Abstract
Various types of cancer pose a notable threat to human health globally. To date, many researchers have undertaken the search for anticancer therapies. However, many anticancer therapeutic approaches accompany many undesirable hazards. In this respect, extracellular vesicles as a whole gained excessive attention from the research community owing to their remarkable potential for delivery of anticancer agents since they are involved in distal intercellular communication via biological cargoes. With the discovery of the fact that tumor cells discharge huge quantities of EVs, new insights have been developed in cancer diagnosis and treatment. Tumor-derived extracellular vesicles (TD-EVs) can be distinguished from the normal cell-derived EVs due to the presence of specific labels on their surface. TD-EVs carry specific oncogenic proteins and the nucleic acids on their surface membrane that participate in tumor progression. Moreover, the proportion of these nucleic acids and the protein greatly varies among malignant and healthy cell-derived EVs. The diagnostic potential of TD-EVs can be implied for the more precise and early-stage detection of cancer that was impossible in the past. This review examines the recent progress in prognostic, diagnostic, and therapeutic potential of the EVs derived from the tumor cells.
Keywords: EVs, Cancer, Diagnosis, Prognosis, Tumor, Therapy
1. Introduction
Extracellular vesicles (EVs) are membrane-bound vesicles secreted by all mammalian cells such as pericytes, endothelial cells, and tumor-associated fibroblasts that are involved in intercellular communication by commuting biological cargoes (Yáñez-Mó et al., 2015). Increasing evidence has manifested that EVs carry microRNAs (miRs), nucleic acids, lipids and, certain proteins which act as anti-cancer agents, propelling for the strategic development of promising anti-cancer therapies. The importance of EVs lies in their ability to transfer information to the other cells thus influencing the recipient cell function. Recently, EVs have grasped excessive attention in the research community owing to their wide-ranging biological applications and their ability to govern certain pathophysiological (immune response, tissue regulation) and physiological processes.
2. Types of extracellular vesicles
EVs have recently gained importance as crucial modulators of immune response concerning cancer progression (Bebelman et al., 2018). For years’ growth factors, chemokines, and cytokines have been attributed to tumor cell-cell communication however, with the progress in cancer therapeutic research EVs have emerged as novel regulators of this process (Zöller, 2009).
EVs can be majorly categorized into 3 classes: (a) Microvesicles/ectosomes/microparticles that are generated by plasma membrane as the result of outward budding and fission. MVs are also known as shedding vesicles or shedding microvesicles (SMVs) (b) Exosomes that are produced within the endosomal network and released upon fusion of multi-vesicular bodies with the plasma membrane; and (c) Apoptotic bodies or apoptotic blebs are released as blebs of cells going through apoptosis (Di Vizio et al., 2012).
3. Microvesicles
Microvesicles (MVs) are the extracellular vesicles that are formed by the outward budding of the plasma membrane. The typical size range for MVs is 100 nm to 1um in diameter (Di Vizio et al., 2012, Zhang and Aravind, 2012, Hood et al., 2011) (Table 1). The formation of MVs is not well understood however, it is presumed that certain cytoskeleton components such as microtubules, actin, kinesins, myosins, and SNAres and tethering factors are required for its assembly (Zaborowski et al., 2015, Rak, 2010). The number of MVs produced and consumed entirely depends on the microenvironment and physiological state of the donor and recipient cell respectively (Di Vizio et al., 2012).
Table 1.
Characteristics of the extracellular vesicles.
| Microvesicles | Exosomes | Apoptotic bodies | References | |
|---|---|---|---|---|
| Size (nm) | 100–1000 | 30–200 | 1000–5000 | (Akers et al., 2013, van Niel et al., 2018, Al-Nedawi et al., 2009, Théry et al., 2009, Minciacchi et al., 2015) |
| Morphological appearance | Various shapes | Cup shaped | heterogeneous | (Mathivanan et al., 2010, Théry et al., 2009, Skog et al., 2008) |
| Protein components | Death receptors such as CD40 ligand, Cell adhesion (integrins, selectins), |
Tetraspanins (CD63, CD9 CD82, CD81), Multivesicular bodies (TSG101, ALIX) | Transcription factors and histones | (Al-Nedawi et al., 2009) |
| Lipid composition | Cholesterol, High phosphatidylserine exposure | Cholesterol, sphingomyelin, lipid rafts, ceramide, low phosphatidylserine exposure | Enriched in Phosphatidylserine | (György et al., 2011, Baj-Krzyworzeka et al., 2007, Jang et al., 2015) |
| Mode of the extracellular release process | Cellular/ constitutive activation | Cellular/ constitutive activation | Cellular/ constitutive activation | (Al-Nedawi et al., 2009) |
| Markers | Selectin, flittilin-2, metalloprotease surface phosphatidylserine, glycophorin, Integrin (B1), MMP, CD34, CD40, CD45 | Rab5a/b, CD63, Alix, CD81, CD82, CD9, HSP70, HSP90, flottlin, GTPasestetraspanins, TSG101, ESCRT, and MHC |
Histones, calnexin, Surface phosphatidylserinehistones, annexin V, C3b, cytochrome C, and TSP | (Al-Nedawi et al., 2009, Mathivanan et al., 2010, Caby et al., 2005, Vojtech et al., 2014, Zlotogorski-Hurvitz et al., 2015) |
| Biogenesis | By outward budding of the plasma membrane | Upon fusion of multi-vesicular bodies within endosomal networks | Produced by cells going through apoptosis | (Caby et al., 2005, Yuan et al., 2018, Dixon et al., 2018) |
Abbreviations: TSG101: tumor susceptibility gene 101; ESCRT: The endosomal sorting complexes required for transport; HSP: heat shock protein; miRNA: microRNA; MMP: matrix metalloproteinase;
As far as biogenesis of MVs is concerned it has been illustrated that proteomic profiles of these extracellular vesicles are mainly dependent on the methods by which these are isolated. Markers proteins are present in MVs regardless of their cell origin as a result of the biogenesis. As MVs are produced by outward budding of the plasma membrane so it is evident that majorly contain cytosolic proteins such as tetraspanins that cluster at the surface of the plasma membrane (Phimister and O’Driscoll, 2015).
Heat shock proteins, cytoskeletal proteins, integrins, and other proteins involved in glycosylation and phosphorylation in the post-transcriptional modification are also thought to be present in Mvs (Minciacchi et al., 2015, György et al., 2011). Initially, it was considered that MVs like other extracellular vesicles are involved in the maintenance of cells by which they dispose of their unwanted material. however, with the advancement in biological processes of these EVs, it has been well demonstrated that these microvesicles are involved in cell-cell communication in a way that they can alter the response of the recipient cell. Other forms of cell-cell communication such as growth factors, hormones, and cytokines are well understood in context to their function and direct interaction with other cells, the uniqueness of the extracellular vesicles lies in their property as biological cargoes (Barrachina et al., 2019).
However, the exact mechanisms by which these EVs cargo certain materials (proteins, nucleic acids, and miRNAs) is remained to be evaluated. From this point of view, one can better understand that if this communication occurs between physiologically healthy cells and their function alters in response to these deliveries so same notion can be extended to the tumor cells (Ståhl et al., 2019).
Tumor cells use these EVs to package and transport their proteins to other healthy cells so does metastasis progress. so, a better understanding of the regulatory mechanisms and the formation of these MVs can lead to great opportunities to develop new theranostic approaches.
4. Exosomes
Exosomes are the smallest subtype of EVs referred to as intraluminal vesicles (ILVs) when contained in multivesicular bodies (MVBs) and have been observed in almost all bodily fluids, such as blood, urine, breast milk, synovial fluid, tears, semen, cerebrospinal fluid, amniotic fluid, lymph, bile and gastric juice (Li et al., 2018, Grigor’eva et al., 2016, Milasan et al., 2016, Yoon and Chang, 2017, Möbius et al., 2002, Babst et al., 2002, Wollert and Hurley, 2010, Borges et al., 2013, Akers et al., 2013). MVBs can go to lysosomes for the degradation of ILVs or upon fusing with plasma membrane they release the vesicles in the extracellular spaces hence knows as exosomes (Table 1). The element that determines the fate of MVBs is not well demonstrated. However, it has been elucidated that the providence of MVBs mainly depends on their cholesterol level. Among morphologically identical vesicles only those will be secreted that are enriched with cholesterol and the rest will be directed to lysosomes for the degradation (Akers et al., 2013). The regulation of MVBs and the formation of exosomes takes place through the ESCRT pathway (Raposo and Stoorvogel, 2013, van Niel et al., 2018).
In the ESCRT dependent biogenesis of the exosomes, four different (ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III) multiprotein complexes are involved which play a stupendous role in MVB production, protein cargo sorting, and vesicular budding process (Buschow et al., 2010, Theos et al., 2006, van Niel et al., 2011). Moreover, this pathway is orchestrated by some supporting molecules, such as ALIX (ALG-2-interacting protein X), ATPase, or VSP4 (vacuolar protein sorting-associated protein) (Arendt et al., 2014). It has also been reported that certain growth factors stimulate the production of MVBs but the final adjustment of the exosome production is dependent on the need of the cell (Stuffers et al., 2009).
Some studies have indicated that there is another mechanism for the release of exosomes that are known as the ESCRT-independent pathway. Compelling evidence has been congregated that CD63 positive exosomes were released by the sphingomyelinase enzyme-mediated pathway in ESCRT depleted cells (Bobrie et al., 2011, Chaput and Théry, 2011, Fauré et al., 2006). The CD63, along with CD81 and CD9 belongs to the tetraspanin family. Exosomes as compared to the cell lysate are found to be enriched with these transmembrane proteins (Krämer-Albers et al., 2007). Initially, tetraspanin proteins were considered as the specific exosomal marker however this notion was changed when these proteins were detected in the MVs and the apoptotic blebs.
Exosomes are thought to be important modulators of the immune response because of their role as antigen-presenting molecules (Lachenal et al., 2011, Bakhti et al., 2011). They play a tremendous role in tumor progression and cell maintenance. Their implicated role in the central nervous system (CNS) cannot be denied as they promote myelin formation, neuronal survival, and dendrite growth resulting in tissue regeneration and repair (Wang et al., 2011, Fevrier et al., 2004). Positives and negatives went side by side whenever extracellular vesicles were discussed concerning their implications in therapeutics. Exosomes in CNS were found to be involved in the disease progression because of the possession of some pathogenic proteins such as synuclein, beta-amyloid peptide, and superoxide dismutase (Alvarez-Llamas et al., 2008, Al-Nedawi et al., 2009, Simpson et al., 2009).
In recent years exosomal research has been a prime focus for scientists due to its wide applications in clinical settings. Their ability to carry biomarkers of certain diseases makes them the point of great attention in clinical settings. Recently, these EVs were proved to be the carrier of biomarkers of certain conditions such as glioblastoma, pancreatic cancer, Parkinson’s disease, lung cancer, and kidney injury (Lai et al., 2013, Mathivanan et al., 2010, Borges et al., 2013, Holmgren et al., 1999) Table 2. Their presence in bodily fluids allows minimal to non-invasive liquid biopsy methods to diagnose and monitor the patients’ response to treatment easily (Lynch et al., 2017). Their immunological applications are far-reaching due to the greater half-life in the human body. Because of their vast inherent properties, exosomes can be used as an efficient drug delivery carrier. Moreover, techniques are still being evolved to inject DNA and proteins into exosomes so that specific cancer cells can be targeted and the desired response can be achieved in these cells (Han et al., 2019).
Table 2.
Diagnostic biomarkers of different cancer conditions.
| Cancer type | Specific biomarkers | Reference |
|---|---|---|
| Breast cancer | HER2, FAK, survivin, and EpCAM, glypican-1 (GPC1), EMMPRIN, fibronectin, EGFR, CD24, developmental endothelial locus-1 (EDIL3) | (Ciravolo et al., 2012, Amorim et al., 2014) |
| Prostate cancer | CD63, EpCAM, PTK7, LZH8, PSA, CA25, HER2, FABP5, ADIRF, VATL | (Pang et al., 2020) |
| CNS cancer | miRNAs (miR‐301a, miR‐182‐5p, miR‐328‐3p, miR‐339‐5p, miR‐340‐5p, miR‐485‐3p, miR‐486‐5p, miR‐543, miR‐320, miR‐301a), mRNAa (RNU6‐1, IDH1, c‐MET, ABCB1, MMP2, ITG‐A9) and proteins(EGFR, EGFRvIII, APOE, C3, FOLR1, PTRF) |
(Testa et al., 2021) |
| Head and neck cancer | miR‐27a‐3p, miR‐184, miR‐223‐3p, Let‐7b‐5p, HOTAIR, CD81, CYPA | (Testa et al., 2021) |
| Lung cancer | miR‐502‐5p, miR‐378a, miR‐379, miR‐151a‐5p, miR‐376a‐5p, miR‐1974, miR‐139‐5p, miR‐200b‐5p, miR‐629, miR‐17, miR‐190b, miR‐30a‐3p, miR‐100, miR‐154‐3p | |
| Oesophageal Cancer | miR‐126‐5p, miR‐192‐5p, miR‐196b‐5p, miR‐146a‐5p, miR‐223‐3p, miR‐409‐3p, miR‐223‐5p, miR‐483‐5p, miR‐22‐3p, miR‐23b‐5p, miR‐203‐5p, miR‐27b‐3p, miR‐149‐5p | (Warnecke-Eberz et al., 2015, Zhou et al., 2017) |
5. Apoptotic bodies
Apoptotic bodies (Abs) are membrane vesicles that are formed during the controlled cell death process and released in the extracellular space (Table 1). As a result of increased hydrostatic pressure, these vesicles are budded off during cell contraction (Hristov et al., 2004). The composition of apoptotic bodies is highly varied that of exosomes and microvesicles in the context that they contain glycosylated proteins, chromatin, and intact organelles such as mitochondria and certain nuclear fragments. Unlike exosomes, higher levels of histone proteins and the proteins associated with Golgi apparatus, endoplasmic reticulum, and mitochondria are present in Abs. Furthermore, a stark difference has been seen in proteomic profiles of exosomes and cell lysate, however, no such remarkable difference is evident in the case of Abs (Atkin-Smith et al., 2015).
It has been found that apoptotic bodies are involved in tumor metastasis and tumor microenvironment formation (Zweemer et al., 2017). Therefore, it can be said that Abs are involved in the modulation of immune response by the transfer of bioactive molecules to their targeted cells (Arendt et al., 2014, Liao et al., 2012, Dong et al., 2014). An increase in tumor cell migration, mediated by the AXL receptor has also been observed in phosphatidylserine (PS) containing apoptotic bodies (Chen et al., 2018, Kreger et al., 2016).
6. Involvement of anti-cancer drugs in EVs’ release
It has been reported that anti-cancer drugs enhance the secretion of EVs from cancer cells (Bandari et al., 2018). Certain drugs for example paclitaxel, carboplatin and irinotecan could upraise the exosomes release from the HepG2 significantly (Lv et al., 2012). Another study demonstrated that CAG human cells released a dramatically high EVS upon treatment with bortezomib, and melphalan and carfilzomi, just after 16 h (Bandari et al., 2018). MDA-MB231 breast cancer cells also showed the same trend of increase in EVs level when compared with untreated control (Kreger et al., 2016, Ab Razak et al., 2019). In contrast to this there are some studies reporting that chemotherapy did not change the EVs level when analyzed by nanoparticle tracking analysis (NTA). The levels of EVs were not greatly differed in ovarian cancer cell lines when treated with cisplatin. However this discrepancy could be explained by the fact that this assessment was done after 2h of treatment. Most of the studies for the analysis of EVs level were conducted in vitro however the biology of EVs differs in in-vivo and in-vitro conditions (Margolis and Sadovsky, 2019). There were some interesting studies describing the lower levels of EVs from head and neck cancer cells upon treatment with anticancer drugs (Ludwig et al., 2017). Acute myeloid leukemia (AML) patients showed significant decrease in the level of exosomal proteins after chemotherapy however, the EVs level was assessed several days after chemotherapy (Boyiadzis et al., 2013, Boyiadzis et al., 2017). It can be postulated that several tumors cells undergo apoptosis process and die after chemotherapy that actually explains the lower levels of EVs (Fleshner and Crane, 2017). To be sure about the involvement of anti-cancer drugs in EVs release, more robust techniques and time limiting factor of the drugs is needed.
7. Tumor-derived extracellular vesicles
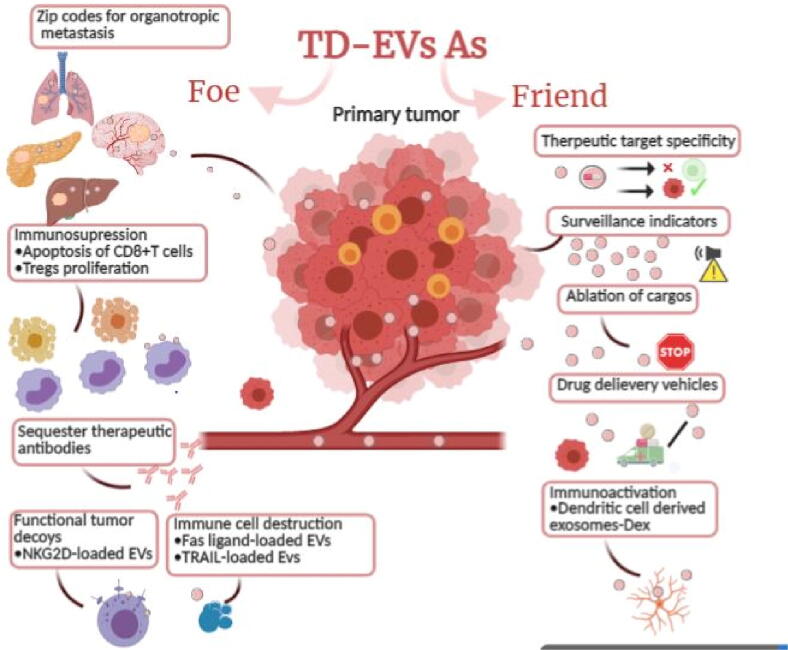
Tumor-derived extracellular vesicles (TD-EVs) can be distinguished from the normal cell-derived EVs due to the presence of specific labels on their surface. It has been reported that TD-EVs carry specific oncogenic proteins and the nucleic acids on their surface membrane that participate in tumor progression. Moreover, the proportion of these nucleic acids and the protein greatly varies among malignant and healthy cell-derived EVs (Akers et al., 2013). An important membrane protein present on MVs, CSE1L (chromosome segregation 1 like) has been shown to play a crucial role in mediating the Ras-triggered MV formation and progression of B16F10 malignant cells (Khan et al., 2012). TD-EVs relay paracrine information to the neighboring tumor cells thus allowing crosstalk between the tumor cells. TD-EVs are considered as potential biomarkers of cancer development because of their role in metastatic function through modulation of the tumor microenvironment (TME) and pre-metastatic niche development as shown in Fig. 2 (Hoshino et al., 2015).
Fig. 2.
TD-EVs as friend and foe: EVs suppress immune cell response via cargo interaction present at their surface such as NKG2D ligands. Fas-ligand-mediated apoptosis is responsible for the destruction of specific immune cells by EVs. They carry the signal for organotropic metastasis thus spreading cancer through pre-metastasis niche formation. The therapeutic and diagnostic potential of these EVs lies in their cargos and the specific proteins present on their surface and absent from EVs derived from healthy cells. TD-EVs can be modified for the target-specific drug delivery thus enhancing the anticancer effects and diminishing the associated side effects of drugs (Created by BioRender.com).
8. Prognostic & diagnostic potential of TD-EVs
Extensive investigation has also been made on cell-free DNAs (cfDNAs) and circulating tumor cells (CTCs) as diagnostic biomarkers of cancer. However certain limitations have limited their use in the clinical utilities (Ye et al., 2014). Rapid clearance of DNAs from the blood and the rarity of CTCs have been indicated as technical problems in their use as diagnostic markers (Vader et al., 2014). Detection methods and capturing techniques need to be more ameliorated for the precision and early-stage diagnosis of cancer.
There is a shred of growing evidence that TD-EVs can be used for the early diagnosis of cancer. The diagnostic information of cancer can be collected from the EVs depending on their amount contents, origin, developmental stage, and response to treatment. Conventional approaches for cancer diagnosis include relatively painful and risk-carrying procedures (Logozzi et al., 2009, Chen et al., 2017).
Biopsies are generally performed by oncologists to confirm the diagnosis after strong suspicion. However, in recent years when EVs became the prime focus of the research, liquid biopsies gained extraordinary attention in the scientific community due to their non-invasive and painless nature (Logozzi et al., 2009, Zhang et al., 2017).
In liquid biopsies, bodily fluids such as plasma, serum, and other biofluids are assessed for disease-associated proteins or nucleic acids. TD-EVs are found in the circulation and the differential expression of certain biomarkers is evaluated in healthy and melanoma cells. in another study, survivin expression was found remarkably higher in plasma exosomes of prostate cancer patients than the healthy controls (Ferreira et al., 2016).
Recently, the organotropic metastasis role of the TD-EVs with integrin expression profile has been elucidated. This study has given a new horizon in cancer research by further implicating the role of integrin profile of exosomes as biomarkers for organotropic metastasis (Taylor and Gercel-Taylor, 2008). EVs derived from the cancer cells were found to be enriched in PD-L1 (major immune response regulator) and CD63, when compared with healthy donor cells, thus proving their implications in cancer metastasis (Logozzi et al., 2009, Cazzoli et al., 2013). Furthermore, phosphoproteome analyses of the TD-EVs can serve as an excellent biomarker against cancer; given the fact that protein phosphorylation is crucial for the functioning of cancer cells. The melanoma cells from the breast cancer patients showed elevated levels of specific phosphoproteins (ERBB2, AXL, EPHA2, EGFR, MET, SRC, PTK2, YES1, MAPK, LYN, ERK1) as compared to the normal cells (Fabbri et al., 2013).
miRNA has also played a substantial role in the early diagnosis of cancer leading to better survival of cancer patients. In the past two decades, much effort has been devoted to the profiling of these miRNAs and their utility in the cancer diagnosis. For example, miR-21, miR-141, miR-200a,b,c, miR-203, miR-205, miR-214, and, miR-373 were found to be higher in concentrations in the exosomes from ovarian cancer patients than that of normal or benign diseased individual (Fabbri et al., 2013). Similarly, a wide range of microRNAs analyses of lung adenocarcinoma patients exhibited that miRNA-200-5p, miRNA-378a, miRNA-379, and miRNA139-5p could excellently serve as early diagnostic markers of this cancer due to their upregulation in the metastatic cells.
Roles of these oncogenic miRNAs in tumor proliferation and aggressiveness have been explicated in several studies. It has been presented that, miRNA-21 and miRNA-29a present in TD-EVs cause the metastasis. It is presumed that these oncogenic miRNAs play an important role in the activation of the NF-κB by toll-like receptors. In turns, the downstream signaling of the immune cells led to the production of the cytokines that helps the tumor cells in cell division and invasion (Sidhu et al., 2004). Onco-miRNAs such as miRNA-891a, miRNA-106a-5p, miRNA-24-3p, and miRNA-20a-5p are released from the tumor-released exosomes and exacerbate the tumor cell proliferation by inhibiting the MARK1 protein pathway (Jorfi and Inal, 2013). Keeping in view the oncogenic role of miRNAs and their abundance in the TD-EVs, their diagnostic potential can be implied for the more precise and early-stage detection of cancer that was impossible in the past.
9. Therapeutic potential of TD-EVs
Contemplating the supporting role of TD-EVs in pre-metastatic niche formation and TME remodeling, they can serve as a potent therapeutic target for cancer treatment. For example, angiogenesis, metastasis and, negative communication between tumor cells can be hindered by inhibition of EVs release, and ultimately invasion of the oncogenic cells to the surrounding healthy cells can be stopped (Wei et al., 2014).
Several reports have been published on the drug resistance of tumor cells, mediated by TD-MVs. Thus, the transition of tumor cells from drug sensitivity to drug resistance limits the efficacy of chemotherapy in several ways (Challagundla et al., 2015, Saari et al., 2015). In this context, TD-MVs elimination can revamp the chemotherapy against tumor cells. It has also been reported that Her-2 positive MVs could considerably subdue the antiproliferation effect of trastuzumab on several breast cancer cell lines. Therefore, the antiproliferation effect of trastuzumab can be enhanced by removing the Her-2 positive MVs from the tumor site (Yang et al., 2015). Moreover, the role of miRNAs has also been extensively investigated regarding the drug resistance in patients on chemotherapy against cancer. miRNAs present in TD-EVs modulate the response of cells against drugs thus lowering the efficacy of the treatment (Saari et al., 2015). To get an insight, recently some specific miRNA profiles such as miR-222 and miR-100 have been explored in TD-Exosomes from drug-resistant patients of breast cancer (Guo et al., 2016). Transfer of miR-221/222 from tamoxifen-resistant cells to tamoxifen-sensitive tumor cells with the help of exosomes resulted in a drug-resistant response in the recipient cell. Drug-resistant response and the deregulation of the cell cycle were attributed to a reduction in expression of the targeted p27 gene and the estrogen receptor (ER-Alpha). Exosomal miRNAs are responsible for the crosstalk between the cancer cells and their TME, resultantly the modulation of growth and their response to certain drugs (Saari et al., 2015).
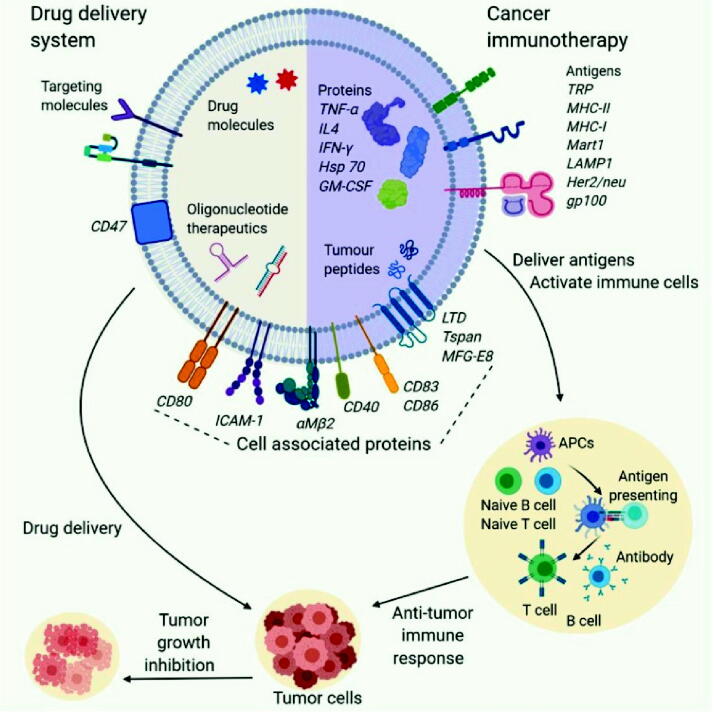
TD-EVs fuse with the recipient cell through the endocytosis and release their contents into donee’s cytoplasm; this ability of these vesicles makes them excellent vectors for the targeted delivery of the antitumor drugs and agents if used against the treatment of cancer (Yang et al., 2015). For example, EVs derived from the PC-3 and LNCap cell lines from prostate cancer were modified for the transport of paclitaxel (PTX) to the recipient cell via the endocytic pathway (Challagundla et al., 2015). Modified EVs showed remarkably increased cytotoxic effects in vitro. Moreover, neuronal glioblastoma (U-87 MG) derived EVs with doxorubicin (DOX) or PTX decreased the growth of recipient U-87 MG cells (Zhang et al., 2017). Targeted drug delivery by the TD-EVs has been proved to show promising results in the context of the efficacy of the drug and reduced cytotoxicity to the healthy cells as shown in Fig. 1. In vitro and in vivo experiments with exoDOX (doxorubicin-loaded exosomes) derived from breast adenocarcinoma (MDA-MB-231) and colorectal carcinoma (HCT-116) showed no reduction in the efficacy of DOX. Concurrently, when nude mice were treated with exoDOX, it showed no cardiotoxicity in contrast to the counterparts treated with exo free DOX. Furthermore, the mass spectrometric analysis showed reduced accumulation of DOX in the heart. Similar results have been reported for in vivo models of STOSE (ovarian cancer) and MDA-MB-231 (breast cancer) (Guo et al., 2016). Thus, TD-EV's positive therapeutic potential can be exploited to enhance the efficacy of anti-cancer treatments in clinical settings.
Fig. 1.
Therapeutic Role of EVs in cancer: Therapeutic substances can be loaded to EVs to modify the native content. Cargoes of EVs are hence transferred to the tumor cells to initiate anti-proliferative effect. EVs decorated with specific antigen cells are responsible for initiation of immune response (Pirisinu et al., 2020).
10. Role of TD-EVs in Immunotherapy
Immunotherapy has shown its tremendous potential for the cancer treatment, oftentimes demonstrating great results when employed in combination with other conventional treatments such as chemotherapy and radiotherapy (Khalil et al., 2016). Till now, many reports have been published on immunological potential of TD-EVs as they modulate the antigen presentation and regulate tumor microenvironment (Parayath et al., 2020). TD-EVs contain MHC-I (major histocompatibility complex class I) and Hsp70 which initiate immune response for tumor cells. It has been reported that HCC derived EVs could trigger potential immune response in DCs resulting in reduced level of FoxP3+ CD25+ CD4+ regulatory T cells and censored tumor growth. The clinical trial for cell-free cancer vaccine was initiated in 2008 and proved to be safe for use of TD-EVs (Dai et al., 2008). Phase I and II clinical trials conducted on NSCLC patients proved the utility of exosomes to stimulate T cell and NK cell-based immune responses in patients (Pitt et al., 2016).
TD-EVs showed no significance in advanced stage colorectal carcinoma (CRC) patients when administered alone however in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF), they activated a CD8 + CTL inducing CEA-specific anti-tumour immune response (Morishita et al., 2016). DCs stuffed with glioma derived EVs initiate tumor-specific T- cell response (Gu et al., 2015). This therapeutic approach triggers the up regulation of CD86, CD80 and MHCII molecules on DCs’ surface (Chen et al., 2006). Moreover, M1 macrophages are responsible for mediation of immuno-stimulatory effect of TD-EVs in tumor microenvironment which ultimately up regulates the release of certain cytokines such as IL-6, TNF-α, IFN-γ, and IL-12, responsible for T-cell mediated immune response. For instance EVs derived from breast cancer, gastric cancer and melanoma cells are up taken by macrophages which initiate NF-κB resulting in higher expression of proinflammatory factors (Marton et al., 2012).
11. Conclusion
Cancer progression is a dynamic and highly evolving process regulated by the tumor microenvironment. To date, much research has been done on the EVs for heterogeneity, biogenesis, cargo profiles, and packaging. Still, the exact mechanisms of the communication between the cancer cells through TD-EVs largely remain to be undetermined. TD-EVs are highly responsible for the negative intercellular communication by transferring the information from the primary tumor to the other cells thereby promoting the metastasis to healthy cells. The role of miRNAs has largely been described in several studies about tumor suppression and progression. Tumor-suppressive miRNAs can also be delivered at the tumor site to suppress metastasis. However, unraveling the exact mechanism by which these vesicles transfer their tumor signatures as cargoes is crucial to use them in any clinical setting. How to make good use of the merits while bypassing the demerits of TD-EVs in cancer diagnostics and therapeutics is attracting the utmost attention of the research community. Paramount functions of TD-EVs in target-specific drug delivery can be exploited; however, it will only be possible by elucidating the exact molecular mechanisms of the biogenesis and release of these EVs.
Funding
This author(s) received no specific funding for this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ab Razak N.S., Ab Mutalib N.S., Mohtar M.A., Abu N. Impact of chemotherapy on extracellular vesicles: Understanding the chemo-EVs. Front. Oncol. 2019;9:1113. doi: 10.3389/fonc.2019.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers J.C., Ramakrishnan V., Kim R., Skog J., Nakano I., Pingle S., Kalinina J., Hua W., Kesari S., Mao Y., Breakefield X.O., Hochberg F.H., Van Meir E.G., Carter B.S., Chen C.C., Chen M. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS ONE. 2013;8(10):e78115. doi: 10.1371/journal.pone.0078115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers J.C., Gonda D., Kim R., Carter B.S., Chen C.C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013;113(1):1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nedawi K., Meehan B., Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8(13):2014–2018. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- Alvarez-Llamas G., de la Cuesta F., Barderas M.E.G., Darde V., Padial L.R., Vivanco F. Recent advances in atherosclerosis-based proteomics: new biomarkers and a future perspective. Expert Rev. Proteomics. 2008;5(5):679–691. doi: 10.1586/14789450.5.5.679. [DOI] [PubMed] [Google Scholar]
- Amorim M., Fernandes G., Oliveira P., Martins-de-Souza D., Dias-Neto E., Nunes D. The overexpression of a single oncogene (ERBB2/HER2) alters the proteomic landscape of extracellular vesicles. Proteomics. 2014;14(12):1472–1479. doi: 10.1002/pmic.201300485. [DOI] [PubMed] [Google Scholar]
- Arendt B.K., Walters D.K., Wu X., Tschumper R.C., Jelinek D.F. Multiple myeloma cell-derived microvesicles are enriched in CD147 expression and enhance tumor cell proliferation. Oncotarget. 2014;5(14):5686–5699. doi: 10.18632/oncotarget.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin-Smith G.K., Tixeira R., Paone S., Mathivanan S., Collins C., Liem M., Goodall K.J., Ravichandran K.S., Hulett M.D., Poon I.K.H. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat. Commun. 2015;6(1) doi: 10.1038/ncomms8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M., Katzmann D.J., Estepa-Sabal E.J., Meerloo T., Emr S.D. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 2002;3(2):271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Baj-Krzyworzeka M., Szatanek R., Węglarczyk K., Baran J., Zembala M. Tumour-derived microvesicles modulate biological activity of human monocytes. Immunol. Lett. 2007;113(2):76–82. doi: 10.1016/j.imlet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Bakhti M., Winter C., Simons M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem. 2011;286(1):787–796. doi: 10.1074/jbc.M110.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandari S.K., Purushothaman A., Ramani V.C., Brinkley G.J., Chandrashekar D.S., Varambally S., Mobley J.A., Zhang Y.i., Brown E.E., Vlodavsky I., Sanderson R.D. Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior. Matrix Biol. 2018;65:104–118. doi: 10.1016/j.matbio.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrachina M.N., Calderón-Cruz B., Fernandez-Rocca L., García Á. Application of extracellular vesicles proteomics to cardiovascular disease: guidelines, data analysis, and future perspectives. Proteomics. 2019;19:1800247. doi: 10.1002/pmic.201800247. [DOI] [PubMed] [Google Scholar]
- Bebelman M.P., Smit M.J., Pegtel D.M., Baglio S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018;188:1–11. doi: 10.1016/j.pharmthera.2018.02.013. [DOI] [PubMed] [Google Scholar]
- Bobrie A., Colombo M., Raposo G., Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- Borges F.T., Melo S.A., Özdemir B.C., Kato N., Revuelta I., Miller C.A., Gattone V.H., LeBleu V.S., Kalluri R. TGF-β1–containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 2013;24(3):385–392. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges F.T., Reis L.A., Schor N. Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013;46(10):824–830. doi: 10.1590/1414-431X20132964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyiadzis, M., Hong, C.-S., Whiteside, T.L., 2013. Exosomes as markers of therapeutic response in patients with acute myeloid leukemia. American Society of Hematology Washington, DC. [DOI] [PMC free article] [PubMed]
- Boyiadzis, M., Hong, C.S., Whiteside, T.L., 2017. Plasma-derived exosomes carrying leukemia-associated antigens associate with leukemia relapse in AML patients after chemotherapy. American Society of Hematology Washington, DC.
- Buschow S.I., Balkom B.W.M., Aalberts M., Heck A.J.R., Wauben M., Stoorvogel W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol. Cell Biol. 2010;88(8):851–856. doi: 10.1038/icb.2010.64. [DOI] [PubMed] [Google Scholar]
- Caby M.-P., Lankar D., Vincendeau-Scherrer C., Raposo G., Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- Cazzoli R., Buttitta F., Di Nicola M., Malatesta S., Marchetti A., Rom W.N., Pass H.I. microRNAs derived from circulating exosomes as noninvasive biomarkers for screening and diagnosing lung cancer. J. Thoracic Oncol. 2013;8(9):1156–1162. doi: 10.1097/JTO.0b013e318299ac32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challagundla K.B., Wise P.M., Neviani P., Chava H., Murtadha M., Xu T., et al. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. JNCI: J. Nat. Cancer Inst. 2015;107 doi: 10.1093/jnci/djv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput N., Théry C. Exosomes: immune properties and potential clinical implementations. Seminars Immunopathol.: Springer. 2011;33(5):419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- Chen G., Huang A.C., Zhang W., Zhang G., Wu M., Xu W., Yu Z., Yang J., Wang B., Sun H., Xia H., Man Q., Zhong W., Antelo L.F., Wu B., Xiong X., Liu X., Guan L., Li T., Liu S., Yang R., Lu Y., Dong L., McGettigan S., Somasundaram R., Radhakrishnan R., Mills G., Lu Y., Kim J., Chen Y.H., Dong H., Zhao Y., Karakousis G.C., Mitchell T.C., Schuchter L.M., Herlyn M., Wherry E.J., Xu X., Guo W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Wang J., Shao C., Liu S., Yu Y., Wang Q., Cao X. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur. J. Immunol. 2006;36(6):1598–1607. doi: 10.1002/eji.200535501. [DOI] [PubMed] [Google Scholar]
- Chen I.-H., Xue L., Hsu C.-C., Paez J.S.P., Pan L.i., Andaluz H., Wendt M.K., Iliuk A.B., Zhu J.-K., Tao W.A. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc. Natl. Acad. Sci. 2017;114(12):3175–3180. doi: 10.1073/pnas.1618088114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciravolo V., Huber V., Ghedini G.C., Venturelli E., Bianchi F., Campiglio M., Morelli D., Villa A., Mina P.D., Menard S. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J. Cell. Physiol. 2012;227(2):658–667. doi: 10.1002/jcp.22773. [DOI] [PubMed] [Google Scholar]
- Dai S., Wei D., Wu Z., Zhou X., Wei X., Huang H., Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008;16(4):782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Vizio D., Morello M., Dudley A.C., Schow P.W., Adam R.M., Morley S., Mulholland D., Rotinen M., Hager M.H., Insabato L., Moses M.A., Demichelis F., Lisanti M.P., Wu H., Klagsbrun M., Bhowmick N.A., Rubin M.A., D'Souza-Schorey C., Freeman M.R. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 2012;181(5):1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C.L., Sheller-Miller S., Saade G.R., Fortunato S.J., Lai A., Palma C., et al. Amniotic fluid exosome proteomic profile exhibits unique pathways of term and preterm labor. Endocrinology. 2018;159:2229–2240. doi: 10.1210/en.2018-00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Pan Q., Jiang L., Chen Z., Zhang F., Liu Y., et al. Tumor endothelial expression of P-glycoprotein upon microvesicular transfer of TrpC5 derived from adriamycin-resistant breast cancer cells. Biochem. Biophys. Res. Commun. 2014;446(1):85–90. doi: 10.1016/j.bbrc.2014.02.076. [DOI] [PubMed] [Google Scholar]
- Fabbri M., Paone A., Calore F., Galli R., Croce C.M. A new role for microRNAs, as ligands of Toll-like receptors. RNA Biol. 2013;10(2):169–174. doi: 10.4161/rna.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauré J., Lachenal G., Court M., Hirrlinger J., Chatellard-Causse C., Blot B., Grange J., Schoehn G., Goldberg Y., Boyer V., Kirchhoff F., Raposo G., Garin J., Sadoul R. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006;31(4):642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Ferreira M.M., Ramani V.C., Jeffrey S.S. Circulating tumor cell technologies. Mol. Oncol. 2016;10:374–394. doi: 10.1016/j.molonc.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevrier B., Vilette D., Archer F., Loew D., Faigle W., Vidal M., Laude H., Raposo G. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. 2004;101(26):9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M., Crane C.R. Exosomes, DAMPs and miRNA: features of stress physiology and immune homeostasis. Trends Immunol. 2017;38(10):768–776. doi: 10.1016/j.it.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigor’eva A.E., Tamkovich S.N., Eremina A.V., Tupikin A.E., Kabilov M.R., Chernykh V.V., Vlassov V.V., Laktionov P.P., Ryabchikova E.I. Exosomes in tears of healthy individuals: Isolation, identification, and characterization. Biochem. (Moscow) Suppl. Ser. B: Biomed. Chem. 2016;10(2):165–172. [Google Scholar]
- Gu X., Erb U., Büchler M.W., Zöller M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int. J. Cancer. 2015;136(4):E74–E84. doi: 10.1002/ijc.29100. [DOI] [PubMed] [Google Scholar]
- Guo P., Yang J., Jia D.i., Moses M.A., Auguste D.T. ICAM-1-targeted, Lcn2 siRNA-encapsulating liposomes are potent anti-angiogenic agents for triple negative breast cancer. Theranostics. 2016;6(1):1–13. doi: 10.7150/thno.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- György B., Szabó T.G., Pásztói M., Pál Z., Misják P., Aradi B., László V., Pállinger É., Pap E., Kittel Á., Nagy G., Falus A., Buzás E.I. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011;68(16):2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Lam E.-F., Sun Y.u. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol. Cancer. 2019;18(1) doi: 10.1186/s12943-019-0980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren L., Szeles A., Rajnavölgyi E., Folkman J., Klein G., Ernberg I., et al. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood, J. Am. Soc. Hematol. 1999;93:3956–3963. [PubMed] [Google Scholar]
- Hood J.L., San R.S., Wickline S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71(11):3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- Hoshino A., Costa-Silva B., Shen T.-L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., Singh S., Williams C., Soplop N., Uryu K., Pharmer L., King T., Bojmar L., Davies A.E., Ararso Y., Zhang T., Zhang H., Hernandez J., Weiss J.M., Dumont-Cole V.D., Kramer K., Wexler L.H., Narendran A., Schwartz G.K., Healey J.H., Sandstrom P., Jørgen Labori K., Kure E.H., Grandgenett P.M., Hollingsworth M.A., de Sousa M., Kaur S., Jain M., Mallya K., Batra S.K., Jarnagin W.R., Brady M.S., Fodstad O., Muller V., Pantel K., Minn A.J., Bissell M.J., Garcia B.A., Kang Y., Rajasekhar V.K., Ghajar C.M., Matei I., Peinado H., Bromberg J., Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov M., Erl W., Linder S., Weber P.C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104:2761–2766. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- Jang S.C., Kim S.R., Yoon Y.J., Park K.-S., Kim J.H., Lee J., Kim O.Y., Choi E.-J., Kim D.-K., Choi D.-S., Kim Y.-K., Park J., Di Vizio D., Gho Y.S. In vivo kinetic biodistribution of nano-sized outer membrane vesicles derived from bacteria. Small. 2015;11(4):456–461. doi: 10.1002/smll.201401803. [DOI] [PubMed] [Google Scholar]
- Jorfi S., Inal J.M. The role of microvesicles in cancer progression and drug resistance. Biochem. Soc. Trans. 2013;41:293–298. doi: 10.1042/BST20120273. [DOI] [PubMed] [Google Scholar]
- Khalil D.N., Smith E.L., Brentjens R.J., Wolchok J.D. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016;13(5):273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Jutzy J.M.S., Valenzuela M.M.A., Turay D., Aspe J.R., Ashok A., Mirshahidi S., Mercola D., Lilly M.B., Wall N.R., Li J. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS ONE. 2012;7(10):e46737. doi: 10.1371/journal.pone.0046737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer-Albers E., Bretz N., Tenzer S., Winterstein C., Möbius W., Berger H., Nave K.A., Schild H., Trotter J. Oligodendrocytes secrete exosomes containing 35 major myelin and stress-protective proteins: Trophic support for axons. Proteomics 36 Clin. Appl. 2007;1:1446–1461. doi: 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- Kreger B., Johansen E., Cerione R., Antonyak M. The enrichment of survivin in exosomes from breast cancer cells treated with paclitaxel promotes cell survival and chemoresistance. Cancers. 2016;8(12):111. doi: 10.3390/cancers8120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachenal G., Pernet-Gallay K., Chivet M., Hemming F.J., Belly A., Bodon G., Blot B., Haase G., Goldberg Y., Sadoul R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 2011;46(2):409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Lai R.C., Yeo R.W.Y., Tan K.H., Lim S.K. Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013;31(5):543–551. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang Y., Xiao K.e., Xiang S., Li Z., Weng X. Emerging role of exosomes in the joint diseases. Cell. Physiol. Biochem. 2018;47(5):2008–2017. doi: 10.1159/000491469. [DOI] [PubMed] [Google Scholar]
- Liao C.-F., Lin S.-H., Chen H.-C., Tai C.-J., Chang C.-C., Li L.-T., Yeh C.-M., Yeh K.-T., Chen Y.-C., Hsu T.-H., Shen S.-C., Lee W.-R., Chiou J.-F., Luo S.-F., Jiang M.-C. CSE1L, a novel microvesicle membrane protein, mediates Ras-triggered microvesicle generation and metastasis of tumor cells. Mol. Med. 2012;18(9):1269–1280. doi: 10.2119/molmed.2012.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logozzi M., De Milito A., Lugini L., Borghi M., Calabrò L., Spada M., Perdicchio M., Marino M.L., Federici C., Iessi E., Brambilla D., Venturi G., Lozupone F., Santinami M., Huber V., Maio M., Rivoltini L., Fais S., Cao Y. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE. 2009;4(4):e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig S., Floros T., Theodoraki M.-N., Hong C.-S., Jackson E.K., Lang S., Whiteside T.L. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin. Cancer Res. 2017;23(16):4843–4854. doi: 10.1158/1078-0432.CCR-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L.-H., Wan Y.-L., Lin Y., Zhang W., Yang M., Li G.-L., Lin H.-M., Shang C.-Z., Chen Y.-J., Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J. Biol. Chem. 2012;287(19):15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C., Panagopoulou M., Gregory C.D. Extracellular vesicles arising from apoptotic cells in tumors: roles in cancer pathogenesis and potential clinical applications. Front. Immunol. 2017;8:1174. doi: 10.3389/fimmu.2017.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis L., Sadovsky Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019;17(7):e3000363. doi: 10.1371/journal.pbio.300036310.1371/journal.pbio.3000363.g001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton A., Vizler C., Kusz E., Temesfoi V., Szathmary Z., Nagy K., Szegletes Z., Varo G., Siklos L., Katona R.L., Tubak V., Howard O.M.Z., Duda E., Minarovits J., Nagy K., Buzas K. Melanoma cell-derived exosomes alter macrophage and dendritic cell functions in vitro. Immunol. Lett. 2012;148(1):34–38. doi: 10.1016/j.imlet.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Mathivanan S., Ji H., Simpson R.J. Exosomes: extracellular organelles important in intercellular communication. J. Proteomics. 2010;73(10):1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Milasan A., Tessandier N., Tan S., Brisson A., Boilard E., Martel C. Extracellular vesicles are present in mouse lymph and their level differs in atherosclerosis. J. Extracell. Vesicles. 2016;5(1):31427. doi: 10.3402/jev.v5.31427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minciacchi V.R., Freeman M.R., Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minciacchi V.R., You S., Spinelli C., Morley S., Zandian M., Aspuria P.-J., Cavallini L., Ciardiello C., Sobreiro M.R., Morello M., Kharmate G., Jang S.C., Kim D.-K., Hosseini-Beheshti E., Guns E.T., Gleave M., Gho Y.S., Mathivanan S., Yang W., Freeman M.R., Di Vizio D. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget. 2015;6(13):11327–11341. doi: 10.18632/oncotarget.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möbius W., Ohno-Iwashita Y., Donselaar E.G.V., Oorschot V.M.J., Shimada Y., Fujimoto T., Heijnen H.F.G., Geuze H.J., Slot J.W. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J. Histochem. Cytochem. 2002;50(1):43–55. doi: 10.1177/002215540205000105. [DOI] [PubMed] [Google Scholar]
- Morishita M., Takahashi Y., Matsumoto A., Nishikawa M., Takakura Y. Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111:55–65. doi: 10.1016/j.biomaterials.2016.09.031. [DOI] [PubMed] [Google Scholar]
- Pang B., Zhu Y., Ni J., Thompson J., Malouf D., Bucci J., Graham P., Li Y. Extracellular vesicles: the next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis. Theranostics. 2020;10(5):2309–2326. doi: 10.7150/thno.39486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parayath N.N., Padmakumar S., Amiji M.M. Extracellular vesicle-mediated nucleic acid transfer and reprogramming in the tumor microenvironment. Cancer Lett. 2020;482:33–43. doi: 10.1016/j.canlet.2020.04.009. [DOI] [PubMed] [Google Scholar]
- Phimister E.G., O’Driscoll L. Expanding on exosomes and ectosomes in cancer. N. Engl. J. Med. 2015;372(24):2359–2362. doi: 10.1056/NEJMcibr1503100. [DOI] [PubMed] [Google Scholar]
- Pirisinu, M., Pham, T.C., Zhang, D.X., Hong, T.N., Nguyen, L.T., 2020. Le MT: Extracellular vesicles as natural therapeutic agents and innate drug delivery systems for cancer treatment: Recent advances, current obstacles, and challenges for clinical translation. In: Seminars in Cancer Biology: 2020: Elsevier. [DOI] [PubMed]
- Pitt J.M., André F., Amigorena S., Soria J.-C., Eggermont A., Kroemer G., Zitvogel L. Dendritic cell–derived exosomes for cancer therapy. J. Clin. Invest. 2016;126(4):1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak, J., 2010. Microparticles in cancer. Seminars in thrombosis and hemostasis: © Thieme Medical Publishers, pp. 888–906. [DOI] [PubMed]
- Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari H., Lázaro-Ibáñez E., Viitala T., Vuorimaa-Laukkanen E., Siljander P., Yliperttula M. Microvesicle-and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Controll. Release. 2015;220:727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Sidhu S.S., Mengistab A.T., Tauscher A.N., LaVail J., Basbaum C. The microvesicle as a vehicle for EMMPRIN in tumor–stromal interactions. Oncogene. 2004;23(4):956–963. doi: 10.1038/sj.onc.1207070. [DOI] [PubMed] [Google Scholar]
- Simpson R.J., Lim J.WE., Moritz R.L., Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev. Proteomics. 2009;6(3):267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- Skog J., Würdinger T., van Rijn S., Meijer D.H., Gainche L., Curry W.T., Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhl A.-L., Johansson K., Mossberg M., Kahn R., Karpman D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatric Nephrol. 2019;34(1):11–30. doi: 10.1007/s00467-017-3816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuffers S., Sem Wegner C., Stenmark H., Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10:925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- Taylor D.D., Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Testa A., Venturelli E., Brizzi M.F. Extracellular Vesicles as a Novel Liquid Biopsy-Based Diagnosis for the Central Nervous System, Head and Neck, Lung, and Gastrointestinal Cancers: Current and Future Perspectives. Cancers. 2021;13(11):2792. doi: 10.3390/cancers13112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos A.C., Truschel S.T., Tenza D., Hurbain I., Harper D.C., Berson J.F., et al. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev. Cell. 2006;10:343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Vader P., Breakefield X.O., Wood M.J.A. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol. Med. 2014;20(7):385–393. doi: 10.1016/j.molmed.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Niel G., Charrin S., Simoes S., Romao M., Rochin L., Saftig P., et al. The tetraspanin CD63 regulates ESCRT-independent and-dependent endosomal sorting during melanogenesis. Dev. Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G., D'Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Vojtech L., Woo S., Hughes S., Levy C., Ballweber L., Sauteraud R.P., et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucl. Acids Res. 2014;42:7290–7304. doi: 10.1093/nar/gku347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Cesca F., Loers G., Schweizer M., Buck F., Benfenati F., Schachner M., Kleene R. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J. Neurosci. 2011;31(20):7275–7290. doi: 10.1523/JNEUROSCI.6476-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke-Eberz U., Chon S.-H., Hölscher A.H., Drebber U., Bollschweiler E. Exosomal onco-miRs from serum of patients with adenocarcinoma of the esophagus: comparison of miRNA profiles of exosomes and matching tumor. Tumor Biol. 2015;36(6):4643–4653. doi: 10.1007/s13277-015-3112-0. [DOI] [PubMed] [Google Scholar]
- Wei Y., Lai X., Yu S., Chen S., Ma Y., Zhang Y., Li H., Zhu X., Yao L., Zhang J. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res. Treat. 2014;147(2):423–431. doi: 10.1007/s10549-014-3037-0. [DOI] [PubMed] [Google Scholar]
- Wollert T., Hurley J.H. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464(7290):864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez-Mó M., Siljander P.-M., Andreu Z., Bedina Zavec A., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., Colás E., Cordeiro-da Silva A., Fais S., Falcon-Perez J.M., Ghobrial I.M., Giebel B., Gimona M., Graner M., Gursel I., Gursel M., Heegaard N.H.H., Hendrix A.n., Kierulf P., Kokubun K., Kosanovic M., Kralj-Iglic V., Krämer-Albers E.-M., Laitinen S., Lässer C., Lener T., Ligeti E., Linē A., Lipps G., Llorente A., Lötvall J., Manček-Keber M., Marcilla A., Mittelbrunn M., Nazarenko I., Nolte-‘t Hoen E.N.M., Nyman T.A., O'Driscoll L., Olivan M., Oliveira C., Pállinger É., del Portillo H.A., Reventós J., Rigau M., Rohde E., Sammar M., Sánchez-Madrid F., Santarém N., Schallmoser K., Stampe Ostenfeld M., Stoorvogel W., Stukelj R., Van der Grein S.G., Helena Vasconcelos M., Wauben M.H.M., De Wever O. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4(1):27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Martin P., Fogarty B., Brown A., Schurman K., Phipps R., Yin V.P., Lockman P., Bai S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015;32(6):2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S.-B., Li Z.-L., Luo D.-H., Huang B.-J., Chen Y.-S., Zhang X.-S., Cui J., Zeng Y.-X., Li J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5(14):5439–5452. doi: 10.18632/oncotarget.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S.B., Chang J.H. Extracellular vesicles in bile: a game changer in the diagnosis of indeterminate biliary stenoses? Hepatobiliary Surg. Nutrition. 2017;6(6):408–410. doi: 10.21037/hbsn.2017.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z., Bedi B., Sadikot R.T. Bronchoalveolar lavage exosomes in lipopolysaccharide-induced septic lung injury. JoVE (J. Visualized Exp.) 2018:e57737. doi: 10.3791/57737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborowski M.P., Balaj L., Breakefield X.O., Lai C.P. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 2015;65:783–797. doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Aravind L. Novel transglutaminase-like peptidase and C2 domains elucidate the structure, biogenesis and evolution of the ciliary compartment. Cell Cycle. 2012;11(20):3861–3875. doi: 10.4161/cc.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Xia W., Lv Z., Ni C., Xin Y., Yang L. Liquid biopsy for cancer: circulating tumor cells, circulating free DNA or exosomes? Cell. Physiol. Biochem. 2017;41(2):755–768. doi: 10.1159/000458736. [DOI] [PubMed] [Google Scholar]
- Zhou X., Wen W., Zhu J., Huang Z., Zhang L., Zhang H., Qi L.-W., Shan X., Wang T., Cheng W., Zhu D., Yin Y., Chen Y., Zhu W., Shu Y., Liu P. A six-microRNA signature in plasma was identified as a potential biomarker in diagnosis of esophageal squamous cell carcinoma. Oncotarget. 2017;8(21):34468–34480. doi: 10.18632/oncotarget.16519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotogorski-Hurvitz A., Dayan D., Chaushu G., Korvala J., Salo T., Sormunen R., Vered M. Human saliva-derived exosomes: comparing methods of isolation. J. Histochem. Cytochem. 2015;63(3):181–189. doi: 10.1369/0022155414564219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer. 2009;9(1):40–55. doi: 10.1038/nrc2543. [DOI] [PubMed] [Google Scholar]
- Zweemer A.J.M., French C.B., Mesfin J., Gordonov S., Meyer A.S., Lauffenburger D.A. Apoptotic bodies elicit Gas6-mediated migration of AXL-expressing tumor cells. Mol. Cancer Res. 2017;15(12):1656–1666. doi: 10.1158/1541-7786.MCR-17-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]