Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) occurs after infection with SARS-CoV-2 and its incidence is likely to depend on multiple factors, including the variant of the preceding SARS-CoV-2 infection and vaccine effectiveness. We aimed to estimate the incidence of MIS-C, and describe the clinical phenotype, following the delta variant of SARS-CoV-2 (B.1.617.2 and sublineages) according to vaccination status. We aimed to compare the incidence and clinical phenotype of MIS-C from our cohort during the pre-delta era.
Methods
This prospective, population-based cohort study included patients aged 0–17 years hospitalised with MIS-C in Denmark, according to the US Centers for Disease Control and Prevention case definition, from Aug 1, 2021, to Feb 1, 2022, a period dominated by the delta variant. We identified MIS-C cases via a nationwide research collaboration involving real-time data collection from all 18 paediatric departments. Aggregated number of SARS-CoV-2 infections by vaccination status was obtained from the Danish COVID-19 surveillance registries. The incidence of MIS-C was calculated using the estimated number of infected individuals by vaccination status. We calculated the incidence of MIS-C per 1 000 000 vaccinated and unvaccinated person-years, and estimated vaccine effectiveness as 1–incidence rate ratio using Poisson regression. Incidence and phenotype of MIS-C were compared with MIS-C cases from the first year of the pandemic. This study is registered at ClinicalTrials.gov, NCT05186597.
Findings
We identified 51 MIS-C cases among unvaccinated individuals and one in a fully vaccinated adolescent. The incidence of MIS-C was one in 3400 unvaccinated individuals (95% CI 2600–4600) with the delta variant and one in 9900 vaccinated individuals (95% CI 1800–390 000) with breakthrough infection. The estimated vaccine effectiveness against MIS-C after the delta variant was 94% (95% CI 55–99; p=0·0061) in individuals aged 5–17 years. The clinical phenotype during the delta wave was comparable to the pre-delta era.
Interpretation
We found the incidence and phenotype of MIS-C in unvaccinated children during the delta wave to be similar to the incidence during the first year of the pandemic. We found vaccine effectiveness to be high against MIS-C, which we suggest was due to protection from infection and, possibly, a decreased incidence of MIS-C after breakthrough infection. Knowledge of the incidence of MIS-C after different SARS-CoV-2 variants and the effect of vaccination might contribute to the elucidation of the extent to which MIS-C is a vaccine-preventable disease.
Funding
National Ministry of Higher Education and Science and Innovation Fund Denmark.
Introduction
Multisystem inflammatory syndrome in children (MIS-C) is a rare, severe, post-infectious hyperinflammatory condition that generally occurs 2–6 weeks after an infection with SARS-CoV-2.1, 2 MIS-C is reported to have occurred in approximately one of 3000–4000 SARS-CoV-2 infections in unvaccinated children and adolescents during the pre-delta (B.1.617.2) SARS-CoV-2 waves.3, 4
The incidence of MIS-C is likely to depend on multiple factors, including the risk after infection with each SARS-CoV-2 variant, COVID-19 vaccine effectiveness against SARS-CoV-2 infection, and COVID-19 vaccine effectiveness against MIS-C after breakthrough infection. Studies from the USA and France have suggested a high vaccine effectiveness against MIS-C in adolescents during the delta wave;5, 6 however, whether these findings are due to vaccine protection from infection, a reduced risk of MIS-C after breakthrough infections, or both, is unclear.5, 6 Furthermore, whether the delta variant implies the same risk of MIS-C as the pre-delta variants has not been explored.
In Denmark, the delta variant caused the majority of SARS-CoV-2 infections from July to December, 2021.7 The BNT162b2 (Pfizer–BioNTech) COVID-19 vaccine was recommended to children and adolescents immediately after the vaccine was approved by the European Medicines Agency on May 15 (16–17 years), July 15 (12–15 years), and Nov 25, 2021 (5–11 years).8 Denmark has comprehensive population registries and a national COVID-19 surveillance system holding nationwide test results from both public and private providers, as well as details of vaccination status for each individual using the unique, personal civil registration number.9 A nationwide paediatric COVID-19 research project was established early on in the pandemic, with prospective real-time data collection of detailed phenotypes of COVID-19-associated diseases, including a cohort of cases of MIS-C.3, 10, 11
Research in context.
Evidence before this study
We searched MEDLINE on Feb 1, 2022, for publications investigating the incidence of multisystem inflammatory syndrome in children (MIS-C) after infection with the delta (B.1.617.2) variant of SARS-CoV-2 and COVID-19 vaccine effectiveness against MIS-C from Feb 1, 2020 to Feb 1, 2022. The search terms used for MIS-C incidence were “(multisystem inflammatory syndrome in children OR paediatric multisystem inflammatory syndrome temporally associated with COVID-19)” and “(incidence OR risk)”. This search yielded two papers investigating the incidence of MIS-C among infected individuals during the first surges of the pandemic dominated by the wildtype strain, one from the USA and one from Denmark. No studies were identified exploring the incidences of MIS-C during the alpha (B.1.1.7) or delta surges. The search terms for vaccine effectiveness against MIS-C were “(multisystem inflammatory syndrome in children OR paediatric multisystem inflammatory syndrome temporally associated with COVID-19)” and “(vaccine effectiveness OR vaccination)”. This search yielded one study from France and one study from the USA, both investigating vaccine effectiveness in case–control designs, but were not population-based.
Added value of this study
To our knowledge, this is the first national population-based study to investigate the incidence and phenotype of MIS-C after infection with the delta variant of SARS-CoV-2 by vaccination status. In Denmark, SARS-CoV-2 vaccination was recommended for children aged 5–17 years immediately after the vaccine approvals by the European Medicines Agency. A nationwide research collaboration with real-time enrolment of cases allowed us to identify and retrieve details of MIS-C cases, and the Danish COVID-19 surveillance database provided us with aggregated numbers of SARS-CoV-2 infections by vaccination status. In the study period, Danish school children were recommended weekly antigen screening tests. We found the incidence of MIS-C after infection with the delta variant to be similar to previous variants.
Implications of all the available evidence
Our population-based study confirms the results of two case–control studies from France and the USA, finding a high vaccine effectiveness against MIS-C during the delta era. We suggest the vaccine effectiveness against MIS-C was due to a high vaccine protection from infection with the delta variant and possibly a direct vaccine effect on the risk of developing MIS-C after breakthrough infection. The study adds important knowledge concerning the incidence of MIS-C depending on the strain of the preceding SARS-CoV-2 infection and the extent to which MIS-C is a vaccine-preventable disease.
We aimed to estimate the incidence of MIS-C and describe the clinical phenotype, following infection with the delta variant of SARS-CoV-2 according to vaccination status. Moreover, we aimed to compare the incidence and clinical phenotype of MIS-C from our cohort during the pre-delta era.
Methods
Study design and population
In this prospective, population-based cohort study, we identified children and adolescents (aged 0–17 years) hospitalised with MIS-C in Denmark from Aug 1, 2021, to Feb 1, 2022. MIS-C cases were included in the study if they had an RT-PCR-confirmed infection with the delta variant, or an infection with SARS-CoV-2, with unknown subtype, from July 15, 2021—the time from which the delta variant caused more than 90% of SARS-CoV-2 infections in Denmark—and until Dec 15, 2021, when omicron (B.1.1.529) became the dominant variant. The multicentre study included all 18 paediatric departments in Denmark, providing 24 h emergency service, and inpatient and outpatient treatment for children and adolescents. As part of the paediatric nationwide COVID-19 research project, all 18 departments had a principal investigator responsible for prospective real-time data collection to REDCap, a secure web application, including details on clinical characteristics, treatment, and outcome of patients with COVID-19-associated disease, including MIS-C, since March 12, 2020.3, 10, 11 To ensure completeness of MIS-C cases identified by the prospective data collection, patients hospitalised with ICD-10 diagnosis codes for MIS-C (DB972B and DB972B1) were obtained through the Danish National Patient Registry.12 We matched cases from the prospective real-time data collection and those identified through ICD-10 diagnosis codes through the unique social security number for each person in Denmark.
Patients' medical records were evaluated by two consultants in paediatric infectious diseases to confirm they met the US Centers for Disease Control and Prevention (US CDC) MIS-C case definition; ie, clinically severe illness requiring hospitalisation in persons aged younger than 21 years; fever of 38°C or more for at least 24 h or report of subjective fever for at least 24 h; laboratory evidence of inflammation; multisystem (≥2) organ involvement; laboratory evidence of acute or previous SARS-CoV-2 infection by RT-PCR, serology, or antigen test, or known COVID-19 exposure within 4 weeks of symptom onset; and no alternative plausible diagnosis.13
To compare the incidence and clinical phenotype of MIS-C after delta variant infection with MIS-C occurring in the period in which the wildtype strain was dominant, we used previously reported data from our prospective nationwide study of MIS-C in Denmark including data from March 1, 2020, to Feb 28, 2021.3
The study was approved by the Ethics Committee of Capital Region of Denmark (H-20028631) and the Danish Data Protection Agency (P-2019-29). Informed oral and written parental consent was provided before participation. A waiver of requirement of informed consent for cases not prospectively enrolled was obtained by the Danish Patient Safety Authority (3-3013-2907/1).
Procedures
To calculate the incidence of MIS-C among infected children and adolescents with SARS-CoV-2, the number of infected children and adolescents was estimated from the number of PCR-confirmed SARS-CoV-2 infections, obtained from the Danish COVID-19 surveillance register.9 Since the number of children and adolescents with laboratory-confirmed SARS-CoV-2 infections is known to be an underestimation of the number of individuals actually infected, a multiplier was applied, as also described by others.4, 14 We applied a multiplier of 1·5 to individuals aged 5–17 years—a multiplier recently estimated from extrapolating the seroprevalence in Danish blood donors to PCR-confirmed SARS-CoV-2 infections in the general Danish population.15 For individuals aged 0–4 years, who had a significantly lower test activity, we assumed the cumulated SARS-CoV-2 incidence during the study period was 13%, as for individuals aged 5–11 years, and adjusted the multiplier accordingly. This resulted in a multiplier of 6·1 for individuals aged 0–4 years. Children and adolescents who tested positive from July 15 to Dec 15, 2021, were included in the denominator. Positive SARS-CoV-2 tests were reported separately among vaccinated and unvaccinated individuals.
To account for the increasing vaccination coverage during the period, we calculated days as unvaccinated (or not fully vaccinated defined as the period until 14 days after the second COVID-19 vaccination) and fully vaccinated for each individual between 5 years and 17 years during the period between July 15 and December 15, summed these and converted them to person-years. We accumulated days from July 15 to Dec 15, 2021, or their 18th birthday (whichever came first) using the Danish COVID-19 surveillance register. A child or adolescent with MIS-C was defined as fully vaccinated if the SARS-CoV-2 infection occurred at least 14 days after the second vaccine dose.
Statistical analysis
Categorical variables are presented as numbers, and percentages and continuous variables as medians with corresponding IQRs, as appropriate. Non-parametric two-tailed Mann-Whitney U tests were used to compare continuous variables and χ2 or Fisher's exact tests to compare categorical variables. We estimated the incidence of MIS-C by dividing the number of reported MIS-C cases by the estimated number of SARS-CoV-2 infections among people younger than 18 years according to vaccination status. CIs were calculated using the exact method for binomial proportions. We estimated the MIS-C incidence rate per 1 000 000 person-years, by dividing the number of reported MIS-C cases by the number of person-years during the period by vaccination status. We calculated vaccine effectiveness as 1–incidence rate ratio using Poisson regression. The study was registered at ClinicalTrials.gov, NCT05186597.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit.
Results
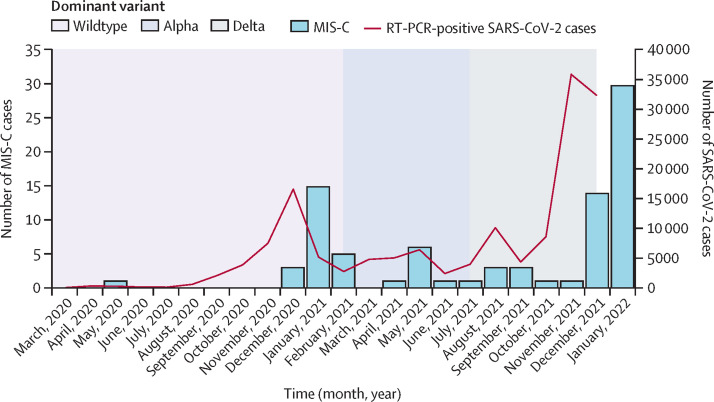
58 patients were reported with MIS-C through the prospective nationwide real-time data collection in the period Aug 1, 2021, to Feb 1, 2022 (figure ). Seven patients, who were initially diagnosed and treated for MIS-C, were subsequently excluded because they did not meet the US CDC case definitions: three due to identification of alternative diagnoses (one with acute Epstein-Barr virus infection and two with systemic juvenile idiopathic arthritis); three due to lack of known exposure and laboratory evidence of SARS-CoV-2 infection (negative nucleocapsid SARS-CoV-2 antibodies and SARS-CoV-2 nasopharyngeal test); and one because MIS-C occurred after vaccination. The patient with MIS-C after vaccination had no known SARS-CoV-2 exposure and negative nucleocapsid antibodies.16 One additional case of MIS-C was identified through the National Patient Register by ICD-10 diagnosis code for MIS-C. The National Patient Register included 11 misclassified cases, and one patient with MIS-C was not identified. Thus, 52 patients with MIS-C were included in the study, including 51 unvaccinated individuals and one fully vaccinated individual (table 1 ). Of these patients, 48 had PCR-confirmed SARS-CoV-2 infection with the delta variant (B.1.617.2 and AY lineages), one had known delta variant exposure among household members, and three had known exposure during the delta wave and positive nucleocapsid SARS-CoV-2 antibodies.
Figure.
Laboratory-confirmed SARS-CoV-2 cases per month in Denmark (March 1, 2020, to Dec 15, 2021) and following MIS-C cases (March 1, 2020, to Feb 1, 2022) in children and adolescents younger than 18 years
MIS-C=multisystem inflammatory syndrome in children.
Table 1.
Clinical phenotypes of multisystem inflammatory syndrome in unvaccinated children and adolescents during the wildtype and delta waves of COVID-19
| Wildtype (n=23) | Delta (n=51) | ||
|---|---|---|---|
| Patient characteristics | |||
| Age, years | 8 (5–14) | 8 (7–11) | |
| Sex | |||
| Female | 9 (39%) | 14 (27%) | |
| Male | 14 (61%) | 37 (73%) | |
| Interval between SARS-CoV-2 infection and hospital admission, weeks | 5·3 (3·4–6·0) | 5·4 (4·4–6·1) | |
| Symptoms* | |||
| Duration of symptoms before hospital admission, days | 3 (2–5) | 3 (2–5) | |
| Hypotension or shock | 13 (57%) | 26 (51%) | |
| Cardiac involvement | 23 (100%) | 46 (90%) | |
| Gastrointestinal involvement | 23 (100%) | 50 (98%) | |
| Haematological involvement | 21 (91%) | 36 (71%) | |
| Dermatological involvement | 19 (83%) | 44 (86%) | |
| Neurological involvement | 0† | 0† | |
| Respiratory involvement | 6 (26%) | 6 (12%) | |
| Renal involvement | 7 (23%) | 12 (24%) | |
| Treatment | |||
| Treatment at intensive care unit | 12 (52%) | 28 (55%) | |
| Vasoactive support | 5 (22%) | 8 (16%) | |
| Mechanical ventilation | 0 | 0 | |
| Extracorporeal membrane oxygenation | 0 | 0 | |
| Intravenous immunoglobulin | 21 (91%) | 38 (75%) | |
| Steroid | 17 (74%) | 48 (94%) | |
| Anakinra | 3 (13%) | 15 (29%) | |
| Length of hospital stay, days | 8·0 (6·0–9·0) | 5·0 (4·0–7·0)‡ | |
Data are median (IQR) or n (%).
Organ system involvement was defined as per the US Centers for Disease Control and Prevention: (1) cardiac involvement (eg, elevated troponin or N-terminal pro B-type natriuretic peptide, abnormal echocardiogram, or arrhythmia, or a combination of these; (2) gastrointestinal involvement (eg, abdominal pain, vomiting, diarrhoea, elevated liver enzymes, ileus, gastrointestinal bleeding); (3) haematological involvement (ie, thrombophilia or thrombocytopenia, elevated D-dimers); (4) dermatological involvement (eg, erythroderma, mucositis, other rash); (5) neurological involvement (ie, seizure, stroke or aseptic meningitis); (6) respiratory involvement (eg, pneumonia, acute respiratory distress syndrome, or pulmonary embolism); or (7) renal involvement (ie, acute kidney injury or renal failure). Shock was defined as persistent blood pressure below the fifth percentile, according to age.
No cases had seizures and no cases were investigated for stroke or aseptic meningitis. However, during the delta and pre-delta waves, headache was reported in 50% and 46% and confusion was observed in 11% and 13% of MIS-C cases.
p=0·0013.
51 individuals with MIS-C were identified among an estimated 175 458 unvaccinated individuals infected with SARS-CoV-2 aged 0–17 years. Thus, the incidence of MIS-C was estimated to be in one in 3400 unvaccinated individuals (95% CI 2600–4600) with the delta variant, equalling 291 per 1 000 000 infected vaccinated children and adolescents (95% CI 216–382; table 2 ). This incidence was similar to that of MIS-C in the period dominated by the wildtype strain, which was estimated to be one in 4100 infected individuals (95% CI 2700–6400); ie, 246 per 1 000 000 infected vaccinated children and adolescents (95% CI 156–369) based on 23 cases among an estimated 93 538 infected individuals. Age-stratified numbers are shown in table 2. Among fully vaccinated adolescents, the estimated incidence of MIS-C was one in 9900 adolescents with breakthrough infections (95% CI 1800–390 000), equalling 101 per 1 000 000 infected vaccinated children and adolescents (95% CI 3–565). No cases of MIS-C occurred among 1504 estimated re-infections during the study period. The incidences based on laboratory-confirmed case counts are shown in the appendix.
Table 2.
Number of MIS-C cases, estimated SARS-CoV-2 infections, and incidence of MIS-C after SARS-CoV-2 infections in children and adolescents in Denmark by age group, vaccination status, and SARS-CoV-2 variant
|
Wildtype (March, 2020, to February, 2021*) |
Delta (August, 2021, to February, 2022) |
|||||||
|---|---|---|---|---|---|---|---|---|
| MIS-C (n) | Estimated SARS-CoV-2 infections† (n) | Incidence of MIS-C per 1 000 000 infected children and adolescents (95% CI) | Incidence of MIS-C in infected children and adolescents | MIS-C (n) | Estimated SARS-CoV-2 infections‡ (n) | Incidence of MIS-C per 1 000 000 infected children and adolescents (95% CI) | Incidence of MIS-C in infected children and adolescents | |
| Not vaccinated | ||||||||
| Total | 23 | 93 397 | 246 (156–369) | 1 in 4100 (2700–6400) | 51 | 175 458 | 291 (216–382) | 1 in 3400 (2600–4600) |
| 0–4 years | 4 | 24 939 | 160 (44–411) | 1 in 6200 (2400–22 700) | 3 | 61 573 | 49 (10–142) | 1 in 20 500 (7000–100 000) |
| 5–11 years | 10 | 35 762 | 280 (134–514) | 1 in 3600 (1900–7500) | 42 | 88 295 | 476 (343–643) | 1 in 2100 (1600–2900) |
| 12–17 years | 9 | 33 183 | 273 (125–519) | 1 in 3700 (2700–6400) | 6 | 25 590 | 234 (86–510) | 1 in 4300 (2000–11 600) |
| Vaccinated§ | ||||||||
| 12–17 years | .. | .. | .. | .. | 1 | 9855 | 101 (3–565) | 1 in 9900 (1800–390 000) |
MIS-C=multisystem inflammatory syndrome in children.
Data regarding incidence of MIS-C after the wildtype strain has been published previously.3
Estimated by serology.3
Estimated cases of SARS-CoV-2 in the period July 15 to Dec 15, 2021 (using a multiplier of 1·5 to laboratory conformed cases in individuals 5–17 years and 6·1 in individuals 0–4 years).
Defined as SARS-CoV-2 infection occurring at least 14 days after the second vaccine dose.
The vaccination coverage among adolescents rose during the study period, and by Dec 15, 2021, 262 300 unvaccinated person-years and 87 400 vaccinated person-years had accumulated in children and adolescents aged 5–17 years. With 48 cases of MIS-C among unvaccinated adolescents during this period, the incidence rate of MIS-C was 183 per 1 000 000 unvaccinated person-years—ie, one in 5500. The incidence of MIS-C was 11 per 1 000 000 vaccinated person-years—ie, one in 87 400 vaccinated adolescents per year, rendering an incidence rate ratio of 0·063 (95% CI 0·009–0·453) and a vaccine effectiveness estimate of 94% (95% CI 55–99; p=0·0061).
Among the 51 unvaccinated patients with MIS-C in the delta wave, 14 (27%) were female and 37 (73%) were male, and the median age was 8 years (range 3–17; table 1). The median interval from infection to hospital admission was 5·4 weeks (IQR 4·4–6·1). 26 (51%) patients had hypotension, 28 (55%) were admitted to the intensive care unit (ICU), and eight (16%) received vasoactive support. One vaccinated female aged 17 years developed MIS-C 4 months after the second dose of the Pfizer–BioNTech vaccine and 5 weeks after a confirmed breakthrough infection. She presented with severe chest pain, but otherwise had a similar clinical phenotype to the unvaccinated cohort, with hypotension and four organs system affected, including reduced left ventricular ejection fraction of 30% and cardiac MRI with subepicardial late gadolinium enhancement, meeting the Lake Louise diagnostic criteria for myocarditis. 38 (75%) patients received intravenous immunoglobulin, 48 (94%) received glucocorticoid therapy, and 15 (29%) received interleukin-1 inhibitor (anakinra); two cases were self-limiting without treatment. All children and adolescents survived, and all had normal echocardiogram at hospital discharge. None had obvious short-term sequelae, except one with uveitis.
When compared with MIS-C cases during the first year of the pandemic in Denmark, which was dominated by the wildtype strain, the MIS-C cases during the period studied herein were similar in terms of sex, age, and time from SARS-CoV-2 infection to hospital admission (table 1). Regardless of variant type, the patients had similar phenotypes concerning organ involvement, hypotension, and need of treatment at ICU (table 1). There was no significant difference in the treatment of MIS-C. The duration of hospital admission decreased significantly, from a median of 8·0 days to 5·0 days (p=0·0013) during the delta wave.
Discussion
In this prospective nationwide study, we found the incidence of MIS-C after infection with the delta variant of SARS-CoV-2 to be one in 3400 infected unvaccinated children and adolescents, similar to the estimated incidence of one in 3000–4000 during the pre-delta era.3, 4 This is, to our knowledge, the first study to estimate the incidence of MIS-C after infection with the delta variant. Furthermore, we found a vaccine effectiveness of two doses of Pfizer–BioNTech vaccines against MIS-C of 94%. This effectiveness is similar to the estimated Pfizer–BioNTech vaccine effectiveness against MIS-C of 91% reported from US data.6 A similar observation has been made in France, where no MIS-C cases were reported in fully vaccinated children and adolescents.5 A decreased incidence of MIS-C in vaccinated children and adolescents might have two explanations. First, vaccination offers protection from infection with SARS-CoV-2, rendering fewer children and adolescents at risk of developing MIS-C, which is compatible with vaccine effectiveness studies showing reduced susceptibility to infection with the delta variant.17, 18 Second, we found an indication of direct vaccine effect on the risk of MIS-C after breakthrough infection, with an estimated occurrence in one in 9900, although our case numbers were too small to allow conclusions to be drawn. To our knowledge, other studies have not explored this risk and the case–control designs of the studies from France and the USA estimating vaccine effectiveness against MIS-C do not allow exploration of the risk of MIS-C after breakthrough infections. A potential explanation for direct vaccine protection against MIS-C after breakthrough infection could be due to vaccine-induced priming of the immune system, preventing the development of hyperinflammation.
Knowledge of the effect of vaccination on MIS-C is important for informing decision makers who set public health vaccination guideance and parents when deciding whether to vaccinate children. Furthermore, both vaccine effectiveness and data on the incidence of MIS-C after different SARS-CoV-2 variants are important to estimate the magnitude of MIS-C cases during future SARS-CoV-2 waves. However, the risk of children and adolescents developing MIS-C possibly depends on several factors, including the SARS-CoV-2 variant. Although we found the incidences of MIS-C to be equal for the delta variant and the wild type, the omicron variant is phylogenetically distant from the previous variants and forms a distant monophyletic class,19 which might impact its power to trigger the immune response to cause MIS-C. A second factor is vaccine effectiveness: details of vaccine effectiveness against MIS-C are still unexplored, including the duration of the effect. Furthermore, the low vaccine effectiveness against the omicron variant will soon allow us to explore the extent to which vaccination reduces the risk of MIS-C after breakthrough infections.17, 18 Another factor is risk of MIS-C after reinfections: due to the size of the omicron wave, a sizable proportion of children have now been twice infected with SARS-CoV-2. The risk of MIS-C after re-infections, which is yet to be explored, will also influence the magnitude of the disease in the time to come.
We found the clinical characteristics of children and adolescents with MIS-C after infection with the delta variant to be similar to patients with MIS-C occurring during the first year of the pandemic, as also reported by Miller and colleagues.20 Cardiac, gastrointestinal, and dermatological involvement was found in more than 80% of cases both during the delta wave and in the pre-delta era. Furthermore, the proportion of patients with hypotension and need of ICU admission was similar. We found a decreased duration of hospitalisation (from a median of 8 days to 5 days), as also found in the USA by Miller and colleagues.20 Among our MIS-C cases, this decreased duration of hospitalisation might partly be explained by prompt use of steroids and anakinra in complicated cases. Male predominance of MIS-C cases has been found to increase over time in the pandemic20—a finding also true in our cohort, with 73% male children and adolescents after infection with the delta variant wave compared with 61% after infection with the wildtype strain. However, the non-significant increase in our cohort occurred in non-vaccinated male children and adolescents, and was therefore not a signal of post-vaccine myocarditis presenting similarly to MIS-C.
The clinical phenotype in the only individual in our cohort with MIS-C after breakthrough infection was similar to the phenotype in unvaccinated individuals, but was remarkable due to presentation with severe chest pain, which is rarely seen in unvaccinated children with MIS-C, but reported as an rare adverse event to vaccination.11 Since the patient had completed the Pfizer–BioNTech vaccine 4 months before the development of MIS-C, vaccine-induced MIS-C was less plausible, despite severe chest pain being reported as the presenting symptom in vaccine-induced MIS-C.16 The other cases of MIS-C reported after breakthrough infection in vaccinated individuals had a similar phenotype as in non-vaccinated children, with hypotension and several organs involved, but not severe chest pain as the initial presentation.6, 21
The estimation of the incidence of MIS-C is dependent on a precise number of MIS-C cases. We assume our MIS-C case number is close to complete for several reasons. First, identification of cases was determined by active surveillance through a nationwide prospective research collaboration with real-time enrolment of cases, including detailed clinical characteristics. Second, case completeness was assured through manual review of medical records of all patients with ICD-10 diagnosis codes of MIS-C identified through the National Patient Register using the unique patient identification number. Third, all cases were re-evaluated by two consultants in paediatric infectious diseases and cases were excluded, despite initial diagnosis and treatment as MIS-C, if alternative diagnoses were identified or if previous SARS-CoV-2 infection was disproven by negative nucleocapsid SARS-CoV-2 antibodies. Fourth, due to the thorough Danish sequencing strategy, all cases had confirmed infection with the delta variant, including all MIS-C cases occurring in January, 2022, when the omicron variant could theoretically have caused cases of MIS-C due to the rapid rise of omicron infections from mid-December, 2021, in Denmark. Despite our case numbers being thought to be near complete, milder self-limiting cases not requiring hospital admission, or not identified as MIS-C, might have been missed because no unequivocal diagnostic criteria exist. Furthermore, hyperinflammatory conditions unrelated to SARS-CoV-2 infections, but occurring in patients with previous SARS-CoV-2 infection, might have been diagnosed as MIS-C.
The ability to estimate the incidence of MIS-C relative to SARS-CoV-2 infections is also dependent on a precise denominator; ie, the number of children and adolescents with SARS-CoV-2 infections. We used the multiplier of 1·5–6·1 to laboratory-confirmed case number of SARS-CoV-2 infections to estimate the true number of infected children and adolescents, which is lower than the multipliers of 7·1–16·0 used for the US population.4, 14 The multiplier of 1·5 was based on a recent study, exploring the seroprevalence in Danish blood donors in January, 2022, estimating that only one-third of all adult cases in the general population were not laboratory confirmed during the delta wave. This translates to a multiplier of 1·5. We used this multiplier for individuals aged 5–17 years, because the test activity in school children has been similar to adults due to recommendation of weekly antigen screening tests in schools. Since the test activity in children aged 0–4 years was substantially lower, we estimated the multiplier assuming the cumulated SARS-CoV-2 incidence in this age group was similar to individuals aged 5–11 years during the delta wave. However, the multiplier, and thus the estimated number of SARS-CoV-2, although encumbered with uncertainty, is our best available to date.
A major limitation of this study is the low number of MIS-C and SARS-CoV-2 cases due to our small population size, making the estimates uncertain. A strength of our study is the nationwide paediatric research set-up with prospective real-time data collection of COVID-19-associated diseases with detailed phenotyping, in combination with the unique Danish national surveillance system concerning test results and vaccination status. Previous studies using the same prospective paediatric nationwide research set-up during the COVID-19 pandemic3, 11 have resulted in estimates of incidences of MIS-C, as well as incidence of myocarditis after vaccination, very similar to estimates from other populations, including the USA.4, 22
In conclusion, our results highlight that MIS-C remains a rare, but serious, complication of SARS-CoV-2 infection in children and adolescents, and that the phenotype has not changed during the pandemic. The incidence of MIS-C after the delta variant was one in 3400 unvaccinated children and adolescents, similar to that of the pre-delta variants. Furthermore, we found a vaccine effectiveness against MIS-C of 94%, which we suggest was due to a protection from infection with the delta variant and, possibly, a direct vaccine protection of developing MIS-C after breakthrough infection. Knowledge of the incidence of MIS-C after different SARS-CoV-2 variants, and the effect of vaccination, might help to elucidate the extent to which MIS-C is a vaccine-preventable disease.
Data sharing
Data will not be made available for others according to Danish data protection legislation.
Declaration of interests
UN received a research grant from the National Ministry of Higher Education and Innovation Fund, Denmark. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The study was funded by a COVID-19 grant from the National Ministry of Higher Education and Science (grant number 0237-00004B) and Innovation Fund Denmark (0176-00020B), to whom we express our gratitude.
Contributors
All co-authors conceptualised the study. UN, MH, UBH, JG, LSS, SBN, ATM, and M-LvL collected MIS-C cases for the study. UN obtained funding for the study. LE provided numbers of SARS-CoV-2-positive cases and vaccinated and unvaccinated person-years accumulated during the study period. UBH, MH, UBH, and LE verified and analysed data for the study. UN, M-LvL, MH, and UBH drafted the first version of the manuscript. All authors contributed data to the study and to the data interpretation, critically reviewed the manuscript, and approved the final manuscript for submission.
Supplementary Material
References
- 1.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holm M, Hartling UB, Schmidt LS, et al. Multisystem inflammatory syndrome in children occurred in one of four thousand children with severe acute respiratory syndrome coronavirus 2. Acta Paediatr. 2021;110:2581–2583. doi: 10.1111/apa.15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne AB, Gilani Z, Godfred-Cato S, et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy M, Recher M, Hubert H, et al. Multisystem inflammatory syndrome in children by COVID-19 vaccination status of adolescents in France. JAMA. 2022;327:281–283. doi: 10.1001/jama.2021.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zambrano LD, Newhams MM, Olson SM, et al. Effectiveness of BNT162b2 (Pfizer–BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 years—United States, July–December, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:52–58. doi: 10.15585/mmwr.mm7102e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danish COVID-19 Genome Consortium Genomic overview of SARS-CoV-2 in Denmark; dominating VOC lineages. 2021. https://www.covid19genomics.dk/statistics
- 8.Statens Serum Institut COVID-19 vaccination, vaccinedata-dashboard. 2022. https://covid19.ssi.dk/
- 9.Schønning K, Dessau RB, Jensen TG, et al. Electronic reporting of diagnostic laboratory test results from all healthcare sectors is a cornerstone of national preparedness and control of COVID-19 in Denmark. Apmis. 2021;129:438–451. doi: 10.1111/apm.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartling UB, Holm M, Glenthoej JP, et al. The need for hospitalization due to SARS-CoV-2 in children: a population-based estimate. Pediatr Infect Dis J. 2021;40:e250–e251. doi: 10.1097/INF.0000000000003127. [DOI] [PubMed] [Google Scholar]
- 11.Nygaard U, Holm M, Bohnstedt C, et al. Population-based Incidence of myopericarditis after COVID-19 vaccination in Danish adolescents. Pediatr Infect Dis J. 2022;41:e25–e28. doi: 10.1097/INF.0000000000003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Multisystem inflammatory syndrome in children (MIS-C) associated with Coronavirus disease 2019 (COVID-19) 2020. https://emergency.cdc.gov/han/2020/han00432.asp
- 14.Reese H, Iuliano AD, Patel NN, et al. Estimated incidence of Coronavirus disease 2019 (COVID-19) illness and hospitalization—United States, February–September, 2020. Clin Infect Dis. 2021;72:e1010–e1017. doi: 10.1093/cid/ciaa1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statens Serum Institut . Statens Serum Institut; Copenhagen: 2022. Seroprævalensundersøgelse af bloddonorer.https://www.ssi.dk/-/media/arkiv/subsites/covid19/overvaagningsdata/moerketal/seropraevalensundersoegelse-af-bloddonorer_runde5.pdf?la=da [Google Scholar]
- 16.Chai Q, Nygaard U, Schmidt RC, Zaremba T, Møller AM, Thorvig CM. Multisystem inflammatory syndrome in a male adolescent after his second Pfizer–BioNTech COVID-19 vaccine. Acta Paediatr. 2022;111:125–127. doi: 10.1111/apa.16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruxvoort KJ, Sy LS, Qian L, et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ. 2021;375 doi: 10.1136/bmj-2021-068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med. 2022;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kandeel M, Mohamed MEM, Abd El-Lateef HM, Venugopala KN, El-Beltagi HS. Omicron variant genome evolution and phylogenetics. J Med Virol. 2022;94:1627–1632. doi: 10.1002/jmv.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller AD, Zambrano LD, Yousaf AR, et al. Multisystem inflammatory syndrome in children—United States, February, 2020–July, 2021. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab1007. published online Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Ardoin SP, Blaney C, et al. Multisystem Inflammatory syndrome in children after breakthrough infection in a COVID-19-vaccinated child. Pediatr Infect Dis J. 2021;41(4):e182–e183. doi: 10.1097/INF.0000000000003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witberg G, Barda N, Hoss S, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will not be made available for others according to Danish data protection legislation.