Highlights
-
•
Using and impact of nanoparticles in agriculture.
-
•
Genotoxicity of nanoparticles in plant.
-
•
Effect of commercial amino zinc nanoparticles on cell division.
-
•
The harmful effect of commercial amino zinc nanoparticles in chromosomal structure.
Keywords: Amino zinc NPs, Mitosis, Mitotic index, Chromosomal aberrations, Root elongation, Cytogenetics, Treatments, Wheat
Abstract
Nanoparticles have a positive impact in several subjects especially in agriculture, while their safety is still being debated. Numerous commercial nano pesticide, insecticides, and fertilizers products are found in the local markets without any intensely studies on the side effect of these products on plant, human as well as environmental effects. The present study aimed to evaluate the genotoxicity of commercial amino zinc nanoparticles (AZ NPs) on Triticum aestivum L. during seeds germination and root elongation using concentration ranges (50, 100, and 150 ppm) at different exposure times (8, 16 and 24 hrs). Long term exposure to AZ NPs, exhibited only slight variation in germination rates and the elongation of roots was affected by AZ NPs treatment ranged from 97.66 to 100%. Significant reduction in the mitotic index was 35.33% after 24 hrs and 150 ppm of AZ NPs, was also observed comparing with control which was 88.0%. Genotoxicity was evaluated at a cytological level in root meristems that revealed sever variations in mitotic activity, chromosomal aberrations, and micronuclei release. Results exhibited that nano amino zinc could enter effortlessly into the cells and inhibit the normal cellular function. The decrease in the emergence of chromosomal aberrations resulting from AZ NPs exposure in a dose-dependent manner was clearly indicated that AZ NPs has induced genotoxic effect on wheat root tips.
1. Introduction
The application of nanotechnology is intensifying quickly in every single field of sciences, together with innumerable sectors of agriculture. Nanoparticles (NPs) are comprised of atomic or molecular aggregates (dimension −1 and 100 nm) (Roco, 2003, Elrys et al., 2020). The destructive nanoparticle effect has created major concern to motivate the advent of nano-toxicology as an exclusive and significant research discipline (Ghio et al., 2009, Abdelsalam et al., 2018, Abdelsalam et al., 2019a). The effect of nanoparticles, zinc oxide and alumina NPs on different plants such as Lolium perenne (ZnO NPs), maize (magnetic NPs), Spinacia oleracea (TiO2 NPs), Phaseolus vulgaris (Al NPs), Onion (Ag NPs), and T. aestivum (Cu and Ag NPs) has been studied (Abdelsalam et al., 2019a, Abdelsalam et al., 2019b, Corredor et al., 2009). Nanoparticles have the potential to alter the physiochemical mechanisms of various substances, which can produce significant biological effects on existing cells (Gaidajis and Angelakoglou, 2009, Atallah and Yassin, 2020). Due to their variable shapes and sizes, it is tough to predict the progressive or adverse effects and their modes of action in the environment and biological systems (Corredor et al., 2009, Chen et al., 2020). Generally, heavy metals exhibit a negative impact on plant biomass by constraining their growth and development by means of inhibiting their biochemical and physiological processes (bin Hussein et all., 2002, Atallah et al., 2021). Another investigation by Raskar and Laware (2014) used different concentrations of ZnO Nps (0.0, 10, 20, 30 and 40 g ml−1) in onion plants to study the effect of ZnO Nps on cell division and seed germination, their results showed a decrease in mitotic index and increase in chromosomal aberrations with the higher treatments. Also, the seed germination increased under the lower concentrations.
In addition, the germination and root growth analyses of plants exposed to nano amino zinc reveal. Also, their phytotoxicity potential. Henceforth, AZ NPs proved their capability to influence plant physiology (Mahmoudi et al., 2009). Also, the extensive use of silver nanoparticles (Ag NPs) in several viable products like antimicrobial, textiles, and detergents etc., have resulted in growing problems in the environment. The addition of micronutrients essential for plant growth in nano particle form to fertilizers has exhibited positive effects in plant development (Pandey et al., 2010). As an important micronutrient, Zinc (Zn) deficiency seriously affects yield in several crops (Taheri et al., 2016). AZ-NP incorporated fertilizers have been applied to meet Zn deficiencies and increase plant yields. To evaluate the genotoxicity of AZ NPs, different dosages of nano amino zinc (AZ NPs) and its genotoxicity influence were evaluated on germination of seeds and root tip cells of T. aestivum.
The movement of the chromosome may be caused by the early terminalization and stickiness of chromosomes due to the movement of chromosome ahead of the rest during anaphase as reviewed by Premjit (1985). Kumari et al. (2011 showed that ZnO-NPs has exerted cytotoxic and genotoxic effects, including lipid peroxidation, reducing the mitotic index, and increasing the micronuclei and chromosomal aberrations indices on root cells of Allium cepa. Manosij (Ghosh et al., 2016) evaluated the genotoxicity and biochemical effects of ZnO nanoparticles in Allium cepa, Nicotiana tabacum, and Vicia faba plants. Their results indicated that the root meristems of Allium cepa cells showed loss of membrane integrity, increased chromosome aberrations, micronucleus formation, and cell-cycle. Newly, Ma et al. (2013) studied the toxicity of ZnO nanoparticles in different plant species. There are few studies discussed this effect, only a limited number were done on plants, such as Manzo et al. (2011). There are comparable studies have revealed significant toxicity of several other nanoparticles, including metals and metal oxides (Kumari et al., 2009, Ghosh et al., 2015). Larue et al. (2011) studied the effects of TiO2NPs on Triticum aestivum, Brassica napus, and Arabidopsis thaliana. They showed that these NPs were absorbed by plants and did not affect their germination and root elongation. The authors also highlighted the need of more studies of NPs toxicity, and on NPs interaction with plants. bin Hussein et al. (2002b) studied the toxicology of Al2O3, SiO2, ZnO, and Fe3O4 on Arabidopsis thaliana and found that that ZnO nanomaterials at 400 mgL−1 capable of inhibiting germination. Sharma et al. (2009) discovered that the toxic effect by ZnO is more significant in seed germinations, root elongations, and the number of leaves, rather than other nanoparticles. Wong et al. (2010) showed that ZnO nanomaterials are one of the most toxic nanomaterials that could terminate root growth of test plants. Therefore, this study was designed to investigate the genotoxicity of amino Zn nanoparticles in wheat (Triticum aestivum L.).
2. Materials and methods
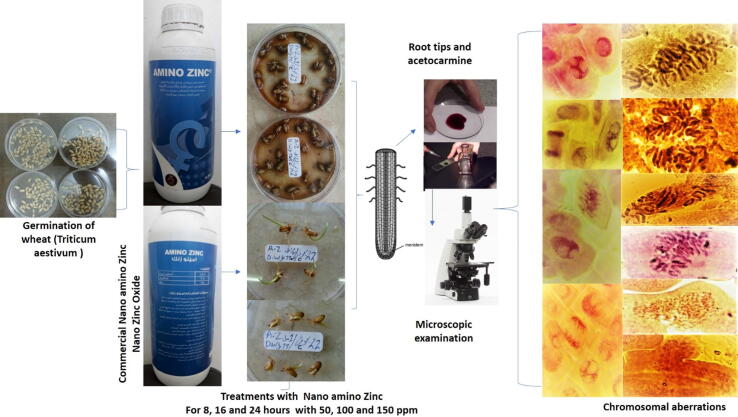
The present experiments were carried out at the Agricultural Botany Department, Faculty of Agriculture, Saba Basha, Alexandria University, Egypt and Department of plant protection, Faculty of Agriculture, Saba Basha, Alexandria, Egypt. These studies were conducted from 2015 up to 2017 to study the effect of nano amino Zn-NPs on chromosomal aberrations and cell division in wheat as designed in Fig. 1.
Fig. 1.
The experimental steps and obtained results (photos were taken by corresponding author).
2.1. Nano amino zinc
Nano amino Zn-NPs was obtained from “Bio Nano Tech” fertilizer development company, Egypt (Amino acids: 10%, Vitamins: 1%, Zinc: 6%). Nano amino Zn-NPs used in the current investigation were in colloidal form (25.6 to 79.0 nm size).
2.2. Transmission electron microscopy
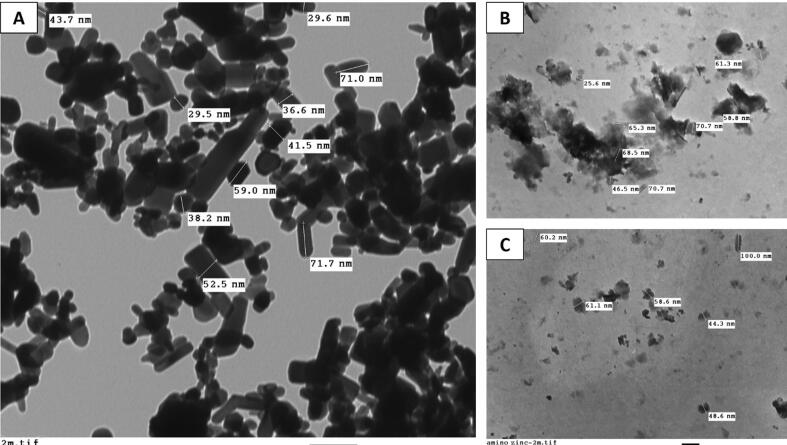
The nanoparticles morphology was analyzed with H-7500 TEM (transmission electron microscope) (Hitachi, Japan; JEOL-TEM 100 CX) with an acceleration voltage of 80 kV, by observing their TEM micrographs (Elavazhagan and Arunachalam, 2011). Nanoparticles were photographed after drying a droplet on a carbon-coated 200-mesh copper grid as found in Fig. 2.
Fig. 2.
Transmission electron microscopy (TEM) of nano amino zinc showing the nano size of current materials (100 nm), (a) 13,000×, (b & c) 7500×.
2.3. Plant materials
The root-tip of T. aestivum L. was used as a test material. “Behooth 22” [CMSS96Y03236M-050M-040M-020M-050Sy-020SY-IM-0Y] was obtained from the Ministry of Agriculture in Iraqi, Agriculture research center, Department of cereals and legumes.
2.4. Germination test and treatment
Twenty wheat grains were planted in a petri dish (diameter, 7.5 cm) at once under stander room temperature. Deionized water was added until roots germinated (after 2 days). The seeds were treated with AZ NPs at time intervals of 8, 16 and 24 hrs, at three different concentrations i.e. 50, 100, and 150 ppm were completed to the total volume of dH2O. The different concentrations of AZ NPs were added for the wheat grains till the root length reached 1.5–2 cm. Alive and dead grains were measured and recorded for calculating the percentage of germination. Also, the percentage of germination was calculated between the different dosages on the selected cultivar “Behooth 22” as found in Table 1.
2.5. Cytological studies
2.5.1. Chromosomal aberration assay and mitosis studies
Mitotic studies were conducted on root tips of primary roots from germinated seeds post treatments. The root tips were washed with dH2O and put in 95% ethanol with glacial acetic acid (3:1, v/v) for 24 h for killing and fixation. Root tips of were separated from the fixative solution and transferred in 70% ethanol then stored in a 4–5 °C until assessment (Ma and Kabir, 1992). The root tips were removed from the 70% alcohol and treated with (1 N) hydrochloric acid at 60 °C for 10 min for hydrolysis. The hydrolysed roots tip were washed with distilled water and placed in a petri dish containing acetocarmine stain for 1–1.5 h. The tips of roots were kept on a clean slide with one drop of acetocarmine and squashed with a rusted needle, then covered with cover slide. The mitotic analysis of root tip cells was performed by acetocarmine as described in many previous studies such as (Raskar and Laware, 2014, Ghosh et al., 2016, Venora et al., 2002, Abdelsalam et al., 2019c). Karyotyping system (FUJITSU, Germany) was used to detect all mitotic cell divisions. Different mitosis stages were observed and assessed for differences corresponding to different nanoparticles concentrations. Mitotic index% (MI) were observed from at least ∼1650 cells for each amino Zn nanoparticles concentration indicating the total divided cell, number of different mitosis stages (Prophase, Metaphase, Anaphase and Telophase) where;
Also, all the visible aberratiZons in the chromosomes of treated samples were recorded and named, including c-metaphase, fragment, bridge, uncoiling, stickiness, nucleotide deletion, distributed anaphase, Ring chromosome, multi-nuclei, elongation, gap chromosome, different multi polar anaphase, multi polar metaphase, erosion, distributed chromosome and lagging chromosome.
2.6. Statistical analysis
The data are displayed as the means ± standard error (SE). One-way analysis of variance (ANOVA) and Duncan's Multiple Range test was performed to analyze the data, showing significant variations among means, which were compared at p ≤ 0.05. Analysis of data was performed by COSTAT computer software (CoHort Software version 6.303, Berkeley, CA, USA).
3. Results
3.1. Cell division and mitotic index
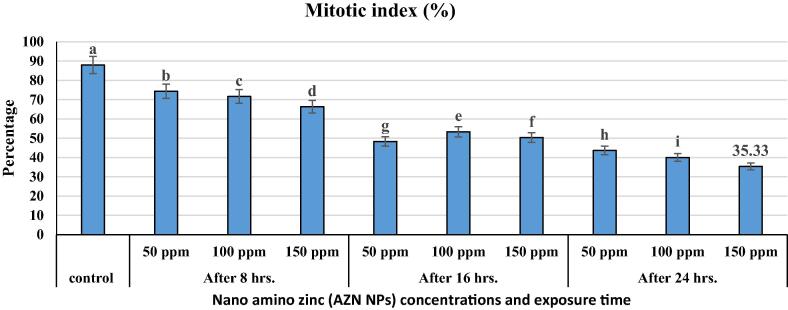
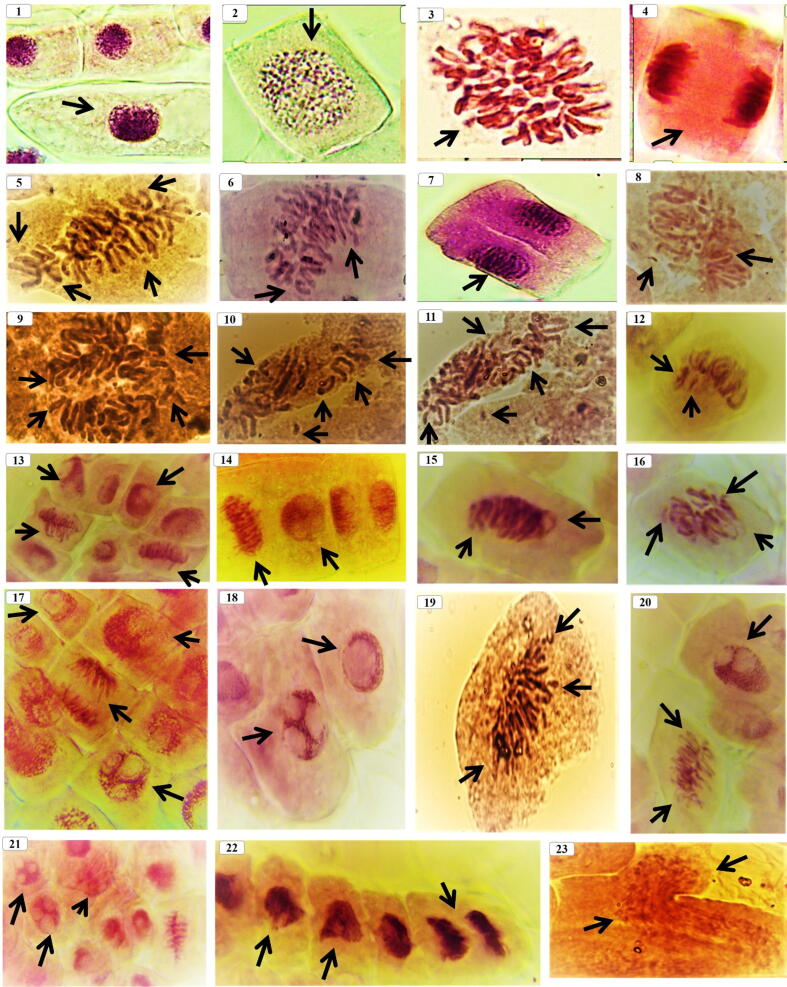
The control grains in all the tested materials displayed 100% germination. All the mitosis stages for wheat “Behoth 22” root tip, control (Fig. 3, Fig. 4, Fig. 5) which includes the normal mitosis division such as prophase, metaphase, polar view metaphase, anaphase, telophase, telophase and late telophase were observed and the cells in each stage were counted (Table 2). The results indicated that, within the total observed cells (∼1838 ± 4.04 cells), the highest divided stage was prophase by 1054 ± 0.58 cells, while, metaphase was 232.5 ± 2.04 cells, anaphase recorded 262.0 ± 1.53 cells and telophase, 75.67 ± 1.86 cells. Data showed that interphase was 208 cells and the index of mitosis was 88.0 ± 0.58%. The data showed just 1.33 ± 0.33% was abnormal cells in control and this percentage may be due to the environmental effects or to the chemical fertilization for the original wheat grains. No huge abnormal stages were observed, we detected the three types as follows: C-metaphase, fragments and stickiness (Fig. 3, Fig. 4, Fig. 5).
Fig. 3.
The decreasing of mitotic index in T. aestivum L. root tips as affected by different commercial nano amino zinc concentrations under different exposure time.
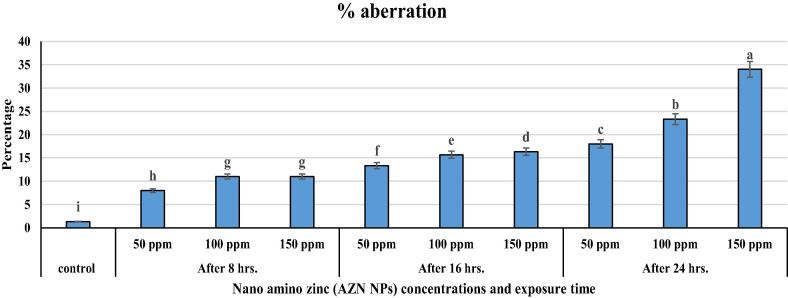
Fig. 4.
The increasing of aberration percentage in T. aestivum L. root tips as affected by different commercial nano amino zinc concentrations under different exposure time.
Fig. 5.
Different mitosis stages of Iraqi wheat “Behoth 22” i.e. (1) Interphase, (2) normal prophase, (3) Metaphase with polar view, (4) normal anaphase, (5–8) showing the effect of different Nano-amino zinc concentrations on chromosomal aberrations i.e. 50 ppm after 8 hrs (5) fragment, C-phase, ring chromosome, distributed chromosome and deletion; 100 ppm after 8 hrs (6) showing irregular metaphase, lagging chromosome, distributed chromosome, stickiness and c-phase; 150 ppm after 8 hrs (7–8) showing undistributed anaphase, erosion, gab chromosome, ring chromosome, fragment and stickiness, (9) showing the chromosomal aberrations under 50 ppm after 16 hrs, were abnormal metaphase (sticky and uncoiling); 100 ppm after 16 hrs, (10) showing irregular anaphase (fragments, lagging, ring, C-phase, distributed chromosome and sticky), 150 ppm after 16 hrs, (11–12) showing abnormal metaphase (sticky, deletion, distributed chromosome, gap chromosome and ring), from (13–18) showing chromosomal aberrations under 50 ppm after 24 hrs were abnormal metaphase and sticky ends with lagging chromosome (13–15) showing irregular metaphase and anaphase with sticky and erosion, (16–18) showing irregular anaphase with sticky, bridge, fragments, erosions, and multinuclei under 100 ppm after 24 hrs (19–23) showing the effect under 150 ppm after 24 hrs of treatments were multinuclei, fragments, erosions, irregular metaphase and anaphase with sticky and erosion and (23) showing explosion in cell wall that cussed chromatids deletion, and ghost cell.
Table 2.
The effect of different nano amino zinc concentrations on mitosis division and chromosomal aberrations under different time treatments.
| Types | Control | Concentration of AZ NPs and time of treatments (Mean + SEM) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| After 8 hrs |
After 16 hrs |
After 24 hrs |
||||||||
| 50 ppm | 100 ppm | 150 ppm | 50 ppm | 100 ppm | 150 ppm | 50 ppm | 100 ppm | 150 ppm | ||
| NOC | 1838 ± 4.4a | 1999.6 ± 6.3a | 1804 ± 3.6a | 1838.6 ± 5.2a | 1996 ± 5.3a | 1642 ± 5.1a | 1716.7 ± 6.2a | 1908.3 ± 5.9a | 2047.3 ± 6.9a | 1948.6 ± 4.2a |
| NDC | 1630 ± 3.2b | 1483.5 ± 4.6b | 1302.5 ± 4.4b | 1206.5 ± 6.5b | 953.5 ± 4.9b | 901 ± 4.8b | 895 ± 5.3b | 860 ± 3.8b | 819 ± 4.6b | 700.0 ± 5.2b |
| Prophase | 1054 ± 5.3c | 962.67 ± 4.3c | 845.67 ± 4.8c | 785.67 ± 4.2c | 618.3 ± 4.2c | 584.33 ± 4.6c | 575.0 ± 4.9c | 558.0 ± 4.2c | 532.33 ± 4.1c | 454.0 ± 3.9c |
| Metaphase | 232.5 ± 2.6e | 192.5 ± 2.9e | 169.0 ± 2.9e | 157.5 ± 3.6e | 124.5 ± 3.6f | 117.5 ± 2.4f | 117.0 ± 2.3e | 112.5 ± 2.9f | 109.5 ± 1.6e | 95.5 ± 2.3f |
| Anaphase | 262.0 ± 2.9d | 220.67 ± 3.6d | 193.67 ± 3.3d | 181.0 ± 4.2d | 141.0 ± 3.4d | 132.33 ± 2.6e | 131.67 ± 2.8d | 130 ± 2.6e | 121.67 ± 2.3e | 106 ± 2.5e |
| Telophase | 75.67 ± 1.7g | 101.67 ± 1.9g | 91.67 ± 2.2g | 82.33 ± 1.8g | 65.0 ± 1.9g | 62.0 ± 1.1g | 61.0 ± 1.4f | 60.0 ± 1.5g | 57.0 ± 1.4f | 50.0 ± 1.8g |
| M.I. | 88.0 ± 1.4f | 74.33 ± 2.2h | 71.66 ± 2.4h | 66.33 ± 3.2h | 48.3 ± 1.1h | 53.33 ± 1.3g | 50.33 ± 1.3g | 43.66 ± 1.6h | 40.0 ± 1.1g | 35.3 ± 1.1h |
| NAC | 20.33 ± 1.1h | 131.33 ± 3.2f | 156.33 ± 2.8f | 142.33 ± 3.6f | 129.6 ± 2.3e | 145 ± 2.3d | 140.66 ± 3.6d | 155 ± 3.3d | 183.66 ± 2.2d | 239.6 ± 2.7d |
| % of aberration | 1.33 ± 0.3i | 8 ± 0.44i | 11 ± 0.58i | 11 ± 0.44i | 13.3 ± 0.52i | 15.6 ± 0.4h | 16.33 ± 0.5h | 18 ± 0.51i | 23.33 ± 0.9h | 34 ± 1.5h |
| LSD | 5.16 | 4.25 | 4.27 | 5.14 | 4.87 | 10.8 | 9.96 | 6.57 | 15.2 | 4.92 |
*NOC: Number of Observed Cells (total cells); NDC: Number of Divided Cells (in different mitosis stages); M.I.: Mitotic Index; NAC: Number of Abnormal Cells (total abnormal cells); % of aberration: the percentage of aberrations in cells.
All the AZ NPs treated wheat grains displayed a high percentage of germination (96.77–100%, Table 1). The rates of germination according to the different exposure times and concentrations of AZ NPs (Table 1).
Table 1.
Germination percentage of the “Behoth 22” wheat cultivar under different nano amino zinc concentrations and different time.
| Cultivar | Concentrations (AZ NPs) |
Average | |||
|---|---|---|---|---|---|
| Time | 50 ppm | 100 ppm | 150 ppm | ||
| Behoth 22 | Control | 100 | 100 | 100 | 100% |
| 8 hrs | 100 | 100 | 100 | 100.0% | |
| 16 hrs | 100 | 98 | 98 | 98.66% | |
| 24 hrs | 100 | 97 | 96 | 97.66% | |
Within the observed cells (1996 ± 4.0–2047.33 ± 13.92 cells, data indicated that the highest number of divided cells were recorded at 50 ppm/8 hrs (1483.5 ± 3.67 cells) and the lowest number were recorded at 150 ppm/24 hrs (700.0 ± 4.08 cells) (Table 1 and Fig. 3). The general mean of divided cells was 1011.88 with a mitotic index, 35.33 ± 0.33%. Different mitosis stages were observed during the cell divisions in the normal way, on other hand, abnormal stages were also observed under different AZ NPs concentrations (see Table 2). Under the high level of AZ NPs, and 24 hrs, prophase showed (454.0 ± 1.0 cells), metaphase (95.5 ± 041 cells), anaphase (106 ± 0.58 cells) and telophase (50.0 ± 0.58 cells) compared with the control group.
3.2. Cell division and chromosomal aberrations
In the total of divided cells, many abnormal cases were observed, including c-metaphase, fragment, bridge, uncoiling chromosomes, stickiness chromosome, deletion, abnormal anaphase, ring chromosome, multi-nuclei, gap chromosome, multi polar anaphase, erosion, distributed chromosome and lagging chromosome (Table 3 and Fig. 3, Fig. 4, Fig. 5). For instance, under 8 hrs of treatment, the numbers of irregular cells varied from 131.33 ± 0.88 cells to 142.33 ± 0.88 cells, while, under 16 h it was 129.66 ± 0.88 cells to 140.66 ± 0.33 cells. However, under 24 h treatment, 155 ± 0.58 cells to 239.66 ± 0.88 cells irregular cells were observed (Table 2). The greatest number of irregular cells were found in the prominent dosage of AZ NPs under 24 h (239.66 ± 0.88 cells) with 34 ± 0.0% MI when compared with the divided cells (700.0 ± 4.08 cells). The least number of damaged cells were recorded in grains treated with 50 ppm of AZ NPs after 16 h (Table 3). With the increase in AZ-NPs doses and times of exposure, the numbers of abnormal cells observed in different stages increased (Table 3 and Fig. 2, Fig. 3, Fig. 4, Fig. 5). Higher dose of AZ NPs and longer time exposure resulted in nucleus erosion that was further degraded by lysosome. Furthermore, it also caused cell burst, releasing all the nuclear contents outside the cell (Fig. 3, Fig. 4, Fig. 5).
Table 3.
Observed of chromosomal aberrations caused by different nano amino zinc concentrations under different time treatments.
| Type of aberrations | Control | Concentration of AZ NPs and time of treatments (Mean + SEM) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| After 8 hrs |
After 16 hrs |
After 24 hrs |
||||||||
| 50 ppm | 100 ppm | 150 ppm | 50 ppm | 100 ppm | 150 ppm | 50 ppm | 100 ppm | 150 ppm | ||
| C-etaphase | 3 ± 0.1b | 10.6 ± 0.5d | 7.33 ± 0.3e | 9 ± 0.6e | 11 ± 1.1e | 14.33 ± 1.3d | 14 ± 0.9d | 14 ± 1.8d | 18.3 ± 1.6d | 26 ± 1.5c |
| Fragments | 3 ± 0.1b | 17.6 ± 0.8c | 19 ± 1.1c | 21.6 ± 1.9c | 22.6 ± 1.2b | 14 ± 1.2 d | 25 ± 2.2a | 22 ± 2.1b | 30 ± 2.6a | 48.3 ± 2.2a |
| Bridge | 0 ± 0.0 | 0 ± 0.0i | 3 ± 0.07g | 8 ± 0.4e | 8 ± 0.7f | 8 ± 0.9e | 7.67 ± 0.3e | 3 ± 0.02 j | 7 ± 0.5ij | 9.33 ± 0.7fg |
| Uncloing | 0 ± 0.0 | 21 ± 1.2b | 25.6 ± 1.3b | 20.3 ± 1.8d | 21 ± 1.5c | 20.3 ± 1.1c | 20.6 ± 1.6c | 3 ± 0.02j | 6 ± 0.2jk | 8 ± 0.6gh |
| Stickiness | 16 ± 0.8a | 22.6 ± 1.1a | 25.3 ± 1.2b | 32 ± 2.1a | 29.3 ± 1.6a | 27 ± 2.2b | 21 ± 1.1c | 20.3 ± 2.0c | 20.6 ± 1.8c | 30.3 ± 2.1b |
| ND | 0 ± 0.0c | 1 ± 0.03h | 1.67 ± 0.03h | 6 ± 0.1f | 0 ± 0.0h | 5.67 ± 0.06f | 7 ± 0.2ef | 8 ± 0.3h | 9 ± 0.5gh | 10.3 ± 0.7f |
| DA | 0 ± 0.0c | 9 ± 0.4e | 8 ± 0.5e | 9 ± 0.5e | 7.66 ± 0.5f | 8 ± 0.4 e | 7.33 ± 0.4e | 10.6 ± 1.6e | 11.3 ± 1.2e | 16.3 ± 0.9d |
| Ring | 0 ± 0.0c | 0 ± 0.0i | 2 ± 0.06h | 3 ± 0.1gh | 0 ± 0.0h | 2 ± 0.0 j | 4 ± 0.06g | 8.33 ± 0.34gh | 8 ± 0.7hi | 10.6 ± 0.8ef |
| Multinuclei | 0 ± 0.0c | 21.3 ± 0.9b | 29.3 ± 1.2a | 25.3 ± 2.2b | 18 ± 1.2d | 29 ± 2.1a | 23 ± 2.1b | 25.6 ± 2.2a | 27 ± 2.7b | 30.6 ± 1.9b |
| Elongation | 0 ± 0.0c | 10.67 ± 0.6d | 5.33 ± 0.1f | 0 ± 0.0i | 1 ± 0.0h | 2 ± 0.0j | 1 ± 0.0hi | 4.6 ± 0.06i | 6.66 ± 0.3j | 7 ± 0.5h |
| GC | 0 ± 0.0c | 0 ± 0.0i | 3.33 ± 0.05g | 0 ± 0.0i | 1 ± 0.0h | 2 ± 0.0 j | 0 ± 0.0 i | 0 ± 0.0 k | 2 ± 0.03 l | 0 ± 0.0j |
| MPA | 0 ± 0.0c | 4.66 ± 0.2f | 8 ± 0.4e | 4 ± 0.2g | 1 ± 0.0h | 0 ± 0.0 k | 0 ± 0.0i | 4 ± 0.03i | 5.33 ± 0.1 k | 5.3 ± 0.3i |
| Erosion | 0 ± 0.0c | 9 ± 0.3e | 15.6 ± 1.2d | 2 ± 0.06h | 5 ± 0.2g | 5 ± 0.07g | 2 ± 0.05h | 9.33 ± 0.6f | 10.3 ± 0.8ef | 10.6 ± 0.8ef |
| DC | 0.33 ± .01c | 0.66 ± 0.01hi | 0.33 ± 0.0i | 0 ± 0.0i | 1 ± 0.0h | 3 ± 0.01i | 0.67 ± 0.0hi | 8.33 ± 0.3gh | 11 ± 1.1e | 12 ± 0.8e |
| LG | 0 ± 0.0c | 2.33 ± 0.1g | 0 ± 0.0i | 0 ± 0.0i | 0.66 ± 0.03h | 3.66 ± 0.03h | 5.67 ± 0.09f | 9 ± 0.5fg | 9.33 ± 0.6fg | 9.6 ± 0.8f |
| LSD | 0.79 | 0.77 | 0.95 | 1.27 | 1.20 | 0.46 | 1.37 | 0.94 | 1.04 | 1.39 |
*ND: Nucleotide Deletion; DA: Distributed Anaphase; GC: Gap Chromosome; MPA: Multi Polar Anaphase; DC: Distributed Chromosome; LG: Lagging Chromosome.
4. Discussion
The growth of plants requires all nutrients and, in the absence, or deficiency of these macro or micro-nutrients constrains plant growth as well as their yields. Zn is one of the major micronutrients that has a pivotal role as catalysts and plant growth regulators Bu et al. (2014). bin Hussein et al. (2002a) reported severe Zn deficiencies in wheat irrigated fields in Iran. To overcome yield losses, fertilizers incorporated with AZ NPs were developed for commercial applications. To evaluate the safety of these AZ NPs, this study was carried out to investigate the cytotoxicity as well as genotoxicity effects in root tips of wheat. Our results were in the same line with Truta et al. (2013) who estimated the range of chromosomal aberrations prompted in Hordeum vulgare L. on the time of germination with diverse dosages (10, 100, 250, 500 µM) of Zn. The results illustrated that the enhanced effect was observed at all dosages of the Zn compounds. Also, the rate of aberrations in anaphase and telophase were 2–3 times more than the untreated and the frequency of metaphase disturbances was five to ten times higher than the control. Other studies reported that, by forming a complex with nucleic acids, Zn can negatively influence their stability, and it may result in genetic data errors Patra et al. (2004). Similarly, higher Zn2+ dosages displayed micronuclei in Vicia faba (Kumari et al., 2012). The interactions among Zn and DNA is slightly known in relation to potential connection in carcinogenesis. The genotoxicity of Zn recorded in various species of herbaceous and woody plants mainly depends on the criteria of dosages, duration of exposure, different class of Zn, and unary or binary treatment solutions (Marcato-Romain et al., 2009).
The different aberrations such as ring chromosomes was also recorded at higher concentrations of AZ NPs, and the formation of R-chromosome can be recognized to the chromosomal breakage and fragmented sticky chromosome ends found as a result toward the effect of AZ NPs. The movement of the chromosome may be caused by the early terminalization, stickiness of chromosomes and, due to the movement of chromosome ahead of the rest during anaphase as reviewed by (Premjit, 1985). Also, the results indicated high frequencies of C-metaphase, disturbed anaphase and abnormal chromosomes at anaphase which indicated partial inhibition of mitotic apparatus due to oxidative stress exerted by higher concentration of AZ NPs. The observed data recorded micronuclei under high concentration and these may have created from the lagging chromosomes at anaphase and telophase stages or as found by (Badr and AG, I. , 1987, Merwad et al., 2018) from chromosome fragments, which reported that the foundation of micronuclei is a mutagenic aspect, and may cause losing of the genetic material Raun and Lilum (1992). Kumari et al. (2012) showed that ZnO NPs exerted cytotoxic and genotoxic effects, including lipid peroxidation, reducing the mitotic index and increasing the micronuclei and chromosomal aberrations indices on root cells of Allium cepa.
However, some previous reports stated that there is no genotoxic effect even at the higher dosages of Zn treatment, which contrasted with the present research (Gómez-Arroyo et al., 2001, Marcato-Romain et al., 2009, Elrys et al., 2018). The aneugenic and clastogenic activity was noticed in a variety of spices such as wheat, black cumin, onion and crops such as sugarcane, post treatment with Zn (Shaymurat et al., 2012). But the exact mechanisms of the Zn treatment and aberration frequency wer not properly studied. In contrast, Ramesh et al. (2014) illustrated that the development and growth rate of wheat were not significantly affected post treatment with the TiO2 NPs and ZnO NPs. bin Hussein et al. (2002a) studied the toxicity of Al2O3, SiO2, ZnO, and Fe3O4 on A. thaliana and the results showed that ZnO NPs at 400mgL−1, were capable of inhibiting seed germination. Also, they suggested that the higher plants generally absorb Zn as a divalent cation (Zn2+), which triggers either the structural, functional, or metal enzymes or are regulatory cofactors of numerous enzymes. Similar to the present study, (Ghosh et al., 2016, Desoky et al., 2020, Mansour et al., 2021) proved that the ZnO nanoparticles (np; diameter, ∼85 nm) exposure modulates the cytological, genetic, and biochemical reactions like prominent chromosome breaks, micronuclei formations, loss of membrane integrity and cell cycle arrest in Allium cepa, Vicia faba and Nicotiana tabacum. Our results also contrasted with (Elrys et al., 2019) who investigated that the prominent dosages of ZnO nanoparticle decreases the onion seed germination and early seedling growth. Also, higher dosages of ZnO drops the Mitotic Index (MI) rate and displayed profound chromosomal damage in the onion seeds. The chromosome stickiness at metaphase and anaphase were very apparent post treatment with Zn. Similarly, (El-Khodary et al., 1990), found chromosome polymerization effect post treatment with ZnO NPs (GI, 1992). Generally, occurrence of chromosome bridges and breaks may lead to damage of genetic material (Salam, 1993). Likewise the above statement the clastogenic aberration ring chromosome and breaks were also observed at higher dosage of amino Zn NPs. The precocious movement of the chromosome might have been caused by stickiness of chromosomes and/or because of the movement of a chromosome ahead of the rest during anaphase (Premjit, 1985). Very high frequencies of c-metaphase, disturbed anaphase and unoriented chromosome at anaphase indicate partial inhibition of mitotic apparatus due to oxidative stress exerted by higher concentrations of amino Zn NPs. Badr and AG (1987), considered that the micronuclei formation to the treatment with a higher dosage of mutagens might have been generated from chromosomes lagging at anaphase and telophase or from chromosome fragments. Micronuclei formation is considered a true mutagenic feature, which may damage the genetic material (Raun and Lilum, 1992).
5. Conclusion
Nano amino Zn has the potency to move freely into the cells and interfere the normal functions. The treated root-tip cells showed assorted chromosomal aberrations, like disorientation at metaphase, chromosomes, alteration of metaphasic plate, spindle dysfunction, chromosomes stickiness, precocious drive at metaphase and bridge, fragmentation in chromosomes, multiple bridges, fragmentation, unequal separation, scattering, laggard and elongation, gap chromosome, multi polar anaphase, erosion, distributed chromosome and lagging chromosome during the experiment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors would like to extend their sincere appreciation to the Researchers Supporting Project (RSP-2021/123) King Saud University, Riyadh, Saudi Arabia.
Funding statement
This research was funded by Researchers Supporting Project number (RSP-2021/123) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelsalam N.R., Abdel-Megeed A., Ali H.M., Salem M.Z.M., Al-Hayali M.F.A., Elshikh M.S. Genotoxicity effects of silver nanoparticles on wheat (Triticum aestivum L.) root tip cells. Ecotoxicol. Environ. Saf. 2018;155:76–85. doi: 10.1016/j.ecoenv.2018.02.069. [DOI] [PubMed] [Google Scholar]
- Abdelsalam N.R., Fouda M.M.G., Abdel-Megeed A., Ajarem J., Allam A.A., El-Naggar M.E. Assessment of silver nanoparticles decorated starch and commercial zinc nanoparticles with respect to their genotoxicity on onion. Int. J. Biol. Macromol. 2019;133:1008–1018. doi: 10.1016/j.ijbiomac.2019.04.134. [DOI] [PubMed] [Google Scholar]
- Abdelsalam N.R., Kandil E.E., Al-Msari M.A.F., Al-Jaddadi M.A.M., Ali H.M., Salem M.Z.M., Elshikh M.S. Effect of foliar application of NPK nanoparticle fertilization on yield and genotoxicity in wheat (Triticum aestivum L.) Sci. Total Environ. 2019;653:1128–1139. doi: 10.1016/j.scitotenv.2018.11.023. [DOI] [PubMed] [Google Scholar]
- Abdelsalam N.R., Awad R.M., Ali H.M., Salem M.Z.M., Abdellatif K.F., Elshikh M.S. Morphological, pomological, and specific molecular marker resources for genetic diversity analyses in fig (ficus carica l.) Hort. Sci. 2019;54(8):1299–1309. [Google Scholar]
- Atallah O., Yassin S. Aspergillus spp. eliminate Sclerotinia sclerotiorum by imbalancing the ambient oxalic acid concentration and parasitizing its sclerotia. Environ Microbiol. 2020;22(12):5265–5279. doi: 10.1111/1462-2920.15213. [DOI] [PubMed] [Google Scholar]
- Atallah O.O., Osman A., Ali M.AS., Sitohy M. Soybean β-conglycinin and catfish cutaneous mucous p22 glycoproteins deteriorate sporangial cell walls of Pseudoperonospora cubensis and suppress cucumber downy mildew. Pest Manag. Sci. 2021;77(7):3313–3324. doi: 10.1002/ps.6375. [DOI] [PubMed] [Google Scholar]
- Badr A., AG I. Effect of herbicide glean on mitosis, chromosomes and nucleic acids in Allium cepa and Vicia faba root meristems. Cytologia. 1987;52(2):293–302. [Google Scholar]
- bin Hussein M.Z., Zainal Z., Yahaya A.H., Foo D.W.V. Microwave-assisted Aging of Organic–inorganic Hybrid Nanocomposite of α-naphthaleneacetate in the Lamella of Zn-al-layered Double Hydroxide. J. Mater. Synth. Process. 2002;10:89–95. [Google Scholar]
- bin Hussein M.Z., Zainal Z., Yahaya A.H., Foo D.W.V. Controlled release of a plant growth regulator, α-naphthaleneacetate from the lamella of Zn–Al-layered double hydroxide nanocomposite. J. Control. Release. 2002;82(2-3):417–427. doi: 10.1016/s0168-3659(02)00172-4. [DOI] [PubMed] [Google Scholar]
- Bu Q., Lv T., Shen H., Luong P., Wang J., Wang Z., Huang Z., Xiao L., Engineer C., Kim T.H., Schroeder J.I., Huq E. Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiol. 2014;164(1):424–439. doi: 10.1104/pp.113.226837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Yu G., Wang Q. Effects of climate and forest age on the ecosystem carbon exchange of afforestation. J. For. Res. 2020;31(2):365–374. [Google Scholar]
- Corredor E., Testillano P.S., Coronado M.-J., González-Melendi P., Fernández-Pacheco R., Marquina C., Ibarra M.R., de la Fuente J.M., Rubiales D., Pérez-de-Luque A., Risueño M.-C. Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification. BMC Plant Biol. 2009;9(1) doi: 10.1186/1471-2229-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoky E.-S., EL-Maghraby L.M.M., Awad A.E., Abdo A.I., Rady M.M., Semida W.M. Fennel and ammi seed extracts modulate antioxidant defence system and alleviate salinity stress in cowpea (Vigna unguiculata) Sci. Hortic. 2020;272:109576. doi: 10.1016/j.scienta.2020.109576. [DOI] [Google Scholar]
- Elavazhagan T., Arunachalam K.D. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int. J. Nanomed. 2011;6:1265–1278. doi: 10.2147/IJN.S18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khodary S., Habib A., Haliem A. Effects of the herbicide tribunil on root mitosis of Allium cepa. Cytologia. 1990;55(2):209–215. [Google Scholar]
- Elrys A.S., Abdo A.I.E., Abdel-Hamed E.M.W., Desoky E.-S. Integrative application of licorice root extract or lipoic acid with fulvic acid improves wheat production and defenses under salt stress conditions. Ecotoxicol. Environ. Saf. 2020;190:110144. doi: 10.1016/j.ecoenv.2019.110144. [DOI] [PubMed] [Google Scholar]
- Elrys A.S., Abdo A.I.E., Desoky E.-S. Potato tubers contamination with nitrate under the influence of nitrogen fertilizers and spray with molybdenum and salicylic acid. Environ. Sci. Pollut. Res. 2018;25(7):7076–7089. doi: 10.1007/s11356-017-1075-y. [DOI] [PubMed] [Google Scholar]
- Elrys A.S., Desoky E.M., Abo El-Maati M.F., Elnahal A.S., Abdo A.I., Raza S., Zhou J. Can secondary metabolites extracted from 1 Moringa seeds suppress ammonia oxidizers to increase nitrogen use efficiency and reduce nitrate contamination in potato tubers? Ecotoxicol. Environ. Saf. 2019;185 doi: 10.1016/j.ecoenv.2019.109689. [DOI] [PubMed] [Google Scholar]
- Gaidajis G., Angelakoglou K. Indoor air quality in university classrooms and relative environment in terms of mass concentrations of particulate matter. J. Environ. Sci. Health Part A. 2009;44(12):1227–1232. doi: 10.1080/10934520903139936. [DOI] [PubMed] [Google Scholar]
- Ghio A.J., Dailey L.A., Richards J.H., Jang M. Acid and organic aerosol coatings on magnetic nanoparticles increase iron concentrations in human airway epithelial cells. Inhalation Toxicol. 2009;21(8):659–667. doi: 10.1080/08958370802406282. [DOI] [PubMed] [Google Scholar]
- Ghosh M., Jana A., Sinha S., Jothiramajayam M., Nag A., Chakraborty A., Mukherjee A., Mukherjee A. Effects of ZnO nanoparticles in plants: cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat. Res./Genet. Toxicol. Environ. Mutagenesi. 2016;1(807):25–32. doi: 10.1016/j.mrgentox.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Ghosh M., Bhadra S., Adegoke A., Bandyopadhyay M., Mukherjee A. MWCNT uptake in Allium cepa root cells induces cytotoxic and genotoxic responses and results in DNA hyper-methylation. Mutat. Res./Fundam. Mol. Mech. Mutagenesis. 2015;774:49–58. doi: 10.1016/j.mrfmmm.2015.03.004. [DOI] [PubMed] [Google Scholar]
- GI B.A. Comparative study of MH and EMS in the induction of chromosomal aberrations on lateral root meristem in Clitoria ternatea L. Cytologia. 1992;57:259–264. [Google Scholar]
- Gómez-Arroyo S., Cortés-Eslava J., Bedolla-Cansino R.M., Villalobos-Pietrini R., Calderón-Segura M.E., Ramírez-Delgado Y. Sister chromatid exchanges induced by heavy metals in Vicia faba. Biol. Plant. 2001;44(4):591–594. [Google Scholar]
- Kumari M., Mukherjee A., Chandrasekaran N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009;407(19):5243–5246. doi: 10.1016/j.scitotenv.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Kumari M., Khan S.S., Pakrashi S., Mukherjee A., Chandrasekaran N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J. Hazard. Mater. 2011;190(1-3):613–621. doi: 10.1016/j.jhazmat.2011.03.095. [DOI] [PubMed] [Google Scholar]
- Kumari N., Abha A., Jha A.M. Genotoxicity testing of food additives by employing Vicia MN assay. J. Phytol. 2012;4(5):42–45. [Google Scholar]
- Larue C., Khodja H., Herlin-Boime N., Brisset F., Flank A.M., Fayard B., Chaillou S., Carrière M. Investigation of titanium dioxide nanoparticles toxicity and uptake by plants. Proc. J. Phys.: Conf. Ser. 2011;304:012057. doi: 10.1088/1742-6596/304/1/012057. [DOI] [Google Scholar]
- Ma H., Williams P.L., Diamond S.A. Ecotoxicity of manufactured ZnO nanoparticles–a review. Environ. Pollut. 2013;172:76–85. doi: 10.1016/j.envpol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Ma S., Kabir G. Interphase nuclear structure and heterochromatin in two species of Corchorus and their F1 hybrid. Cytologia. 1992;57(1):21–25. [Google Scholar]
- Mahmoudi M., Simchi A., Milani A.S., Stroeve P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J. Colloid Interface Sci. 2009;336(2):510–518. doi: 10.1016/j.jcis.2009.04.046. [DOI] [PubMed] [Google Scholar]
- Mansour E., Desoky E.-S., Ali M.M.A., Abdul-Hamid M.I., Ullah H., Attia A., Datta A. Identifying drought-tolerant genotypes of faba bean and their agro-physiological responses to different water regimes in an arid Mediterranean environment. Agric. Water Manag. 2021;247:106754. doi: 10.1016/j.agwat.2021.106754. [DOI] [Google Scholar]
- Manzo S., Rocco A., Carotenuto R., De Luca Picione F., Miglietta M.L., Rametta G., Di Francia G. Investigation of ZnO nanoparticles’ ecotoxicological effects towards different soil organisms. Environ. Sci. Pollut. Res. 2011;18(5):756–763. doi: 10.1007/s11356-010-0421-0. [DOI] [PubMed] [Google Scholar]
- Marcato-Romain C.E., Pinelli E., Pourrut B., Silvestre J., Guiresse M. Assessment of the genotoxicity of Cu and Zn in raw and anaerobically digested slurry with the Vicia faba micronucleus test. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis. 2009;672(2):113–118. doi: 10.1016/j.mrgentox.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Merwad A.-R., Desoky E.-S., Rady M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018;228:132–144. [Google Scholar]
- Pandey A.C., S. Sanjay S., S. Yadav R. Application of ZnO nanoparticles in influencing the growth rate of Cicer arietinum. J. Exp. Nanosci. 2010;5(6):488–497. [Google Scholar]
- Patra M., Bhowmik N., Bandopadhyay B., Sharma A. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ. Exp. Bot. 2004;52(3):199–223. [Google Scholar]
- Premjit K. Cytological effect of some organophosphorus pesticides II effect. Cytologia. 1985;50:199–211. [Google Scholar]
- Ramesh M., Palanisamy K., Babu K., Sharma N.K. Effects of bulk & nano-titanium dioxide and zinc oxide on physio-morphological changes in Triticum aestivum L. J. Global Biosci. 2014;3(2):415–422. [Google Scholar]
- Raskar S., Laware S. Effect of zinc oxide nanoparticles on cytology and seed germination in onion. Int. J. Curr. Microbiol. Appl. Sci. 2014;3(2):467–473. [Google Scholar]
- Raun C., Lilum J. Application of micronucleus test in Vicia faba root tips in the rapid detection of mutagenic environmental pollutants. Chin. J. Environ. Sci. 1992;4:56–58. [Google Scholar]
- Roco M.C. Nanotechnology: convergence with modern biology and medicine. Curr. Opin. Biotechnol. 2003;14(3):337–346. doi: 10.1016/s0958-1669(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Salam A. The mutagenic potentialities of three pesticides on three biological systems. Egypt. J. Genet. Cytology. 1993;22:109–128. [Google Scholar]
- Sharma V., Shukla R.K., Saxena N., Parmar D., Das M., Dhawan A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett. 2009;185(3):211–218. doi: 10.1016/j.toxlet.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Shaymurat T., Gu J., Xu C., Yang Z., Zhao Q., Liu Y., Liu Y. Phytotoxic and genotoxic effects of ZnO nanoparticles on garlic (Allium sativum L.): a morphological study. Nanotoxicology. 2012;6(3):241–248. doi: 10.3109/17435390.2011.570462. [DOI] [PubMed] [Google Scholar]
- Taheri P., Wang J., Xing H., Destino J.F., Arik M.M., Zhao C., Kang K., Blizzard B., Zhang L., Zhao P., Huang S., Yang S., Bright F.V., Cerne J., Zeng H. Growth mechanism of largescale MoS2 monolayer by sulfurization of MoO3 film. Mater. Res. Express. 2016;3(7):075009. doi: 10.1088/2053-1591/3/7/075009. [DOI] [Google Scholar]
- Truta E., Gherghel D., Bara I.C.I., Vochita G.V. Zinc-induced genotoxic effects in root meristems of barley seedlings. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2013;41(1):150–156. [Google Scholar]
- Venora G., Blangiforti S., Castiglione M.R., Pignone D., Losavio F., Cremonini R. Chromatin organisation and computer aided karyotyping of Triticum durum Desf. cv. Timilia. Caryologia. 2002;55(1):91–98. [Google Scholar]
- Wong S.W.Y., Leung P.T.Y., Djurišić A.B., Leung K.M.Y. Toxicities of nano zinc oxide to five marine organisms: influences of aggregate size and ion solubility. Anal. Bioanal. Chem. 2010;396(2):609–618. doi: 10.1007/s00216-009-3249-z. [DOI] [PubMed] [Google Scholar]