Abstract
Ovulation failure was associated with a reduction in pre-mating concentrations of oestradiol-17β and prolactin (PRL). The present study aimed to evaluate whether pre-mating PRL levels have a role in the reproductive efficiency of doe rabbits. A total of 78 multiparous California does (2nd parity) were divided, according to plasma pre-mating PRL, into five categories, >20–25, >25–30, >30–35, >35–40, and >40–45 ng/ml. Does in all categories were naturally mated and kindled, then their reproductive measurements and progesterone (P4) levels were determined. Results show that pre-mating PRL averaged 23.60 ± 0.78, 28.00 ± 0.83, 33.46 ± 0.43, 38.17 ± 0.49 and 41.98 ± 0.68 ng/ml in five categories (p < 0.05), respectively, representing the highest distribution (38.5%) in the 3rd-category. Live body weight of doe rabbits, at mating, pregnancy, and parturition increased (p < 0.05) with increasing pre-mating PRL level. The number of services, litter size, and pregnancy rate increased (p < 0.05) by increasing PRL levels. Reproductive traits and P4 level at mid-pregnancy of does, and the average weight of kits at birth increased (p < 0.05) by increasing PRL levels. The pre-mating PRL profile is important for the identification of reproductive performance in doe rabbits.
Keywords: Litter size, Prolactin, Progesterone, Pregnancy, Rabbits
1. Introduction
Prolactin (PRL) is a 199-amino-acid single-chain protein generated in the anterior pituitary glands lactotrophs (Nett, 1993). It is principally regulated by dopamine produced from the hypothalamus and has many biological functions in many species (Hadley, 2000). Dopamine is a PRL inhibiting factor (Ben-Jonathan,1985). Although prolactin is associated with pregnancy and lactation, it has lately been discovered to play an important function in reproducing non-pregnant females of several species. PRL levels fluctuate dramatically during puerperal breastfeeding due to their release in response to suckling Hwang et al. (1971). PRL levels in the blood steadily rise during pregnancy, peaking at term Ajibola et al. (2012). PRL levels fall after birth, but baseline concentrations do not reach non-pregnant levels until at least two weeks after delivery. (McNeilly, 1965).
Ovulation failure was linked to rabbits with lower pre-mating levels of oestradiol-17β and PRL. Rabbits that did not ovulate showed lower pre-mating PRL concentrations than others who ovulated at this time. Despite an apparent identical suckling stimulation, litter size, and weight, no significant changes in PRL concentrations were identified throughout the pre-ovulatory period between rabbits ovulating on different post-partum days Lamb et al. (1991).
Previous research has shown that PRL is important in reproduction in mares in non-pregnant animals. During the shift from the anovulatory to the breeding season, the level of PRL rose, peaking during the breeding season Thompson et al. (1991). In another experiment, daily delivery of recombinant porcine PRL to seasonally anovulatory pony mares resulted in ovulation or the formation of the corpus luteum (CL) Thompson et al. (1986).
Given the positive response of reproduction in mares to the profile of PRL during the breeding season, the role of a pre-mating profile of PRL is of limited value for identifying possible variability in the fertility of doe rabbits. These findings prompted us to evaluate the potential effects of pre-mating prolactin profile on the reproductive efficiency of doe rabbits and the litter characteristics of their offspring.
2. Materials and methods
The experimental work was carried out in a private Rabbit Production Farm in Zian Region, Dakahlia governorate, Egypt, from December 2019 to March 2020.
2.1. Animals and experimental design
The experimental animals included multiparous California doe rabbits (n = 78) having 6–7 months of age and weighing 2.8–3.1 kg, and fertile California rabbit bucks (n = 20) with 10–13 months of age and weighing 3.5–4.1 kg. All experimental animals were housed in flat deck cages (50–60-40 cm) with natural ventilation in a building. Automatic water dispensers and feeders were installed in all cages, but only the does' cages were included inside nest boxes.
A complete feed diet (2750 kcal ME/kg, 18.5% CP, and 12.5% CF) was used as a commercial diet for feeding rabbits ad-libitum according to their physiological stage. The experimental animals were kept under the same managerial factors. Before kindling, cages and nest boxes were regularly cleaned and disinfected. Cleaning the floor of the cages from the urine and feces was achieved daily in the morning.
At the beginning of the experimental period, blood samples were collected from does (n = 78) immediately following the 1st parity. Within 24 h of parturition, receptive doe rabbits were mated, and plasma PRL concentration was determined for all doe rabbits. Doe rabbits were divided, according to plasma PRL levels, into five PRL categories (A-E), involving >20–25, >25–30, >30–35, >35–40 and >40–45 ng/ml. Pregnancy diagnosis was performed on days 10–12 post-mating, and doe rabbits that failed to conceive were remated.
2.2. Reproductive traits
Doe rabbits were weighed on mating, transferred to the buck's cage, then returned to their cage after mating. To diagnose pregnancy and compute the pregnancy rate, each mated doe was palpated 10–12 days after mating. Rabbits who did not conceive after the first mating were remated, and the number of services per conception was recorded. On day 27 of pregnancy, wooden straw was placed in the nest boxes to assist the doe in creating a warm, comfy nest for the kits. Following the kindling, at birth and weaning, each doe's gestation time and litter size (total and live) were calculated. Does live body weight on days 9, 14, and 28 of pregnancy and at the time of fertilization (within 12 h). The viability rate at birth and weaning, kit weight at birth, and sex ratio at weaning were all documented.
2.3. Blood samples and hormonal assay
Blood samples were taken from the ear vein and promptly centrifuged (3000 rpm for 15 min), after which blood plasma was separated and stored at −20 °C until the hormonal testing. Blood samples were taken from all does (n = 78) before mating for PRL determination and from seven does proven pregnant in each PRL category on Days 15 and 27 of gestation for the progesterone (P4) assay El‐Ratel et al. (2020).
Pre-mating PRL concentration in blood plasma was determined using PRL kit (DiaMetra S.r. I Headquarter: Via Garibaldi, 18-20090 SEGRATE “MI,” Italy) according to Shome and Parlow (1974). P4 plasma concentrations were measured using a P4 kit (Oxis International, Inc. 323 Vintage Park Dr. Foster City, CA 94404) on Days 15 and 27 of pregnancy Ross et al. (1981). According to manifactorial kits used in this study for the PRL and P4 assays, the intra- and inter-assay coefficients of variation were 51; 8–3 percent and 6; 13%, respectively.
2.4. Statistical analysis
The data were statistically examined with the (SAS, 2004) computer program, which used one-way analysis of variance (ANOVA) techniques with GLM procedures. Duncan Multiple Range Test was used to distinguish the significant differences, using a level of p < 0.05. The pregnancy rate, viability rate, and sex ratio were all statistically analyzed using the Chi-square test. The pre-mating PRL level was correlated with the reproductive features of does use Pearson correlation coefficients.
3. Results
3.1. Pre-mating prolactin profile
Pre-mating plasma PRL ranged between >20 and 45 ng/ml in five categories, averaging the lowest values in category A and the highest values in category E (p < 0.05). Does in category C (>30–35 ng/ml) represented the highest frequency distribution (38.5%), while those in category E (>40–45 ng/ml) had the lowest distribution (12.8%, Table 1).
Table 1.
Pre-mating plasma PRL profile of doe rabbits.
| Pre-mating PRL category | Doe rabbits |
Pre-mating PRL (ng/ml) |
||
|---|---|---|---|---|
| n | % | Range | Mean ± SE | |
| A | 14 | 17.9 | >20–25 | 23.60 ± 0.78e |
| B | 12 | 15.4 | ≥25-30 | 28.00 ± 0.83d |
| C | 30 | 38.5 | >30–35 | 33.46 ± 0.43c |
| D | 12 | 15.4 | >35–40 | 38.17 ± 0.49b |
| E | 10 | 12.8 | >40–45 | 41.98 ± 0.68a |
a,b …e: Means denoted within the same column with different superscripts are significantly different at p < 0.05.
3.2. Live body weight of does
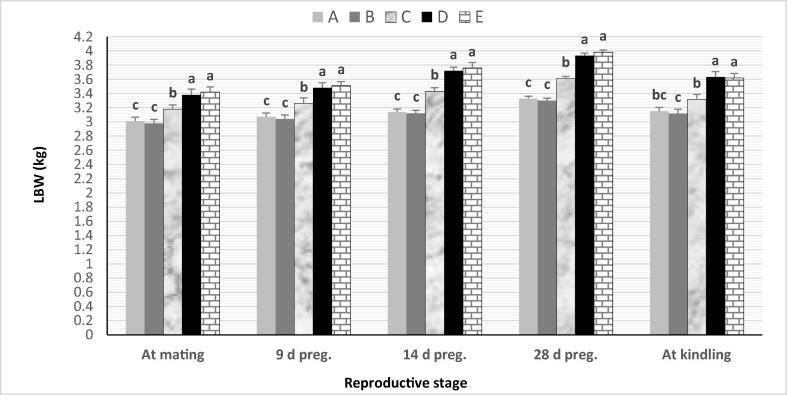
Live body weight (LBW) of does gradually increased at mating and during the gestation period with a marked drop at kindling, being the heaviest in categories D and E, moderate in category C, and the lightest in categories A and B (p < 0.05, Fig. 1). The obtained results cleared pre-mating PRL levels of different categories between 23.60 ± 0.78 and 41.98 ± 0.68 ng/ml, and the differences among categories were significant (p < 0.05). Category C of PRL level of 33.46 ± 0.43 ng/ml represented the highest distribution among categories. Also, a significant relationship was cleared between pre-mating PRL level and LBW of does. Does in all PRL categories showed a similar trend of increased LBW from mating up to kindling by increasing pre-mating PRL. Does were heavier with high than low pre-mating PRL categories, whereas does were the heaviest in categories D and E with the highest PRL levels (>35–45 ng/ml), and the lightest in categories A and B with the lowest PRL levels (>20–30 ng/ml).
Fig. 1.
Change in live body weight of doe rabbits from mating up to kindling. (a,b,c: significant category differences at p < 0.05).
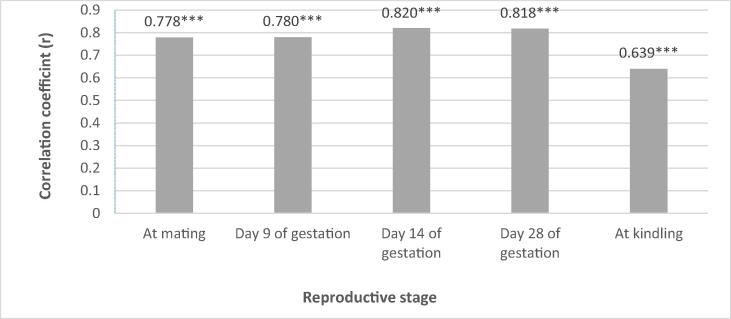
The correlation coefficients between pre-mating PRL level and LBW of does were significantly positive at all reproductive stages, being the highest on Day 14 and 28 of gestation (r = 0.820; r = 0.818; p < 0.05) and the lowest at kindling (r = 0.639, p < 0.05) (Fig. 2).
Fig. 2.
Correlation coefficients between PRL level and doe live body weight at different reproductive stages. (*** significant at p < 0.001).
3.3. Reproductive performance of does
Reproductive traits including the number of mating cases per conception, pregnancy rate after the 1st mating, litter size (live and dead) of does, and viability rate and average body weight of their kits increased (p < 0.05) by increasing PRL category, being the best in categories D and E, and the lowest in category A. However, gestation period length was nearly similar in all PRL categories (Table 2).
Table 2.
Reproductive traits of doe rabbits as affected by the PRL category.
| PRL category | Mating cases per conception (n) | Pregnancy rate+ | Gestation period (d) |
Litter size at birth |
Average kit weight at birth (g) | |
|---|---|---|---|---|---|---|
| Total | Live | |||||
| A | 1.14 ± 0.16ab | 85b | 31.3 ± 0.39 | 5.71 ± 0.31c | 5.14 ± 0.28b | 55.71 ± 2.227c |
| B | 1.00 ± 0.00b | 100a | 31.0 ± 0.47 | 5.80 ± 0.39c | 5.00 ± 0.58b | 55.00 ± 0.000c |
| C | 1.20 ± 0.10a | 80b | 31.1 ± 0.20 | 6.80 ± 0.27b | 6.40 ± 0.40b | 60.47 ± 0.581b |
| D | 1.00 ± 0.00b | 100a | 30.8 ± 0.18 | 8.40 ± 0.22a | 7.80 ± 0.45a | 66.00 ± 0.931a |
| E | 1.00 ± 0.00b | 100a | 31.6 ± 0.22 | 8.60 ± 0.37a | 8.40 ± 0.47a | 65.00 ± 0.000a |
: Means denoted within the same column with different superscripts are significantly different at p < 0.05. + Following the 1st mating.
3.4. Litter characteristics
Litter characteristics at weaning in litter size and the number of males and females born (p < 0.01) increased by increasing PRL level, being the highest with does in categories D and E. However, the sex ratio of kits at weaning was not affected by PRL level (Table 3, Table 4) .
Table 3.
Litter characteristics and sex ratio of does at weaning in different PRL categories.
| PRL category | Litter size at weaning | Number of males/doe | Number of females/doe | Sex ratio (Male: female) |
|---|---|---|---|---|
| A | 4.42 ± 0.222c | 2.42 ± 0.222b | 2.00 ± 0.000b | 54.3 |
| B | 4.60 ± 0.425c | 2.60 ± 0.425b | 2.00 ± 0.000b | 54.7 |
| C | 6.07 ± 0.333b | 4.00 ± 0.340a | 2.07 ± 0.274b | 65.1 |
| D | 7.80 ± 0.456a | 4.20 ± 0.684a | 3.60 ± 0.559ab | 53.3 |
| E | 8.00 ± 0.294a | 4.00 ± 0.294a | 4.00 ± 0.294a | 50.0 |
: Means denoted within the same column with different superscripts are significantly different at p < 0.05.
Table 4.
Personal correlation coefficients of PRL profile or LBW at mating with different reproductive traits.
| Item | Correlation coefficient (r) |
|
|---|---|---|
| Pre-mating PRL | Pre-mating LBW | |
| Pregnancy rate | 0.387* | 0.175 |
| Total litter size at birth | 0.779*** | 0.859*** |
| Live litter size at birth | 0.670*** | 0.754*** |
| Weaning litter size | 0.772*** | 0.813*** |
| Average kit weight at birth | 0.772*** | 0.806*** |
| P4 at mid-pregnancy+ | 0.809*** | 0.810*** |
| P4 at late pregnancy++ | −0.546*** | −0.454** |
On day 15 of pregnancy;
on day 27 of pregnancy;
significant at p < 0.05;
significant at p < 0.01;
significant at p < 0.001.
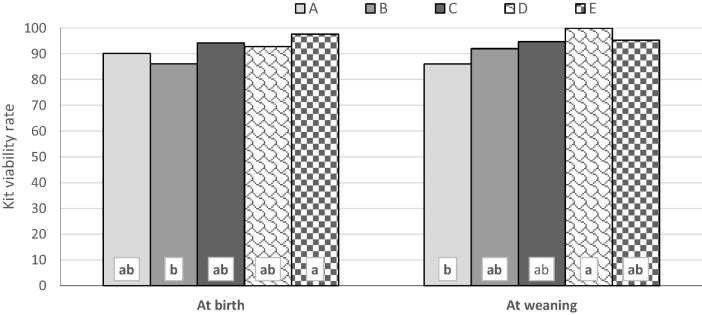
Kit viability rate at birth showed an inconsistent trend (p < 0.05) of change with pre-mating PRL level. The viability rate at weaning showed a gradual increase (p < 0.05) by increasing PRL level up to category D, then decreased (p ≥ 0.05) with category E (Fig. 3).
Fig. 3.
Kit viability rate at birth and weaning as affected by PRL category. (a,b: significant category differences at p < 0.05).
3.5. Progesterone profile during gestation
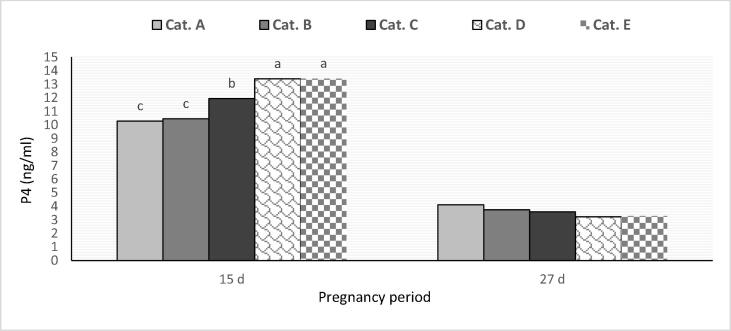
The average plasma P4 concentration at mid-pregnancy (15 days) increased (p < 0.05) by increasing pre-mating PRL level up to the maximal level with categories D and E. Concentration P4 of at late pregnancy (27 days) was not affected by pre-mating PRL category. In all PRL categories, the P4 level was higher at mid-pregnancy than at late pregnancy (Fig. 4).
Fig. 4.
The concentration of progesterone in the blood plasma of does in different PRL categories at mid-and late pregnancy. (a,b,c: significant category differences at p < 0.05.
4. Discussion
Rabbit production is one of the most important successful economic projects due to the short time of the production cycle and the high productivity of does (Khelfa et al., 2012, Khelfa et al., 2015, Abdelnour et al., 2020a, Sheiha et al., 2020). Rabbit meat also contributes to bridging the nutritional gap and provides the consumer with meat of great nutritional value and distinct because it contains a proportion of protein, vitamins and minerals equivalent to red meat with low cholesterol level (Khelfa et al., 2012a, Khelfa et al., 2012b, Abdelnour et al., 2020b, Abou-Kassem et al., 2022). Viral, bacterial, parasitic and fungal diseases are among the most important risks threatening the poultry and rabbit industry all over the world (Setta et al., 2018, Abd El Hamid et al., 2019, Salem et al., 2019, Marouf et al., 2020, Morsy et al., 2020, Abd El-Hack et al., 2021b). Recently, the world is moving to find natural and safe products to resist these diseases and raise the productive efficiency of birds and rabbits and provide a rich and safe food source for human consumption (Abd El-Hack et al., 2021a, Abou-Kassem et al., 2021a, Arif et al., 2022, El-Shall et al., 2022).
The current study aimed to identify the possible effects of pre-mating PRL profile on reproductive efficiency of multiparous doe rabbits (2nd parity) and litter characteristics of their offspring. Pre-mating PRL and LBW were 33.46 ng/ml and 3.18 kg in 38.5% of does (category C), and 41.98 ng/ml and 3.41 kg in 12.8% of does (category E). This relationship was indicated by the recorded positive correlation between pre-mating PRL level, and LBW of does at mating (r = 0.778, p < 0.001). Moreover, pre-mating PRL correlated with LBW of does during gestation (r = 0.780–0.818, p < 0.001) and kindling (r = 0.639, p < 0.001). These findings may suggest that LBW of does at mating is important to reflect a higher PRL profile at this stage of reproduction.
Regarding the relationship between pre-mating PRL category and reproductive performance, our results indicated remarkable improvement in most reproductive traits by increasing pre-mating PRL associated with increasing doe LBW at mating. This was proved in categories D and E with the highest pre-mating PRL profile and heaviest does at mating, whereas does in these categories showed the best reproductive traits and weight of their kits compared to categories A, B and C. In accordance with the present results, Rosell et al. (2020) found that pregnancy rate was increased in overweight than in underweight (73.1 vs. 82.6%) doe rabbits. These results may indicate an association between high pre-mating PRL and improving pregnancy rate and litter size with increasing LBW and PRL of does at mating. These results may indicate an association between high pre-mating PRL and improving pregnancy rate and litter size. However, improving litter size may be associated with PRL level, and LBW of does at mating. This relation was indicated by highly significant (p < 0.001) and strong correlations between LBW of does at mating and litter size as total (r = 0.859), live at birth (r = 0.754), and weaning (r = 0.813).
Our results showed that increasing kit viability rate at weaning was observed in high PRL categories (D and E) in comparison with low PRL categories. These findings may suggest an association of PRL concentration at mating and during the suckling period. Importantly, increasing PRL for initiation of lactation and consequently higher viability of kits during the suckling period.
Several publications assessed the impact of PRL throughout the suckling phase in terms of pronounced negative effects on rabbit reproductive performance. Also, during the first and last days of lactation, when plasma PRL levels were low, an increase in sexual receptivity was found, which is linked to receptivity inhibition. The antagonism between elevated plasma PRL concentrations during the suckling period and this impact could be the cause. Ubilla et al. (1992), and the estrogenic effect on receptivity described Hudsonet al. (1990). About the beneficial effects of PRL level at mating on reproductive performance of doe rabbits, plasma pre-ovulatory PRL concentration was higher for ovulating than in non-ovulating and nulliparous doe rabbits Lamb et al. (1991). This observation is consistent with our findings of doe pre-mating PRL.
After parturition, the level of PRL decreased but do not reach the non-pregnant range (basal level) 2 weeks post-parturition at least (McNeilly, 1965). Therefore, a high PRL concentration was expected at mating immediately after parturition compared to a few days post-parturition. Increasing PRL level immediately post-parturition and pre-mating may positively affect the reproductive performance of doe rabbits. In this respect, the PRL level has a wide range of biological actions in different species. At puberty, the level of PRL seems to increase, but only to a minor degree in comparison with gonadotrophins (LH and FSH); estrogen experienced at puberty is stimulatory to the secretion of PRL.
Moreover, PRL was found to increase receptors of LH on granulosa and luteal cells of the ovarian follicles (Jones and Hsueh, 1981, Advis et al., 1981). In rats, PRL suppression retarded the normal development of the ovarian follicles and may delay the incidence of puberty (Besognet et al., 1997). In mares, PRL has active involvement in reproduction. Horses have higher PRL levels during the breeding season, with mares having the highest levels. Experience increases in many hormones, included PRL, was observed during the transition interval of mares (from the anovulatory season to the breeding season Thompson et al. (1991). The level of PRL increases in mares during winter, aiding in follicular development and may lead to ovulation. In this context, an increase in PRL level was reported after the dopamine antagonist's treatment (Van Straalen and Zeilmaker, 1982). Stimulating follicular growth positively and rapidly was observed to increase PRL level by treatment of dopamine antagonist or by bovine PRL injection Nequin et al. (1993). Also, a daily injection of seasonally anovulatory pony mares with a recombinant porcine PRL leads to ovulation or the formation of CL Thompson et al. (1986).
The absence of the pre-ovulatory LH surge and a decrease in pre-mating concentrations of estradiol-17β and PRL were linked with ovulation failure in rabbits. Does who failed to ovulate on day 14 postpartum had no preovulatory LH surge and significantly (p < 0.05) lower pre-mating oestradiol-17β and prolactin concentrations than those who ovulated at this period. In the blood plasma of ovulating, non-ovulating, and unmated rabbit does, the average PPRL was 41.6, 15.5, and 16.3 ng/ml, respectively. However, no significant variations in PRL concentrations were found between doe rabbits ovulating on days 1 and 14 post-partum during the pre-ovulatory period. Lamb et al. (1991). In intact rabbits, chronic prolactin therapy stimulates cholesterol accumulation and increases the ovary's sensitivity to LH. Chronic prolactin therapy produces ovarian interstitial tissue enlargement in these animals but does not stimulate progestin release in the absence of estrogen or LH. Prolactin works specifically on the rabbit ovary to check the steroid-producing capability and interstitial tissue shape Hilliard et al. (1968). The immunological activity of PRL on the health and immunity of doe rabbits at mating may explain why high PRL levels improve reproductive performance. In this context, PRL activates thymocytes and lymphocytes and the secretion of the thymic hormone “thymulin” (Kruger et al., 1989, Spangelo and Gorospe, 1995).
It is of interest to note that P4 level during mid-pregnancy increased by increasing PRL level associated with litter size (number of CLs) of each PRL category. This trend reflected a strong and positive correlation of premating PRL with P4 at mid-pregnancy (r = 0.809, p < 0.001). At late pregnancy, P4 level decreased by increasing PRL associated with the incidence of CL regression at the end of gestation period in all categories. Still, the differences were not significant, reflecting a negative correlation with P4 at late pregnancy (r = −0.546, p < 0.001).
Based on the initial findings and correlations, a pre-mating PRL profile is important for identifying the reproductive performance of doe rabbits with different PRL levels. Pre-mating PRL level increased by advancing parity, and threshold level of pre-mating PRL in rabbits is required for fertility prediction.
Data availability statement
The data sets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Compliance with ethical standards and animal welfare
The experiments were carried out following the European Parliament and Council Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes, which came into effect on September 22, 2010. Only blood samples were subjected to ethical review. The authors affirm that the journal's ethical principles were followed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We appreciate and thank Taif University for the financial support for Taif University Researchers Supporting Project (TURSP-2020/105), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Abo Ghanima M.M., Shukry M., Swelum A.A., Taha A.E., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult. Sci. 2021:101584. doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., El-Shall N.A., Saad A.M., Salem H.M., El-Tahan A.M., Khafaga A.F., Taha A.E., AbuQamar S.F., El-Tarabily K.A. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives – a comprehensive review. Poult. Sci. 2021:101590. doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El Hamid M.I., Abd El-Moaty D.A.M., El-Sergany E.F., Salem H.M., El-Sawy H., Abbas A.M. Utility of molecular biology tools for identification and characterization of egyptian riemerella anatipestifer duck isolates. Inter. J. Vet. Sci. 2019;8(4):335–341. [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-shargi O.Y.A., Al-Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240:104220. doi: 10.1016/j.livsci.2020.104220. [DOI] [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19(1):1046–1056. doi: 10.1080/1828051X.2020.1815598. [DOI] [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20(1):896–910. [Google Scholar]
- Abou-Kassem, D.E., El-Abasy, M.M., Al-Harbi, M.S., Abol-Ela, S., Salem, H.M., El-Tahan, A.M., El-Saadony, M.T., Abd El-Hack, M.E., Ashour, E.A., 2022. Influences of total sulfur amino acids and photoperiod on growth, carcass traits, blood parameters, meat quality and cecal microbial load of broilers. Saudi J. Biol. Sci. 29 (3), 1683–1693. doi: https://doi.org/10.1016/j.sjbs.2021.10.063. [DOI] [PMC free article] [PubMed]
- Advis J.P., White S.S., Ojeda S.R. Delayed puberty induced by chronic suppression of prolactin release in the female rat. Endocrinol. 1981;109(5):1321–1330. doi: 10.1210/endo-109-5-1321. [DOI] [PubMed] [Google Scholar]
- Ajibola A., Chamunorwa J.P., Erlwanger K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. (Lond) 2012;9(1):61. doi: 10.1186/1743-7075-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif, M., Baty, R. S., Althubaiti, E. H., Ijaz, M. T., Fayyaz, M., Shafi, M. E., Albaqami, N. M., Alagawany, M., Abd El-Hack, M. E., Taha, A. E., Salem, H. M., El-Tahan, A. M., Elnesr, S. S., 2022. The impact of betaine supplementation in quail diet on growth performance, blood chemistry, and carcass traits. Saudi J. Biol. Sci. 29 (3), 1604–1610. https://doi.org/10.1016/j.sjbs.2021.11.002. [DOI] [PMC free article] [PubMed]
- Ben-Jonathan, N.J.E., 1985. Dopamine: a prolactin-inhibiting hormone. Endocr. Rev. 6, Issue 4, 564–589, doi.org/10.1210/edrv-6-4-564. [DOI] [PubMed]
- Besognet B., Hansen B.S., Daels P.F. Induction of reproductive function in anestrous mares using a dopamine antagonist. Theriogenology. 1997;47(2):467–480. doi: 10.1016/s0093-691x(97)00005-8. [DOI] [PubMed] [Google Scholar]
- El‐Ratel I.T., Abdel‐Khalek A.-K., Gabr S.A., Hammad M.E., El‐Morsy H.I. Influence of allicin administration on reproductive efficiency, immunity and lipid peroxidation of rabbit does under high ambient temperature. J. Anim. Physiol. Anim. Nutr. 2020;104(2):539–548. doi: 10.1111/jpn.13316. [DOI] [PubMed] [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2022;101(1):101542. doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley, M.E., 2000. Endocrinology, Prentice Hall, Inc., Upper Saddle River, New Jersey, pp 97-99, 517-518, 525, www.biblio.com/9780130803566.
- Hilliard, J., Spies, H.G., Lucas, L., Sawyer, C.H.J.E., 1968. Effect of prolactin on progestin release and cholesterol storage by rabbit ovarian interstitium. Endocrinology, 82, 122-131. doi:10.1210/endo-82-1-122. [DOI] [PubMed]
- Hudson, R., González-Mariscal, G., Beyer, C.J.H., 1990. Chin marking behavior, sexual receptivity, and pheromone emission in steroid-treated, ovariectomized rabbits. Horm. Behav., 24, 1-13, doi.org/10.1016/0018-506X(90)90022-P. [DOI] [PubMed]
- Hwang, P., Guyda, H., Friesen, H., 1971. A radioimmunoassay for human prolactin. PNAS August 1, 68 (8) 1902-1906, doi.org/10.1073/pnas.68.8.1902. [DOI] [PMC free article] [PubMed]
- Jones, P.B., Hsueh, A.J.J.E., 1981. Regulation of progesterone-metabolizing enzyme by adrenergic agents, prolactin, and prostaglandins in cultured rat ovarian granulosa cells. Endocrinology, 109,1347-1354, https ://doi.org/10.1210/endo-109-5-1347. [DOI] [PubMed]
- Khelfa D.E., Abd El-Ghany W.A., Salem H.M. Serological and molecular typing of clostriduim perfringens and its toxins recovered from weaned rabbit’s flocks in Egypt. Life Sci. 2012;9(4):2263–2271. [Google Scholar]
- Khelfa D.E., Abd El-Ghany W.A., Salem H.M. Recent status of clostridial enteritis affecting early weaned rabbits in Egypt. Life Sci. 2012;9(4):2272–2279. [Google Scholar]
- Khelfa D.E., Abd El-Ghany W.A., Salem H.M. The effect of Clostridium difficile experimental infection on the health status of weaned rabbits. J. Appl. Sci. Res. 2012;8(8):4672–4677. [Google Scholar]
- Khelfa D.G., Madian K., El-Meneisy A. A., Faten F. M., Salem, H. M., 2015. Field and Laboratory Diagnosis of C. perfringens Enteric Infection among Rabbit Flocks in Egypt. Middle East Journal of Applied Sciences (MEJAS). ISSN 2077-4613. 5(1): 252-261.
- Kruger T.E., Smith L.R., Harbour D.V., Blalock J.E. Thyrotropin: an endogenous regulator of the in vitro immune response. J. Immunol. 1989;142:744–747. PMID: 2492328. [PubMed] [Google Scholar]
- Lamb, I., Strachan, W., Henderson, G., Atkinson, T., Lawson, W., Partridge, G., Fuller, M., Racey, P., 1991. Effects of reducing the remating interval after parturition on the fertility and plasma concentrations of luteinizing hormone, prolactin, oestradiol-17β and progesterone in lactating domestic rabbits. J. Reprod. Fert., 92, 281-289, doi.org/10.1530/jrf.0.0920281. [DOI] [PubMed]
- Marouf, S., Moussa, I.M., Salem, H., Sedeik, M., Elbestawy, A. H. A. Hemeg, T. M. Dawoud A. S. Mubarakb, H. Mahmouda, R. A. Alsubki, Ali H. Bahkali. 2020. A picture of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Egypt: Phenotypic and genotypic characterization. J. King Saud Univ. Sci. 32: 2263–2268., https://doi.org/10.1016/j.jksus.2020.02.036.
- McNeilly, A.S., 1965. Lactation and the physiology of prolactin secretion. Postgrad. Med. J., 51, 231-235, doi.org/10.1136/pgmj.51.594.231. [DOI] [PMC free article] [PubMed]
- Morsy E.A., Salem H.M., Khattab M.S., Hamza D.A., Abuowarda M.M. Encephalitozoon cuniculi infection in farmed rabbits in Egypt. Acta Vet Scand. 2020;62:11. doi: 10.1186/s13028-020-0509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nequin, L., King, S., Johnson, A.L., Gow, G., 1993. Ferreira-Dias, G.M. Prolactin may play a role in stimulating the equine ovry during the spring reproductive transition. J. Equine Vet. Sci., 13, 631-635, doi.org/10.1016/S0737-0806(07)80391-1.
- Nett, T.M., 1993. Reproductive peptide and protein hormones. in: Equine Reproduction. A. McKinnon and J. Voss, ed. Williams & Wilkins, Media, PA, pp. 110.
- Rosell J.M., de la Fuente L.F., Carbajo M.T., Fernández X.M. Reproductive diseases in farmed rabbit does. Animals. 2020;10:1873. doi: 10.3390/ani10101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, G.T., Vande Wiele, R.L., and Frantz, A.G., 1981. The ovaries and the breasts. In: Williams, R.H. (ed.) Textbook of Endocrinology, W.B. Saunders Co., Philadelphia, 355–411.
- Salem H.M., Morsy E.A., Hassanen E.I., Shehata A.A. Outbreaks of myxomatosis in Egyptian domestic rabbit farms. World Rabbit Sci. 2019;27:85–91. doi: 10.4995/wrs.2019.10585. [DOI] [Google Scholar]
- SAS, 2004. SAS/STAT User’s Guide: Version 9.1.3., SAS Institute, Cary, NC.
- Setta A., Salem H.M., Elhady M., El-Hussieny A., Abdel Satar Arafa A. Molecular and genetic characterization of infectious bronchitis viruses isolated from commercial chicken flocks in Egypt between 2014 and 2016. J. World Poult. Res. 2018;8(1):01–08. [Google Scholar]
- Sheiha, A.M., Abdelnour, S.A., El-Hack, A., Mohamed, E., Khafaga, A. F., Metwally, K. A., S. Ajarem, J.S. Maodaa, S.N., Allam, A.A., El-Saadony, M. T. 2020. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals, 10(3), 430. [DOI] [PMC free article] [PubMed]
- Shome, B., Parlow, A., 1974. Human follicle stimulating hormone (hFSII): first proposal for the amino acid sequence of the a-subunit of human luteinizing hor-mone (hLHa). J. Clin. Endocrinol. Metab., 39, 199-202, doi.org/10.1210/jcem-39-1-199. [DOI] [PubMed]
- Spangelo B.L., Gorospe W.C. Role of the cytokines in the neuroendocrine-immune system axis. Front. Neuroendocrinol. 1995;16:1–22. doi: 10.1006/frne.1995.1001. [DOI] [PubMed] [Google Scholar]
- Thompson D., Garza, F., George, R.L., Rabb, M., Barry, B., French, D.D., 1991. Relationships among LH, FSH, and prolactin secretion, storage, and response to secretagogue and hypothalamic GnRH content in ovariectomized pony mares administered testosterone, dihydrotestosterone, estradiol, progesterone, dexamethasone, or follicular fluid. Domest. Anim. Endocrinol., 8, 189-199, doi.org/10.1016/0739-7240(91)90055-o. [DOI] [PubMed]
- Thompson, D., Johnson, L., George, R.L., Garza F., 1986. Concentrations of prolactin, luteinizing hormone and follicle-stimulating hormone in pituitary and serum of horses: effect of sex, season and reproductive state. J. Anim. Sci., 63, 854-860, doi.org/10.2527/jas1986.633854x. [DOI] [PubMed]
- Ubilla, E., Alvarin, J., Esquifino, A., Agrasal, C., 1992. Effects of induction of parturition by administration of a prostaglandin F2α analogue in rabbits: possible modification of prolactin, LH and FSH secretion patterns. Anim. Reprod. Sci., 27, 13-20. doi.org/10.1016/0378-4320(92)90066-M.
- Van Straalen, R., Zeilmaker, G.J., 1982. Observations on the effects of prolactin on LH-receptors and steroidogenesis in corpus luteum and testis of the hypophysectomized rat. Eur. J. Endocrinol., 99, 437-442, doi.org/10.1530/acta.0.0990437. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.