Abstract
Heavy metal stress is one of the major abiotic stresses that cause environmental pollution in recent decades. An elevated concentration of these heavy metals is highly toxic to plant. Chromium (Cr) is one of the heavy metals whose concentration in the environment is still increasing alarmingly. It is harmful for plant growth and achene yield. To check out the growth and protein alternation towards pollutants, two sunflower varieties (RA-713 and AHO-33) were subjected to different chromium concentrations (control, 200 ppm, 400 ppm) by soil application. This study has elaborated that 400 ppm Cr resulted in a reduction of various growth parameters. Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was used to enhance the understanding of plant proteomic modulation under Cr stress. Different protein bands like 48 and 49, 26 kDa have newly appeared, and three 60, 47, and 42 kDa, and two protein bands 49 and 13 kDa were up-regulated in seeds of RA-713 and AHO-33, respectively. Some proteins (52, 16 kDa) are down-regulated in leaf tissues of both varieties. Only 6 and 81 kDa protein showed up-regulation and 154 kDa down-regulation behavior in the shoot in response to stress. The finding s of study might support the selection of tolerant genotype under Cr contamination and the discovery of new protein biomarkers that can use as monitoring tools in heavy metal stress biology.
Keywords: Chromium, Heavy metal, Helianthus annuus, Proteomics, Physiological alterations
1. Introduction
Environmental pollution is a major threat to our modern society, mostly caused by heavy metals (Berni et al., 2019, Jabeen et al., 2009, Keyster et al., 2020, Stambulska et al., 2018, Zafar-ul-Hye et al., 2018). Currently, Cr has been a very common environmental pollutant amongst all heavy metals (Ali et al., 2015, Eleftheriou et al., 2015, Kumar et al., 2019, Ozdemir et al., 2005, Shahid et al., 2017, Sytar et al., 2019), which is being happened due to its use on a large scale in many industries, including tanning, wood preservation, metallurgical, pulp, and paper production, electroplating, and production of paints and pigments (Amin et al., 2013). In Pakistan, tanning is one of the oldest industries, and the wet process of tanning is also one of the potential sources of heavy metals, particularly Cr, which pollute water and soil environments (Danish et al., 2019, Ejaz et al., 2020, Shafiq et al., 2020). Its presence in surplus amount cause stunted growth (Faisal and Hasnain, 2005) and disturbs the pattern of nutrient uptake in the plant because of nutrient metal interaction (Anjum et al., 2017, Rafiullah et al., 2020b, Zupančič et al., 2004). It adversely affects several morphological and biochemical parameters, seed germination, protein contents, inhibiting enzyme activity, photosynthesis, and causing chlorosis and necrosis (Dube et al., 2003, Ertani et al., 2017, Ma et al., 2016, Nath et al., 2005, Stambulska et al., 2018, Tang et al., 2012).
Sunflower (Helianthus annuus L.) is the fifth important source of edible. With a worldwide seed production of sunflower, about 25.8 million tones are destined almost exclusively to oil extraction, providing 8.2% of total world volume (Cantamutto and Poverene, 2007). It can accumulate high concentrations of metals in its various tissues with a reasonable tolerance. Rendering is a suitable candidate for the phytoremediation process (Salt et al., 1998), based on a hyperaccumulator plant’s phytoextraction mechanism like a sunflower for eliminating, destroying, or sequencing hazardous substances from the environment (Ma et al., 2001, Sytar et al., 2020).
Most of scientists suggest organic and biofertilizers to over the problem of poor growth, nutrients uptake and yield under stress and normal environment (Abbas et al., 2020, Ahmed et al., 2020, Danish and Zafar-ul-Hye, 2020, Rafiullah et al., 2020a, Ullah et al., 2020, Wahid et al., 2020, Zafar-ul-Hye et al., 2020). However, recently, proteomics is becoming an essential tool in understanding fundamental processes in plant growth and development (Cánovas et al., 2004, Jorrín et al., 2006, Rossignol, 2001). It helps understand crop nutritional values, crop yield, responses to stresses and identifies key molecular markers for their use in crop improvement through classical plant breeding or biotechnology (Castillejo et al., 2008). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is undoubtedly the most widely used biochemical method for extracting and separating proteins based on their molecular mass and charge. The processes involved in heavy metal uptake, distribution, oxidative stress induction, accumulation, and detoxification have been investigated in a wide range of studies on plants, but the mechanisms involved are still only partially understood. Therefore the proteomic approach can help elucidate new aspects of plant metal stress (Cvjetko et al., 2010, Gratão et al., 2008, Horvat et al., 2007). This research aimed to investigate the growth and protein expression alternation in two sunflower varieties (RA-713 and AHO-33) in response to chromium stress.
2. Materials and methods
2.1. Plant material and experimental design
Certified seeds of two sunflower (Helianthus annuus) varieties RA-713 and AHO-33; (named as S1 and S2 varieties) were obtained courtesy of the Federal Seed Certification and Registration Department, Lahore. The experiment was conducted in the wirehouse of the Department of Botany, University of the Punjab, Lahore. The pots (6-inch) were filled with a weighed amount (1 kg) of sieved and crushed soil taken from Botanical Garden, University of the Punjab, Lahore. Six seeds were sown in each pot. Seeds were sown at 2.5 cm soil depth. The temperature of wirehouse was 28–34 °C. Gentle surface digging of soil was carried out periodically with a knife for proper aeration of the soil. All the pots were irrigated periodically during germination. The emergence of seeds started after three days. After five days of sowing, plants were transplanted in Cr-treated soil.
2.2. Chromium treatment
The weighted amount of soil (1 kg) was placed in plastic pots of a diameter of 6-inches. Chemical grade potassium dichromate (K2CrO4) was used as a source of Cr (VI). Both varieties were subjected to three different Cr concentrations (0, 200, and 400 ppm). Three replicates per treatment were prepared. Three plants per replicate were transplanted into Cr treated soil. Seedlings were allowed to grow for 25 days.
2.3. Sowing of seeds for laboratory experiment
Seeds of both varieties were grown for three days in Petri plates, washed thoroughly, and sterilized in an autoclave (Fig. 1a, Fig. 1b). A single layer of filter paper was placed on each Petri plate. Three seeds were placed in each Petri plate and subjected to three Cr concentrations (0, 200 ppm, and 400 ppm). These Petri plates were incubated at 28 °C for 72 h followed by protein extraction.
Fig. 1a.
Seeds of S1 variety subjected to different concentrations of Cr (Control, 200 ppm, 400 ppm) for three days.
Fig. 1b.
Seeds of S2 variety subjected to different concentrations of Cr (Control, 200, 400 ppm) for three days.
2.4. Growth parameters
Sunflower plants were gently harvested after 25 days and prepared for measurements. Plants were cleaned with distilled water to remove soil residues. Various growth parameters such as root length shoot length, fresh and dry weight of root and shoot, and the total number of leaves plant−1 were recorded.
2.5. Extraction and quantification of total protein
2.5.1. Extraction of protein from leaves and stem
After 25 days, old sunflower seedlings, 1 g leaves, and stems of both varieties were crushed in pre-chilled pestle and mortar using liquid nitrogen. Proteins were isolated using lysis buffer (370 µl of 1 mol/L Tris-HCl (pH 6.8), 600 µl of 10% (w/v) SDS, 300 µl of conc. glycerol, 150 µl of conc. β-mercaptoethanol and 1580 µl of ml High-purity water). After 30 min of vortexing, centrifuge for 5 min at 4 °C. The supernatant was collected and stored at −80 °C until further use (Verbi et al., 2005).
2.5.2. Extraction of protein from seeds
The whole seed was crushed and ground to a fine powder in pestle and mortar using liquid nitrogen to extract seed proteins. Around 0.1-gram seed powder was put into a 1.5 ml micro-tube. To extract proteins from powder, 50 mM phosphate extraction buffer (700 μl) of pH 7.0 was added to powder as an extraction liquid. The slurry was then vortexed for 5 min and centrifuged at 14,000 rpm in eppendorf centrifuge 5417R for 10 min. The supernatant was stored at −20 °C for further use in electrophoresis. The protein concentrations were estimated by Bradford method (Hameed et al., 2009).
2.5.3. Protein purification and quantification
Plant tissue lysates (leaves, stem, and seed) were treated with pre-cooled 10% TCA in acetone containing 10 mM DTT for purification. Total protein content was estimated using standard Bradford Assay taking Bovine Serum Albumin as standard (Bradford, 1976).
2.5.4. Protein separation using SDS-PAGE
SDS-PAGE was carried out in Mini PROTEAN® Tetra Cell system from Bio-Rad using a modification procedure (Laemmli, 1970). Therefore, 10 μg of extracted proteins were subjected to 12 % sodium dodecyl sulfate-polyacrylamide gel. The two vertical glass plates for gel were fixed together. The separating gel consisted of 1% by weight N.N-methylene-acrylamide in 0.5 M Tris-HCl buffer (pH 8.8) with 10% SDS and 29% acrylamide. The gel was polymerized by adding tetramethylene-diamine (TEMED) and 10 % ammonium persulphate. The stacking gel comprised 1 % N.N-methylene-acrylamide in the 0.5 M Tris-Hcl buffer (pH 6.8) with 10% SDS. The gel was polymerized by pouring TEMED and 10 % APS). Then gels were run in tris–glycine buffer (its 1x working solution containing 25 mM Tris-Cl, 250 mM glycine, and 0.1% SDS) at 60 V until the sample enters, resolving the voltage was increased to 120 V until the dye reaches the bottom of the gel.
After that, gels were kept in fixative (30% ethanol, 10% acetic acid) overnight. Then protein bands were visualized by staining with colloidal Coomassie blue stain (100 g ammonium sulphate, 1.2 g Coomassie blue R 250, 100 ml 85% phosphoric acid in H2O to final volume 800 ml, methanol was added upon use in 1:4v/v, i.e., methanol: dye and the gels were destined (5% methanol and 7% acetic acid). The gel image was scanned, and the image Quant TL (GE Healthcare) version 1.1 was used to analyze all 1-D gels. An unstained protein marker of molecular weight ranging from 10 to 200 kDa (Fermentas life sciences) was used as a reference to determine the molecular weight of polypeptide bands.
2.5.5. Image analysis
Protein bands were analyzed, and each protein band was quantified using Image Quant TL (GE Healthcare) version 1.1 software.
2.6. Statistical analysis
Data were analyzed using SPSS Version 20.0. Using descriptive statistics, Mean and Standard Deviation for each growth parameter was calculated for each treatment. The physiological parameters were average of n = 3 (Steel et al., 1997).
3. Results and discussion
3.1. Growth parameter
Different growth parameters of two sunflower varieties were checked under Cr stress. Both varieties' growth was suppressed under high chromium concentration (400 ppm), whereas in low concentration of Cr, slight inhibition of growth was noticed (Fig. 2). S1 variety has proved to be more tolerant than S2 variety because of decreased percentage variation in plant leaves at 400 ppm (31% in S1 and 34 % in S2 variety compared to control) (Table 1). The reduction in plant leaves numbers has also been reported by Shanker et al. (2004) due to chromium toxicity. Fresh weight of shoot and root had shown 21% and 36% decline in 400 ppm in S1 variety and 30% and 41% in S2 variety respectively. The decreases in similar results were found by Maiti et al. (2012) with chromium concentrations of 0–300 ppm. However, the parameters like plant height, root length, shoot length, the number of leaves per plant, and dry weight of shoot and root decreased by different degree of a percent (21, 58, 76.66, 63.04, 83.42, 45.83 and 34, 64%, respectively) in 400 ppm of Cr when compared to control plants (Fig. 2, Fig. 3; Table 1). The results conform to Amin et al. (2013), who observed major changes of the morphological parameter in Hibiscus esculentus L. plant in response to an enhancing Cr concentration.
Fig. 2.
Effect of different Cr treatments on the sunflower varieties S1 (A) and S2 (B) after 25 days.
Table 1.
Analysis of growth parameter of sunflower plants against Cr.
| Parameters | Varieties |
Treatments |
||
|---|---|---|---|---|
| Control | 200 ppm | 400 ppm | ||
| Number of Leaves | S1 | 7.33 ± 1.15 | 7.00 ± 1.00 | 5.00 ± 1.00 |
| S2 | 21.00 ± 1.80 | 19.33 ± 1.04 | 16.50 ± 0.50 | |
| Plant height (cm) | S1 | 1.28 ± 0.244 | 1.10 ± 0.33 | 0.90 ± 0.14 |
| S2 | 1.31 ± 0.41 | 1.16 ± 0.11 | 1.04 ± 0.05 | |
| Root length (cm) | S1 | 1.28 ± 0.244 | 1.10 ± 0.33 | 0.90 ± 0.14 |
| S2 | 18.83 ± 2.08 | 17.33 ± 1.04 | 12.40 ± 2.29 | |
| Shoot fresh weight (g) | S1 | 22.00 ± 2.00 | 14.66 ± 1.25 | 9.33 ± 0.76 |
| S2 | 19.66 ± 2.25 | 12.33 ± 1.75 | 7.00 ± 1.50 | |
| Root fresh weight (g) | S1 | 0.70 ± 0.15 | 0.60 ± 0.39 | 0.45 ± 0.06 |
| S2 | 0.59 ± 0.12 | 0.48 ± 0.05 | 0.35 ± 0.05 | |
| Root dry weight(g) | S1 | 0.08 ± 0.01 | 0.05 ± 0.00 | 0.03 ± 0.00 |
| S2 | 0.07 ± 0.01 | 0.06 ± 0.00 | 0.04 ± 0.00 | |
| Shoot dry weight(g) | S1 | 0.39 ± 0.28 | 0.34 ± 0.10 | 0.26 ± 0.37 |
| S2 | 0.29 ± 0.02 | 0.21 ± 0.02 | 0.18 ± 0.01 | |
The data represents the mean of mean ± SD replicates (n = 3).
Fig. 3.
Seedlings of sunflower varieties taken for Growth parameters; A (S1) and B (S2).
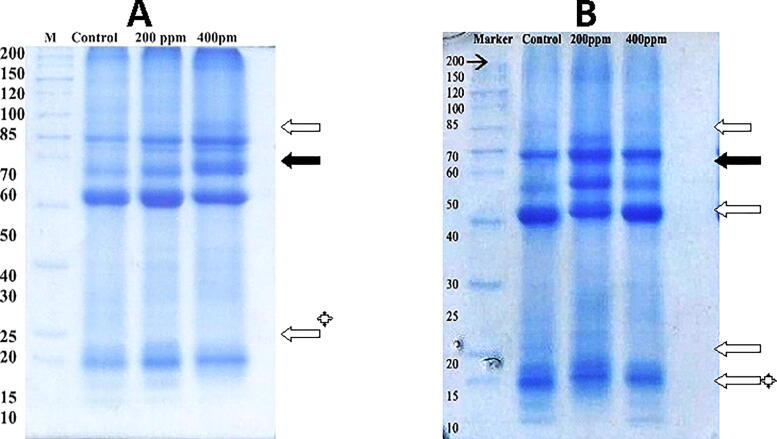
3.2. Protein expression in seed of S1 and S2varieties
SDS-PAGE analysis of the protein profile in seeds of S1 and S2 varieties of Cr treated plants revealed major changes than control (Table 2). Out of 12 differentially expressed proteins, five proteins (60, 48, 47, 42, and 12 kDa) in S1 variety and three protein of 59, 49, 27, and 13 kDa showed significant differential expression, i.e., >1.5-fold change in S2 variety (Fig. 4), seemed to be expressed in response to increased Cr concentration. Plants alter the protein expression pattern (up-regulated or newly induced proteins) in response to stress (Bagheri et al., 2013). A 112 kDa protein only appeared in response to a high dose of Cr. A protein of 49 kDa was up-regulated one-fold in 200 ppm, and 26 kDa protein was 1.59-fold up-regulated in 200 ppm in the S2 variety. A similar 26 kDa protein was reported by Rani and Reddy (1994) in rice which is associated with salt stress. This protein is named osmotin which was also reported in many crops. The findings of our study were consonant with Alirezia (2014). Who demonstrated legumin-like seed storage protein in sunflower ranging from 45 to 66.2 kDa using 2-DE (Alireza, 2014). Guljun et al. (2006), observed the proteome changes in sunflower seeds induced by N+ implantation. These dramatic proteome changes were at 16–34 kDa and 94, 199, 279, 280 kDa proteins.
Table 2.
Protein profile alternation in control and Cr treated seed of S1 (A) and S2 (B) varieties; where fold change 1 = difference between control and 200 ppm and Fold change 2 = difference between control and 400 ppm, plus sign indicate an increase and minus sign indicate a decrease in expression as compared to reference gel.
|
For S1 (A) |
Expression of total protein concentration load per sample (10 µg) |
||||||
|---|---|---|---|---|---|---|---|
| Sr No. | Mol.Wt (kDa) | Rf value | Control | 200 ppm | 400 ppm | Fold change (1) | Fold change (2) |
| 1 | 122 | 0.045 | 0.5 | – | – | −0.5 | −0.5 |
| 2 | 116 | 0.054 | – | 0.97 | – | +0.97 | +0.97 |
| 3 | 112 | 0.060 | – | – | 1.50 | – | +1.50 |
| 4 | 66 | 0.210 | – | – | 1.21 | – | +1.21 |
| 5 | 60 | 0.249 | 0.49 | 0.89 | 0.82 | +1.81 | +1.67 |
| 6 | 55 | 0.275 | – | 1.4 | 1.03 | +1.4 | +1.03 |
| 7 | 48 | 0.323 | – | – | 1.59 | – | +1.59 |
| 8 | 47 | 0.332 | 0.53 | 1.24 | – | +2.33 | −0.53 |
| 9 | 42 | 0.404 | 0.49 | 1.40 | 1.55 | +2.85 | +3.16 |
| 10 | 27 | 0.722 | – | 1.26 | – | +1.26 | – |
| 11 | 20 | 0.859 | 7.48 | 1.61 | 2.31 | − 4.64 | −3.23 |
| 12 | 12 | 0.955 | 0.51 | 1.23 | – | +2.41 | −0.51 |
| For S2 (B) | Expression of total protein concentration load per sample (10 µg) | ||||||
| Sr No. | Mol.Wt (kDa) | Rf value | Control | 200 ppm | 400 ppm | Fold change (1) | Fold change (2) |
| 1 | 120 | 0.048 | 1.73 | 1.21 | 1.34 | −1.42 | −1.29 |
| 2 | 68 | 0.203 | – | 1.18 | – | +1.18 | – |
| 3 | 59 | 0.251 | 1.26 | 0.72 | – | −1.75 | +1.38 |
| 4 | 57 | 0.256 | – | – | 1.31 | 0.00 | +1.31 |
| 5 | 49 | 0.316 | – | 1.51 | – | +1.55 | – |
| 6 | 47 | 0.331 | 1.47 | – | 1.43 | −1.47 | +1.02 |
| 7 | 42 | 0.403 | 1.51 | 1.56 | 1.57 | +0.96 | −1.03 |
| 8 | 26 | 0.719 | – | 1.59 | – | +1.59 | – |
| 9 | 21 | 0.848 | 2.38 | 2.22 | 2.87 | −1.07 | −1.20 |
| 10 | 13 | 0.940 | 1.64 | – | 1.49 | −1.64 | +1.1 |
Fig. 4.
Changes in protein profile in the seed of S1 and S2 varieties after 25 days of applied treatment with Cr. The black and white arrows show newly synthesize and increase expression of protein, respectively. White arrows with an asterisk are protein disappeared upon Cr stress.
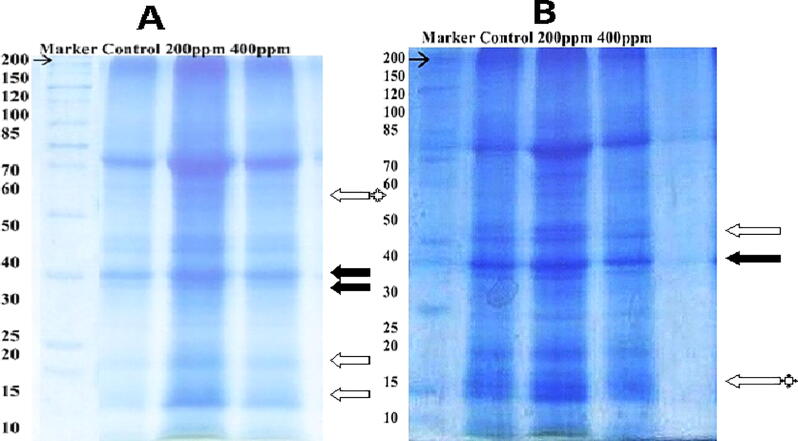
3.3. Protein expression in leaves of S1 and S2 variety
Three proteins (52, 23, and 16 kDa) showed significant differential expression >1.5-fold change, and all these protein bands showed a range of fold change between 1.61 to 14.33 in S1, and 52 and 16 kDa in S2 variety leaves were suppressed in response to Cr concentration (Table 3). Two proteins (34, 32 kDa) seemed to be up-regulated (1.62, 1.79 respectively) in S1variety. Whereas one protein band 32 kDa expressed against 400 ppm in S2 variety (Fig. 5). The results confirm the result of Kiribandage and Hasintha (2012). He found that 32 kDa protein was expressed in Sundance and Sunflower Teddy Bear L. when treated with heavy metal Arsenic. Further, he confirmed this protein band as chitinase (Kiribandage, 2012). A protein of 34 kDa was up-regulated 1.62 times in 200 ppm, while 32 and 23 kDa protein showed up-regulation in increasing Cr (400 ppm) in the S1 variety. Lower molecular weight protein 15 kDa was down-regulated 1.60 times in 400 ppm in S2 variety. A protein of 52 kDa was found to have a high percentage volume expression in both S1 and S2 varieties (1.76 and 1.87, respectively). Garcia et al. (2006) also found 54 kDa protein bands in sunflower leaves in response to Cr, further correlated this protein band by analysis database to large chain of ribulose-bisphosphate-carboxylase (54.07 kDa). This protein, rubisco, is present in leaf, which participate in Calvin cycle (fixation of CO2) during the photosynthetic processes. The carboxylation ratio is dependent on the amount of this protein (Berg et al., 2002). In our results, a protein of 34 kDa was up-regulated which is inconsonant with Gracia et al. (2006) finding in sunflower leaves in response to heavy metal stress. This protein band is reported as 1-aminocyclopropane-1-carboxylic acid oxidized (34.89 kDa), which participates in ethylene biosynthesis (Kasai et al., 1998). That is an important parameter bused as a stress indicator. Hagemeyer and Breckle (1996) commented that ethylene response depended on concentration and interacting metals ions.
Table 3.
Protein profile alternation in control and Cr treated leaves of S1 (A) and S2 (B) varieties; where fold change 1 = difference between control and 200 ppm and Fold change 2 = difference between control and 400 ppm, plus sign indicate an increase and minus sign indicate a decrease in expression as compared to reference gel.
|
For S1 (A) |
Expression of total protein concentration load per sample (10 µg) |
||||||
|---|---|---|---|---|---|---|---|
| Sr No. | Mol.Wt (kDa) | Rf value | Control | 200 ppm | 400 ppm | Fold change (1) | Fold change (2) |
| 1 | 135 | 0.055 | 0.86 | 0.86 | – | 0.86 | −0.86 |
| 2 | 140 | 0.049 | – | – | 0.86 | – | +0.86 |
| 3 | 70 | 0.175 | – | – | 0.98 | – | +0.98 |
| 4 | 69 | 0.181 | 0.89 | 0.83 | – | −1.07 | −0.89 |
| 5 | 52 | 0.277 | 1.76 | 0.76 | 0.76 | −2.31 | −2.31 |
| 6 | 44 | 0.334 | 0.89 | – | 0.73 | −0.89 | −1.21 |
| 7 | 43 | 0.353 | 0.78 | – | – | −0.78 | −0.78 |
| 8 | 41 | 0.300 | 1.08 | 0.76 | 0.95 | −1.42 | −1.13 |
| 9 | 37 | 0.463 | 0.90 | – | – | −0.90 | −0.90 |
| 10 | 36 | 0.471 | 0.86 | 0.76 | – | −1.13 | −0.86 |
| 11 | 34 | 0.505 | 0.50 | 0.82 | 0.47 | +1.62 | −1.06 |
| 12 | 32 | 0.569 | 0.69 | 0.92 | 1.24 | +1.33 | +1.79 |
| 13 | 29 | 0.596 | 0.86 | 0.86 | 0.63 | – | −1.36 |
| 14 | 27 | 0.698 | 0.86 | 0.90 | 1.20 | +1.04 | +1.39 |
| 15 | 23 | 0.794 | 0.92 | 0.69 | 0.57 | −1.33 | −1.61 |
| 16 | 16 | 0.909 | 0.86 | 0.06 | 0.76 | −14.33 | −1.13 |
| For S2 (B) | Expression of total protein concentration load per sample (10 µg) | ||||||
| Sr No. | Mol.Wt (kDa) | Rf value | Control | 200 ppm | 400 ppm | Fold change (1) | Fold change (2) |
| 1 | 144 | 0.047 | 0.84 | – | – | −0.84 | −0.84 |
| 2 | 137 | 0.052 | – | 0.92 | – | +0.92 | – |
| 3 | 132 | 0.058 | 0.9 | – | – | −0.9 | −0.9 |
| 4 | 69 | 0.181 | 0.96 | 0.96 | 0.96 | – | – |
| 5 | 54 | 0.263 | – | – | 0.96 | – | +0.96 |
| 6 | 52 | 0.275 | 1.87 | 0.87 | – | −2.14 | −1.87 |
| 7 | 44 | 0.338 | – | 0.69 | 1.25 | +0.96 | +1.25 |
| 8 | 41 | 0.387 | 0.95 | 0.95 | – | – | −0.95 |
| 9 | 37 | 0.467 | 0.81 | – | 0.93 | −0.81 | −1.15 |
| 10 | 36 | 0.475 | – | 0.73 | – | +0.73 | – |
| 11 | 35 | 0.503 | 0.80 | 0.66 | 0.64 | −1.22 | −1.25 |
| 12 | 32 | 0.566 | 0.57 | 0.57 | 0.61 | – | +1.67 |
| 13 | 29 | 0.596 | 0.60 | 0.65 | 0.65 | 0.01 | +0.01 |
| 14 | 27 | 0.703 | 1.12 | 1.12 | 1.25 | – | +1.12 |
| 15 | 23 | 0.799 | 1.01 | 0.89 | 1.07 | −1.13 | +1.06 |
| 16 | 16 | 0.909 | 1.44 | 1.04 | 0.9 | −1.38 | −1.60 |
Fig. 5.
Changes in protein profile in leaves of S1 (A) and S2 (B) variety after 25 days of applied treatment with Cr. The black and white arrows show newly synthesize and increase expression of protein, respectively. White arrows with an asterisk are protein disappeared upon Cr stress.
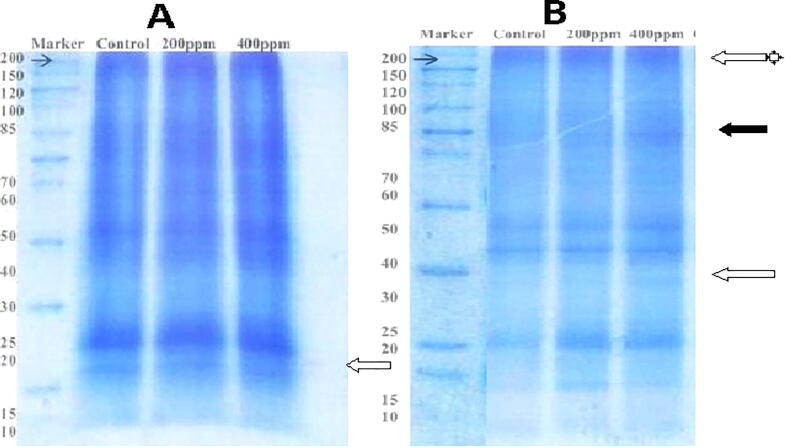
3.4. Protein expression in shoot of S1 and S2 variety
One protein exhibited a significant increase in abundance (up-regulated), and one protein had a decline in abundance (down-regulated) in the S2 variety shoot (Table 4). A low dose of Cr resulted in only one band's appearance compared to control in the S1 variety shoot. Proteins having a molecular mass of 154, 81 kDa was expressed significantly in the S2 variety shoot. In contrast, a 15 kDa protein had shown 1.88 folds up-regulation in low treatments of Cr in S2 variety (Fig. 6). The alteration in this protein is reported by Gomathy et al. (2013) in response to salinity stress. A protein of 154 kDa was found to be down-regulated 1.06 times in 400 ppm. 164, 81, and 55 kDa bands seemed to be suppressed in response to Cr stress. A band of 81 kDa proteins showed a one-fold increase in 400 ppm compared to control. Another 15 kDa protein showed 1.88 times up-regulation in 200 ppm, whereas it was not expressed in control and at 400 ppm. The excess of heavy metal ions causes oxidative stress in sunflowers and reduces protein levels. The metal ions disturb metabolism in the sunflower and generate toxic species that cause the degradation of proteins (Palma et al., 2002). The decrease in the number of protein bands in Cr treated leaves compared to control indicated that the cellular proteins are the main targets of heavy metal treatments (El-Gam, 2008).
Table 4.
Protein profile alternation in control and Cr treated shoot of S1 (A) and S2 (B) varieties; where fold change 1 = difference between control and 200 ppm and Fold change 2 = difference between control and 400 ppm, plus sign indicate an increase and minus sign indicate a decrease in expression as compared to reference gel.
|
For S1 (A) |
Expression of total protein concentration load per sample (10 µg) |
||||||
|---|---|---|---|---|---|---|---|
| Sr No. | Mol.Wt (kDa) | Rf value | Control | 200 ppm | 400 ppm | Fold change (1) | Fold change (2) |
| 1 | 89 | 0.119 | 1.4 | 1.06 | 1.55 | −1.32 | +1.11 |
| 2 | 64 | 0.230 | 2.63 | 2.36 | 1.93 | −1.11 | −1.36 |
| 3 | 39 | 0.445 | 1.55 | 1.58 | 1.42 | – | −1.09 |
| 4 | 36 | 0.500 | 1.66 | 1.55 | 2.19 | −1.07 | +1.32 |
| 5 | 20 | 0.717 | 2.13 | 2.24 | 2.26 | +1.05 | +1.06 |
| 6 | 15 | 0.836 | 0.66 | 1.24 | 0.66 | +1.88 | – |
| For S2 (B) | Expression of total protein concentration load per sample (10 µg) | ||||||
| Sr No. | Mol.Wt (kDa) | Rf value | Control | 200 ppm | 400 ppm | Fold change (1) | Fold change (2) |
| 1 | 164 | 0.034 | – | – | 1.36 | – | +1.36 |
| 2 | 154 | 0.043 | 1.63 | 1.35 | – | −1.21 | −1.63 |
| 3 | 83 | 0.139 | – | 0.77 | – | +0.77 | – |
| 4 | 81 | 0.147 | 0.61 | – | 1.08 | −0.61 | +1.77 |
| 5 | 55 | 0.221 | 0.85 | 1.06 | 1.18 | +0.80 | +1.39 |
| 6 | 46 | 0.303 | 1.05 | 1.22 | 1.08 | +1.16 | +1.03 |
| 7 | 30 | 0.473 | 3.24 | 2.87 | 2.71 | −1.13 | −1.20 |
| 8 | 17 | 0.743 | 1.22 | 1.27 | 1.4 | +1.04 | +1.15 |
| 9 | 16 | 0.817 | 0.82 | – | – | −0.82 | −0.82 |
| 10 | 15 | 0.833 | – | 0.95 | 0.69 | +0.95 | +0.69 |
| 11 | 10 | 0.933 | 0.58 | 0.51 | 0.49 | −1.14 | +1.18 |
Fig. 6.
Changes in protein profile in the shoot of S1 (A) and S2 (B) variety after 25 days of applied treatment with Cr. The black and white arrows show newly synthesize and increase expression of protein, respectively. White arrows with an asterisk are protein disappeared upon Cr stress.
4. Conclusions
The findings lead us to conclude that SDS-PAGE provides a useful method for identifying differentially expressed proteins in different plant tissues (seed, leaves, and shoot), against different concentrations of Cr (0, 200 ppm, 400 ppm). Various growth parameters and alternation in protein expression suggested that plants modify their proteome to adapt to Cr stress. Among the two varieties, the overall Cr stress response in AHO-33 is comparatively more altered than sunflower variety RA-713. These differentially expressed proteins, when validated, can act as candidate biomarkers in response to Cr stress. However, for further validation of this study 2-DE combined with mass spectrometric analysis (MALDI-TOF/TOF) approaches is required.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This project was supported by Researchers Supporting Project number (RSP-2021/315) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Anis Ali Shah, Email: anisalibot@gmail.com, anisalibot@gmail.com.
Subhan Danish, Email: sd96850@gmail.com.
Shah Fahad, Email: shah_fahad80@yahoo.com.
Rahul Datta, Email: rahulmedcure@gmail.com.
References
- Abbas M., Anwar J., Zafar-ul-Hye M., Khan R.I., Saleem M., Rahi A.A., Danish S., Datta R. Effect of seaweed extract on productivity and quality attributes of four onion cultivars. Horticulturae. 2020;6:28. [Google Scholar]
- Ahmed N., Ahsen S., Ali M.A., Hussain M.B., Hussain S.B., Rasheed M.K., Butt B., Irshad I., Danish S. Rhizobacteria and silicon synergy modulates the growth, nutrition and yield of mungbean under saline soil. Pakistan J. Bot. 2020;52:9–15. doi: 10.30848/PJB2020-1(16). [DOI] [Google Scholar]
- Ali S., Bharwana S.A., Rizwan M., Farid M., Kanwal S., Ali Q., Ibrahim M., Gill R.A., Khan M.D. Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ. Sci. Pollut. Res. 2015;22:10601–10609. doi: 10.1007/s11356-015-4271-7. [DOI] [PubMed] [Google Scholar]
- Alireza S. Differential proteomics analysis in sunflower (Helianthus annuus L.) Biotechnology. 2014;13:245–247. doi: 10.3923/biotech.2014.245.247. [DOI] [Google Scholar]
- Amin H., Arain B.A., Amin F., Surhio M.A. Phytotoxicity of chromium on germination, growth and biochemical attributes of Hibiscus esculentus L. Am. J. Plant Sci. 2013;04:2431–2439. doi: 10.4236/ajps.2013.412302. [DOI] [Google Scholar]
- Anjum S.A., Ashraf U., Khan I., Tanveer M., Shahid M., Shakoor A., Wang L. Phyto-toxicity of chromium in maize: oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere. 2017;27:262–273. doi: 10.1016/S1002-0160(17)60315-1. [DOI] [Google Scholar]
- Bagheri R., Bashir H., Ahmad J., Baig A., Qureshi M.I. Effects of cadmium on leaf proteome of spinacia oleracia (spinach) Int. J. Agric. Food Sci. Technol. ISSN. 2013;4:33–36. [Google Scholar]
- Berg J.M., Tymoczko J.L., Stryer L. W.H Freeman and Company; New York City, NY, USA: 2002. Biochemistry. [Google Scholar]
- Berni R., Luyckx M., Xu X., Legay S., Sergeant K., Hausman J.F., Lutts S., Cai G., Guerriero G. Reactive oxygen species and heavy metal stress in plants: impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019;161:98–106. doi: 10.1016/j.envexpbot.2018.10.017. [DOI] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cánovas F.M., Dumas-Gaudot E., Recorbet G., Jorrin J., Mock H.P., Rossignol M. Plant proteome analysis. Proteomics. 2004:285–298. doi: 10.1002/pmic.200300602. [DOI] [PubMed] [Google Scholar]
- Cantamutto M., Poverene M. Genetically modified sunflower release: opportunities and risks. F. Crop. Res. 2007;101(2):133–144. [Google Scholar]
- Castillejo M.A., Maldonado A.M., Ogueta S., Jorrin J.V. Proteomic analysis of responses to drought stress in sunflower (Helianthus annuus) leaves by 2DE gel electrophoresis and mass spectrometry. Open Proteomics J. 2008;1:59–71. doi: 10.2174/1875039700801010059. [DOI] [Google Scholar]
- Cvjetko P., Tolić S., Šikić S., Balen B., Tkalec M., Vidaković-Cifrek Ž., Pavlica M. Effect of copper on the toxicity and genotoxicity of cadmium in duckweed (Lemna Minor L.) Arh. Hig. Rada Toksikol. 2010;61:287–296. doi: 10.2478/10004-1254-61-2010-2059. [DOI] [PubMed] [Google Scholar]
- Danish S., Tahir F.A., Rasheed M.K., Ahmad N., Ali M.A., Kiran S., Younis U., Irshad I., Butt B. Effect of foliar application of Fe and banana peel waste biochar on growth, chlorophyll content and accessory pigments synthesis in spinach under chromium (IV) toxicity. Open Agric. 2019;4 doi: 10.1515/opag-2019-0034. [DOI] [Google Scholar]
- Danish S., Zafar-ul-Hye M. Combined role of ACC deaminase producing bacteria and biochar on cereals productivity under drought. Phyton-International Journal of Experimental Botany. 2020;89:217–227. doi: 10.32604/phyton.2020.08523. [DOI] [Google Scholar]
- Dube B.K., Tewari K., Chatterjee J., Chatterjee C. Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere. 2003;53:1147–1153. doi: 10.1016/S0045-6535(03)00570-8. [DOI] [PubMed] [Google Scholar]
- El-Gam A.-D.-E.-G. Protein profile changes in Chrococcus dispersus, Microcystis flos-aquae and Microcoleus steenstruqii in response to cadmium treatments. J. Sci. 2008;20:131–148. [Google Scholar]
- Ejaz F., Nawaz M.F., Dasti Z.A., Gul S., Islam U., Waqar M. Risk assessment of heavy metal and microbial contamination in commercially available salad vegetables of Faisalabad, Pakistan. Pakistan J. Botany. 2020;52(4):1397–1403. [Google Scholar]
- Eleftheriou E.P., Adamakis I.D.S., Panteris E., Fatsiou M. Chromium-induced ultrastructural changes and oxidative stress in roots of Arabidopsis thaliana. Int. J. Mol. Sci. 2015;16:15852–15871. doi: 10.3390/ijms160715852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertani A., Mietto A., Borin M., Nardi S. Chromium in agricultural soils and crops: a review. Water. Air. Soil Pollut. 2017;228:190. doi: 10.1007/s11270-017-3356-y. [DOI] [Google Scholar]
- Faisal M., Hasnain S. Chromate resistant Bacillus cereus augments sunflower growth by reducing toxicity of Cr (VI) J. Plant Biol. 2005;48:187–194. doi: 10.1007/BF03030407. [DOI] [Google Scholar]
- Garcia J.S., Gratão P.L., Azevedo R.A., Arruda M.A.Z. Metal contamination effects on sunflower (Helianthus annuus L.) growth and protein expression in leaves during development. J. Agric. Food Chem. 2006;54:8623–8630. doi: 10.1021/jf061593l. [DOI] [PubMed] [Google Scholar]
- Gomathi R., Yukashini K., Shiyamala S., Vasantha S., Suganya A., Rakkiyappan P. Induced response of sugarcane variety Co 86032 for thermotolerance. Sugar Tech. 2013;15:17–26. doi: 10.1007/s12355-012-0192-7. [DOI] [Google Scholar]
- Gratão P.L., Monteiro C.C., Antunes A.M., Peres L.E.P., Azevedo R.A. Acquired tolerance of tomato (Lycopersicon esculentum cv. Micro-Tom) plants to cadmium-induced stress. Ann. Appl. Biol. 2008;153:321–333. doi: 10.1111/j.1744-7348.2008.00299.x. [DOI] [Google Scholar]
- Guijun D., Weidong P., Gongshe L. The analysis of proteome changes in sunflower seeds induced by N + implantation. J. Biosci. 2006;31:247–253. doi: 10.1007/BF02703917. [DOI] [PubMed] [Google Scholar]
- Hagemeyer J., Breckle S.W. In: Plant Roots: The Hidden Half. Waisel Y., Eshel A., Kafkafi U., editors. Marcel Dekker; New York: 1996. Growth under trace elements stress; pp. 415–433. [Google Scholar]
- Hameed A., Tariq M.S., Babar M.A., Iqbal A., Haq M.A., Ali H. Comparative seed storage protein profiling of kabuli chickpea genotypes. Pakistan J. Bot. 2009;41:703–710. [Google Scholar]
- Horvat T., Vidaković-Cifrek Ž., Oreščanin V., Tkalec M., Pevalek-Kozlina B. Toxicity assessment of heavy metal mixtures by Lemna minor L. Sci. Total Environ. 2007;384:229–238. doi: 10.1016/j.scitotenv.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Jabeen R., Ahmad A., Iqbal M. Phytoremediation of heavy metals: physiological and molecular mechanisms. Bot. Rev. 2009;75:339–364. doi: 10.1007/s12229-009-9036-x. [DOI] [Google Scholar]
- Jorrín J.V., Rubiales D., Dumas-Gaudot E., Recorbet G., Maldonado A., Castillejo M.A., Curto M. Proteomics: a promising approach to study biotic interaction in legumes. A review. Euphytica. 2006;147:37–47. doi: 10.1007/s10681-006-3061-1. [DOI] [Google Scholar]
- Kasai Y., Hyodo H., Ikoma Y., Yano M. Characterization of 1-aminocyclopropane-1-carboxylate (ACC) oxidase in broccoli florets and from Escherichia coli cells transformed with cDNA of broccoli ACC oxidase. Bot. Bull. Acad. Sin. 1998;39:225–230. [Google Scholar]
- Keyster M., Niekerk L.A., Basson G., Carelse M., Bakare O., Ludidi N., Klein A., Mekuto L., Gokul A. Decoding heavy metal stress signalling in plants: towards improved food security and safety. Plants. 2020;9(12):1781. doi: 10.3390/plants9121781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiribandage C.H.W. ProQuest LLC; 789 East Eisenhower Parkway: 2012. Proteomic Approaches to Elucidate Molecular Mechanisms of Metal Accumulation in Plants with Focus on Arsenic. UMI.3534934. [Google Scholar]
- Kumar V., Sharma A., Kaur P., Singh Sidhu G.P., Bali A.S., Bhardwaj R., Thukral A.K., Cerda A. Pollution assessment of heavy metals in soils of India and ecological risk assessment: a state-of-the-art. Chemosphere. 2019;216:449–462. doi: 10.1016/j.chemosphere.2018.10.066. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ma J., Lv C., Xu M., Chen G., Lv C., Gao Z. Photosynthesis performance, antioxidant enzymes, and ultrastructural analyses of rice seedlings under chromium stress. Environ. Sci. Pollut. Res. 2016;23:1768–1778. doi: 10.1007/s11356-015-5439-x. [DOI] [PubMed] [Google Scholar]
- Ma L.Q., Komar K.M., Tu C., Zhang W., Cai Y., Kennelley E.D. A fern that hyperaccumulates arsenic. Nature. 2001;409:579. doi: 10.1038/35054664. [DOI] [PubMed] [Google Scholar]
- Maiti S., Ghosh N., Mandal C., Das K., Dey N., Adak M.K. Responses of the maize plant to chromium stress with reference to antioxidation activity. Brazilian J. Plant Physiol. 2012;24:203–212. doi: 10.1590/S1677-04202012000300007. [DOI] [Google Scholar]
- Nath K., Saini S., Sharma Y.K. Chromium in tannery industry effluent and its effect on plant metabolism and growth. J. Environ. Biol. 2005;26:197–204. [PubMed] [Google Scholar]
- Ozdemir C., Karatas M., Dursun S., Argun M.E., Dogan S. Effect of MnSO4 on the chromium removal from the leather industry wastewater. Environ. Technol. 2005;26:397–400. doi: 10.1080/09593332608618551. [DOI] [PubMed] [Google Scholar]
- Palma J.M., Sandalio L.M., Javier Corpas F., Romero-Puertas M.C., McCarthy I., Del Río L.A. Plant proteases, protein degradation, and oxidative stress: role of peroxisomes. Plant Physiol. Biochem. 2002:521–530. doi: 10.1016/S0981-9428(02)01404-3. [DOI] [Google Scholar]
- Rafiullah, Jamal Khan, M., Muhammad, D., Fahad, S., Adnan, M., Wahid, F., Alamri, S., Khan, F., Muhammad Dawar, K., Irshad, I., Danish, S., Arif, M., Amanullah, Saud, S., Khan, B., Ahmad Mian, I., Datta, R., Zarei, T., Ali Shah, A., Ramzan, M., Zafar Ul Hye, M., Mussarat, M., Siddiqui, M.H., 2020a. Phosphorus nutrient management through synchronization of application methods and rates in wheat and maize crops. Plants 9, 1389. 10.3390/plants9101389. [DOI] [PMC free article] [PubMed]
- Rafiullah, Tariq, M., Khan, F., Shah, A.H., Fahad, S., Wahid, F., Ali, J., Adnan, M., Ahmad, M., Irfan, M., Zafar-ul-Hye, M., Battaglia, M.L., Zarei, T., Datta, R., Saleem, I.A., Hafeez-u-Rehman, Danish, S., 2020b. Effect of micronutrients foliar supplementation on the production and eminence of plum. Qual. Assur. Saf. Crop. Foods 12, 32–40. 10.15586/qas.v12iSP1.793.
- Rani U.R., Reddy A.R. Salt stress responsive polypeptides in germinating seeds and young seedlings of Indica Rice (Oryza sativa L.) J. Plant Physiol. 1994;143:250–253. doi: 10.1016/S0176-1617(11)81696-2. [DOI] [Google Scholar]
- Rossignol M. Analysis of the plant proteome. Curr. Opin. Biotechnol. 2001;12(2):131–134. doi: 10.1016/S0958-1669(00)00186-5. [DOI] [PubMed] [Google Scholar]
- Salt D.E., Smith R.D., Raskin I. Phytoremediation. Annu. Rev. Plant Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Shafiq M., Bakht J., Iqbal A., Shafi M. Growth, protein expression and heavy metal uptake by tobacco under heavy metals contaminated soil. Pakistan J. Bot. 2020;52(5):1569–1576. [Google Scholar]
- Shahid M., Shamshad S., Rafiq M., Khalid S., Bibi I., Niazi N.K., Dumat C., Rashid M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere. 2017;178:513–533. doi: 10.1016/j.chemosphere.2017.03.074. [DOI] [PubMed] [Google Scholar]
- Shanker A.K., Djanaguiraman M., Sudhagar R., Chandrashekar C.N., Pathmanabhan G. Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram (Vigna radiata (L.) R.Wilczek. cv CO 4) roots. Plant Sci. 2004;166:1035–1043. doi: 10.1016/j.plantsci.2003.12.015. [DOI] [Google Scholar]
- Stambulska U.Y., Bayliak M.M., Lushchak V.I. Chromium(VI) toxicity in legume plants: modulation effects of rhizobial symbiosis. Biomed Res. Int. 2018;2018:1–13. doi: 10.1155/2018/8031213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel R.G., Torrie J.H., Dickey D.A. third ed. McGraw Hill Book International Co.; Singapore: 1997. Principles and Procedures of Statistics: A Biometrical Approach. [Google Scholar]
- Sytar O., Ghosh S., Malinska H., Zivcak M., Brestic M. Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol. Plant. 2020;173(1):148–166. doi: 10.1111/ppl.13285. [DOI] [PubMed] [Google Scholar]
- Sytar O., Kumari P., Yadav S., Brestic M., Rastogi A. Phytohormone priming: regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019;38(2):739–752. [Google Scholar]
- Tang J., Xu J., Wu Y., Li Y., Tang Q. Effects of high concentration of chromium stress on physiological and biochemical characters and accumulation of chromium in tea plant (Camellia sinensis L.) African J. Biotechnol. 2012;11:2248–2255. doi: 10.5897/ajb11.2402. [DOI] [Google Scholar]
- Ullah A., Ali M., Shahzad K., Ahmad F., Iqbal S., Habib Ur Rahman M., Ahmad S., Mazhar Iqbal M., Danish S., Fahad S., Alkahtani J., Soliman Elshikh M., Datta R. Impact of seed dressing and soil application of potassium humate on cotton plants productivity and fiber quality. Plants. 2020;9:1444. doi: 10.3390/plants9111444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbi F.M., Arruda S.C.C., Rodriguez A.P.M., Pérez C.A., Arruda M.A.Z. Metal-binding proteins scanning and determination by combining gel electrophoresis, synchrotron radiation X-ray fluorescence and atomic spectrometry. J. Biochem. Biophys. Methods. 2005;62:97–109. doi: 10.1016/j.jbbm.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Wahid F., Fahad S., Danish S., Adnan M., Yue Z., Saud S., Siddiqui M.H., Brtnicky M., Hammerschmiedt T., Datta R. Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture. 2020;10:334. doi: 10.3390/agriculture10080334. [DOI] [Google Scholar]
- Zafar-ul-Hye M., Shahjahan A., Danish S., Abid M., Qayyum M.F. Mitigation of cadmium toxicity induced stress in wheat by ACC-deaminase containing PGPR isolated from cadmium polluted wheat rhizosphere. Pakistan J. Bot. 2018;50:1727–1734. [Google Scholar]
- Zafar-ul-Hye M., Zahra M.B., Danish S., Abbas M., Rehim A., Akbar M.N., Iftikhar A., Gul M., Nazir I., Abid M., Tahzeeb-ul-Hassan M., Murtaza M. Multi-strain inoculation with PGPR producing ACC deaminase is more effective than single-strain inoculation to improve wheat (Triticum aestivum) growth and yield. Phyton-International Journal of Experimental Botany. 2020;89:405–413. doi: 10.32604/phyton.2020.08918. [DOI] [Google Scholar]
- Zupančič M., Bukovec N., Milačič R., Ščančar J. Comparison of various phosphate stabilisation agents for the immobilisation of Ni and Zn in sewage sludge. Water. Air. Soil Pollut. 2004;156:57–69. doi: 10.1023/B:WATE.0000036789.07619.b6. [DOI] [Google Scholar]