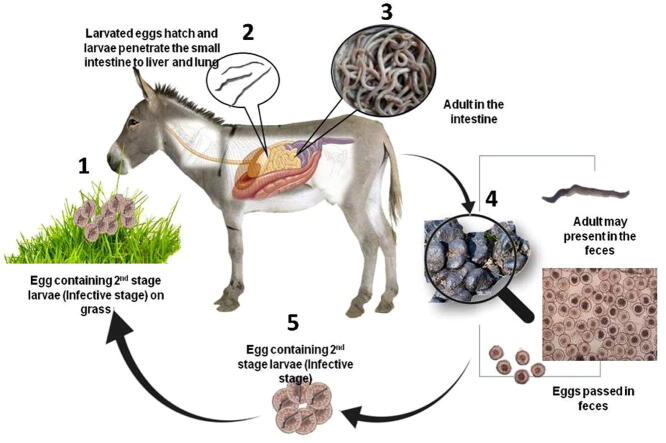
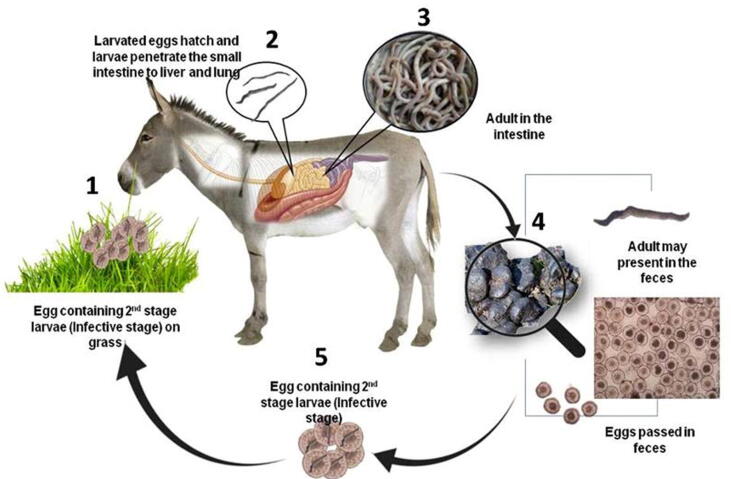
Eighteen examined donkeys were positive for P. equorum adult or larvae by postmortem examination of the intestine and abdomen with prevalence rate of 36% (Fig. 1, Fig. 2).
Fig. 1.
Life cycle of P. equorum; the adult worms inhabit the small intestine of donkey. The worm lay eggs that passed into the feces, Eggs have a thick shell as appeared under microscope. Eggs are then ingested as in the infective form as egg containing second stage larvae within the contaminated grass or drinking water by the donkey. After that, the ingested infective stage become a free larva that penetrate the small intestines and migrate into blood stream to the liver and further to the lung to irritate the animal to coughed up and re-swallowed again.
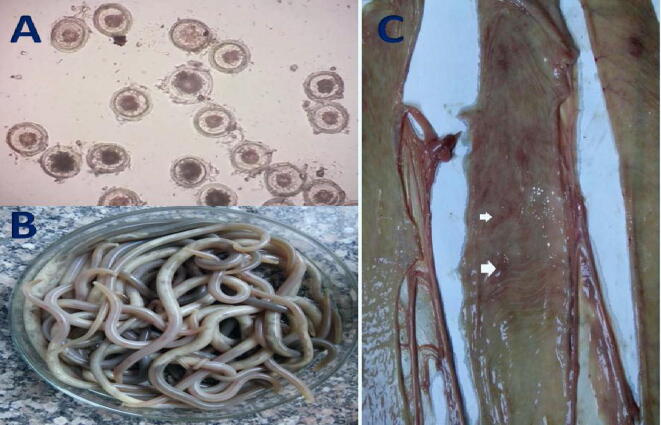
Fig. 2.
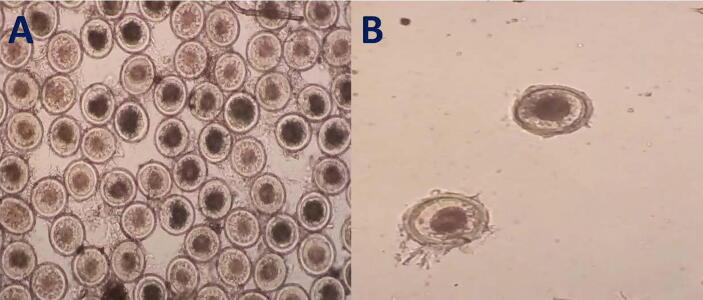
A: Microscopical appearance of P. equorum eggs which present in fecal samples; the eggs appear round with small embryo. B: Adult P. equorum worms. C: Opened intestine of donkey infected with P. equorum showing mucosal surface congestion with presence of hemorrhagic streaks showed by arrows.
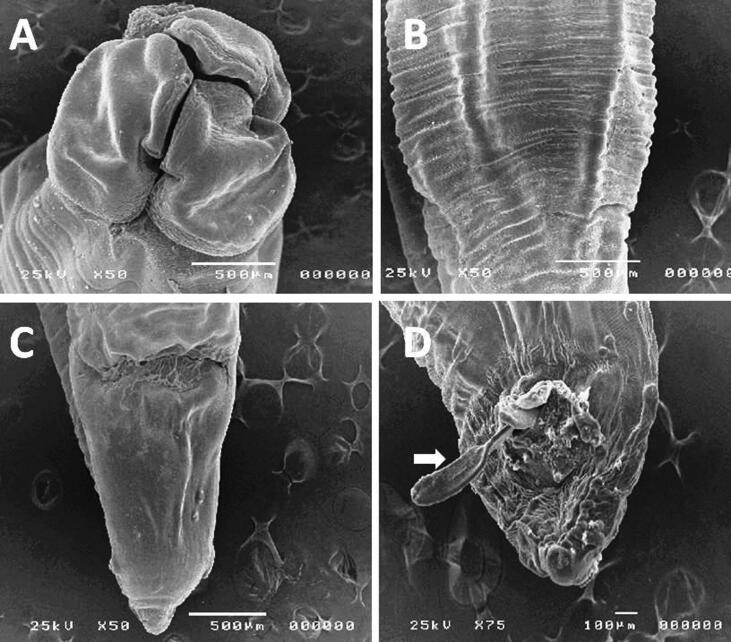
The adult P. equorum were whitish in color, and cylindrical; the anterior end had 3 large lips with deep transverse groove, lips were large; crown and prominent, 3 in number, one dorsal and two sub-ventrals. The cuticle was with finely striated annulation. The tail of the male was relatively long, and often having a small button-like termination. The tail of the female was conical and slightly attenuated at its distal end with spicule (Fig. 3).
Fig. 3.
Scanning electron microscopic micrograph of P. equorum showing; A: the anterior end had 3 large lips with deep transverse groove the lips were large; crown and prominent, 3 in number, one dorsal and two sub-ventrals. B: the cuticle was with finely striated annulation. C: The tail of the female was conical and slightly attenuated at its distal end. D: The tail of the male was relatively long, and often having a small button-like termination with spicule.
The examined infected donkeys with P. equorum showed significantly high in TAC levels 0.57 ± 0.68 and the serum MDA was 2.45 ± 0.53, p = 0.001 levels than that in non-infected control donkeys. The levels of AST enzymes were 278.54 ± 0.45 while ALT was 14.97 ± 0.87 which was significantly higher than that of control negative donkeys (Table 2).
Table 2.
Mean fecal egg count per gram feces within month observation period.
| *Treated group | *Un-treated group | |
|---|---|---|
| 1st sample (zero time) | 120 | 122 |
| 1st week | 40 | 112 |
| 2nd week | 18 | 120 |
| 3rd week | 11 | 106 |
| 4th week | 9 | 130 |
*Mean fecal egg count per gram feces.
In the infected donkeys with 20 worms of P. equorum significantly showed upregulation of the TNF-α by 18 than that of control non infected donkeys while the gene expression analysis of TNF-α increased by 25 in donkeys with 21–30 collected worms; the TNF-α raised to 28-fold when the donkeys harbored more than 30 worms (Table 3).
Table 3.
Biochemical parameters changes in donkeys infected with P. equorum (the data expressed as Mean ± SE).
| Parameters | Infected donkeys (n = 18) | Control donkeys (n = 5) |
|---|---|---|
| AST (U/l) | 278.54 ± 0.45* | 240.5 ± 0.18 |
| ALT (U/l) | 14.97 ± 0.87* | 8.98 ± 0.96 |
| TAC (mmol/L) | 0.57 ± 0.68* | 0.78 ± 0.57 |
| MDA (mmol/L) | 2.45 ± 0.53* | 0.88 ± 0.67 |
In the infected donkeys with 20 worms of P. equorum showed significantly upregulation of the IL-1β by 10 than that of control non infected donkeys while the gene expression analysis of IL-1β increased by 17 in donkeys with 21–30 collected worms; the IL-1β raised to 26-fold when the donkeys harbored more than 30 worms (Table 3).
Naturally infected donkeys with P. equorum shed at least 100 eggs per gram feces. The results of fecal samples examination are summarized in Table 4. The fecal egg count was marked decreased after treatment with moxidectin as seen in Fig. 4.
Table 4.
Genetic parameters (TNF-α and IL-1β) changes in donkeys infected with P. equorum (the data expressed as Mean ± SE).
| Parameters | Infected donkeys |
Control donkeys | ||
|---|---|---|---|---|
| 20 worms | 21–30 | >30 worms | ||
| TNF-α | 18 | 25 | 28 | 3.00 ± 0.04 |
| IL-1β | 10 | 17 | 26 | 2.5 ± 0.07 |
Fig. 4.
A: Microscopic appearance of P. equorum eggs showing large number of P. equorum eggs during fecal examination using saturated salt solution with floatation technique before treatment. B: Microscopic appearance showing few numbers of P. equorum eggs after treatment.
4. Discussion
P. equorum is a common ascarid nematode which inhabits the small intestine of family Equidae mainly the young animals which is highly susceptible. the female adult Nematoda lays its eggs in small intestine; these eggs develop into eggs with larva (L2) in the environment full mature eggs takes 10 days to become infective in temperature between 25 and 35 °C. Donkeys are prone to endemic infection with gastrointestinal helminths (Jajere et al., 2016). From our results, the prevalence of P. equorum infection was 36 % among examined donkeys. This prevalence was higher than that recorded by Attia et al., 2018, Shrikhande et al., 2009, Fikru et al., 2005 as they found P. equorum with prevalence 25%; 29.26% and 17.3% in donkeys, While Ayele et al., (2006) found that prevalence rate was 43%. This difference is due to different grazing pasture and the climatic condition.
The infected donkeys with P. equorum showed significant increase in TAC levels and the serum MDA levels than that in non-infected control donkeys. In a parallel study conducted by Elmeligy et al., (2021) found that level of MDA was marked increased while, total antioxidant capacity (TAC) level was lowered in donkeys infected with Strongylus species. In another study Mousa and Soliman, (2016) found that the levels of superoxidase dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT) were significantly increased (p ≤ 0.05) while vitamins C and E levels were significant decrease (p ≤ 0.05) in pneumonic goats.
Cellular immune responses against parasitism were studied previously on different parasites as Oestrus ovis (Attia et al., 2020) and in Rhinoestrus usbekistanicus infection (Attia and Mahdy, 2021) as an example of nasal myiasis. The parasites provoke many immunological cells which secreted by the body at site of infection. The immunological cells were (macrophages; eosinophils; different lymphocytes).
This study concentrates in the evaluation of mRNA in the intestine of infected donkeys with P. equorum, through measurements of different produced cytokines during infection with the examined round worms versus control non infected donkeys.
The activation of the macrophage produces two different cytokines (Interleukin-1types). This interleukin is the most important proinflammatory product which used specially in immunity. It had two types of IL-1 α and IL-1 β produced from macrophages and monocytes. IL-1 is associated in different parasitic infections which promotes and regulate the function of several immunological cells such as neutrophils; lymphocytes, macrophages; monocytes and Th2 activation (Gatkowska, 2009).
The two examined genes were expressed regularly against the infection with P. equorum; the infection initiate the production of different immunological cells as macrophages; mast cell; eosinophils; and lymphocytes. These results are due to the first produced cytokines is TNF which secrete from the activated APC (antigen presenting cell) with subsequent release of IL-1β which was important in activatation of the subsequent cascade of the immunological cells. the released cells are (eosinophils; neutrophils; macrophage; NK cells. Mast cell).
From our results, moxidectin is an effective treatment for P. equorum in donkeys. This result agreed with Cobb and Boeckh, (2009) as they found combination between moxidectin and praziquantel is effective broad spectrum, prolonged acting, enabling prolonged treatment interval against intestinal helminths in horses, while Reinemeyer and Marchiondo, (2007) recorded that moxidectin failed to reduce fecal egg count of P. equorum in horses. Handling the parasitic problem in animals should be achieved using the biosecurity measures as well as the improved health status of the animals using environment-friendly products such as probiotics (Abd El-Hack et al., 2021a, Alagawany et al., 2021a, El-Saadony et al., 2021a), prebiotics (Abd El-Hack et al., 2021b, Yaqoob et al., 2021), essential oil (El-Tarabily et al., 2021, Alagawany et al., 2021b), bioactive peptides (El-Saadony et al., 2021b, El-Saadony et al., 2021c), herbal extracts (Abou-Kassem et al., 2021, Saad et al., 2021, Abd El-Hack et al., 2021c), green synthesized nanoparticles (Attia et al., 2017, El-Saadony et al., 2021d, El-Saadony et al., 2021e, El-Saadony et al., 2021f) should be adopted in the animal facility.
5. Conclusions
The development of resistance against anthelmintic against parasites at broad and P. equorum as well means that traditional selection of anthelmintics must be shifted routinely. In the future, each drug class's resistance status should be assessed, and efficacy should be confirmed on a regular basis. Also, it is highly recommended that periodical deworming with different molecules is more economic and lifesaving than a single anthelmintic treatment every 12 months to combat and control parasitic infection in equine in Egypt parallel with parasitic testing to identify the risky animals that considered as source of infection.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Taif University Researchers Supporting Project number (TURSP-2020/57), Taif University. Taif Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, Zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11(7):1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., et al. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021;19:1–10. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.-S., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. 2021;28(5):4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abdelsalam M., Attia M.M., Mahmoud M.A. Comparative morphomolecular identification and pathological changes associated with Anisakis simplex larvae (Nematoda: Anisakidae) infecting native and imported chub mackerel (Scomber japonicus) in Egypt. Reg. Stud. Mar. Sci. 2020;39:101469. doi: 10.1016/j.rsma.2020.101469. [DOI] [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20(1):896–910. doi: 10.1080/1828051X.2021.1926348. [DOI] [Google Scholar]
- Abu-Elala N.M., Attia M.M., Abd-Elsalam R.M. Chitosan-silver nanocomposites in goldfish aquaria: A new perspective in Lernaea cyprinacea control. Int. J. Biol. Macromol. 2018;111:614–622. doi: 10.1016/j.ijbiomac.2017.12.133. [DOI] [PubMed] [Google Scholar]
- Ahmed N.E., El-Akabawy L.M., Ramadan M.Y., Radwan A.M.M. Studies on helminthe parasites in necropsied donkeys in Egypt. BENHA Vet. Med. J. 2011:153–162. [Google Scholar]
- Aktas M.S., Kandemir F.M., Kirbas A., Hanedan B., Aydin M.A. Evaluation of Oxidative Stress in Pigeon Infected with Psoroptes Ovis using Total Antioxidant Capacity, Total Oxidant Status, and Malondialdehyde Level. J. Vet. Res. 2017;61(2):197–201. doi: 10.1515/jvetres-2017-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F.M. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100(6):101172. doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276:114920. doi: 10.1016/j.anifeedsci.2021.114920. [DOI] [Google Scholar]
- Ali B.A., El Sayed M.A., Matoock M.Y., Fouad M.A., Heleski C.R. Comparative efficacy of three anthelmintic programs in working equids in Egypt. J. Vet. Sci. Med. Diagn. 2015;4 [Google Scholar]
- Attia M.M., El-Gameel S.M., Ismael E. Evaluation of tumor necrosis factor-alpha (TNF-α); gamma interferon (IFN-γ) genes and oxidative stress in sheep: immunological responses induced by Oestrus ovis (Diptera: Oestridae) infestation. J. Parasit. Dis. 2020;44(2):332–337. doi: 10.1007/s12639-020-01220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Salem H.M. Morphological and molecular characterization of Pseudolynchia canariensis (Diptera: Hippoboscidae) infesting domestic pigeons. J. Trop. Insect Sci. Int. 2021 [Google Scholar]
- Attia M.M., Khalifa M.M., Atwa M.T. The prevalence and intensity of external and internal parasites in working donkeys (Equus asinus) in Egypt. Vet. World. 2018;11(9):1298–1306. doi: 10.14202/vetworld.2018.1298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Mahdy O.A. Evaluation of the cell mediated immune responses using quantitative real-time polymerase chain reaction during the infestation of Rhinoestrus usbekistanicus (Diptera: Oestridae) in equine. Int. J. Trop. Insect Sci. 2021;41(4):3147–3153. doi: 10.1007/s42690-021-00509-4. [DOI] [Google Scholar]
- Attia M.M., Soliman S.M., Khalf M.A. Hydrophilic nanosilica as a new larvicidal and molluscicidal agent for controlling of major infectious diseases in Egypt. Vet. World. 2017;10(9):1046–1051. doi: 10.14202/vetworld.2017.1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Soliman S.M., Saleh N.M.K. Advanced and rapid serodiagnosis of oestrosis (Oestrus ovis; Diptera: Oestridae) in sheep using indirect and dot-ELISA. Jordan J. Biol. Sci. 2019;12(3):275–281. [Google Scholar]
- Ayele G., Feseha G., Bojia E., Joe A. Prevalence of gastrointestinal parasites of donkeys in Dugda Bora District, Ethiopia. Livest. Res. Rural Dev. 2006;18:2–6. [Google Scholar]
- Aytekin I., Unubol Aypak S. Levels of selected minerals, nitric oxide, and vitamins in aborted Sakis pigeon raised under semitropical conditions. Trop. Anim. Health Prod. 2011;43:511–514. doi: 10.1007/s11250-010-9724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazik E.L., Luquire J.T., Little D. Pyrantel pamoate resistance in horses receiving daily administration of pyrantel tartrate. J. Am. Vet. Med. Assoc. 2006;228(1):101–103. doi: 10.2460/javma.228.1.101. [DOI] [PubMed] [Google Scholar]
- Cobb R., Boeckh A. Moxidectin: a review of chemistry, pharmacokinetics and use in horses. Parasit. Vectors. 2009;2(2):S5. doi: 10.1186/1756-3305-2-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drudge J.H., Lyons E.T., Tolliver S.C., Lowry S.R., Fallon E.H. Piperazine resistance in population-B equine strongyles: a study of selection in Thoroughbreds in Kentucky from 1966 through 1983. Am. J. Vet. Res. 1983;49:986–994. [PubMed] [Google Scholar]
- Elmeligy E., Abdelbaset A., Elsayed H.K., Bayomi S.A., Hafez A., Abu-Seida A.M., El-Khabaz K.H.A., Hassan D., Ghandour R.A., Khalphallah A. Oxidative stress in Strongylus spp. infected donkeys treated with piperazine citrate versus doramectin. Open Vet. J. 2021;11(2):238–250. doi: 10.5455/OVJ.2021.v11.i2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O.S., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20(1):762–776. doi: 10.1080/1828051X.2021.1926346. [DOI] [Google Scholar]
- El-Saadony M.T., Alagawany M., Patra A.K., Kar I., Tiwari R., Dawood M.A.O., Dhama K., Abdel-Latif H.M.R. The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol. 2021;117:36–52. doi: 10.1016/j.fsi.2021.07.007. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., El. Abdel-Hamid S., Taha T.F., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28(8):4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., S. F. Khalil O., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28(8):4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Shafi M.E., Hassan M.A. The use of biological selenium nanoparticles in controlling Triticum aestivum L. crown root and rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021;28:4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., et al. Selenium nanoparticles, from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi, as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28(12):6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A.M., Elnesr S.S., E. Abd El-Hack M. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28(9):5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikru R., Reta D., Teshale S., Bizunesh M. Prevalence of equine gastrointestinal parasites in western highlands of Oromia, Ethiopia. Bull. Anim. Health Prod. Afr. 2005;53(3):161–171. [Google Scholar]
- Gatkowska J. arious aspects of IL-1 biological activity. I. The role of IL-1 in parasitic infections (Review) Wiad. Parazytol. 2009;55(2):109–114. [PubMed] [Google Scholar]
- Getachew A.M., Innocent G.T., Trawford A.F., Feseha G., Reid S.J.W., Love S. Equine parascarosis under the tropical weather conditions of Ethiopia: a coprological and postmortem study. Vet. Rec. 2008;162(6):177–180. doi: 10.1136/vr.162.6.177. [DOI] [PubMed] [Google Scholar]
- Getahun T.K., Kassa T.Z. Prevalence and species of major gastrointestinal parasites of donkeys in Tenta Woreda, Amhara Regional State, Ethiopia. J. Vet. Med. Anim. Health. 2017;9(2):23–31. [Google Scholar]
- Hilali M.A., Mahdy O.A., Attia M.M. Monthly variations of Rhinoestrus spp. (Diptera: Oestridae) larvae infesting donkeys in Egypt Morphological and molecular identification of third stage larvae. J. Adv. Res. 2015;6(6):1015–1021. doi: 10.1016/j.jare.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhao Y., Bai D., Shiraigol W., Li B., Yang L., Dugarjaviin M. Donkey genome and insight into the imprinting of fast karyotype evolution. Sci. Rep. 2015;5(1):1–10. doi: 10.1038/srep14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jajere S.M., Lawal J.R., Bello A.M., Wakil Y., Turaki U.A., Waziri I. Risk Factors Associated with the Occurrence of Gastrointestinal Helminths among Indigenous Donkeys (Equus asinus) in Northeastern Nigeria, Hindawi Publishing Corporation. Scientifica. 2016;2016 doi: 10.1155/2016/3735210. Article ID 3735210, 7 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R.M., Klei T.R., Lyons E.T., Lester G., Courtney C.H., French D.D., Tolliver S.C., Vidyashankar A.N., Zhao Y. Prevalence of anthelmintic resistant cyathostomes on horse farms. J. Am. Vet. Med. Assoc. 2004;225(6):903–910. doi: 10.2460/javma.2004.225.903. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20(10):477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Mahdy O.A., Attia M.M. Comparative micro-morphological and phylogenetic analysis between Rhinoestrus purpureus and Rhinoestrus usbekistanicus (Diptera: Oestridae) larvae and its adults“. Int. J. Trop. Insect Sci. 2021;41(1):241–250. [Google Scholar]
- Mousa S., Soliman M.S. Oxidant and Antioxidant Status in Pneumonic Goats with Special Reference to Bacterial Etiology. Int. J. Livest. Res. 2016;6(5):15–23. doi: 10.5455/ijlr.20160417045508. [DOI] [Google Scholar]
- Nielsen M.K., Pfister K., von Samson-Himmelstjerna G. Selective therapy in equine parasite control–application and limitations. Vet. Parasitol. 2014;202(3-4):95–103. doi: 10.1016/j.vetpar.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Parsani H., Momin R., Lateef A., Das H. Studies on gastrointestinal helminths of Equus acinus in North Gujarat, India. Egypt. J. Biol. 2013;15(1) [Google Scholar]
- Peregrine A.S., Molento M.B., Kaplan R.M., Nielsen M.K. Anthelmintic resistance in important parasites of horses: does it really matter? Vet. Parasitol. 2014;201(1-2):1–8. doi: 10.1016/j.vetpar.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Pereira J.R., Vianna S.S. Gastrointestinal parasitic worms in equines in the Paraiba Valley, State of Sao Paulo, Brazil. J. Vet. Parasitol. 2006;140:289–295. doi: 10.1016/j.vetpar.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Raynaud J.P. Etude de l’efficacité d’une technique de coproscopie quantitative pour le diagnostic et le contrôle des infestations parasitaires des bovins, ovins, équins et porcins. Ann. Parasitol. Hum. Comp. 1970;85:321–342. [PubMed] [Google Scholar]
- Reinemeyer C.P., Marchiondo A.A. Proceedings of American Association of Veterinary Parasitology, 52nd Annual Meeting. 2007. Efficacy of pyrantel pamoate in horses against a macrocyclic lactone-resistant isolate of Parascaris equorum; p. 78. [DOI] [PubMed] [Google Scholar]
- Reinemeyer C.R. Diagnosis and control of anthelmintic-resistant Parascaris equorum. Parasit. Vectors. 2009;2(2):S8. doi: 10.1186/1756-3305-2-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, R.J., Hodgson, D.R., 1993. Manual of Equine Practice. Saunders. ISBN 0 7216 3739 6.
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT - Food Sci. Technol. 2021;148:111668. doi: 10.1016/j.lwt.2021.111668. [DOI] [Google Scholar]
- Salem H.M., Attia M.M. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: Muscidae) larvae in broiler chickens: a field study. Int. J. Trop. Insect Sci. 2021 doi: 10.1007/s42690-021-00492-w. [DOI] [Google Scholar]
- Salem N.Y., Yehia S.Y., Farag H.S., Soliman M.S. Evaluation of Hepcidin Level and Clinico-Pathological Modifications in Canine Parvovirus Enteritis. Inter. J. Vet. Sci. 2018;7(2):93–96. [Google Scholar]
- Shalaby H.A., Abdel-Aziz M.M., Abdel-Shafy S. Diagnostic value of some Parascaris equorum antigens in foals. Assiut Vet. Med. J. 2008;54(118):1–16. [Google Scholar]
- Shrikhande G., Rewatkar S., Deshmukh S., Maske D., Raghorte Y. The incidence of helminth parasites in donkeys. Vet. World. 2009;2(6):224. [Google Scholar]
- Soulsby E.J.L. seventh ed. Bailliere Tindall; London: 1986. Helminths, Arthropods & Protozoa of Domesticated Animals. [Google Scholar]
- Swiderski C.E., Klei T.R., Horohov D.W. Quantitative measurement of equine cytokine mRNA expression by polymerase chain reaction using target-specific standard curves. J. Immunol. Methods. 1999;222(1-2):155–169. doi: 10.1016/s0022-1759(98)00193-8. [DOI] [PubMed] [Google Scholar]
- Urqhart, G. M., Armour, J., et al., 1988. Veterinary Parasitology. Longmann Scientific and Technical. ISBN 0 5824 0906 3.
- Yaqoob M.U., El-Hack M.E.A., Hassan F., El-Saadony M.T., Khafaga A.F., Batiha G.E., Yehia N., Elnesr S.S., Alagawany M., El-Tarabily K.A., Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;100(7):101143. doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis N.A., Laban S.E., Al-Mokaddem A.K., Attia M.M. Immunological status and histopathological appraisal of farmed Oreochromis niloticus exposed to parasitic infections and heavy metal toxicity. Aquaculture Int. 2020;28(6):2247–2262. doi: 10.1007/s10499-020-00589-y. [DOI] [Google Scholar]
- Zaki A.A., Attia M.M., Ismael E., Mahdy O.A. Prevalence, genetic, and biochemical evaluation of immune response of police dogs infected with Babesia vogeli. Vet. World. 2021;14(4):903–912. doi: 10.14202/vetworld.10.14202/vetworld.2021.410.14202/vetworld.2021.903-912. [DOI] [PMC free article] [PubMed] [Google Scholar]