Abstract
A proper vaccination against avian influenza viruses in chicken can significantly reduce the risk of human infection. Egypt has the highest number of recorded humans highly pathogenic avian influenza (HPAI)-H5N1 infections worldwide despite the widespread use of homologous vaccines in poultry. Enhancing H5N1 vaccine efficacy is ultimately required to better control HPAI-H5N1. The aim of this study is to boost chicken immunity by combined with inactivated HPAI-H5N1 with selenium nanoparticles (SeNPs). The chickens groups 1–3 were fed diets supplemented with SeNPs concentrations (0.25, 0.5, and 1 mg/kg) for 3 weeks and then vaccinated (inactivated HPAI-H5N1). while groups 4,5 and 6 were fed with SeNPs free diets and administered with 0.5 ml of the vaccine combined with 0.02, 0.06, and 0.1 mg/dose of SeNPs and then all groups were challenged with homologous virus 3 weeks post-vaccination (WPV). Group 7, 8 were used as control positive and negative respectively. At 4, 5, and 6 WPV, antibody titer was considerably higher in the group fed a meal supplemented with 1 mg SeNPs/kg. In contrast, both methods of SeNPs supplementation significantly increased the Interleukin 2 (IL2), Interleukin 6 (IL6), and Interferon γ (IFNγ) expressions in the blood cells in a dose-dependent manner, with a higher expression observed in the group that was vaccinated with 0.1 mg/dose. After the challenge, all groups that received SeNPs via diet or vaccines dose showed significant reduction in viral shedding and milder inflammation in lung, trachea, spleen, and liver in addition to higher expression of IL2, IL6, and IFNγ, with the highest expression observed in the group that was vaccinated with 0.1 mg/dose compared the plain vaccinated group. The groups of 1 mg SeNPs/kg and combined vaccinated with 0.1 mg/dose showed the best vaccine efficacy. However, the group vaccinated with 0.1 mg/dose showed the earliest reduction in viral shedding. Overall, SeNPs supplementation in the diet and the administration of the vaccine formula with SeNPs could enhance vaccine efficacy and provide better protection against HPAI-H5N1 in chickens by enhancing cellular immunity and reducing inflammation. We recommend using SeNPs as a vaccine combination or feeding with diet to increase the immunity and vaccine efficacy against H5N1.
Keywords: Avian influenza, HPAI (H5N1), Vaccine, Nanoselenium, Humoral immunity
1. Introduction
The highly pathogenic avian influenza virus (HPAIV) (H5N1A/goose/Guangdong/1996 [Gs/GD] lineage) has been reported in 84 countries throughout Asia, Africa, Europe, and North America (Criado et al., 2020), causing severe economic losses and increase human infection (Zhang et al., 2020). In 2005, 2006, an H5N1 highly pathogenic avian influenza (HPAI) of clade 2.2 was reported and spread in Central Asia, Europe, Africa, and the Middle East. It was introduced into Egypt in early 2006 (Aly et al., 2008). Despite all the control measures including extensive vaccination programs, the virus spread all over Egypt causing massive economic losses threatening human health and was declared to be enzootic in 2008 (Peyre et al., 2009, Abdelwhab and Hafez, 2011). After 1 year of extensive vaccination, a genetic and antigenic variant 2.2.1.1 emerged as a vaccine escape mutant that circulated in vaccinated commercial farms, whereas 2.2.1c circulated in backyard farms and was responsible for most of the human cases (Grund et al., 2011). Further evolution of 2.2.1c led to the emergence of the 2.2.1.2 clade that was associated with a surge of HPAI-H5N1 human infections and became the dominant lineage circulating in Egypt (Arafa et al., 2015).
In Egypt, after a few weeks of HPAI-H5N1 virus introduction, the veterinary authorities adopted a massive vaccination strategy (Aly et al., 2008, Abdelwhab et al., 2016). Since then, at least 24 influenza H5 vaccines were licensed for use in Egypt (Kayali et al., 2016). According to experimental infection, vaccines with seed viruses with a high degree of genetic homology (88% or more) given better clinical protection against H5N1 virus (Abdelwhab et al., 2016). However, under field conditions, partial protection or even vaccination failure was also reported due to antigenic variation, concurrent infections, maternally derived antibodies, or rearing various species especially in the backyard system (Kandeil et al., 2017). Moreover, the efficacy of the H5 vaccine used in Egypt, even the ones with homologous vaccinal seeds, need to be improved.
In the present study, selenium nanoparticles (SeNPs) were evaluated as immunomodulatory agents in the HPAI-H5N1 homologous vaccine for enhancing vaccinal immune response by delivering long-lasting humoral and cellular immune responses (Chan et al., 2009), which enhances the immune response to the infectious agent's long-term viability as well as disease infection resistance (Firenzuoli et al., 2008, Weickert and Pfeiffer, 2008). The selenium (Se) is antioxidants that protect cells from reactive oxygen (RO) by reducing free radicals and preventing lipid peroxidation (Harsini et al., 2012). Selenium (Se) is needed for the activity of Se-dependent glutathione peroxidases, which are enzymes that reduce hydrogen peroxide and lipid hydroperoxides (Vakili and Daliri, 2010). Furthermore, selenium is a key component in a number of compounds that boost immune system response by altering the production of certain cytokines or improving immune cells' resistance to oxidative stress (Mohamed et al., 2015). In comparison to sodium selenite, nanoselenium appears to be less toxic and more biocompatible, as well as possessing a variety of useful properties such as catalytic performance, adsorption strength, surface activity, and chemical stability and high antioxidant effect (Wang et al., 2007, Zhang et al., 2008, Boostani et al., 2015a, Boostani et al., 2015b, Skalickova et al., 2017). It is also increasing the humoral immunity (Cai et al., 2012).
The selenium nanoparticles also had antiviral activity in recent years. It inhibits the activity of hemagglutinin and neuraminidase that is important in the binding of influenza virus in the host cell (Li et al., 2017). Also, Se NPs inhibiting p53 signalling and ROS-mediated AKT pathways so it inhibits apoptosis induced by H1N1 influenza virus (Wang C. et al., 2020).
The aim of this study was evaluating the effect of the different doses of SeNPs incorporated in the chickens’ diet or vaccine formula on the immunogenicity and efficiency of H5N1 whole inactivated vaccine against the homologous HPAI-H5N1 challenge strain.
2. Material and methods
2.1. Selenium nanoparticle preparation, characterization
20 ml freshly prepared ascorbic acid (400 mg ml−1) was added dropwise to 200 ml of Na2SeO3 (10 mmol) with constant stirring for 30 min at room temperature to become red colored. Then, the mixture was centrifuged and washed several times with double-distilled water (El-Saadony et al., 2021a, Sheiha et al., 2020, Abdel-Moneim et al., 2022, Alagawany et al., 2021b). The surface plasmon resonance absorbance peak of SeNPs was evaluated using the Laxco™ dual-beam spectrophotometer (USA) (El-Saadony et al. 2020). The active compounds in both soluble and powdered SeNPs were evaluated by collecting the Fourier transform infrared (FTIR) spectrum of SeNPs on KBr plates cast by using a Bruker Tensor 37, Kaller (Germany). To identify the size, distribution, and morphology of the SeNPs, transmission electron microscopy (TEM) was used (Abd El-Hack et al., 2021, El-Ashry et al., 2022, Saad et al., 2021). The particle size, polydispersity index (PDI), and zeta potential of the SeNPs were measured using the dynamic light scattering (DLS) technique (DLS, Zetasizer Ver. 6.32, Malvern, UK) (El-Saadony et al., 2021b, El-Saadony et al., 2021c, El-Saadony et al., 2021d).
2.2. Virus propagation and vaccine preparation
The HPAI-H5N1 isolate clade 2.2.1.2 (A/chicken/Egypt/1575S/2015, EPI573317) was used as a challenge virus which has accession no.: EPI579780 was propagated and titrated in SPF-ECE.
The vaccinal seed (A/chicken/Egypt/1575S/2015, EPI573317) was produced by plasmid-based reverse genetics with the HA and NA genes from the Egyptian HPAI-H5N1 strain (1575S) and internal gene segments from a laboratory-adapted strain (A/PR8/1934) (Webby et al., 2004). The reverse genetic-based vaccinal strain used in the present study was developed in the National Research Centre (NRC), Egypt. The reverse genetic-based vaccinal strain used in the present study was developed in the National Research Centre (NRC), Egypt. The viruses were propagated in a 10-day-old specific pathogen free embryonated chicken egg (SPF-ECE) in accordance with the standard procedure (Swayne and Brown, 2015). For vaccine formulation and testing, allantoic fluid was harvested from infected eggs, tested for sterility, and then the inactivation process was carried out according to Beard, (1989). Preparation of 25 ml of formalin solution 10%, as follows: 2.5 ml formalin (40%) (Sigma-Aldrich, Inc., Germany) was added to 22.5 ml saline. Twenty-five ml of formalin solution 10% was added to each liter of the virus (infectious allantoic fluid), to give a final concentration of formalin 0.1%. The harvested allantoic fluid was treated with formalin at a final concentration of 1:1000 and put on magnetic stirrer for continuous stirring during inactivation process. Final concentration of 2% sodium bisulfite was added as a residual formalin neutralizing agent. It used as an antigen for vaccine production and serological assays. The inactivation was confirmed by the absence of hemagglutination test (HA) after three successive passages in SPF-ECE. The vaccine was adjuvanted with Montanide ISA 70 VG (Seppic, France) in accordance with the manufacturer’s instructions. Using a high-shear mixer, three concentrations of SeNPs (0.02, 0.06, and 0.1 mg/dose) were added to prepare three vaccine formulas. The antigens were adjusted to have 512 hemagglutinating units HAU per 0.5-ml dose. Moreover, vaccine safety and sterility were evaluated (Swayne and Brown, 2015).
2.3. Genetic relationship between recent field strains, challenged virus and vaccine used
In the present study, we tested the most recent samples from broiler chicken farms from five governorates (10 Giza, 8 Cairo, 5Alexandria, 5Assuit and 2El- fayum) during2016, The extraction of viral RNA of pooled collected swabs was done by using a QIAmp viral RNA mini kit (Qiagen, Hilden, Germany), as directed by manufacturer and tested by reverse transcriptase real time- polymerase chain reaction (RRT-PCR) for detection of matrix (M) and Hemagglutine (HA) genes typing by using the real-time PCR step one plus System (Applied Biosystems, Foster City, CA, USA) and the AgPath Real-time Kit (Ambion) (Fereidouni et al., 2012, Hoffmann et al., 2016). The positive samples were isolated in SPF-ECE and allantoic sac was collected and tested by rapid HA test according to (OIE manual, 2008). We select 5 positive isolates for HA gene sequencing using First-Strand Synthesis method SuperScript™ III (Thermo Fisher Scientific, MA, USA) using specific primer (Hoper et al. 2009) then purification carried out by the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany). By using Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, California, USA), the National Center for Biotechnology Information has published the descriptions of the strains used in this study. The accession number (from MW454822 to MW454826).
The nucleotide sequence alignment and analysis were carried out using studying strains and other related strains and challenged virus used in this study ((A/Duck/Egypt/CLEVEB-24-N00238/2015, EPI579780) and (A/chicken/Egypt/1575S/2015, EPI573317) vaccine seed. The CLUSTAL-W tool and the DNASTAR software Meg-Align module were used to align the sequences (Lasergene version 7.2; DNASTAR, Madison, WI, USA). Mega7 software was used to create a phylogenetic tree using maximum likelihood methods (Tamura et al., 2013). DNASTAR software was used to calculate pair-wise nucleotide percent identity.
2.4. Experimental design
2.4.1. Experiment 1: Study the safety of different concentration of SeNPs in chicken
The animal experiment was conducted in the animal experimental facility of the Poultry Dept., Faculty of Agriculture, Zagazig University, Egypt. The experimental procedures were performed in accordance with the Local Experimental Animal Care Committee. Total 70one-day-old Ross 308 broilers were purchased from a local commercial provider and kept for 1 week on basal diet. One week later, the birds were randomly assigned to six groups: one control group (basic diet) and Groups 2, 3, 4, 5, and 6 fed with basal diet supplemented with 25, 50, 75, 100, and 125 mg SeNPs/kg, respectively. Moreover, all birds had free access to water. Four weeks post-treatment (5 weeks old), blood samples were collected from six birds of each group. The serum samples tested for the concentration of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphate (ALP) activities were evaluated using the commercially available kits (Bio Diagnostic Co.; Giza, Egypt) (Reda et al., 2021, Alagawany et al., 2021c, Alagawany et al., 2021a). The markers of oxidative stress were estimated in the blood serum, including malondialdehyde (MDA) and reduced glutathione (GSH), using kits manufactured by Merck (KGaA, Darmstadt, Germany).
2.4.2. Experiment 2: Study the effect of the different doses of SeNPs incorporated in the chicken’s diet or vaccine formula on H5N1 whole inactivated vaccine immunogenicity and efficiency
The animal experimental designs and procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Central Laboratory for Evaluation of Veterinary Biologics, Egypt (CLEVB), in accordance with the guidelines of the Ministry of Agriculture and Land Reclamation of Egypt and the European Union Directive 2010/63/EU regarding the protection of animals used for scientific purposes.
Total 200 SPF one-day-old chicks were obtained from the national project for the production of SPF eggs, Kom-Oshim, Fayom, Egypt. The chicks were randomly assigned to eight groups with access to food and water throughout the experiment. Each group (25 chicks) was housed in a biosafety level 3 isolator at the CLEVB animal experimental facility, Agricultural Research Centre, Egypt. The first three groups were fed with diets supplemented with SeNPs at a concentration of 0.25, 0.5, and 1 mg SeNPs/kg, respectively, whereas the remaining groups were fed with diets without SeNPs supplementation. On 3 weeks, Groups 1, 2, 3, and 7 received 0.5 ml of the prepared plain vaccine subcutaneously, whereas Groups4, 5, and6 were subcutaneously injected with 0.5 ml of the vaccine combined with 0.02, 0.06, and 0.1 mg/dose of SeNPs, respectively. Group 8remained as the unvaccinated control group. Three weeks after vaccination, all birds were challenged intranasally via the choanal cleft with 106Egg Infective Dose 50 (EID50) per 0.1 ml of challenge virus. Then, the birds were monitored daily for clinical signs and mortality for 2 weeks.
2.5. Evaluation of the immunogenicity and protective effects
2.5.1. Hemagglutination inhibition (HI)
To evaluate the specific antibodies titers, the blood samples were collected pre-and post challenge on a weekly basis and the sera were separated and tested via HI test using a vaccine virus antigen. The titers were expressed as the reciprocal of the serum dilution of the positive samples and were converted to log2. The HI assay was performed in accordance with the reference procedure (Swayne and Brown, 2015).
2.5.2. Viral shedding
Oropharyngeal swabs were collected at 1, 3, 5, 7, and 10 days post challenge (DPC) from three birds in each group and processed using quantitative real-time PCR (qRT-PCR) (Lee and Suarez, 2004) to quantify viral shedding. The standard curve was generated using tenfold serial dilution of the challenge virus to correlate between the qRT-PCR cycle threshold (CT) and virus titer (expressed in EID50/ml). A CT value of 35 was used as a cutoff point between positive and negative shedding.
2.5.3. Cytokine gene expression
Peripheral blood mononuclear cells (PBMCs) were separated from whole blood collected at 2 and 3 weeks post vaccination using Ficoll-Paque PLUS (GE HealthcareBio-Sciences AB, Sweden), as previously described (Samy et al., 2016a). The lung and spleen were aseptically collected from live or freshly dead birds at 2 weeks DPC. The PBMCs, lung, and spleen were collected from three birds in each group and stored in RNA later solution (Ambion, USA) in accordance with the manufacturer’s instruction until RNA extraction was done to measure the cytokine gene expression. The gene express ions of Interleukin 2 (IL2), Interleukin 6 (IL6), and Interferon γ (IFNγ) were relatively quantified as previously described (Samy et al., 2016b).
2.6. Histopathology
At the end of the experiment (2 weeks DPC), the trachea, lungs, spleen, cecal tonsils, and liver tissue specimens were collected from five birds / each experimental group and fixed in 10% neutral buffered formalin. Then, they were sectioned at a thickness of 5 µm and stained with hematoxylin and eosin (H&E) for subsequent histopathological examination by a light microscope (Olympus BX50, Tokyo, Japan) under x100 and x200 magnification power (Suvarna et al., 2012). The histopathological lesions were scored through the determination of the percentage of lesions in five randomly examined microscopic fields per bird (n = 5) as follows: (−) no lesions of 0–10%, (+) mild lesions of 10–20%, (++) moderate lesions of 20–60% and (+++) severe lesions of greater than 60%. (Eladl et al., 2019, Rohaim et al., 2021).
2.7. Statistical analysis
Data analysis was performed using the GraphPad Prism version 8.0 software (GraphPad Software Inc., San Diego, CA, USA). HI titer, viral shedding, and cytokine fold change were expressed as mean value ± SD. The Mann-Whitney test and Tukey one-way ANOVA were performed to determine the significant differences among various groups. The difference between the means was considered significant at P < 0.05. Fold changes in the cytokine gene expression level were calculated using the ΔΔCt method (Livak and Schmittgen, 2001), with 28 s ribosomal RNA being the endogenous reference gene to normalize the level of the target gene expression and control group (vaccinated without “SeNPs” treatment) being the calibrator.
3. Results
3.1. Characterization of SeNPs
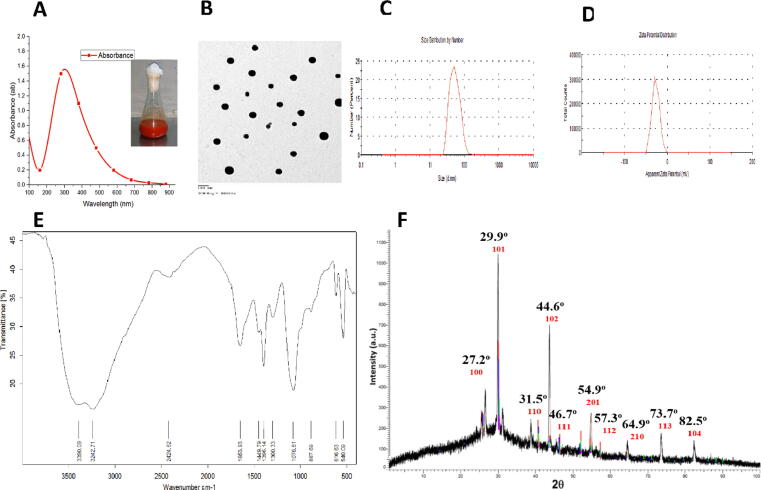
The reduction of selenium ions into selenium nanoparticles was demonstrated by the visual color change from yellow to reddish with a maximum absorption peak at 280 nm (Fig. 1a). The TEM characterization of SeNPs particles revealed a 10–25 nm size range with spherical morphology (Fig. 1b). Furthermore, the PDI and zeta potential of SeNPs were 65.32 nm, 0.193 (Fig. 1c), and −21.3 mV (Fig. 1d), respectively, indicating a higher stability. The FTIR spectrum analysis revealed the displayed IR absorbance peaks at 2425 cm−1and 3243 cm−1corresponding to the conjugated C O and O—H groups in water and alcohol (Fig. 1e). The XRD analysis of the crystal structure was in the range of 10°–90° (Fig. 1f).
Fig. 1.
Characterization of SeNPs:(a) UV spectrum, (b) TEM image, (c) size distribution in aqueous solutions, (d) zeta potential, (e) FTIR, and (f) XRD.
3.2. Vaccine preparation, safety, and potency
The reassortant H5N1 vaccine seed (1575S) was propagated in the SPF egg, and the HAU and EID50 of the harvested allantoic fluid were 512 HAU/50 μL and 109.0/ml, respectively. The inactivation was confirmed by the absence of HA reaction after three blind passages of the inactivated virus in the SPF egg. All vaccinated SPF chickens showed no local or systemic reaction after vaccination within 4 weeks before challenge.
3.3. Genetic relationship between recent field strains, challenged virus and vaccine used
The tested broiler chicken flocks suffered from respiratory signs, including dyspnea, swelling of the ocular and nasal sinus, coughing, cyanosis in leg with high mortality rate reach 80–100%. The post-mortem lesions recorded congestion of muscles and haemorrhage in pancreas and spleen and confirmed positive by RRT-PCR in 20 flocks in (8Giza, 5Cairo, 2 Alexandria, 3Assuit and 2 El- fayum) during 2016.
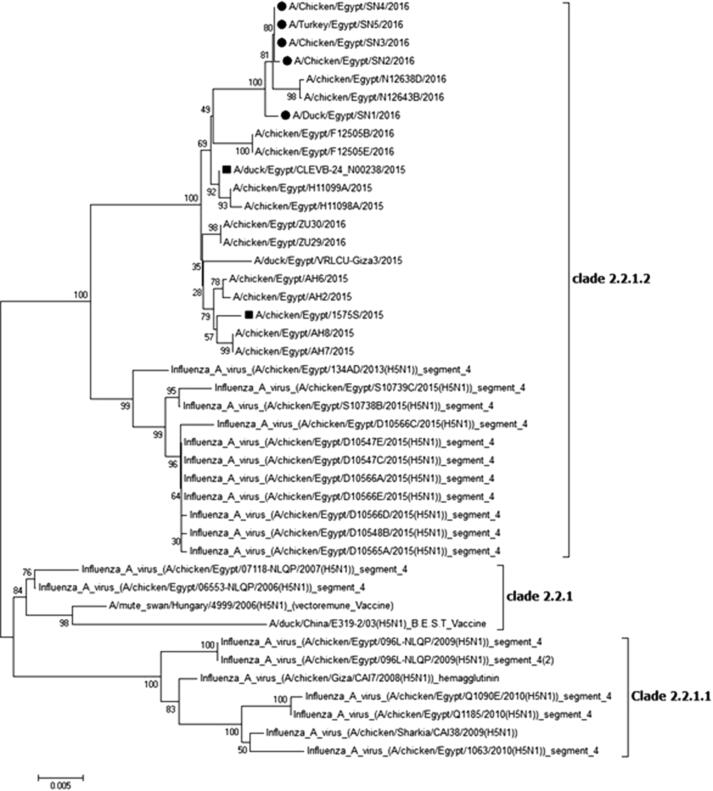
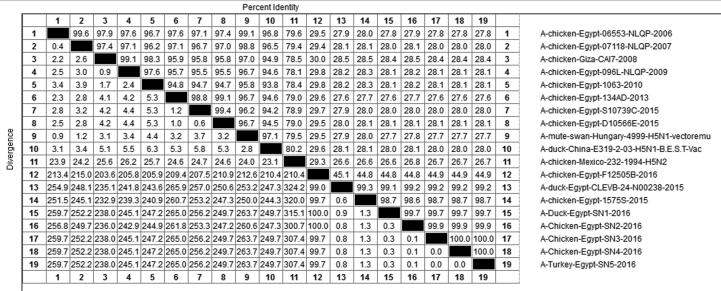
Phylogenetic analysis indicated that all tested viruses belonged to clade 2.2.1.2 and were clustered with HA gene sequences of viruses isolated in Egypt from -2015-2016 (Fig. 2). Full HA gene sequence indicated 99.1 to 99.2 % similarity with the vaccinal seed used and 98.6%-98.7% with the used challenge virus with a typical polybasic cleavage site sequence (PQGEKRRKKR/G) (Fig. 3). All tested viruses possessed antigenic sites A, B, and E, which were identical to the challenge and vaccine seed viruses.
Fig. 2.
Phylogenetic tree of the nucleotide sequences of the HA gene of Egyptian HPAI (H5N1) viruses representing different clades. The tree was generated using the maximum likelihood method and bootstrapped with 1000 replicates using the GTR substitution model with gamma distribution. The viruses characterized in this study are indicated with a black circle and other vaccinal and challenge viruses with a black rectangle.
Fig. 3.
Nucleotide identities of sequenced H5N1 virus related to other selected strains (vaccine strains used in this study and challenged virus).
3.4. Animal experiment
3.4.1. Experiment 1: Safety of different concentration of SeNPs in chicken
The levels of ALT, AST, and ALP were normal in the groups fed with diets supplemented with 25, 50, and 75 mg SeNPs/kg compared with the control group fed with a diet without SeNPs supplementation. Moreover, the group fed with a diet supplemented with a higher concentration of SeNPs (100 and 125 mg SeNPs/kg) showed significantly higher levels of ALT, AST, and ALP, with a relative increase of about 87% compared with safe concentrations. In contrast, the oxidative markers were in normal ranges in the lowest concentrations (25, 50, and 78 mg SeNPs/kg), but increased concentration led to a significant decrease in GSH and increase in MDA (Table 1).
Table 1.
Serum biochemical and oxidation markers of chickens administered with different concentrations of chemical selenium nanoparticles (25–125 mg kg−1).
| Parameters | Control | SeNPs (mg mL−1) |
||||
|---|---|---|---|---|---|---|
| 25 | 50 | 75 | 100 | 125 | ||
| ALT (U/lit) | 11.2 ± 0.9d | 11.30 ± 0.7 cd | 10.60 ± 0.9 cd | 11.22 ± 1.3c | 70.3 ± 0.2b | 85.4 ± 0.6a |
| AST (U/lit) | 121.9 ± 0.2e | 122.22 ± 1.2d | 121.8 ± 1.3 cd | 121.12 ± 1.5c | 190.22 ± 0.3b | 210.33 ± 0.7a |
| ALP (U/lit) | 1.7 ± 0.3d | 1.6 ± 1.3 cd | 1.65 ± 1.9 cd | 1.6 ± 2.1c | 15 ± 0.5b | 30.44 ± 0.5a |
| GSH (µmol/g protein) | 4.9 ± 0.5a | 4.8 ± 0.9ab | 4.75 ± 0.2b | 4.67 ± 0.3bc | 3.8 ± 0.4c | 3.5 ± 0.6 cd |
| MDA (µmol/g protein) | 13.2 ± 0.4d | 13.9 ± 0.5 cd | 13.5 ± 1.2c | 14.2 ± 0.8c | 17.2 ± 0.9b | 18.9 ± 0.4a |
The values are mean ± SD. Values in the same raw with different lowercase letters are significantly different (p ≤ 0.05). ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase.
3.4.2. Experiment 2: The effect of the different doses of SeNPs incorporated in the chickens diet or vaccine formula on H5N1 whole inactivated vaccine immunogenicity and efficiency
.
3.5. Clinical protection mortality
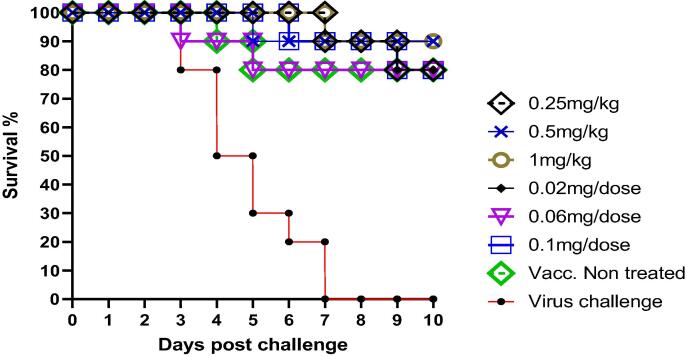
All birds challenged with HPAI-H5N1 without previous vaccination showed typical clinical signs with 100% mortality (Table 2 and Fig. 4), whereas all vaccinated birds with and without SeNPs treatment showed no observable clinical signs. Higher dietary supplementation of SeNPs (0.5 and 1 mg) improved the survival rate of vaccinated challenged chickens to 90% compared with 80% in other treated groups. Note that the SeNPs treatment relatively delays the onset of death in all treated groups (Fig. 4, Table 2).
Table 2.
Daily clinical observation and mortality for 10 days post-challenge.
| Group | SeNPsmg/kg | Vaccine | SeNPs/vaccine dose | Challenge virus |
Days post-challenge* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||
| 1 | 0.25 mg | H5N1 HA-PR8a | NA | HPAI-H5N1 (2.2.1.2) | Normal | Normal | Normal | Normal | Normal | Normal | Normal (9/10) | Normal | Normal (8/10) | Normal |
| 2 | 0.5 mg | NA | Normal | Normal | Normal | Normal | Normal (9/10) | Normal | Normal | Normal | Normal | Normal | ||
| 3 | 1 mg | NA | Normal | Normal | Normal | Normal | Normal | Normal | Normal (9/10) | Normal | Normal | Normal | ||
| 4 | NA | 0.02 mg | Normal | Normal | Normal | Normal | Normal | Normal | Normal (9/10) | Normal | Normal (8/10) | Normal | ||
| 5 | NA | 0.06 mg | Normal | Normal | Normal (9/10) | Normal | Normal (8/10) | Normal | Normal | Normal | Normal | Normal | ||
| 6 | NA | 0.1 mg | Normal | Normal | Normal | Normal | Normal | Normal | Normal (9/10) | Normal | Normal (8/10) | Normal | ||
| 7 | NA | NA | Normal | Normal | Normal | Normal (9/10) | Normal (8/10) | Normal | Normal | Normal | Normal | Normal | ||
| 8 | NA | NA | NA | Sick | Sick (9/10) | Sick (7/10) | Sick (5/10) | Sick (3/10) | All dead | |||||
The vaccinal seed was produced by plasmid-based reverse genetics with the HA and NA genes from the Egyptian HPAI-H5N1 strain (1575S) and internal gene segments from a laboratory-adapted strain (A/PR8/1934).
Fig. 4.
The survival curve of the vaccinated (with and without SeNPs treatment) and unvaccinated (Sham) chickens challenged at 4 weeks of age with H5N1 HPAIV (clade 2.2.1.2) at a dose of 106 EID50/0.1 ml.
3.6. Evaluation of the immunogenicity and protective effects
3.6.1. Hemagglutination inhibition (HI)
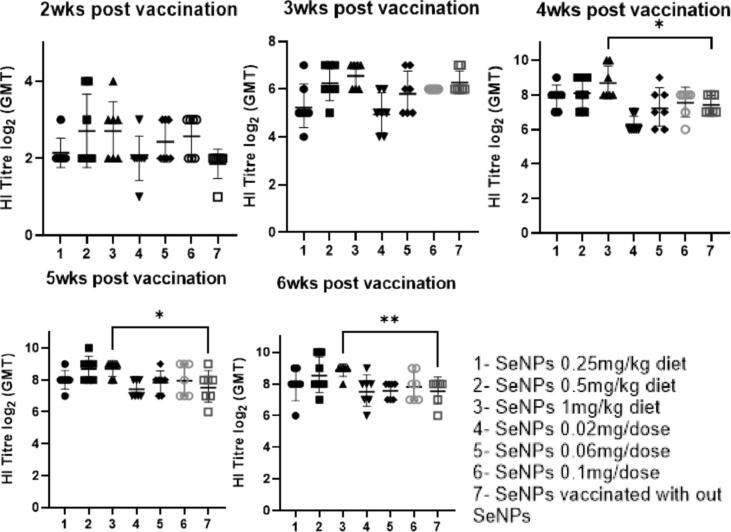
All vaccinated birds, with and without SeNPs treatment, showed an increase in protective HA neutralizing antibodies against homologous H5N1 antigens. However, a significantly higher antibody titer was observed in chickens fed with diets supplemented with a high dose of SeNPs (1 mg/Kg) during the 4th (8.6 ± 1.1),5th (8.7 ± 0.5), and 6th (8.7 ± 0.7) week post-vaccination (WPV). Moreover, a significantly higher antibody titer was observed in chickens fed with diets supplemented with a lower dose of SeNPs (0.5 mg/kg) during the 5th (8.5 ± 0.9) and 6th (8.6 ± 1.1) WPV, and in the vaccinated nontreated group during the 4th (7.5 ± 0.9), 5th (7.6 ± 0.9), and 6th (7.6 ± 0.7) WPV, but not statistically significant. Furthermore, the groups that were administered with SeNPs incorporated in the vaccine formula showed no difference (Fig. 5).
Fig. 5.
Serum HI titers pre-challenge (2, 3, and 4 weeks post-vaccination) and post-challenge (5 and 6 weeks post-vaccination). The HI titters were expressed as geometric mean titters (GMT-log2) of sera collected from seven birds with error bars included (standard deviation). The different groups are represented by the numbers as shown in the legend. The asterisks indicate p < 0.05 compared with the vaccinated non-treated chickens (Group 7).
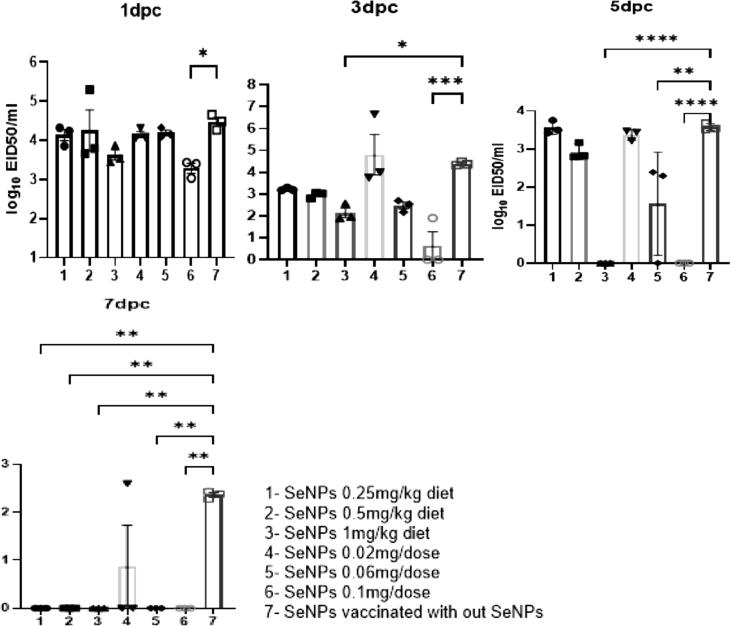
3.6.2. Viral shedding
A significant reduction of viral shedding was observed in all vaccinated groups compared with the challenged nonvaccinated group at all time points (Table 3). An earlier significant reduction of viral shedding at 1 and 3 DPC in Group 6 (0.1 mg/dose) and 3 DPC in Group 3(1mgSeNPs/kg) compared with the vaccinated nontreated group with the absence of any detectable viral shedding at5 and 7 DPC was observed. Moreover, the birds in Group 5 (0.06 mg/dose) showed a significant reduction of viral shedding at 5 DPC, whereas the remaining groups showed a significant reduction of viral shedding at 7 DPC (Fig. 6).
Table 3.
Viral shedding titers in vaccinated and nonvaccinated challenged control groups. Titers expressed as mean ± SD of three birds from each group.
| Days post challenge | H5N1 (2.2.1.2) vaccine |
Challenged control group |
||||||
|---|---|---|---|---|---|---|---|---|
| SeNPs in diet (per kg) |
SeNPs with vaccine (per dose) |
No SeNPs | ||||||
| 0.25 mg | 0.5 mg | 1 mg | 0.02 mg | 0.06 mg | 0.1 mg | |||
| 1 | 4.1 ± 0.25 | 4.2 ± 0.9 | 3.6 ± 0.2 | 4.2 ± 0.1 | 4.2 ± 0.1 | 3.3 ± 0.2 | 4.5 ± 0.2 | 5.3 ± 0.2 |
| 3 | 3.2 ± 0.1 | 3 ± 0.1 | 2.1 ± 0.4 | 4.6 ± 1.6 | 2.5 ± 0.3 | 0.6 ± 1.1 | 4.4 ± 0.1 | 5.9 ± 0.1 |
| 5 | 3.6 ± 0.2 | 2.9 ± 0.2 | 0 | 3.4 ± 0.1 | 1.6 ± 1.4 | 0 | 3.6 ± 0.1 | 6.3 ± 0.2 |
| 7 | 0 | 0 | 0 | 0.9 ± 1.5 | 0 | 0 | 2.4 ± 0.1 | All dead |
Fig. 6.
Oropharyngeal viral shedding in vaccinated birds with and without SeNPs treatment. Viral shedding measured using quantitative real-time PCR analysis and expressed as EID50/ml ± SD. The different groups are represented by numbers as shown in the legend. The asterisks indicate p < 0.05, compared with the vaccinated non-treated chickens (Group 7).
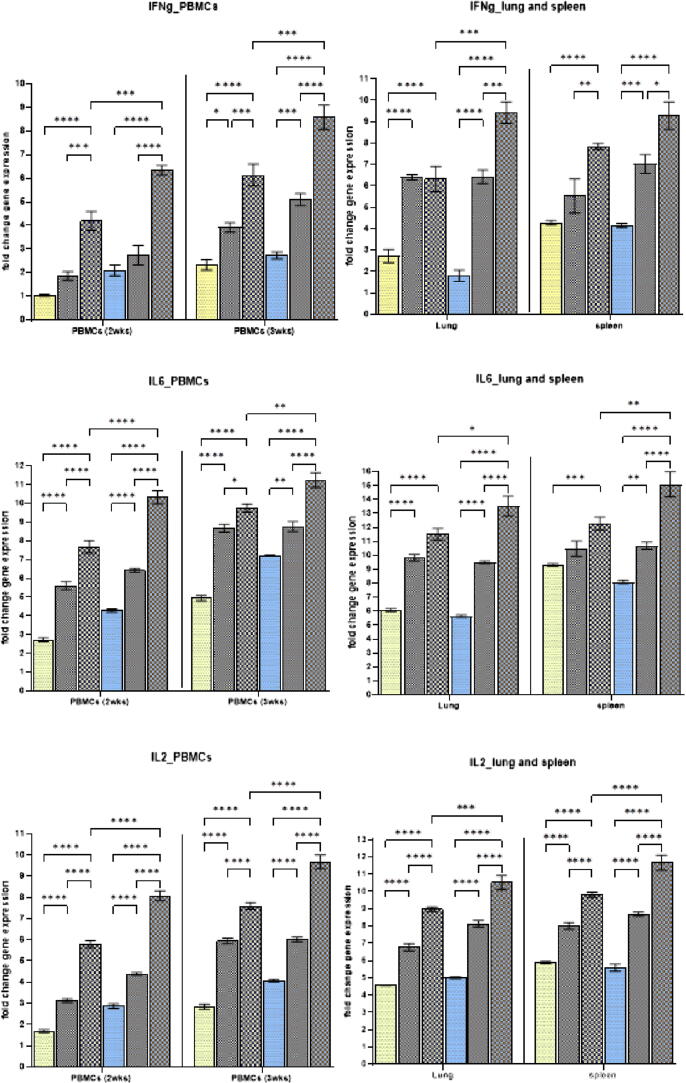
3.6.3. Cytokine gene expression
To determine whether the SeNPs treatments used in the present study can impact cellular immunity, we examined the expression of IFNγ, IL2, and IL6 in PBMCs at 2 and 3 WPV and in the spleen and lung at 14 DPC. The expression was calculated relative to the vaccinated nontreated group. As shown in Fig. 6, the results revealed that the IFNγ, IL2, and IL6 mRNA expression levels were upregulated in a dose-dependent manner inall groups. However, a significantly higher expression was observed when 0.1 mg of SeNPs per vaccine dose was administered compared with in the other groups except IFNγ in the spleen. Moreover, no significant difference was observed between the group fed with a diet supplemented with 1mgSeNPs/kg and the group vaccinated with0.06 mg/dose (Fig. 7).
Fig. 7.
Effect of the different concentrations of SeNPs incorporated in the chickens’diet and vaccine formula on the mRNA gene expressions of IFNγ, IL6, and IL2. Data are expressed as mean ± SD (n = 3). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.001.
3.7. Histopathological findings
The trachea, lungs, spleen, cecal tonsils, and liver tissue specimens were collected from live or freshly dead birds under aseptic conditions. All samples collected from the control chickens revealed normal microscopical. In contrast, challenged nonvaccinated birds showed severe inflammatory response in all tested organs (Fig. 8i−Fig. 11i).
Fig. 8.
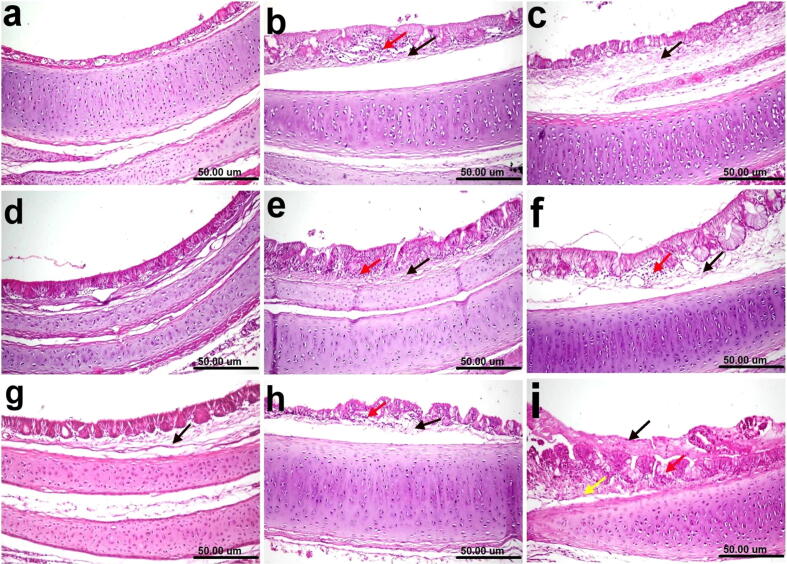
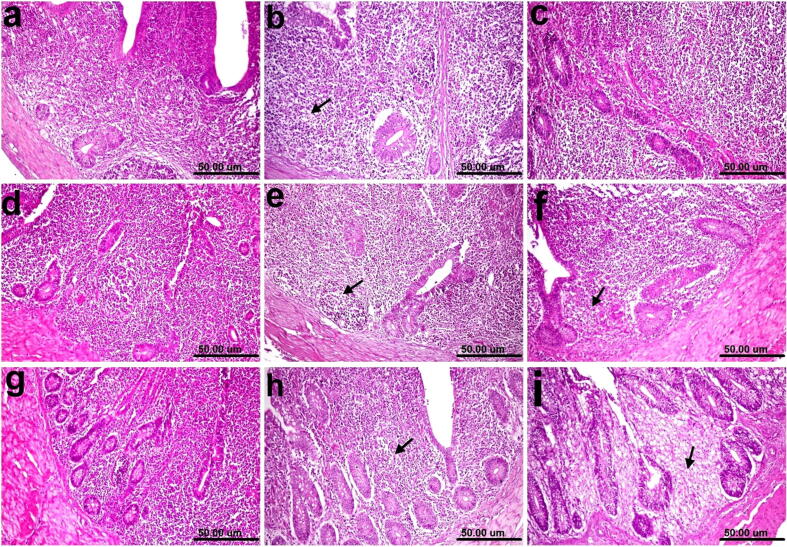
Photomicrographs of histological H&E-stained tracheal sections of chickens. (a) Normal control showing the normal histological architecture of tracheal layers. (b) Group 1 (0.25 mg selenium/kilogram) showing edema in the lamina propria/submucosa (black arrow) associated with the infiltration of a few inflammatory cells (red arrow). (c) Group 2 (0.5 mg/kilogram) showing edema in the lamina propria/submucosa (black arrow). (d) Group 3 (1 mg/kilogram) showing apparently a normal histological structure. (e) and (f) Groups 4 and 5 showing edema in the lamina propria/submucosa (black arrow) associated with the infiltration of inflammatory cells (red arrow). (g) Group 6 (vaccine + o.1 mg/dose) showing edema in the lamina propria/submucosa (black arrow). (h) Group 7 (raw vaccine) showing edema in the lamina propria/submucosa (black arrow) associated with the infiltration of inflammatory cells (red arrow). (i) Non-vaccinated and challenged group showing the activation of mucous-secreting glands (red arrow), intraluminal accumulation of mucous (black arrow), and edema in the lamina propria/submucosa (yellow arrow) (scale bar, 50 μm).
Fig. 11.
Photomicrographs of histological H&E-stained cecal tonsil sections of chickens. (a) Normal control showing the normal histological structure. (b) Group 1 (0.25 mg selenium/kilogram) showing slight lymphocytic necrosis and depletion (arrow). (c) and(d) Groups 2 and 3 (0.5 and 1 mg selenium/kilogram, respectively) showing no histopathological alterations. (e) and (f) Groups 4 and 5 (vaccine + 0.02 and 0.06 mg/dose, respectively) showing slight lymphocytic necrosis and depletion. (g) Group 6 (vaccine + 0.1 mg/dose) showing no histopathological changes. (h) Group 7 (raw vaccine) showing lymphocytic necrosis and depletion (arrow). (i) Non-vaccinated and challenged group showing lymphocytolysis with severe lymphocytic depletion and fibrinoid necrosis (arrow) (scale bar, 50 μm).
The histopathological findings of the trachea revealed that edema in the lamina propria/submucosa was associated with the infiltration of a few inflammatory cells in Group1 (Fig. 8b). No changes were observed except the edema in the lamina propria/submucosa in Group 2 (Fig. 8c) Apparently, Group 3 showed anormal histological structure (Fig. 8d). Edema in the lamina propria/submucosa associated with the infiltration of inflammatory cells in Groups 4 and 5 (Fig. 8e and f). Moreover, edema in the lamina propria/submucosa was the only change observed in the trachea of the chickens in Group 6 (Fig. 8g). Edema in the lamina propria/submucosa and the infiltration of a few inflammatory cells were observed in Group 7 (Fig. 8g). In contrast, severe histopathological changes were observed in the trachea of nonvaccinated and challenged chickens, due to the activation of mucous-secreting glands, intraluminal accumulation of mucous, and edema in the lamina propria/submucosa (Fig. 8i).
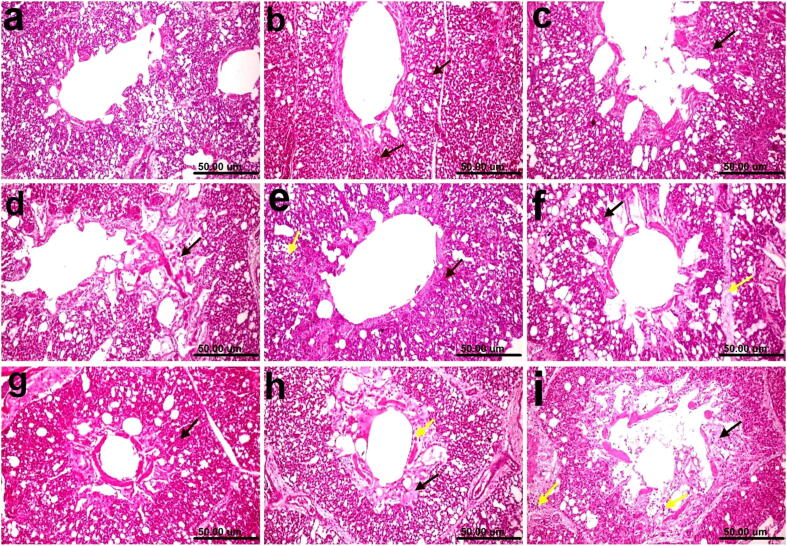
The histopathological findings of the lungs revealed a congestion of the pulmonary blood vessels in Group1 (Fig. 9b). Infiltration of a few heterophils (Fig. 9c) and slight edema (Fig. 9d) in Groups 2 and 3, respectively, were observed. The congestion of the pulmonary blood vessels was associated with the infiltration of a few inflammatory cells in Group 4 (Fig. 9e). Congestion and interlobular edema were observed in Group 5 (Fig. 9f). In Group 6, only congestion was observed in the lungs (Fig. 9g). In Group 7, the lungs (raw vaccine) revealed marked edema in the wall of parabronchus associated with the infiltration of heterophils (Fig. 9h). In contrast, the lungs of nonvaccinated and challenged chickens showed severe alterations, such as necrosis in the mucosa of the parabronchus and intraluminal and interlobular inflammatory exudates (Fig. 9i).
Fig. 9.
Photomicrographs of the histological H&E-stained lung sections of chickens. (a) Normal control showing the normal histological architecture of the parabronchus and air capillaries. (b) Group 1 (0.25 mg selenium/kilogram) showing the congestion of pulmonary blood vessels (arrow). (c) Group 2 (0.5 mg/kilogram) showing the infiltration of a few heterophils (arrow). (d) Group 3 (1 mg/kilogram of diet) showing slight edema (arrow). (e) Group 4 (vaccine + 0.02 mg/dose) showing the congestion of pulmonary blood vessels (black arrow) associated with the infiltration of a few inflammatory cells (yellow arrow). (f) Group 5 (vaccine + 0.06 mg/dose) showing congestion (black arrow) and interlobular edema (yellow arrow). (g) Group 6 (vaccine + 0.1 mg/dose) showing congestion (arrow). (h) Group 7 (raw vaccine) showing marked edema in the wall of the parabronchus (black arrow) associated with the infiltration of heterophils (yellow arrow). (i) Non-vaccinated and challenged group showing necrosis in the mucosa of the parabronchus (black arrow) and intraluminal and interlobular inflammatory exudate (yellow arrows) (scale bar, 50 μm).
The histopathological findings of the spleen revealed no histopathological alterations in Groups 1, 2, 3, 5, and 6 (Fig. 10b, c, d, f, and g). Slight lymphocytic necrosis and depletion were observed in Group4 (Fig. 10e). Hyperplasia of the reticular cells and infiltration of a few heterophils were observed in the spleen of chickens from Group 7 (raw vaccine) (Fig. 10h). Moreover, severe histopathological lesions described as lymphocytolysis with severe lymphocytic depletion, fibrinoid necrosis, and infiltration of heterophils were observed in Group 8 (Fig. 10i).
Fig. 10.
Photomicrographs of histological H&E-stained spleen sections of chickens. (a) Normal control showing the normal histological architecture of the white pulp. (b), (c), and (d) Groups 1, 2, and 3 (0.25, 0.5, and 1 mg selenium/kilogram, respectively) showing no histopathological alterations. (e) Group 4 (vaccine + 0.02 mg/dose) showing lymphocytic necrosis and depletion. (f) and(g) Groups 5 and 6 (vaccine + 0.06 and 0.1 mg/dose) showing no histopathological changes. (h) Group 7 (raw vaccine) showing hyperplasia of reticular cells (black arrow) and infiltration of a few heterophils (yellow arrow). (i) Non-vaccinated and challenged group showing lymphocytolysis with severe lymphocytic depletion (black arrow), fibrinoid necrosis (red arrow), and infiltration of heterophils (yellow arrow) (scale bar, 25 μm).
Histopathological findings of the cecal tonsils revealed slight lymphocytic necrosis and depletion in Group 1 (Fig. 11b). No histopathological alterations were observed in Groups 2, 3, and 6 (Fig. 11c, d, and g). Slight lymphocytic necrosis and depletion were observed in Groups 4 and 5 (Fig. 11eand f). Lymphocytic necrosis and depletion were observed in Group 7 (Fig. 11h). Moreover, lymphocytolysis with severe lymphocytic depletion and fibrinoid necrosis were observed in Group 8 (Fig. 11i).
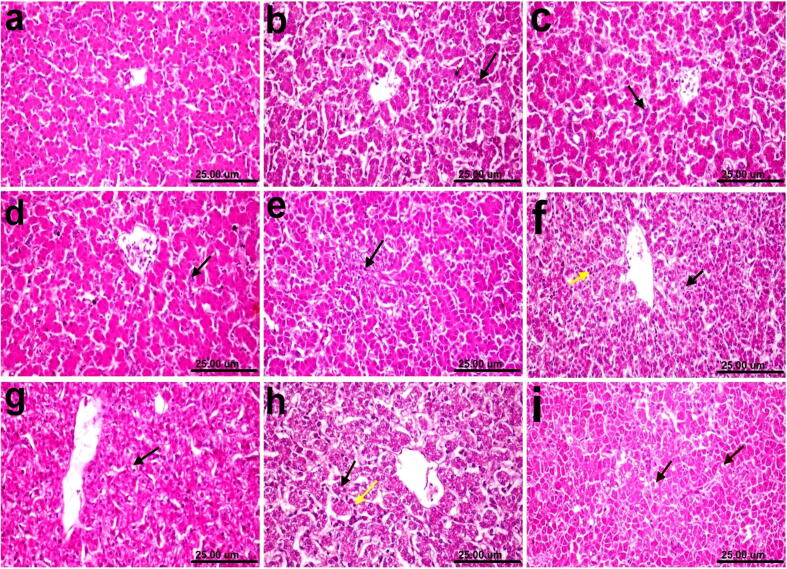
Histopathological findings of the liver revealed no histopathological lesions except a slight congestion of the hepatic sinusoids in Groups 1, 2, and 3 (Fig. 12b, c, and d). Small focal hepatocellular necrosis was associated with the infiltration of inflammatory cells in Group 4 (Fig. 12e). A slight vacuolization of hepatocytes and infiltration of a few heterophils were observed in Group 5 (Fig. 12f). A slight vacuolar degeneration of some hepatocytes was observed in Group 6 (Fig. 12g). A slight vacuolar degeneration of hepatocytes and infiltration of a few heterophils were observed in Group7 (Fig. 12h). Moreover, a marked cytoplasmic vacuolar degeneration of hepatocytes and focal hepatocellular necrosis were associated with the infiltration of inflammatory cells in Group 8(Fig. 12i). Table 4 shows the histopathological scoring of the lesions.
Fig. 12.
Photomicrographs of histological H&E-stained liver sections of chickens. (a) Normal control showing the normal histological architecture of hepatic parenchyma. (b), (c), and (d) Groups 1, 2, and 3 (0.25, 0.5, and 1 mg selenium/kilogram, respectively) showing a slight congestion of hepatic sinusoids (arrow). (e) Group 4 (vaccine + 0.02 mg/dose) showing small focal hepatocellular necrosis associated with the infiltration of inflammatory cells (arrow). (f) Group 5 (vaccine + 0.06 mg/dose) showing a slight vacuolization of hepatocytes (black arrow) and infiltration of a few heterophils (yellow arrow). (g) Group 6 (vaccine + 0.1 mg/dose) showing a slight vacuolar degeneration of some hepatocytes (arrow). (h) Group 7 (raw vaccine) showing a slight vacuolar degeneration of hepatocytes (black arrow) and infiltration of a few heterophils (yellow arrow). (i) Non-vaccinated and challenged groups showing a marked cytoplasmic vacuolar degeneration of hepatocytes (arrow) (scale bar, 25 μm).
Table 4.
Histopathological scoring of lesions in the different organs of five birds/group (n = 5). The histopathological lesions were scored through the determination of the percentage of lesions in five randomly examined microscopic fields per bird (n = 5) as follows: (−) no lesions of 0–10%, (+) mild lesions of 10–20%, (++) moderate lesions of 20–60% and (+++) severe lesions of greater than 60%.
| Treatments |
Trachea |
Lungs |
Spleen |
Cecal tonsils |
Liver |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Edema in the lamina propria/submucosa | Infiltration of inflammatory cells | Activation of mucous-secreting glands | Congestion | Edema | Infiltration of inflammatory cells | Exudate in the parabronchus | Lymphocytic necrosis and depletion | Fibrinoid necrosis | Infiltration of heterophils | Lymphocytic necrosis and depletion | Congestion | Vacuolar degeneration of hepatocytes | Hepatocellular necrosis | |
| Group 1 (0.25 mg/kg) | + | + | − | ++ | − | + | − | − | − | − | + | + | − | − |
| Group 2 (0.5 mg/kg) | + | − | − | − | + | + | − | − | − | − | − | + | − | − |
| Group 3 (1 mg/kg) | − | − | − | − | + | − | − | − | − | − | − | + | − | − |
| Group 4 (0.02 mg /dose) | ++ | + | − | ++ | − | + | − | + | − | + | + | + | − | + |
| Group 5 (0.06 mg /dose) | ++ | + | − | + | + | − | − | − | − | − | + | − | + | − |
| Group 6 (0.1 mg /dose) | + | − | − | + | + | − | − | − | − | − | − | − | + | − |
| Group 7 (vac. Only) | ++ | + | + | ++ | + | + | − | ++ | − | + | + | + | + | − |
| Group 8 (H5N1) | ++ | ++ | +++ | +++ | +++ | ++ | ++ | +++ | ++ | ++ | +++ | ++ | +++ | + |
4. Discussion
Viral diseases affect the health of animals, causing severe damage to them. These diseases are expensive to treat, in addition, they cause high mortality in animals, causing high economic losses for breeders (Swelum et al., 2020). Despite the observed reduction of HPAI-H5N1 outbreaks and high prevalence of HPAI-H5N8 in Egypt since late 2016 (Kandeil et al., 2019), vaccination against H5N1 virus should still be required. Moreover, the reduction of the virus outbreaks does not rely on an efficient control plan nor adequate surveillance procedure and avian influenza remains a major public health concern in Egypt (Gomaa et al., 2020). Due to several industrial logistic considerations, egg-based whole inactivated vaccine against avian influenza virus is still the primary technological platform in use especially in developing countries (Rao et al., 2009). Whole inactivated vaccines with seed viruses with ahigh degree of genetic homology with the circulating viruses showed good protectively and significant reduction of viral shedding (Abdelwhab et al., 2016). However, considerable viral shedding is still released from vaccinated birds especially under field conditions with mixed infection or mixed age and species of birds (Kandeil et al., 2017). This leads to an increase in environmental load and enhances the risk of infection of susceptible hosts, including wild birds, which explains the endemicity of influenza viruses in Egypt despite the massive vaccination strategy (Abdelwhab et al., 2016). Highlighting the importance of continuous increase of the inactivated vaccine efficiency even that with high genetic homology.
In the present study, we evaluated the use of different concentrations of SeNPs in feed and vaccine formula to enhance whole inactivated HPAI-H5N1 vaccine efficacy. To this end, we incorporated different concentrations of SeNPs in the chickens’ diet and vaccine formula and evaluated the immune response post vaccination and protectively post challenge by using vaccine seed and challenge virus of high genetic similarity (>98%) to the most recent isolated viruses.
The avian influenza H5N1 were circulated in high incidence till 2016 then the incidence rate was decrease till the H5N8 was recorded in 2017 (Kandeil et al., 2019, Kandeil et al., 2017). In our study we detected 20 out of 30 swab samples collected with 4 governorates with 66% incidence rate during 2016 and we selected the challenged strain that grouping with recent viruses during 2016. Chickens fed with a higher concentration of SeNPs (1 mg/kg) demonstrated significantly enhanced humeral immunity, indicated by HI titer as well as cellular immunity indicated by higher expressions of IL2, IL6, and IFNγ in PBMCs. Moreover, the high concentration of SeNPs (0.1 mg/dose) incorporated in the vaccine did not lead to any significant change in HI titer but resulted in a significantly high expression of IL2, IL6, and IFNγ among all tested groups, indicating different immune responses based on the method of application. Our data supports the ability of nanoselenium to boost both cellular and antibody-mediated immune response as well as the studied impact of selenium deficiency associated with the reduction in circulated T lymphocytes and immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies (Chang et al., 1994, Boostani et al., 2015a, Boostani et al., 2015b). Selenium supplementation enhances both cellular and humeral immune response (Rao et al., 2013, Boostani et al., 2015a, Boostani et al., 2015b). Furthermore, it was shown that the SeNPs application method and the dose differentially affected various types of immune responses (Hoffmann and Berry, 2008, Lin et al., 2021).
After the challenge, Groups 3 (1 mg/kg) and 6 (0.1 mg/dose) showed an early significant reduction of viral shedding and early clearance of the virus (5DPC) compared with the other groups. However, the group vaccinated with0.1 mg/dose showed a significant reduction of viral shedding at 1DPC comparing with the group supplemented with 1mgSeNPs/kg at 3 DPC. Our results are consistent with those of Shojadoost et al., (2020) who showed a significant reduction of H9N2 shedding in vaccinated birds fed with diets supplemented with organic and inorganic selenium. However, in contrast to our study, Shojadoost et al., (2020) showed that both higher- and lower-level selenium supplementation reduced oropharyngeal virus titers to the same degree. This difference could be attributed to the fact that SeNPs have a higher retention in the body, good intestinal absorption, and higher safety range compared with other sources of selenium (Hu et al., 2012).
It has been reported that Se supplementation supports immunity by reducing inflammation (Fan et al., 2020). Inflammatory response is an important mechanism of HPAI-H5N1 pathogenicity (Suzuki et al., 2009). In the present study, all treated groups showed a reduction of pathological findings in all tested tissues compared with vaccinated non treated group, indicating a reduction in inflammation. These findings were more prominent in the group fed with diets supplemented with 1mgSeNPs/kg and the group vaccinated with 0.1 mg/dose, which showed a normal and mild inflammatory response in the trachea, respectively. This finding supports the lower viral shedding and early clearance in both groups as well as the higher IL2, IL6, and IFNγ gene expressions in the spleen and lung at 14DPC. IL2 and IFNγ are associated with the initiation and regulation of cellular immune responses in birds (Göbel et al., 2003), whereas IL6 (pro-inflammatory cytokine) is associated with the final maturation of B lymphocytes into antibody-secreting plasma cells (Jones, 2005) and development of T cell memory to influenza virus (Longhi et al., 2008). The significant upregulation of IL2, IL6, and IFNγ in the lungs along with mild inflammatory response upon SeNPs supplementation is indicative of the localized role of cellular immunity in the reduction of viral shedding and reduction of inflammatory reaction indicated by histopathology. Furthermore, SeNPs supplementation is associated with the higher expression of IL2, IL6, and IFNγ in the spleen which acts as a secondary lymphoid organ where in the maturation and activation of B and T lymphocytes take place (Yiming et al., 2020). It is worth noting that the incorporation of SeNPs in the vaccine formula (0.1 mg/dose) induced a significantly higher expression of tested cytokines, which might be attributed to the direct exposure of circulating immune cells to the injected SeNPs.
5. Conclusions
The incorporation of SeNPs in the chickens’ diet and vaccine formula enhanced the protectivity of homologous whole inactivated H5N1 vaccine in a dose-dependent manner. The high protectivity induced by SeNPs treatment without significant enhancement in the HI titers (in all groups except 1 mg/kg) indicated that humeral immune enhancement is not the primary derivative of a higher protectivity induced by SeNPs. In contrast, the anti-inflammatory impact along with cellular immunity is associated with enhanced protectivity induced by SeNPs. Based on the amount of SeNPs administered to the different groups and the vaccine efficacy outcomes, the vaccination effectiveness was highest in the groups that were fed a diet supplemented with 1mgSeNPs/kg and vaccinated with 0.1 mg/dose. The group vaccinated with 0.1 mg/dose, on the other hand, demonstrated the most rapid reduction in virus shedding. Further studies should investigate the amount and time of retention of a higher concentration of SeNPs in different organs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We would like to thank our colleagues at the CLEVB animal experimental facilities, and Department of Agricultural Microbiology, Faculty of Agriculture, Zagazig University, for their technical help.
Disclosures: Authors declare no conflict of interests.
Funding
This study was supported by Taif University Researchers Supporting Project (TURSP-2020/09), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11(7):1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Moneim A.M.E., El-Saadony M.T., Shehata A.M., Saad A.M., Aldhumri S.A., Ouda S.M., Mesalam N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022;29(2):1197–1209. doi: 10.1016/j.sjbs.2021.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelwhab E.M., Hassan M.K., Abdel-Moneim A.S., Naguib M.M., Mostafa A., Hussein I.T.M., Arafa A., Erfan A.M., Kilany W.H., Agour M.G., El-Kanawati Z., Hussein H.A., Selim A.A., Kholousy S., El-Naggar H., El-Zoghby E.F., Samy A., Iqbal M., Eid A., Ibraheem E.M., Pleschka S., Veits J., Nasef S.A., Beer M., Mettenleiter T.C., Grund C., Ali M.M., Harder T.C., Hafez H.M. Introduction and enzootic of A/H5N1 in Egypt: Virus evolution, pathogenicity and vaccine efficacy ten years on. Infect. Genet. Evol. 2016;40:80–90. doi: 10.1016/j.meegid.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Abdelwhab E.M., Hafez H.M. An overview of the epidemic of highly pathogenic H5N1 avian influenza virus in Egypt: epidemiology and control challenges. Epidemiol. Infect. 2011;139(5):647–657. doi: 10.1017/S0950268810003122. [DOI] [PubMed] [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F.M. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100(6):101172. doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: Antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276:114920. doi: 10.1016/j.anifeedsci.2021.114920. [DOI] [Google Scholar]
- Alagawany M., Qattan S.Y.A., Attia Y.A., El-Saadony M.T., Elnesr S.S., Mahmoud M.A., Madkour M., Abd El-Hack M.E., Reda F.M. Use of chemical nano-selenium as an antibacterial and antifungal agent in quail diets and its effect on growth, carcasses, antioxidant, immunity and caecal microbes. Animals. 2021 doi: 10.3390/ani11113027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M.M., Arafa A., Hassan M.K. Epidemiological findings of outbreaks of disease caused by highly pathogenic H5N1 avian influenza virus in poultry in Egypt during 2006. Avian Dis. 2008;52(2):269–277. doi: 10.1637/8166-103007-Reg.1. [DOI] [PubMed] [Google Scholar]
- Arafa A.S., Naguib M.M., Luttermann C., Selim A.A., Kilany W.H., Hagag N., Harder T.C. Emergence of a novel cluster of influenza A (H5N1) virus clade 2.2. 1.2 with putative human health impact in Egypt, 2014/15. Eurosurveillance. 2015;20(13):21085. doi: 10.2807/1560-7917.es2015.20.13.21085. [DOI] [PubMed] [Google Scholar]
- Boostani A., Sadeghi A.A., Mousavi S.N., Chamani M., Kashan N. Effects of organic, inorganic, and nano-Se on growth performance, antioxidant capacity, cellular and humoral immune responses in broiler chickens exposed to oxidative stress. Livest. Sci. 2015;2015(178):330–336. [Google Scholar]
- Boostani A., Sadeghi A.A., Mousavi S.N., Chamani M., Kashan N. The effects of organic, inorganic, and nanoselenium on blood attributes in broiler chickens exposed to oxidative stress. Acta Sci. Vet. 2015;43:1264. [Google Scholar]
- Cai S.J., Wu C.X., Gong L.M., Song T., Wu H., Zhang L.Y. Effects of nanoselenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult. Sci. 2012;91(10):2532–2539. doi: 10.3382/ps.2012-02160. [DOI] [PubMed] [Google Scholar]
- Chan G.C., Chan W.K., Sze D.M. The effects of ß-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009;2(1):5–7. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W.P., Hom J.S., Dietert R.R., Combs G.F., Marsh J.A. Effect of dietary vitamin E and selenium deficiency on chicken splenocyte proliferation and cell surface marker expression. Immunopharmacol. Immunotoxicol. 1994;16:203–223. doi: 10.3109/08923979409007091. [DOI] [PubMed] [Google Scholar]
- Criado M.F., Sá e Silva M., Lee D.-H., Salge C.A.d.L., Spackman E., Donis R., Wan X.-F., Swayne D.E., Parrish C.R. Cross-Protection by Inactivated H5 Prepandemic Vaccine Seed Strains against Diverse Goose/Guangdong Lineage H5N1 Highly Pathogenic Avian Influenza Viruses. J. Virol. 2020;94(24) doi: 10.1128/JVI.00720-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eladl A.H., Arafat N., El-Shafei R.A., Farag V.M., Saleh R.M., Awadin W.F. Comparative immune response and pathogenicity of the H9N2 avian influenza virus after administration of Immulant®, based on Echinacea and Nigella sativa, in stressed chickens. Comp. Immunol. Microbiol. Infect. Dis. 2019;65:165–175. doi: 10.1016/j.cimid.2019.05.017. [DOI] [PubMed] [Google Scholar]
- El-Ashry R.M., El-Saadony M.T., Elsobkia A.E.A., El-Tahan A.M., Al-Otaibid S., El-Shehawi A.M., Saad A.M., Elshaera N. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne Incognita in eggplants. Saudi J. Biol. Sci. 2022;29(2):920–932. doi: 10.1016/j.sjbs.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., Abdel-Hamid S.E., Taha T.F., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28(8):4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Desoky E.-S., Saad A.M., Eid R.S.M., Selem E., Elrys A.S. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 2021;106:1–14. doi: 10.1016/j.jes.2021.01.012. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Taha A.E., Fouda M.M.G., Ajarem J.S., Maodaa S.N., Allam A.A., Elshaer N. Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials. 2020;10(3):587. doi: 10.3390/nano10030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Shafi M.E., Hassan M.A. The use of biological selenium nanoparticles in controlling Triticum aestivum L. crown root and rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi. J. Biol. Sci. 2021;28(8):4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69:102645. doi: 10.1016/j.ifset.2021.102645. [DOI] [Google Scholar]
- Fan R.-F., Liu J.-X., Yan Y.-X., Wang L., Wang Z.-Y. Selenium relieves oxidative stress, inflammation, and apoptosis within spleen of chicken exposed to mercuric chloride. Poult. Sci. 2020;99(11):5430–5439. doi: 10.1016/j.psj.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fereidouni S.R., Harder T.C., Gaidet N., Ziller M., Hoffmann B., Hammoumi S., Globig A., Starick E. Saving resources: Avian influenza surveillance using pooled swab samples and reduced reaction volumes in real-time RT-PCR. J. Virol. Methods. 2012;186(1-2):119–125. doi: 10.1016/j.jviromet.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Firenzuoli F., Gori L., Lombardo G. The medicinal mushroom Agaricus blazei murrill: review of literature and pharmaco-toxicological problems. Evid. Based complement. Alternat. Med. 2008;5(1):3–15. doi: 10.1093/ecam/nem007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel T.W., Schneider K., Schaerer B., Mejri I., Puehler F., Weigend S., Kaspers B. IL-18 stimulates the proliferation and IFN-gamma release of CD4+ T cells in the chicken: conservation of a Th1-like system in a nonmammalian species. J. Immunol. 2003;171:1809–1815. doi: 10.4049/jimmunol.171.4.1809. [DOI] [PubMed] [Google Scholar]
- Gomaa M.R., El Rifay A.S., Abu Zeid D., Elabd M.A., Elabd E., Kandeil A., Shama N.M.A., Kamel M.N., Marouf M.A., Barakat A., Refaey S., Naguib A., McKenzie P.P., Webby R.J., Ali M.A., Kayali G. Incidence and seroprevalence of avian influenza in a cohort of backyard poultry growers, Egypt, August 2015-March 2019. Emerg. Infect. Dis. 2020;26(9):2129–2136. doi: 10.3201/eid2609.200266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grund C., Abdelwhab E.-S., Arafa A.-S., Ziller M., Hassan M.K., Aly M.M., Hafez H.M., Harder T.C., Beer M. Highly pathogenic avian influenza virus H5N1 from Egypt escapes vaccine-induced immunity but confers clinical protection against a heterologous clade 2.2.1 Egyptian isolate. Vaccine. 2011;29(33):5567–5573. doi: 10.1016/j.vaccine.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Habibian M., Sadeghi G., Ghazi S., Moeini M.M. Selenium as a feed supplement for heat-stressed poultry: a review. Biol. Trace Elem. Res. 2015;165(2):183–193. doi: 10.1007/s12011-015-0275-x. [DOI] [PubMed] [Google Scholar]
- Harsini S.G., Habibiyan M., Moeini M.M., Abdolmohammadi A.R. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol. Trace Elem. Res. 2012;148(3):322–330. doi: 10.1007/s12011-012-9374-0. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Hoffmann D., Henritzi D., Beer M., Harder T.C. Riems influenza a typing array (RITA): An RT-qPCR-based low-density array for subtyping avian and mammalian influenza a viruses. Sci. Rep. 2016;6:27211–32721. doi: 10.1038/srep27211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P.R., Berry M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008;52:1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höper D., Hoffmann B., Beer M. Simple, sensitive, and swift sequencing of complete H5N1 avian influenza virus genomes. J. Clin. Microbiol. 2009;47(3):674–679. doi: 10.1128/JCM.01028-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.H., Li Y.L., Xiong L., Zhang H.M., Song J., Xia M.S. Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim. Feed Sci. Technol. 2012;177(3-4):204–210. [Google Scholar]
- Jones S.A. Directing transition from innate to acquired immunity: defining a role for IL-6. J. Immunol. 2005;175(6):3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- Kandeil A., Hicks J.T., Young S.G., El Taweel A.N., Kayed A.S., Moatasim Y., Kutkat O., Bagato O., McKenzie P.P., Cai Z., Badra R., Kutkat M., Bahl J., Webby R.J., Kayali G., Ali M.A. Active surveillance and genetic evolution of avian influenza viruses in Egypt, 2016–2018. Emerg. Microbes Infect. 2019;8(1):1370–1382. doi: 10.1080/22221751.2019.1663712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeil A., Mostafa A., El-Shesheny R., El-Taweel A.N., Gomaa M., Galal H., Kayali G., Ali M.A. Avian influenza H5N1 vaccination efficacy in Egyptian backyard poultry. Vaccine. 2017;35(45):6195–6201. doi: 10.1016/j.vaccine.2017.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayali G., Kandeil A., El-Shesheny R., Kayed A.S., Maatouq A.M., Cai Z., McKenzie P.P., Webby R.J., El Refaey S., Kandeel A., Ali M.A. Avian Influenza A(H5N1) Virus in Egypt. Emerg. Infect. Dis. 2016;22(3):379–388. doi: 10.3201/eid2203.150593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-W., Suarez D.L. Application of real-time RT-PCR for the quantitation and competitive replication study of H5 and H7 subtype avian influenza virus. J. Virol. Methods. 2004;119(2):151–158. doi: 10.1016/j.jviromet.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Li Y., Lin Z., Guo M., Xia Y., Zhao M., Wang C., Zhu B. Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int. J. Nanomed. 2017;12:5733–5743. doi: 10.2147/IJN.S140939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Zhang J., Xu J.F., Pi J. The Advancing of Selenium Nanoparticles Against Infectious Diseases. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.682284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Longhi M.P., Wright K., Lauder S.N., Nowell M.A., Jones G.W., Godkin A.J., Jones S.A., Gallimore A.M., Garcia-Sastre A. Interleukin-6 Is Crucial for Recall of Influenza-Specific Memory CD4+ T Cells. PLoS Pathog. 2008;4(2):e1000006. doi: 10.1371/journal.ppat.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyre M., Samaha H., Makonnen Y.J., Saad A., Abd-Elnabi A., Galal S., Ettel T., Dauphin G., Lubroth J., Roger F., Domenech J. Avian influenza vaccination in Egypt: Limitations of the current strategy. J. Mol. Genet. Med. 2009;3:198–204. doi: 10.4172/1747-0862.1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.S., Styles D., Kong W., Andrews C., Gorres J.P., Nabel G.J. A gene-based avian influenza vaccine in poultry. Poult. Sci. 2009;88(4):860–866. doi: 10.3382/ps.2008-00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.V.R., Prakash B., Raju M.V.L.N., Panda A.K., Poonam S., Murthy O.K. Effect of supplementing organic selenium on performance, carcass traits, oxidative parameters and immune responses in commercial broiler chickens. Asian-Australas. J. Anim. Sci. 2013;26(2):247–252. doi: 10.5713/ajas.2012.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100(8):101266. doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohaim M.A., El Naggar R.F., Madbouly Y., AbdelSabour M.A., Ahmed K.A., Munir M. Comparative infectivity and transmissibility studies of wild-bird and chicken-origin highly pathogenic avian influenza viruses H5N8 in chickens. Comp. Immunol. Microbiol. Infect. Dis. 2021;74:101594. doi: 10.1016/j.cimid.2020.101594. [DOI] [PubMed] [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A.M., Taha A.E., Taha T.F., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate Sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021;28(10):5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samy A.A., El-Enbaawy M.I., El-Sanousi A.A., Nasef S.A., Naguib M.M., Abdelwhab E.M., Hikono H., Saito T. Different counteracting host immune responses to clade 2.2.1.1 and 2.2.1.2 Egyptian H5N1 highly pathogenic avian influenza viruses in naïve and vaccinated chickens. Vet. Microbiol. 2016;183:103–109. doi: 10.1016/j.vetmic.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Samy A., El-Enbaawy M.I., El-Sanousi A.A., Nasef S.A., Hikono H., Saito T. Initiation and regulation of immune responses to immunization with whole inactivated vaccines prepared from two genetically and antigenically distinct lineages of Egyptian influenza A virus subtype H5N1. Arch. Virol. 2016;161(10):2797–2806. doi: 10.1007/s00705-016-2989-2. [DOI] [PubMed] [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd El-Hack M.E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10(3):430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojadoost B., Taha-Abdelaziz K., Alkie T.N., Bekele-Yitbarek A., Barjesteh N., Laursen A., Smith T.K., Shojadoost J., Sharif S. Supplemental dietary selenium enhances immune responses conferred by a vaccine against low pathogenicity avian influenza virus. Vet. Immunol. Immunopathol. 2020;227:110089. doi: 10.1016/j.vetimm.2020.110089. [DOI] [PubMed] [Google Scholar]
- Skalickova S., Milosavljevic V., Cihalova K., Horky P., Richtera L., Adam V. Selenium nanoparticles as a nutritional supplement. Nutrition. 2017;33:83–90. doi: 10.1016/j.nut.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Suvarna K.S., Layton C., Bancroft J.D. Online and Print. Elsevier Health Sciences; 2012. Bancroft's theory and practice of histological techniques: expert consult. [Google Scholar]
- Suzuki K., Okada H., Itoh T., Tada T., Mase M., Nakamura K., Kubo M., Tsukamoto K. Association of increased pathogenicity of Asian H5N1 highly pathogenic avian influenza viruses in chickens with highly efficient viral replication accompanied by early destruction of innate immune responses. J. Virol. 2009;83(15):7475–7486. doi: 10.1128/JVI.01434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne, D., Brown, I., 2015. Manual of diagnostic tests and vaccines for terrestrial animal, chapter 2.3. 4. Avian Influenza.
- Swelum A.A., Shafi M.E., Albaqami N.M., El-Saadony M.T., Elsify A., Abdo M., Taha A.E., Abdel-Moneim A.-M., Al-Gabri N.A., Almaiman A.A., Saleh Al-wajeeh A., Tufarelli V., Staffa V.N., Abd El-Hack M.E. COVID-19 in human, animal, and environment: a review. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biological Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakili R., Daliri R. The effect of different levels of vitamin E on humoral immunity, and performance in broiler chicks. J. Vet. Res. 2010;65(3):239–244. [Google Scholar]
- Wang C., Chen H., Chen D., Zhao M., Lin Z., Guo M., Xu T., Chen Y.i., Hua L., Lin T., Tang Y., Zhu B., Li Y. The inhibition of H1N1 influenza virus-induced apoptosis by surface decoration of selenium nanoparticles with β-thujaplicin through Reactive Oxygen species-mediated AKT and p53 signaling pathways. ACS omega. 2020;5(47):30633–30642. doi: 10.1021/acsomega.0c04624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.L., Zhang J.S., Yu H.Q. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007;42:1524–1533. doi: 10.1016/j.freeradbiomed.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Webby R.J., Perez D.R., Coleman J.S., Guan Y., Knight J.H., Govorkova E.A., McClain-Moss L.R., Peiris J.S., Rehg J.E., Tuomanen E.I., Webster R.G. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet. 2004;363(9415):1099–1103. doi: 10.1016/S0140-6736(04)15892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert M.O., Pfeiffer A.F. Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr. 2008;138(3):439–442. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- Yiming Z., Qingqing L., Hang Y., Yahong M., Shu L.i. Selenium deficiency causes immune damage by activating the DUSP1/NF-κB pathway and endoplasmic reticulum stress in chicken spleen. Food Funct. 2020;11(7):6467–6475. doi: 10.1039/d0fo00394h. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen Y., Shan N., Wang X., Lin S., Ma K., Li B., Li H., Liao M., Qi W. Genetic diversity, phylogeography, and evolutionary dynamics of highly pathogenic avian influenza A (H5N6) viruses. Virus Evol. 2020;6(2):veaa079. doi: 10.1093/ve/veaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.S., Wang X.F., Xu T.W. Elemental selenium at nano size (nano-Se) as a potential chemo preventive agent with reduced risk of selenium toxicity: Comparison with Se-methyl selenocysteine in mice. Toxicol. Sci. 2008;101:22–31. doi: 10.1093/toxsci/kfm221. [DOI] [PubMed] [Google Scholar]