Abstract
Background
The present investigation aims to determine the chemical structure and protoscolicidal effects of Elettaria cardamomum L. essential oil (ECEO) and its main compounds 1–8 cineole alone and along with albendazole (ALZ) against Echinococcus granulosus protoscoleces in vitro and ex vivo. We also decided to evaluate some cellular mechanisms such as the apoptotic activity and the permeability of plasma membrane of protoscoleces treated with ECEO and 1–8 cineole.
Methods
Hydatid cyst protoscoleces were divided into seven groups including protoscoleces treated with ECEO 50 µl/mL (T1), protoscoleces treated with ECEO 100 µl/mL (T2), protoscoleces treated with ECEO 200 µl/mL (T3), protoscoleces treated with 1–8 cineole 100 µg/mL (T4), protoscoleces treated with 1–8 cineole 200 µg/mL (T5), protoscoleces treated with 1–8 cineole 100 µg/mL + albendazole 50 µg/mL (T6), and protoscoleces treated with 1–8 cineole 200 µg/mL + albendazole ALZ-50 µg/mL (T7). The viability of protoscoleces were recorded by eosin staining examination. Moreover, the induction of apoptosis and the plasma membrane permeability of the protoscoleces treated with ECEO and 1–8 cineole were evaluated.
Results
The highest protoscolicidal effect of ECEO was observed at the dose of 200 µl/ml (T3). 1,8-Cineole alone and combined with ALZ, particularly at the dose of 200 µg/ml (T5 and T7), destroyed the 100% protoscolices after 10 min incubation. The ECEO (T1-T3) and 1–8 cineole alone (T4 and T5) and in combination with ALZ (T6 and T7) took longer to display their protoscolicidal effect ex vivo. The obtained results of relative fuorescent items exhibited that the protoscoleces incubated with ECEO and 1,8-Cineole, alter the permeability of plasma membrane by Sytox Green with increasing the concentration. The findings revealed exhibited that ECEO and 1,8-Cineole increasingly and dose-dependently induced activation of caspase-3 enzyme ranging from 6.8 to 23.3%.
Conclusion
Our obtained results revealed that ECEO and its main compound, 1,8-Cineole exhibited the potent protoscolicidal in vitro and ex vivo; and if more research is done on their efficacy and toxicity in animal models and even clinical setting, it can be suggested as a protoscolicidal agent to use during hydatid cyst surgery.
Keywords: Herbal medicines, Cardamom, Apoptosis, Plasma membrane, Cystic echinococcosis
1. Introduction
Hydatidosis (hydatid cyst disease) which found around the world and considered as a serious public well-being concern, is triggered by Echinococcus granulosus (a tapeworm parasite) larval stage (Moro and Schantz, 2009, Eckert and Thompson, 2017). Dogs are considered the definitive host of the parasite and as an accidental host, humans become infected through ingestion of food, water, and vegetables contaminated with the eggs of worm (Siracusano et al., 2009). While the adult worms are not life-threatening in dogs, however, in the intermediate host the larvae form of parasite can result in serious disease through producing cysts in numerous tissues, including liver, lung, heart., etc. (Solomon et al. 2017). The beginning of the hydatidosis has no specific symptoms, but over time, depending on the site and dimeter of the cyst, clinical signs appear. In slight and inactive cysts, the use of benzimidazole family drugs is a good treatment strategy, but for therapy of big and active cysts, surgery is measured as the favored plan (Agudelo Higuita et al., 2016).
The rupture of cysts or release of hydatid fluid containing protoscoleces during surgery is the most important cause of recurrence (Stojkovic et al., 2009, Velasco-Tirado et al., 2018). To date, the use of protoscolicidals such as hypertonic salt 20%, silver nitrate and formalin with the aim of reducing the risk of recurrence of the disease has strongly recommended during hydatid cyst surgery (Ya-Min et al., 2018, Junghanss et al., 2008). However, many studies have reported that these materials do not have high safety and can result in some problems including liver cirrhosis, biliary fibrosis, and necrosis (Sharafi et al., 2017, Rajabi, 2009, Besim et al., 1998). Hence the requirement to discovery a new protoscolicidal agents with better efficiency and effectiveness and fewer side effects has always been of interest to researchers.
Historically, plants and plant derived compounds can help treat or prevent many diseases (Alnomasy et al., 2021). In recent years, many beneficial drugs have been industrialized from main constituents originally derived from medicinal herbs (Cheraghipour et al., 2021, Sedighi et al., 2017).
Elettaria cardamomum L. (green cardamom), belonging to the Zingiberaceae family, also called “Hael” in Saudi Arabia is a flavor agent (spice) which broadly used in foods, perfumes, as well as traditional remedy (Ashokkumar et al., 2020). Green cardamom is rich in volatile oils, which mainly contain phenolic and flavonoid compounds such as catechins, gallic acid, quercetin. While starch, protein, waxes and sterols are other compounds in green cardamom extract (Ashokkumar et al., 2021). Previous studies demonstrated that cardamom essential oil contains more than 20 compounds, the most important of which are 1–8 cineole, alpha-terpinol acetate, linalyl acetate, borneol, camphor and alpha-tripineol (Ashokkumar et al., 2020, Ashokkumar et al., 2021). Recently, Alam et al., have reported that the main components of essential oils of E. cardamomum grown in India and Guatemala were α-terpinyl acetate and 1,8-Cineole, respectively (Alam et al., 2021). In the study conducted by Goudarzvand Chegin et al. (2016), the major components of E. cardamomum seeds were terpinenyl acetate (36.61%), 1,8-cineole (30.42%), linalyl acetate (5.79%) and sabinene (4.85%), respectively (Goudarzvand Chegini and Abbasipour, 2017). However, it has been proven that the yield and chemical composition of the E. cardamomum essential oil are vary depending on the variety, plant parts and extraction methods used (Ashokkumar et al., 2020).
In traditional medicine, E. cardamomum have been used for a broad spectrum of diseases and conditions such as culinary and traditional medicine applications including improving asthma, treating the oral mouth infections, gastrointestinal disorders, cardiac ailments, nausea, diarrhea, etc. (Sharma, 2011). In recent years, in addition to traditional uses, numerous therapeutic properties such as antidiabetic, antinociceptive, anti-inflammatory, anticancer, anti-obesity, cardioprotective, antiulcerogenic, and insecticidal activities of E. cardamomum have been broadly proven (Sharma, 2011, Garg et al., 2016, Saeed et al., 2014). In addition, several studies have been proven the antimicrobial effects of E. cardamomum against some pathogenic bacteria (e.g., Escherichia coli and Pseudomonas aeruginosa, Salmonella typhimurium, Klebsiella pneumoniae as gram-negative bacteria and Staphylococcus aureus and Enterococcus faecalis as gram-positive bacteria), fungi (Aspergillus terreus, Candida spp. and Saccharomyces cerevisiae), parasites (Plasmodium falciparum and Trypanosoma evansi) (Ashokkumar et al., 2020).
The present investigation aims to determine the chemical structure and protoscolicidal effects of E. cardamomum essential oil (ECEO) and its main compounds, 1–8 cineole alone and along with albendazole (ALZ) against hydatid cyst protoscoleces in vitro and ex vivo. Also, as a secondary objective of this study, we decided to evaluate some cellular mechanisms such as apoptotic activity and the plasma membrane permeability of the protoscoleces treated with ECEO and its main compounds 1–8 cineole.
2. Materials and methods
2.1. Ethics
This study was approved by the Ethical Committee of the Scientific Research, Department of Biological Sciences, Faculty of Sciences and Humanities, Shaqra University, Saudi Arabia under the number SH 31-2020.
2.2. Plant collections
Cardamom seeds of were obtained from a market in Riyadh, Saudi Arabia. The collected fruits were then recognized by a botanist and a voucher sample of the herbs fruits was archived at the herbarium of Department of Biological Science, Faculty of Science and Humanities, Shaqra University, Ad-Dawadimi, Saudi Arabia (Fig. 1).
Fig. 1.
Map of study site in Riyadh Province, Saudi Arabia.
2.3. Preparing of essential oil
Hydro-distillation method was used to extract essential oil of green cardamom by Clevenger apparatus. According to this method, 100 g powdered green cardamom and 700 ml of distilled water was put to the glass Clevenger apparatus to extract the essential oil. Extraction time of essential oil from green cardamom lasted 4 h, whereas the maximum volume of extracted essential oil was in the first hour. After extracting the essential oil, it was separated from the water surface and dewatered with sodium sulfate. Until the chemical and protoscolicidal tests, the extracted essential oil was reserved in clean containers wrapped in aluminum foil at 4 °C (Mahmoudvand et al., 2016b, Shaapan et al., 2021).
2.4. Gas chromatography–mass spectrometry (GC-MS)
To recognize the compounds in green cardamom essential oil, a Hewlett-Packard 6890 (Palo Alto, CA, USA) device was used to perform the GC analysis equipped with a HP-5MS column (30 m × 0.25 mm, film thickness 0.25 mm). To do this, 0.1 μl of essential oil was inserted into the gas chromatography apparatus and the column temperature programming (225–80 °C) for adjusted for separation. Furthermore, the percentage of constituents of essential oil was calculated. Normal C28 -C8 alkanes were injected under the same conditions to calculate the inhibition index of essential oil components. Helium gas was used at a rate of 1.1 ml/min and ionization energy of 70 electrons was used. Finally, the constituents of green cardamom essential oil were recognized in comparison with the standard mass spectra available in the Willy-Library available in the software and in comparison with the standard numbers available in the references (Adams, 2004).
2.5. Collection of protoscoleces
The sheep livers infected with hydatid cysts were collected and then transferred to the Parasitology Laboratory, Department of Biological Science, Faculty of Science and Humanities, Shaqra University, Ad-Dawadimi, Saudi Arabia (Fig. 1). Under sterile conditions, all contents (protoscoleces) of the cyst were put into Falcon tubes using a sterile syringe. If the aspirated fluid is cloudy, the presence of bacteria and white blood cells in the microscopic view, the cyst fluid was discarded. While, the fluid of the cysts that had the highest rate of live protoscoleces (minimum 90% viability) was selected for the study. The prepared protoscoleces were adjusted as 5 × 103 protoscoleces in a 0.9% NaCl solution based on the method explained elsewhere (Ezzatkhah et al., 2021).
2.6. In vitro protoscolicidal activity
Initially, 500 µl of ECEO at the doses of 50, 100, and 200 µl/ml and its components, 1,8-Cineole alone (100 and 200 µg/ml) and along with ALZ-50 µg/ml was added to the tubes contain the 500 µl of protoscoleces (5 × 103/ml) and then incubated for 5, 10, 20, 30, and 60 min. After the exposure time, the superior part of each tube was cautiously discarded and then 50 μl of 0.1% eosin stain was put to the residual with protoscoleces and mixed slowly. Next, smears were prepared from the sediment of protoscoleces on a glass slide, covered with a cover glass, and studied by a light microscope. The rate of mortality of protoscoleces was considered through calculating 300 parasites through eosin exclusion examination. Normal saline and Ag nitrate were measured as control groups (Alyousif et al., 2021, Mahmoudvand et al., 2016a).
2.7. Eosin exclusion test
This examination was according the permeability of eosin stain (Sigma-Aldrich, St. Louis, MO, USA) solution (0.1%) in the protoscoleces and also the movement of flame cell. Dead protoscoleces are seen in red; nevertheless, the protoscoleces that are live do not absorb any color and will be seen colorless and typical activity of muscular and flame cell.
2.8. Ex vivo protoscolicidal activity
In order to determine the Ex vivo protoscolicidal activity of ECEO, practically 50% of the contents of viable hydatid cysts was removed to evaluate the rate of the mortality of protoscoleces by means of eosin test. Three hydatid cysts were used for each concentration of ECEO (50, 100, and 200 µl/ml) and 1,8-Cineole alone (100 and 200 µg/ml) and combined with ALZ (50 µg/ml); whereas each concentration was separately injected into the cysts. Next, after 5–60 min incubation, some protoscoleces-containing cysts were aspirated and mixed with 0.1% eosin dye. At the end, the rate of the mortality of parasites was measured like to the in vitro assay (Niazi et al., 2019, Mahmoudvand et al., 2019).
2.9. Plasma membrane permeability
To assess the permeability of plasma membrane of protoscoleces, 200 µl of protoscoleces (5 × 103/ml) were incubated with different concentrations of ECEO (5, 10, and 20 µl/ml) and its components, 1,8-Cineole alone (10 and 20 µg/ml) based on the Sytox green stain technique. Non-treated protoscoleces and the protoscoleces treated with 2.5% of Triton X-100 (Sigma-Aldrich) were measured as the negative and positive control, respectively. The permeability of plasma membrane was carried out through a microplate reader (BMG Labtech, Germany) for 4 h (Saeed et al., 2014).
2.10. Evaluating the caspase-3 like activity
The activity of the Caspase-3 enzyme of protoscoleces treated with ECEO was determined according to the colorimetric protease (Sigma, Germany) protocols. This method was performed according to the spectrophotometric color formed by the discharge of a molecule (pNA attached to the substrate) by the enzyme caspase-3 activity. Briefly, the protoscoleces were exposed with ECEO (2.5, 5, and 10 µl/ml) and its components, 1,8-Cineole alone (2.5 and 5 µg/ml) days and were centrifuged at 650 rpm for 6 min at 4 °C, the cell residue was lysed, and centrifuged at 20,000 rpm for 10 min. Then, 5 μg of superior phase was mixed with buffer (85 μl) and caspase 3 (10 μl) (pNA-DEVD-Ac) solution and was kept for 2 h at 37 °C. Lastly, the absorption was assessed by the ELISA reader at 405 nm (Ezzatkhah et al., 2021).
2.11. Statistical analysis
To analyze the data and compare the study groups, first the normality of the data was measured using the Shapiro-Wilk test. Due to the normality of the data, one-way ANOVA and Repeated Measure Anova were used. All statistical tests were performed using SPSS software ver.22 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered as a significant level.
3. Results
3.1. GC/MS analysis
The percentage yield of essential oil extracted from E. cardamomum was 4.1 % (v/w). According to the acquired showed in GC/MS, sixteen constituents were detected, representing 97.4% of the whole essential oil (Table 1). The main constituents were 1,8-Cineole (42.6%), α-pinene (24.1%), and α-Terpinyl acetate (19.3%), respectively.
Table 1.
GC/MS analysis of chemical compositions of E. cardamomum essential oil.
| No | Compound | RIa | Composition (%) |
|---|---|---|---|
| 1. | α-Pinene | 950 | 24.1 |
| 2. | Sabinene | 985 | 6.25 |
| 3. | α-Thujene | 1022 | 0.65 |
| 4. | β-Myrcene 1,6-octadiene, 7 | 1045 | 0.4 |
| 5. | 1,8-Cineole | 1066 | 42.6 |
| 6. | Camphene | 1112 | 0.74 |
| 7. | γ-Terpinen | 1180 | 1.1 |
| 8. | 4-Terpinen-4-ol | 1196 | 0.81 |
| 9. | Geraniol | 1207 | 0.65 |
| 10. | 1-Phellandrene | 1210 | 0.7 |
| 11. | Trans-sabinene hydrate | 1232 | 0.4 |
| 12. | α-Terpinyl acetate | 1244 | 19.3 |
| 13. | Linalool | 1265 | 0.9 |
| 14. | α-Terpineol | 1465 | 1.8 |
| 15. | Linalyl acetate | 1523 | 1.3 |
| 16. | E-citral 2, 6-octadienal, 3,7-dim | 1725 | 0.7 |
| Total | 97.4 | ||
Retention index (relative retention times).
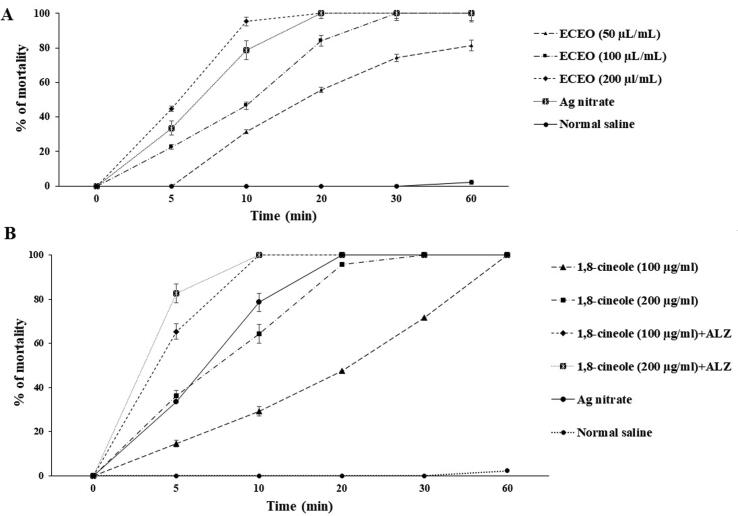
3.2. In vitro protoscolicidal effects
The in vitro protoscolicidal effect of different doses of ECEO on protoscoleces of hydatid cyst in different times is shown in Fig. 2A. Based on the in vitro findings, ECEO at the dose of 50 µl/ml showed the activity with killing 81.3% of protoscoleces after 60 min incubation. While, the ECEO at the concentrations 100 and 200 µl/ml killed 100% of protoscoleces after 30 and 20 min, respectively (p < 0.05). Fig. 2B showed that in vitro protoscolividal effects of various concentrations of 1,8-Cineole alone and in combination with ALZ (50 µg/ml) against protoscoleces. The findings confirmed that 1,8-Cineole at the dose of 100 and 200 µg/ml completely destroyed protoscoleces after 60 and 30 min incubation; whereas 1,8-Cineole (100 and 200 µg/ml) in combined with ALZ significantly (p < 0.05) reduced the viability of protoscoleces by 100% after 10 min incubation. The mortality rate of protoscoleces in the negative and positive control group was 2.3% and 100% after 60 and 20 min of exposure, respectively.
Fig. 2.
In vitro protoscolicidal activity of (A) various concentration of E. cardamomum essential oil (ECEO) and its main components, (B) 1,8-Cineole alone and in combined with albendazole (ALZ, 50 µg/ml) against hydatid cyst protoscoleces following various exposure times. Data are expressed as mean ± SD. (n = 3).
3.3. Ex vivo protoscolicidal effects
As displayed in Fig. 3A, injection of ECEO at the concentrations of 50, 100 and 200 μl/ml into hydatid cysts significantly reduced the viability of protoscoleces; but, in comparison to in vitro assay, various concentrations of ECEO took longer time to display their protoscolicidal effect. So that, ECEO at the highest concentration (200 µg/ml) killed just 100% of protoscoleces after 30 min incubation. Ex vivo, 1,8-Cineole (100 and 200 µg/ml) alone and in combined with ALZ showed slightly weaker effects than in vitro because they needed more time to affect the protoscoleces; but at the 200 µg/ml + ALZ in same incubation time similar in vitro showed the highest protoscolicidal effect after 10 min incubation (p < 0.001) (Fig. 3 B). This weaker ex vivo perotoscolicidal effect of tested drugs may be due to the presence of certain proteins, enzymes and some inhibitory compounds in the hydatid cyst fluid, which reduces their effect against hydatid cyst protoscoleces.
Fig. 3.
Ex vivo protoscolicidal activity of (A) various concentration of E. cardamomum essential oil (ECEO) and its main components, (B) 1,8-Cineole alone and in combined with albendazole (ALZ, 50 µg/ml) against hydatid cyst protoscoleces following various exposure times. Data are expressed as mean ± SD. (n = 3).
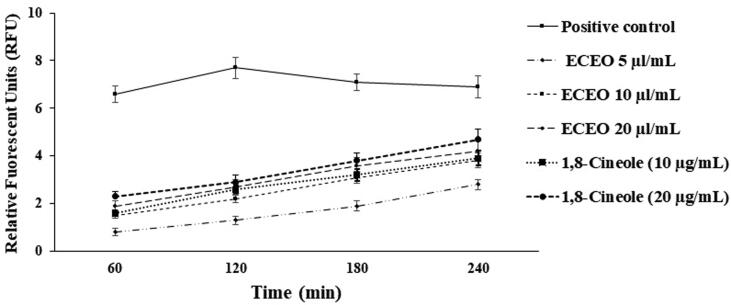
3.4. The plasma membrane permeability
The obtained results of relative fuorescent units exhibited that the protoscoleces treated with ECEO and 1,8-Cineole, alter the permeability of plasma membrane by Sytox Green as a dose dependent manner (Fig. 4); while in positive control permeabilized protoscoleces through increasing in the detected fuorescence.
Fig. 4.
The plasma membrane permeability of the protoscoleces treated with various concentration of E. cardamomum essential oil (ECEO) and its main components, 1,8-Cineole. Data are expressed as mean ± SD. (n = 3).
3.5. Evaluating the caspase-3 like activity
The results represented that the ECEO at the concentrations of 2.5, 5, and 10 μl/ml (p < 0.001) induced caspase-3 activation by 6.8%, 14.3%, and 23.3%, respectively; on the other hand, its main components 1,8-Cineole at the concentrations of 2.5 and 5 μg/ml (p < 0.001) induced caspase-3 activation by 12.6%, and 18.6%, respectively (Fig. 5).
Fig. 5.
Evaluation of the induction of apoptosis in hydatid cyst protoscoleces by assess Caspase-3 activity of protoscoleces treated with various concentration of E. cardamomum essential oil and its main components, 1,8-Cineole. Data show as mean ± SD from three experiments in duplicate. * p < 0.05 difference was significant compared with control group.
4. Discussion
Historically, plants and plant derived compounds can help treat or prevent many diseases (Alnomasy et al., 2021). In recent years, many beneficial drugs have been industrialized from main constituents originally derived from medicinal herbs (Cheraghipour et al., 2021, Sedighi et al., 2017). The present investigation aims to determine the chemical structure and protoscolicidal effects of ECEO and its main compounds 1–8 cineole alone and along with ALZ against hydatid cyst protoscoleces in vitro and ex vivo. Also, as a secondary objective of this study, we decided to evaluate some cellular mechanisms such as apoptotic activity and the plasma membrane permeability of the protoscoleces treated with ECEO and its main compounds 1–8 cineole.
By GC/MS, the main constituents were 1,8-Cineole (42.6%), α-pinene (24.1%), and α-Terpinyl acetate (19.3%), respectively. According to the previous studies, the chemical composition of essential oils is partly different depending on a number of reasons such as the place where the plant grew place, the part of the herbs that is used, time of harvesting the herbs, and the technique of isolating the essential oil from the herbs (Dhifi et al., 2016, Saedi Dezaki et al., 2016). Considering the chemical constituents of E. cardamomum, Alam et al. (2021) have demonstrated that in the essential oil of E. cardamomum fruits purchased in Saudi Arabia, the main components were α-Terpinyl acetate (19.3%) and 1,8-Cineole (42.6%) (Alam et al., 2021). Goudarzvand Chegini et al. (2016) have described that the key constituents of E. cardamomum essential oil in GC/MS analysis were terpinenyl acetate (36.61%), 1,8-cineole (30.42%), and linalyl acetate (5.79%), respectively (Goudarzvand Chegini and Abbasipour, 2017). Masoumi-Ardakani et al (2016) have revealed the obtained results of GC/MS analysis exhibited that the 1,8-cineole (45.6%), α-terpinyl acetate (33.7%), and sabinene (3.8%), were the main composites ECEO (Masoumi-Ardakani et al., 2016). However, it has been proven that the yield and chemical composition of the E. cardamomum essential oil are vary depending on the variety, plant parts and extraction methods used (Ashokkumar et al., 2020).
Based on the in vitro findings, ECEO at the concentrations 100 and 200 µl/ml killed 100% of protoscoleces after 30 and 20 min, respectively (p < 0.05). 1,8-Cineole at the dose of 100 and 200 µg/ml completely destroyed protoscoleces after 60 and 30 min incubation; whereas 1,8-Cineole (100 and 200 µg/ml) in combined with ALZ significantly (p < 0.05) reduced the viability of protoscoleces by 100% after 10 min incubation. Ex vivo showed that ECEO at the highest concentration (200 µg/ml) killed just 100% of protoscoleces after 30 min incubation. 1,8-Cineole (100 and 200 µg/ml) alone and in combined with ALZ showed slightly weaker effects than in vitro because they needed more time to affect the protoscoleces; but at the 200 µg/ml + ALZ in same incubation time similar in vitro showed the highest protoscolicidal effect after 10 min incubation (p < 0.001). This weaker ex vivo perotoscolicidal effect of tested drugs may be due to the presence of certain proteins, enzymes and some inhibitory compounds in the hydatid cyst fluid, which reduces their effect against hydatid cyst protoscoleces.
Today, protoscolicidal activity a number of medicinal plant essential oils such as Ferula gummosa, Zataria multifliora, Lepidium sativum, Nigella sativa, Pelargonium roseum, Pistacia vera, Bunium persicum, etc., have been reported (Ali et al., 2020). For example, Alyousif et al. (2021) have reported that Ferula macrecolea essential oil at the concentrations of 150 and 300 µl/mL, completely killed protoscoleces were after 30 and 20 min of exposure, respectively (Alyousif et al., 2021). Mahmoudvand et al. (2020) have also reported that Curcuma zadoaria essential oil at the concentration of 300 and 150 µl/ml entirely eliminates the hydatid cyst protoscoleces after 5 and 10 min; whereas, more time is required to show a potent protoscolicidal activity ex vivo (Mahmoudvand et al., 2020). Considering the antiparasitic effects of E. cardamom, according to our knowledge, there is few studies; Farrag et al. (2021) have demonstrated that E. cardamomum aqueous extracts at the doses of 1000, 2000, and 2500 µg/ml considerably decreased the parasite load, infectivity rate and motility of Trypanosoma evansi in vitro; whereas this plant significantly reduced the parasitemia load and improving the symptoms in Wistar male rats infected with T. evansi (Farrag et al., 2021). In addition, antibacterial effects of ECEO against some pathogenic bacterial strains such as Staphylococcus spp, Listeria monocytogenes, Escherichia coli, Salmonella enteric subsp. Bacillus cereus, Candida spp, and Aspergillus terreus with zones of inhibitions ranging from 5.7 to 11.6 mm (Abdullah et al., 2017, Noshad and Behbahani, 2019, Singh et al., 2008). Monoterpenes, including hydrocarbons, are well-known as one of the important class of herb secondary metabolites which broadly observed in essential oils (Kozioł et al., 2014). These phytochemicals and their derivatives are main constituents in the design strategies and invention of novel biologically active agents (Wojtunik-Kulesza et al., 2019). Previous studies have demonstrated the antimicrobial effects of monoterpene phytochemicals such as 1,8-Cineole, α-pinene, α-Terpinyl acetate., etc against numerous of bacterial, fungal, viral, and pathogens (Zielińska-Błajet and Feder-Kubis, 2020). For example, Goulart et al have demonstrated the potent antiparasitic activity of some terpens such as linalool, farnesol, nerolidol, limonene, and S-farnesylthiosalicylic acid against Plasmodium falciparum in vitro (Rodrigues Goulart et al., 2004). Considering the antimicrobial mechanisms of monoterpene phytochemicals, although the accurate antimicrobial mechanisms are not clearly understood; however, some investigations have proven that these constituents displayed antimicrobial effects through the cell membrane disruption, restricting the oxygen intake, hanging-up of virulence factors, etc. (Anand et al., 2019). Therefore, we can relate the potent protoscolicidal effect of this plant to the presence of monoterpene phytochemicals such as 1,8-Cineole, α-pinene, α-Terpinyl acetate., etc.
Currently, it has been confirmed that the rupture and/or cross plasma membrane is one of the key cellular mechanisms to inhibit the growth microbial pathogens (Karar et al., 2016); in the present study, the obtained results of relative fuorescent units exhibited that the protoscoleces treated with ECEO and 1,8-Cineole, alter the permeability of plasma membrane by Sytox Green as a dose dependent manner. Recently, Souissi et al. (2020) have reported that E. cardamomum extract disrupted the cell membrane of Porphyromonas gingivalis, as one of the main agents of periodontal infections (Souissi et al., 2020).
One of the key process that obviously links microbe’s survival to its ability to provoke controlled death is apoptosis (Elmore, 2007). Among the main mediators involved in apoptosis, Caspases and especially Caspase-3 are considered as frequently activated death protease which induce the cell death (Budihardjo et al., 1999, Porter and Jänicke, 1999). Thus, because the induction of apoptosis is well-known as one of the main promising antimicrobial mechanisms of tested drugs, we aimed to determine the Caspase-3 like activity of protoscoleces of treated with ECEO and its main components, 1,8-Cineole by means of the colorimetric protease approach. The results represented that the ECEO at the concentrations of 2.5, 5, and 10 μl/ml (p < 0.001) induced caspase-3 activation by 6.8%, 14.3%, and 23.3%, respectively; on the other hand, its main components 1,8-Cineole at the concentrations of 2.5 and 5 μg/ml (p < 0.001) induced caspase-3 activation by 12.6%, and 18.6%, respectively. In consistent with our results (Fig. 5), Almeer et al have exhibited that E. cardamomum significantly increased the mRNA expression of caspase3, 8 and 9 in Ehrlich solid tumor bearing mice (Almeer et al., 2021). Therefore, the increase of caspase-3 enzyme and the consequent increase of stimulation of apoptosis in protoscoleces can be considered as one of the main protoscoliocidal mechanisms of ECEO and its main compound, 1,8-Cineole.
5. Conclusion
Our obtained results revealed that ECEO and its main compound, 1,8-Cineole exhibited the potent protoscolicidal in vitro and ex vivo as an intraperitoneal model of administration of agents to hydatid cyst treatment; but, additional surveys are mandatory to evaluate the efficacy and safety ECEO and its main compound, 1,8-Cineole as a promising protoscolicidal agent in clinical setting. The results also demonstrated that while the promising protoscolicidal mechanisms of ECEO and its main compound, 1,8-Cineole are not apparently understood, but, the increasing of permeability of plasma membrane and the induction of apoptosis by the activation of caspase-3 enzyme might be measured as the main mechanisms.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors deeply acknowledge the Researchers Supporting Program (TUMA-Project-2021-33), Almaarefa University, Riyadh, Saudi Arabia for supporting steps of this work. Also, the authors thanks the staff members of the Biological Science Department, Faculty of Science and Humanities, Shaqra University, and the staff members of the Department of Biology, Faculty of Science, University of Tabuk, Saudi Arabia for their technical support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdullah A.A., Butt M.S., Shahid M., Huang Q. Evaluating the antimicrobial potential of green cardamom essential oil focusing on quorum sensing inhibition of Chromobacterium violaceum. J. Food Sci. Technol. 2017;54(8):2306–2315. doi: 10.1007/s13197-017-2668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R.P. Allured Publishing Corporation; Carol Stream, IL: 2004. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. [Google Scholar]
- Agudelo Higuita N.I., Brunetti E., McCloskey C. Cystic Echinococcosis. J. Clin. Microbiol. 2016;54(3):518–523. doi: 10.1128/JCM.02420-15. Epub 2015 Dec 16. PMID: 26677245; PMCID: PMC4767951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A., Rehman N.U., Ansari M.N., Palla A.H. Effects of Essential Oils of Elettaria cardamomum Grown in India and Guatemala on Gram-Negative Bacteria and Gastrointestinal Disorders. Molecules. 2021;26(9):2546. doi: 10.3390/molecules26092546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali R., Khan S., Khan M., Adnan M., Ali I., Khan T.A., Haleem S., Rooman M., Norin S., Khan S.N. A systematic review of medicinal plants used against Echinococcus granulosus. PLoS ONE. 2020;15(10):e0240456. doi: 10.1371/journal.pone.0240456. PMID: 33048959; PMCID: PMC7553295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeer R.S., Alnasser M., Aljarba N., AlBasher G.I. Effects of Green cardamom (Elettaria cardamomum Maton) and its combination with cyclophosphamide on Ehrlich solid tumors. BMC Complement. Med. Therapies. 2021;21(1):1–3. doi: 10.1186/s12906-021-03305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnomasy S., Al-Awsi G.R., Raziani Y., Albalawi A.E., Alanazi A.D., Niazi M., Mahmoudvand H. Systematic review on medicinal plants used for the treatment of Giardia infection. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyousif M.S., Al-Abodi H.R., Almohammed H., Alanazi A.D., Mahmoudvand H., Shalamzari M.H., Salimikia I. Chemical Composition, Apoptotic Activity, and Antiparasitic Effects of Ferula macrecolea Essential Oil against Echinococcus granulosus Protoscoleces. Molecules. 2021;26(4):888. doi: 10.3390/molecules26040888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U., Jacobo-Herrera N.J., Altemimi A.B., Lakhssassi N. A Comprehensive Review on Medicinal Plants as Antimi-crobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites. 2019;9:258. doi: 10.3390/metabo9110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashokkumar K., Murugan M., Dhanya M.K., Warkentin T.D. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]–A critical review. J. Ethnopharmacol. 2020;10(246) doi: 10.1016/j.jep.2019.112244. rontiers in Sustainable Food Systems. 2021 Apr 29;5:137. [DOI] [PubMed] [Google Scholar]
- Ashokkumar K., Vellaikumar S., Murugan M., Dhanya M.K., Ariharasutharsan G., Aiswarya S., Akilan M., Warkentin T.D., Karthikeyan A. Essential oil profile diversity in cardamom accessions from southern India. Front. Sustainable Food Syst. 2021;29(5):137. [Google Scholar]
- Besim H.K., Karayalcin K., Hamamci O., Güngör C., Korkmaz A. Scolicidal agents in hydatid cyst surgery. HPB Surg. 1998;10(6):347–351. doi: 10.1155/1998/78170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budihardjo I., Oliver H., Lutter M., Luo X., Wang X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999;15(1):269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Cheraghipour K., Masoori L., Ezzatpour B., Roozbehani M., Sheikhian A., Malekara V., Niazi M., Mardanshah O., Moradpour K., Mahmoudvand H. The Experimental Role of Medicinal Plants in Treatment of Toxoplasma gondii Infection: A Systematic Review. Acta Parasitologica. 2021;66(2):303–328. doi: 10.1007/s11686-020-00300-4. [DOI] [PubMed] [Google Scholar]
- Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines. 2016;3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert J., Thompson R.C. Historical Aspects of Echinococcosis. Adv Parasitol. 2017;95:1–64. doi: 10.1016/bs.apar.2016.07.003. Epub 2016 Sep 29 PMID: 28131361. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzatkhah F., Khalaf A.K., Mahmoudvand H. Copper nanoparticles: Biosynthesis, characterization, and protoscolicidal effects alone and combined with albendazole against hydatid cyst protoscoleces. Biomed. Pharmacother. 2021;1(136):111257. doi: 10.1016/j.biopha.2021.111257. [DOI] [PubMed] [Google Scholar]
- Farrag H.M., Yones D.A., Hassanin E.S., Ibraheim Z.Z., Eaehm H. Thymus vulgaris, Mentha piperita and Elettaria cardamomum against Trypanosoma evansi in vitro and in an animal model with new insights for the treatment of trypanosomosis. Ann. Parasitol. 2021;67(1):19–29. doi: 10.17420/ap6701.308. [DOI] [PubMed] [Google Scholar]
- Garg G., Sharma S., Dua A., Mahajan R. Antibacterial potential of polyphenol rich methanol extract of Cardamom (Amomum subulatum) J. Innov. Biol. 2016;3:271–275. [Google Scholar]
- Goudarzvand Chegini S., Abbasipour H. Chemical composition and insecticidal effects of the essential oil of cardamom, Elettaria cardamomum on the tomato leaf miner. Tuta absoluta. Toxin Rev. 2017;36:12–17. doi: 10.1080/15569543.2016.1250100. [DOI] [Google Scholar]
- Junghanss T., da Silva A.M., Horton J., et al. Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am. J. Trop. Med. Hyg. 2008;79(3):301–311. [PubMed] [Google Scholar]
- Karar M.E., Quiet L., Rezk A., Jaiswal R., Rehders M., Ullrich M.S., Brix K., Kuhnert N. Phenolic profile and in vitro assessment of cytotoxicity and antibacterial activity of Ziziphusspina-christi leaf extracts. Med chem. 2016;6(3):143–156. [Google Scholar]
- Kozioł A., Stryjewska A., Librowski T., Sałat K., Gaweł M., Moniczewski A., Lochynski S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Med. Chem. 2014;14:1156–1168. doi: 10.2174/1389557514666141127145820. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H., Fallahi S., Mahmoudvand H., Shakibaie M., Harandi M.F., Dezaki E.S. Efficacy of Myrtus communis L. to inactivate the hydatid cyst protoscoleces. J. Invest. Surg. 2016;29(3):137–143. doi: 10.3109/08941939.2015.1088601. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H., Tavakoli Oliaei R., Mirbadie S.R., Kheirandish F., Tavakoli Kareshk A., Ezatpour B., Mahmoudvand H. Efficacy and safety of Bunium persicum (Boiss) to inactivate protoscoleces during hydatid cyst operations. Surgical infect. 2016;17(6):713–719. doi: 10.1089/sur.2016.010. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H., Pakravanan M., Aflatoonian M.R., Khalaf A.K., Niazi M., Mirbadie S.R., Kareshk A.T., Khatami M. Efficacy and safety of Curcuma longa essential oil to inactivate hydatid cyst protoscoleces. BMC Complement. Alternative Med. 2019;19(1):1–7. doi: 10.1186/s12906-019-2527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudvand H., Pakravanan M., Kheirandish F., Jahanbakhsh S., Sepahvand M., Niazi M., Rouientan A., Aflatoonian M.R. Efficacy and Safety Curcuma zadoaria L. to Inactivate the Hydatid Cyst Protoscoleces. Curr. Clin. Pharmacol. 2020;15(1):64–71. doi: 10.2174/1574884714666190918155147. PMID: 31533603; PMCID: PMC7366002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoumi-Ardakani Y., Mandegary A., Esmaeilpour K., Najafipour H., Sharififar F., Pakravanan M., Ghazvini H. Chemical composition, anticonvulsant activity, and toxicity of essential oil and methanolic extract of Elettaria cardamomum. Planta Med. 2016;82(17):1482–1486. doi: 10.1055/s-0042-106971. 12-7. [DOI] [PubMed] [Google Scholar]
- Moro P., Schantz P.M. Echinococcosis: a review. Int. J. Infect. Dis. 2009;13(2):125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Niazi M., Saki M., Sepahvand M., Jahanbakhsh S., Khatami M., Beyranvand M. In vitro and ex vivo scolicidal effects of Olea europaea L. to inactivate the protoscolecs during hydatid cyst surgery. Ann. Med. Surgery. 2019;1(42):7–10. doi: 10.1016/j.amsu.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshad M., Behbahani B.A. Identification of chemical compounds, antioxidant activity, and antimicrobial effect of Elettaria cardamomum essential oil on a number of pathogenic microorganisms in vitro. Qom Univ. Medical Sci. J. 2019;13(2):57–69. [Google Scholar]
- Porter A.G., Jänicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Rajabi M.A. Fatal reactions and methaemoglobinaemia after silver nitrate irrigation of hydatid cyst. Surg Pract. 2009;13:2–7. [Google Scholar]
- Rodrigues Goulart H., Kimura E.A., Peres V.J., Couto A.S., Aquino Duarte F.A., Katzin A.M. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob. Agents Chemother. 2004;48(7):2502–2509. doi: 10.1128/AAC.48.7.2502-2509.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedi Dezaki E., Mahmoudvand H., Sharififar F., Fallahi S., Monzote L., Ezatkhah F. Chemical composition along with anti-leishmanial and cytotoxic activity of Zataria multiflora. Pharm. Biol. 2016;54(5):752–758. doi: 10.3109/13880209.2015.1079223. [DOI] [PubMed] [Google Scholar]
- Saeed A., Sultana B., Anwar F., Mushtaq M., Alkharfy K.M., Gilani A.-H. Antioxidant and antimutagenic potential of seeds and pods of green cardamom (Elettaria cardamomum) Int. J. Pharmacol. 2014;10:461–469. doi: 10.3923/ijp.2014.461.469. [DOI] [Google Scholar]
- Sedighi M., Bahmani M., Asgary S., Beyranvand F., Rafieian-Kopaei M. A review of plant-based compounds and medicinal plants effective on atherosclerosis. J Res Med Sci. 2017;15(22):30. doi: 10.4103/1735-1995.202151. PMID: 28461816; PMCID: PMC5390544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaapan R.M., Al-Abodi H.R., Alanazi A.D., Abdel-Shafy S., Rashidipour M., Shater A.F., Mahmoudvand H. Myrtus communis Essential Oil; Anti-Parasitic Effects and Induction of the Innate Immune System in Mice with Toxoplasma gondii Infection. Molecules. 2021;26(4):819. doi: 10.3390/molecules26040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharafi S.M., Sefiddashti R.R., Sanei B., Yousefi M., Darani H.Y. Scolicidal agents for protoscolices of Echinococcus granulosus hydatid cyst: review of literature. J. Res. Med. Sci.: Off. J. Isfahan Univ. Medical Sci. 2017;22 doi: 10.4103/jrms.JRMS_1030_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Sharma J., Kaur G. Therapeutic uses of Elettaria cardomum. Int. J. Drgug Res. 2011:102–108. [Google Scholar]
- Singh G., Kiran S., Marimuthu P., Isidorov V., Vinogorova V. Antioxidant and antimicrobial activities of essential oil and various oleoresins of Elettaria cardamomum (seeds and pods) J Sci Food Agric. 2008;88:280–289. doi: 10.1002/jsfa.3087. [CrossRef] [Google Scholar] [Ref list]. [DOI] [Google Scholar]
- Siracusano A., Teggi A., Ortona E. Human cystic echinococcosis: old problems and new perspectives. Interdiscip. Perspect. Infect. Dis. 2009;1:2009. doi: 10.1155/2009/474368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon N., Fields P.J., Tamarozzi F., Brunetti E., Macpherson C.N.L. Expert Reliability for the World Health Organization Standardized Ultrasound Classification of Cystic Echinococcosis. Am. J. Trop. Med. Hyg. 2017;96(3):686–691. doi: 10.4269/ajtmh.16-0659. Epub 2017 Apr 6. PMID: 28070008; PMCID: PMC5361546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souissi M., Azelmat J., Chaieb K., Grenier D. Antibacterial and anti-inflammatory activities of cardamom (Elettaria cardamomum) extracts: Potential therapeutic benefits for periodontal infections. Anaerobe. 2020;1(61):102089. doi: 10.1016/j.anaerobe.2019.102089. [DOI] [PubMed] [Google Scholar]
- Stojkovic M., Zwahlen M., Teggi A., Vutova K., Cretu C.M., Virdone R., Nicolaidou P., Cobanoglu N., Junghanss T. Treatment response of cystic echinococcosis to benzimidazoles: a systematic review. PLoS Negl Trop Dis. 2009;3(9):e524. doi: 10.1371/journal.pntd.0000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco-Tirado V., Alonso-Sardón M., Lopez-Bernus A., Romero-Alegría Á., Burguillo F.J., Muro A., Carpio-Pérez A., Bellido J.L., Pardo-Lledias J., Cordero M., Belhassen-García M. Medical treatment of cystic echinococcosis: systematic review and meta-analysis. BMC Infect. Dis. 2018;18(1):1–9. doi: 10.1186/s12879-018-3201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtunik-Kulesza K.A., Kasprzak K., Oniszczuk T., Oniszczuk A. Natural monoterpenes: Much more than only a scent. Chem. Biodivers. 2019;16:e1900434. doi: 10.1002/cbdv.201900434. [DOI] [PubMed] [Google Scholar]
- Ya-Min G., Wen-Jun Z., Shun-Yun Z., Xiu-Min H., Zheng-Guang X. [Surgical treatment strategy for complex hepatic echinococcosis: a review] Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018;30(6):705–708. doi: 10.16250/j.32.1374.2018169. PMID: 30891993. [DOI] [PubMed] [Google Scholar]
- Zielińska-Błajet M., Feder-Kubis J. Monoterpenes and their derivatives—Recent development in biological and medical applications. Int. J. Mol. Sci. 2020;21(19):7078. doi: 10.3390/ijms21197078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- Albalawi A.E., Khalaf A.K., Alyousif M.S., Alanazi A.D., Baharvand P., Shakibaie M., Mahmoudvand H. Fe3O4@ piroctone olamine magnetic nanoparticles: Synthesize and therapeutic potential in cutaneous leishmaniasis. Biomed. Pharmacother. 2021;1(139):111566. doi: 10.1016/j.biopha.2021.111566. [DOI] [PubMed] [Google Scholar]