Graphical abstract
Keywords: Biological control, Mosquito, Bacteria, Fungi, Viruses
Abstract
Malaria is a disease caused by protozoan species of the genus Plasmodium. It is widespread and becoming a challenge in several African countries in the tropical and subtropical regions. In 2010, a report was published showing that over 1.2 million death cases were occurred globally due to malaria in just one year. The transmission of the disease from one person to another occurs via the bite of the Anopheles female. It is known that Plasmodium ovale, P. vivax, P. malariae, P. falciparum, and P. knowlesi are the highly infective malaria species. The problem of this disease is the absence of any effective medical treatment or vaccine, making the mosquito control is the only feasible way for disease prevention. Pesticides are currently the most widely used method for mosquito control, despite its well-known negative effects, including health hazards on human, the increasing insecticidal resistance, and the negative impact on the environment and beneficial organisms. Biological control (also called: biocontrol) of insects has been a promising method to overcome the negative effects of using chemical insecticides, as it depends on just using the natural enemies of pests to either minimize their populations or eradicate them. This article provides an overview of the recent and effective biological means to control malaria, such as bacteria, fungi, viruses, larvivorous fish, toxorhynchites larva and nematodes. In addition, the importance, advantages, and disadvantages of the biocontrol methods will be discussed in comparison with the traditionally used chemical methods of malaria control with special reference to nanotechnology as a novel method for insects’ control.
1. Introduction
Malaria is the most prevailing mosquito-borne human disease in the tropical and subtropical regions on earth, with huge negative impacts on medical, economic, and social levels (Asia, 2007). It was reported in 2010 that in a global scale, there was more than 1.2 million death cases because of Malaria in only one year (which include both adults and kids) (Murray et al., 2012). Malaria transmission from infected individual to another healthy one is mediated by the bite of an Anopheles female (Mullen and Durden, 2002). Parasites that are very common in causing malaria are: Plasmodium vivax, P. falciparum, P. ovale and P. malariae. Recently, P. knowlesi was also discovered, which mainly infects monkeys, but sometimes can also infect human beings (Kamareddine, 2012, Mullen and Durden, 2002). This disease is notorious for the absence of any effective medical treatment or vaccine, which makes mosquito control the only feasible way of disease prevention (Benelli et al., 2016a, Benelli et al., 2016b). Mosquito species (reaching over 4500) are grouped in 34 genera under the Culicidae family (Chandra et al., 2013). Species of Anopheles, Aedes, Culex, Mansonia, Haemagogus, Psorophora and Sabethes, are the most common vectors of malaria (Mullen and Durden, 2002). The problems of mosquitoes are not only limited to malaria transmission, since it also serves as vector for some of the most life-threatening human diseases that can infect more than two billion humans in tropical region (Odalo et al., 2005). Examples of those diseases are dengue fever, yellow fever, filariasis, chikungunya, and encephalitis (Bence, 1988, Benelli et al., 2016a, Benelli et al., 2016b, Ghosh et al., 2005, Sarwar, 2015).
Currently, the most extensively used method in controlling mosquito is chemical pesticides (in addition to personal protection methods), despites its well-known negative effects. Most clearly, are the health hazards on human, the increasing insecticide resistance, and the negative impact on the ecological system and beneficial organisms (Moraga et al., 2006). Therefore, various studies demonstrated several Eco-friendly natural compounds which may have insecticidal activity among these compounds are bioactive peptides (Saad et al., 2020a, Saad et al., 2021a, El-Saadony et al., 2021a, El-Saadony et al., 2021b), polyphenolic extracts (Saad et al., 2020b, Saad et al., 2021b, Saad et al., 2021c), essential oils (El-Tarabily et al., 2021, Abd El-Hack et al., 2021, Alagawany et al., 2021), and nanomaterials (Saad et al., 2021d, El-Ashry et al., 2022, El-Saadony et al., 2021c), additionally microbial control is a safe and an effective attitude in controlling heavy metals and insects (Desoky et al., 2020). These techniques were used to circumvent such problems, biological control (also known as: biocontrol) was introduced as an alternative in order to control mosquito vectors. Fruthermore, genetic engeneering can be used to develop the plants' autoresistance aganist insects (Saad et al., 2020). Biocontrol is an environmentally friendly and effective approach of controlling pests and their damages via the use of their natural enemies (Timmins, 1988). Most, if not all pests are known to have their own biological enemies, which can be exploited to control even more than one pest at once (Bence, 1988, Ghosh et al., 2005, Sarwar, 2015). Due to its economical efficiency and the health and environmental benefits, biocontrol should be the highest recommended method for malaria control (Mahar and Ridgway, 1993). Biocontrol can even work more efficiently if employed as part of a more comprehensive control management, using multiple methods of pest control in concert, a process known as “integrated pest management”. Insects and mosquito natural enemies can be pathogens, parasites, or predators, which include fishes, nematodes, bacteria, fungi, and viruses. Those organisms have a wide variation in their infection mode, replication site, and pathogenicity mechanisms (Porter et al., 1993). There are several mechanisms for biocontrol pathogenicity, such as: vector killing, creating behavioral-based increasing self-mortality, creating infertile vectors or incapable of disease transmission (Benelli et al., 2016a, Benelli et al., 2016b). Recently, nanotechnology tended to find new toles for insects’ control for instance, external parasites of pigeons are a nuisance to birds and considered as a mean for diseases transmission either mechanically or biologically (Attia and Salem, 2021, Salem et al., 2022, Soliman et al., 2022) therefore, chitosan-silver nanocomposite was used for the control of experimentally infested pigeons with Pseudolynchia canariensishas (P. canariensis) and the used nanocomposite revealed a promising effect in the elimination of P. canariensis infestation in pigeons as well as, ameliorated the negative effects of insects infestation (Attia et al., 2022). Therefore, the use of these natural practices is safer, environmentally friendly, and more effective than chemical formulations and limits the spread of diseases (Swelum et al., 2020).
2. Biocontrol agents
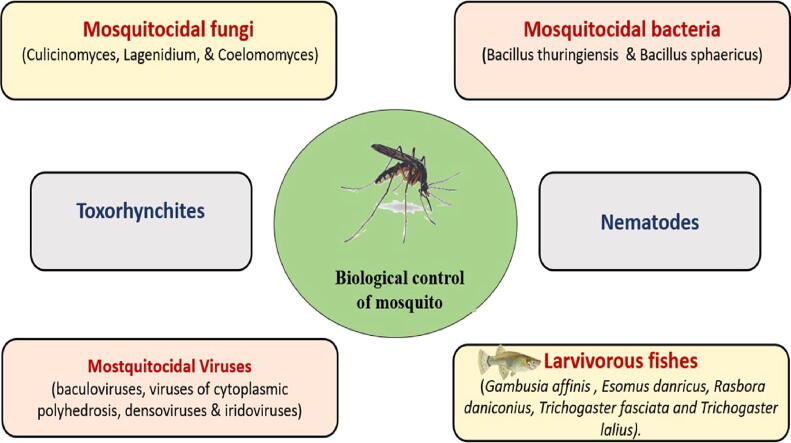
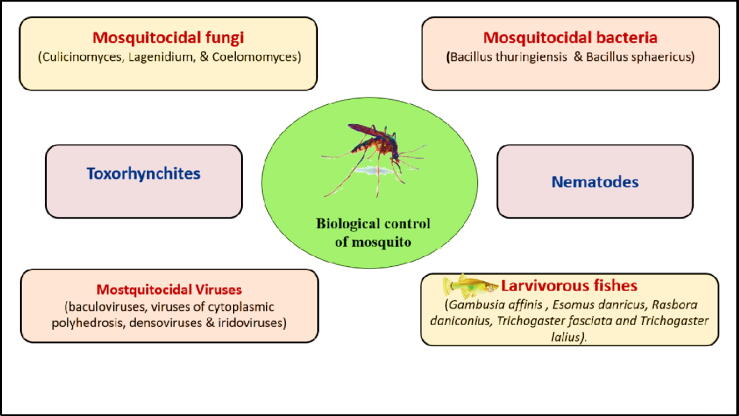
Biocontrol has expanded slowly over time from a limited practice to a more effective and enlarged biocontrol methods. The main development has occurred in the number and varieties of the biocontrol agents used to fight different pests and mosquito vectors (Vail et al., 2001). Biocontrol methods have already played a major role in minimizing the population of mosquito, mainly through the control of vector. The following parts of this review will discuss the role of different biocontrol agents in killing mosquitoes using different mechanisms. different biological control methods are seen in Table 1 and Fig. 1.
Table 1.
Different types of biological control of mosquito.
| Biological control type | Examples | Mechanism of action | References |
|---|---|---|---|
| Predator fish |
Gambusia affinis, Aphanius dispar Cynolebias species Fundulus species Clarias fuscus Cyprinus carpio Nothobranchius species Poecilia reticulate Tilapia cyprinids |
They have capability of to feed on developmental stages of mosquito | (Chandra et al., 2008, Das and Prasad, 1991, Louca et al., 2009, Subramaniam et al., 2015, Subramaniam et al., 2016, Van dam and Walton, 2007) |
| Amphibians |
Tadpoles, Frogs, Toads |
They have capability of to feed on developmental stages of mosquito | (Bowatte et al., 2013, Brodman and Dorton, 2006, Murugan et al., 2015) |
| Omnivorous copepods |
Cyclops vernalis, Megacyclops formosanus, Mesocyclops |
They have capability of to feed on developmental stages of mosquito | (Benelli et al., 2016a, Benelli et al., 2016b, Schaper, 1999, Vu et al., 1998) |
| Odonate young instars | Libellula forensis | They have capability of to feed on developmental stages of mosquito | Singh et al. (2003) |
| Water bugs | Lethocerus americanus | They have capability of to feed on developmental stages of mosquito | Bailey, 1989, Shaalan et al., 2007 |
| Larvae of another mosquito species |
Toxorhynchites spp |
They have capability of to feed on developmental stages of mosquito | Steffan and Evenhuis, 1981, Focks et al., 1985 |
| Bacteria |
Bacillus thuringiensis Streptomyces avermitilis Bacillus sphaericus |
Bacterial as biocontrol agent has been proven as environmentally friendly method and is a promising alternative for controlling mosquitoes using chemical insecticides | Ingabire et al., 2017, Kendie, 2020 |
| Fungi |
Microsporidia Coelomomyces Metarhizium |
Fungi that are pathogenic to arthropods are widespread in the tropical area, and play a major role in the arthropod population balance | Evans et al. (2018) |
| Virus |
Densoviruses Baculoviruses Iridoviruses |
The number of entomopathogenic viruses that have known active insecticidal effects reach up to tens of thousands, despite the very limited number of its commercially available products, probably because of our limited knowledge or experiments testing the viral field effects | Becnel and White (2007) |
| Toxorhynchites | Toxorhynchites splendens | Toxorhynchites is a mosquito genus known for the capability of its larvae to feed on species of mosquito and other aquatic organisms living in either natural or artificial habitats | Focks (2007) |
| Nematode |
Rhabditidae Heterorhabditidae Steinernematidae |
Nematode having species that can parasite, kill, or affect host growth. The infection style of nematodes is either by entering during insect feeding, penetrating throughout the cuticle, or entering via anus or spiracles | Petersen (1985) |
Fig. 1.
Different methods for biological control of mosquito.
2.1. Bacteria
On the international scale, there is a great and continuous efforts to identify new mosquitocidal strains of bacteria from the environment. That is because using bacteria as biocontrol agent has been proven as environmentally friendly method and is a promising alternative for controlling mosquitoes using chemical insecticides (Poopathi et al., 2014). Some of the prominent examples of mosquitocidal bacteria are Bacillus thuringiensis (Bt) and Bacillus sphaericus (Bs), as both have been used as biolarvicides with a wide range of insect hosts. Both species are working under variable conditions, with almost undetectable adverse ecological effects, such as human safety concerns, affecting non-target organisms. That makes them highly beneficial in reducing pesticide residues in the environment, and thus improving the activities of other natural enemies and enhancing the biodiversity in natural environments. Those two species are not only used as living biocontrol agents but are now mostly used as preparations of non-living microbial insecticide (in the form of toxin) (Mullen and Durden, 2002).
Globally, the most frequently used microbial insecticides are the preparations of Bacillus thuringiensis (Bt) (Ramírez-Lepe and Ramírez-Suero, 2012). It is by far, the most efficient pathogen, due to its ease of mass production, high safety level to human and environment (Ingabire et al., 2017), and its obvious variation in the host range specificity (for mosquito larvae) of its species (Poopathi, 2012).
Bacillus thuringiensis has a crystal endotoxin protein attached to the spore. This protein remains nontoxic until it reaches the insect's gut, where it is processed and becomes toxic due to high pH level in the insect gut. That same mechanism is their point of strength, when it comes to human safety aspects, since the pH of the human stomach is acidic, which is not suitable for converting the toxin to its toxic form (Reyaz et al., 2021). There is abundance of studies regarding the effects and benefits of using Bt as insecticidal, with wide variation in the level of effectiveness (Land and Miljand, 2014). The most common form of Bt is a slow-release preparation with a lasting effect for up to one month. Another form of Bt application is a soluble powder, applied to the larval area. After spraying the bacteria, it will not be able to reproduce, therefore, repeating the application is necessary. One of the other toxin-producing bacteria is Streptomyces avermitilis, which produces avermectins toxin, known to be highly effective against classes of Insecta, Nematode and Arachinida (Pirali-Kheirabadi, 2012). Bacillus sphaericus is like Bt in its killing mechanism, however its capable of multiplication in the larval environment. In addition, it is specifically effective in controlling Culex mosquito (Mullen and Durden, 2002). It is worth mentioning that, even though few species of mosquitoes (e.g, Culex quinquefasciatus in the laboratory and Culex pipiens in the field) have developed resistance against Bt and Bs, other species like Aedes vexans has never developed resistance for Bt even after 25 years of exposure. Therefore, Bt and Bs are still maintaining their reliability in the field of microbial insecticides.
Recently, genetic engineering technology has increased the potential of microbial insecticides, for example by recombining the important genes from the mentioned species, therefore, broadening their host range of insects. In other cases, endotoxin-producing genes can be transferred to other bacteria that are more adaptive to the environment, highly reproducible, or even transferring the genes to the target plant itself (Mullen and Durden, 2002).
2.2. Fungi
Fungi that are pathogenic to arthropods are widespread in the tropical area, and play a major role in the arthropod population balance. The variation of fungal spore forms, in addition to the different abnormal behaviors that they cause to the host, both increase the efficiency and rate of the infection (Evans et al., 2018). It is very common that insects are infected by fungi, which usually cause definite death. Almost all insects are susceptible to fungal infections, including mosquitoes. Many fungal species can infect and destroy mosquitoes at different stages, some of which are Culicinomyces, Lagenidium, and Coelomomyces which infect mosquito vectors, and have attracted a lot of researcher's attentions (Scholte et al., 2004). Several fungi are capable of attacking A. aegypti in some African regions, which can be used as economic, as well as environmentally friendly tools for controlling the pandemics of flavivirus in north and south America (Evans et al., 2018). Metarhizium anisopliae, an entomopathogenic fungus, has shown promising laboratory results in its infection of different mosquitoes. In addition, different concentrations of the fungus were tested for infectivity against Culex pipiens (fourth instar larvae), where the mosquito mortality rate reached up to 96% by increasing the concentration of fungal conidia. These results introduce Metarhizium anisopliae as a promising candidate for biocontrol of Culex pipiens, and thus, it deserves further studies and research focus (Benserradj and Mihoubi, 2014). Beauveria bassiana is another entomopathogenic fungus that was reported to decrease the endurance of mosquito vectors, and it required further virulence studies against mosquitoes. It has been reported that the isolate numbers of entomopathogenic fungi is 93, classified under six species. Those species: Isaria flavovirescens, I. fumosorosea, I. farinosa, M. anisopliae, B. bassiana, and Lecanicillium spp., can be a suitable potential source for biocontrol fungi against Aedes aegypti (Darbro et al., 2011). The genera of Coelomycidium and Coelomomyces, under the phylum Chytridiomycota, showed high mortality rate for haematophagous diptera, and had considerable attention as biocontrol agent against mosquitoes and black flies. It is worthy of note that the same phylum (Chytridiomycota) has several other entomopathogenic fungi that deserve attention (Tanada and Kaaya, 1993). The genera related to entomophthoraleans fungi that received most attention in the field of pest control are Conidiobolus, Neozygites, Entomophthora, and Erynia, while the least number of entomopathogenic fungi were reported in Basidiomycota (McCoy et al., 1988). On contrary, mitosporic fungi contains various prominent entomopathogenic species, and many of them are most frequently used as biocontrol agents for insect vectors (Pirali-Kheirabadi, 2012). Similarly, Microsporidia are known as one of the largest and highly versatile parasitic fungal group against mosquitoes, and it is highly possible that mosquitoes are considered hosts for at least one, if not more, parasitic microsporidia. This group also include many effective parasites for other eukaryotes and have a very specific system for penetrating host cells through their spores (Andreadis, 2007).
2.3. Virus
Mosquitoes are vulnerable for infection by too many viruses, which are mainly classified under four main groups (Huang et al., 2017), including: Baculoviruses, (genus: Nucleopolyhedrovirus, under family: Baculoviridae), viruses of cytoplasmic polyhedrosis (genus: Cyprovirus, under family: Reoviridae), densoviruses (genus: Brevidensovirus, under family: Parvoviridae), and iridoviruses (genus: Chloriridovirus, under family: lridoviridae) (Becnel and White, 2007, Federici, 1995). The number of entomopathogenic viruses that have known active insecticidal effects reach up to tens of thousands, in spite of the very limited number of its commercially available products, probably because of our limited knowledge or experiments testing the viral field effects (Pirali-Kheirabadi, 2012). There are two major viral pathogenic groups that infect mosquitoes, namely: occluded (baculoviruses and cyproviruses) and non-occluded (densoviruses and iridoviruses). Those are also divided into DNA viruses (Iridoviruses, Baculoviruses, and Densoviruses), and RNA viruses (cyproviruses) (Becnel, 2006). The weak transmission efficiency of pathogenic viruses to the mosquito larvae is a major obstacle that hindered the advancement of using them in mosquito control. However, recent researches have shown that incorporation of divalent cations has greatly facilitated the transmission of cyproviruses and baculoviruses to mosquito larvae. The presence of magnesium ions with cyproviruses or baculoviruses in the larvae feeding, increases the viral infection rate substantially, while the presence of calcium ions inhibits the infection rate (Becnel, 2006).
2.4. Larvivorous fishes
Using fishes to control mosquito's larval stage have started in nearly 1937. Raising larvivorous fish in water is a highly economical method of mosquito control, and it also has a sustained long-term effect as it is a natural and auto-reproducing enemy (by means of predation) for insects (Das et al., 2018). This method, however, seems to be effective and practical when the areas of mosquito breeding are fairly few, and can be identified easily (Chandra et al., 2008). Moreover, to achieve the highest mosquito control efficiency, an integrated biocontrol method must be followed (Al-Akel and Suliman, 2011). While several types of fishes can be used, it is best to use the native fish, as it is highly sustainable in its environment (Chandra et al., 2008). Gambusia affinis is one of the most fishes being used widely as a biocontrol agent (Wickramasinghe and Costa, 1986, Walton, 2007, Sarwar, 2015). This fish was basically native to southern USA and northern Mexico and is capable of living in warm water. However, after only few years, it was used in almost 60 countries for the purpose of controlling mosquito larvae. Those countries are located in Europe, the Pacific islands, India, Middle East, Africa, and South Asia (Mullen and Durden, 2002). Earlier studies have proven that mosquito fishes are the greatest enemies of the Anopheles stephensi and Aedes aegypti larvae. Their predation efficacy focuses mostly on the 3rd instar larvae (Singaravelu et al., 1997, Arijo et al., 2017). Bano and Serajuddin (2017) studied five fish species to evaluate their larvicidal efficiency; four of which were indigenous, and one was foreign. They found that these fishes have considerable larvicidal effect, with some differences in the level of efficiency. The highest predation success was found in the exotic (Gambusia affinis), followed by Esomus danricus, Rasbora daniconius, Trichogaster fasciata and Trichogaster lalius. Different results were found from another laboratory study, where Aphanius was found to be a better mosquito predator than Gambusia for 3rd and 4th instars and mosquito pupal stage. Opposite results, however, were found for the 1st and 2nd instar larvae. In addition, when more Aphanius fish exist in a shallow water, they attack more larvae (2nd instar) in water environment (Homski et al., 1994). Another study focused on collecting and identifying larvivorous fish, found that the efficient larvicidal fishes were only 22 out of 58 screened fish species (Rao, 2014). Aphanius dipar (a killifish type) has the capacity to reproduce, either naturally or artificially, so that it can maintain a reasonable population, which can be a major control for the disease-causing mosquitoes (Al-Akel and Suliman, 2011). There are other fish species that are not as aggressive as mosquito fish, however, they can be suitable for water polluted with organic contaminants and is also more adapted for hot environment than Gambusia affinis. One widely used example is Poecilia reticulate, a South American guppy fish (Mullen and Durden, 2002). There are other fish species suitable for controlling Aedes aegypti in water tanks, where they feed on mosquito larvae, some of which are: Ctenopharyngodon idella and Cyprinus carpio (carp fishes), and Clarias fuscus (edible catfish) (Mullen and Durden, 2002). Oreochromis niloticus (previously named Tilapia nilotica) is an example of larvivorous fishes that is not only used for mosquito control, but is also being raised for eating in Egypt, Sudan and Kenyan highlands. Whereas, there was a major adverse effect on biological control that occurred after introducing other tilapia species (Tilapia cyprinids) into the water system (Howard et al., 2007). Other research has shown that black molly was proven successful as mosquito control agent for all species, since almost all its stages has shown substantial efficacy against mosquito larvae (Sumithra et al., 2014). Out of the different species of black molly, Trichogaster trichopterus was the only one where both male and female were able to feed on mosquito larvae. On contrary, the males of Poecilia reticulate were much more effective against larvae, unlike the females of that species (Cavalcanti et al., 2007). Other species were verified as effective agents for mosquito control in southern Iran, such as Aphanius sp., Aphanius dispar and Gambusia holbrooki (Shahi et al., 2015). Some larvivorous fishes (e.g, Fundulus sp., and Aphanius dispar) are inhabitants of saltwater, hence, they can be used for mosquito biocontrol in marine water ecosystems.
Despite all the positives of using fish as biocontrol agent, one downside is that they are not suitable for mosquito control in small water bodies or pools that may frequently dry out. Even though, there are some fish species such as Cynolebias and Nothobranchius, known as annual fish, since their eggs can resist drought. Such aspect makes them more suitable for the previously mentioned water containers (Mullen and Durden, 2002). It is obvious that there is a number of advantages for mosquito fish biocontrol over the traditional chemical mosquito control. However, still there is a problem with using exotic mosquito fish as they can harm the native fishes and local habitats, therefore, a great deal of precaution should be taken in this regard (Mullen and Durden, 2002). In addition, such exotic fishes may also harm and diminish some important aquatic invertebrates (e.g., other zooplanktons and predators) (Bence, 1988). Consequently, only environmentally friendly mosquito fishes that do not adversely affect the local habitat and fish fauna can be used for mosquito larvae biocontrol.
2.5. Toxorhynchites mosquito
Toxorhynchites is a mosquito genus known for the capability of its larvae to feed on species of mosquito and other aquatic organisms living in either natural or artificial habitats. Since mosquito species living in such habitats comprise most of the medically significant mosquitoes, Toxorhynchites mosquito has been considered as promising biological control agent for mosquitoes living in variable environments (Collins and Blackwell, 2000, Focks, 2007).
First trials to use Toxorhynchites were as early as the beginning of the 19th century, with no much success at the time (Collins and Blackwell, 2000). Toxorhynchites mosquitoes feed on other mosquito larvae with a number reaching up to 400 larvae throughout their larval stage, with higher numbers and efficiency in containers of a small size, and also feeding usually occurs as turn cannibalistic type (Goettle and Adler, 2005). When using carnivorous larvae in combination with harmless adults, more biocontrol efficiency is achieved, however, there is no enough research showing sustainable larval control using Toxorhynchites. Despite of that, Toxorhynchites was used as biological control agent successfully in countries like: United States, Japan, the Caribbean and Southeast Asia (Goettle and Adler, 2005). It was reported that T. splendens is one of the most successful species of Toxorhynchites biocontrol agents and was used as part of an integrated mosquito biocontrol system. T. splendens can eat 20–25 larvae of A. aegypti daily. In order to test this species against Culex quinquefasciatus, A. albopictus and A. aegypti it was used in their water environment, where mosquitoes were almost totally destroyed in less than four days (Pantuwatana et al., 1979).
2.6. Nematodes
Nematodes are classified as parasitic insects that are either obligate or facultative in their feeding style. There are five orders under the Phylum nematode, and 14 obligative parasitic families under those orders. Out of those, the only family found in the mosquito natural habitat is Mermithidae (Platzer, 1981, Pirali-Kheirabadi, 2012). Several nematodes have been reported to be suitable candidates for biocontrol of insects. Beside Mermithidae, there are eight other families of nematode having species that can parasite, kill, or affect host growth. The infection style of nematodes is either by entering during insect feeding, penetrating throughout the cuticle, or entering via anus or spiracles (Pirali-Kheirabadi, 2012). Those families are: Allantonematidae, Diplogasteridae, Rhabditidae, Sphaerulariidae, Heterorhabditidae, Neotylenchidae, Steinernematidae and Tetradonematidae (Petersen, 1985). Mermithids has the largest nematode species used for controlling mosquito larvae. The main host of Mermithids is insects (feeding as obligate parasites), but still, they also attack spiders, crustaceans, mollusks, leeches, and earthworms. As they cause the infection, they usually kill the host, and they can be specific for single species or a hole family of insects. This family has gained much interest since they cause so little, if any ecological hazard, they are not threatened by other competitive beneficial organisms, and a long-term existence for its individuals in the target control site can be achieved based on the conditions of inoculation (Petersen, 1985). Even though Mermithids have been reported to infect more than 60 mosquito species, they still did not attract as much attention. These nematodes can be highly promising candidates as biocontrol agent since they are specific to their host, can kill the host at certain growth stage, can be handled easily, highly effective parasites, reproduce in a high rate, and are also swimming efficiently (Petersen, 1973).
3. Advantages of biocontrol
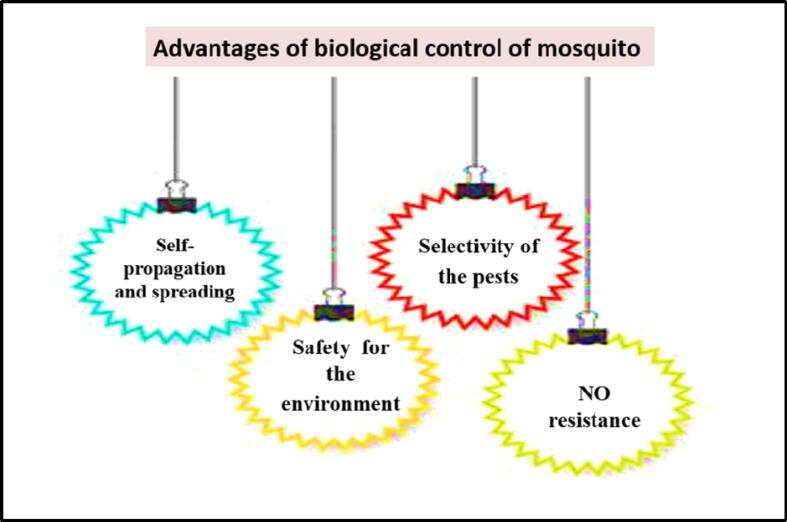
Biocontrol of pest insects has several advantages over the traditional chemical pest control methods. First and most important is the safety for the environment, since chemical pesticides are major pollutants (Kok and Kok, 1999). Second advantage is the selectivity of the pests, as the non-target insects will not be deteriorated, as is the case with the traditional chemical pesticides (Tebit, 2017). In fact, there was almost no side effects for using biological control throughout the past years (Emden 2004). The principal advantage is selectivity, as it sustains the balance between different living organisms in the agricultural environment, since any harm to the non-target organism can minimize the numbers of natural pest enemies (Kok and Kok, 1999). Another benefit for the biocontrol approach is the ability for self-propagation and spreading through the environment which is very important from the economic point of view (Reichelderfer, 1981). Additional benefit for the biocontrol approach is the difficulty, and probably incapability of the pest to develop biocontrol resistance (Tebit, 2017). On the other hand, the target insect might be able to develop a defense mechanism to escape the attack by the biocontrol agent. It might be possible that an effective biocontrol, may push the pest for a strong selection to develop a scape or tolerance mechanism against the attack of the biocontrol agent (Holt and Hochberg, 1997). Luckily, there is no evidence to support this scenario as of yet, especially for the large biocontrol agents such as mosquito fish. The advantage of the cost effectiveness is mainly due to its sustained propagation. Thus, if a biocontrol agent is established in a certain area, the population of the target pest would be minimized to a threshold of acceptable level for a long period of time (Kok and Kok, 1999). In addition to the previous advantages, a biocontrol agent can start with a small number and then grow to a fairly high population to develop an extended pest control over a wide distance. Taking into account the cost of preparing the biocontrol agent, it is generally more economic than the cost of chemical pesticide production (Reichelderfer, 1981). Advantages of the usage of biological control of mosquito are summarized in Fig. 2.
Fig. 2.
Advantages of biological control pf mosquito.
4. Disadvantages of biocontrol
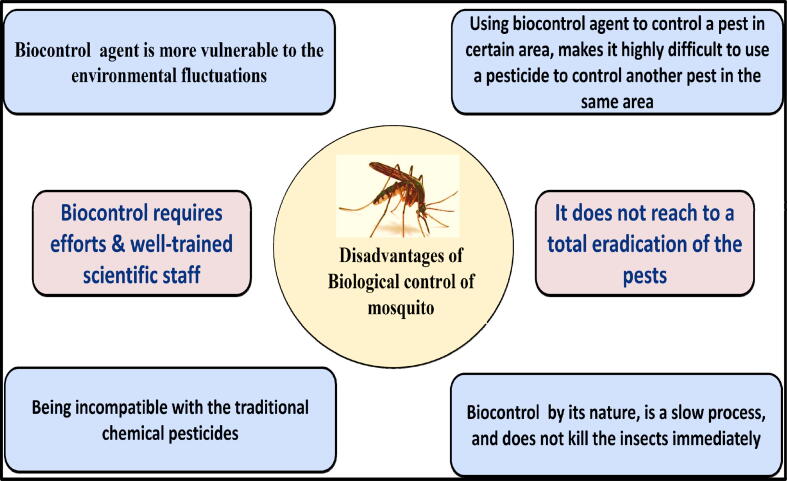
Disadvantages of the usage of biological control of mosquito are summarized in Fig. 3.
Fig. 3.
Disadvantages of biological control of mosquito.
The major disadvantage of biological control approach is the possibility of income instability for the agriculture producer. Besides, the biocontrol agent is more vulnerable to the environmental fluctuations compared with the insecticidal methods, which in turn affects the efficacy of the method. Also, using chemical pesticides is obviously easier than the biocontrol approach. Being incompatible with the traditional chemical pesticides, is another main problem. Emden, (2004) stated that using biocontrol agent to control a pest in certain area, makes it highly difficult to use a pesticide to control another pest in the same area. In addition, biocontrol by its nature, is a slow process, and does not kill the insects immediately. It might take days, or more likely few weeks, in order to diminish mosquito populations to an acceptable level (Mullen and Durden, 2002). Moreover, natural competition is highly dependent on the environment, which makes it sometimes unpredictable (Emden, 2004). To get desirable results from using biocontrol approach in a new environment, one should apply much research because of the limitations in such environment. It is also known that it does not reach to a total eradication of the pests and is acceptable to minimize their numbers to the Economic Injury Level (EIL) (Tebit, 2017). Thus, biocontrol systems can be used with the vegetables and fruits, even though the incomplete pest extermination is highly undesirable, since the lowest level of damage of the product shape is undesirable by the agricultural producer (Reichelderfer, 1981). Also, one of the advantages that was stated before, which is selectivity, could probably be a disadvantage, as the biocontrol agent specificity for a single pest, could allow another pest to flourish and cause harm (Reichelderfer, 1981). In addition, biocontrol requires well-trained scientific staff, which would raise the cost of its production (Tebit, 2017). Another drawback is the inconsistency of production batches. This would be because applying high-quality measures for rearing biocontrol agent, raises the production cost. Such higher cost pushes some mass-production companies to ease on some production measures and sacrifice some level of quality (Lenteren, 2003). Biocontrol is most appropriate for using with exotic pest that is closely related to the native beneficial species (Kok and Kok, 1999).
5. Conclusions and future directions
The different approaches used for malaria control are either targeting the plasmodium development inside the mosquito or repress the mosquito as the vector for the plasmodium. Nonetheless, some factors related to the strategies of chemical control of vector, such as the incomplete infrastructure, lack of resources, and imperfect management plans, reduce the efficiency of malaria control. Besides, chemical control of mosquito fails because of the environmental variations, and the different behaviors of some mosquito species, for example, the building up of insecticidal resistance in some mosquito strains, and the subsequent pest re-emergence. The previously mentioned reasons made it necessary to develop different strategy for vector control. Biological control was a promising alternative, which has a minimal harmful side effect. Despite being relatively difficult to apply and maintain as a strategy for pest management, biocontrol approach has several advantages over the traditional chemical pesticide approach. The most outstanding advantage is the environmental safety, as it does not release any pollutants to the environment. Consequently, further research is required to discover more potential candidates as biocontrol agents to minimize the drawbacks of biocontrol and to further extend its advantages. The following targets can be suggested to minimize the disadvantages of biocontrol and to maximize the advantages. First, is to use indigenous biocontrol agents in order to avoid harming the native beneficial organisms. Second, is to use biocontrol agents that have efficient reproduction rate and high adaptation level to the surrounding environment. Third, is to rear and release large quantities of highly efficient biocontrol agents to achieve a high killing rate and mosquito reduction. Fourth, is to search for biocontrol agents that has tolerance to the traditional pesticides to allow for using both at the same time.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors extend their appreciation to the deanship of Scientific Research at King Khalid University, Abha KSA for supporting this work under grant number (R.G.P.2/61/42).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Abo Ghanima M.M., Shukry M., Swelum A.A., Taha A.E., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poultry Sci. 2021:101584. doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F.M. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poultry Sci. 2021;100(6):101172. doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Akel A.S., Suliman E.M. Biological control agent for mosquito larvae: Review on the killifish, Aphanius dispar dispar (Rüppel, 1829) Afr. J. Biotechnol. 2011;10:8683–8688. [Google Scholar]
- Andreadis T.G. Microsporidian parasites of mosquitoes. J. Am. Mosq. Con. Assoc. 2007;23(sp2):3–29. doi: 10.2987/8756-971X(2007)23[3:MPOM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Arijo A., Sethar A., Ahmad L., Muhammad F. Biological control of mosquito larvae using edible fish. Int. J. Innov. Appl. res. 2017;5:1–6. [Google Scholar]
- Asia, 2007. Anopheline species complexes in South and South-east Asia, vol. 57 (World Health Organization).
- Attia M.M., Salem H.M. Morphological and molecular characterization of Pseudolynchia canariensis (Diptera: Hippoboscidae) infesting domestic pigeons. Int. J. Trop. Insect Sci. 2021 doi: 10.1007/s42690-021-00597-2. [DOI] [Google Scholar]
- Attia M.M., Yehia N., Soliman M.M., Shukry M., El-Saadony M.T., Salem H.M. Evaluation of the antiparasitic activity of the chitosan-silver nanocomposites in the treatment of experimentally infested pigeons with Pseudolynchia canariensis. Saudi J. Biol. Sci. 2022;29(3):1644–1652. doi: 10.1016/j.sjbs.2021.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P.C.E. The effect of water temperature on the functional-response of the water stick insect Ranatra dispar (Heteroptera, nepidae) Aust. J. Ecol. 1989;14(4):381–386. [Google Scholar]
- Bano F., Serajuddin M. Comparative study of larvicidal efficacy of four indigenous fish with an exotic top water minnow, Gambusia affinis. J. Ecophysiol. Occup. Health. 2017;16:7–12. [Google Scholar]
- Becnel J.J. Transmission of viruses to mosquito larvae mediated by divalent cations. J. Invertebr. Pathol. 2006;92(3):141–145. doi: 10.1016/j.jip.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Becnel J.J., White S.E. Mosquito pathogenic viruses—the last 20 years. J. Am. Mosq. Control Assoc. 2007;23(sp2):36–49. doi: 10.2987/8756-971X(2007)23[36:MPVLY]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bence J.R. Indirect effects and biological control of mosquitoes by mosquitofish. J. Appl. Ecol. 1988;25(2):505. doi: 10.2307/2403840. [DOI] [Google Scholar]
- Benelli G., Jeffries C., Walker T. Biological control of mosquito vectors: past, present, and future. Insects. 2016;7(4):52. doi: 10.3390/insects7040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli G., Jeffries C., Walker T. Biological control of mosquito vectors: past, present, and future. Insects. 2016;7(4):52. doi: 10.3390/insects7040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benserradj O., Mihoubi I. Larvicidal activity of entomopathogenic fungi Metarhizium anisopliae against mosquito larvae in Algeria. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:54–62. [Google Scholar]
- Bowatte G., Perera P., Senevirathne G., Meegaskumbura S., Meegaskumbura M. Tadpoles as denguemosquito (Aedes aegypti) egg predators. Biol. Control. 2013;67(3):469–474. [Google Scholar]
- Brodman R., Dorton R. The effectiveness of pond-breeding salamanders as agents of larval mosquito control. J. Freshw. Ecol. 2006;21(3):467–474. [Google Scholar]
- Cavalcanti L.P.D.G., Pontes R.J.S., Regazzi A.C.F., Júnior P., Frutuoso R.L., Sousa E.P., Dantas Filho F.F., Lima J.W.D.O. Efficacy of fish as predators of Aedes aegypti larvae, under laboratory conditions. Revista de saude publica. 2007;41 doi: 10.1590/s0034-89102006005000041. [DOI] [PubMed] [Google Scholar]
- Chandra G., Bhattacharjee I., Chatterjee S., Ghosh A. Mosquito control by larvivorous fish. Indian J. Med. Res. 2008;127:13. [PubMed] [Google Scholar]
- Chandra G., Anupam Ghosh A.G., Indranil Bhattacharjee I.B., Ghosh S.K. In: Biological and Environmental Control of Disease Vectors. Cameron M.M., Lorenz L.M., editors. CABI; Wallingford: 2013. Use of larvivorous fish in biological and environmental control of disease vectors; pp. 25–41. [DOI] [Google Scholar]
- Collins L.E., Blackwell A. The biology of Toxorhynchites mosquitoes and their potential as biocontrol'agents. Biol. Control. News Inform. 2000;21:105–116. [Google Scholar]
- Darbro J.M., Graham R.I., Kay B.H., Ryan P.A., Thomas M.B. Evaluation of entomopathogenic fungi as potential biological control agents of the dengue mosquito, Aedes aegypti (Diptera: Culicidae) Biocontrol. Sci. Technol. 2011;21(9):1027–1047. [Google Scholar]
- Das M.K., Prasad R.N. Evaluation of mosquito fish Gambusia affinis in the control of mosquito breeding in rice fields. Indian J. Malariol. 1991;28:171–177. [PubMed] [Google Scholar]
- Das ManojKumar, Rao M.K., Kulsreshtha A.K. Native larvivorous fish diversity as a biological control agent against mosquito larvae in an endemic malarious region of Ranchi district in Jharkhand, India. J. Vector Borne Dis. 2018;55(1):34. doi: 10.4103/0972-9062.234624. [DOI] [PubMed] [Google Scholar]
- Desoky E.S.M., Merwad A.R.M., Semida W.M., Ibrahim S.A., El-Saadony M.T., Rady M.M. Heavy metals-resistant bacteria (HM-RB): Potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol. Environ. Saf. 2020;198(15):110685. doi: 10.1016/j.ecoenv.2020.110685. [DOI] [PubMed] [Google Scholar]
- El-Ashry R.M., El-Saadony M.T., El-Sobki A.E.A., El-Tahan A.M., Al-Otaibi S., El-Shehawi A.M., Saad A.M., Elshaer N. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J. Biol. Sci. 2022;29(2):920–932. doi: 10.1016/j.sjbs.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O.S., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20(1):762–776. doi: 10.1080/1828051X.2021.1926346. [DOI] [Google Scholar]
- El-Saadony M.T., Khalil O.S., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28(8):4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar S.F., El-Tarabily K.A., Salama A. Selenium nanoparticles, from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi, as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28(12):6782–6784. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A.M., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28(9):5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emden V.H.F. Cambridge University Press; 2004. Pest and Vector Control. [Google Scholar]
- Evans H.C., Elliot S.L., Barreto R.W. Entomopathogenic fungi and their potential for the management of Aedes aegypti (Diptera: Culicidae) in the Americas. Memórias do Instituto Oswaldo Cruz. 2018;113(3):206–214. doi: 10.1590/0074-02760170369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici B.A. The future of microbial insecticides as vector control agents. J. Am. Mosq. Control Assoc. 1995;11:260–268. [PubMed] [Google Scholar]
- Focks D.A. Toxorhynchites as biocontrol agents. J. Am. Mosq. Control Assoc. 2007;23:118–128. doi: 10.2987/8756-971X(2007)23[118:TABA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Focks D.A., Sackett S.R., Dame D.A., Bailey D.L. Effect of weekly releases of Toxorhynchites amboinensis (Doleschall) on Aedes aegypti (L.) (Diptera: Culicidae) in New Orleans, Louisiana. J. Econ. Entomol. 1985;78:622–626. doi: 10.1093/jee/78.3.622. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Mandal S., Bhattacharjee I., Chandra G. Biological control of vector mosquitoes by some common exotic fish predators. Turk. J. Biol. 2005;29:167–171. [Google Scholar]
- Goettle B.J., Adler P.H. South Carolina State Documents Depository; 2005. Elephant (or Treehole) Predatory mosquito. [Google Scholar]
- Holt R.D., Hochberg M.E. When is biological control evolutionarily stable (or is it)? Ecology. 1997;78(6):1673–1683. [Google Scholar]
- Homski D., Goren M., Gasith A. Comparative evaluation of the larvivorous fish Gambusia affinis and Aphanius dispar as mosquito control agents. Hydrobiologia. 1994;284(2):137–146. [Google Scholar]
- Howard A.F., Zhou G., Omlin F.X. Malaria mosquito control using edible fish in western Kenya: preliminary findings of a controlled study. BMC Public Health. 2007;7:199. doi: 10.1186/1471-2458-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.J., Higgs S., Vanlandingham D. Biological control strategies for mosquito vectors of arboviruses. Insects. 2017;8:21. doi: 10.3390/insects8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingabire C.M., Hakizimana E., Rulisa A., Kateera F., Van Den Borne B., Muvunyi C.M., Mutesa L., Van Vugt M., Koenraadt C.J.M., Takken W., Alaii J. Communitybased biological control of malaria mosquitoes using Bacillus thuringiensis var. israelensis (Bti) in Rwanda: community awareness, acceptance and participation. Malar. J. 2017;16(1) doi: 10.1186/s12936-017-2046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamareddine L. The biological control of the malaria vector. Toxins. 2012;4(9):748–767. doi: 10.3390/toxins4090748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendie F.A. Potential biological control agents against mosquito vector in the case of larvae stage: a review. World News Nat. Sci. 2020;28:34–50. [Google Scholar]
- Kok L.T., Kok V. Cabi Publishing; 1999. Biological control for the public. [Google Scholar]
- Land, M., Miljand, M., 2014. Biological control of mosquitoes using Bacillus thuringiensis israelensis: a pilot study of effects on target organisms, non-target organisms and humans. Mistra EviEM, Stockholm, Sweden (www.eviem.se).
- Lenteren J.C.V., editor. Quality control and production of biological control agents: theory and testing procedures. CABI; Wallingford: 2003. [Google Scholar]
- Louca V., Lucas M.C., Green C., Majambere S., Fillinger U., Lindsay S.W. Role of fish as predators of mosquito larvae on the floodplain of the Gambia river. J. Med. Entomol. 2009;46:546–556. doi: 10.1603/033.046.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar D., Ridgway N. An introduction to beneficial natural enemies and their use in pest management. N. Cen. Regional Pub. 1993;48l:36. [Google Scholar]
- McCoy, G., Samson, R., Boucias, D., 1988. Entomogenous fungi in ‘CRC Handbook of Natural Pesticides, Part A. Entomogenous Protozoa and fungi’ (CM Ignoffo, ed).
- Moraga E.Q., Carrasco-Diaz J.-A., Santiago-Alvarez C. Insecticidal and antifeedant activities of proteins secreted by entomopathogenic fungi against Spodoptera littoralis (Lep., Noctuidae) J. Appl. Entomol. 2006;130(8):442–452. [Google Scholar]
- Mullen G.R., Durden L.A. Academic Press; Elsevier: 2002. Medical and Veterinary Entomology. [Google Scholar]
- Murray C.JL., Rosenfeld L.C., Lim S.S., Andrews K.G., Foreman K.J., Haring D., Fullman N., Naghavi M., Lozano R., Lopez A.D. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379(9814):413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- Murugan K., Priyanka V., Dinesh D., Madhiyazhagan P., Panneerselvam C., Subramaniam J., Suresh U., Chandramohan B., Roni M., Nicoletti M., Alarfaj A.A., Higuchi A., Munusamy M.A., Khater H.F., Messing R.H., Benelli G. Predation by Asian bullfrog tadpoles, Hoplobatrachus tigerinus, against the dengue vector, Aedes aegypti, in an aquatic environment treated with mosquitocidal nanoparticles. Parasitol. Res. 2015;114(10):3601–3610. doi: 10.1007/s00436-015-4582-0. [DOI] [PubMed] [Google Scholar]
- Odalo J.O., Omolo M.O., Malebo H., Angira J., Njeru P.M., Ndiege I.O., Hassanali A. Repellency of essential oils of some plants from the Kenyan coast against Anopheles gambiae. Acta tropica. 2005;95(3):210–218. doi: 10.1016/j.actatropica.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Pantuwatana, S., Premabutr, P., Bhumiratana, A., 1979. Biological control of mosquitoes by Toxorhynchites splendens and Bacillus thuringiensis.
- Petersen J.J. Role of mermithid nematodes in biological control of mosquitoes. Exp. Parasitol. 1973;33(2):239–247. doi: 10.1016/0014-4894(73)90030-1. [DOI] [PubMed] [Google Scholar]
- Petersen J.J. Nematodes as biological control agents: Part I. Mermithidae. Adv. Parasitol. 1985:307–344. doi: 10.1016/s0065-308x(08)60565-5. [DOI] [PubMed] [Google Scholar]
- Pirali-Kheirabadi K. Parasitology. InTech; 2012. [DOI] [Google Scholar]
- Platzer E. Biological control of mosquitoes with mermithids. J. Nematol. 1981;13:257. [PMC free article] [PubMed] [Google Scholar]
- Poopathi S. Current trends in the control of mosquito vectors by means of biological larvicides. J. Biofertil. Biopestic. 2012;3:1–14. [Google Scholar]
- Poopathi S., Thirugnanasambantham K., Mani C., Ragul K., Sundarapandian S. Isolation of mosquitocidal bacteria (Bacillus thuringiensis, B. sphaericus and B. cereus) from excreta of arid birds. Indian J. Exp. Biol. 2014;52(7):739–747. [PubMed] [Google Scholar]
- Porter A.G., Davidson E.W., Liu J.W. Mosquitocidal toxins of bacilli and their genetic manipulation for effective biological control of mosquitoes. Microbiol. Mol. Biol. Rev. 1993;57(4):838–861. doi: 10.1128/mr.57.4.838-861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Lepe, M., Ramírez-Suero, M., 2012. Biological control of mosquito larvae by Bacillus thuringiensis subsp. israelensis. In: Insecticides-Pest Engineering. Intech Open. 10.5772/29139. [DOI]
- Rao R. A study on larvivorous fish species efficacy of lower Manair dam at Karimnagar, Andhra Pradesh, India. Adv. Appl. Sci. Res. 2014;133–143 [Google Scholar]
- Reichelderfer K.H. Economic feasibility of biological control of crop pests. Biol. Control Crop Prod. 1981;12:403–418. [Google Scholar]
- Reyaz A.L.N., Balakrishnan N., Udayasuriyan V. A novel Bacillus thuringiensis isolate toxic to cotton pink bollworm (Pectinophora gossypiella Saunders) Microb. Pathog. 2021;150:104671. doi: 10.1016/j.micpath.2020.104671. [DOI] [PubMed] [Google Scholar]
- Saad A.M., Bardisi E.A., Salama A., Sitohy M.Z. Transfer of anthocyanin accumulating delila and rosea1 genes from the transgenic tomato micro-tom cultivar to moneymaker cultivar by conventional breeding. J Agric. Food Chem. 2020;68(39):10741–10749. doi: 10.1021/acs.jafc.0c03307. [DOI] [PubMed] [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A.M., Taha A.E., Taha T.F., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021;28(10):5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., El-Saadony M.T., Mohamed A.S., Ahmed A.I., Sitohy M.Z. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour-based noodles. Int. J. Food Sci. Technol. 2021;2021(56):3255–3268. [Google Scholar]
- Saad A.M., Mohamed A.S., Ramadan M.F. Storage and heat processing affect flavors of cucumber juice enriched with plant extracts. Int. J. Vegetable Sci. 2020:1–11. [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT - Food Sci. Technol. 2021;148:111668. doi: 10.1016/j.lwt.2021.111668. [DOI] [Google Scholar]
- Saad A.M., Osman A.O.M., Mohamed A.S., Ramadan M.F. Enzymatic hydrolysis of Phaseolus vulgaris protein isolate: characterization of hydrolysates and effect on the quality of minced beef during cold storage. Int. J. Pept. Res. Ther. 2020;26(1):567–577. [Google Scholar]
- Saad A.M., Sitohy M.Z., Ahmed A.I., Rabie N.A., Amin S.A., Aboelenin S.M., Soliman M.M., El-Saadony M.T. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules. 2021;26(15):4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Yehia N., Al-Otaibi S., El-Shehawi A.M., Elrys A.A.M.E., El-Saadony M.T., Attia M.M. The prevalence and intensity of external parasites in domestic pigeons (Columba livia domestica) in Egypt with special reference to the role of deltamethrin as insecticidal agent. Saudi J. Biol. Sci. 2022;29(3):1825–1831. doi: 10.1016/j.sjbs.2021.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar M. Reducing dengue fever through biological control of disease carrier Aedes Mosquitoes (Diptera: Culicidae) Int. J. Prev. Med. 2015;1:161–166. [Google Scholar]
- Schaper S. Evaluation of Costa Rican copepods (Crustacea: Eudecapoda) for larval Aedes aegypti control with special reference to Mesocyclops thermocyclopoides. J. Am. Mosq. Control Assoc. 1999;15:510–519. [PubMed] [Google Scholar]
- Scholte E.-J., Knols B.G.J., Samson R.A., Takken W. Entomopathogenic fungi for mosquito control: a review. J. Insect Sci. 2004;4(19):1–24. doi: 10.1093/jis/4.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaalan E.-S., Canyon D.V., Muller R., Younes M.W.F., Abdel-Wahab H., Mansour A.-H. A mosquito predator survey in Townsville, Australia, and an assessment of Diplonychus sp. and Anisops sp. predatorial capacity against Culex annulirostris mosquito immatures. J. Vector Ecol. 2007;32(1):16–21. doi: 10.3376/1081-1710(2007)32[16:ampsit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Shahi M., Kamrani E., Salehi M., Habibi R., Hanafi-Bojd A.A. Native larvivorous fish in an endemic malarious area of southern Iran, a biological alternative factor for chemical larvicides in malaria control program. Iran. J. Public Health. 2015;44:1544. [PMC free article] [PubMed] [Google Scholar]
- Singaravelu G., Mahalingam S., Bharathi K.J. Predatory efficiency of larvivorous fish, Gambusia affinis on the mosquito larvae of Aedes aegypti and Anopheles stephensi. Curr. Sci. 1997;72:512–514. [Google Scholar]
- Singh R.K., Dhiman R.C., Singh S.P. Laboratory studies on the predatory potential of dragon-fly Nymphs on mosquito larvae. J. Commun. Dis. 2003;35:96–101. [PubMed] [Google Scholar]
- Soliman S.M., Attia M.M., Al-Harbi M.S., Saad A.M., El-Saadony M.T., Salem H.M. Low host specificity of Hippobosca equina infestation in different domestic animals and pigeon. Saudi J. Biol. Sci. 2022;29(4):2112–2120. doi: 10.1016/j.sjbs.2021.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan W.A., Evenhuis N.L. Biology of Toxorhynchites. Annu. Rev. Entomol. 1981;26(1):159–181. [Google Scholar]
- Subramaniam J., Murugan K., Panneerselvam C., Kovendan K., Madhiyazhagan P., Dinesh D., Kumar P.M., Chandramohan B., Suresh U., Rajaganesh R., Alsalhi M.S., Devanesan S., Nicoletti M., Canale A., Benelli G. Multipurpose effectiveness of Couroupita guianensis-synthesized gold nanoparticles: High antiplasmodial potential, field efficacy against malaria vectors and synergy with Aplocheilus lineatus predators. Environ. Sci. Pollut. Res. Int. 2016;23(8):7543–7558. doi: 10.1007/s11356-015-6007-0. [DOI] [PubMed] [Google Scholar]
- Subramaniam J., Murugan K., Panneerselvam C., Kovendan K., Madhiyazhagan P., Kumar P.M., Dinesh D., Chandramohan B., Suresh U., Nicoletti M., Higuchi A., Hwang J.-S., Kumar S., Alarfaj A.A., Munusamy M.A., Messing R.H., Benelli G. Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: towards an integrative approach? Environ. Sci. Pollut. Res. Int. 2015;22(24):20067–20083. doi: 10.1007/s11356-015-5253-5. [DOI] [PubMed] [Google Scholar]
- Sumithra V., Janakiraman A., Altaff K. Biocontrol of mosquito larvae through the black molly, Poecilia sphenops. Int. J. Pure Appl. Zool. 2014;2:270–274. [Google Scholar]
- Swelum A.A., Shafi M.E., Albaqami N.M., El-Saadony M.T., Elsify A., Abdo M., Mohamed E. COVID-19 in human, animal, and environment: a review. Front. Vet. Sci. 2020;7:578. doi: 10.3389/fvets.2020.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanada Y., Kaaya H.K. Academic Press; San Diego, USA: 1993. Insect Patology. [Google Scholar]
- Tebit E.K. Natural Remedies in the Fight Against Parasites. INTECH Open Science; 2017. Biological control of parasites; pp. 24–58. [Google Scholar]
- Timmins S.M. Science and Research Directorate, Department of Conservation; 1988. Biological Control in Protected Natural Areas. [Google Scholar]
- Vail P.V., Coulson W.C., Kauffman D. History of biological control programs in the United States department of agriculture. Am. Entomol. 2001;47:24–49. [Google Scholar]
- Van dam A.R., Walton W.E. Comparison of mosquito control provided by the Arroyo chub (Gila orcutti) and the mosquitofish (Gambusia affinis) J. Am. Mosq. Control Assoc. 2007;23(4):430–441. doi: 10.2987/5620.1. [DOI] [PubMed] [Google Scholar]
- Vu S.N., Nguyen T.Y., Kay B.H., Marten G.G., Reid J.W. Eradication of Aedes aegypti from a village in Vietnam, using copepods and community participation. Am. J. Trop. Med. Hyg. 1998;59:657–660. doi: 10.4269/ajtmh.1998.59.657. [DOI] [PubMed] [Google Scholar]
- Walton W.E. Larvivorous fish including Gambusia. J. Am. Mosq. Control Assoc. 2007;23(sp2):184–220. doi: 10.2987/8756-971X(2007)23[184:LFIG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe M.B., Costa H.H. Mosquito control with larvivorous fish. Parasitol Today. 1986;2(8):228–230. doi: 10.1016/0169-4758(86)90089-x. [DOI] [PubMed] [Google Scholar]