Abstract
Background
Autism spectrum disorder (ASD) is a neurodevelopmental condition that causes disability in social interaction, communication, and restrictive and repetitive behaviors. Common environmental factors like prenatal, perinatal, and/or postnatal factors play a key role in ASD etiologies. Moreover, specific metabolic disorders can be associated with ASD.
Subjects and methods
We performed a retrospective case-control study in child psychiatry clinics, involving 51 children with ASD and 40 typical development controls (TDC).
Results
We found a correlation between children being breastfed for less than 6 months, having fathers more than 40 years old at childbirth in ASD compared to TDC group. Our study also associated low blood cholesterol and low erythrocyte magnesium levels with increased risk for ASD.
Conclusion
Findings support the implication of total cholesterol (TC) and erythrocyte magnesium level in defining autism outcome.
Keywords: ASD, Breastfeeding duration, Advanced paternal age, Lipid profile, Erythrocyte magnesium
1. Introduction
Autism spectrum disorders (ASD) are a group of neurodevelopmental disabilities. The main symptoms of autism include impairments in social interaction and communication, as well as the presence of restricted and repetitive behaviors (Kocsis, 2013).
Approximately 52 million people worldwide suffer from ASD (Baxter et al., 2015). Based on the latest Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition DSM-IV, the prevalence of ASD has risen dramatically in the recent years as 1 in 68 children have been diagnosed (Baio et al., 2018).
In Middle East and North Africa region, there are limited data about ASD prevalence. However recent retrospective descriptive study conducted between 2011 and 2018 estimated the prevalence of ASD at 20.35 per 10.000 in Oman and that this prevalence increased 15 times since 2011 (Al-Mamari et al., 2019). More recently, in Saudi Arabia, a cross-sectional work that included 37 ASD centers and schools in Makkah and Jeddah between January and March 2020 showed that prevalence of ASD was 2.81 per 1,000 children for both cities (Sabbagh et al., 2021).
This expansion necessitates exploring the role of genetic and environmental factors associated with ASD. Despite the highly reported heritability of ASD, some environmental factors can influence the risk of ASD, including advanced paternal age at childbirth or breastfeeding period, for instance (Hultman et al., 2011, Tseng et al., 2019).
One of the major perinatal risks for ASD is parental age. Having children at later paternal ages increases the risk and is commonly called “paternal age effect disorders” (PAE) (Sharma et al., 2015). PAE was confirmed in Omani study conducted between January 2015 and June 2016 showing that both maternal and paternal increased age are correlated with higher risk of ASD (Al-Mamari et al., 2021).
Current evidences indicate that breastfeeding (BF) is a protective postnatal factor for childhood cognitive development. BF could impact cognitive development via the nutritional or hormonal content of breastmilk, or via the social contact between mother and child during the act of nursing (Belfort, 2017). Moreover, high intelligence quotient (IQ) in childhood is highly correlated with BF (Horta et al., 2018). According to recent data from 2018, Tunisia has a breastfeeding rate of 13.5%, among the lowest in the world, with less than a third of newborns put to the breast during the first hour of life. ASD prevalence in Tunisia is ~ 35/10000 (Gaddour et al., 2012).
Recently, lipid metabolism has been suggested to have important roles in ASD etiology (Luo et al., 2020). Abnormal lipid profiles inducing autism pathogenesis have been proposed, however mechanisms remain unclear. Recent studies demonstrated decreases in total cholesterol (TC) (Tierney et al., 2006), high-density lipoprotein cholesterol (HDL-C) and an increase in total triglyceride (TG) (Luçardo et al., 2021) in the blood plasma of children with ASD. Recent Egyptian hospital-based study confirmed that children with autism syndrome have lowest level of cholesterol compared to control group (Hassan et al., 2019). Other studies also demonstrated that the Smith–Lemli–Opitz syndrome (SLOS), which is a malformation syndrome resulting from an inborn error of cholesterol biosynthesis, is associated with ASD (Bukelis et al., 2007).
In order to investigate certain ASD risk factors, in a cohort of Tunisians patients, this case study focused on two key factors.
The first part of the study aimed to explore prenatal, perinatal, and postnatal factors potentially associated with ASD. The second part explored biochemistry parameters in plasma and erythrocyte of children with ASD.
2. Subjects and methods
2.1. Description of the population
Fifty-one children with ASD age range between 3 and 16 years old (40 boys. and 11 girls; with a mean age of 7.30 ± 3.2 years) were recruited from August 2018 to April 2019 at the Department of Child and Adolescent Psychiatry in Fattouma Bourguiba University Hospital, Monastir, Tunisia and at the Department of Child and Adolescent Psychiatry in Farhat Hached University Hospital, Sousse, Tunisia.
Forty typical development controls age range between 3 and 14 years old (25 boys. and 15 girls; with a mean age of 5.98 ± 3.12 years) were recruited in the same period. Control participants were randomly selected. They were not known to have any neurodevelopmental or behavioral disruptions that might be related to ASD. The control group consisted exclusively of patients diagnosed with simple conditions like flu or a simple routine physical examination.
Intervention and control groups were heterogenic in terms of etiologic characteristics. The initial diagnoses of ASD were established by an experienced psychiatrist based on the classification system stated in the Diagnostic and Statistical Manual of Mental Disorders fifth edition DSM-V criteria (American Psychiatric Association and Association, 2013).
The following references were applied to confirm the diagnosis of ASD: The Childhood Autism Rating Scale CARS (Schopler et al., 1980), Autism Diagnostic Observation Schedule, Second Edition ADOS-2 (Lord et al., 2012), and Autism Diagnostic Interview-Revised ADI-R (Lord et al., 1994).
Information regarding use of psychotropic medication (antiepileptic. antipsychotics and stimulants) was obtained from participant medical charts.
2.2. Sampling protocol
All the families of participants received information about the protocol from child psychiatrist and the first author. They also signed a consent form. The study was approved by the University of Monastir Ethical Committee.
Patients’ parents who agreed to participate had appointments for their children for a fasting blood test. On the day of the blood test, the parents fill out an information sheet that contains information about them and their children. Data were kept private and confidential and parents were informed of test results by the author and the child psychiatrist.
Blood samples (4 ml) were obtained from each child after a 12-h overnight fast. We measured plasma lipids and lipoprotein concentrations including Total-Cholesterol (T-Chol), High-density lipoprotein (HDL), and Triglycerides (TG). Low-density lipoprotein (LDL) concentration was calculated as [LDL-C = T-Chol − (TG/5 + HDL-C)]. We also measured fasting blood glucose (FBG), renal profile (urea, uric acid, creatinine), hepatic profile (ASAT, ALAT), sodium, potassium, chloride, calcium, iron, as well as magnesium. To determine erythrocyte magnesium, we centrifuged the samples at 2000 rpm for 5 min, thrawed the supernatant, and kept the red blood cell pellet for hemolysis. Deproteinization was achieved with sodium tungstate. All metabolites were quantified using a routine clinical biochemistry automatic analyzer Unicel DxC600 synchron Clinical Systems (Beckman Coulter).
2.3. Statistical analysis
Data distributions are reported as mean ± standard deviation for continuous variables and as number (percentage) for categorical variables. The chi-square test was used to measure the statistical differences between classes of categorical data. In the case of a small sample, the non-parametric Fisher exact test replaced the chi-square test. Logistic regression analyses were used to evaluate the impact of environmental factors on ASD risk and the adjusted odds ratios (ORs) assessed with 95% confidence intervals (CIs). Metabolic parameters for both groups were compared to age and gender-based normative data from the Tunisian population study. All statistical analyses were performed using SPSS 25.0 with a cutoff p-value < 0.05 was used for all tests of statistical significance.
3. Results
3.1. Population characteristics
The anthropologic characteristics of cases and controls were compared in Table 1.
Table 1.
ASD and TDC group clinical Data.
| Clinical Profile | ASD N(%) | TDC N(%) | P value |
|---|---|---|---|
| Gender | 0.095a | ||
| Male | 40 (78.4) | 25 (62.5) | |
| Female | 11 (21.6) | 15 (37.5) | |
| Class of age (years) | 0.141a | ||
| ≤ 4 | 14 (27.5) | 19 (47.5) | |
| 5 to 9 | 27 (52.9) | 15 (37.5) | |
| ≥ 10 | 10 (19.6) | 6 (15) | |
| Birthweight (kg) | 0.399b | ||
| < 2.5 | 2 (3.9) | 4 (10) | |
| > = 2.5 | 49 (96.1) | 36 (90) | |
| PregnancyNatural | 3 (5.9) | 40 (1 0 0) | 0.253b |
| In vitro fertilization (IVF) | 48 (94.1) | ||
| Prematurity | 0.460b | ||
| Yes | 5 (9.8) | 2 (5) | |
| No | 46 (90.2) | 38 (95) | |
| Delivery | 0.305a | ||
| Vaginal delivery | 36 (70.6) | 32 (80) | |
| Cesarean section | 15 (29.4) | 8 (20) | |
| Breastfeeding (months) | 0.004a* | ||
| < 6 | 18 (35.3) | 3 (7.5) | |
| 6 to 12 | 14 (27.5) | 14 (35) | |
| >12 | 19 (37.3) | 23 (57.5) | |
| Mother age at child’s birth (years) | 0.341a | ||
| < 35 | 38 (76.5) | 27 (67.5) | |
| ≥ 35 | 12 (23.5) | 13 (32.5) | |
| Father age at child’s birth (years) | 0.001a* | ||
| < 40 | 31 (60.8) | 37 (92.5) | |
| ≥ 40 | 20 (39.2) | 3 (7.5) | |
| Difference in age between parents (years) | 0.364a | ||
| < 10 | 41 (80.4) | 35 (87.5) | |
| ≥ 10 | 10 (19.6) | 5 (12.5) | |
| Consanguinity | 0.542b | ||
| Not related | 42 (82.4) | 37 (92.5) | |
| First degree | – | 2 (5) | |
| Second degree | 3 (5.9) | 1 (2.5) | |
| Third degree | 6 (11.8) | – | |
| Risperidone treatment | – | ||
| Yes | 8 (15.7) | – | |
| No | 43 (84.3) | ||
| Family risk factorsNo Family history of ASD | 22 (43.1) | – | – |
| Sibling history of ASD | 9 (17.6) | ||
| Family history of ASD | 5 (9.8) | ||
| Other psychiatric family history | 15 (29.4) |
aChi-square test, bFisher’s exact test, * p < 0.05
As expected, the majority of ASD participants were males (78.4%). Indeed, is has been reported that males are more frequently diagnosed with ASD than females with a ratio of 4:1 (Fombonne, 2005). The descriptive statistics of the ASD group showed that 15.7% of children with ASD were treated with Risperidone, a serotonin antagonist neuroleptic that effectively reduces aggressive behavior and irritability. The mean age of fathers at childbirth and breastfeeding duration were significantly different between cases and controls (P = 0.001; P = 0.004 respectively).
In the logistic regression analysis in which no covariates were included, a positive and significant correlation was found between children who were breastfed for less than 6 months and risk for ASD. A similar observation was found for children whose fathers were older than 40 years. The adjustment with mother’s age at childbirth increased the risk with RR = 3.16 (p = 0.001) compared to the TDC group (Table 2).
Table 2.
Logistic regression analysis of breastfeeding and parental age in children with ASD.
| RR | B | Sig | Exp(B) | 95% CI | |
|---|---|---|---|---|---|
| ASD*FA ≥ 40 years | 1.91 | 2.074 | 0.002* | 7.957 | (2.160–29.312) |
| ASD*FA ≥ 40 years and MA ≥ 35 years | 3.16 | 2.420 | 0.001* | 11.251 | (2.723–46.494) |
| ASD *BF < 6 months vs 6 ≤ BF < 12 months | 1.73 | 1.846 | 0.011* | 6.333 | (1.523–26.341) |
| ASD*BF < 6 months vs BF ≥ 12 months | 1.97 | 2.091 | 0.003* | 8.093 | (2.067–31.687) |
Sig: significance following logistic regression. TDC as reference group *: significance < 0.05.
FA: father’s age, MA: mothers age, BF: breastfeeding, RR: relative risk.
3.2. Plasma metabolic profile
The metabolic profile of patients receiving Risperidone was assessed and compared to untreated cases within the ASD group. No association was found between lipid profile, FBG, and erythrocyte magnesium levels in those receiving Risperidone treatment (result not shown). Also, both conditions (with and without treatment) were compared as one ASD group with the control group TDC.
Plasma biochemical profiles of the two study groups are shown in Table 3. We compared the levels of biochemical parameters of each group with the reference range of the Tunisian population after adjustment for age. Only total cholesterol (TC), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C) levels were adjusted for age and gender. Each continuous variable was transformed in to a categorical variable of 3 classes: Normal, Low, and High. There was a significant difference in total cholesterol (TC) (P = 0.032) and erythrocyte magnesium levels (p = 0.020) between the study groups.
Table 3.
levels of biochemical parameters in the blood plasma and erythrocyte of children with ASD (n = 51) and TDC (n = 40) expressed as mean ± SD.
| TDC group | ASD group | Sig. | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Total cholesterol TC (mmol/l) | 3.54 ± 0.561 | 3.80 ± 0.927 | 0.032b* |
| Triglycerides (mmol/l) | 0.62 ± 0.216 | 0.81 ± 0.432 | 0.199b* |
| HDL-C (mmol/l) | 1.14 ± 0.313 | 1.17 ± 0.344 | 0.872b |
| LDL-C (mmol/l) | 2.12 ± 0.439 | 2.28 ± 0.710 | 0.256b |
| Urea (mmol/l) | 3.98 ± 1.084 | 3.92 ± 0.965 | 0.502b |
| Uric acid (mmol/l) | 211.30 ± 55.419 | 226.37 ± 55.859 | 0.593b |
| Creatinine (µmol/l) | 27.83 ± 4.031 | 30.71 ± 6.685 | 0.143a |
| ALT (U/l) | 13.65 ± 3.424 | 14.61 ± 5.020 | – |
| AST (U/l) | 29.70 ± 5.954 | 33.57 ± 9.281 | 0.502b |
| FBG (mmol/l) | 4.69 ± 0.466 | 4.95 ± 0.494 | 0.077b |
| Sodium Na+ (mmol/l) | 137.85 ± 2.155 | 137.59 ± 2.816 | 0.477b |
| Potassium K+ (mmol/l) | 4.39 ± 0.344 | 4.55 ± 0.457 | 0.140a |
| Chloride Cl- (mmol/l) | 103.63 ± 1.970 | 103.33 ± 2.479 | 0.502b |
| Calcium Ca (mmol/l) | 2.38 ± 0.103 | 2.34 ± 0.142 | 0.502b |
| Iron Fe (µmol/l) | 10.55 ± 5.804 | 11.18 ± 5.881 | 0.672a |
| Magnesium Mg (mmol/l) | 0.823 ± 0.066 | 0.802 ± 0.096 | 0.176bb |
| Erythrocyte Mg (mmol/l) | 1.78 ± 0.447 | 1.48 ± 0.417 | 0.020b* |
| Total Protein Pr (mmol/l) | 71.60 ± 3.045 | 73.04 ± 3.709 | – |
a: Chi-square test b: Fisher’s exact test, SD: standard deviation, * Sig < 0.05
HDL-C: High density lipoprotein-cholesterol, LDL-C: Low density lipoprotein-cholesterol.
ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, FBG: fast blood glucose.
-: no statistics were computed because all the categories were in the normal range.
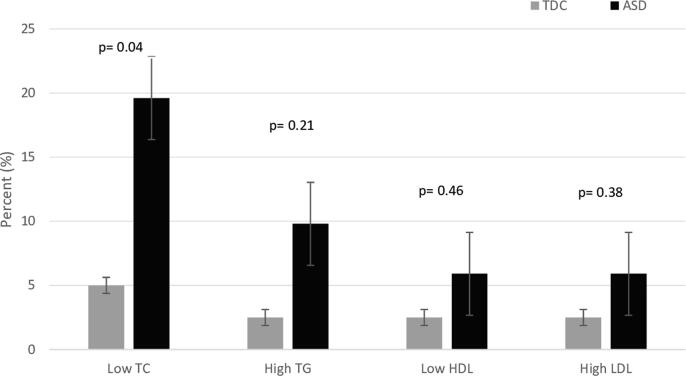
As showed in Fig. 1, the logistic regression analysis showed that the ASD group had lower levels of total cholesterol (TC) (p = 0.04) compared to the TDC group.
Fig. 1.
Lipid profile level in blood plasma. The distribution of lipid profiles is expressed as percentage of number of individuals. The distributions are illustrated with box and whisker plots for the TDC (light gray) and ASD (dark gray) group. P-value is significantly different at p < 0.05, according to logistic regression test.
There was no significant difference in the mean plasma TG, HDL-cholesterol and LDL-cholesterol levels between the two groups (P = 0.199, P = 0.872, P = 0.256, respectively) as reported in Table 3.
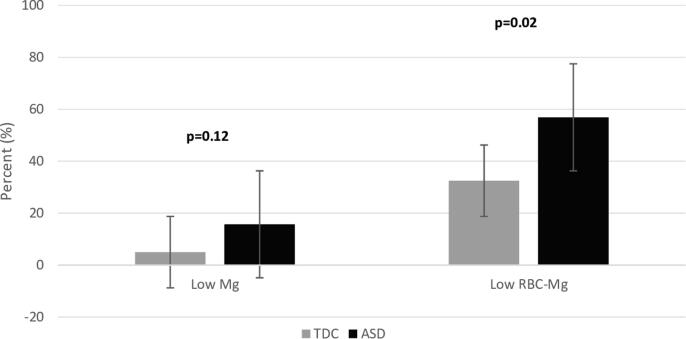
The adjustment for age showed a lower erythrocyte magnesium concentration (P = 0.02) in ASD children compared to the control group (Fig. 2).
Fig. 2.
Magnesium level in blood plasma and in erythrocytes. Distribution of magnesium profile is expressed as percentage of number of individuals. The distributions are illustrated with box and whisker plots for the TDC (light gray) and ASD (dark gray) group. P-value is significantly different at p < 0.05, according to logistic regression test.
Other measures like urea, uric acid, creatinine, Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), fast blood glucose (FBG), sodium (Na + ), potassium (K + ), chloride (Cl-), iron (Fe), and proteins (Pr) concentrations did not differ among the study groups (Table 2)
4. Discussion
4.1. Association between breastfeeding and ASD
World Health Organization (WHO) recommends to mothers to breastfeed their children exclusively for the first 6 months. After, mothers can complement with safe and adequate food with continued breastfeeding up to at least 2 years old. Before 6 months, breast milk is sufficient for the majority of children’s needs. It contains more than 200 components that provide the energy and nutrients required. After age 6 months, complementary feeding becomes necessary to fill energy and nutrient gaps (Organization, 2010).
Regarding its potential etiologic role in ASD, breastfeeding has been the subject of multiple studies that showed that it is not only beneficial for protection against many diseases and also plays a protective role for risk of autism (Mortensen et al., 2002, Sacker et al., 2006, Schultz et al., 2006, Gallup and Hobbs, 2011).
This case-control study shows that BF for less than 6 months was significantly higher in children with ASD than the control group. Correlation is even higher when the breastfeeding period exceeded 12 months compared to children breastfed for less than 6 months with 1.97 more likely to develop ASD compared to the control group (TDC). Similar data were found in a case control study with more than 860 children with ASD and it confirmed a strong correlation between BF less than 6 month and developing autistic spectrum disorder (Schultz et al., 2006).
It has been reported also that prolonging breastfeeding is associated with better cognitive development and reduced autistic features in a multicenter study from Spain (Boucher et al., 2017).
Another study investigated optimal breastfeeding practices, and found that immediate breastfeeding after birth, increasing the exclusive breastfeeding period, and continued breastfeeding decrease the risk of ASD (Al-Farsi et al., 2012).
Several hypotheses were proposed to explain the protective role of breastfeeding. Some researchers investigated breast milk composition and claimed that compared to infant formula, breast milk is richer in nutrients which can support emotional and cognitive growth of infants and protects them from many diseases (Belfort, 2017). Insulin-like growth factor IGF-1 stimulates neuron cell proliferation, promotes tissue growth and development and may improve cognitive development of babies and protect them against autistic traits (Steinman and Mankuta, 2013). Other studies suggested that breastfeeding promotes mother-infant connection through the transmission of oxytocin in breast milk (Lee et al., 2015). It contributes to social recognition, social bonding, and neurodevelopment in the infant, reduces the mother’s stress, and may reduce autism risks.
4.2. Association between advanced paternal age and ASD
Parent age at birth remains an important issue to take into consideration when studying the risk for ASD. In this study, children with fathers age older than 40 years old at childbirth were 1.91 more likely to develop ASD compared to the TDC group. Children whose father’s age exceeded 40 years and mother’s age higher than 35 years at childbirth were 3.16 more likely to develop ASD. Similarly, a large study in Swedish population showed an increased risk for ASD in children whose fathers were older than 45 years (hazard ratio [HR] = 3.45; 95% CI, 1.62–7.33) compared to children born to fathers 20–24 years old (D’Onofrio et al., 2014).
To explain the association between parental age and psychiatric disorders, two hypotheses are postulated.
The first hypothesis suggests that older men pass more genetic disease-causing mutations to their offspring, compared to younger fathers, because occunence of the “de novo mutations”. It’s has been reported that the older men are at the time of childbearing, the more spontaneous mutations they pass on to their children. Some of these mutations are believed to be involved in autistic disorders and schizophrenia (Kong et al., 2012). Of note, this hypothesis does not consider the mother age’s, because, unlike spermatozoa which are produced throughout adulthood, women ovarian is set from birth.
The second hypothesis concerns impairments in epigenetic modifications causing damage to chromatin structure and DNA-methylation patterns, which lead to altered brain gene expressions and subsequent behavioral problems (Milekic et al., 2015).
4.3. Lipid profile and magnesium profile
In this study, total cholesterol was determined as lower in children with ASD compared to the normal range in the Tunisian population adjusted for age and gender. It has been reported that children with ASD have a significantly lower blood cholesterol level suggesting that sterol metabolism or homeostasis disorders may be associated with ASD (Tierney et al., 2006). In a cross-sectional study, the blood serum of 60 children with ASD was assayed for triglycerides by a colorimetric enzymatic reaction and 50% of the sample was found to have elevated triglycerides (Luçardo et al., 2021). Other researchers have reported associations between hypercholesterolemia and Asperger syndrome (Monteleone et al., 2005, Dziobek et al., 2007).
According to a genome study (Luo et al., 2020), there are different subtypes of ASD with each subtype affected by mutations in specific sets of genes. Interestingly, some of these genes are involved in lipid metabolism. Further studies are needed to identify which ASD subtypes are associated with abnormal blood lipid profiles.
Previous studies associated dyslipidemia and autism in patients with Smith-Lemli-Opitz syndrome (SLOS) (Sikora et al., 2006), a rare congenital disorder caused by mutations in 7-dehydrocholesterol reductase (DHCR7) genes, and consequent perturbation in the encoded reductase enzyme, a precursor of cholesterol biosynthesis. Blood analysis of children with SLOS reveal high concentrations of 7-dehydrocholesterol and low cholesterol levels. Furthermore, 75% of children with SLOS have common symptoms with ASD (Sikora et al., 2006, Bukelis et al., 2007). Dietary cholesterol supplementation has shown to improve autistic behavior in children with SLOS (Sikora et al., 2006, Luo et al., 2020).
Some of the patients was taken the Risperidone as medication, and a contrario to a study suggesting that psychoactive medications including antipsychotics can increase triglycerides, LDL-C, and decrease HDL-C (Jacobson et al., 2014), no correlation between Risperidone intake and lipid profile levels was detected in the current study.
Magnesium (Mg) is the second most abundant intracellular mineral in the body and an essential factor for cellular activity (Volpe, 2013). In this study, the concentration of Mg in the plasma of children with ASD was lower in comparison to TD children. In line with this data, published studies showed a correlation between low Mg concentrations and ASD (Tschinkel et al., 2018, Wu et al., 2019, Guo et al., 2020). Magnesium is involved in several metabolic functions, hence, a diminution in magnesium may be associated with many clinical diseases including neuropsychiatric problems. Some evidence suggest that chronic low blood levels of Mg might also lead to growth retardation and behavioral changes (Johnson, 2001). It may also be implicated in ASD, attention-deficit/hyperactivity disorder and developmental delay (Adams et al., 2004). An interesting study investigated the implication of magnesium in learning and memory in the rat hippocampus. The results suggests that increasing magnesium improves learning capabilities, short-term synaptic facilitation and long-term memory through induction of synaptic plasticity and potentiation of synaptic transmission (Slutsky et al., 2010).
Additionally, we determinate that erythrocyte magnesium was notably lower in children with ASD compared to the control group, similarly to previously reported data, were low erythrocyte magnesium levels were found in 33 children with clinical symptoms of ASD (Mousain-Bosc et al., 2004). The same group reported that treatment with a combination of magnesium and vitamin B6 for 6 months significantly reduced autism symptoms in 23 children with correction of erythrocyte magnesium concentrations (Mousain-Bosc et al., 2006). Also, lower erythrocyte magnesium levels were found in patients with schizophrenia (Nechifor, 2011). Two hypotheses could explain the erythrocyte Mg reduction in children with ASD:
The first hypothesis suggests that metabolic inhibition of membrane Na+/K+ ATPase is associated with an increase in intracellular calcium and a reduction in intracellular Mg2+ (Kurup and Kurup. 2003). The second hypothesis suggests a genetic defect in magnesium transport that may occur through the plasma membrane Na+-Mg2+ exchanger (Ebel and Günther, 2005).
5. Conclusion
This case-control study provides support a protective role of breastfeeding against risk for ASD, and that advanced paternal age is a risk factor for developing ASD. In parallel, some metabolic profiles of children with ASD, especially blood cholesterol and erythrocyte magnesium levels differ from controls. Small limitations of this study include the sample size and the lack of age and sex normalization between the two groups.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
SS: Statistical analysis, design and drafting of manuscript; WB: Drafting of manuscript, AD: Controls screening and referral, IS: statistical analysis AC & NG: Patients screening and referral; SF: Project leader.
Ethical approval
Approval for use of human subjects was obtained from the research and ethics committees for the University of Monastir and Fattouma Bourguiba University Hospital.
Informed consent
All volunteers provided informed written consent before blood sampling.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors want to thank each person having participated in this work. Special thanks to pedopsychiatry service in Fattouma Bourguiba university hospital center in Monastir. The blood analyses were supported by the laboratory of biochemistry in Farhat Hached university hospital center in Sousse.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adams J.B., Holloway C.J.J.o.A., Medicine C. Pilot study of a moderate dose multivitamin/mineral supplement for children with autistic spectrum disorder. J. Alternat. Complement. Med. 2004;10(6):1033–1039. doi: 10.1089/acm.2004.10.1033. [DOI] [PubMed] [Google Scholar]
- Al-Farsi, Y.M., Al-Sharbati, M.M., Waly, M.I., Al-Farsi, O.A., Al-Shafaee, M.A., Al-Khaduri, M.M., Trivedi, M.S., Deth, R.C.J.N., 2012. Effect of suboptimal breast-feeding on occurrence of autism: A case–control study. [DOI] [PubMed]
- Al-Mamari, W., Idris, A.B., Aala’A, A.-Z., Jalees, S., Murthi, S., Al-Jabri, M., Gabr, A., Fombonne, E.J.S.Q.U.M.J., 2021. Parental age and the risk of autism spectrum disorder in Oman: A case-control study. [DOI] [PMC free article] [PubMed]
- Al-Mamri, W., Idris, A.B., Dakak, S., Al-Shekaili, M., Al-Harthi, Z., Alnaamani, A.M., Alhinai, F.I., Jalees, S., Al Hatmi, M., El-Naggari, M.A.J.S.Q.U.M.J., 2019. Revisiting the prevalence of autism spectrum disorder among Omani children: A multicentre study. [DOI] [PMC free article] [PubMed]
- American Psychiatric Association, D., Association, A.P., 2013. Diagnostic and statistical manual of mental disorders: Dsm-5, Washington, DC: American psychiatric association.
- Baio J., Wiggins L., Christensen D.L., Maenner M.J., Daniels J., Warren Z., Kurzius-Spencer M., Zahorodny W., Robinson C., Rosenberg, White T., Durkin M.S., Imm P., Nikolaou L., Yeargin-Allsopp M., Lee L.-C., Harrington R., Lopez M., Fitzgerald R.T., Hewitt A., Pettygrove S., Constantino J.N., Vehorn A., Shenouda J., Hall-Lande J., Van K., Naarden, Braun, Dowling N.F. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018;67(6):1–23. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A.J., Brugha T.S., Erskine H.E., Scheurer R.W., Vos T., Scott J.G. The epidemiology and global burden of autism spectrum disorders. Psychol. Med. 2015;45(3):601–613. doi: 10.1017/S003329171400172X. [DOI] [PubMed] [Google Scholar]
- Belfort M.B. The science of breastfeeding and brain development. Breastfeed. Med. 2017;12(8):459–461. doi: 10.1089/bfm.2017.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O., Julvez J., Guxens M., Arranz E., Ibarluzea J., Sánchez de Miguel M., Fernández-Somoano A., Tardon A., Rebagliato M., Garcia-Esteban R., O’Connor G., Ballester F., Sunyer J. Association between breastfeeding duration and cognitive development, autistic traits and ADHD symptoms: a multicenter study in Spain. Pediatr. Res. 2017;81(3):434–442. doi: 10.1038/pr.2016.238. [DOI] [PubMed] [Google Scholar]
- Bukelis I., Porter F.D., Zimmerman A.W., Tierney E. Smith-lemli-opitz syndrome and autism spectrum disorder. AJP. 2007;164(11):1655–1661. doi: 10.1176/appi.ajp.2007.07020315. [DOI] [PubMed] [Google Scholar]
- D’Onofrio B.M., Rickert M.E., Frans E., Kuja-Halkola R., Almqvist C., Sjölander A., Larsson H., Lichtenstein P. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiat. 2014;71(4):432. doi: 10.1001/jamapsychiatry.2013.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziobek I., Gold S.M., Wolf O.T., Convit A. Hypercholesterolemia in Asperger syndrome: Independence from lifestyle, obsessive–compulsive behavior, and social anxiety. Psychiat. Res. 2007;149(1-3):321–324. doi: 10.1016/j.psychres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Ebel, H., Günther, T.J.M.r., 2005. Na+/mg 2+ antiport in erythrocytes of spontaneously hypertensive rats: Role of mg 2+ in the pathogenesis of hypertension. [PubMed]
- Fombonne, E., 2005. Epidemiological studies of pervasive developmental disorders.
- Gaddour N., Bedoui A., Soltani M.S., Gaha L. Autism screening during the second year of life in Tunisia. Neuropsychiatrie de l'Enfance et de l'Adolescence. 2012;60(5):S204. doi: 10.1016/j.neurenf.2012.04.415. [DOI] [Google Scholar]
- Gallup G.G., Hobbs D.R. Evolutionary medicine: bottle feeding, birth spacing, and autism. Med. Hypothes. 2011;77(3):345–346. doi: 10.1016/j.mehy.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Guo, M., Li, L., Zhang, Q., Chen, L., Dai, Y., Liu, L., Feng, J., Cai, X., Cheng, Q., Chen, J.J.N.n., 2020. Vitamin and mineral status of children with autism spectrum disorder in Hainan province of china: Associations with symptoms. [DOI] [PubMed]
- Hassan M.H., Desoky T., Sakhr H.M., Gabra R.H., Bakri A.H. Possible metabolic alterations among autistic male children: clinical and biochemical approaches. J. Mol. Neurosci. 2019;67(2):204–216. doi: 10.1007/s12031-018-1225-9. [DOI] [PubMed] [Google Scholar]
- Horta, B.L., de Sousa, B.A., de Mola, C.L.J.C.o.i.c.n., care, m., 2018. Breastfeeding and neurodevelopmental outcomes. [DOI] [PubMed]
- Hultman C.M., Sandin S., Levine S.Z., Lichtenstein P., Reichenberg A. Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Mol. Psychiat. 2011;16(12):1203–1212. doi: 10.1038/mp.2010.121. [DOI] [PubMed] [Google Scholar]
- Jacobson T.A., Ito M.K., Maki K.C., Orringer C.E., Bays H.E., Jones P.H., McKenney J.M., Grundy S.M., Gill E.A., Wild R.A., Wilson D.P., Brown W.V. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1 – executive summary. J. Clin. Lipidol. 2014;8(5):473–488. doi: 10.1016/j.jacl.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Johnson S. The multifaceted and widespread pathology of magnesium deficiency. Med. Hypothes. 2001;56(2):163–170. doi: 10.1054/mehy.2000.1133. [DOI] [PubMed] [Google Scholar]
- Kocsis R.N. Sage Publications; Los Angeles, CA: 2013. Book review: Diagnostic and statistical manual of mental disorders: (dsm-5) [Google Scholar]
- Kong A., Frigge M.L., Masson G., Besenbacher S., Sulem P., Magnusson G., Gudjonsson S.A., Sigurdsson A., Jonasdottir A., Jonasdottir A., Wong W.S.W., Sigurdsson G., Walters G.B., Steinberg S., Helgason H., Thorleifsson G., Gudbjartsson D.F., Helgason A., Magnusson O.T., Thorsteinsdottir U., Stefansson K. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488(7412):471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup R.K., Kurup P.A. A hypothalamic digoxin-mediated model for autism. Int. J. Neurosci. 2003;113(11):1537–1559. doi: 10.1080/00207450390231482. [DOI] [PubMed] [Google Scholar]
- Lee S.Y., Lee A.R., Hwangbo R., Han J., Hong M., Bahn G.H. Is Oxytocin Application for Autism Spectrum Disorder Evidence-Based? Exp. Neurobiol. 2015;24(4):312–324. doi: 10.5607/en.2015.24.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P., Risi S., Gotham K., Bishop S.J.L.A. second ed. Western Psychological Corporation; CA: 2012. Autism diagnostic observation schedule. (ados-2) [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luçardo J.d.C., Monk G.F., Dias M.d.S., Martins-Silva T., Fernandes M.P., Maia J.C., Valle S.C., Vaz J.D.S. Interest in food and triglyceride concentrations in children and adolescents with autistic spectrum disorder. J. de Pediatria. 2021;97(1):103–108. doi: 10.1016/j.jped.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Eran A., Palmer N., Avillach P., Levy-Moonshine A., Szolovits P., Kohane I.S. A multidimensional precision medicine approach identifies an autism subtype characterized by dyslipidemia. Nat. Med. 2020;26(9):1375–1379. doi: 10.1038/s41591-020-1007-0. [DOI] [PubMed] [Google Scholar]
- Milekic M.H., Xin Y., O’Donnell A., Kumar K.K., Bradley-Moore M., Malaspina D., Moore H., Brunner D., Ge Y., Edwards J., Paul S., Haghighi F.G., Gingrich J.A. Age-related sperm DNA methylation changes are transmitted to offspring and associated with abnormal behavior and dysregulated gene expression. Mol. Psychiat. 2015;20(8):995–1001. doi: 10.1038/mp.2014.84. [DOI] [PubMed] [Google Scholar]
- Monteleone P., Santonastaso P., Pannuto M., Favaro A., Caregaro L., Castaldo E., Zanetti T., Maj M. Enhanced serum cholesterol and triglyceride levels in bulimia nervosa: Relationships to psychiatric comorbidity, psychopathology and hormonal variables. Psychiat. Res. 2005;134(3):267–273. doi: 10.1016/j.psychres.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Mortensen E.L., Michaelsen K.F., Sanders S.A., Reinisch J.M.J.J. The association between duration of breastfeeding and adult intelligence. JAMA. 2002;287(18):2365. doi: 10.1001/jama.287.18.2365. [DOI] [PubMed] [Google Scholar]
- Mousain-Bosc, M., Roche, M., Polge, A., Pradal-Prat, D., Rapin, J., Bali, J.J.M.r., 2006. Improvement of neurobehavioral disorders in children supplemented with magnesium-vitamin b6. [PubMed]
- Mousain-Bosc M., Roche M., Rapin J., Bali J.-P. Magnesium VitB6 Intake Reduces Central Nervous System Hyperexcitability in Children. J. Am. College Nutr. 2004;23(5):545S–548S. doi: 10.1080/07315724.2004.10719400. [DOI] [PubMed] [Google Scholar]
- Nechifor, M.J.M.i.t.C.N.S.A.U.o.A.P., Australia, 2011. Magnesium in psychoses (schizophrenia and bipolar disorders). [PubMed]
- Organization, W.H., 2010. Indicators for assessing infant and young child feeding practices part 3: Country profiles.
- Sabbagh H.J., Al-Jabri B.A., Alsulami M.A., Hashem L.A., Aljubour A.A., Alamoudi R.A. Prevalence and characteristics of autistic children attending autism centres in 2 major cities in Saudi Arabia: A cross-sectional study. SMJ. 2021;42(4):419–427. doi: 10.15537/smj.2021.42.4.20200630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacker, A., Quigley, M.A., Kelly, Y.J.J.P., 2006. Breastfeeding and developmental delay: Findings from the millennium cohort study. [DOI] [PubMed]
- Schopler, E., Reichler, R.J., DeVellis, R.F., Daly, K.J.J.o.a., disorders, d., 1980. Toward objective classification of childhood autism: Childhood autism rating scale (cars). [DOI] [PubMed]
- Schultz, S.T., Klonoff-Cohen, H.S., Wingard, D.L., Akshoomoff, N.A., Macera, C.A., Ji, M., Bacher, C.J.I.b.j., 2006. Breastfeeding, infant formula supplementation, and autistic disorder: The results of a parent survey. [DOI] [PMC free article] [PubMed]
- Sharma R., Agarwal A., Rohra V.K., Assidi M., Abu-Elmagd M., Turki R.F. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod. Biol. Endocrinol. 2015;13(1) doi: 10.1186/s12958-015-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora D.M., Pettit-Kekel K., Penfield J., Merkens L.S., Steiner R.D. The near universal presence of autism spectrum disorders in children with Smith–Lemli–Opitz syndrome. Am. J. Med. Genet. 2006;140A(14):1511–1518. doi: 10.1002/ajmg.a.31294. [DOI] [PubMed] [Google Scholar]
- Slutsky I., Abumaria N., Wu L.-J., Huang C., Zhang L., Li B.o., Zhao X., Govindarajan A., Zhao M.-G., Zhuo M., Tonegawa S., Liu G. Enhancement of Learning and Memory by Elevating Brain Magnesium. Neuron. 2010;65(2):165–177. doi: 10.1016/j.neuron.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Steinman G., Mankuta D. Breastfeeding as a possible deterrent to autism – a clinical perspective. Med. Hypothes. 2013;81(6):999–1001. doi: 10.1016/j.mehy.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Tierney E., Bukelis I., Thompson R.E., Ahmed K., Aneja A., Kratz L., Kelley R.I. Abnormalities of cholesterol metabolism in autism spectrum disorders. Am. J. Med. Genet. 2006;141B(6):666–668. doi: 10.1002/ajmg.b.30368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschinkel, P.F.S., Bjørklund, G., Conón, L.Z.Z., Chirumbolo, S., Nascimento, V.A.J.B., Pharmacotherapy, 2018. Plasma concentrations of the trace elements copper, zinc and selenium in brazilian children with autism spectrum disorder. [DOI] [PubMed]
- Tseng, P.-T., Chen, Y.-W., Stubbs, B., Carvalho, A.F., Whiteley, P., Tang, C.-H., Yang, W.-C., Chen, T.-Y., Li, D.-J., Chu, C.-S.J.N.n., 2019. Maternal breastfeeding and autism spectrum disorder in children: a systematic review and meta-analysis. [DOI] [PubMed]
- Volpe, S.L.J.A.i.n., 2013. Magnesium in disease prevention and overall health. [DOI] [PMC free article] [PubMed]
- Wu L.-L., Mao S.-S., Lin X.u., Yang R.-W., Zhu Z.-W. Evaluation of whole blood trace element levels in chinese children with autism spectrum disorder. Biol. Trace Elem. Res. 2019;191(2):269–275. doi: 10.1007/s12011-018-1615-4. [DOI] [PubMed] [Google Scholar]