Abstract
The development of agriculture requires the use of microorganisms in the management of phytopathogens as a way to compensate for the use of chemical pesticides, in order to produce healthy crops. The objective of this study was to characterize a new isolate of Trichoderma sp. based on morphological and molecular features, and its potential ability to control the pathogen Alternaria sp. The antagonistic isolate was isolated from soil samples of potato fields in Guasave Sinaloa, Mexico, whereas the pathogen was collected from infected apple leaves in the orchard “La Escondida” in Guerrero County, Chihuahua, Mexico. For morphological characterization both fungi were grown on solid PDA medium. DNA of Trichoderma sp. was isolated using the CTAB method and PCR analyses were done using ITS1, ITS4 primers resulting in amplified products of 600 bp. These were sequenced, submitted to Genbank (acc. no. MN950427) and used for further phylogenetic analysis through Bayesian inference approach. Five clades were identified and the polytome topography recovered from clade 4 indicates a high genetic similarity with T. asperellum. A BLAST examination of the resulting sequence in GenBank showed 98.11% similarity with T. asperellum. This result together with the morphological and the phylogenetic analyses indicates that the isolate belongs to Trichoderma asperellum Samuels, Lieckfeldt & Nirenberg. Biocontrol tests of this isolate showed inhibition of Alternaria sp. between 50% and 93%. These results are essential for biodiversity research and give some new possibilities for pest management.
Keywords: Antagonistic microorganisms, Biotechnology, Biocontrol, Trichoderma asperellum
1. Introduction
The genus Malus Mill. gathers 25 to 33 species (Ma et al., 2017) and includes one of the most economically important fruit trees worldwide: the apple (Malus domestica Borkh.) (Posadas-Herrera et al., 2018). Apple production is constantly affected by various biotic stresses such as Alternaria alternata (Fries) Keissler, the fungus predominantly associated with the moldy heart of the apple fruit (Reuveni et al., 2006).
The disease caused by A. alternata is considered the most important of Red Delicious cultivars in northern Mexico and is characterized by the growth of mycelium within the locules, with or without penetration into the mesoderm of the fruit (Reuveni et al., 2003). The external symptoms of diseased fruits are difficult to perceive, although some may acquire color and fall prematurely (Spotts, 1990). Chemical control is the most widely used method, with efficient results, but with residual effects, which causes chemicals to accumulate in water bodies, soil, plants and animals. This is one of the main reasons for the application of ecological strategies such as microorganisms (Michel-Aceves et al., 2009).
The use of antagonistic microorganisms that are naturally present in the soil offers options to reduce environmental contamination by use of chemical pesticides. Biological control implies the use of natural enemies for the regulation of pest populations. These enemies include bacteria, viruses, nematodes and fungi. The genus Trichoderma is one of the most studied fungus and is widely used as biocontrol since it includes species which are versatile, adaptable to various environments and are easy to manipulate (Duarte-Leal et al., 2017).
The mechanisms used by Trichoderma sp. to control plant-pathogens include antibiosis, mycoparasitism and competition for space and nutrients (Harman et al., 2004, Bailey et al., 2008). In addition, when it interacts with the roots, Trichoderma promotes growth and increased resistance, producing positive effects on the development of the plant, due to the presence of growth-regulating hormones and formation of iron chelating siderophores, stimulating the primary meristematic tissues in young parts (Candelero et al., 2015). Because the plasticity of morphological traits in Trichoderma species is not enough to make a precise taxonomic diagnosis, it is essential to characterize them with accurate molecular tools (Hermosa et al., 2000).
Previous research used molecular techniques to characterize 16 biological control strains formerly known as T. harzianum and one strain identified as T. viride. Hybridizations using a mitochondrial DNA probe, showed a certain degree of polymorphism and sequence analysis of internal transcribed spacers 1 and 2 (ITS1 and ITS2) revealed three different ITS lengths and four different sequence forms (Hermosa et al., 2000). García-Núñez et al. (2017) characterized by morphology and molecularly 10 native strains of Trichoderma (TL2, TL4, TL5, TL6, TX7, TX8, TT6, TF8, TF10 and TJ6) and their phylogenetic relationship, as well as their biocontrol capacity against Phytophthora infestans. This study found that six of the analyzed strains corresponded to T. asperellum (TF8, TT6, TX7, TX8, TL2 and TL4) and four to T. harzianum (TJ6, TF10, TL5 and TL6). In addition, the phylogenetic analysis showed a close correlation among the strains of these two groups.
According to the data above, it is inferred that the use of antagonistic microorganisms such as Trichoderma sp. could be a viable option for the management of fungal diseases for different crops, and by increasing the availability with autochthonous strains, integrated control strategies would become more efficient. The morphological and molecular studies of isolates around the world may reveal novel species or strains. This may constitute a framework for future taxonomic, phylogenetic and biological investigations. Therefore, the objective of the present research was to characterize a novel isolate of Trichoderma sp. based on morphological and molecular approaches, as well as to evaluate its potential biocontrol against Alternaria sp., the causing agent of moldy heart of the apple fruit.
2. Materials and methods
2.1. Purification of isolates
A native isolate of Trichoderma sp., was obtained from 15 soil samples from a potato field in Guasave, Sinaloa located at 108° 19′ 8.5″ West and 25° 32′ 25″ North, at an altitude of 21 m above sea level (masl). These samples were taken from a surface of 1 ha. Soils were taken at a depth of 20 cm and close to the roots. The samples (≈500 g) were stored in plastic bags and taken to the laboratory under refrigeration at approximately 18 °C until processing (Sadeghian, 2018).
Each sample was added to sterile distilled water (1 g 100 mL−1), from which 1 mL was diluted to 10−3, afterwards, 100 µl were placed in Petri dishes with PDA dextrose agar medium (Potato Dextrose Agar medium). After spreading the suspension, the plates were incubated at 26 °C for 7 days until the Trichoderma sp. colonies developed. Afterwards they were purified and transferred to Petri dishes with PDA medium (Girard, 1964, Maniscalco and Dorta, 2015).
The pathogen (Alternaria sp.) was isolated from infected apple leaves from the orchard “La Escondida” which is located in the Mesa of Miñaca, Guerrero County, Chihuahua, Mexico located at 107° 27′ 02.6″ West and 28° 28′ 11.7″ North, at an altitude of 21.50 masl. The collected leaves were disinfected with 1% sodium hypochlorite and washed three times with sterile distilled water to proceed to the direct plating of leaf portions (half healthy and half diseased) in PDA medium (BD Bioxon) at pH 5.0–5.5. The Petri dishes were incubated in the dark at 25 ± 2 °C during 7 days.
2.2. Morphological characterization
2.2.1. Macroscopic identification
The morphological characterization was done after obtaining a pure isolate of Trichoderma sp. The isolate was identified following the method of Barnett and Hunter (1972), considering concentric rings, conidia development and pigmentation, and mycelium texture.
2.2.2. Microscopic identification
The microscopic identification was implemented through the recognition of structures observed in culture, such as, phialides hyphae, shape of conidia and number of conidiophores. Once of the fungal structures were visualized, microcultures were done on slides with agar water at 2%, and they were incubated at 25 °C. Subsequently at 48–72 h, microcultures were observed with an optical microscope (VELAB). The Trichoderma sp. isolate was identified using the codes and descriptions of Barnett and Hunter (1972), for fungi genera and species. The Alternaria sp. isolate was characterized using the guide of Morales-Mora et al. (2020).
2.3. Molecular characterization
The DNA extraction of the isolate was carried out from the mycelium using the method developed by Rajendrakumar et al. (2006). The extracted DNA was observed in agarose gel at 1%. To amplify the internal transcribed spacer region (600–1400 bp), the oligonucleotide pair ITS-1 (TCCGTAGGTGAACCTGCGG) and ITS-4 (TCCTCCGCTTATTGATATGC) (White et al., 1990) were used. The PCR (Polymerase Chain Reaction) was done in a thermocycler (BioRad, CA, EU.), with 25 µl of a reaction combination including 0.2 mM dNTP, 2 mM MgCl2, 0.5 µM of each oligonucleotide and 1.25 µl of recombinant Taq DNA polymerase (Invitrogen). The amplification program was one cycle at 95 °C for 4 min, 30 cycles at 95 °C for 1 min, 60 °C for 60 min and 72 °C by 2 min, with a final cycle at 72 °C for 5 min. The amplification was determined on 1% agarose gel (electrophoresis).
The PCR product was purified using the Wizard SV Gel Kit and PCR Clean-Up System (Promega) and sequenced in the Chemistry DNA laboratory of the CINVESTAV Irapuato, using the Dye Terminator Cycle Sequencing Ready Reaction kit, and the ABI PRISM 377 PERKIN-ELMER Sequencer (Cetus, Norwalk, CT). Forward and reverse sequences were reviewed and aligned in BioEdit 7.0.9 (Hall, 1999) and Clustal W (Thompson et al., 1994) to obtain a consensus sequence for further analysis.
The ITS sequence obtained (GenBank acc. no. MN950427), was compared with sequences available in the GenBank Database (Benson et al., 2018) such as Trichoderma asperellum AY380912, T. longibrachatium EU401556, T. brunneoviride EU518659, T. hamatum MK765015, T. atrobrunneum MH459162, T. tawa KC847184, T. piluliferum KF985185, T. lixii KT588249, T. virens KT588282 and T. guizhouense MN258612, using ITS sequences from Beauveria bassiana MK246940 and Metarhizium rileyi MG637450 as external group. The sequences were aligned with Clustal W and the final matrix containing 600 bp was used for distance analysis performed in PAUP* 4.0–10 (Swofford, 1998), using the neighbor union method (NJ) (Saitou and Nei, 1987) and Bootstrap (Felsenstein, 1985) with 1000 repetitions, with 100 cycles of random addition each to evaluate the internal branch support and finally the tree was edited in FigTree 1.4.3 (Rambaut, 2016).
2.4. In vitro antagonistic test
The antagonistic test was evaluated in Petri dishes with PDA medium containing a disc (5 mm in diameter) with mycelium of Trichoderma asperellum placed at one end, and at the opposite end, another disc of the same size containing the pathogen Alternaria sp. A control was included which consisted in a Petri dish inoculated with only the disc with mycelium of Alternaria sp. The inoculum of the pathogen was prepared 72 h before the test in PDA medium. The antagonistic test was carried out for 7 days. Petri dishes were incubated at 25 ± 2 °C in the dark. The diameter of each fungal colony was measured daily, until one of these (either the antagonist or the pathogen) completed its growth in the Petri dish.
The inhibition rate of their radial growth was determined using the formula of Samaniego et al. (1989), PIRG = [(R1-R2)/R1 × 100] where R1 is the radial growth of the control and R2 the radial growth of the pathogen confronted against T. asperellum in the dual culture.
A randomized experiment was performed with three repetitions and the potential of the isolate as a biocontrol agent of Alternaria sp. at the different times was done by analysis of variance and multiple media comparison (Tukey, p < 0.05), using the SAS (Statistical Analysis System) software version 9.4.
3. Results
3.1. Morphological characterization
3.1.1. Macroscopic identification of Trichoderma sp.
A pure culture was obtained and the isolate presented white mycelium of spongy consistency that spread throughout the plate, two to three concentric rings, with yellow-green color (Fig. 1).
Fig. 1.
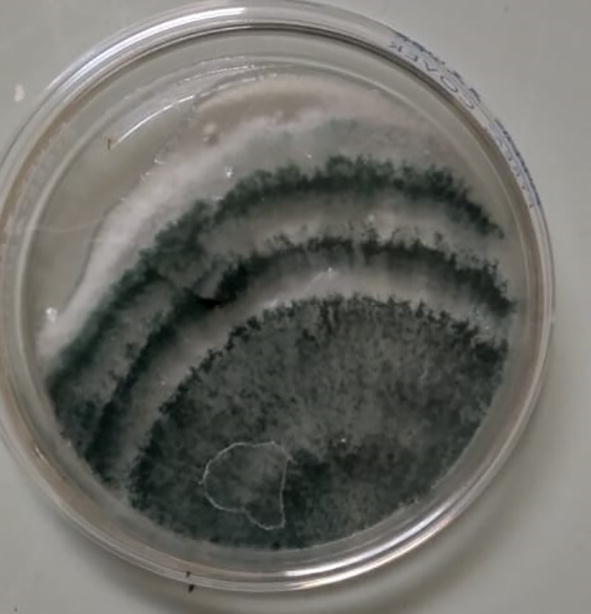
Colony of the native strain of Trichoderma sp. in PDA medium.
García-Núñez et al. (2017) described several Trichoderma isolates that developed profuse fluffy mycelium and two to three fine defined concentric mycelium (white) and conidia (green) rings. Other characteristics that define the genus Trichoderma are a fast growth in culture medium and development of conidia with green-yellow color (Chaverri et al., 2015). These characteristics are similar to several Trichoderma species, so it was difficult to identify the isolate based only on morphological data (Gupta et al., 2013).
3.1.2. Microscopic morphological characterization of Trichoderma sp. and Alternaria sp.
Results from the microscopic observation of Trichoderma sp. isolate showed dense conidia, branched conidiophores, ampuliform phialides, slightly globose conidia with yellow-green pigmentation (Fig. 2).
Fig. 2.
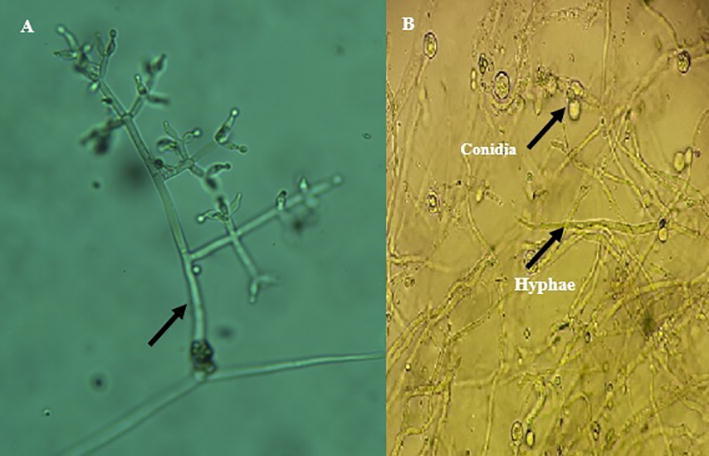
Microscopic structures of Trichoderma sp. (a) Conidiogenic cells (x40); (b) Hyphae and conidia (x100). Scale bars: 10 µm.
Regarding the Alternaria sp. isolate, it showed septated hyaline hyphae, and its conidia are oval to oblong-shaped, transversally septated with three to five divisions, with dark color when mature (Fig. 3).
Fig. 3.
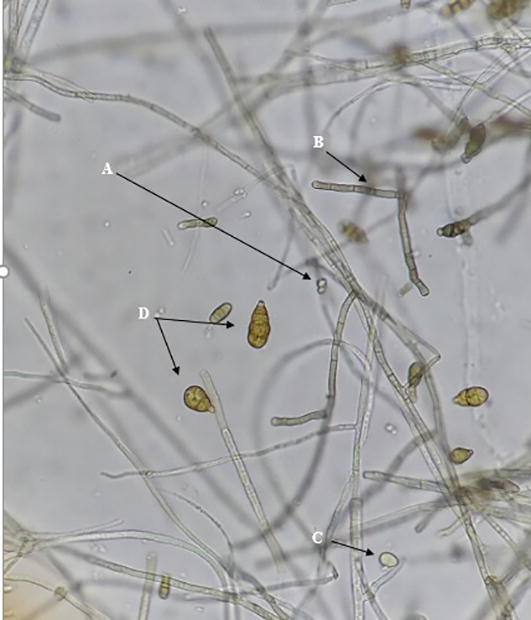
Microscopic structures of Alternaria sp. (A) Conidia (x40); (B) Septate hyphae; (C) Young conidia, and (D) Mature conidia (x100). Scale bars: 10 µm.
3.2. Molecular characterization
A fragment of 600 bp was visualized after DNA extraction and PCR amplification. A consensus sequence was obtained for this region and deposited in GenBank with accession number MN95047. The PCR amplification done with primers ITS-1 and ITS-4 is showed in Fig. 4.
Fig. 4.
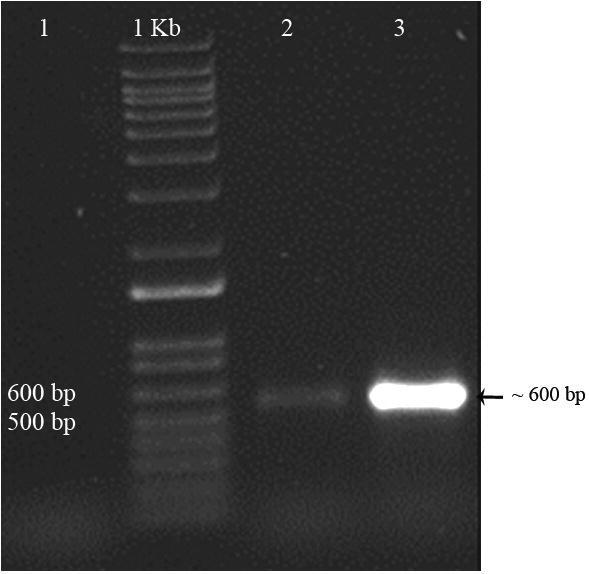
PCR products amplification of the fungal isolate PCR: (1): negative; (1 kb): molecular weight marker; (2): positive and (3): isolate fragment.
The phylogenetic analysis showed that this isolate matched with Trichoderma sp. (GenBank acc. no. MN950427), which clustered by 98.11% identity the species Trichoderma asperellum (accession number AY380912) from Indonesia, thus confirming by molecular and bioinformatics means that the isolate corresponds to T. asperellum (Fig. 5).
Fig. 5.
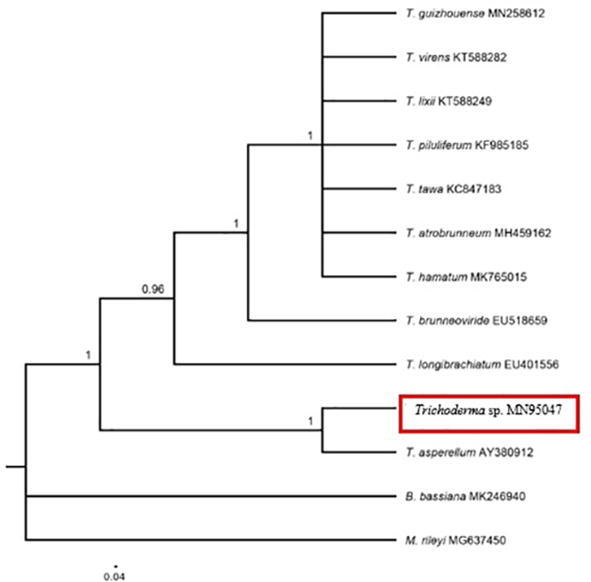
Neighboring tree of Trichoderma sp. MN950427 accessions, based on ITS sequences. The new isolate is marked with a red box. The numbers on the branches indicate Bootstrap compatibility (96%) and the scale bar indicates nucleotide substitutions per site.
Using the ITS sequence information, four clades have been identified, and the polytomous topography suggests a high genetic similarity among T. asperellum (clade 4).
These molecular results support the morphological description of the isolate and confirm that the analysis used lead to a precise and reliable taxonomic diagnosis.
3.3. In vitro confrontation
The beginning of the antagonistic activity in the dual culture of T. asperellum and Alternaria sp. isolates was observed with a marked difference in growth speed in favor of the antagonist, which grew at an average speed of = 0.79 mm h−1. Contact between the colonies began at 48 h, which was most noticeable at 96 h (Fig. 6).
Fig. 6.
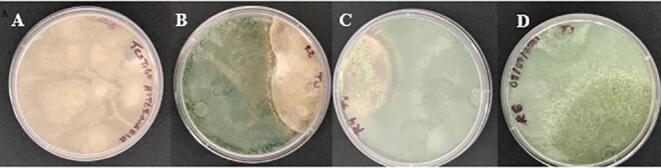
Interaction between Trichoderma asperellum and Alternaria sp. isolates where: A. Alternaria sp.; B. Dual culture at 48 h; C. Dual culture at 96 h; D. Dual culture at day six.
The maturation of the spores of T. asperellum and the total coating of the pathogenic colony by them was observed on the 5th and 6th day of the confrontation.
The PIRG varied between 50% and 93% in the evaluated time points with significant differences (Table 1), which indicate an increase of mycelial growth of T. asperellum on the pathogenic fungus.
Table 1.
Analysis of the mycelial growth of Trichoderma asperellum on the pathogen.
| Evaluation Time | R1 (mm h−1) | R2 (mm h−1) | PIRG (%) |
|---|---|---|---|
| 48 h | 0.4b | 0.2a | 50c |
| 96 h | 1.4a | 0.1a | 92b |
| 6th day | 1.2a | 0.1a | 93c |
4. Discussion
The previous results demonstrated the importance of the morphological and molecular studies for species identification. Regarding the Alternaria sp. isolate the microscopic characteristics are in agreement with those reported by Morales-Mora et al. (2020).
In contrast, the macroscopic features described for the novel Trichoderma sp. isolate agree with the taxonomic identification criteria of Barnett and Hunter (1972). These include profuse fluffy mycelium and two to three well defined concentric white (mycelium) and green (conidia) rings. It also was observed fast growth in culture medium and development of conidia with green-yellow color (Chaverri et al., 2015, García-Núñez et al., 2017). Likewise, the microscopic characteristics of the isolate were similar to those reported for the species T. asperellum (Barnett and Hunter, 1972). These included the presence of ampulliform phialides, oval-round conidia with greenish-yellow pigmentation and branched conidiophores (Samuels et al., 1999). These features agree with the observations done with the isolate from the present study, as well as with the T2 colony reported by Gamboa-Villa et al. (2020).
Due to the abundant homoplasia of phenetic characters in Trichoderma species (Gupta et al., 2013), it is necessary to make additional molecular analyses besides morphology in order to determine the species accurately (Schuster and Schmoll, 2010; García-Núñez et al., 2017).
Previous studies have shown that ITS primers are valuable to effectively identify Trichoderma isolates (Torres-De la Cruz et al., 2015). In the present study, molecular analyses showed a PCR product of ≈600 bp of the amplified ITS region of the Trichoderma isolate. This result agrees with findings of several other studies. Ten isolates of Trichoderma spp., were analyzed with the same primers ITS1 and ITS4, obtaining amplified DNA fragment sizes of 600 bp (García-Núñez et al., 2017). Another study obtained a single PCR product of approximately 560–600 bp from 17 biocontrol isolates of Trichoderma spp. using primers ITS1 and ITS4 (Hermosa et al., 2000). The results of the present study are similar to those obtained by Hermosa et al., (2000).
The phylogenetic analysis of this work supports the data on morphological characteristics found in this investigation and allowed the identification of the isolate as T. asperellum. The use of ITS sequences and the phylogenetic analysis enabled to identify as new records several species of Trichoderma (T. asperellum, T. brevicompactum, T. koningiopsis/H. koningiopsis, T. pleuroticola, T. reesei/H. jecorina and T. spirale) in the cacao agrosystem in Tabasco, Mexico (Torres-De la Cruz et al., 2015). Similar results were reported by García-Núñez et al. (2017) using a BLAST analysis of the amplified ITS sequences, where 6 out of 10 isolates tested were highly identical (99%) to T. asperellum.
Considering the PIRG results obtained in the present study (antagonistic efficacy between 50% and 93%), it is very possible that T. asperellum be an effective control against Alternaria sp. Results found by Camacho-Luna et al. (2021) reported the growth inhibition of A. porri in 56% using two isolates of T. asperellum; while T. atroviridae showed only 20% inhibition of the mycelial growth of the pathogen. At 48 h after the confrontation, the value was classified as class III according to the scale proposed by Bell et al. (1982) and from 96 h on, the value was classified as class I, meaning that T. asperellum showed a high antagonistic capacity.
Pandey (2010) found that T. harzianum inhibited A. alternata at 67% after 10 days of incubation, while T. viride inhibited the pathogen at 66.7% and the biocontrol process started at the 5th day of incubation. Other authors found different percentages of Alternaria sp. growth inhibition by several species of Trichoderma. Examples include 52.9% PIRG of A. alternata by T. harzianum (Zehra et al., 2017), 53.8–82.8% growth inhibition of A. alternata by T. harzianum (Kayım et al., 2018), 43.6 ± 6.7% PIRG of A. niger by T. harzianum (Morales-Mora et al., 2020), and T. aggressivum f. europaeum showed high antagonistic activity (≥80%) for different phytopathogens (Sánchez-Montesinos et al., 2021). In the present study, the dual culture of T. asperellum colonized half of the medium at 48 h and at the sixth day, this antagonist completely covered the whole medium surface with 93% inhibition of the pathogen radial growth. Hence, this isolate showed a higher biocontrol capacity than all the studies reported with several Trichoderma species.
5. Conclusions
This study identified a new autochthonous Trichoderma sp. isolate as Trichoderma asperellum through morphological and molecular analyses, and it was registered at the National Center for Biotechnology Information (NCBI) database with the accession No. MN95047. The in vitro confrontation of T. asperellum versus Alternaria sp. showed a prominent antagonist capacity (50–93%), indicating that this isolate can be a good candidate to control this phytopathogen in apple fields. Therefore, it is important to carry out research with this system under controlled conditions (greenhouse) and in the field, using various application methods.
This research reports for the first time a Trichoderma asperellum isolate from potato fields in Guasave, Sinaloa, Mexico. This fungus could be used as a biocontrol agent against some phytopathogenic fungi for the benefit of several types of crops and the environment.
6. Funds
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
To Melissa Madrid Molina for providing the apple leaves infected with Alternaria sp.
Footnotes
Peer review under responsibility of King Saud University.
References
- Bailey A., Bae H., Strem M., Crozier J., Thomas S., Samuels G., Vinyard B., Holmes K. Antibiosis, mycoparasitism and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao. Biocontrol. 2008;46(1):24–35. http://doi:10.1016/j.biocontrol.2008.01.003 [Google Scholar]
- Barnett H., Hunter B. Burgess Publ; Co., EE. UU: 1972. Ilustrated genera of imperfect fungi. http://dx.doi:10.2307/3757954. [Google Scholar]
- Bell D.K., Wells H.D., Markham C.R. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathol. 1982;72:379–382. doi: 10.1094/Phyto-72-379. [DOI] [Google Scholar]
- Benson D.A., Cavanaugh M., Clark K., Karsch-Mizrachi I., Ostell J., Pruitt K.D., Sayers E.W. GenBank. Nucl. Acids Res. 2018;46(D1):D41–D47. doi: 10.1093/nar/gkx1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Luna V., Flores-Moctezuma H.E., Rodríguez-Monroy M., Montes-Belmont R., Sepúlveda-Jiménez G. Induction of the defense response of onion plants in interaction with Trichoderma asperellum and Alternaria porri. Rev. Mexicana Cienc. Agric. 2021;12(4):685–698. [Google Scholar]
- Candelero D.J., Cristobal A.J., Reyes R.A., Tun S.J.M., Gamboa A.M.M., Ruiz S.E. Trichoderma spp. promotoras del crecimiento en plántulas de Capsicum chinense Jacq. y antagónicas contra Meloidogyne incógnita. PHYTON. 2015;84:113–119. [Google Scholar]
- Chaverri P., Branco-Rocha F., Jaklitsch W., Gazis R., Degenkolb T., Samuels G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycol. 2015;107(3):558–590. doi: 10.3852/14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Leal Y., Lamz-Piedra A., Martínez-Coca B. Antagonismo in vitro de aislamientos de Trichoderma asperellum Samuels, Lieckfeldt y Nirenberg frente a Sclerotium rolfsii Sacc. Rev. Prot. Veg. 2017;32(3):1–11. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- Gamboa-Villa L.C., Martínez-Fernández E., Martínez-Jaimes P., Suárez-Rodríguez R., Ramírez-Trujillo J.A. Biocontrol de Trichoderma spp. hacia patógenos de la raíz de caña de azúcar (Saccharum officinarum) Agrociencia. 2020;54(7):955–966. doi: 10.47163/agrociencia.v54i7.2245. [DOI] [Google Scholar]
- García-Núñez, H.G., Martínez-Campos, A.R., Hermosa-Prieto, M.R., Monte-Vázquez, E., Aguilar-Ortigoza, C.J., González-Esquivel, C.E., 2017. Morphological and molecular characterization of native isolates of Trichoderma and its potential biocontrol against Phytophthora infestans. Rev. Mex. Fitopatol. 35, 58-79. <https://doi.org/ 10.18781/R.MEX.FIT.1605-4>.
- Girard B.R. Acribia; Zaragoza-España: 1964. Técnicas de Microbiología Agrícola. [Google Scholar]
- Gupta G., Schmoll M., Herrera-Estrella A., Upadhyay R., Druzhinina I., Tuohy M. Biotechnology and biology of Trichoderma. Elsevier, Amsterdam, Belgium. 2013 doi: 10.1016/B978-0-444-59576-8.00001-1. [DOI] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999;41:95–98. doi: 10.14601/PHYTOPATHOL_MEDITERR-14998U1.29. [DOI] [Google Scholar]
- Harman G., Howell C., Viterbo A., Chet I., Lorito M. Trichoderma species opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. http://dx.doi:10.1038/nrmicro797 [DOI] [PubMed] [Google Scholar]
- Hermosa R., Grondona I., Iturriaga E., Díaz M., Castro C., Monte E., García A. Molecular characterization and identification of biocontrol isolates of Trichoderma spp. Appl. Environ. Microbiol. 2000;66:1890–1898. doi: 10.1128/aem.66.5.1890-1898.2000. http://dx.doi.10.1128/AEM.66.5.1890-1898.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayım M., Yones A.M., Endes A. Biocontrol of Alternaria alternata causing leaf spot disease on faba bean (Vicia faba L.) using some Trichoderma harzianum ısolates under in vitro condition. Harran Tarım ve Gıda Bilimleri Dergisi. 2018;22(2):169–178. doi: 10.29050/harranziraat.329976. [DOI] [Google Scholar]
- Ma B., Liao L., Peng Q., Fang T., Zhou H., Korban S.S., Han Y. Reduced representation genome sequencing reveals patterns of genetic diversity and selection in apple. J. Integr. Plant Biol. 2017;59(3):190–204. doi: 10.1111/jipb.12522. [DOI] [PubMed] [Google Scholar]
- Maniscalco D.P., Dorta B. Diversidad del hongo Trichoderma spp. en plantaciones de maíz de Venezuela. Interciencia. 2015;40(1):23–31. [Google Scholar]
- Michel-Aceves A., Otero S., Solano P., Ariza F., Barrios A., Rebolledo M. Biocontrol in vitro con Trichoderma spp., Fusarium subglutinans, (Wollenweb y Reinking) Nelson, Toussoun y Marasas y F. oxysporum Schlecht., agentes causales de la “Escoba de bruja” del Mango (Mangifera indica L.) Rev. Mex. Fitopatol. 2009;27:18–26. [Google Scholar]
- Morales-Mora L.A., Andrade-Hoyos P., Valencia-de Ita M.A., Romero-Arenas O., Silva-Rojas H.V., Contreras-Paredes C.A. Characterization of strawberry associated fungi and in vitro antagonistic effect of Trichoderma harzianum. Rev. Mex. Fitopatol. 2020;38(3):434–449. doi: 10.18781/R.MEX.FIT.2005-7. [DOI] [Google Scholar]
- Pandey A. Antagonism of two Trichoderma species against Alternaria alternata on Capsicum frutescens. J. Exp. Sci. 2010;1(5):18–19. [Google Scholar]
- Posadas-Herrera B., López P., Gutiérrez-Rangel N., Díaz-Cervantes R., Ibáñez-Martínez A. La diversidad fenotípica de manzano en Zacatlán, Puebla, México es amplia y es aportada principalmente por características de fruto. Rev. Fitotec. Mex. 2018;41(1):49–58. doi: 10.35196/rfm.2018.1.49-58. [DOI] [Google Scholar]
- Rajendrakumar P., Sujatha K., Rao K.S., Kumar N.P., Viraktamath B.C., Balachandran S.M., Biswal A.K., Sundaram R.M. A protocol for isolation of DNA suitable for rapid seed and grain purity assessments in rice. Rice Genet. Newsl. 2006;23:92–95. [Google Scholar]
- Rambaut, A., 2016. FigTree v 1.4.3. Available at: <http://tree.bio.ed.ac.uk/software/figtree/>.
- Reuveni M., Sheglov D., Cohen Y. Control of moldy–core decay in apple fruits by β amynobutiric acids and potassium phosphites. Plant Dis. 2003;87:933–936. doi: 10.1094/PDIS.2003.87.8.933. [DOI] [PubMed] [Google Scholar]
- Reuveni M., Sheglov N., Eshel D., Prusky D., Ben-Arie R. Virulence and the production of Endo–1,4 β–glucanase by isolates of Alternaria alternata involved in the moldy–core disease of apples. J. Phytopathol. 2006;155:50–55. doi: 10.1111/j.1439-0434.2006.01201.x. [DOI] [Google Scholar]
- Sadeghian S.K. Interpretación de los resultados de análisis de suelo. Avances Técnicos, ISSN -0120–0178. 2018;2p:497. [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Samaniego G., Ulloa S., Herrera S. Hongos del suelo antagonistas de Phymatotrichum omnivorum. Rev. Mex. Fitopatol. 1989;8:86–95. [Google Scholar]
- Samuels G.J., Lieckfeldt E., Nirenberg H.I. Trichoderma asperellum, a new species with warted conidia and redescription of T. viride. Sydowia. 1999;51:71–88. [Google Scholar]
- Sánchez-Montesinos B., Santos M., Moreno-Gavíra A., Marín-Rodulfo T., Gea F.J., Diánez F. Biological control of fungal diseases by Trichoderma aggressivum f. europaeum and its compatibility with fungicides. J. Fungi. 2021;7:598. doi: 10.3390/jof70805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster A., Schmoll M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010;87(3):787–799. doi: 10.1007/s00253-010-2632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spotts R.A. In: Compendium of Apple and Pear Diseases. Jones A.L., Aldwinckle H.B., editors. APS Press; St. Paul Minnesota, USA: 1990. Moldy core and core rot; pp. 29–30. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts: 1998. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. ClustalW: improving the sensivity of progressive multiple sequence alignment through sequence weighting, position specific gap penaltiesand weight matrix choice. Nucl. Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-De la Cruz M., Ortiz-García C.F., Bautista-Muñoz C., Ramírez-Pool J.A., Ávalos-Contreras N., Cappello-García S., De la Cruz-Pérez A. Diversidad de Trichoderma en el agroecosistema cacao del estado de Tabasco. México. Rev. Mex. Biodivers. 2015;86(4):947–961. doi: 10.1016/j.rmb.2015.07.012. [DOI] [Google Scholar]
- White T.J., Bruns T., Lee S., Taylor J. In: PCR Protocols: A Guide to Methods and Applications. Innis M., Gelfand D., Sninsky J., White T., editors. Academic Press; San Diego: 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics; pp. 315–322. [Google Scholar]
- Zehra A., Dubey M.K., Meena M., Upadhyay R.S. Effect of different environmental conditions on growth and sporulation of some Trichoderma species. J. Environ. Biol. 2017;38(2):197–203. [Google Scholar]


