Graphical abstract
Keywords: Biological control, Parasitic nematodes, Piper nigrum, Yield, Meloidogyne incognita
Abstract
Black pepper (Piper nigrum L.) is one of the oldest spices in the world, additionally, it is highly demanded. Several biotic and abiotic variables pose black pepper production worldwide. Plant-parasitic nematodes play a key role among biotic factors, causing considerable economic losses and affecting the production. Different synthetic nematicides were used for controlling plant nematodes, however the majority of pesticides have been pulled from the market due to substantial non-target effects and environmental risks. As a result, the search for alternative eco-friendly agents for controlling plant-parasitic nematodes populations. Microbial agents are a precious option. In this review the bacterial and fungal agents used as an alternative nematicides, they were studied and confirmed as essential anti-microbial agents against plant nematodes which infected Piper nigrum L. This work examines the most common plant nematodes infected Piper nigrum L., with a focus on root knot and burrowing nematodes, in addition, how to control plant parasitic nematodes using microorganisms.
1. Introduction
Black pepper (Piper nigrum L.) is an Indian origin. It is grown on an estimated 1,37,378 ha in India, with an evaluated yield production of 61,000 tons in 2019–2020 (Subha and Balamurugan, 2020). Karnataka produces the majority of pepper in India, which has been evaluated to deliver about 30 thousand metric tons of pepper annually. It was expected that the perennial woody vine of pepper is going to grow in about 40 ha of land (Statista, 2021). Many factors were affected the black pepper crop for several years (Karmawati et al., 2020). One of the most earnest restrictions is parasitic nematodes, which cause massive yield losses and lower productivity as a result of biotc and abiotic stressors such as in (Desoky et al., 2020a). Burrowing nematode, Radopholus similis and Root knot nematode, Meloidogyne incognita are the most common plant parasitic nematodes infesting black pepper crops (Gómez-Rodríguez et al., 2017; Pervez, 2018). Table 1 presented 250 species from 43 genera that meet one or more of the criteria for being classified as a phytosanitary risk. The majority of these species affected the economically important crops, and the others were vectors for viruses (Singh et al., 2013).
Table 1.
Nematode’s genera and number of species that badly affect black pepper crops.
| Genera | Number of species |
|---|---|
| Achlysiella | 1 |
| Anguina | 8 |
| Aphasmatylenchus | 1 |
| Aphelenchoides | 12 |
| Aphelenchus | 1 |
| Belonolaimus | 2 |
| Bitylenchus | 3 |
| Bursaphelenchus | 4 |
| Cactodera | 3 |
| Ditylenchus | 8 |
| Dolichodorus | 1 |
| Globodera | 3 |
| Helicotylenchus | 7 |
| Hemicriconemoides | 3 |
| Hemicycliophora | 3 |
| Heterodera | 25 |
| Hirschmanniella | 5 |
| Hoplolaimus | 5 |
| Ibipora | 3 |
| Longidorus | 10 |
| Macroposthonia | 2 |
| Meloidogyne | 38 |
| Merlinius | 3 |
| Nacobbus | 1 |
| Neodolichodorus | 2 |
| Paralongidorus | 2 |
| Paratrichodorus | 11 |
| Paratylenchus | 3 |
| Pratylenchus | 24 |
| Punctodera | 3 |
| Quinisulcius | 3 |
| Radopholus | 5 |
| Rotylenchulus | 3 |
| Rotylenchus | 1 |
| Scutellonema | 5 |
| Sphaeronema | 1 |
| Subanguina | 3 |
| Trichodorus | 5 |
| Tylenchorhynchus | 8 |
| Tylenchulus | 2 |
| Vittatidera | 1 |
| Xiphinema | 15 |
| Zygotylenchus | 1 |
| Achlysiella | 1 |
2. Plant parasitic nematodes in black pepper
Black pepper was infected with plant 29 genera and 48 spp, of parasitic nematodes. Seventeen genera have been linked to the crop thus far. Meloidogyne incognita, Rhadopholus similis, and Holicotylenchus spp. usually infest black pepper in Kerala (Subila and Suseela Bhai, 2020) M. incognita is the most prevalent species. Trophotylenchulus piperis also attack black pepper (Ravindra et al, 2015). Meloidogyne piperi sp. is a new species of root-knot nematode discovered in the roots of Piper nigrum growing in Kerala, India, has been described and illustrated (Nisha et al., 2019). The varities, Radopholus similis, Meloidogyne incognita, and Trophotylenchulus piperis attack the roots of Piper nigrum. In Vietnam, 35 plant-parasitic nematodes from 19 sp. and 11 families hurt pepper plants (Thuy et al., 2012). Meloidogyne spp., Tylenchus sp., Rotylenchulus reniformis, Ditylenchus ausafi, and Aphelenchus avenae are five plant-parasitic nematodes found in all provinces studied. Meloidogyne spp, were the common taxon found, and all Meloidogyne were recognized as M. incognita. In Brazil, the check results revealed that Meloidogyne arenaria, M. incognita, M. javanica, Hoplolaimus seinhorsti, and Xiphinema ifacolum affect the growth of black pepper. Plant height and root development were both lowered by all nematode species, where the leaves of stunted plants attacked with H. seinhorsti and M. incognita revealed yellow color (De Souza et al, 2021). Additionally, Criconemoides sp., Xiphinema sp. Aphelenchus sp., Longidorus sp., Trophotylenchulus piperis, Radopholus similis, Hoplolaimus sp., Meloidogyne incognita, Pratylenchus sp., Scutellonema sp., Helicotylenchus sp. Rotylenchulus reniformis, Tylenchorhynchus sp., and Acontylus sp. (Ramana et al., 1994). The gradual wilt/yellows disease in the black pepper is caused by M. incognita and R. similis (Brooks, 2021). The slow decline infection in black pepper is induced by M. incognita, Phytophthora capsica, and R. similis, revealing feeder root damage. Generally the bad effects of nematodes were briefed in Fig. 2.
Fig. 2.
RKN Infection and the benefit of biocontrol.
2.1. Root knot nematode
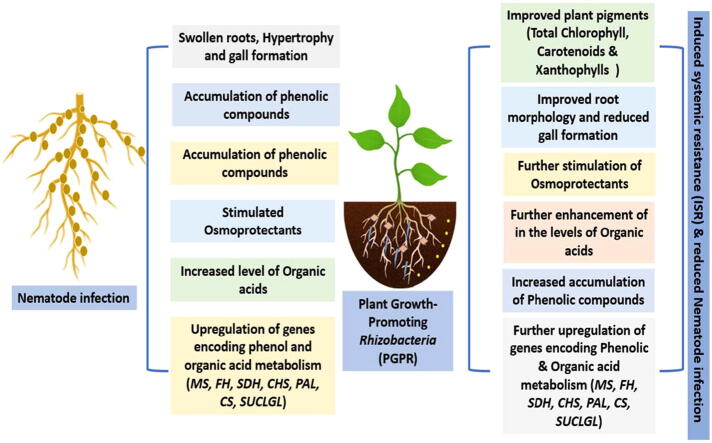
Meloidogyne incognita (Kofoid and White) Chitwood, a root-knot nematode (RKN), is one of the highly persistent pests because of its damage-revealing potential (Ravindra et al., 2014) see Fig. 2, the infection levels exceed to 90% in Brazil and India. Meloidogyne spp. has been implicated in the decreased unprofitable development of black pepper in India (Narayana et al., 2018), Malaysia (Leong et al., 2021), and Brazil (De Souza et al., 2021). Under potts conditions, ten juveniles per plant can reduce black pepper growth by 16%. The root-knot nematode is an obligatory endoparasite that lives entirely within the roots of plants. The RKN causes “giant cells” bloating in the vascular tissues after entering the plant roots (Jones and Goto, 2011). The root-knot nematode's feeding disrupts water and nutrients intake via plant roots, while the gigantic cells are metabolic active cells which consider as feed sinks to meet the nematode females' rising nutritional demands for reproduction (Mhatre et al., 2015). The yellowing and wilt disease that affects pepper plants is caused by M. incognita. Wilting appears 2-3 months after a severe infection in conditions of sunny, warm, and dry weather (Shahnazi et al., 2012).
2.2. Burrowing nematode
Radopholus similis (Cobb) Thorne, a burrowing nematode, found to be infesting over 300 plant species (Sathyan et al, 2020). Via the host variable analysis using multiple Citrus sp. and cytological research in India, proved that R. similis infected black pepper was known as the ‘banana race' (Ramana, 1992). R. similis population increase in black pepper gardens throughout the year except in the summer, when they are estimated to be more than 250 nematodes/gram of roots. The nematode growth population begins in June/July and reached the peak in September/October. R. similis prefers black pepper to white pepper as a host of R. similis. Yellowing symptoms, root necrosis, and canopy dieback are all signs of burrowing nematode infestation in pepper crops (Kumar et al., 2018).
3. Impact of plant parasitic nematodes
Plant-parasitic nematodes posed 15% of annual crop yield estimating losses of 100–157 billion USD all over the world (Abd-Elgawad and Askary, 2015, Phani et al., 2021). Due to the complicated relationship between plants, nematodes, soil organisms and soils, yield loss data is difficult to acquire (Coyne and Plowright, 2000). Several biotic and abiotic stressors are threatening black pepper production (Thangaselvabal et al., 2008, Negi et al., 2021). Plant parasitic nematodes are one of the principal limiting factors among the biotic stressors, causing yield losses of up to 15–35% (Abd-Elgawad and Askary, 2015). Nematodes have been identified as a primary cause of early decrease in black pepper output (Thuy et al, 2012). Slow wilt is thought to be caused by a combination of nematode fungal infection and nutritional inadequacy in many pepper growing areas (Naik et al, 2017). M. incognita and R. similis produced considerable reductions in black pepper growth and productivity in pathogenicity experiments conducted under simulated field conditions (Mohandas and Ramana, 1991). Nematode feeding combined by secondary diseases such as fungus and bacteria, in addition to direct feeding and migratory harm (Mitiku, 2018). More crucially, just 0.2% of the infested crops were used to fund nematological research to remedy these losses (Pervez and Eapen, 2015). The root-knot nematode's relationship with black pepper vines was first observed in 1906 (Butler, 1906), followed by the fungus Fusarium sp. connected with the 'wilt sickness' (Krishna Menon, 1949). Considerable decline infection was thought to be caused by a combination of worm and fungus disease, as well as nutrients deficiencies (Abd-Elgawad and Askary, 2018). Both organisms had a synergistic pathogenic effect on plants, causing the plants wilting, and the presence of an initial nematode infection increased the growth inhibition. The decline in black pepper yield is not only due to nematodes or fungi, but also the synergy of nematodes and fungi causes a massive decrease in pepper yield (Ramana et al., 1992, Usman et al., 2020).
4. Biological control of nematodes in black pepper
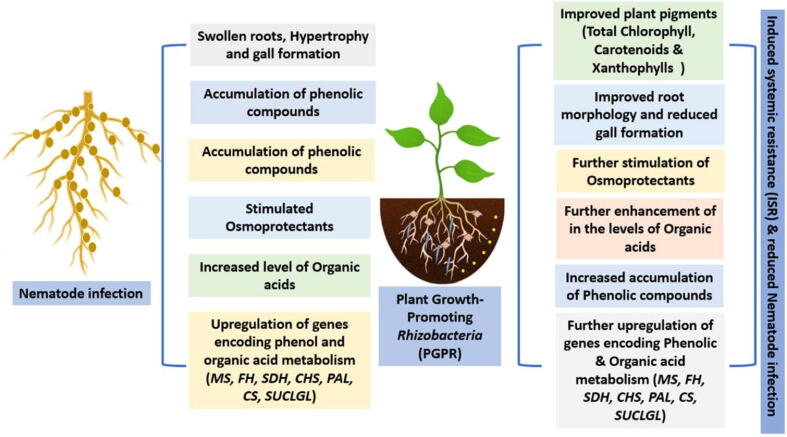
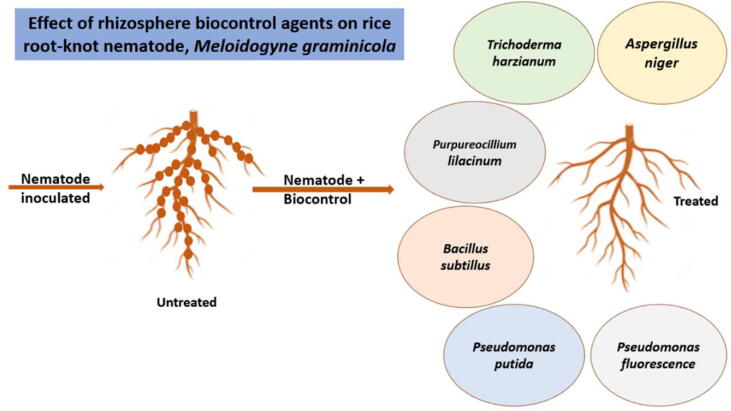
Because nematodes primarily block the soil and attack the plants’ roots, nematode treatment is more complex than other pests (Mian, 1998). Chemical nematocidies have been used to control plant parasitic nematodes for decades, but they are progressively being re-evaluated due to health and environmental concerns, as well as restricted availability in developing countries. Various studies demonstrated several techniques i.e., convential breeding for black pepper (Hassanin et al., 2020),eco-friendly natural compounds which may have nematocidial activity among these compounds are bioactive peptides (Saad et al., 2020a, El-Saadony et al., 2021a, El-Saadony et al., 2021b, Saad et al., 2021a), polyphenolic extracts (Saad et al., 2020b, Saad et al., 2021b, Saad et al., 2021c), essential oils (El-Tarabily et al., 2021, Abd El-Hack et al., 2022, Alagawany et al., 2021), and nanomaterials (Saad et al., 2021d, El-Ashry et al., 2022, El-Saadony et al., 2021c), additionally microbial control is a safe and an effective attitude in controlling parasitic nematodes (Dong and Zhang, 2006, Mukhtar and Pervaz, 2003). Biological control is a cost-effective and environmentally acceptable alternative to chemical control for worm management, as well as a safer crop protection technique to combat nematode stress (Mhatre et al., 2019). When considering the microbial-based efficacy inside a suppressive soil, the effectiveness of pathogens, endophytes, and opportunities for biological management of Meloidogyne spp and other stressors, is high (Desoky et al., 2020b, Hallmann et al., 2009). Therefore, the use of these natural practices is safer, environmentally friendly, and more effective than chemical formulations and limits the spread of diseases (El-Saadony et al., 2020, Swelum et al., 2020). Many studies have shown that numerous culturable microorganisms, such as bacteria and fungi, can be used as biocontrol agents against plant parasitic nematodes in a variety of crops (Mukhtar et al., 2013, Rahanandeh et al., 2012). Table 2, Fig. 1, Fig. 2 showed recent brief about microbial control of nematodes and their mechanisms of action.
Table 2.
Microbial control of black pepper parasite nematodes.
| Microorganisms | Mode of action | References | |
|---|---|---|---|
| Fungi | Paecliomyces lilacinus | extensively infested root-knot nematode-infested black pepper plantations where colonised 90% of female nematodes, inhibited egg hatching kept in spore suspension | Ahmad et al., 2019, Sivakumar et al., 2020, Youssef et al., 2020 |
| Pochonia halamydosporia | reduced the eggs hatching of RKN by 41.4% in five days, indicating that it could be used to manage root knot nematodes in spice crops | Ghahremani et al., 2019, Manzanilla-López et al., 2017 | |
| Arthrobotrys oligospora | The fungal activity in the soil lead to a drop in nematodes count, minimizing the nematode's destruction | Liu et al., 2021, Soliman et al., 2021 | |
| Vesicular Arbuscular Mycorrhizae (VAM) | Pre-inoculating pepper vines with VAM will help to reduce the severity of M. incognita root infestation. Glomus fasciculatum had a reduction in root-knot index of 32.4%, whereas Glomus etunicalum had a reduction of 36%. In black pepper plants | Bhale et al., 2018, da Silva Campos, 2020, Nair et al., 2022, Vallejos-Torres et al., 2021 | |
| Bacteria | Bacillus subtilis | revealed the strongest inhibition on root knot nematode egg hatching by 92% where, Chitinase and protease were found to be highly linked to egg hatching suppression, while natural chemicals with thermal stability were recently identified to be important in killing J2 nematodes |
Cao et al., 2019, Mazzuchelli et al., 2020 |
| Bacillus thuringiensis | Freshly born 2nd stage juveniles (J2) of Meloidogyne javanica were killed by B. thuringiensis culture by 80% | Leong et al., 2021, Sidhu, 2018, Khanh, 2020 | |
| Pasteuria spp | One of the most promising bacterial biocontrol agents for many worm species because they can totally limit nematode reproduction by functioning as an ovarian parasite. It is found to be specific to M. incognita from completed its life cycle | Öztürk et al., 2020, Perrine-Walker and Le, 2021, Sidhu et al., 2021 | |
| Bacteria | Pseudomonas fluorescens | is attributed to bind the root surface with carbohydrate and lectin, competing with the host, made it a promising biocontrol agent against root knot nematode |
Senthilkumar and Ananthan, 2018, Sharma et al., 2021, Azam et al., 2018 |
| Entophytic bacteria | Serratia spp. | reduced nematode populations in soil by over 70% while simultaneously producing over 65% nematode-free plantlets | Daulagala, 2021, Maulidia et al., 2020, Nguyen et al., 2021, Tran et al., 2019, Maris et al., 2020, Munif et al., 2020 |
| Pseudomonads spp. | |||
| Arthrobacter spp | |||
| Curtobacterium spp. | |||
| Micrococcus spp. | |||
| Bacillus spp. | |||
Fig. 1.
Effect of rhizosphere biocontrol against RKN.
4.1. Fungus
Nematopathogenic fungi are carnivorous fungi, which trap vermiform nematodes with their spores, mycelial structures or hyphal tips to parasitize nematode cysts and eggs or create toxins to assault nematodes (Khan and Haque, 2011). More than 200 fungus species from six different classes have been found to parasitize nematode eggs, juveniles, adults, and cysts (Mukhtar et al., 2013).
4.1.1. Paecliomyces lilacinus
Paecilomyces lilacinus (Thorn.) Samson, a soil-dwelling hypomycetes is potent against root-knot nematodes recently, it has piqued the interest of different scientists because of its effectiveness in controlling phytonematode propagations. It has been discovered in most agricultural soils with repeated occurrence in the tropics and subtropics (Chen et al., 1996). Ten local strains of Paecilomyces lilacinus (PL) obtained from two extensively infested root-knot nematode-infested black pepper plantations (Pau et al., 2012), colonized female nematodes to varied degrees. Two indigenous strains out of ten showed highly substantial colonization (90%) on RKN females and dramatically inhibited egg hatching kept in spore suspension. Paecilomyces did not entirely suppress nematodes, but it can reduce nematode infection and enhance root mass in black pepper (Hano and Khan, 2016). The fungus was more effective On Meloidogyne incognita than Rhadopholus similis. The low values of Root Knot Index (RKI) in plants treated with P. lilacinus, while M. incognita was attributed to the specificity of P. lilacinus parasites RKN eggs. In pots under greenhouse conditions, the potency of P. lilacinus to overcome of M. incognita on black pepper was investigated. In the treatment of RKN in black pepper, P. lilacinus was more effective than P. penetrans, although the usage of both species together was beneficial (Sosamma and Koshy, 1997).
4.1.2. Pochonia chalamydosporia
Pochonia chlamydosporia (Goddard) (Verticillium chlamydosporium) was reported as a parasite of nematode eggs in 1974. For the first time (Sreeja et al., 1996), Verticillium chlamydosporium was isolated and identified from infected black pepper by a semi-endoparasitic nematode. In an in vitro experiment, the fungus reduced the eggs hatching of RKN by 41.4% in five days, indicating that it could be used to manage root knot nematodes in spice crops. Owing to the mass population, saprophytic character, and durability of chlamydospores, only Pochonia chlamydosporia demonstrated valuable control of root knot nematodes attacking black pepper (Eapen et al., 2009). Organic soils have proven to be a better substrate for P. chlamydosporia growth than mineral soils (Kerry et al., 1993). It was discovered that the tritrophic relationship between root-knot nematodes, P. chlamydosporia, and the host plant is complex (Kerry, 2001).
4.1.3. Arthrobotrys oligospora
Arthrobotrys oligospora is a species of Arthrobotrys. The widely isolated and the most ubiquitous nematode-catching fungus in the environment, the first reported nematode trapping fungus (Farrell et al., 2006, Jaffee, 2004, Wachira et al., 2009). Arthrobotrys (53 sp.), Dactylellina (28 sp.), and Drechslerella are the three primary genus of nematophagous fungi (14 sp.). The fungal activity in the soil lead to a drop in nematodes count, minimizing the nematode's distruction (Jaffee et al., 1996). They are made up of about 200 species of taxonomically varied fungi that all have the potential to feed on living nematodes (juveniles, adults, and eggs) and use them as nutrients (Nordbring-Hertz et al., 2006). Three strains of nematophagous fungus Arthrobotrys oligospora were isolated from sixty coffee and pepper planted soil samples have multi-trap effect for different nematode sp., especially Meloidogyne incognita and Pratylenchus coffea, which harmed pepper and coffee in Vietnam (Hiep et al., 2019).
4.1.4. Vesicular arbuscular mycorrhizae (VAM)
VAM's contribution to minimizing the damaging effects of root invasion by several parasitic nematodes in crop plants is now widely acknowledged. Four kinds of vesicular arbuscular mycorrhizae were equally potent as phorate in controlling worm infections in black pepper. Pre-inoculating pepper vines with VAM will help to reduce the severity of M. incognita root infestation. Glomus fasciculatum had a reduction in root-knot index of 32.4%, whereas Glomus etunicalum had a reduction of 36%. In black pepper plants, the highest plant development was recorded in the shape of vine length, nodes numbers, existing leaves number, shoot and root weight in plants, which received only AMF (Koshy et al., 2003). The multiplication of burrowing and root knot nematodes was decreased when AMF was administered prior to nematode inoculation, lowering root-knot indices and the root-lesion indices.
4.2. Bacteria
Host-plant tissues, soil and nematodes, as well as their eggs and cysts, have all yielded a range of nematopathogenic bacterial groupings (Tian et al., 2007). To manage populations of plant parasite nematodes in natural conditions, they build a net with intricate interactions between the environment, bacteria, nematodes and plants (Tian et al., 2007, Rahanandeh et al., 2012).
4.2.1. Bacillus subtilis
Bacillus subtilis (Ehrenberg) Cohn is useful in increasing plant vigour while also being toxic to plant diseases and nematodes. Active nematocidal rhizobacteria, Bacillus subtilis strain (RB.DL.28) was isolated from black pepper roots in Vietnam, revealed the strongest inhibition on root knot nematode egg hatching with 82% (Nguyen et al., 2019). Chitinase and protease were found to be highly linked to egg hatching suppression, while natural chemicals with thermal stability were recently identified to be important in killing J2 nematodes. Prophylactic application of B. subtilis, P. fluorescens, T. viride, and AMF resulted in a soil environment capable of suppressing nematode population formation in the soil and roots of Piper longum, as well as keeping infection at a lower level. P. longum treated with B. subtilis showed the greatest reduction in root knot index (Subhagan, 2008). additionaly, Bacillus thuringiensis Berliner (Bt)had nematicidal effects for insect management (Brar et al., 2006), also, it examined against commercially important phyto parasitic nematodes (El-Sherif et al., 2007, Khan et al., 2010, Mohammed et al., 2008). Freshly born 2nd stage juveniles (J2) of Meloidogyne javanica were killed by B. thuringiensis culture (Carneiro et al., 1998). After in vitro treatment with Bt was reduced the nematodes population with 80% (Mozgovaya et al., 2002). Pasteuria is a Gram positive, endospore forming bacterial parasite of a high range of invertebrates that was first discovered parasitizing Daphnia spp., a type of water flea. Pasteuria parasitizes plant parasitic nematodes in six species (Mohan et al., 2012) and one parasitizes bacterivorous nematodes in one species (Mohan et al., 2012). Pasteuria spp. are one of the most promising bacterial biocontrol agents for many worm species because they can totally limit nematode reproduction by functioning as an ovarian parasite (Perrine-Walker and Le, 2021). Black pepper is a perennial crop that has been reported to be an excellent host for P. penetrans on M. incognita (Sosamma and Koshy, 1997). Under greenhouse conditions, the efficiency of P. penetrans in controlling RKN in black pepper was considerably reduced nematode propagation, root-gall indexes, and improve development and root mass productivity (Sosamma and Koshy, 1997). The Pasteuria strain found to be specific to M. incognita and ruined its life cycle (Mhatre et al., 2020). Pasteuria prevented nematode fecundity by preventing infected females from laying eggs or egg masses.
4.2.2. Pseudomonas fluorescens
The capacity of Pseudomonas fluorescens Migula is attributed to bind the root surface with carbohydrate and lectin, competing with the host, acheving a promising biocontrol agent against root knot nematode (Oostendrop and Sikora, 1990). Different biological agents, as Bacillus subtilis (Bbv 57), Pseudomonas fluorescens (Pfbv 22), Trichoderma viridi, AM fungi and Biodynamic compost were discovered to have the ability to boost considerable plant growth in terms of number of leaves and plant biomass in black pepper (Senthilkumar and Ananthan, 2018). In terms of nematode population decrease in black pepper, FYM enriched Pseudomonas fluorescens is considered the best of all bio-control agents (Bina and Sarodee, 2019).
4.3. Endophytic bacteria
One of the antagonist species commonly used in biological control is endophytic bacteria (Ryan et al., 2008). It like endoparasitic nematodes, colonizes inside plant tissue, making them great candidates for pathogen control (Hallmann et al., 2009). When compared to chemical control, endophytes are more effective since they migrate to internal plant tissue and detect pathogen on their own (Ryan et al., 2008). Endophytic bacteria consortiums (Serratia, Pseudomonads, Arthrobacter spp., Curtobacterium sp., Micrococcus spp., and Bacillus spp.) reduced nematodes, Radopholus similis and M. incognita (Aravind et al., 2009). Endophytic bacteria isolated from black pepper plant roots were studied for their biocontrol characteristics against root knot nematodes, as well as their activity against Fusarium oxysporum and Meloidogyne incognita (Wiratno et al., 2019). Nine endophytic bacteria isolated from pepper plants were safe and potent against F. oxysporum and M. incognita. Protease and chitinase are among the enzymes produced by the bacteria, which also play a role in nitrogen fixation and phosphate solubilization (Wiratno et al., 2019). Phytophthora capsici and Radopholus similis were discovered to have strong antagonistic effects against naturally existing endophytic bacteria from black pepper vines. On cut shoots, stem bacterisation with endophytic Pseudomonas spp., suppressed P. capsici infection (almost 90% lowering the length of the lesion) (Aravind et al., 2012). The majority of plantlets which inoculated with Pseudomonas aeruginosa were free of P. capsici infection on roots. Pseudomonas putida, and Bacillus megaterium. Curtobacterium luteum and Bacillus megaterium reduced nematode populations in soil by over 70% while simultaneously producing over 65% nematode-free plantlets. The bacteria were discovered to boost the growth of rooted cuttings in addition to protecting the plants against infections (Aravind et al., 2012). Curtobacterium luteum TC 10 was found to have much greater nematode suppression than Bacillus megaterium BP 17 regardless of the black pepper cultivars evaluated (Aravind et al., 2012).
5. Conclusion
Because of the possible risk of environmental and health issues, the alternatives for managing root knot nematodes are becoming increasingly limited. Several synthetic compounds have been employed to control plant parasitic nematodes; however, most of them have been pulled from the market due to substantial non-target effects. Several nematode-controlling nematicides are potent in controlling nematode infections in black pepper, but their use is minimized due to large costs and pollution. In places like India, where plant parasitic nematodes represent a severe danger to spice production, nematodes with adequate label claims are lacking. There is an immediate need for farmers to accept an alternative, cost-effective, and environmentally acceptable nematode management technique. As a result, the demand for comprehensive nematode management programs is pressing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors extend their appreciation to the deanship of Scientific Research at King Khalid University, Abha KSA for supporting this work under grant number (G.R.P/78/41).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Abo Ghanima M.M., Shukry M., Swelum A.A., Taha A.E., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poultry Sci. 2022;101(2):101584. doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-Elgawad M.M.M., Askary T.H. In: Biocontrol agents of phytonematodes. Askary T.H., Martinelli P.R.P., editors. CABI; Wallingford: 2015. Impact of phytonematodes on agriculture economy. In Biocontrol agents of phytonematodes; pp. 3–49. [DOI] [Google Scholar]
- Abd-Elgawad M.M., Askary T.H. Fungal and bacterial nematicides in integrated nematode management strategies. Egypt J. Biol. Pest Control. 2018;28(1):1–24. [Google Scholar]
- Ahmad R.Z., Sidi B.B., Endrawati D., Ekawasti F. Paecilomyces lilacinus and P.variotii as a predator of nematode and trematode eggs. IOP Conf. Ser.: Earth Environ. Sci. 2019;299(1):012056. doi: 10.1088/1755-1315/299/1/012056. [DOI] [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F.M. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100(6):101172. doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind R., Antony D., Eapen S., Kumar A., Ramana K. Isolation and Evaluation of Endophytic Bacteria against Plant Parasitic Nematodes Infesting Black Pepper (Piper nigrum L.) Indian J. Nematol. 2009;39(2):211–217. [Google Scholar]
- Aravind R., Kumar A., Eapen S.J. Pre-plant bacterisation: a strategy for delivery of beneficial endophytic bacteria and production of disease-free plantlets of black pepper (Piper nigrum L.) Arch. Phytopathol. Plant Protect. 2012;45(9):1115–1126. [Google Scholar]
- Azam, F., ul Haq, M. I., Mehmood, U., Shahzad, T., Latif, U., Saeed, M. 2018. Evaluation of Biocontrol Potential of Pseudomonas fluorescens against Root-Knot Nematode (Meloidogyne Javanica) Infecting Chili. Plant Protection, 2(2), 57-62.
- Bhale U.N., Bansode S.A., Singh S. In: Fungi and their Role in Sustainable Development: Current Perspectives. Gehlot P., Singh J., editors. Springer; Singapore: 2018. Multifactorial role of arbuscular mycorrhizae in agroecosystem; pp. 205–220. [DOI] [Google Scholar]
- Bina G.B., Sarodee B. Bio-management of root knot nematode in long pepper. Ann. Plant Sci. 2019;27(1):156–160. [Google Scholar]
- Brar S.K., Verma M., Tyagi R.D., Val E’ro J.R., Surampalli R.Y. Efficient centrifugal recovery of Bacillus thuringiensis from fermented waste water and waste water sludge. Water Res. 2006;40:1310–1320. doi: 10.1016/j.watres.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Brooks F. Symptoms and signs. Phytopathology News. 2021 [Google Scholar]
- Butler E.J. The wilt disease of pigeon pea and pepper. Agric. J India. 1906;1:23–36. [Google Scholar]
- Cao H., Jiao Y., Yin N., Li Y., Ling J., Mao Z., Xie B. Analysis of the activity and biological control efficacy of the Bacillus subtilis strain Bs-1 against Meloidogyne incognita. Crop Prot. 2019;122:125–135. [Google Scholar]
- Carneiro, R.M.D.G, Souza, I.S.D., Belarmino, L.C., 1998. Nematicidal activity of Bacillus spp. strains on juveniles of Meloidogyne javanica. Nematologia Brasileira. 1998, 22:12-19.
- Chen Z.X., Dickson D.W., Sorley R., Mitchell D.J., Hewlett T.E. Suppression of Meloidogyne arenaria race 1 by soil application of endospores od Pasteuria penetrans. J. Nematol. 1996;28:159–168. [PMC free article] [PubMed] [Google Scholar]
- Coyne D.L., Plowright R.A. Heterodera sacchari: field population dynamics and damage to upland rice in Cote d’Ivoire. Nematol. 2000;2:541–550. [Google Scholar]
- da Silva Campos M.A. Bioprotection by arbuscular mycorrhizal fungi in plants infected with Meloidogyne nematodes: a sustainable alternative. Crop Prot. 2020;135:105203. doi: 10.1016/j.cropro.2020.105203. [DOI] [Google Scholar]
- Daulagala P.W.H.K.P. Chitinolytic endophytic bacteria as biocontrol agents for phytopathogenic fungi and nematode pests: a review. Asian J. Res. Botany. 2021:14–24. [Google Scholar]
- De Souza R.A., Alves F.R., De Oliveira C.M.G., Rosa J.M.O., Pinheiro J.D.A., De Aguiar G.P., Moraes W.B. Occurrence of Meloidogyne arenaria in black pepper (Piper nigrum L.) in the extreme south of the State of Bahia. Brazil. Helminthologia. 2021;58(2):213–216. doi: 10.2478/helm-2021-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoky E.S.M., Merwad A.R.M., Semida W.M., Ibrahim S.A., El-Saadony M.T. Heavy metals-resistant bacteria (HM-RB): Potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol. Environ. Saf. 2020;198(15):110685. doi: 10.1016/j.ecoenv.2020.110685. [DOI] [PubMed] [Google Scholar]
- Desoky E.S.M., Saad A.M., El-Saadony M.T., Merwad A.R.M., Rady M.M. Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal. Agric. Biotechnol. 2020;30:101878. [Google Scholar]
- Dong L.Q., Zhang K.Q. Microbial control of plant parasitic nematodes: a five party interaction. Plant and Soil. 2006;288(1-2):31–45. [Google Scholar]
- Eapen J.S., Beena B., Ramana K. Field evaluation of Trichoderma harzianum, Pochonia chlamydosporia and Pasteuria penetrans in a root knot nematode infested black pepper (Piper nigrum L.) garden in India. J. Plant Crops. 2009;37(3):196–200. [Google Scholar]
- El-Ashry, R. M., El-Saadony, M. T., El-Sobki, A. E., El-Tahan, A. M., Al-Otaibi, S., El-Shehawi, A. M., ... & Elshaer, N., 2022. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J. Biol. Sci. 29 (2), 920–932. https://doi.org/10.1016/j.sjbs.2021.10.013. [DOI] [PMC free article] [PubMed]
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O.S., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20(1):762–776. doi: 10.1080/1828051X.2021.1926346. [DOI] [Google Scholar]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods. 2020;9(5):639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Khalil O.S.F., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28(8):4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar S.F., El-Tarabily K.A., Salama A. Selenium nanoparticles, from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi, as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28(12):6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherif A.G., Refaei A.R., El-Nagar M.E., Salem H.M.M. Integrated management of Meloidogyne incognita infecting eggplant by certain organic amendments, Bacillus thuringiensis and Oxamy with reference to N P K and total chlorophyll status. Plant Pathol. J. 2007;6:147–152. [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A.M., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28(9):5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell F.C., Jaffee B.A., Strong D.R. The nematode trapping fungus Arthrobotrys oligospora in soil of the Bodega marine reserve: distribution and dependence on nematode-parasitized moth larvae. Soil Biol Biochem. 2006;38:1422–1429. [Google Scholar]
- Ghahremani Z., Escudero N., Saus E., Gabaldón T., Sorribas F.J. Pochonia chlamydosporia induces plant-dependent systemic resistance to Meloidogyne incognita. Front. Plant Sci. 2019;10:945. doi: 10.3389/fpls.2019.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Rodríguez O., Corona-Torres T., Aguilar-Rincón V.H. Differential response of pepper (Capsicum annuum L.) lines to Phytophthora capsici and root-knot nematodes. Crop protection. 2017;92:148–152. [Google Scholar]
- Hallmann J., Davies K.G., Sikora R. In: Root-Knot Nematodes. Perry R.N., Moens M., Starr J.L., editors. CABI; UK: 2009. Biological control using microbial pathogens, endophytes and antagonists. [Google Scholar]
- Hano P., Khan M.R. Evaluation of fungal (Paecilomyces lilacinus) formulations against root knot nematode infecting tomato. Bangladesh J. Bot. 2016;45(5):1003–1013. [Google Scholar]
- Hassanin A.A., Saad A.M., Bardisi E.A., Salama A., Sitohy M.Z. Transfer of anthocyanin accumulating delila and rosea1 genes from the transgenic tomato micro-tom cultivar to moneymaker cultivar by conventional breeding. J Agric. Food Chem. 2020;68(39):10741–10749. doi: 10.1021/acs.jafc.0c03307. [DOI] [PubMed] [Google Scholar]
- Hiep N.V., Ha N.T., Thuy T.T.T., Van Toan P. Isolation and selection of Arthrobotrys nematophagous fungi to control the nematodes on coffee and black pepper plants in Vietnam. Arch. Phytopathol. Plant Prot. 2019;52(7-8):825–843. [Google Scholar]
- Jaffee B.A. Wood nematodes, and the nematode-trapping fungus Arthrobotrys oligospora. Soil Biol. Biochem. 2004;36(7):1171–1178. [Google Scholar]
- Jaffee B.A., Strong D.R., Muldoon A.E. Nematode trapping fungi of natural shrubland: tests for food chain involvement. Mycologia. 1996;88(4):554–1364. [Google Scholar]
- Jones M.G., Goto D.B. In: Genomics and molecular genetics of plant-nematode interactions. Jones J., Gheysen G., Fenoll C., editors. Springer; Dordrecht: 2011. Root-knot Nematodes and Giant Cells. [Google Scholar]
- Kerry, B.R., 2001. Exploitation of the nematophagous fungal Verticillium chlamydosporium Goddard for the biological control of root-knot nematodes (Meloidogyne spp.): 155- 167. In: Fungi as Biocontrol Agents. (Eds.) Butt, T.M., Jackson, C. and Magan, N. CAB International, Oxon. UK.
- Karmawati E., Ardana I.K., Soetopo D. Factors effecting pepper production and quality in several production center. IOP Conference Series: Earth Environ. Sci. 2020;418(1):12051. [Google Scholar]
- Kerry B.R., Kirkwood I.A., de Leij F.A.A.M., Barba J., Leijdens M.B., Brookes P.C. Growth and survival of Verticillium chlamydosporium Goddard, a parasite of nematodes in soil. Biocontrol Sci. Tech. 1993;3(3):355–365. [Google Scholar]
- Khan M.Q., Abbasi M.W., Zaki M.J., Khan S.A. Evaluation of Bacillus thuringiensis isolates against root-knot nematodes following seed application in okra and mungbean. Pak. J. Bot. 2010;42:2903–12010. [Google Scholar]
- Khan M.R., Haque Z. Soil application of Pseudomonas fluorescens and Trichoderma harzianum reduces rootknot nematode, Meloidogyne incognita, on tobacco. Phytopathol. Mediterr. 2011;50:257–266. [Google Scholar]
- Khanh, T. L. V., 2020. Selection of Bacillus thuringiensis against Pathogenic Nematodes Attacking Pepper Tree. Biotechnol. 36(3).
- Koshy P.K., Sosamma V.K., Samuel R. Bio-control of Radopholus similis on Black Pepper, Piper nigrum L. under Field Conditions. Indian. J. Nematol. 2003;33(1):43–46. [Google Scholar]
- Krishna Menon K. The survey of pollu and root diseases of pepper. Indian J. Agric. Sci. 1949;19:89–136. [Google Scholar]
- Kumar N.U., Ravichandra N.G., Nataraja A. Ecofriendly Management of Wilt Complex in Black Pepper (Piper nigrum L.). Indian. J. Nematol. 2018;48(2):146–155. [Google Scholar]
- Leong S.C.T., Pau C.G., Beattie G.A.C., Leong S.S. In Vitro Bioassay of Purpureocillium lilacinum and Bacillus thuringiensis for Control of Meloidogyne incognita on Black Pepper (Piper nigrum L.) in Sarawak, Malaysia, Northern Borneo. jers. 2021;1(23):41–59. [Google Scholar]
- Liu T., Long X.i., Zhou J.-P., Tian D.-W., Yang Y.-H., Zou C.-G., Xu J.-P., Mo M.-H., Zhang K.-Q., Schadt C.W. Fungistatic mechanism of ammonia against nematode-trapping fungus Arthrobotrys oligospora, and strategy for this fungus to survive ammonia. Msystems. 2021;6(5) doi: 10.1128/mSystems.00879-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanilla-López R.H., Esteves I., Devonshire J. In: Perspectives in Sustainable Nematode Management Through Pochonia chlamydosporia Applications for Root and Rhizosphere Health. Manzanilla-López R.H., Lopez-Llorca L.V., editors. Springer International Publishing; Cham: 2017. Biology and management of Pochonia chlamydosporia and plant-parasitic nematodes. In Perspectives in sustainable nematode management through Pochonia chlamydosporia applications for root and rhizosphere health; pp. 47–76. [DOI] [Google Scholar]
- Maris, P., Wahyuno, T.E., Djiwanti, S.R., 2020. Control Strategies of Plant Parasitic Nematodes in Black Pepper Plantation. In IOP Conference Series: Earth and Environmental Science (Vol. 418, No. 1, p. 012053). IOP Publishing.
- Maulidia V., Soesanto L., Syamsuddin S., Khairan K., Hamaguchi T., Hasegawa K., Sriwati R. Secondary metabolites produced by endophytic bacteria against the Root-Knot Nematode (Meloidogyne sp.) Biodiversitas J. Bio. Diversity. 2020;21(11) [Google Scholar]
- Mazzuchelli R.D.C.L., Mazzuchelli E.H.L., de Araujo F.F. Efficiency of Bacillus subtilis for root-knot and lesion nematodes management in sugarcane. Biol. Control. 2020;143:104185. [Google Scholar]
- Mhatre P.H., Eapen S.J., Chawla G., Pervez R., N A.V., Tadigiri S., M N. Isolation and characterization of Pasteuria parasitizing root-knot nematode, Meloidogyne incognita, from black pepper fields in India. Egypt J. Biol. Pest Control. 2020;30(1) doi: 10.1186/s41938-020-00296-z. [DOI] [Google Scholar]
- Mhatre P.H., Karthik C., Kadirvelu K., Divya K.L., Venkatasalam E.P., Srinivasan S., Ramkumar G., Saranya C., Shanmuganathan R. Plant growth promoting rhizobacteria (PGPR): a potential alternative tool for nematodes bio-control. Biocatal. Agric. Biotechnol. 2019;17:119–128. [Google Scholar]
- Mhatre P.H., Pankaj M.S.K., Kaur S., Singh A.K., Mohan S., Sirohi A. Histopathological changes and evaluation of resistance in Asian rice (Oryza sativa) against rice root knot nematode, Meloidogyne graminicola. Indian J. Genet. Plant Breed. 2015;75(1):41–48. [Google Scholar]
- Mian I.H. IPSA; Gazipur, Bangladesh: 1998. Introduction to Nematology; pp. 29–66. [Google Scholar]
- Mitiku, M., 2018. Plant-parasitic nematodes and their management: A review. Agric. Res. Technol. 16.
- Mohammed, S.H., Saedy, A.E.M., Enan, M.R., Ibrahim, N.E., Ghareeb, A., Moustafa, S.A., 2008. Biocontrol efficiency of Bacillus thuringiensis toxins against root knot nematode, Meloidogyne incognita. J. Mol. Cell Biol. 7, 57-66.
- Mohan S., Mauchline T.H., Rowe J., Hirsch P.R., Davies K.G. Pasteuria endospores from Heterodera cajani (Nematoda: Heteroderidae) exhibit inverted attachment and altered germination in cross-infection studies with Globodera pallida (Nematoda: Heteroderidae) FEMS Microbiol. Ecol. 2012;79(3):675–684. doi: 10.1111/j.1574-6941.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- Mohandas C., Ramana K.V. Pathogenicity of Meloidogyne incognita and Radopholus similis on black pepper (Piper nigrum L.) J. Plant Crops. 1991;19(1):41–53. [Google Scholar]
- Mozgovaya I.N., Byzov B.A., Ryabchenko N.F., Romanenka N.D., Zvyagintsev D.G. Nematicidal effects of entomopathogenic bacteria, Bacillus thuringiensis in soil. Pedobiologia. 2002;46:558–572. [Google Scholar]
- Mukhtar T., Hussain M.A., Kayani M.Z. Biocontrol potential of Pasteuria penetrans, Pochonia chlamydosporia, Paecilomyces lilacinus and Trichoderma harzianum against Meloidogyne incognita in okra. Phytopathol. Mediterr. 2013;52(1):66–76. [Google Scholar]
- Mukhtar T., Pervaz I. In vitro evaluation of ovicidal and larvicidal effects of culture filtrates of Verticillium chlamydosporium against Meloidogyne javanica. Int. J. Agric. Biol. 2003;4:576–579. [Google Scholar]
- Munif, A., Putri, D., Mutaqin, K.H., 2020. Induced resistance and plant growth promotion by endophytic bacteria Bacillus sp. AA2 against Meloidogyne sp. on pepper. In IOP Conference Series: Earth and Environmental Science (Vol. 468, No. 1, p. 012040). IOP Publishing.
- Naik B.G., Manu T.G., Nagamma G., Balagar M. Survey for the incidence of slow wilt of black pepper in southern parts of Karnataka. Trends Biosci. 2017;10(4):1162–1164. [Google Scholar]
- Nair A., Rao A.S., Bhanu L., More V.S., Anantharaju K.S., More S.S. Arbuscular mycorrhizae, a treasured symbiont to agriculture. Elsevier; 2022. pp. 45–62. [Google Scholar]
- Narayana R., Thomas S., Sheela M.S. Management of root-knot nematode meloidogyne incognita infecting black pepper. Indian J. Nematol. 2018;48(1):51–55. [Google Scholar]
- Negi A., George Kokkat J., Jasrotia R.S., Madhavan S., Jaiswal S., Angadi U.B., Kumar D. Drought responsiveness in black pepper (Piper nigrum L.): Genes associated and development of a web-genomic resource. Physiologia. Plantarum. 2021;172(2):669–683. doi: 10.1111/ppl.13308. [DOI] [PubMed] [Google Scholar]
- Nguyen S.D., Trinh T.H.T., Tran T.D., Nguyen T.V., Chuyen H.V., Ngo V.A., Nguyen A.D. Combined application of rhizosphere bacteria with endophytic bacteria suppresses root diseases and increases productivity of black pepper (Piper nigrum L.) Agriculture. 2021;11(1):15. [Google Scholar]
- Nguyen V.B., Wang S.-L., Nguyen T.H., Nguyen T.H., Trinh T.H.T., Nong T.T., Nguyen T.U., Nguyen V.N., Nguyen A.D. Reclamation of rhizobacteria newly isolated from black pepper plant roots as potential biocontrol agents of root-knot nematodes. Res. Chem. Intermed. 2019;45(11):5293–5307. [Google Scholar]
- Nisha M.S., Anusree S.S., Sooraj S. Efficacy of Andrographis paniculata (Burm. f.) Nees against root-knot nematode in pepper, Piper nigrum L. J. Entomol Zool. Stud. 2019;7(4):539–545. [Google Scholar]
- Nordbring-Hertz B., Jansson H.B., Tunlid A. Encyclopedia of life sciences. Macmillan; London: 2006. Nematophagous fungi. [Google Scholar]
- Oostendrop M., Sikora R.A. In vitro inter relationship between rhizosphere bacteria and Heterodera schachtii. Rev. Nematol. (English) 1990;13:269–274. [Google Scholar]
- Öztürk L., Behmand T., Avcı G.G., Bozbuğa R., Mirik M., Elekcioğlu İ.H. Survey of Pasteuria, the parasitic bacterial group to plant parasitic nematodes in Turkey. Egypt J. Biol. Pest Control. 2020;30:1–7. [Google Scholar]
- Pau, C.G., Leong, S., Teck, C., Wong, S.K., Eng, L., Jiwan, M., 2012. Isolation of indigenous strains of paecilomyces lilacinus with antagonistic activity against Meloidogyne incognita. Int. J. Agric. Biol. 14(2),197-203.
- Perrine-Walker F., Le K. Propidium iodide enabled live imaging of Pasteuria sp.-Pratylenchus zeae infection studies under fluorescence microscopy. Protoplasma. 2021;258(2):279–287. doi: 10.1007/s00709-020-01567-0. [DOI] [PubMed] [Google Scholar]
- Pervez R., Eapen S. Distribution of plant parasitic nematodes associated with black pepper in Idukki District. India. Ann. Plant Protect. Sci. 2015;23:188–199. [Google Scholar]
- Pervez R. Indian Spices. Springer International Publishing; Cham: 2018. pp. 205–247. [DOI] [Google Scholar]
- Phani V., Khan M.R., Dutta T.K. Plant-parasitic nematodes as a potential threat to protected agriculture: current status and management options. Crop Protect. 2021;144:105573. doi: 10.1016/j.cropro.2021.105573. [DOI] [Google Scholar]
- Rahanandeh H., Khodakaramian G., Hassanzadeh N., Seraji A., Asghari S.M., Tarang A.R. Inhibition of tea root lesion nematode, Pratylenchus loosi, by rhizosphere bacteria. J. Ornam. Hortic. Plants. 2012;2(4):243–250. [Google Scholar]
- Ramana K.V., Mohandas C., Eapen S.J. Technical bulletin; Director, National Research Centre for Spices, Calicut, Kerala, India: 1994. Plant parasitic nematodes and slow decline disease of black pepper; p. 14. [Google Scholar]
- Ramana, K.V., Sarma, Y.R., Mohandas, C. 1992. Slow decline disease of black pepper (Piper nigrum L.) and role of plant parasitic nematodes and Phytophthora capsici in the disease complex. J Plant. Crops. 20(Suppl.):65-68.
- Ravindra, H., Sehgal, M., Manu, T.G., Murali, R., Latha, M., Narasimhamurthy, H.B., 2014. Incidence of root-knot nematode (Meloidogyne incognita) in black pepper in Karnataka. J. Entomol. Nematol. 6(4), 51- 55.
- Ravindra, H., Sehgal, M., Manu, T.G., Murali, R., Narasimhamurthy, H.B., Khan, I., Latha, M., 2015. Status of plant parasitic nematode problems of Karnataka and their management with special reference to genus Meloidogyne.
- Ryan R.P., Germaine K., Franks A., Ryan D.J., Dowling D.N. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett. 2008;278(1):1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A.M., Taha A.E., Taha T.F., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021;28(10):5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., El-Saadony M.T., Mohamed A.S., Ahmed A.I., Sitohy M.Z. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour-based noodles. Int. J. Food Sci. Technol. 2021;2021(56):3255–3268. [Google Scholar]
- Saad A.M., Mohamed A.S., Ramadan M.F. Storage and heat processing affect flavors of cucumber juice enriched with plant extracts. Int. J. Veg. Sci. 2020:1–11. [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT - Food Sci. Technol. 2021;148:111668. doi: 10.1016/j.lwt.2021.111668. [DOI] [Google Scholar]
- Saad A.M., Osman A.O.M., Mohamed A.S., Ramadan M.F. Enzymatic hydrolysis of Phaseolus vulgaris protein isolate: Characterization of hydrolysates and effect on the quality of minced beef during cold storage. Int. J. Pept. Res. Ther. 2020;26(1):567–577. [Google Scholar]
- Saad A.M., Sitohy M.Z., Ahmed A.I., Rabie N.A., Amin S.A., Aboelenin S.M., Soliman M.M., El-Saadony M.T. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules. 2021;26(15):4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyan T., Elanchezhyan K., Murugesan N. Pests of black pepper and their management. Biotica, Res. Today. 2020;2(4):87–89. [Google Scholar]
- Senthilkumar T., Ananthan M. Study on the efficacy of biological agents on black pepper (Piper nigrum L.) against root knot nematode, Meloidogyne incognita. Int. J Curr. Microbiol. App. Sci. 2018;7(07):3693–3696. [Google Scholar]
- Shahnazi S., Meon S., Vadamalai G., Ahmad K., Nejat N. Morphological and molecular characterization of Fusarium spp. associated with yellowing disease of black pepper (Piper nigrum L.) in Malaysia. J. General Plant Pathol. 2012;78(3):160–169. [Google Scholar]
- Sharma M., Saini I., Kaushik P., Al Dawsari M.M., Al Balawi T., Alam P. Mycorrhizal Fungi and Pseudomonas fluorescens application reduces root-knot nematode (Meloidogyne javanica) infestation in Eggplant. Saudi J. Biol. Sci. 2021;28(7):3685–3691. doi: 10.1016/j.sjbs.2021.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu H.S. Potential of plant growth-promoting rhizobacteria in the management of nematodes: a review. J. Entomol. Zool. Stud. 2018;6(3):1536–1545. [Google Scholar]
- Sidhu H.S., Kanwar R.S., Kumar A. Compatibility of predatory nematode, fictor composticola and bacterial parasite, Pasteuria penetrans for the management of root-knot nematode. Curr. Microbiol. 2021;78(6):2400–2405. doi: 10.1007/s00284-021-02514-9. [DOI] [PubMed] [Google Scholar]
- Singh S.K., Hodda M., Ash G.J. Plant-parasitic nematodes of potential phytosanitary importance, their main hosts and reported yield losses. EPPO Bull. 2013;43(2):334–374. [Google Scholar]
- Sivakumar T., Renganathan P.B.P., Sanjeevkumar K. Bio efficacy of bio-nematon (Paecilomyces lilacinus 1.15% wp) against root-knot nematode (Meloidogyne incognita) in cucumber crop. Plant Arch. 2020;20(2):3805–3810. [Google Scholar]
- Soliman M.S., El‐Deriny M.M., Ibrahim D.S.S., Zakaria H., Ahmed Y. Suppression of root-knot nematode Meloidogyne incognita on tomato plants using the nematode trapping fungus Arthrobotrys oligospora Fresenius. J. Appl. Microbiol. 2021;131(5):2402–2415. doi: 10.1111/jam.15101. [DOI] [PubMed] [Google Scholar]
- Sosamma V.K., Koshy P.K. Biological control of Meloidogyne incognita on black pepper by Pasteuria penetrans and Paecilomyces lilacinus. J. Plant. Crops. 1997;25(1):72–76. [Google Scholar]
- Subhagan, S.R., 2008. Management of root knot nematode in thippali (Piper longum L.). M.Sc. (Ag.) thesis, Kerala Agricultural University, Thrissur, 80.
- Subila, K.P., Suseela Bhai, R., 2020. Pythium deliense, a pathogen causing yellowing and wilt of black pepper in India. New Dis. Rep. 42, 6-6.
- Sreeja T.P., Eapen S.J., Ramana K.V. Occurrence of Verticillium chlamydosporium Goddard in a black pepper (Piper nigrum L.) garden in Kerala. J. Spices Aroma. crops. 1996;5(2):143–147. [Google Scholar]
- Subha S.P., Balamurugan S. Economic analysis of pepper cultivation in India. SSRG International Journal of Economics and Management Studies. 2020:1–5. [Google Scholar]
- Swelum A.A., Shafi M.E., Albaqami N.M., El-Saadony M.T., Elsify A., Abdo M., Taha A.E., Abdel-Moneim A.-M., Al-Gabri N.A., Almaiman A.A., Saleh Al-wajeeh A., Tufarelli V., Staffa V.N., Abd El-Hack M.E. COVID-19 in human, animal, and environment: a review. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaselvabal T., Justin C.G.L., Leelamathi M. Black pepper (Piper nigrum l.) ‘The King of Spices’ – a review. Agric. Rev. 2008;29(2):89–98. [Google Scholar]
- Thuy T.T.T., Yen N.T., Tuyet N.T.A., Te L.L., De Waele D. Plantparasitic nematodes and yellowing of leaves associated with black pepper plants in Vietnam. Arch. Phytopathol. Plant Protect. 2012;45(10):1183–1200. [Google Scholar]
- Tian B., Yang J., Zhang K.-Q. Bacteria used in the biological control of plant parasitic nematodes: populations, mechanisms of action and future prospects. FEMS Microbiol. Ecol. 2007;61(2):197–213. doi: 10.1111/j.1574-6941.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- Tran T.P.H., Wang S.L., Nguyen V.B., Tran D.M., Nguyen D.S., Nguyen A.D. Study of novel endophytic bacteria for biocontrol of black pepper root-knot nematodes in the central highlands of Vietnam. Agronomy. 2019;9(11):714. [Google Scholar]
- Usman M., Gulzar S., Wakil W., Wu S., Piñero J.C., Leskey T.C., Shapiro-Ilan D. Virulence of Entomopathogenic Fungi to Rhagoletis pomonella (Diptera: Tephritidae) and Interactions with Entomopathogenic Nematodes. J. Econ. Entomol. 2020;113(6):2627–2633. doi: 10.1093/jee/toaa209. [DOI] [PubMed] [Google Scholar]
- Vallejos-Torres G., Espinoza E., Marín-Díaz J., Solis R., Arévalo L.A. The role of arbuscular mycorrhizal fungi against root-knot nematode infections in coffee plants. J. Soil Sci. Plant Nutr. 2021;21(1):364–373. [Google Scholar]
- Wachira P., Mibey R., Okoth S., Kimenju J., Kiarie J. Diversity of nematode destroying fungi in Taita Taveta. Kenya. Fungal Ecol. 2009;2(2):60–65. [Google Scholar]
- Wiratno, W., Syakir M., Sucipto, I., Pradana, A.P., 2019. Isolation and characterization of endophytic bacteria from roots of Piper nigrum and their activities against Fusarium oxysporum and Meloidogyne incognita. Biodiversitas 20, 682-687.
- Youssef M.M., El-Nagdi W.M., Lotfy D.E. Evaluation of the fungal activity of Beauveria bassiana, Metarhizium anisopliae and Paecilomyces lilacinus as biocontrol agents against root-knot nematode, Meloidogyne incognita on cowpea. Bull. Natl. Res. Cent. 2020;44(1):1–11. [Google Scholar]
- 2021. https://www.statista.com/statistics/744331/india-pepper-production-volume-by-state/.
Further Reading
- Koenning S.R., Overstreet C., Noling J.W., Donald P.A., Becker J.O., Fortnum B.A. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. J. Nematol. 1999;31:587–618. [PMC free article] [PubMed] [Google Scholar]
- Koshy P.K., Bridge J. In: Plant Parasitic Nematodes in Subtropical and Tropical Agriculture. Luc M., Sikora R.A., Bridge J., editors. CAB International; Wallingford, UK: 1990. Nematode parasites of spices; pp. 557–582. [Google Scholar]
- Paul S.K., Ahmed M., Mamun M.S.A. Biopesticides: a potential tool for the management of Plant parasitic nematodes in tea. Tea J. Bangladesh. 2014;43:24–33. [Google Scholar]
- Rumbos C.I., Kiewnick S. Effect of plant species on persistence of Paecilomyces lilacinus strain 251 in soil and on root colonization by the fungus. Plant and Soil. 2006;283(1-2):25–31. [Google Scholar]