Abstract
Stevia (Stevia rebaudiana Bertoni) is an alternative sugar crop currently gaining importance in several geographic regions of the world. Generally, crop is propagated by seeds; however, low seed germination hinders the adaptability of the crop in different cropping systems. Seed priming with different compounds improves germination of several arable crops under stressful and benign environmental conditions. This study evaluated the role of different seed priming agents and priming durations on stand establishment, allometric and yield-related traits, and steviol glycosides contents of Stevia in two different experiments. The first experiment consisted of five different seed priming agents (i.e., hydropriming with distilled water, ascorbic acid, potassium chloride, benzyl amine purine and unprimed seeds) and five priming durations (0, 8, 16, 24 and 32 h). Seed priming with potassium chloride (KCl) and benzyl amine purine (BAP) for 32 h improved stand establishment and biochemical attributes of Stevia seeds. Therefore, these two seed priming agents along with unprimed seeds were included in the second experiment to quantify their impact on allometric and yield-related traits and steviol glycosides contents. Seed priming with both KCl and BAP resulted in better allometric traits (plant height, number of leaves per plant, leaf area and chlorophyll index) compared with unprimed seeds. Similarly, seed priming with KCl resulted in higher fresh and dry biomass production of stem and leaves. Nonetheless, higher stevioside and rebaudioside A contents were recorded for the seeds primed with KCl, whereas unprimed seeds resulted in the lowest values. It is concluded that Stevia seeds must be primed with KCl for 32 h before sowing to get higher seed germination, leaf yield and steviol glycosides contents. Nonetheless, the role of KCl priming in improving abiotic stress tolerance of Stevia must be explored in the future studies.
Keywords: Stevia, Stand establishment, Allometric traits, Yield, Steviol glycosides
1. Introduction
Stevia rebaudiana Bertoni, commonly known as ‘Stevia’ is a perennial plant cultivated to produce steviol glycosides (SG), which are 100–400 times sweeter than sucrose (Rajasekaran et al., 2007, Yadav et al., 2011, Gupta, 2013). The plant is branched bushy shrub, native to the northeastern region of Paraguay (Jeppesen et al., 2002, Jeppesen et al., 2003, Srimaroeng et al., 2005), also grows in the neighboring parts of Brazil and Argentina (Kinghorn, 2001). Stevia can be grown as annual or perennial crop in temperate and tropical regions, respectively.
Stevia naturally propagates by seeds. Unfortunately, its seeds have low germination ability, which hinder its propagation on commercial scale (Gorzi et al., 2020, Jain et al., 2020, Simlat et al., 2020). Although distinct reasons of low germination are still unknown, low humidity and high temperature are generally related with the poor germination (Murdoch and Ellis, 2000). Besides, heavy rainfall during pollination could negatively affect the seed germination and yield (Madan et al., 2010). Tissue culture and stem cutting are the alternative propagation source of Stevia; however, these techniques are not applicable on commercial scale due to heavy costs (Yadav et al., 2011). Some earlier works have been conducted on optimizing seed germination of Stevia, and 25 °C has been reported as optimum (Simlat et al., 2016, Simlat et al., 2018, Simlat et al., 2019, Simlat et al., 2020). Similarly, light has been reported to have positive influence on seed germination (Simlat et al., 2016).
Seed germination requires specific soil and environmental conditions, which vary for different plant species. Temperature plays an important role in species’ seed germination and every plant species has a distinct temperature requirement for germination. The optimum temperature for seed germination depends on the nature of the crop and environmental conditions faced by maternal plants. The optimum temperature of seed germination of Stevia has been reported as 25 °C (Carneiro and Guedes, 1992, Simlat et al., 2019).
Seed priming is a partial hydration of seeds, which eases seed germination of several crops (Hussain et al., 2006, Lutts et al., 2016). The seeds are partially hydrated in seed priming, which start germination process; however, radical is not emerged (Farooq et al., 2006a, Farooq et al., 2006b, Hussain et al., 2006). Since most of the metabolic changes occur during lag phase, it is regarded as the most important phase of seed germination. The lag phase is strongly influenced by seed priming. Seed priming reduces the time between seed sowing and seedling emergence, which results in uniform crop stands.
Different seed priming agents and distilled water have improved the seed germination of different field crops under stressful and benign environmental conditions (Khan et al., 2015, Farooq et al., 2015, Farooq et al., 2017, Farooq et al., 2020). Thus, these agents could potentially be used for improving the seed germination of Stevia as well. However, optimization of different doses of these seed priming agents and duration of priming needs to be thoroughly tested. Different studies have reported that seed priming could be used to boost the germination of Stevia (Gorzi et al., 2020, Gorzi et al., 2018, Hossa et al., 2017, Shahverdi et al., 2017a, Shahverdi et al., 2017b, Simlat et al., 2020, Simlat et al., 2018, Simlat et al., 2016). Similarly, phytohormones applied through seed priming have been proved to improve seed germination (Liopa-Tsakalidi, 2012, Hossa et al., 2017) of Stevia. Germination percentage and rate, germination energy, seedling length, germination uniformity and value had strong positive correlation. However, these studies have mostly focused on boric acid, melatonin, nutripriming and methyl jasmonate, whereas the conventional seed priming agents have rarely been tested for improving seed germination of Stevia. Nonetheless, duration of seed priming has merely been explored in these studies. Besides, the influence of conventional seed priming agents on allometric and yield related traits and steviol glycosides have merely been tested.
Therefore, the current study was designed to optimize the priming duration for different conventional seed priming agents and find the best performing seed priming agent for improving stand establishment and allometric traits of Stevia. It was hypothesized that increasing priming duration will improve stand establishment and biochemical attributes of primed seeds. Similarly, seed priming agents with better stand establishment and biochemical attributes would have better allometric and yield related traits and steviol glycosides contents.
2. Materials and methods
2.1. Experimental site and soil
The current study was conducted at Agronomic Research Farm, Department of Agronomy, Bahauddin Zakariya University, Multan, Pakistan during spring seasons of two consecutive years, i.e., 2018–19 and 2019–20. Physio-chemical analysis of experimental soil was conducted each year prior to sowing and presented in Table 1.
Table 1.
Physical and chemical properties of experimental soil before initiating the experiment during 2018–19 and 2019–20.
| Soil determination | Unit |
Years |
Unit |
Years |
|||
|---|---|---|---|---|---|---|---|
| 2018–19 | 2019–20 | 2018–19 | 2019–20 | ||||
| Chemical Analysis | Physical analysis | ||||||
| Organic matter content | % | 0.61 | 0.63 | Silt | % | 51.16 | 56.10 |
| Total nitrogen (N) | % | 0.07 | 0.08 | Sand | % | 27.74 | 25.20 |
| Available phosphorus (P) | mg kg−1 | 8.11 | 9.32 | Clay | % | 21.10 | 18.70 |
| Available potassium (K) | mg kg−1 | 234.65 | 243.31 | Textural class | Silty-clay | Silty-clay | |
| pH | 8.34 | 8.45 | |||||
| EC | dS m−1 | 4.67 | 4.60 | ||||
2.2. Experimental details
Two different experiments were conducted to infer the influence of different seed priming agents and priming durations on stand establishment and biochemical attributes of primed and unprimed seeds, and yield related attributes of Stevia. The first experiment comprised of five seed priming agents and priming durations. Seed priming agents and priming duration with superior stand establishment and biochemical attributes were selected for the second experiment where allometric and yield related traits and steviol glycosides were studied.
2.3. Experiment 1
This experiment comprised of two factors, i.e., seed priming agents and priming duration. Four different seed priming agents, i.e., hydropriming (with distilled water), ascorbic acid, potassium chloride (KCl) and benzyl amine purine (BAP), along with a control (unprimed seeds) were included in the experiment. Similarly, five different seed priming durations, i.e., 0, 8, 16, 24 and 32 h were used for seed priming with respective solution. For control treatment, seeds were aerated dry for respective time, whereas the seeds were dipped in respective aerated solutions for the required time according to the treatment. Seeds were dried under shade after priming near to their original weight and used in the experiment. The experiment was conducted in plastic trays and repeated over time. Seed priming agents and duration with the highest stand establishment traits were selected for quantifying their impacts on agronomic and yield-related attributes of Stevia. The KCl and BAP with 32 h priming duration had superior stand establishment and biochemical attributes; therefore, used in the second experiment. The first experiment was laid out according to randomized complete block design with split plot arrangements. Seed priming agents were kept in main plot, whereas priming durations were randomized in sub-plots. Each treatment had four replications and 50 seeds were used per replication.
2.4. Experiment 2
This experiment consisted of KCl and BAP priming for 32 h along with a control treatment. Allometric and yield related traits were studied in pot experiment. Free-draining 8.8 l plastic pots filled with 2.8 kg soil were used in the experiment. The nursery was first raised and then transplanted to pots to ensure 100% seedling emergence. Initially, five plants per pot were transplanted and then thinned to three keeping uniform and healthy plants. The pots were irrigated on alternate days to avoid the risk of drought stress. Morphological and yield-related attributes were recorded 5 months after transplantation following Simlat et al. (2018). Only one plant per pot was used for morphological observations, whereas the rest of the plants were used for the determination of yield and SG contents. The experiment was laid out according to completely randomized design. All treatments had four replications and the experiment was repeated. Two pots were considered as a single replication.
2.5. Data collection
2.5.1. Stand establishment traits
The methods described in Seedling Evaluation Handbook (Association of Official Seed Analysts, 1990) were followed for recording stand establishment traits. Time taken to 50% emergence (T50) was calculated from the daily emergence count according to the following formula:
where N is the final number of emerged seedlings, and ni and nj are the cumulative number of seedlings emerged on two adjacent days when ni < N/2 < nj.
Ellis and Roberts (1981) were followed to calculate mean emergence time (MET). The total number of seedlings emerged at the final count was considered the final emergence count.
2.5.2. Biochemical attributes of seeds
Biochemical attributes of primed and unprimed seeds were determined following the methods of Blackig et al. (1996) for α-amylase and total dehydrogenase activity and Dubois et al. (1956) for total soluble sugars.
2.5.3. Allometric traits
Plant height, number of leaves per plant, leaf area, and fresh and dry biomass of leaves and stem were recorded at the time of harvest from 2nd experiment. All plants in each pot were measured from the base to the tip of the plant to measure plant height. Total number of leaves present on each plant in each pot were carefully counted and averaged.
2.5.4. Analysis of steviol glycosides
For stevioside and rebaudioside A analysis, 500 mg of dry biomass was extracted with 2 ml of methanol in an ultrasonic bath for 1 h at 30 °C. Obtained extracts were filtered through a PTFE, 0.22 μm Millipore filter. Reversed-phase (RP)-HPLC analysis was conducted as described by Bayraktar et al. (2016).
2.6. Statistical analysis
The collected data of both experiments were analyzed and presented separately. The differences among experimental runs were tested by paired t-test, which indicated non-significant differences. Therefore, data for both experimental runs of each experiment was pooled before analysis. Normality of the data was assessed with the Shapiro-Wilk normality test and parameters with non-normal distribution were normalized by arcsine transformation to meet the normality assumption of Analysis of Variance (ANOVA). Two-way and one-way ANOVA was conducted to infer significance in the data of first and second experiment, respectively (Steel et al., 1997). The least significant difference test at 95% probability level was used as a post-hoc test to separate the means. Linear correlation was computed for allometric and biochemical attributes of the seeds in first experiment. All statistical analyses were performed using SPSS version 20.0 (IBM, 2012) and graphs were produced in Microsoft Excel.
3. Results
The stand establishment and biochemical attributes of Stevia seeds were significantly altered by different seed priming agents and priming duration (Table 2). The highest emergence percentage was recorded for KCl and BAP priming for 32 h, whereas unprimed seeds aerated for 32 h recorded the lowest emergence percentage (Table 3). Generally, seedling emergence percentage was increased with increasing priming duration for all seed priming techniques except for unprimed seeds. Mean emergence time (MET) and time to complete 50% emergence (T50) were reduced with increasing priming duration with the highest MET and T50 observed for unprimed seeds, whereas lowest MET and T50 were recorded for hydroprimig for 32 h. The increasing priming duration linearly increased mean daily germination in all seed priming techniques except unprimed seeds. The highest and the lowest mean daily germination were noted for KCl priming for 32 h and unprimed seeds aerated for 32 h (Table 3).
Table 2.
Analysis of variance for stand establishment and biochemical traits of Stevia seeds subjected to different seed priming agents and duration of seed priming.
| Source | DF | Sum of squares | Mean squares | F value | P value |
|---|---|---|---|---|---|
| Seedling emergence percentage | |||||
| Seed priming agents (A) | 4 | 11079.23 | 2769.81 | 1373.47 | <0.0001* |
| Seed priming duration (D) | 4 | 8444.33 | 2111.08 | 1046.83 | <0.0001* |
| A × D | 16 | 3701.14 | 231.32 | 114.71 | <0.0001* |
| Mean emergence time | |||||
| Seed priming agents (A) | 4 | 17.98 | 4.50 | 595.95 | <0.0001* |
| Seed priming duration (D) | 4 | 2.87 | 0.72 | 95.09 | <0.0001* |
| A × D | 16 | 4.41 | 0.28 | 36.54 | <0.0001* |
| Time to complete 50% emergence | |||||
| Seed priming agents (A) | 4 | 12.64 | 3.16 | 360.30 | <0.0001* |
| Seed priming duration (D) | 4 | 2.10 | 0.52 | 59.79 | <0.0001* |
| A × D | 16 | 3.52 | 0.22 | 25.11 | <0.0001* |
| Mean daily germination | |||||
| Seed priming agents (A) | 4 | 49.24 | 12.31 | 1373.47 | <0.0001* |
| Seed priming duration (D) | 4 | 37.53 | 9.38 | 1046.83 | <0.0001* |
| A × D | 16 | 16.45 | 1.03 | 114.71 | <0.0001* |
| α-amylase activity | |||||
| Seed priming agents (A) | 4 | 304.92 | 76.23 | 2167.74 | <0.0001* |
| Seed priming duration (D) | 4 | 164.16 | 41.04 | 1167.09 | <0.0001* |
| A × D | 16 | 37.35 | 2.33 | 66.38 | <0.0001* |
| Dehydrogenase activity | |||||
| Seed priming agents (A) | 4 | 1331.91 | 332.98 | 2819.06 | <0.0001* |
| Seed priming duration (D) | 4 | 717.09 | 179.27 | 1517.75 | <0.0001* |
| A × D | 16 | 163.14 | 10.20 | 86.32 | <0.0001* |
| Soluble sugars | |||||
| Seed priming agents (A) | 4 | 880.37 | 220.09 | 1478.94 | <0.0001* |
| Seed priming duration (D) | 4 | 473.98 | 118.50 | 796.24 | <0.0001* |
| A × D | 16 | 107.83 | 6.74 | 45.29 | <0.0001* |
DF = degree of freedom, * = significant.
Table 3.
The influence of different seed priming agents and duration of seed priming on stand establishment traits of Stevia.
| Seed priming agents | Seed priming duration (hours) | ||||
|---|---|---|---|---|---|
| 0 | 8 | 16 | 24 | 32 | |
| Seedling emergence percentage | |||||
| Control | 10.11 mn | 8.05 no | 8.97 mn | 9.66 mn | 6.44 o |
| Hydropriming | 13.56 l | 22.07 ij | 41.84 e | 46.90 d | 52.41 c |
| Ascorbic acid priming | 10.57 m | 19.31 k | 23.45 hi | 29.89 fg | 31.49 f |
| BAP priming | 19.77 jk | 24.60 h | 45.52 d | 51.26 c | 60.00 ab |
| KCl priming | 19.08 k | 28.97 g | 32.18 f | 57.70 b | 62.30 a |
| LSD 0.05 | 2.32 | ||||
| Mean emergence time (days) | |||||
| Control | 13.27 c | 13.71 b | 13.70 b | 13.57 b | 14.07 a |
| Hydropriming | 13.36 c | 12.44 g | 12.46 fg | 12.28 h | 12.01 j |
| Ascorbic acid priming | 13.02 d | 12.71 e | 12.52 fg | 12.20 hi | 12.15 hi |
| BAP priming | 13.07 d | 12.69 e | 12.59 ef | 12.46 fg | 12.19 hi |
| KCl priming | 12.57 efg | 12.19 hi | 12.24 hi | 12.19 hi | 12.13 ij |
| LSD 0.05 | 0.14 | ||||
| Time to complete 50% emergence (days) | |||||
| Control | 12.94 d | 13.28 b | 13.32 b | 13.18 bc | 13.60 a |
| Hydropriming | 13.10 c | 12.33 fg | 12.30 fg | 12.29 g | 11.90 i |
| Ascorbic acid priming | 12.84 de | 12.43 fg | 12.30 fg | 11.97 hi | 11.92 hi |
| BAP priming | 12.72 e | 12.42 fg | 12.45 f | 12.33 fg | 12.06 h |
| KCl priming | 12.32 fg | 11.92 hi | 12.01 hi | 12.04 hi | 12.01 hi |
| LSD 0.05 | 0.15 | ||||
| Mean daily germination (seeds) | |||||
| Control | 0.67 mn | 0.54 no | 0.60 mn | 0.64 mn | 0.43 o |
| Hydropriming | 0.90 l | 1.47 ij | 2.79 e | 3.13 d | 3.49 c |
| Ascorbic acid priming | 0.70 m | 1.29 k | 1.56 hi | 1.99 fg | 2.10 f |
| BAP priming | 1.32 jk | 1.64 h | 3.03 d | 3.42 c | 4.00 ab |
| KCl priming | 1.27 k | 1.93 g | 2.15 f | 3.85 b | 4.15 a |
| LSD 0.05 | 0.16 | ||||
Means sharing same letters within a column or row are statistically similar (p > 0.05).
Different seed priming techniques and duration of priming significantly affected the biochemical traits of Stevia seeds (Table 2). The increasing priming duration linearly increased biochemical traits, i.e., α-amylase activity, dehydrogenase activity and soluble sugars. Overall, unprimed seeds had the lowest α-amylase and dehydrogenase activities and soluble sugars, whereas seeds subjected to KCl exhibited higher α-amylase and dehydrogenase activities and soluble sugars (Table 4). The highest and the lowest higher α-amylase and dehydrogenase activities and soluble sugars were recorded for seeds subjected to KCl priming for 32 h and unprimed seeds aerated for 0 h, respectively (Table 4).
Table 4.
The influence of different seed priming agents and duration of seed priming on biochemical traits of Stevia.
| Seed priming agents | Seed priming duration (hours) |
||||
|---|---|---|---|---|---|
| 0 | 8 | 16 | 24 | 32 | |
| α-amylase activity (IU mg−1 protein) | |||||
| Control | 5.29 s | 5.97 r | 6.64 q | 7.16 p | 8.30 n |
| Hydropriming | 7.85 o | 8.32 n | 8.92 lm | 9.47 k | 10.39 i |
| Ascorbic acid priming | 8.71 m | 9.11 l | 9.82 j | 10.75 gh | 11.07 ef |
| BAP priming | 9.13 l | 9.76 jk | 10.85 fg | 12.62 d | 15.02 b |
| KCl priming | 9.79 j | 10.52 hi | 11.37 e | 14.22 c | 16.45 a |
| LSD 0.05 | 0.30 | ||||
| Dehydrogenase activity (µg Formazan per 100 seeds) | |||||
| Control | 8.81 s | 10.22 r | 11.62 q | 12.72 p | 15.09 n |
| Hydropriming | 14.15 o | 15.14 n | 16.40 lm | 17.53 k | 19.46 i |
| Ascorbic acid priming | 15.94 m | 16.79 l | 18.28 j | 20.21 gh | 20.89 f |
| BAP priming | 16.82 l | 18.15 j | 20.42 fg | 24.12 d | 29.14 b |
| KCl priming | 18.21 j | 19.73 hi | 21.51 e | 27.46 c | 32.12 a |
| LSD 0.05 | 0.56 | ||||
| Soluble sugars (mg g−1) | |||||
| Control | 5.25 r | 6.39 q | 7.53 p | 8.43 o | 10.35 m |
| Hydropriming | 9.59 n | 10.39 m | 11.42 kl | 12.34 ij | 13.91 h |
| Ascorbic acid priming | 11.05 l | 11.73 jk | 12.95 i | 14.52 fgh | 15.07 ef |
| BAP priming | 11.76 jk | 12.84 i | 14.69 fg | 17.69 d | 21.78 b |
| KCl priming | 12.89 i | 14.12 gh | 15.57 e | 20.41 c | 24.20 a |
| LSD 0.05 | 0.63 | ||||
Means sharing same letters within a column or row are statistically similar (p > 0.05).
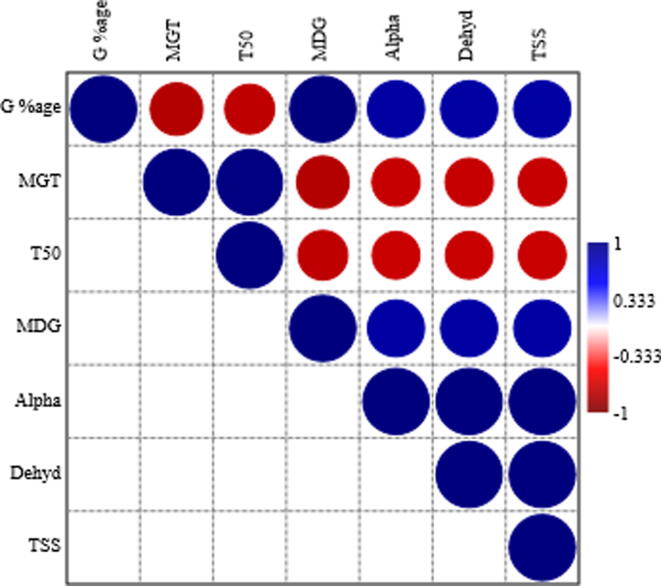
Significant positive and negative correlations were noted among different stand establishment and biochemical attributes of Stevia seeds subjected to different seed priming techniques and priming durations (Fig. 1). Final emergence percentage was negatively correlated with MET and T50, whereas had positive correlation with mean daily germination, α-amylase and dehydrogenase activities and soluble sugars. Similarly, MET and T50 had significant negative correlation with mean daily germination, α-amylase and dehydrogenase activities and soluble sugars. All other stand establishment and biochemical traits were positively correlated with each other (Fig. 1).
Fig. 1.
Correlation matrix of different stand establishment and biochemical traits of Stevia seeds subjected to different seed priming agents and duration of priming.
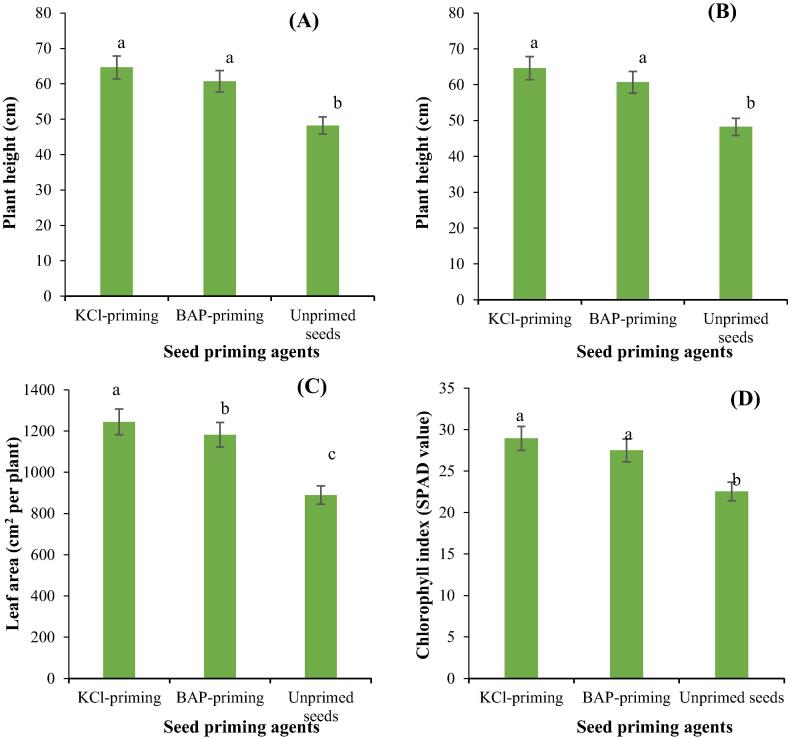
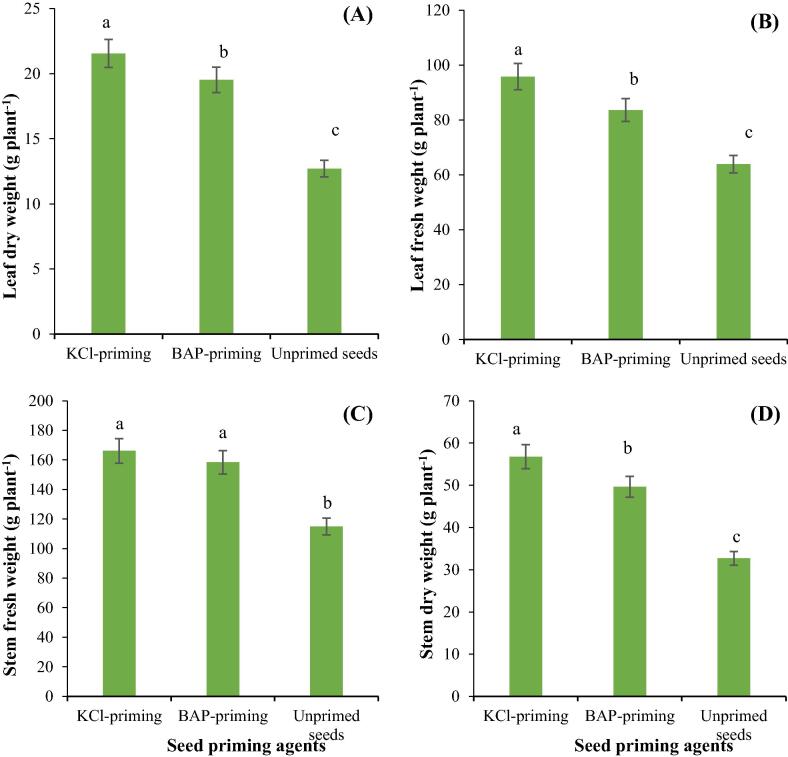
Different seed priming techniques significantly altered allometric and yield related traits of Stevia (Table 5). Both seed priming techniques significantly improved allometric and yield related attributes of Stevia compared to unprimed seeds. The highest plant height, number of leaves, leaf area and chlorophyll index were recorded for both seed priming agents, whereas unprimed seeds had the lowest values of these allometric traits (Fig. 2A, B, C, D). Seed priming with KCl resulted in the highest fresh and dry biomass production of leaves and stems, whereas the lowest fresh and dry biomass of leaves and stem was recorded for unprimed seeds (Fig. 3A, B, C, D).
Table 5.
Analysis of variance of different allometric and yield related traits grown with different seed priming techniques.
| Source | DF | Sum of squares | Mean squares | F value | P value |
|---|---|---|---|---|---|
| Plant height | |||||
| Seed primimg techniques | 2 | 440.4262 | 220.2131 | 29.24 | 0.0008* |
| Number of leaves per plant | |||||
| Seed primimg techniques | 2 | 8793.56 | 4396.78 | 49.59 | 0.0002* |
| Leaf area | |||||
| Seed primimg techniques | 2 | 215842.67 | 107921.33 | 202.52 | <0.0001* |
| Chlorophyll index | |||||
| Seed primimg techniques | 2 | 67.87 | 33.94 | 27.01 | 0.0010* |
| Leaf fresh weight | |||||
| Seed primimg techniques | 2 | 1558.86 | 779.43 | 49.91 | 0.0002* |
| Leaf dry weight | |||||
| Seed primimg techniques | 2 | 128.84 | 64.42 | 73.50 | <0.0001* |
| Stem fresh weight | |||||
| Seed primimg techniques | 2 | 4575.02 | 2287.51 | 78.82 | <0.0001* |
| Stem dry weight | |||||
| Seed primimg techniques | 2 | 917.05 | 458.52 | 155.17 | <0.0001* |
| Stevioside in young leaves | |||||
| Seed primimg techniques | 2 | 19.78 | 9.89 | 221.98 | <0.0001* |
| Stevioside in old leaves | |||||
| Seed primimg techniques | 2 | 21.21 | 10.61 | 358.48 | <0.0001* |
| Rebaudioside in young leaves | |||||
| Seed primimg techniques | 2 | 266.66 | 133.33 | 329.18 | <0.0001* |
| Rebaudioside in old leaves | |||||
| Seed primimg techniques | 2 | 244.17 | 122.08 | 215.90 | <0.0001* |
DF = degree of freedom, * = significant.
Fig. 2.
The influence of different seed priming agents on plant height (A), number of leaves per plant (B), leaf area (C) and chlorophyll index (D) of Stevia.
Fig. 3.
The influence of different seed priming agents on leaf fresh weight per plant (A), leaf dry weight per plant (B), stem fresh weight per plant (C) and stem dry weight per plant (D) of Stevia.
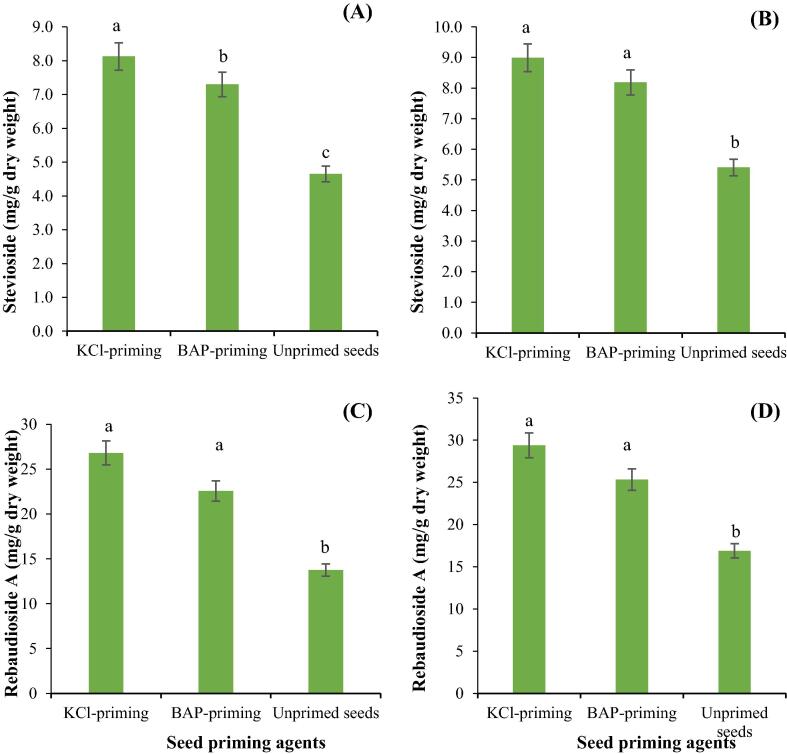
The seed priming agents included in the study significantly improved stevioside and rebaudioside A contents in young and old leaves of Stevia. Overall, old leaves had higher stevioside and rebaudioside A contents compared to young leaves. Seeds primed with KCl had the highest stevioside and rebaudioside A contents, whereas unprimed seeds recorded the lowest stevioside and rebaudioside A contents in both old and young leaves of Stevia (Fig. 4A, B, C, D). The superiority of in KCl-priming over other priming techniques may be because in KCl-primed seeds imbibed more water, which enabled better stand establishment.
Fig. 4.
The influence of different seed priming agents on stevioside in young leaves (A), stevioside in old leaves (B), rebaudioside A in young leaves (C) and rebaudioside A in old leaves (D) of Stevia.
4. Discussion
The stand establishment and biochemical attributes of Stevia seeds were significantly altered by different seed priming agents and priming duration (Table 2). The highest emergence percentage was recorded for KCl and BAP priming for 32 h, whereas unprimed seeds aerated for 32 h recorded the lowest emergence percentage. Seed germination requires specific soil and environmental conditions, which vary for different plant species. Temperature plays an important role in species’ seed germination and every plant species has a distinct temperature requirement for germination. The optimum temperature for seed germination depends on the nature of the crop and environmental conditions faced by maternal plants. The optimum temperature of seed germination of Stevia has been reported as 25 °C in previous studies (Carneiro and Guedes, 1992, Simlat et al., 2019).
Improved germination and stand establishment are owed to starch metabolism (Hossain et al., 2015), whereas lower α-amylase and dehydrogenase activities, and less accumulation of soluble sugars is responsible for poor stand establishment traits (Silva-Neta et al., 2015). Seed priming with different salts exerts moderate stress to seeds, which better equips them for future environmental conditions (Gallardo et al., 2003). Seed priming with different salts results in the accumulation of various stress proteins, such as late embryogenesis proteins and heat shock proteins (Gurusinghe et al., 2002, Gallardo et al., 2003, Farooq et al., 2020), which probably increased α-amylase and dehydrogenase activities to improve germination and stand establishment.
Different conventional seed priming agents significantly improved stand establishment and biochemical attributes of Stevia. Similarly, the increasing priming duration, as hypothesized, improved stand establishment and biochemical attributes. Shortening the lag phase and increasing the metabolism of seed reserves by activating hydrolytic enzymes during seed priming (Kato-Noguchi and Macías, 2005, Farooq et al., 2006a) also improve stand establishment. Different studies have reported that seed priming could be used to boost the germination of Stevia (Gorzi et al., 2020, Gorzi et al., 2018, Hossa et al., 2017, Shahverdi et al., 2017a, Shahverdi et al., 2017b, Simlat et al., 2020, Simlat et al., 2018, Simlat et al., 2016). Similarly, phytohormones applied through seed priming have been proved to improve seed germination (Liopa-Tsakalidi, 2012, Hossa et al., 2017) of Stevia. Germination percentage and germination rate, germination energy, seedling length, germination uniformity and germination value had strong positive correlation.
The present study confirms that conventional seed priming agents can be effectively used to improve stand establishment traits of Stevia. The results of priming duration are also interesting, indicating that priming for longer duration in place of conventional short-term priming is better for Stevia seeds. Stevia seeds probably require a longer water imbibition period for metabolism, DNA, and mitochondria repair during lag phase.
Improved patterns of dry matter accumulation in KCl-primed seeds was reflected in better SGs accumulation. This suggests that vigorous seedlings resulted from in KCl-primed seeds could result in earlier and enhanced mobilization of reserves than noted for poor seedlings from unprimed seeds.
5. Conclusion
Seed priming with KCl and BAP resulted in better allometric traits compared with unprimed seeds. Similarly, seed priming with KCl resulted in higher fresh and dry biomass production of stem and leaves. Nonetheless, higher stevioside and rebaudioside A contents were recorded for the seeds primed with KCl, whereas unprimed seeds resulted in the lowest values. It is concluded that Stevia seeds must be primed with KCl for 32 h before sowing to get higher seed germination, leaf yield and steviol glycosides contents.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Association of Official Seed Analysts Rules for testing seeds. J. Seed Technol. 1990;12:1–112. [Google Scholar]
- Bayraktar M., Naziri E., Akgun I.H., Karabey F., Ilhan E., Akyol B., Bedir E., Gurel A. Elicitor induced stevioside production, in vitro shoot growth, and biomass accumulation in micropropagated Stevia rebaudiana. Plant Cell, Tissue Organ Cult. 2016;127(2):289–300. [Google Scholar]
- Blackig M., Corbineau F., Grzesikit M., Guyi P., Côme D. Carbohydrate metabolism in the developing and maturing wheat embryo in relation to its desiccation tolerance. J. Exp. Bot. 1996;47(2):161–169. [Google Scholar]
- Carneiro J.W.P., Guedes T.A. Influence of the contact of stevia seeds with the substrate, evaluated by means of the Wiebull function. Rev. Bras. Sementes. 1992;14(1):65–68. [Google Scholar]
- Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28(3):350–356. [Google Scholar]
- Ellis R.H., Roberts E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981;9(2):373–409. [Google Scholar]
- Farooq M., Barsa S.M.A., Wahid A. Priming of field-sown rice seed enhances germination, seedling establishment, allometry and yield. Plant Growth Regul. 2006;49(2–3):285–294. [Google Scholar]
- Farooq M., Hussain M., Habib M.M., Khan M.S., Ahmad I., Farooq S., Siddique K.H.M. Influence of seed priming techniques on grain yield and economic returns of bread wheat planted at different spacings. Crop Pasture Sci. 2020;71(8):725. [Google Scholar]
- Farooq M., Basra S.M.A., Tabassum R., Afzal I. Enhancing the performance of direct seeded fine rice by seed priming. Plant Prod. Sci. 2006;9(4):446–456. [Google Scholar]
- Farooq S., Hussain M., Jabran K., Hassan W., Rizwan M.S., Yasir T.A. Osmopriming with CaCl2 improves wheat (Triticum aestivum L.) production under water-limited environments. Environ. Sci. Pollut. Res. 2017;24(15):13638–13649. doi: 10.1007/s11356-017-8957-x. [DOI] [PubMed] [Google Scholar]
- Farooq S., Shahid M., Khan M.B., Hussain M., Farooq M. Improving the productivity of bread wheat by good management practices under terminal drought. J. Agron. Crop. Sci. 2015;201(3):173–188. [Google Scholar]
- Gallardo, K., Job, C., Groot, S.P., Puype, M., Demol, H., Vandekerckhove, J., Job, D., 2003. Proteomics of Arabidopsis seed germination and priming. In: Nicolas, G. (Ed.), Biol. Seeds Recent Adv., pp. 199–209.
- Gorzi A., Omidi H., Bostani A. Effect of stevia (Stevia rebaudiana) seed priming treatments with salicylic acid, iron, and zinc on some germination traits and photosynthetic pigments under drought stress. Iran J. Seed Res. 2020;6(2):125–135. [Google Scholar]
- Gorzi A., Omidi H., Bostani A.B. Morpho-physiological responses of Stevia (Stevia rebaudiana Bertoni) to various priming treatments under drought stress. Appl. Ecol. Environ. Res. 2018;16(4):4753–4771. [Google Scholar]
- Gupta P. Plant tissue culture of Stevia rebaudiana (Bertoni): a review. J. Pharmacogn. Phyther. 2013;5(2):26–33. [Google Scholar]
- Gurusinghe S., Powell A.L.T., Bradford K.J. Enhanced expression of BiP is associated with treatments that extend storage longevity of primed tomato seeds. J. Am. Soc. Hortic. Sci. 2002;127(4):528–534. [Google Scholar]
- Hossa K.R., Pedroza Carneiro J.W., Guedes T.A., Braccini A.L. Stevia rebaudiana (Bert) Bertoni: influence of osmotic stress and seed priming on seed germination under laboratory conditions. Acta Sci. Agro. 2017;39(3):379. [Google Scholar]
- Hossain M.A., Bhattacharjee S., Armin S.M., Qian P., Xin W., Li H.Y., Burritt D.J., Fujita M., Tran L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front. Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Farooq M., Basra S.M.A., Ahmad N. Influence of seed priming techniques on the seedling establishment, yield and quality of hybrid sunflower. Int. J. Agric. Biol. 2006;8(1):14–18. [Google Scholar]
- IBM C, 2012. SPSS Statistics for Windows. IBM Corp Released 2012. Version 20, pp.1–8.
- Jain P., Farooq B., Lamba S., Koul B. Foliar spray of Moringa oleifera Lam. leaf extracts (MLE) enhances the stevioside, zeatin and mineral contents in Stevia rebaudiana Betoni. South African J. Bot. 2020;132:249–257. [Google Scholar]
- Jeppesen P.B., Gregersen S., Alstrup K.K., Hermansen K. Stevioside induces antihyperglycaemic, insulinotropic and glucagonostatic effects in vivo: studies in the diabetic Goto-Kakizaki (GK) rats. Phytomedicine. 2002;9(1):9–14. doi: 10.1078/0944-7113-00081. [DOI] [PubMed] [Google Scholar]
- Jeppesen P.B., Gregersen S., Rolfsen S.E.D., Jepsen M., Colombo M., Agger A., Xiao J., Kruhøffer M., Ørntoft T., Hermansen K. Antihyperglycemic and blood pressure-reducing effects of stevioside in the diabetic Goto-Kakizaki rat. Metabolism. 2003;52(3):372–378. doi: 10.1053/meta.2003.50058. [DOI] [PubMed] [Google Scholar]
- Kato-Noguchi H., Macías F.A. Effects of 6-methoxy-2-benzoxazolinone on the germination and α-amylase activity in lettuce seeds. J. Plant Physiol. 2005;162(12):1304–1307. doi: 10.1016/j.jplph.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Khan M.B., Hussain M., Abid R., Farooq S., Jabran K. Seed priming with CaCl2 and ridge planting for improved drought resistance in maize. Turkish J. Agric. For. 2015;39(2):193–203. [Google Scholar]
- Kinghorn A.D. CRC Press; 2001. Stevia: the genus Stevia. [Google Scholar]
- Liopa-Tsakalidi A. Effect of salicylic acid (SA) and gibberellic acid (GA3) pre-soaking on seed germination of stevia (Stevia rebaudiana) under salt stress. J. Med. Plants Res. 2012;6(3):416–423. [Google Scholar]
- Lutts, S., Benincasa, P., Wojtyla, L., Kubala, S., Pace, R., Lechowska, K., Quinet, M., Garnczarska, M., 2016. Seed priming: New comprehensive approaches for an old empirical technique. In: Araújo, S., Balestrazzi, A. (Eds.). New Challenges Seed Biol – Basic Transl Res Driv Seed Technol. InTech. pp. 1–46.
- Madan S., Ahmad S., Singh G.N., Kohli K., Kumar Y., Singh R., Garg M. Stevia rebaudiana (Bert.) Bertoni-a review. Indian J. Nat. Prod. Resour. 2010;267–286 [Google Scholar]
- Murdoch A.J., Ellis R.H. Dormancy, viability and longevity. Seeds Ecol. Regen Plant Comm. 2000;2:183–214. [Google Scholar]
- Rajasekaran T., Giridhar P., Ravishankar G. Production of steviosides inex vitro andin vitro grown Stevia rebaudiana Bertoni. J. Sci. Food Agric. 2007;87(3):420–424. [Google Scholar]
- Shahverdi M.A., Omidi H., Tabatabaei S.J. Effect of nutri-priming on germination indices and physiological characteristics of stevia seedling under salinity stress. J. Seed Sci. 2017;39(4):353–362. [Google Scholar]
- Shahverdi M.A., Omidi H., Tabatabaei S.J. Determination of optimum duration and concentration of Stevia (Stevia rebaudiana Bert.) seed priming with boric acid (H3BO3) Türkiye Tarımsal Araştırmalar Derg. 2017;4(1):24–30. [Google Scholar]
- Silva-Neta I.C., Pinho E.V., Veiga A.D., Pìnho R.G., Guimarães R.M., Caixeta F., Santos H.O., Marques T.L. Expression of genes related to tolerance to low temperature for maize seed germination. Genet. Mol. Res. 2015;14(1):2674–2690. doi: 10.4238/2015.March.30.28. [DOI] [PubMed] [Google Scholar]
- Simlat M., Ptak A., Skrzypek E., Warchoł M., Morańska E., Piórkowska E. Melatonin significantly influences seed germination and seedling growth of Stevia rebaudiana Bertoni. PeerJ. 2018;6:e5009. doi: 10.7717/peerj.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simlat M., Skrzypek E., Warchoł M., Maciaszek I., Ptak A. Evaluation on Stevia rebaudiana Bertoni seed germination and seedling development under phytohormones treatment. Sci. Hortic. 2019;257:108717. [Google Scholar]
- Simlat M., Ślęzak P., Moś M., Warchoł M., Skrzypek E., Ptak A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Sci. Hortic. 2016;211:295–304. [Google Scholar]
- Simlat M., Szewczyk A., Ptak A. Melatonin promotes seed germination under salinity and enhances the biosynthesis of steviol glycosides in Stevia rebaudiana Bertoni leaves. PLoS One. 2020;15(3):e0230755. doi: 10.1371/journal.pone.0230755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srimaroeng C., Chatsudthipong V., Aslamkhan A.G., Pritchard J.B. Transport of the natural sweetener stevioside and its aglycone steviol by human organic anion transporter (hOAT1; SLC22A6) and hOAT3 (SLC22A8) J. Pharmacol. Exp. Ther. 2005;313(2):621–628. doi: 10.1124/jpet.104.080366. [DOI] [PubMed] [Google Scholar]
- Steel R.G.D., Torrie J.H., Dickey D.A. McGraw-Hill; 1997. Principles and Procedures of Statistics: A Biological Approach. [Google Scholar]
- Yadav A.K., Singh S., Dhyani D., Ahuja P.S. A review on the improvement of stevia [Stevia rebaudiana (Bertoni)] Can. J. Plant Sci. 2011;91(1):1–27. [Google Scholar]
Further Reading
- Farooq, M., Basra, S.M.A., Wahid, A., Khaliq, A., Kobayashi, N., 2009. Rice seed invigoration: a review. In: Org Farming, Pest Control Remediat Soil Pollut. Springer, pp. 137–175.