Abstract
A butter-enriched high-fat diet changes lipid metabolism, resulting in fat storage, hyperlipidemia and obesity. Effects of cinnamon powder were investigated in butter-fed mice. 40 Swiss Albino mice, aged 28 to 30 days, were randomly assigned into two groups. Group A was an untreated control group (n = 8) and another group (n = 32) was a butter-treated group fed 10% butter. In the fifth week, mice of the butter-fed group were further divided into four equal groups: B, C, D, and E (n = 8), fed 10% butter with cinnamon 200 mg, 400 mg, and 600 mg powder per liter drinking water, respectively for 10 weeks. The butter-fed group was gained the most weight. Cinnamon supplementation significantly normalized weight gain and had no harmful effects on hematological parameters. Butter supplementation significantly increased total cholesterol (TC), triglycerides, and LDL cholesterol (LDL-c) whereas, cinnamon powder significantly reduced TC, LDL-c and glucose levels. In butter-fed mice, a significant increase was observed in the liver enzymes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels with subsequent fat deposition in the liver. Excitingly, these enzymes were decreased and no fat depositions were observed in the liver of cinnamon-treated mice. Applying different concentrations of cinnamon powder improved the lipid profile in butter-fed female albino mice.
Keywords: Cinnamon, Blood biomarkers, Butter, Swiss albino mice
1. Introduction
Hyperlipidemia occurs when the concentration of cholesterol or triglyceride-carrying lipoproteins in the blood exceeds an arbitrary standard limit. It is primarily caused by excess lipids or fatty substances in the blood and considered as a major risk factor for developing atherosclerosis and heart disease (Vela‐Vásquez et al., 2021). Lipids in the blood include cholesterol, triglycerides, and lipoproteins, which are fat and cholesterol molecules linked to proteins. Lipoproteins are classified into three types: very-low-density lipoproteins (VLDL), low-density lipoproteins (LDL), and intermediate-density lipoproteins (IDL) (IDL). Chylomicrons are lipoproteins that are made up of triglycerides, cholesterol, and protein (Phogat et al., 2010). Although drugs such as niacin, fibrates, HMG-CoA reductase inhibitors, and bile acid-binding resins are available for the treatment of hyperlipidemia, but associated with numerous side effects. As a result, herbal treatment for hyperlipidemia has gained popularity due to fewer side effects, lower cost, ease of availability, and safety. To avoid the side effects of synthetic drugs, various indigenous plants were being used as potential alternatives for treating a variety of diseases (Javed et al., 2009). Certain cholesterol-lowering functional foods and powder of traditional plants are gaining popularity for their exciting ability to lower total plasma cholesterol levels. Hence, such foods and plant extracts could provide an alternative therapy for treating mild hypercholesterolemic patients rather than treating patients with extremely high cholesterol levels and cardiovascular diseases (Gründemann et al., 2011).
Cinnamon (Cinnamomum zeylanicum, and Cinnamon cassia), the eternal tree of tropical medicine, belongs to the Lauraceae family. Cinnamon is the inner stem bark of Cinnamomum cassia family Lauraceae. It is a very popular condiment in many cultures. The use of cinnamon was found to be quite effective in hypertension (Preuss et al., 2006) and diabetes (Anderson et al., 2016). Researchers have investigated cinnamon for its antimicrobial (Carmo et al., 2008) acaricidal (Fichi et al., 2007), antimutagenic and antioxidant (Jayaprakasha et al., 2007) properties. Cinnamon effectively prevented obesity caused by high-fat diets (Tuzcu et al., 2017). The administration of cinnamon to mice positively affected the lipid profile (Kim & Choung, 2010). Another study reported a reduction in the total cholesterol, triglycerides, and low-density lipoproteins in rats administered Cinnamomum cassia (15%) for 35 days (Rahman et al., 2013). The administration of cinnamon at 1, 3, and 6 g doses per day caused a reduction in serum glucose, triglyceride, total cholesterol, and LDL cholesterol levels in humans (Khan et al., 2003). By using a mice model, this study investigated the effects of different concentrations of cinnamon powder on the alterations of hemato-biochemical parameters and liver histostructure in response to butter-enriched feed intake.
2. Materials and methods
2.1. Experimental animals
The experiment was carried out at the Department of Physiology of Bangladesh Agricultural University (BAU), Mymensingh. The study used forty adult Swiss Albino mice (Mus musculus) weighing 30–34 g. The mice were divided into two groups at random. The control group (n = 8) was fed standard mice pellets and fresh drinking water. The remaining mice (n = 32) were allocated in the butter group, which was fed a standard mice pellet enriched with 10% butter for five weeks before being divided further into four groups of eight mice each. Group B was kept as a butter group fed a standard mice pellet enriched with 10% butter. Groups C (Cn-200), D (Cn-400), and E (Cn-600) were fed standard mice pellets enriched with 10% butter plus cinnamon 200 mg, 400 mg, and 600 mg per liter drinking water, respectively. The experiment was carried out for another ten weeks.
2.2. Ethical approval
The current study, as well as all experimental protocols, were approved and carried out in accordance with the guidelines for animal care and use established by the Animal Welfare and Experimentation Ethics Committee at Bangladesh Agricultural University, Mymensingh, Bangladesh [AWEEC/BAU/2020-24].
2.3. Preparation of cinnamon powder
The cinnamon powder was prepared following a slightly modified version of the protocol used by Ostroschi et al. (2018). At first cinnamon barks were purchased from the local market, then were cleaned & dried well. Dried barks were ground by mortar & pastel followed by a blender machine to make powder form. Three different concentration of cinnamon was mixed with 1 L water (200 mg, 400 mg and 600 mg) and provided to the experimental mice in drinking water. The preparation was made fresh every day.
2.4. Management practices
The mouse cages were kept in a well-ventilated experimental animal room. The feeds were stored in an airtight poly-packed sac to prevent spoilage. Each cage was cleaned on a regular basis during the experiment and proper hygienic and sanitary measures were implemented.
2.5. Body weight
The initial body weight of each mouse was determined using an electric balance. Body weight was assessed on day 0 (the first day of the experiment) and on every 15 days until the trial was completed. Body weight gain was calculated as the difference between the final body weight and the initial body weight (weight gain (g) = final body weight (g) -initial weight (g)). In addition, the percentage body weight gain was computed as percentage weight gain (g) equals to final body weight (g) minus initial body weight (g) divided by initial body weight × 100.
2.6. Hematological studies
Blood samples were collected according to the standard published protocol (Sarker et al., 2019). The mice were fasted overnight before blood collection and then placed one by one in an airtight container containing diethyl ether presoaked cotton. The unconscious mice were removed, and blood was drawn directly from the heart with a sterile syringe. About 1.5 ml of blood was collected and half of it was transferred to an anticoagulant-containing Eppendorf tube, while the other half was transferred to a non-anticoagulant-containing tube for serum preparation. The blood-containing tubes were kept upright and slanted at room temperature for 6 h. They were then placed overnight in the refrigerator (4 °C). Centrifugation was used to separate the serum samples and collected by using micro pipette. Serum samples were kept in the refrigerator at −20 °C for biochemical analysis. Total erythrocyte count (TEC), hemoglobin (Hb) concentration and packed cell volume (PCV) were evaluated according to the procedure described (Rakib et al., 2021).
2.7. Serum biochemical studies
Total serum cholesterol, triglycerides and HDL cholesterol, LDL cholesterol, serum glucose and ALT and AST were determined using Humalyzer 2000 (Human type, Germany) in accordance with the instructions provided. Briefly, total cholesterol (TC) and triglycerides were measured after enzymatic hydrolysis and oxidation with enzymatic colorimetric test for cholesterol and triglycerides with lipid clearing factor (RANDOX kit, UK). Similarly HDL-C and LDL-c were measured with RANDOX kit (UK), using Humalyzer 2000 analyzer. Glucose was measured after enzymatic oxidation in the presence of glucose oxidase with respective diagnostics kit. ALT and AST were measured accordingly with respective kits using Humalyzer 2000.
2.7.1. Histopathology of liver tissues
The livers of each group of mice were collected after the blood was completely removed by perfusion with phosphate- buffered saline and stored in 10% neutral buffered formalin for 15 days. The well-fixed tissues were processed, sectioned, and stained with Hematoxylin and Eosin according to the standard procedure.
2.8. Statistical analysis
All data were statistically analyzed using one-way ANOVA with post-hoc Tukey's test by GraphPad Prism 8 software.
3. Results
3.1. Body weight
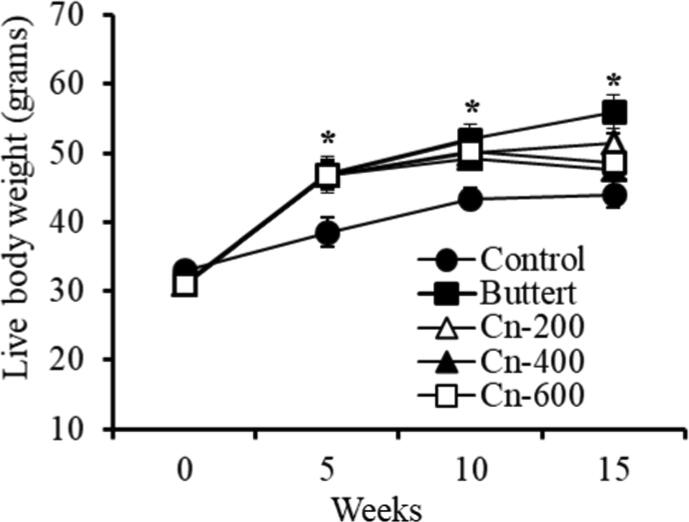
Table 1 shows the effects of butter on body weight gain in female mice after 5 weeks of supplementation. The body weight within all groups was gradually increased with the mice in the butter fed group gaining the most weight after 5 weeks. All of the butter-supplemented mice gained more weight compared to the non-butter-supplemented mice (Table 1). Mice in the butter group were again divided into four groups at the fifth week, and three groups were supplemented with 200 mg, 400 mg, and 600 mg cinnamon per liter drinking water, respectively. The administration of various concentrations of cinnamon powder to butter-fed mice prevented increased in weight gain which caused by the butter (Fig. 1).
Table 1.
Comparison of average body weight gain at 5th week of experiments in two treatment groups of mice.
| Treatment group | Body weight (g) |
Body weight gain at 5th week (g) | % body weight gain at 5th weeks | |
|---|---|---|---|---|
| Initial | 5th week | |||
| Group A (Control) | 34.61 ± 0.79 | *40.16 ± 0.86 | 5.55 ± 0.82 | 15.58% |
| Group B (Butter) | 31.60 ± 0.58 | *45.60 ± 1.11 | 14.00 ± 0.85 | 45.77% |
Fig. 1.
Effects of different concentration of cinnamon powder on live body weight in butter fed mice. Swiss Albino female mice were supplemented with 10% butter followed by administration of different concentration of cinnamon (details in Materials and Method). Body weights were monitored at different time points. *p < 0.05 (Butter versus control and butter versus cinnamon group), n.s., not significant.
3.2. Hematological parameters
After 15 weeks of experiments, hematological parameters such as total erythrocyte count (TEC), hemoglobin concentration (Hb conc.), and packed cell volume (PCV) were measured in different treated groups of mice, and RBC indices were calculated (Table 2). The mean values of RBCs, Hb and PCVs were slightly higher in the butter-fed mice compared to the control group, but the difference was statistically insignificant. After administering different concentrations of cinnamon powder, these values improved slightly but did not differ significantly. When compared to the control group, RBC indices (MCV, MCH, MCHC) of different groups did not differ (Table 2), while MCV values gradually increased in cinnamon-treated groups. White blood cell count (WBC) counts in mice treated with different concentrations of cinnamon with butter were higher compared to the control and butter-treated mice (Table 2).
Table 2.
Effects of different concentration of cinnamon powder on hematological parameters in butter fed mice at 15th week.
| Parameters | Group-A (control) | Group-B (Butter) | Group-C (Cn-200) | Group-D (Cn-400) | Group-E (Cn-600) |
|---|---|---|---|---|---|
| Hemoglobin (Hb) (g%) | 8.45 ± 0.34 | 8.95 ± 0.47 | 8.45 ± 0.59 | 8.68 ± 0.22 | 8.90 ± 0.38 |
| Total Erythrocyte Count (TEC) (106/µL) | 7.11 ± 0.46 | 7.55 ± 0.33 | 7.07 ± 0.31 | 6.99 ± 0.56 | 7.11 ± 0.24 |
| Packed Cell Volume (PCV) (%) | 34.75 ± 2.98 | 36.5 ± 3.10 | 37.0 ± 2.58 | 38.5 ± 2.38 | 38.0 ± 2.16 |
| Mean Corpuscular Volume (MCV) (fl) | 49.22 ± 7.46 | 49.46 ± 5.25 | 52.52 ± 5.95 | 55.48 ± 7.19 | 53.40 ± 1.66 |
| Mean cCorpuscular Hemoglobin (MCH) (pg) | 11.92 ± 0.99 | 12.13 ± 1.33 | 11.96 ± 0.91 | 12.46 ± 0.931 | 12.52 ± 0.63 |
| Mean Corpuscular Hemoglobin Concentrationb (MCHC) (%) | 24.42 ± 1.79 | 24.63 ± 2.11 | 22.92 ± 2.44 | 22.61 ± 1.90 | 23.46 ± 1.38 |
| Total Leukocyte Count (TLC) (103/µL) | 7.85 ± 0.72 | 8.23 ± 0.33 | 8.05 ± 0.67 | 8.50 ± 0.43 | 8.87 ± 0.67 |
Values in a column don’t differ significantly (P > 0.05).
3.3. Biochemcial parameters
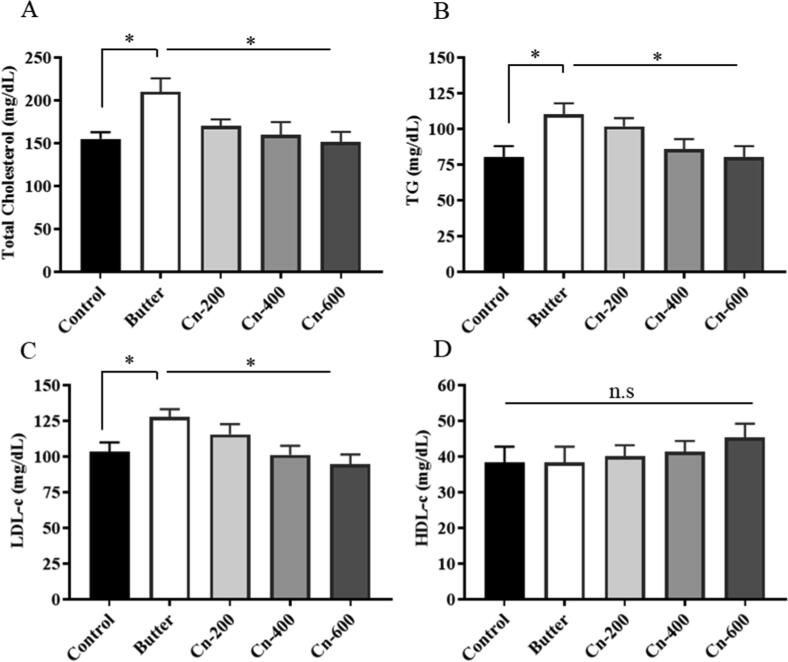
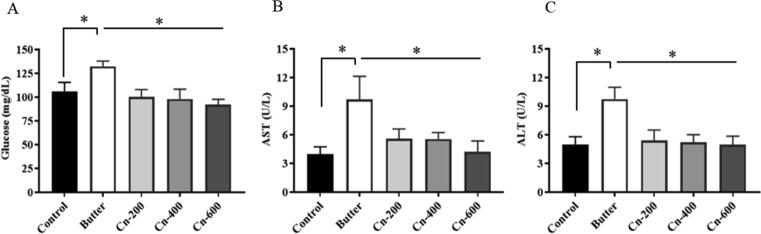
The effect of butter and cinnamon on lipid profile is shown in Fig. 2. The results showed that adding butter to the feed significantly increased total cholesterol (205.00 ± 15.00 mg/dL), triglycerides (111.67 ± 10.41 mg/dL), and LDL-c (125.00 ± 7.00 mg/dL) values in group-B, but not HDL –c values (40.00 ± 5.00 mg/dL) values (Fig. 2). However, a significant increase in HDL-c level was observed in cinnamon -treated groups (C, D, and E) compared to groups (A and B), with group-E having the highest value (55.33 ± 4.53 mg/dL). In the case of serum glucose (Fig. 3), the butter-fed group had significantly (P < 0.01) higher values in comparison with the control group. Interestingly, Cinnamon-treated mice had significantly lower glucose levels than the butter-treated mice while group E had the lowest values, followed by groups D and C. Serum liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were significantly increased in the butter-treated group. On the contrary, the levels of these enzymes in cinnamon extract-treated and non-treated control mice were normal or not increased (Fig. 3).
Fig. 2.
Effects of different concentrations of cinnamon powders on lipid profile in butter-fed mice at 15th week. Swiss Albino female mice were supplemented with 10% butter followed by administration of different concentrations of cinnamon (details in Materials and Method). Serum lipid profile was analyzed to see the effect. A, Total Cholesterol; B, Triglycerides; C, LDL-c and D, HDL-c. *p < 0.05 (Butter versus control and butter versus cinnamon group), n.s., not significant.
Fig. 3.
Effects of different concentration of cinnamon powders on serum glucose and liver function tests in butter fed mice at 15th week. Swiss Albino female mice were supplemented with 10% butter followed by administration of different concentration of cinnamon (details in Materials and Method). Blood samples were collected and serum glucose, AST and ALT analyzed. A, serum gluocse; B, serum aspartate aminotransfearse, AST; C, serum alanin aminotransferase. *p < 0.05 (Butter versus control and cinnamon group).
3.4. Histology of liver
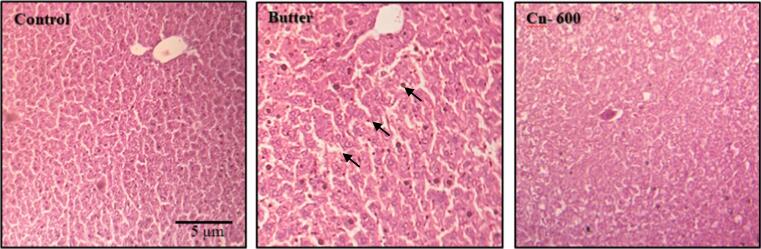
Excess saturated fats gradually accumulate in the liver, resulting in fatty liver syndromes. The current study found that butter-fed mice had higher cholesterol and triglyceride levels, which were reduced when the cinnamon powder was added (Fig. 2). Histology of the liver revealed that the control group's liver section had normal tissue structures without any detectable alterations (Fig. 4). However, the butter-fed group had little fatty changes as well as pyknotic nuclei of hepatocytes. Interestingly, after the cinnamon treatment, there was no evidence of fat deposition and the liver histo-structure resembled the non-butter treated control (Fig. 4).
Fig. 4.
Photomicrograph of histopathological sections of liver tissues. Swiss Albino female mice were supplemented with 10% butter followed by administration of cinnamons (details in Materials and Method). On the 15th of the week, liver tissues were collected from each group of mice, processed and stained with Hematoxylin and Eosin stain (details in Materials and Methods).
4. Discussion
The experiment was carried out to determine the effects of cinnamon on butter-fed albino female mice on growth and blood biomarker abnormalities. The findings revealed that eating high fat diets is strongly and positively associated with being overweight or obese. It is reported previously that consuming a high fat diet causes excessive weight gain (Sarker et al., 2019, Hoefel et al., 2011). Cinnamon extract supplementation significantly reduced excess weight gain caused by a high fat diet (butter), as reduced body weight gain was found in cinnamon supplemented group E, followed by groups D and C. In the current experimental methodology, 200 mg of cinnamon per liter water was sufficient to prevented weight loss. The current findings are consistent with previous findings (Mousavi et al., 2020) that cinnamon supplementation has a significant effect on obesity measures. It could be recommended as a weight-loss supplement in the treatment of obesity. About hematological parameters, cinnamon combined with butter had no obvious effects. The current study's findings differed slightly from previous reports (Ekanem and Yusuf, 2008, Sarker et al., 2019). The findings were quite similar to previously reported data showed increased cholesterol and triglyceride levels in butter supplemented group animals compared to control group values (Alaam et al., 2012). However, administration of cinnamon at varying concentrations to the butter-fed group significantly reduced total cholesterol, LDL-cholesterol, and triglyceride levels when compared to the butter-fed group values (P < 0.05) (Fig. 2). Liver function tests including ALT, AST were altered significantly (p < 0.05) in the butter treated groups. These increased liver enzymes indicated increased liver activity, which could be due to excess lipid (cholesterol and triglyceride) depositions in the liver, leading to fatty liver syndromes. Such elevation in the serum activity of ALT and AST indicated the hepatic dysfunction and damage (Sidhu et al., 2004) which caused increase in the permeability of the cell membrane facilitating the release of transaminase in the blood stream (Deivanayagam et al., 2014). In butter-fed mice, we found elevated cholesterol and triglyceride levels, fat deposition in the liver which were reduced when cinnamon powder was added (Fig. 2). It has also been reported that adult obese individuals have a significant reduction in lipid profile and lipase activity by cinnamon (Khedr et al., 2020). Serum glucose values also increased significantly (p < 0.05) in the butter-fed mice compared to the other groups (Fig. 3). Hyperglycemia may be due to enhanced gluconeogenesis and glycolgenolysis and decreased glucose utilization under oxidative stress enzymes (Sheikh et al., 2011). Cinnamon significantly reduced blood glucose level and liver enzymes. The most important way to reduce blood glucose concentrations is by means of glucose transporters. Cinnamon exerts its effects by enhancing the expression of glucose-transporter, GLUT4 in tissues, such as the muscles and the adipose tissues (Vallianou et al., 2014).
5. Conclusion
Based on the findings of this study, it could be concluded that butter fats have a negative impact on weight gain and lipid profile while having little effect on hematological values. Cinnamon can prevent hyperlipidemia and fat deposition in the liver. Cinnamon, at a concentration of 200 mg, appeared to be sufficient in alleviating fat-induced physiological changes. To fill the gaps and laps, more concise studies involving a different mouse strain and other laboratory animals are required. In addition to that, dissecting the intricate mechanisms involved in the process would further broaden our understanding on the use of such plants as treatment options for hyperlipidemia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are thankful to Department of Pathology and Mohammad Hossain Central Laboratory, Bangladesh Agricultural University, Mymensingh, Bangladesh for providing laboratory supports.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alaam M.H., Yasin N., Hafez S., Mohammed H.H. Biological and histological evaluations of palm oil and its fractions. World J. Dairy Food Sci. 2012;7:120–130. [Google Scholar]
- Anderson R.A., Zhan Z., Luo R., Guo X., Guo Q., Zhou J., Kong J., Davis P.A., Stoecker B.J. Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. J. Traditional Complement. Med. 2016;6(4):332–336. doi: 10.1016/j.jtcme.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo ES, Lima de Oliveira E, de S. EL, FB, de S., 2008. Effect of cinnamomum zeylanicum blume essential oil on the growth and morphogenesis of some potentially pathogenic Aspergillus species. Brazil. J. Microbiol. 39, 91–97. [DOI] [PMC free article] [PubMed]
- Deivanayagam C., Asokan S., Rajasekar S. The Effect of Lufenuron on biochemical parameters in serum of mice Musmusculus species. Int. J. ChemTech Res. 2014;6:5353–5360. [Google Scholar]
- Ekanem J.T., Yusuf O.K. Some biochemical and haematological effects of black seed (Nigella sativa) oil on Trypanosoma brucei-infected rats. Afr. J. Biotechnol. 2008;7:153–157. [Google Scholar]
- Fichi G., Flamini G., Zaralli L.J., Perrucci S. Efficacy of an essentifal oil of Cinnamomum zeylanicum against Psoroptes cuniculi. Phytomedicine. 2007;14(2-3):227–231. doi: 10.1016/j.phymed.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Gründemann C., Papagiannopoulos M., Lamy E., Mersch-Sundermann V., Huber R. Immunomodulatory properties of a lemon-quince preparation (Gencydo®) as an indicator of anti-allergic potency. Phytomedicine. 2011;18(8-9):760–768. doi: 10.1016/j.phymed.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Hoefel A.L., Hansen F., Rosa P.D., Assis A.M., Silveira S.L., Denardin C.C., Pettenuzzo L., Augusti P.R., Somacal S., Emanuelli T., Perry M.L.S., Wannmacher C.M.D. The effects of hypercaloric diets on glucose homeostasis in the rat: Influence of saturated and monounsaturated dietary lipids. Cell Biochem. Funct. 2011;29(7):569–576. doi: 10.1002/cbf.1789. [DOI] [PubMed] [Google Scholar]
- Javed I., Zia-Ur-Rahman N., Khan M.Z., Muhammad F., Aslam B., Iqbal Z., Sultan J.I., Ahmad I. Antihyperlipidaemic efficacy of Trachyspermum ammi in albino rabbits. Acta Veterinaria Brno. 2009;78(2):229–236. [Google Scholar]
- Jayaprakasha G.K., Negi P.S., Jena B.S., Jagan Mohan Rao L. Antioxidant and antimutagenic activities of Cinnamomum zeylanicum fruit extracts. J. Food Composit. Anal. 2007;20:330–336. [Google Scholar]
- Khan A., Safdar M., Khan M. Effect of Various Doses of Cinnamon on Lipid Profile in Diabetic Individuals. Pak. J. Nutrit. 2003;2:312–319. [Google Scholar]
- Khedr N.F., Ebeid A.M., Khalil R.M. New insights into weight management by orlistat in comparison with cinnamon as a natural lipase inhibitor. Endocrine. 2020;67(1):109–116. doi: 10.1007/s12020-019-02127-0. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Choung S.Y. Antihyperglycemic and antihyperlipidemic action of Cinnamomi Cassiae (Cinnamon bark) extract in C57BL/Ks db/db mice. Arch. Pharmacal Res. 2010;33(2):325–333. doi: 10.1007/s12272-010-0219-0. [DOI] [PubMed] [Google Scholar]
- Mousavi S.M., Rahmani J., Kord-Varkaneh H., Sheikhi A., Larijani B., Esmaillzadeh A. Cinnamon supplementation positively affects obesity: A systematic review and dose-response meta-analysis of randomized controlled trials. Clin. Nutrit. 2020;39(1):123–133. doi: 10.1016/j.clnu.2019.02.017. [DOI] [PubMed] [Google Scholar]
- Ostroschi L.C., Brito de Souza V., Echalar-Barrientos M.A., Tulini F.L., Comunian T.A., Thomazini M., Baliero J.C.C., Roudaut G., Genovese M.I., Favaro-Trindade C.S. Production of spray-dried proanthocyanidin-rich cinnamon (Cinnamomum zeylanicum) extract as a potential functional ingredient: Improvement of stability, sensory aspects and technological properties. Food Hydrocolloids. 2018;79:343–351. [Google Scholar]
- Phogat P., Deep A., Sharma P.C., Mittal S.K., Kakkar S., Goyal R., Thakral K. Introduction to Hyperlipidemia and Its Management. A Review. Pharmacologyonline. 2010;02:251–266. [Google Scholar]
- Preuss H.G., Echard B., Polansky M.M., Anderson R. Whole Cinnamon and Aqueous Extracts Ameliorate Sucrose-Induced Blood Pressure Elevations in Spontaneously Hypertensive Rats. J. Am. Coll. Nutr. 2006;25(2):144–150. doi: 10.1080/07315724.2006.10719525. [DOI] [PubMed] [Google Scholar]
- Rahman S., Begum H., Rahman Z., Ara F., Iqbal M.J., Yousuf A.K.M. Effect of Cinnamon (Cinnamomum cassia) as a Lipid Lowering Agent on Hypercholesterolemic Rats. J. Enam Med. College. 2013;3(2):94–98. [Google Scholar]
- Rakib M.S., Sujan K.M., Miah M.A. Deleterious effects of mercuric chloride on blood biochemistry, liver and kidney histology in female albino mice. J. Agric. Food Environ. 2021;02(02):18–23. [Google Scholar]
- Sarker S., Haque M.I., Sujan K.M., Talukder M.I., Miah M.A. Curcumin attenuates butter fat induced hyperlipidemia in mice. J. Bang. Agric. Univ. 2019;17(2):220–225. [Google Scholar]
- Sheikh T.J., P B., J D. Electrolytes alterations in plasma and urine after 28 days repeated oral dose toxicity of mercuric chloride in wistar rat. J. Appl. Pharm. Sci. 2011;2011:150–153. [Google Scholar]
- Sidhu P., Garg M.L., Dhawan D.K. Protective role of zinc in nickel induced hepatotoxicity in rats. Chem. Biol. Interact. 2004;150(2):199–209. doi: 10.1016/j.cbi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Tuzcu Z., Orhan C., Sahin N., Juturu V., Sahin K. Cinnamon Polyphenol Extract Inhibits Hyperlipidemia and Inflammation by Modulation of Transcription Factors in High-Fat Diet-Fed Rats. Oxid. Med. Cell. Longevity. 2017;2017:1–10. doi: 10.1155/2017/1583098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallianou N.G., Evangelopoulos A., Kollas A., Kazazis C., Cicero A.F.G. Hypoglycemic and hypolipidemic effects of cinnamon. Curr. Top. Nutraceut. Res. 2014;12:127–134. [Google Scholar]
- Vela‐Vásquez D.A., Sifuentes‐Rincón A.M., Delgado‐Enciso I., Delgado‐Enciso O.G., Ordaz‐Pichardo C., Arellano‐Vera W., Treviño‐Alvarado V. Improvement of serum lipid parameters in consumers of Mexican Wagyu-Cross beef: A randomized controlled trial. J. Food Sci. 2021;86(6):2713–2726. doi: 10.1111/1750-3841.15739. [DOI] [PubMed] [Google Scholar]