Abstract
Silver nanoparticles are well received in the cosmeceutical industry due to their broad spectrum of pharmacology applications. Research on the therapeutic properties exhibited by silver nanoparticles revealed that the antimicrobial and anti-inflammatory properties are the main attraction in the establishment of nanocosmeceutical products whereby their mechanisms of action are reviewed in this paper. In addition, studies on other uses of silver nanoparticles acknowledged that the particles act as antifungal agents in nail polishes and pigments in coloured beauty products such as lipsticks and eye shadows. Despite the extensive use of silver nanoparticles in the cosmetic line, there are still limited resources on the mechanism of actions and the effect of the particles on the bio-functionality of the body. The safety of silver nanoparticles could be comprehended from their skin penetration ability and toxicity to the human body in which it could be justified that both features are mainly influenced by the morphology of the particles and the method of application. This article summarizes exclusively on the synthesis of silver nanoparticles, the biomedical mechanisms and applications as well the limitations with respect to skin penetration ability and toxicity effects which will contribute significantly to the vast research on the association of nanotechnology and cosmetics.
Keywords: Silver nanoparticles, Nanotechnology, Pharmacology, Nanocosmeceutical, Nanotoxicity
1. Introduction
With the growing demand of skincare and beauty products in the market, the cosmetic industry has expanded exponentially over the years. However, the current demand has diverted towards products that improve overall skin quality. Consumers have been showing interest in products that provide long-term effects and numerous benefits to the skin. Hence, the invention of the term ‘cosmeceutical’ which implies the overlapping of pharmaceuticals and cosmetics (Cavinato, 2019). A cosmeceutical product is said to contain biologically active ingredients that not only treat skin problems but also preserve skin health (Draelos, 2019). The global cosmeceuticals market has garnered about USD 46.93 billion in 2017, with a forecast of USD 80.36 billion by 2023, registering a compound annual growth rate (CAGR) of 9.38% from 2016 to 2022. Evidently, consumers are willing to pay a higher price for products that enhance their skin functionality and thus encourage cosmetic manufacturers to develop and offer more variety of these products (Draelos, 2014).
Nanotechnology has been introduced in the cosmeceutical market in the early 1980 s, with reference to the term ‘nanocosmeceutical’. As the competition in the cosmeceutical market continue to grow immensely, new cosmetic formulations have emerged and manufacturers are constantly improvising to deliver products with higher efficacy. The cosmetic field is one of the early adopters of nanotechnology as its role significantly contributes to superior cosmetic effects from improved skin penetration and stability as well as effective release of ingredients through the skin barrier. Nanotechnology is widely incorporated in the production of various cosmeceutical line such as sunscreens, nail care, lip care, face cleansers, hair repair shampoos, mosturizing and whitening creams as well as anti-aging products (Lohani et al., 2014). The two common types of engineered nanomaterials are titanium oxide and zinc oxide. However, other nanosized materials such as gold and silver metals, metal oxides, liposomes, nanocapsules, cubosomes, dendrimers, niosomes, nanocrystals and solid lipid nanoparticles have begun to be used in cosmetic applications (Cao et al., 2016).
Silver nanoparticles (AgNPs) are one of the most widely researched metallic nanoparticles as they are industrialized for multiple purposes. As stated by Pirtarighat et al. (2019), the strong inhibitory effect of AgNPs on a large spectrum of microbial species is one of their most highly regarded property. As a result, AgNPs received much attention not just in the pharmaceutical industry as a potential substitute for antibiotics but also in the textile industry particularly water filtration (Haider and Kang, 2015, Pirtarighat et al., 2019). Furthermore, AgNPs possess impressive anti-inflammatory effect which is vital in woung healing and medical applications. With those critically acclaimed therapeutic effects, AgNPs are exploited for the formulation of skin cleansers, lotions, creams, deodorants, shampoos and toothpastes (seen in Table 1) (Gajbhiye and Sakharwade, 2016). Besides its antimicrobial and anti-inflammatory effect, AgNPs are proven to have anti-cancer properties as reported on human cell lines as well as animal models whereby AgNPs could inhibit cancer cell growth and induce apoptosis (Jeong et al., 2016, Yang et al., 2016).
Table 1.
Patent review on the incorporation of silver nanoparticles in cosmeceutical products.
| Title | Publication number | Publication date | Applicant |
|---|---|---|---|
| Cosmetic pigment composition containing gold or silver nano-particles | WO2007011103A1 | 2007–01-25 | Korea Research Institute of Bioscience and Biotechnology |
| Skin lotion comprising aqueous dispersion of ultra-fine noble metal particles | HU0401663A2 | 2005–09-28 | Phild Co., Ltd. |
| Anti-microbial body care product | US20020122832A1 | 2002–09-05 | Bernhard Hanke |
| Method for treating human keratin fibers with organomodified metallic particles | US7186274B2 | 2007–03-06 | L'oreal |
| Formulations including silver nanoparticles and methods of using the same | WO2015057983A1 | 2015–04-23 | University of South Alabama |
| Colored nanoparticles for cosmetic and its manufacturing method | JP2009221140A | 2009–10-01 | National Institute Of Advanced Industrial & Technology |
| Colloidal silver, honey, and helichrysum oil antiseptic composition and method of application | US5785972A | 1998–07-28 | Tyler; Kathleen A |
| Toothpaste or tooth gel containing silver nano particles coated with silver oxide | US20130017236A1 | 2013–01-17 | Robert Johnson Holladay |
The application of nanocosmeceutical has soared rapidly yet there are limited research available regarding the mechanism of action of AgNPs and the safety aspects. Therefore in this review, the main features of AgNPs are highlighted and their respective means of interaction to produce pharmaceutical effect leading to their impeccable roles in the cosmeceutical field are elucidated comprehensively. The controversial element of AgNPs which is their toxicity towards the biological functions of humans or animals were also deeply considered and outlined in this paper.
2. Synthesis of silver nanoparticles
2.1. Chemical-reduction synthesis
Just like every other nanoparticles, AgNPs are synthesised via several different means (Fig. 1) with chemical reduction method as one of them. Chemical synthesis of AgNPs comprised of the reduction of silver ions (Ag+) in aqueous or non-aqueous solutions into metallic silver by organic or inorganic reducing agents in the presence of hydroxyl groups (Siegel et al., 2012). Evidently, chemical synthesis requires three fundamentals; Ag precursors, reducing agents and stabilizing agents. Sodium citrate, ascorbate, sodium borohydride (NaBH4), elemental hydrogen, Tollens reagent, N, N-dimethylformamide (DMF) and glucose are examples of reducing agents used in this procedure (Iravani, 2014). Although there are other techniques recommended for chemical syntehesis of AgNPs such as polyol method (Tarek and El-Aziz, 2019) and radiolytic process (Uttayarat et al., 2015), chemical reduction is the preferred method as it promotes high yield with less cost and prevents aggregation of the particles formed (Gudikandula and Maringanti, 2016). The process and nature of reducing agents in the synthesis of AgNPs can significantly affect the size and shape of the particles and therefore altering the chemical and physical properties of the particles. The reactivity of the reducing agent to the redox potential of the metal should be adjusted during the process in order to control the reaction rate. If the reaction rate is accelerated with high speed, the particles formed will be too small due to excessive production of vast amount of metal nuclei. Meanwhile, if the reaction rate is too low, agglomeration of the particles will occur (Suriati et al., 2014).
Fig. 1.
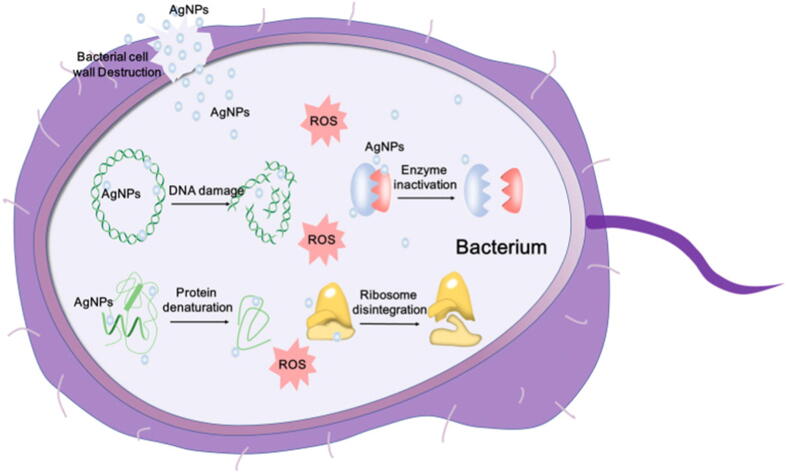
Schematic representation of the mechanisms of AgNPs against bacteria, depicting ROS-dependent pathway, DNA damage, protein denaturation and enzyme inactivation for antibacterial action of AgNPs from Xu et al. (2020) under the terms of the Creative Commons CC BY License, https://creativecommons.org/licenses/by/4.0/.
2.2. Physical synthesis
Besides chemical synthesis, physical method is another alternative adopted by researchers. Among the preeminent physical methods involved in the synthesis of AgNPs are the use of laser ablation, evaporation–condensation and ultraviolet (UV) radiation. However, most of the physical methods are not only time consuming but also require high energy and thus most costly. For instance, a large space is needed for the tube-furnace evaporation–condensation method along with the usage of high energy, heat released to its surroundings and a long time to achieve thermal stability. With that being said, more substitutes have been developed in order to counteract those complications. To illustrate, Jung et al. (2006) attempted the synthesis in a small ceramic heater where nanoparticles are formed through nucleation and growth. In this method, surface temperature achieved thermal stability in a shorter time and maintained uniformly leading to production of small particles in high concentration. Another improved method that had been explored is the thermal decomposition whereby monodispersed silver nanocrystallite were formed from reaction of silver nitrate (AgNO3) with sodium oleate at elevated temperature of 290 °C (Lee and Kang, 2004). The uniformity of size and shape of AgNPs are mostly determined by the thermal, ac power and arc discharge that are employed in physical synthesis (Haider and Kang, 2015).
2.3. Bio-synthesis
Nevertheless, the chemical synthesis of AgNPs involves chemical compounds such as sodium borohydride and hydrazine that may adhere to the surface of the particles and could contribute harmful effects contained in the end product (Rauwel et al. 2015). Furthermore, the conventional way of synthesizing AgNPs are costly and requires high energy expenditure as heat treatment is also involved (Ahmed et al., 2016). With the current trend of ‘going green’, researchers have been exploring a more cost-effective and environmental-friendly way of producing nanoparticles. Hence, assorted biological resources such as plants and microbes (yeast, fungi and bacteria) are used in the green synthesis of silver nanoparticles. The presence of biomolecules such as amino acids, proteins, vitamins, enzymes and polysaccharides as well as secondary metabolites in the extracts of these sources contribute to the reduction reaction in the process (Keat et al., 2015, Siddiqi et al., 2018). For instance, Sahoo et al. (2020) had utilized cyanobacterium Chroococcus minutus as a biological reducing agent to synthesize AgNPs as novel antibacterial agents against upper respiratory tract infection. In addition, cubical, spherical and truncated triangular shaped silver nanoparticles with average size of 88.87 nm were synthesized by green algae (Botryococcus braunii) in another experimental study (Arya et al., 2019). An endangered medicinal plant known as Withania coagulans was used as in the formation of AgNPs with average size 14 nm and spherical in shape. Results had shown that these particles were capable of exerting antioxidant and antibacterial effect on tested microorganisms (Tripathi et al., 2019).
3. Roles of silver nanoparticles in cosmetic products
3.1. Antibacterial
One of the exceptional abilities of AgNPs is their strong antibacterial property. Researchers utilize this property by incorporating AgNPs as active ingredients in the preparation of skincare products, toothpastes and deodorants [Raj et al., 2012, Effiong et al., 2020]. As shown in Fig. 1, AgNPs have the ability to exercise antibacterial features via several mechanisms. According to Dos Santos et al. (2014), AgNPs exert antimicrobial activities through the mechanism of a bactericidal whereby AgNPs adhere to the cell wall and react with the membrane proteins. This is further supported by Qing et al. (2018) whom stated that the adheration allows AgNPs to infiltrate and damage the cell membrane, leading to leakage of cellular contents. Structural damage of bacteria caused by AgNPs were observed by Huq et al. (2020). In their study, morphological changes could be seen on Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa) that were treated with AgNPs in which both cells had distorted and deformed inner and outer surfaces (Huq, 2020). Furthermore, in the cell, the silver ions (Ag+) are released to attack the respiratory chain and causing oxidative stress with an increase in production of reactive oxygen species (ROS). This ultimately leads to protein damage and inhibiton of cell growth. This can be evidently proven from a study conducted by Yan et al. 2018 on P. aeruginosa (Yan et al., 2018). As a result, cell growth is disrupted and eventually leads to cell necrosis. Besides the respiratory chain, research have stated that AgNPs also attack the DNA mechanism in the cells. According to a study, microscopic analysis of Escherichia coli (E. coli) and S. aureus revealed that the DNA of the bacteria cells had shrunk and condensed after treatment with AgNPs (Chatterjee at al., 2015). Likewise, Widdatallah et al. (2020) manifested the inhibition of E. coli and S. aureus by AgNPs synthesised by Nigella sativa seeds (Widdatallah et al., 2020). AgNPs hinder the transcription and translation process by attaching to the phosphorus and sulphur groups of the nucleotide bases and induce the unwinding of DNA (Prabhu and Poulose, 2012; Liao et al, 2019). Alteration of the DNA leads to interference of the replication process as well as gene expression of essential proteins and enzymes. Consequently, the deactivation of respiratory enzyme by AgNPs at the cytoplasmic membrane will essentially interrupt the production of adenosine triphosphates (ATPs) [36]. Additionally, cell enzymatic function can be inactivated via the interaction between AgNPs and released Ag+ with the thiol and sulfhydryl groups of the enzymes respectively (Prabhu and Poulose, 2012).
The effectivity and efficiency of this particular property are shape and size dependent. Several studies have been conducted to prove the effect of the shape and size of AgNPs on the antibacterial activity as displayed in Table 2. Based on the studies conducted, it could be deduced that smaller-sized AgNPs had higher effectivity in microbial inhibition. This is due to the large surface to volume ratio that enable the AgNPs to penetrate through the membrane more efficiently and actively disrupt the structure as well as the cell metabolism in the bacteria (Duran et al., 2010). Another factor to be considered that is the shape of the nanoparticles. For instance, Pal et al. 2007 evaluated the bactericidal action of truncated triangular, spherical and rod-shaped AgNPs on gram-negative bacterium E. coli. From their observations, it could be deduced that truncated triangular-shaped AgNPs performed better antibacterial activity compared to the other two shapes. AgNPs with the same size or surface areas could also exert different antibacterial capacity due to the difference in shape as the effective surface areas and active facets of the AgNPs may vary (Dakal et al., 2016) Table 3.
Table 2.
Studies on the effect of size and shape of AgNPs on the antimicrobial acitvity.
| Journal | Size | Shape | Antimicrobial activity |
|---|---|---|---|
| Jeong et al., 2014, Dong et al., 2019, Helmlinger et al., 2016, Martinez-Castanon et al., 2008, Osonga et al., 2020 | 10 nm and 100 nm 10 nm, 30 nm, 60 nm and 90 nm 40–80 and 120–180 nm, 20–60 nm, 140–180 nm, 80–120 nm 7 and 29 nm, 89 nm 9, 16, 30, 35 nm and 21, 27 nm |
Spherical Spherical Spherical PlateletsCubes Rods Spherical Pseudospherical Spherical Quasi-spherical |
10 nm nanoparticles showed more antimicrobial activity than 100 nm particles on Methylobacterium spp. Both MIC and MBC values for 10 nm size AgNPs were the lowest while 90 nm sized AgNPs had the highest values. Silver nanoplatelets showed the highest antibacterial effect on S. aureus, followed by spheres, rods, and then cubes 7-nm silver nanoparticles present the best antibacterial against E. coli and S. aureus. 7 nm silver nanoparticles showed the highest antibacterial effect against E. coli and S. aureus. Smaller sized spherical (9 nm) and quasi-spherical (21 nm) AgNPs exhibited highest inhibition on tested fungi (Aspergillus nidulans, Trichaptum biforme, Penicillium italicum, Fusarium oxysporum, and Colletotrichum gloeosporioides) and bacteria (Pseudomonas aeruginosa, Aeromonas hydrophila, Escherichia coli, Citrobacter freundii, Listeria monocytogenes and Staphylococcus epidermidis) |
Table 3.
Effect of synthesis on the size and shape of AgNPs.
| Types of synthesis | Physical properties of AgNPs (size,shape) | Source | |
|---|---|---|---|
| Physical | a) Laser ablation in – ethylene glycol − distilled water − chitosan − aqueous monomers isobutyl acrylate b) Inert gas condensation using helium |
Average size of 22.08 nm, spherical shaped Average size of 27.41 nm, spherical shaped Average size of 10.50 nm, spherical shaped Size < 25 nm, spherical shaped Size range from 9 to 32 nm, spherical shaped |
Tajdidzadeh et al., 2014, Tajdidzadeh et al., 2014, Tajdidzadeh et al., 2014, Christopher et al., 2011, Raffi et al., 2007 |
| Chemical reduction | a) 3-hydrazino-isatin b) 1-benzyl-3-hydrazino-isatin c) Hydrazine hydrate d) Sodium borohydride e) Trisodium citrate |
Size range 18–21 nm, monodispersed spheres Size range of 17–20 nm, monodispersed spheres Size range 8–50 nm, agglomerates of small grains Size < 20 nm, small spherical shaped grains Size range of 100–200 nm, agglomerates of small grains Size range of 35–80 nm, quasi spherical/polygonal |
El-Faham., Elzatahry, A., Alothman, Z., Elsayed, E. , 2014, El-Faham., Elzatahry, A., Alothman, Z., Elsayed, E. , 2014, Guzman et al., 2008, Szczepanowicz et al., 2010, Khatoon et al., 2011, Suriati et al., 2014 |
| Green/Bio- synthesis |
|
Size range of 20–40 nm, spherical and oval-shaped Size range of 5–20 nm, spheroidal shaped Diameter range of 200–223 nm, spherical shaped Size range of 30–70 nm, spherical shaped Average diameter around 34 nm, spherical shaped Average size of 9 nm, monodipersed spheres Size range of 19.8–92.8 nm, spherical shaped Size range of 10–30 nm, spherical shaped Size range of 8–24 nm, spherical shaped Size range of 7–31 nm, single/aggregated spherical shaped granules Size range of 5–20 nm, small and spherical shaped Average size of 9.46 ± 2.64 nm, spherical shaped Size range of 30–200 nm, spherical shaped |
Al-Ghamdi, 2018, Qais et al., 2019, Erdogan et al., 2019, Behravan et al., 2019, Ahmed et al., 2016, Bindhu and Umadevi, 2013, Masum et al., 2019, Singh et al., 2015, Huq, 2020, Deljou and Goudarzi, 2016, Niknejad et al., 2015, AbdelRahim et al., 2017, Saravanan et al., 2017 |
This property of AgNPs is deeply acknowledged in the cosmetic industry and therefore they are used as preservatives in various cosmetic line in order to prevent growth of pathogenic microorganism which could affect the quality of the products and human health as well as the integrity of the brand. On top of that, this elemental feature enables AgNPs to be implemented as a treatment for bacterial-induced skin complications such as acne in the form of cream of gel. Some skin cleansers and body soaps also contain AgNPs and are used for acne proned skin or sun-damanged skin. An example of these cleansers are the nanocleanser pink soap produced by Nano Cyclic Inc. that helps to reduce age spots, bacteria and fungi colonization as well as to treat acne (Nafisi and Maibach, 2017). AgNPs are also used in combination with fluoride in toothpaste to prevent formation of carries and promote self-cleaning against plaques (García-Contreras et al., 2011). In a patent review, AgNPs are added into toothpaste or oral care gels at a concentration between 0.0005% and 0.004% as to inhibit bacteria growth that leads to unpleasant oral smells and dental cavities (Holladay, 2011). Silver nanoparticles (AgNPs) are incorporated in anti-dandruff shampoos in order to prevent the buildup of Malassezia species responsible for the itching and scaling of the scalp (Sathishkumar et al., 2016).
3.2. Anti-inflammatory
Other than the ability to inhibit microbial growth, the role of AgNPs in the wound healing process has become a crucial area in therapeutic research and modern medical practice. Agarwal et al. (2019) disclosed that AgNPs substantially disrupt the Vascular Endothelial Growth Factor (VEGF) pathway which is responsible for the T-helper type-2 (TH2) cell-mediated inflammation promoted by secretion of pro-inflammatory cytokines like IL-4, IL-5, IL-9 and IL-13.
Several literature evidence based on animal studies reported that AgNPs alter the inflammatory response via various mechanisms. For instance, one of them suggested that AgNPs lower wound inflammation and regulate the release of fibrogenic cytokines in a thermal injury animal model thus promoting rapid healing. Additionally, a decrease in inflammation markers such tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 was observed in animals treated with AgNPs (Wong et al., 2009, Seung et al., 2018). The ability of green-synthesised AgNPs by Prunus serrulate fruit extract to reduce the expression levels of other inflammatory mediators such as nitric oxide, prostaglandin E2 and inducible nitric oxide synthase in lipopolysaccharide (LPS)-induced RAW264.7 cells, derived from murine macrophage cells was demonstrated by Singh et al. (2017). Apart from these mediators, the study revealed that AgNPs exerted inhibitory influence on NF-κB (Nuclear Factor-kβ) signalling which is responsible for various inflammatory diseases via p38 MAPK (Mitogen-activated protein kinase) pathway. The potentiality of AgNPs as anti-inflammatory agents had been proven from an another animal study on the healing process of anastomosed intestinal tissue of mice using AgNP-coated suture. Macroscopic observation showed a more effective and long-term tissue healing process with a resemblance of a normal intestinal tissue along with reduced inflammatory response using the AgNP-coated suture compared to the control and antibiotic-suture groups (Liu et al., 2017). Quantitative evaluation displayed less macrophage infiltration and reduced expression levels of proinflammatory cytokines even when the tissue healing process reached the later stage. Inhibition of enzyme cyclooxygenase which then supresses prostaglandin (PG) synthesis in the inflammatory response was demonstrated by bio-synthesised AgNPs on carrageenan-induced hind paw oedema model in rats (El-Rafie and Hamed, 2014).
Human cell lines have also been used extensively to determine the role of AgNPs in the inflammatory response sytem. For instance, Aparna Mani et al. (2015) had found that AgNPs synthesised by Piper Nigrum extract were able to reduce the expression levels of TNF-α, IL-1β and IL-6 in human PBMC cells. In another study, it was concluded that AgNPs were able to downregulate the production of a vital proinflammatory mediator known as cyclooxygenase-2 (COX-2) in normal human dermal fibroblasts (NHDFs) and normal human epidermal keratinocytes (NHEKs) (Frankova et al., 2016). Moreover, the anti-inflammatory effect of AgNPs could be observed in tumor necrosis factor-α (TNFα)-induced inflammatory response in human pulmonary epithelial cell line (NCI-H292) in a dose-dependent manner. Gene expression levels of TNFα-induced IL-1β and IL-18 were significantly reduced due to exposure of AgNPs with concentrations of 10 and 100 µg/ml as reported in the study by Fehaid et al. (2020).
The ability of AgNPs to act as a new source of anti-inflammatory drug is essential in the manufacturing of skin ointments and creams. With the combination of both antibacterial and anti-inflammatory property, AgNPs can be a potential active ingredient in the formulation of skin care products used to treat acne. They can perform by minimizing not only the growth of Propionibacterium acnes (P.acnes) and other bacteria but also the inflammation induced by P.acnes (Prabhu and Poulose, 2012, Patil and Muthusamy, 2020). Such impressive characteristic possessed by AgNPs was tested on patients with psoriasis vulgaris and was proved that to be more effective than hydrocortisone (David et al., 2014). In accordance to that, greater amount of studies have been conducted to demonstrate that AgNPs are capable of treating wounds, cuts and burns. In relation to that matter, treatment for ultraviolet (UV) radiation-induced damaged skin with the application of AgNPs in the formulation of various compositions such as sunscreens, sunburn relief formulations, hand, body and/or facial moisturizers, topical analgesic have been patented due to their healing properties and anti-inflammatory effect (Singh et al., 2005).
3.3. Other roles of silver nanoparticles in cosmetic
One of the new strategies which may have great potential in the cosmeceuticals is the incorporation of nanoparticles having antifungal activity (silver and metal oxide nanoparticles) in nail polish to treat fungal toenail infections (Lohani et al., 2014). The use of nanoparticles like silver as cosmetic pigment has been patented whereby it is stated that AgNPs in combination of gold nanoparticles provide yellow–red pigment in the manufacturing of diverse cosmetic products such as foundations, eye shadows, powders, lipsticks, inks, varnishes or eyebrow pencils (Ha et al., 2005, Pulit-Prociak and Banach, 2016).
3.4. Skin penetration of silver nanoparticles
Skin serves as the main exposure to nanoparticles especially when incorporated into topical applications in cosmectic products. However, the skin itself act as a barrier against foreign objects through its layers known as the stratum corneum (the horny layer), the dermis and the subcutaneous wherein the stratum corneum has the highest barrier function. Theoretically, the smaller the size of the particles, the higher the rate of penetration (Yokota and Kyotani, 2018). Cardoso et al. (2017) demonstrated that a higher amount of smaller sized AgNPs were able to penetrate through the skin on the back of a pig’s ear as compared to silver nitrate (AgNO3) due to its larger size. Another reported factor on the penetration ability of AgNPs is the condition of the skin (Staron et al., 2020). Larese et al. (2007) had conducted an in vitro study regarding the penetration of AgNPs (25 nm) on damaged and intact skin whereby they verified that AgNPs absorbed through the intact skin was low but detectable (0.46 ng/cm2) whereas there was an increase in amount of AgNPs penetrated through the damaged skin (2.32 ng/cm2). On the contrary, several papers have also disclosed that AgNPs were able to be absorbed through the skin and preciptated at the stratum corneum. The authors also reported the presence of aggregrates of the particles at stratum corneum which could potentially prevent further penetration into deeper layers (Bianco et al., 2016, Wang et al., 2016). However, an in vivo analysis had been carried out on 16 healthy with normal intact skin and it had been observed that AgNPs were able to penetrate deeper than stratum corneum and into the reticular dermis (George et al., 2014). Nonetheless, none were found in the systemic circulation.
Some researchers had also concluded in past studies that the skin penetration of AgNPs could be shape-dependent. According to a study by Tak et al. (2015) on the skin permeability of the spherical silver nanoparticles (SNPs), rod-shaped silver nanoparticles (RNPs) and triangular silver nanoparticles (TNPs), it was found that RNPs had the highest penetration capability whereas TNPs recorded the lowest penetration capability. From Table 2, it could be justified that the method of synthesis of AgNPs and the reduction agents used contribute to the variety of sizes and shapes of the particles. Along with size and shape of the nanoparticles, the surface properties (polarity/charge), the formulation of the particles (hydrophobocity) and the application method can affect the penetration behaviour of a AgNPs (Liang et al., 2013). In another context, Bianco et al. (2014) documented an experiment on the permeation of AgNPs on three human skin graft samples (fresh, cryopreserved and glycerol-preserved) that are most commonly used in burns recovery, all of which has decreasing cell viability. From the results obtained, it could be observed that more AgNPs could penetrate through glycerol-preserved skin (non-viable cells) compared to fresh and cryopreserved (64% viability) skin samples. Nonetheless, the difference in the amount of AgNPs found in both fresh and cryopreserved skin were insignificant and thus indicating that cell viability is not a factor in the percutaneous absorption of AgNPs and suggesting that the absorption occurs through passive diffusion (Chen et al., 2013).
3.5. Cellular and toxicity effects of silver nanoparticles
As the exploitation of AgNPs continue to magnify due to their intrinsic biomedical applications, their toxicity eefects on human health as displayed in Fig. 2 has become a more apparent concern with the number of studies conducted progressively by researchers to explore this grey area. While it is known that AgNPs has cytotoxic effect on different bacteria species and viruses such as HIV-1 and canine distemper virus (CDV), many in vivo and in vitro studies were performed on various animal models and human cell lines to determine the adverse effects of AgNPs on normal and cancer cells (Chen et al., 2013, Bogdanchikova et al., 2016). Some studies illustrated different toxicity effect of AgNPs at various concentrations. For instance, Kokura et al. (2010) stated that AgNPs at concentration of 0.002–0.02 ppm had no effect on human keratinocytes or UVB-induced cell death. Meanwhile, another recent analysis done by Vazquez-Munoz et al. (2017) showed that at cellular level, the toxicity effect of AgNPs was similar for all biological systems tested; Rift Valley Fever Virus (RVFV), bacteria (Staphylococcus aures and Esherichia coli), microalgae, Candida albicans (yeast), Fusarum oxysporum (filamentous), mammalian cell lines (Vero and Murine Antigen-presenting Dendritic Cells) and human cancer cell lines (HeLa and MDA-MB-231) whereby the inhibitory concentration range was 10 µg/mL.
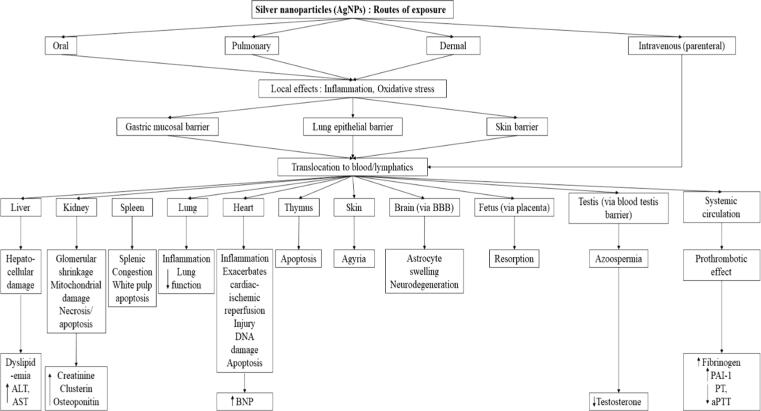
Fig. 2.
Schematic representation of the biodistribution and toxicity of silver nanoparticles (AgNPs) following various routes of exposure from Ferdous and Nemmar (2020) under the terms of the Creative Commons CC BY License, https://creativecommons.org/licenses/by/4.0/
Several genotoxicity studies have also been run by researchers on human and animal cell lines to further elaborate of the mechanism of AgNPs. DNA damage in the form of DNA strand breaks in mouse lymphoma cells and HK-2 immortalized human proximal tubule cells were most evident when incubated with AgNPs compared to several other nanomaterials (Mei et al., 2012, Kermanizadeh et al., 2013). Oxidative DNA damage by AgNPs was also imposed on three human cells, human colorectal adenocarcinoma (HT-29), human epithelial cell line (A549) and human hepatic cell line (HepG2) as reported by Kruszewski et al. (2013). Haase et al. (2012) analyzed the effects of AgNPs on primary neural cells from Wistar rats and found that AgNPs induced the production of ROS along with acute calcium dysregulation. This justified that prolonged exposure to AgNPs may disrupt the central nervous system. This finding is further supported by a later study whereby it was proven that AgNPs induced inflammatory response on mouse neuron cells and deposition of amyloid-β (Aβ) plaques which is a cause of nerurogenerative disorder, Alzheimer's disease (AD) (Huang et al., 2015). A comparative study between the cytotoxic effect of AgNPs on normal and cancer cells were conducted by AshaRani et al. (2008). Exposure to AgNPs led to formation of ROS, reduction of ATP content and DNA damage on both normal human lung fibroblast cells (IMR-90) and human glioblastoma cells (U251). Therefore, it is evident that AgNPs posed cytotoxicty and genotoxicity effect not only on unhealthy, cancerous cells but also on normal cells.
The toxicity effect of AgNPs are determined and influenced by multiple factors hence the inconsistent data reported. According to literature, the cytotoxicity effect of AgNPs are associcated with the release of silver ions from the surface whereby human cell lines showed a much greater vulnerability towards silver salts as compared to AgNPs (Koch et al., 2012, Barbasz et al., 2017). Additionally, the size and morphology of the nanoparticles can strongly affect the mode of actions of the AgNPs. Size-dependent toxicity effect of AgNPs was proven in a study whereby 10 nm of particles portrayed significantly greater toxicity compared to 50 nm, 100 nm and 200 nm particles (Wildt et al., 2016). Similarly, Williams et al. (2016) observed most significant effect on human T84 epithelial cells by 10 nm AgNPs compared to 20 nm, 75 nm and 110 nm. In a review, it was stated that the surface charge developed by surface functionalization can significantly affect the toxicity of mechanism of AgNPs in which their uptake into cells are determined by the magnitude of surface charge measured as zeta potential (Burdusel et al., 2018). Meanwhile, the shape-dependent toxicity of AgNPs was illustrated in a comparative study on the effect of silver wires (diameter 100–160 nm), spherical silver nanoparticles (30 nm) and silver microparticles (<45 μm) synthesized by wet chemical methods on human alveolar epithelial cells (A549). Based on the results reported, reduction of cell viability and increased lactic acid dehydrogenase (LDH) release by silver wires were observed while spherical AgNPs showed no such effects (Stoehr et al., 2011). The physicochemical properties of AgNPs such as their chemical nature, reactivity, composition as well as the presence of aggregation are other factors affecting the toxicity of the particles that should be considered (Korani et al., 2015). Galandakova et al. (2016) had claimed that ionic silver was more cytotoxic compared to AgNPs on NHDF and NHEK. Based on their study, the authors have concluded that the concentration of AgNPs that is suitable to be included in wound healing formulation was till 25 µg/mL.
Put simply, it is established that AgNPs are able to exert toxicity by inducing apoptosis, increasing the production of reactive oxygen species (ROS) leading to oxidative stress and causing DNA damage on a broad spectrum of bacteria species, viruses as a well as human cancer cell lines (Dos Santos et al., 2014, Kora and Sashidhar, 2018). These beneficial effects encourage AgNPs to be explored and used extensively as a novel material in the modern therapeutic applications and devices. However, it is not entirely coherent on whether AgNPs exert similar effects on cellular membrane and organelles such as the nucleus, mitochondria, and lysosomes on normal human cell lines which could potentially lead to the disruption of human biofunctionality.
4. Conclusion
To summarise the above, silver nanoparticles (AgNPs) are undoubtedly critical and valuable addition to the cosmeceutical industry due to their advantageous, therapeutic attributes. With that said, AgNPs contribute enormously to the emergence of new and diverse formulations in the cosmetic line that could prevent or treat prevalent skin diseases, heal damaged skin and subsequently preserve overall skin health and quality for long-term.
The efficiency of these nanoparticles to deliver their beneficial effects are influenced by their physical and chemical properties which in turn, are affected during the synthesis of these particles. It is undeniable that research and literature review on the toxicity of AgNPs are limited and therefore the exact concentration of AgNPs that is safe for human is still not established as results obtained and presented by different studies showed dual effects of AgNPs. Hence, further studies must be conducted to interpret comprehensively on the toxicity aspects of AgNPs to ensure safe use in the cosmeceutical field. The understanding on the mechanism of actions possessed by the particles will provide an opportunity for cosmetic manufacturers to venture into different and versatile applications for their inventions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Financial support of this work by the Ministry of Higher Education through the Fundamental Research Grant Scheme (FRGS/1/2018/WAB01/UCSI/02/1) is gratefully acknowledged.
Footnotes
Peer review under responsibility of King Saud University.
References
- AbdelRahim K., Mahmoud S.Y., Ali A.M., Almaary K.S., Mustafa A.E.Z.M.A., Husseiny S.M. Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi Journal of Biological Sciences. 2017;24(1):208–216. doi: 10.1016/j.sjbs.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal H., Nakara A., Shanmugam V.K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomedicine and Pharmacotherapy. 2019;109:2561–2572. doi: 10.1016/j.biopha.2018.11.116. [DOI] [PubMed] [Google Scholar]
- Ahmed S., Saifullah A., M., Swami, B. L., Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. Journal of Radiation Research and Applied Science. 2016;9(1):1–7. [Google Scholar]
- Al-Ghamdi A.Y. Antibacterial Activity of Green Synthesis Silver Nanoparticles Using Some Wild Edible Plants Commonly Used in Al Baha. Saudi Arabia. Advances in Microbiology. 2018;08(12):938–949. [Google Scholar]
- Aparna Mani K.M., Seethalakshmi S., Gopal V. Evaluation of in-vitro anti-inflammatory activity of silver nanoparticles synthesised using Piper nigrum extract. Journal of Nanomedicine and Nanotechnology. 2015;6(2):1000268. [Google Scholar]
- Arya A., Mishra V., Chundawat T.S. Green synthesis of silver nanoparticles from green algae (Botryococcus braunii) and its catalytic behavior for the synthesis of benzimidazoles. Chemical Data Collections. 2019;20 [Google Scholar]
- AshaRani P.V., Low K.M., G., Hande, M. P., Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano. 2008;3(2):279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- Barbasz A., Oćwieja M., Walas S. Toxicological effects of three types of silver nanoparticles and their salt precursors acting on human U-937 and HL-60 cells. Toxicology Mechanisms and Methods. 2017;27(1):58–71. doi: 10.1080/15376516.2016.1251520. [DOI] [PubMed] [Google Scholar]
- Behravan M., Hossein Panahi A., Naghizadeh A., Ziaee M., Mahdavi R., Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. International Journal of Biological Macromolecules. 2019;124:148–154. doi: 10.1016/j.ijbiomac.2018.11.101. [DOI] [PubMed] [Google Scholar]
- Bianco C., Adami G., Crosera M., Larese F., Casarin S., Castagnoli C., Stella M., Maina G. Silver percutaneous absorption after exposure to silver nanoparticles: A comparison study of three human skin graft samples used for clinical applications. Burns. 2014;40(7):1390–1396. doi: 10.1016/j.burns.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Bianco C., Visser M.J., Pluut O.A., Svetličić V., Pletikapi G., Jakasa I., Riethmuller C., Adami G.L., Filon F., Schwegler-Berry D., Stefaniak A.B., Kezic S. Characterization of silver particles in the stratum corneum of healthy subjects and atopic dermatitis patients dermally exposed to a silver-containing garment. Nanotoxicology. 2016;10(10):1480–1491. doi: 10.1080/17435390.2016.1235739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindhu M.R., Umadevi M. Synthesis of monodispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2013;101:184–190. doi: 10.1016/j.saa.2012.09.031. [DOI] [PubMed] [Google Scholar]
- Bogdanchikova N., Vázquez-Muñoz R., Huerta-Saquero A., Pena-Jasso A., Aguilar-Uzcanga G., Picos-Díaz P.L., Pestryakov A., Burmistrov V., Martynyuk O., Luna-Vazquez-Gomez R., Almanza H. Silver nanoparticles composition for treatment of distemper in dogs. International Journal of Nanotechnology. 2016;13(1–3):227–237. [Google Scholar]
- Burdușel A.C., Gherasim O., Grumezescu A.M., Mogoantă L., Ficai A., Andronescu E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials. 2018;8(9):681. doi: 10.3390/nano8090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M., Li J., Tang J., Chen C., Zhao Y. Gold Nanomaterials in Consumer Cosmetics Nanoproducts: Analyses. Characterization, and Dermal Safety Assessment, Small. 2016;12(39):5488–5496. doi: 10.1002/smll.201601574. [DOI] [PubMed] [Google Scholar]
- Cardoso V.S., de Carvalho Filgueiras M., Dutra Y.M., Teles R.H.G., de Araújo A.R., Primo F.L., Mafud A.C., Batista L.F., Mascarenhas Y.P., Paino I.M.M., Zucolotto V., Tedesco A.C., Silva D.A., Leite J.R.S.A., dos Santos J.R. Collagen-based silver nanoparticles: Study on cell viability, skin permeation, and swelling inhibition. Materials Science and Engineering C. 2017;74:382–388. doi: 10.1016/j.msec.2016.12.025. [DOI] [PubMed] [Google Scholar]
- Cavinato M. In: Encyclopedia of Biomedical Gerontology. Rattan S.I.S., editor. Elsevier; 2019. Cosmetics and cosmeceuticals; pp. 446–461. [Google Scholar]
- Chatterjee T., Chatterjee B.K., Majumdar D., Chakrabarti P. Antibacterial effect of silver nanoparticles and the modeling of bacterial growth kinetics using a modified Gompertz model. Biochimica et Biophysica Acta (BBA) 2015;1850(2):299–306. doi: 10.1016/j.bbagen.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Chen N., Zheng Y., Yin J., Li X., Zheng C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. Journal of Virological Methods. 2013;193(2):470–477. doi: 10.1016/j.jviromet.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Christopher D., Alee K.S., Rao D.N. Synthesis and characterization of silver nanoparticles produced by laser ablation technique in aqueous monomer solution. Trends in Chemistry. 2011;2(1):1–5. [Google Scholar]
- Dakal T.C., Kumar A., Majumdar R.S., Yadav V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Frontiers in Microbiology. 2016;7:1–17. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L., Moldovan B., Vulcu A., Olenic L., Perde-Schrepler M., Fischer-Fodor E., Florea A., Crisan M., Chiorean I., Clichici S., Filip G.A. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids and Surfaces B: Biointerfaces. 2014;122:767–777. doi: 10.1016/j.colsurfb.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Deljou A., Goudarzi S. Green extracellular synthesis of the silver nanoparticles using Thermophilic Bacillus Sp. AZ1 and its antimicrobial activity against several human pathogenetic bacteria. Iranian Journal of Biotechnology. 2016;14(2):25–32. doi: 10.15171/ijb.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Zhu H., Shen Y., Zhang W., Zhang L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS ONE. 2019;14(9) doi: 10.1371/journal.pone.0222322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos C.A., Seckler M.M., Ingle A.P., Gupta I., Galdiero S., Galdiero M., Gade A., Rai M. Silver Nanoparticles: Therapeutical Uses, Toxicity, and Safety Issues. Journal of Pharmaceutical Science. 2014;103(7):1931–1944. doi: 10.1002/jps.24001. [DOI] [PubMed] [Google Scholar]
- Draelos Z.D. Cosmeceuticals: Efficacy and Influence on Skin Tone. Dermatologic Clinics. 2014;32(2):137–143. doi: 10.1016/j.det.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Draelos Z.D. Cosmeceuticals: What’s Real, What’s Not. Dermatologic Clinics. 2019;37(1):107–115. doi: 10.1016/j.det.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Duran N., Marcato P.D., De Conti R., Alves O.L., Costa F.T.M., Brocchi M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. Journal of the Brazilian Chemical Society. 2010;21(6):949–959. [Google Scholar]
- Effiong D., Uwah T., Jumbo E., Akpabio A. Nanotechnology in Cosmetics: Basics, Current Trends and Safety Concerns—A Review. Advances in Nanoparticles. 2020;9:1–22. [Google Scholar]
- El-Faham., Elzatahry, A., Alothman, Z., Elsayed, E. Facile method for the synthesis of silver nanoparticles using 3-hydrazino-isatin derivatives in aqueous methanol and their antibacterial activity. International Journal of Nanomedicine. 2014;9(1):1174. doi: 10.2147/IJN.S58571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Rafie H.M., Hamed M.-A.-A. Antioxidant and anti-inflammatory activities of silver nanoparticles biosynthesized from aqueous leaves extracts of four Terminalia species. Advances in Natural Sciences: Nanoscience and Nanotechnology. 2014;5(3) [Google Scholar]
- Erdogan O., Abbak M., Demirbolat G.M., Birtekocak F., Aksel M., Pasa S., Cevik O. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE. 2019;14(6) doi: 10.1371/journal.pone.0216496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehaid A., Fujii R., Sato T., Taniguchi A. Silver Nanoparticles Affect the Inflammatory Response in a Lung Epithelial Cell Line. The Open Biotechnology Journal. 2020;14:113–123. [Google Scholar]
- Ferdous Z., Nemmar A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. International Journal of Molecular Science. 2020;21(7):2375. doi: 10.3390/ijms21072375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franková J., Pivodová V., Vágnerová H., Juráňová J., Ulrichová J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. Journal of Applied Biomaterials & Functional Materials. 2016;14(2):e137–e142. doi: 10.5301/jabfm.5000268. [DOI] [PubMed] [Google Scholar]
- Gajbhiye S., Sakharwade S. Silver Nanoparticles in Cosmetics. Journal of Cosmetics, Dermatological Sciences and Applications. 2016;6(1):48–53. [Google Scholar]
- Galandáková A., Franková J., Ambrožová N., Habartová K., Pivodová V., Zálešák B., Ulrichová J. Effects of silver nanoparticles on human dermal fibroblasts and epidermal keratinocytes. Human & Experimental Toxicology. 2016;35(9):946–957. doi: 10.1177/0960327115611969. [DOI] [PubMed] [Google Scholar]
- García-Contreras R., Argueta-Figueroa L., Mejia-Rubalcava C., Jiménez-Martínez R., Cuevas-Guajardo S., Sánchez-Reyna P.A., Mendieta-Zeron H. Perspectives for the use of silver nanoparticles in dental practice. International Dental Journal. 2011:297–301. doi: 10.1111/j.1875-595X.2011.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R., Merten S., Wang T.T., Kennedy P., Maitz P. In vivo analysis of dermal and systemic absorption of silver nanoparticles through healthy human skin. Australian Journal of Dermatology. 2014;55(3):185–190. doi: 10.1111/ajd.12101. [DOI] [PubMed] [Google Scholar]
- Gudikandula K., Maringanti S.C. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. Journal of Experimental Nanoscience. 2016;11(9):714–721. [Google Scholar]
- Guzman M., Dille J., Godet S. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. International Journal of Metallurgical & Materials Engineering. 2008;2(7):91–98. [Google Scholar]
- Haase A., Rott S., Mantion A., Graf P., Plendl J., Thünemann A.F., Meier W.P., Taubert A., Luch A., Reiser G. Effects of Silver Nanoparticles on Primary Mixed Neural Cell Cultures: Uptake, Oxidative Stress and Acute Calcium Responses. Toxicological Sciences. 2012;126(2):457–468. doi: 10.1093/toxsci/kfs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider A., Kang I.-K. Preparation of Silver Nanoparticles and Their Industrial and Biomedical Applications: A Comprehensive Review. Advances in Materials Science and Engineering. 2015;2015:1–16. [Google Scholar]
- Ha, T. H., Jeong, J. Y., Jung, B. H., Kim, J. K., Lim, Y.T., 2005. Cosmetic pigment composition containing gold or silver nano-particles, EP1909745A1.
- Helmlinger J., Sengstock C., Groß-Heitfeld C., Mayer C., Schildhauer T.A., Köller M., Epple M. Silver nanoparticles with different size and shape: equal cytotoxicity, but different antibacterial effects. RSC Advances. 2016;6(22):18490–18501. [Google Scholar]
- Holladay., R J., Toothpaste or tooth gel containing silver nano particles coated with silver oxide. US. 2011;20130017236:A1. [Google Scholar]
- Huang C.L., Hsiao I.L., Lin H.C., Wang C.F., Huang Y.J., Chuang C.Y. Silver nanoparticles affect on gene expression of inflammatory and neurodegenerative responses in mouse brain neural cells. Environmental Research. 2015;136:253–263. doi: 10.1016/j.envres.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Huq M.A. Green Synthesis of Silver Nanoparticles Using Pseudoduganella eburnea MAHUQ-39 and Their Antimicrobial Mechanisms Investigation against Drug Resistant Human Pathogens. International Journal of Molecular Sciences. 2020;21(4):1510. doi: 10.3390/ijms21041510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani S., Korbekandi H., Mirmohammadi S., Zolfaghari B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Research in Pharmaceutical Science. 2014;9(6):385–406. [PMC free article] [PubMed] [Google Scholar]
- Jeong J.K., Gurunathan S., Kang M.H., Han J.W., Das J., Choi Y.J. Hypoxia-mediated autophagic flux inhibits silver nanoparticle-triggered apoptosis in human lung cancer cells. Scientific Reports. 2016;6(1):1–13. doi: 10.1038/srep21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y., Lim D.W., Choi J. Assessment of Size-Dependent Antimicrobial and Cytotoxic Properties of Silver Nanoparticles. Advances in Materials Science and Engineering. 2014;2014:1–6. [Google Scholar]
- Jung J.H., Hyun C.O., Hyung S.N., Ji J.H., Sang S.K. Metal nanoparticle generation using a small ceramic heater with a local heating area. Journal of Aerosol Science. 2006;37(12):1662–1670. [Google Scholar]
- Keat C.L., Aziz A., Eid A.M., Elmarzugi N.A. Biosynthesis of nanoparticles and silver nanoparticles. Bioresources and Bioprocessing. 2015;2(1):47. [Google Scholar]
- Kermanizadeh A., Kermanizadeh A., Vranic S., Boland S., Moreau K., Baeza-Squiban A., Gaiser B.K., Andrzejczuk L.A., Stone V. An in vitro assessment of panel of engineered nanomaterials using a human renal cell line: Cytotoxicity, pro-inflammatory response, oxidative stress and genotoxicity. BMC Nephrology. 2013;14(1):96. doi: 10.1186/1471-2369-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon U.T., Rao K.V., Rao J.V.R., Aparna Y. Synthesis and characterization of silver nanoparticles by chemical reduction method. IEEE; India, Chennai: 2011. pp. 97–99. [Google Scholar]
- Koch M., Kiefer S., Cavelius C., Kraegeloh A. Use of a silver ion selective electrode to assess mechanisms responsible for biological effects of silver nanoparticles. Journal of Nanoparticle Research. 2012;14(2):646. [Google Scholar]
- Kokura S., Handa O., Takagi T., Ishikawa T., Naito Y., Yoshikawa T. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomedicine. 2010;6(4):570–574. doi: 10.1016/j.nano.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Kora A.J., Sashidhar R.B. Biogenic silver nanoparticles synthesized with rhamnogalacturonan gum: Antibacterial activity, cytotoxicity and its mode of action. Arabian Journal of Chemistry. 2018;11(3):313–323. [Google Scholar]
- Korani M., Ghazizadeh E., Korani S., Hami Z., Mohammadi-Bardbori A. Effects of silver nanoparticles on human health. European Journal of Nanomedicine. 2015;7(1):51–62. [Google Scholar]
- Kruszewski M., Grądzka I., Bartłomiejczyk T., Chwastowska J., Sommer S., Grzelak A., Zuberek M., Lankoff A., Dusinka M., Wojewódzka M. Oxidative DNA damage corresponds to the long-term survival of human cells treated with silver nanoparticles. Toxicology Letters. 2013;219(2):151–159. doi: 10.1016/j.toxlet.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Larese F.F., D’Agostin F., Crosera M., Adami G., Renzi N., Bovenzi M., Maina G. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology. 2007;255(1–2):33–37. doi: 10.1016/j.tox.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Lee D.K., Kang Y.S. Synthesis of silver nanocrystallites by a new thermal decomposition method and their characterization. Electronics and Telecommunications Research Institute (ETRI) Journal. 2004;26(3):252–256. [Google Scholar]
- Liang X., Xu Z., Grice J., Zvyagin A., Roberts M., Liu X. Penetration of nanoparticles into human skin. Current Pharmaceutical Design. 2013;19(35):6353–6366. doi: 10.2174/1381612811319350011. [DOI] [PubMed] [Google Scholar]
- Liu X., Gao P., Du J., Zhao X., Wong K.K.Y. Long-term anti-inflammatory efficacy in intestinal anastomosis in mice using silver nanoparticle-coated suture. Journal of Pediatric Surgery. 2017;52(12):2083–2087. doi: 10.1016/j.jpedsurg.2017.08.026. [DOI] [PubMed] [Google Scholar]
- Lohani A., Verma A., Joshi H., Yadav N., Karki N. Nanotechnology-Based Cosmeceuticals. ISRN Dermatology. 2014:1–14. doi: 10.1155/2014/843687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Castanon G.A., Nino-Martinez N., Martinez-Guetierrez F., Martinez-Mendoza J.R., Ruiz F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. Journal of Nanoparticle Research. 2008;10:1343–1348. [Google Scholar]
- Masum M.I., Siddiqa M., Ali K.A., Zhang Y., Abdallah Y., Ibrahim E., Qiu W., Yan C., Li B. Biogenic synthesis of silver nanoparticles using phyllanthus emblica fruit extract and its inhibitory action against the pathogen acidovorax oryzaestrain RS-2 of rice bacterial brown stripe. Frontiers in Microbiology. 2019;10:820. doi: 10.3389/fmicb.2019.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N., Zhang Y., Chen Y., Guo X., Ding W., Ali S.F., Biris A.S., Rice P., Moore M.a., M., Chen, T. Silver nanoparticle-induced mutations and oxidative stress in mouse lymphoma cells. Environmental and Molecular Mutagenesis. 2012;53(6):409–419. doi: 10.1002/em.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafisi S., Maibach H.I. In: Cosmetic Science and Technology. Sakamoto K., Lochhead R.Y., Maibach H.I., Yamashita Y., editors. Elsevier; 2017. Chapter 22 - Nanotechnology in Cosmetics; pp. 337–369. [Google Scholar]
- Niknejad F., Nabili M., Daie Ghazvini R., Moazeni M. Green synthesis of silver nanoparticles: Another honor for the yeast model Saccharomyces cerevisiae. Current Medical Mycology. 2015;1(3):17–24. doi: 10.18869/acadpub.cmm.1.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osonga F.J., Akgul A., Yazgan I., Akgul A., Eshun G.B., Sakhaee L., Sadik O.A. Size and Shape-Dependent Antimicrobial Activities of Silver and Gold Nanoparticles: A Model Study as Potential Fungicides. Molecules. 2020;25:2682. doi: 10.3390/molecules25112682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S., Tak Y.K., Song J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli, Applied and Environmental Microbiology. 2007;73(6):1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S., Muthusamy P. A bio-inspired approach of formulation and evaluation of Aegle marmelos fruit extract mediated silver nanoparticle gel and comparison of its antibacterial activity with antiseptic cream’. European Journal Integrative Medicine. 2020;33 [Google Scholar]
- Pirtarighat S., Ghannadnia M., Baghshahi S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. Journal of Nanostructure in Chemistry. 2019;9(1):1–9. [Google Scholar]
- Prabhu S., Poulose E.K. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. International Nano Letters. 2012;2(1):32. [Google Scholar]
- Pulit-Prociak J., Banach M. Silver nanoparticles - a material of the future...?. Open. Chemistry. 2016;14(1):76–91. [Google Scholar]
- Qais F.A., Shafiq A., Khan R.A., Alenazi B., Ahmad I. Antibacterial Effect of Silver Nanoparticles Synthesized Using Murraya koenigii (L.) against Multidrug-Resistant Pathogens. Bioinorganic Chemistry and Applications. 2019;2019:1–11. doi: 10.1155/2019/4649506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing Y., Cheng L., Li R., Liu G., Zhang Y., Tang X., Wang J., Liu H., Qing Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. International Journal of Nanomedicine. 2018;13:3311–3327. doi: 10.2147/IJN.S165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffi M., Rumaiz A.K., Hasan M.M., Shah S.I. Studies of the growth parameters for silver nanoparticle synthesis by inert gas condensation. Journal of Materials Research. 2007;22(12):3378–3384. [Google Scholar]
- Raj S., Jose S., Sumod U.S., Sabitha M. Nanotechnology in cosmetics: Opportunities and challenges. Journal of Pharmacy and Bioallied Science. 2012;4(3):186–193. doi: 10.4103/0975-7406.99016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauwel P., Kuunal S., Ferdov S., Rauwel E. A Review on the Green Synthesis of Silver Nanoparticles and Their Morphologies Studied via TEM. Advances in Materials Science and Engineering. 2015;2015:1–9. [Google Scholar]
- Sahoo C.R., Maharana S., Mandhata C.P., Bishoyi A.K., Paidesetty S.K., Padhy R.N. Biogenic silver nanoparticle synthesis with cyanobacterium Chroococcus minutus isolated from Baliharachandi sea-mouth, Odisha, and in vitro antibacterial activity. Saudi Journal of Biological Science. 2020;27(6):1580–1586. doi: 10.1016/j.sjbs.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan C., Rajesh R., Kaviarasan T., Muthukumar K., Kavitake D., Shetty P.H. Synthesis of silver nanoparticles using bacterial exopolysaccharide and its application for degradation of azo-dyes. Biotechnology Reports. 2017;15:33–40. doi: 10.1016/j.btre.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar P., Preethi J., Vijayan R., Mohd Yusoff A.R., Ameen F., Suresh S. Anti-acne, anti-dandruff and anti-breast cancer efficacy of green synthesised silver nanoparticles using Coriandrum sativum leaf extract. Journal of Photochemsitry and Photobiology. B: Biology. 2016;163:69–76. doi: 10.1016/j.jphotobiol.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Seung H.S., Mi K.Y., Mi K.C., Dong W.L. Anti-Inflammatory Effect of Nano Silver in Chronic Rhinosinusitis Mouse Model. Biomedical Journal of Scientific and Techology Research. 2018;11(1):8287–8292. [Google Scholar]
- Siddiqi K.S., Husen A., Rao R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. Journal of Nanobiotechnology. 2018;16(1):1–28. doi: 10.1186/s12951-018-0334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J., Kvitek O., Ulbrich P., Kolska Z., Slepika P., Svorcik V. Progressive approach for metal nanoparticle synthesis. Material Letters. 2012;89:47–50. [Google Scholar]
- Singh, A. P., Arora, S., Singh, S., 2014. Formulations including silver nanoparticles and methods of using the same, WO2015057983A1.
- Singh P., Ahn S., Kang J.-P., Veronika S., Huo Y., Singh H., Chokkaligam M., Farh M.-A., Aceituno V.C., Kim Y.J., Yang D.-C. In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: a green synthetic approach. Artificial Cells, Nanomedicine, and Biotechnology. 2017:1–11. doi: 10.1080/21691401.2017.1408117. [DOI] [PubMed] [Google Scholar]
- Singh P., Kim Y.J., Singh H., Wang C., Hwang K.H., Farh M.E.A., Yang D.C. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. International Journal of Nanomedicine. 2015;10:2567–2577. doi: 10.2147/IJN.S72313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staroń A., Długosz O., Pulit-Prociak J., Banach M. Analysis of the Exposure of Organisms to the Action of Nanomaterials. Materials. 2020;13(2):349. doi: 10.3390/ma13020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoehr L.C., Gonzalez E., Stampfl A., Casals E., Duschl A., Puntes V., Oostingh G.J. Shape matters: effects of silver nanospheres and wires on human alveolar epithelial cells. Particle and Fibre Toxicology. 2011;8(1):36. doi: 10.1186/1743-8977-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriati G., Mariatti M., Azizan A. Synthesis of silver nanoparticles by chemical reduction method: effect of reducing agent and surfactant concentration. International Journal of Automotive and Mechanical Engineering. 2014;10:1920–1927. [Google Scholar]
- Szczepanowicz K., Stefanska J., Socha R.P., Warszynski P. Preparation of silver nanoparticles via chemical reduction and their antimicrobial activity. Physicochemical Problems of Mineral Processing. 2010;45:85–98. [Google Scholar]
- Tajdidzadeh M., Azmi B.Z., Yunus W.M.M., Talib Z.A., Sadrolhosseini A.R. Synthesis of silver nanoparticles dispersed in various aqueous media using laser ablation. The Scientific World Journal. 2014;2014:1–7. doi: 10.1155/2014/324921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak Y.K., Pal S., Naoghare P.K., Rangasamy S., Song J.M. Shape-dependent skin penetration of silver nanoparticles: does it really matter? Scientific Reports. 2015;5(1):1–11. doi: 10.1038/srep16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarek M., El-Aziz A.M. Synthesis and Characterization of Silver Nanoparticles Using Simple Polyol Method. Minerals, Metals and Materials Series; Springer International Publishing. 2019;Switzerland:185–194. [Google Scholar]
- Tripathi D., Modi A., Narayan G., Rai S.P. Green and cost-effective synthesis of silver nanoparticles from endangered medicinal plant Withania coagulans and their potential biomedical properties. Materials Science and Engineering C. 2019;100:152–164. doi: 10.1016/j.msec.2019.02.113. [DOI] [PubMed] [Google Scholar]
- Uttayarat P., Eamsiri J., Tangthong T., Suwanmala P. Radiolytic synthesis of colloidal silver nanoparticles for antibacterial wound dressings. Advances in Materials Science and Engineering. 2015;2015:1–6. [Google Scholar]
- Vazquez-Muñoz R., Borrego B., Juárez-Moreno K., García-García M.a., Mota Morales J.D., Bogdanchikova N., Huerta-Saquero A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicology Letters. 2017;276:11–20. doi: 10.1016/j.toxlet.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Wang M., Marepally S.K., Vemula P.K., Xu C. In: Nanoscience in Dermatology. Hamblin M.R., Prow T.W., Avci P., editors. Elsevier; 2016. Chapter 5 - Inorganic Nanoparticles for Transdermal Drug Delivery and Topical Application; pp. 57–72. [Google Scholar]
- Widdatallah M., Mohamed A., Alrasheid A., Widatallah H., Yassin L., Eltilib S., Ahmed S. Green Synthesis of Silver Nanoparticles Using Nigella sativa Seeds and Evaluation of Their Antibacterial Activity. Advances in Nanoparticles. 2020;9:41–48. [Google Scholar]
- Wildt B.E., Celedon A., Maurer E.I., Casey B.J., Nagy A.M., Hussain S.M., Goering P.L. Intracellular accumulation and dissolution of silver nanoparticles in L-929 fibroblast cells using live cell time-lapse microscopy. Nanotoxicology. 2016;10(6):710–719. doi: 10.3109/17435390.2015.1113321. [DOI] [PubMed] [Google Scholar]
- Williams K.M., Gokulan K., Cerniglia C.E., Khare S. Size and dose dependent effects of silver nanoparticle exposure on intestinal permeability in an in vitro model of the human gut epithelium. Journal of Nanobiotechnology. 2016;14(1):62. doi: 10.1186/s12951-016-0214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.K.Y., Cheung S.O.F., Huang L., Niu J., Tao C., Ho C.M., Che C.M., Tam P.K.H. Further evidence of the anti-inflammatory effects of silver nanoparticles. ChemMedChem. 2009;4(7):1129–1135. doi: 10.1002/cmdc.200900049. [DOI] [PubMed] [Google Scholar]
- Xu L.i., Wang Y.-Y., Huang J., Chen C.-Y., Wang Z.-X., Xie H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics. 2020;10(20):8996–9031. doi: 10.7150/thno.45413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Yao Q., Cao F., Liu Q., Liu B., Wang X.H. Silver nanoparticles inhibit the function of hypoxia-inducible factor-1 and target genes: Insight into the cytotoxicity and antiangiogenesis. International Journal of Nanomedicine. 2016;11:6679–6692. doi: 10.2147/IJN.S109695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., He B., Liu L., Qu G., Shi J., Hu L., Jiang G. Antibacterial mechanism of silver nanoparticles. In: Pseudomonas aeruginosa: Proteomics approach. Metallomics. 2018;10(4):557–564. doi: 10.1039/c7mt00328e. [DOI] [PubMed] [Google Scholar]
- Yokota J., Kyotani S. Influence of nanoparticle size on the skin penetration, skin retention and anti-inflammatory activity of non-steroidal anti-inflammatory drugs. Journal of Chinese Medicine Association. 2018;81(6):511–519. doi: 10.1016/j.jcma.2018.01.008. [DOI] [PubMed] [Google Scholar]