Abstract
Rainbow trout Oncorhynchus mykiss has a great nutritional value and delicious taste. A 90-days experimental trial was conducted to investigate the effect of dietary leaf extract of neem tree Azadirachta indica as a feeding supplement on the growth performance and proximate composition of O. mykiss. Four experimental diets were designed as T1 (with 5% A. indica leaf extract), T2 (with 7% of A. indica leaf extract), T3 (with 10% A. indica leaf extract), and T4 (control group feed with a regular diet with 0% A. indica leaf extract). The average initial weight of fry 0.4 ± 0.14 g was stocked at 25 fish/tank with two replicates per treatment (4 × 2 = 8). After 90 days of the experimental trial, One-way ANOVA showed significant differences in final body weight, weight gain, specific growth rate, feed conversion ratio, and survival rate among the treatment groups (p < 0.05). The highest final body weight (48.10 g) and weight gain (47.70 g) was observed in T2 with 7% A. indica leaf extract, which was significantly different from the other treatments (p < 0.05). The lowest FCR was recorded in T2 (1.90), which was significantly different compared to other treatment groups (p < 0.05). Inclusion of A. indica leaf extract in formulated feed for rainbow trout had significant effects in the hepatosomatic index, viscerosomatic index and Fulton’s condition factor (p < 0.05), but there was no significant difference in the survival rate of rainbow trout fry treated with different experimental diets (p > 0.05). The phenomenal regression indicates that 7.5% A. indica inclusion is optimum for best growth performance for rainbow trout under a controlled environment. Thus, the present study suggests that the dietary leaf extract has performed an excellent nutritional supplement by enhancing growth performance and health conditions of rainbow trout in the hatchery conditions.
Keywords: Rainbow trout, Azadirachta indica, Leaf extract, Growth performance, Proximate composition
1. Introduction
The world population is increasing at an alarming rate and a 30% increase is expected by 2050. When all the people at all the times have easy access physically and economically to safe, sufficient and healthy, good nutritionally balanced food to fulfill their requirement needs for healthy and productive life (FAO, 2009, Hassan et al., 2021a). Currently, aquaculture actually plays a crucial role in the culture of aquatic species for food and nutritional reliability. It is the fastest increase in the food production industry to reduce wild stock overexploitation and keep the ecosystem from declining. (Fao, 2014, Hassan et al., 2021c, Hussain et al., 2021). Inland aquaculture production has been increased from 29.9 to 41.9 million tons from 2012 to 2017. Rainbow trout, Oncorhynchus mykiss is one of the most suitable aquaculture species with high profit and nutritional value. This cold-water species has a high customer preference due to its taste and flesh quality (Adel and Khara, 2016). Rainbow trout is an exotic carnivore species that can be kept at 0 to 25 °C, while 13 to 18 °C is most favorable for their growth and feeding. Rainbow trout flesh contains high proteins and low carbohydrates, which is good for human health (Polakof et al., 2012).
During the early life stages, larvae and juveniles are particularly susceptible to certain diseases or infections due to various environmental stresses, producing significant economic losses for the fish hatchery. Using synthetic chemicals in fish farms or hatcheries to control some parasitic diseases and predators might cause ecological damage and threaten aquatic animal health. The inclusion of different herbal products as a dietary supplement plays a crucial role in maintaining the health status and survival of fish species. Herbs are currently being used as growth-promoting substances, antimicrobial agents, immunestimulants and nutrient sources in commercially formulated feeds for fish and shellfish species (Citarasu, 2010, AftabUddin et al., 2017). Therefore, researchers are searching for cheap and environment-friendly bioactive compounds from herbs, which have very low or non-toxic impacts on most aquaculture species. Recently, many plants, including Aloe vera, Andrographis pariculata, Annona squamosa, Neem tree Azadirachta indica, Citrus aurantifolia, Coriandrum sativum, Ocimum sanctum, Ollium cepa and Psidium guajava, and different seaweeds have been used as a natural immunostimulant for preventing several fish and shellfish diseases (Nya and Austin, 2009, Pedge and Ahirrao, 2012, AftabUddin et al., 2017, Kaur et al., 2020, AftabUddin et al., 2021, Syed et al., 2021). Among these various herbs, A. indica is the most promising medicinal and commercially exploitable plant widely available in most tropical and sub-tropical regions of the world.
A. indica is a large evergreen tree with aromatic leaves and edible fruits and has widely been used for various purposes because of its immunological, anti-inflammation, and anti-ulcer properties (Shah et al., 2009, Talpur and Ikhwanuddin, 2013, Kaur et al., 2020). Moreover, every part of A. indica tree contain a wide range of pharmacological properties against certain fungal, bacterial, and viral infections and boost antioxidant properties (Talpur and Ikhwanuddin, 2013, Adamu et al., 2018). It can be used as a natural insecticide, pesticides, and molluscicide to kill or control these aquatic parasites (El-Badawi et al., 2015). Similarly, several neem-based products have been widely used in fish farms as an alternative to some toxic pesticides or antibiotics for controlling various fish parasites and fish fry predators during their culture in lakes, rivers, or streams. Hence, using A. indica plant extracts in the fish industry has been encouraging due to their operative role in enhancing immunity against certain fish diseases and suppressing specific pathogens (FAO, 2007, Binh, 2016). However, the potential effects of A. indica leaf extract on the growth and survival of cold-water species, e.g., rainbow trout, have been less studied. Therefore, the objective of the present study was to investigate the effects of A. indica leaf extracts on the growth performance and survival of rainbow trout fry.
2. Materials and methods
2.1. Experimental trials and diet preparation
This trial was conducted in the Trout Fish Hatchery of Madyan valley of Swat District of Khyber Pakhtunkhwa province (35°14′0732″N, 72°54′9529″E) of Pakistan (Fig. 1) from August to October 2020. The current study was conducted in eight tanks. There were four treatments designated as T1 (with 5% A. indica leaf extract), T2 (with 7% A. indica leaf extract), T3 (with 10% A. indica leaf extract) and T4 (Control group without A. indica leaf extract) were fed with experimental diets for 90 days. Each treatment had two replications. Rainbow trout fry had an average initial body weight of 0.4 ± 0.14 g and an average body length of 2.2 ± 0.03 cm. These fish were acclimatized for two weeks in the rearing tank before the experiment. Two hundred individuals were randomly distributed at a stocking density of 25 fish/tank with 1000 L of water capacity and feeding rate adjusted according to fish biomass on a weekly basis. The dietary protein level was 35%, and feeding frequency was four times per day. The ingredients used for these formulated feeds include, i.e., (a) Butylated hydroxytoluene (BHT), (b) wheat flour, (c): Vitamin minerals, (d) Vitamin premix, (e) bone meal, (f) Soya bean meal, (g) dried milk, (h) Brewer yeast, (i) rice bran, (j) soya bean oil, (k) fish meal, (l) Choline chloride, (m) meat meal, (n) Vitamin C (see Table 1 and Table 2). The daily water exchange rate was approximately 30%. Continuous aeration was provided using an air blower and submerged air diffusers.
Fig. 1.
Study area of Madyan valley of Swat in Khyber Pakhtunkhwa province of Pakistan.
Table 1.
Ingredients used to prepare experimental diets for rainbow trout, O. mykiss.
| Ingredients | T1 (g/100 g) | T2 (g/100 g) | T3 (g/100 g) | Control T4 (g/100 g) |
|---|---|---|---|---|
| Butylated hydroxytoluene (BHT) | 0.1 | 0.1 | 0.1 | 0.1 |
| Wheat flour | 20.0 | 20.0 | 20.0 | 20.0 |
| Vitamin Mineral | 0.075 | 0.075 | 0.075 | 0.075 |
| Vitamin premix | 0.55 | 0.55 | 0.55 | 0.55 |
| Bone meal | 1.0 | 1.0 | 1.0 | 1.0 |
| Soya bean meal | 12.0 | 10.0 | 7.0 | 12.0 |
| Dry milk | 5.0 | 5.0 | 5.0 | 5.0 |
| Brewer yeast | 2.0 | 2.0 | 2.0 | 2.0 |
| Rice bran | 3.0 | 3.0 | 3.0 | 8.0 |
| Oil (soya bean) | 4.0 | 4.0 | 4.0 | 4.0 |
| Fish meal | 35.0 | 35.0 | 35.0 | 35.0 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 |
| Meat meal | 12.0 | 12.0 | 12.0 | 12.0 |
| Vitamin C | 0.075 | 0.075 | 0.075 | 0.075 |
| Azadirachta indica , leaf extract | 5 | 7 | 10 | 0 |
| Total | 100 g | 100 g | 100 g | 100 g |
Table 2.
Proximate composition of experimental feed for different treatments.
| Treatments | Crude protein (%) | Crude lipid (%) | Crude fiber (%) | Moisture (%) | Ash (%) |
|---|---|---|---|---|---|
| T1 | 34.90 ± 0.22 | 06.22 ± 0.32 | 08.95 ± 0.22 | 08.11 ± 0.11 | 09.60 ± 0.33 |
| T2 | 35.01 ± 0.01 | 07.66 ± 0.38 | 09.39 ± 0.04 | 08.32 ± 0.22 | 10.11 ± 0.12 |
| T3 | 35.03 ± 0.02 | 07.88 ± 0.08 | 09.48 ± 0.08 | 09.21 ± 0.02 | 10.25 ± 0.33 |
| T4 | 34.95 ± 0.05 | 06.01 ± 0.06 | 07.88 ± 0.89 | 09.38 ± 0.03 | 10.33 ± 0.24 |
2.2. Physicochemical parameters
To maintain the water pH of these tanks, sodium hydroxide (NaOH) was added to raise the pH, and Hydrochloric acid (HCl) was added to reduce pH if required. The total water hardness was maintained by adding the Calcium sulfate (CaSO4) salts to increase its hardness and magnesium sulfate (MgSO4) to decrease the hardness. The scheduled photoperiod during the experimental period was 12L: 12D. The water quality parameters were determined at twelve hours intervals during the experiment. Total dissolved oxygen (mg/L−1) was measured using a portable digital DO-meter (Model: HI 9146, Hanna, UK), the water temperature was determined with a Celsius glass thermometer, pH with a digital pen pH meter (Orion, model 201, USA), and salinity with a handheld refractometer (Hanna, UK). Total hardness (mg L−1), electric conductivity (mScm1) and total ammonia (mg L−1) were measured with a ProPlus multiparameter (YSI, USA). After acclimation, 25 healthy O. mykiss fry of the same size was randomly stocked in each experimental tank.
2.3. Calculation of growth parameters, survival rate and health indices
The growth parameters, survival rate and health indices were calculated by the following equations (Hassan et al., 2021c).
| Weight gain (WG, g) = Final weight - Initial weight |
| Specific growth rate (SGR, %/day) = [(ln FBW − In IBW)/t] × 100 |
where ln = natural logarithm, FBW = final body weight and IBW = initial body weight, t = time in days.
| Feed conversion ratio (FCR) = Feed intake/Weight gain |
| Survival rate (SR, %) = (Number of fish at the end/Initial number of fish) × 100 |
| Viscerosomatic index (VSI, %) = (Weight of viscera/Gutted weight) × 100 |
| Hepatosomatic index (HSI, %) = (Liver weight/Gutted weight) × 100 |
| Fulton’s condition factor (K) = (BW/L3) × 100 |
where BW = total body weight and L = total body length.
2.4. Proximate composition of carcass
After the experimental feeding trials, about five fish samples were collected from each treatment group and then subjected for their proximate composition analysis. Total ash contents, carbohydrate, crude fats, total fibres, crude proteins, dry matters, and moisture contents were analyzed following the methods of Aoac, 2000, Siddique et al., 2012, Khan et al., 2013.
2.5. Statistical analysis
In the present study, data were tested for normality and homogenous variance using a Shapiro-Wilk and Levene’s test, respectively. A one-way (ANOVA) using (SAS) version 9.1 were performed to determine the effects of Neem leaf extracts on the growth parameters of rainbow trout fry treated with different formulated feeds. A one-way ANOVA was also performed to compare the water quality parameters in fry-rearing tanks that were treated with different formulated feeds. Data were presented as mean ± SD. Alpha was set at p < 0.05.
3. Results
3.1. Growth performance and morphological indices
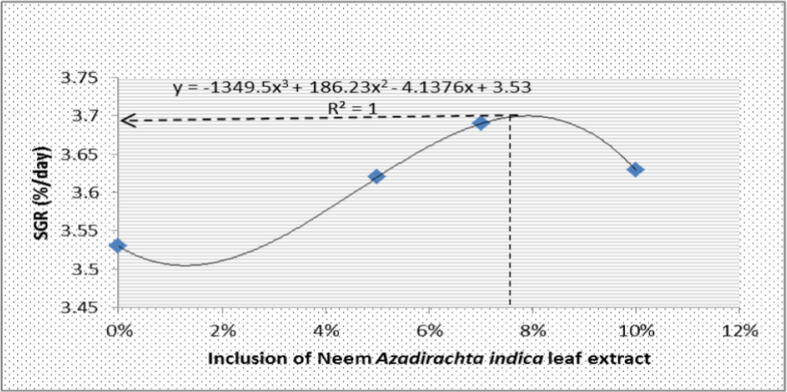
After 90 days of the experimental trial, One-way ANOVA showed significant differences in final body weight, weight gain, specific growth rate, feed conversion ratio, and survival rate among the treatment groups (p < 0.05) (Table 3). The highest final body weight (48.10 g) and weight gain (47.70 g) was observed in T2 with 7% A. indica leaf extract, which was significantly different from the other treatments (p < 0.05). The lowest FCR was recorded in T2 (1.90), which was also significantly different compared to other treatment groups (p < 0.05). In the present study, 5 to 10% inclusion of A. indica leaf extract in formulated feed showed significantly higher SGR in T1, T2 and T3, respectively (p < 0.05) compared to the control group (with no A. indica leaf extract). Inclusion of A. indica leaf extract in formulated feed for rainbow trout had significant effects in the hepatosomatic index, viscerosomatic index and Fulton’s condition factor (p < 0.05, see Table 3), but there was no significant difference in the survival rate of rainbow trout fry treated with different experimental diets (p > 0.05). On the other hand, the maximum point of SGR carve was 7.8%, while the determination coefficient (r2) = 1. The phenomenal regression indicated that the inclusion of 7.5% A. indica extract is optimum for best growth performance and survival of rainbow trout under a controlled environment (see Fig. 2).
Table 3.
Effects of Azadirachta indica on growth, feed utilization and survival indices of Oncorhynchus mykiss. Values with different superscript in the same raw indicate significant differences (p < 0.05).
| Attributes | Inclusion of neem A. indica leaf extract |
|||
|---|---|---|---|---|
| T1 (5%) | T2 (7%) | T3 (10%) | T4 (0%) | |
| Initial body weight (g) | 0.40 ± 0.01 | 0.40 ± 0.08 | 0.42 ± 0.11 | 0.41 ± 0.11 |
| Final body weight (g) | 46.10 ± 2.50b | 48.10 ± 0.50a | 47.28 ± 0.35a | 44.20 ± 3.10c |
| Weight gain (g) | 45.10 ± 0.80b | 47.70 ± 0.70a | 46.60 ± .80b | 43.80 ± 0.50c |
| Feed conversion ratio | 2.90 ± 0.0a | 1.90 ± 0.0d | 2.60 ± 0.0b | 2.10 ± 0.0c |
| Specific growth rate (%/day) | 3.62 ± 0.03 a | 3.68 ± 0.02a | 3.65 ± 0.01 a | 3.50 ± 0.02b |
| Hepatosomatic index (%) | 1.20 ± 0.15c | 1.30 ± 0.14b | 1.50 ± 0.14a | 1.10 ± 0.13c |
| Viscerosomatic index (%) | 3.40 ± 0.31b | 3.80 ± 0.21a | 3.70 ± 0.41a | 3.10 ± 0.21c |
| Fulton’s condition factor | 2.80 ± 0.0a | 2.90 ± 0.1a | 2.80 ± 0.0a | 2.20 ± 0.0b |
| Survival rate (%) | 100.00 ± 0.0 | 100.00 ± 0.0 | 98.00 ± 0.0 | 100.00 ± 0.0 |
Fig. 2.
The optimum supplementation of Neem extract in the dietary protein level of rainbow trout determined by the phenomenal regression.
3.2. Proximate composition of carcass
The proximate composition of fish samples was performed after 12 weeks of the experimental trial. Four fish specimens (whole carcass) were collected from each treatment group to determine the crude proteins, crude lipids, carbohydrates, moisture, dry matter, crude fibre and ash contents (see Table 4). The highest percentage of crude proteins was 70.18 in fishes treated with 5% A. indica leaf extract diets, which was significantly different from the other treatments (p < 0.05). Neem A. indica leaf extract containing diets had significant effects on carcass total carbohydrates, crude lipids, ash, and fibre, among other treatments (p < 0.05, see Table 4).
Table 4.
Proximate composition of rainbow trout O. mykiss (whole carcass) treated with different formulated feeds. Data are represented as mean ± standard values for all treatments. Values with different superscript in the same column indicate significant differences (p < 0.05).
| Treatments | |||||||
|---|---|---|---|---|---|---|---|
| Crude proteins (%) | Carbohydrates (%) | Total lipids (%) | Ash (%) | Total fibre (%) | Moisture (%) | Dry matter (%) | |
| T1 | 70.18 ± 2.70a | 8.83 ± 0.10c | 9.17 ± 1.00c | 11.45 ± 0.90c | 0.37 ± 0.05a | 0.35 ± 0.04c | 99.65 ± 0.08a |
| T 2 | 68.00 ± 1.70b | 9.05 ± 0.90c | 8.53 ± 1.30c | 14.21 ± 0.80b | 0.21 ± 0.01b | 0.64 ± 0.06b | 99.36 ± 0.06a |
| T 3 | 65.84 ± 1.60b | 13.63 ± 0.30a | 10.59 ± 0.90b | 9.53 ± 0.60d | 0.41 ± 0.02a | 0.17 ± 0.04d | 99.82 ± 0.04a |
| T4 (Control) | 61.9 ± 1.20c | 10.55 ± 0.08b | 11.20 ± 0.50a | 16.11 ± 0.40a | 0.24 ± 0.03b | 1.50 ± 0.34a | 99.98 ± 0.03a |
3.3. Physicochemical parameters of experimental trials
During the experimental period, physicochemical parameters of water samples in all tanks were recorded daily. The mean values of every fortnight were calculated for water temperature, pH, total hardness, ammonia, DO, electrical conductivity (Table 5). The overall physicochemical parameters, i.e., water temperature, pH, total hardness, ammonia, DO, and electric conductivity for different treatments groups (T1, T2, T3 and T4) were ranged from 14.5–15.0 °C, 6.9–7.1, 110.7–122.6, 0.4–0.8 mg L−1, 6.2–6.7 mg L−1, and 2.5–32.9 mScm−1, respectively. Among the four treatment groups, a significant difference (p < 0.05) was observed in total hardness and total ammonia concentration.
Table 5.
Physicochemical parameters of water samples collected from different treatment groups of rainbow trout, O. mykiss. Data are presented as Mean ± Standard deviation. Values with different superscript in the same raw indicate significant differences (p < 0.05).
| Parameters | T1 (5%) | T2 (7%) | T3 (10%) | Control (T4) |
|---|---|---|---|---|
| Temperature (°C) | 14.9 ± 0.3a | 15.0 ± 0.3a | 15.0 ± 0.2a | 14.5 ± 0.2a |
| pH | 7.0 ± 0.1a | 7.1 ± 0.1a | 7.1 ± 0.1a | 6.9 ± 0.1a |
| Total hardness | 110.7 ± 1.3b | 110.9 ± 1.8b | 116.0 ± 1.3ab | 122.6 ± 1.0a |
| Total ammonia (mg L−1) | 0.4 ± 0.3b | 0.5 ± 0.2b | 0.8 ± 0.3a | 0.5 ± 0.1b |
| Dissolved oxygen (mgL−1) | 6.5 ± 0.3a | 6.5 ± 0.2a | 6.7 ± 0.4a | 6.2 ± 0.1a |
| Electrical conductivity (mScm1) | 2.9 ± 0.2a | 2.7 ± 0.1a | 2.6 ± 0.1a | 2.5 ± 0.1a |
4. Discussion
The present study showed that A. indica leaf extract is beneficial for the growth of rainbow trout. The maximum weight gain was observed with a mean value of 48.1 g in T2, while the minimum weight gain was recorded at 43.80 g in control (T4). The maximum and minimum ingestion of food was observed in the T1 and T4 (control group) with average values of 90.3 ± 1.0 g and 33.99 ± 0.65 g, respectively. The feed conversion ratio (FCR) was recorded as poor in T1 (2.9), while better for T2 (1.9), respectively. Significant differences were recorded in Fulton’s condition factor (CF) throughout the experiment, which was analogous to the findings of Zeng and Naylor (1996). Zeng and Naylor (1996) reported that adding 50 mg/kg formed allicin (a component of garlic) for tilapia fish helped increase 2–3% of weight gain and survival rates after 45 days of trial. The present study revealed that the inclusion of A. indica leaf extract in formulated diets of rainbow trout positively influenced the growth of the rainbow trout, which was opposite to the statement given by Omorgerie and Okpanachi (1997), who stated that a low quantity of A. indica extracts delayed the growth of cichlid fish. The proximate composition analysis showed significantly higher percentages of proteins, crude fat, fibre, and carbohydrates due to A. indica leaf extract supplementation. The mean percentages of total crude proteins, crude lipids, carbohydrates, and ash contents were very close to Craft et al., 2016, Naeem et al., 2016. Şahin et al. (2011) observed the proximate composition of cultured brook trout (Salvelinus fontinalis) and black sea trout (Salmo trutta labrax) in comparison with their hybrid, which was also supported our present results. Kaur et al. (2020) observed the effects of dietary supplementations of A. indica extracts on the feeding efficiency, growth, survival, immunological, and reproduction properties of certain carp species.
The inclusion of A. indica extracts showed a non-toxicity impact on most non-target aquatic species (Oniovosa et al., 2017); therefore, its extract is mostly with fish feed as dietary supplements are considered safe for the culture of most fish species. Oniovosa et al. (2017) investigated that most fish diseases, mainly dragon-fly larval stages that act as fish fry predators, could be easily controlled by using the aqueous extract of A. indica leaves in the farming of African catfish, Clarias gariepinus. In addition, the haematological and biochemical analysis of Clarias gariepinus was found within normal range, and the level of total proteins, globulin, and lymphocytes increases, which indicates immune stimulation against specific pathogens. Hence, using A. indica extracts in the fish industry has been encouraging due to their operative role in enhancing immunity against certain fish diseases and suppressing particular pathogen growth. Binh (2016) has also reported the beneficial or eco-friendly impact of A. indica leaf extract on the phytoplankton community and water quality of catfish Pangasiushy pophthalmus ponds located in Binh Duong province of Vietnam. A comprehensive study has shown that all water quality parameters were found unchanged except the reduction of dissolved carbon dioxides and no change in the species composition and frequency of occurrence in whole phytoplankton communities located in these fishponds. Dinda et al., 2020, Kaur et al., 2020 observed that 1.0 g/kg of a dietary leaf extract from A. indica when incorporated in the basal diet like rice bran and mustard meal in 1:1 ratio) of Cyprinus carpio revealed the high reproductive capacity by increasing fecundity rates or ova diameter and increasing its feeding efficiency, growth performance, and immunological response against certain diseases. Thus, it increases the growth and survival rates of C. carpio.
Several studies revealed that most extracts from various parts of A. indica plants might have significant antioxidant properties that can considerably protect the animals from oxidative stress. Nowadays, A. indica has successfully used aquaculture systems to control certain fish parasites or predators. Though the Neem-based extracts are mostly considered target-specific and non-toxic to untargeted aquatic life, the aqueous extracts of the bark from A. indica might sometimes produce respiratory issues in tilapia upon its long exposure (El-Badawi et al., 2015). Even low concentrations of crude extracts from the A. indica can delay the growth performance of cichlid fishes (El-Badawi et al., 2015). It has been observed that various Neem-based pesticides, particularly ‘Achook’ were toxic for the zebrafish (Martinez, 2002, Kumar et al., 2010).
More recently, A. indica has been used for increasing aquaculture production because of its potential benefits. The successful usage of A. indica indicating aquaculture practices mostly depends upon various factors, including, i.e., parts of plant selected for treatment, methods of the preparation of plant extract as well as the procedure of its different application for the treatments of certain diseases, its dosages according to fish age or species. From all recent studies, it was proved that A. indica leaf and its extracted products are effective in varying purposes and helpful in controlling predators and parasites during fish culture systems. It has been reported that the extracts of A. indica flower can increase the total WBCs count, which can provide protection against myelosuppression or have a stimulatory impact on the activity of bone marrow (Shah et al., 2009). The leaf extract of A. indica has been reported to increase red and white blood cells and lymphocyte counts, thus enhancing the immune response and antibodies production against most pathogenic diseases. Martinez (2002) examined the impact of leaf extracts on the water quality and protozoa community during the culture of freshwater catfish. Kaur et al. (2020) observed the effects of dietary supplementations of A. indica extracts on the feeding efficiency, growth, survival, immunological, and reproduction properties of certain carp species.
A. indica could also play a vital role in controlling or preventing various fish diseases (Fao, 2014, Oniovosa et al., 2017). Obaroh and Achionye-Nzeh (2011) observed the impact of different percentages of dietary supplements of crude leaf extracts of A. indica as an antifertility agent for controlling the overproduction of tilapia Oreochromis niloticus, which leads to shut the growth during culture. It has been proved that 2.0 to 8.0 g/kg diet of leaf extracts of A. indica could be useful in controlling the overpopulation and sustainable development in tilapia fish farming (Obaroh and Achionye-Nzeh, 2011). Talpur and Ikhwanuddin (2013) also observed the impact of dietary leaf extract of A. indica on enhancing the immune response in fingerlings of Asian seabass (Lates calcarifer) against viral infections. They proved that A. indica leaf extract in diet could significantly increase the phagocytic activity of WBCs and affect the haematological and immunological parameters and survival rate of fingerlings of that species against viral infection.
5. Conclusions
Present study would be helpful to develop the artificial feed with variable composition to increase the healthy production of O. Mykiss in the aquaculture system of Pakistan. During the present research the rainbow trout were fed with four treatments groups, it was concluded that the best diet was Neem leaf plant extract 7% which was the high growth of fish. The optimal supplementation of Neem enhances growth and lowers operating costs. Therefore, further studies are necessary for effective use of Aloe vera extract with optimal dose and suitable duration
Data availability statement
All data analyzed during this study are included in this published article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/144), King Saud University, Riyadh, Saudi Arabia.
Author contributions
ZUA, HUH and ZM designed the study and executed the experimental work. NR and MIA performed chemical analysis and formulated feed. KB and AG helped in data analysis. AU and TZ helped in literature search. HUH, BAP and MAMS conceive the concept of the review. HUH wrote the article.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adamu K.M., Aliyu-Paiko M., Abdullahi F., Mustapha A.Y. Effects of Azadirachta indica leaf powder on some biochemical parameters of the African Catfish (Clarias gariepinus) Nig. J. Basic Appl. Sci. 2018;25(2):41. [Google Scholar]
- Adel A., Khara H. The effects of different dietary vitamin c and iron levels on the growth, haematological and immunological parameters of rainbow trout Oncorhynchus mykiss fingerlings. Iran. J. Fish. Sci. 2016;15(2):886–897. [Google Scholar]
- AftabUddin S., Siddique M.A.M., Habib A., Akter S., Hossen S., Tanchangya P., Abdullah Al M. Effects of seaweeds extract on growth, survival, antibacterial activities, and immune responses of Penaeus monodon against Vibrio parahaemolyticus. Italian J. Anim. Sci. 2021;20(1):243–255. [Google Scholar]
- AftabUddin S., Siddique M.A.M., Romkey S.S., Shelton W.L. Antibacterial function of herbal extracts on growth, survival and immunoprotection in the black tiger shrimp Penaeus monodon. Fish Shellfish Immunol. 2017;65:52–58. doi: 10.1016/j.fsi.2017.03.050. [DOI] [PubMed] [Google Scholar]
- Aoac . 16th ed. Arlington; Virginia, USA: 2000. Official Methods of Analysis. Association of Official Analytical Chemists. [Google Scholar]
- Binh N.T. Survey of the effects of neem leaf extract on the water quality and phytoplankton community in Freshwater Catfish Pond in Binh Duong Province. Vietnam J. Sci. Technol. 2016;54(2A):329–335. [Google Scholar]
- Citarasu T. Herbal biomedicines: a new opportunity for aquaculture industry. Aquacult. Int. 2010;18(3):403–414. [Google Scholar]
- Craft C.D., Ross C., Sealey W.M., Gaylord T.G., Barrows F.T., Fornshell G., Myrick C.A. Growth, proximate composition, and sensory characteristics of Rainbow Trout Oncorhynchus mykiss consuming alternative proteins. Aquaculture. 2016;459:223–231. [Google Scholar]
- Dinda R., Mandal A., Das S.K. Neem (Azadirachta indica A. Juss) supplemented biofloc medium as alternative feed in common carp (Cyprinus carpio var. communis Linnaeus) culture. J. Appl. Aquac. 2020;32(4):361–379. [Google Scholar]
- El-Badawi A.A., Alkhateib Y.G., Abbas H.H., Authman M.M.N. Toxic effects of Neem seeds oil on Nile Tilapia (Oreochromis niloticus) and application of different trials of control. Res. J. Pharm. Biol. Chem. Sci. 2015;6(1):645–658. [Google Scholar]
- FAO, 2009. Food Security and Agricultural Mitigation in Developing Countries: Options for Capturing Synergies. www.fao.org/docrep/012/i1318e/i1318e00.
- FAO, 2007. Universal Software for Fisheries Statistical Time Series, Food and Agriculture Organization of the United Nations, Rome, Italy, vol. 2, pp. 32.
- Fao . Italy; Rome: 2014. The State of World Fisheries and Aquaculture; p. 223. [Google Scholar]
- Hassan H.U., Ali Q.M., Khan W., Masood Z., Abdel-Aziz M.F.A., Shah M.I.A., Gabol K., Wattoo J., Mahmood Chatta A., Kamal M., Zulfiqar T., Hossain M.Y. Effect of feeding frequency as a rearing system on biological performance, survival, body chemical composition and economic efficiency of Asian Seabass Lates calcarifer (Bloch, 1790) reared under controlled environmental conditions. Saudi J. Biol. Sci. 2021;28(12):7360–7366. doi: 10.1016/j.sjbs.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H.U., Ali Q.M., Siddique M.A.M., Hasan M.R., Hossain M.Y. Effects of dietary protein levels on growth, nutritional utilization, carcass composition and survival of Asian Seabass Lates calcarifer (Bloch, 1790) fingerlings rearing in net cages. Thalassas: Int. J. Mar. Sci. 2021 doi: 10.1007/s41208-021-00371-8. [DOI] [Google Scholar]
- Hassan H.U., Mohammad Ali Q., Ahmad N., Masood Z., Yeamin Hossain M.d., Gabol K., Khan W., Hussain M., Ali A., Attaullah M., Kamal M. Assessment of growth characteristics, the survival rate and body composition of Asian Sea bass Lates calcarifer (Bloch, 1790) under different feeding rates in closed aquaculture system. Saudi J. Biol. Sci. 2021;28(2):1324–1330. doi: 10.1016/j.sjbs.2020.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Hassan U.H., Siddique M.A.M., Mahmood K., Abdel-Aziz V., Laghari M.Y., Abro N.A., Gabol K., Nisar, Rizwan S., Halima Effect of varying dietary protein levels on growth performance and survival of milkfish Chanos chanos fingerlings reared in brackish water pond ecosystem. Egypt. J. Aquat. Res. 2021;47(3):329–334. doi: 10.1016/j.ejar.2021.05.001. [DOI] [Google Scholar]
- Kaur Y., Dhawan A., Naveenkumar B.T., Tyagi A., Shanthanagouda A.H. Immuno stimulatory and antifertility effects of Neem (Azadirachta indica) leaf extract on common carp (Cyprinus carpio Linnaeus) Indian J. Anim. Res. 2020;54(2):196–201. [Google Scholar]
- Khan M.S.K., Siddique M.A.M., Zamal H. Replacement of fish meal by plant protein sources in Nile tilapia (Oreochromis niloticus) diet: growth performance and utilization. Iran. J. Fish. Sci. 2013;12(4):864–872. [Google Scholar]
- Kumar A., Prasad M.R., Srivastava K., Tripathi S., Srivastav A.K. Branchial histopathological study of Catfish Heteropneustes fossilis following exposure to purified neem extract, Azad irachtin. World J. Zool. 2010;5(4):239–243. [Google Scholar]
- Martinez, S.O. 2002. NIM – Azadirachta indica: natureza, usosmúltiploseprodução. Instituto Agronômicodo Paraná (IAPAR), Londrina, PR.2002.
- Naeem M., Salam A., Zuberi A. Proximate composition of freshwater rainbow trout (Oncorhynchus mykiss) in relation to body size and condition factor from Pakistan. Pak. J. Agric. Sci. 2016;53(02):468–472. [Google Scholar]
- Nya E.J., Austin B. Use of dietary ginger, Zingiberofficinale Roscoe, as an immunostimulant to control Aeromonashydrophila infections in rainbow trout, Oncorhynchus mykiss (Walbaum) J. Fish Dis. 2009;32(11):971–977. doi: 10.1111/j.1365-2761.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- Obaroh I.O., Achionye-N G.C. Effects of crude extract of Azadirachta indica leaves at controlling prolific breeding in Oreochromis niloticus (Linnaeus, 1758) Asian J. Agric. Res. 2011;5(5):277–282. [Google Scholar]
- Omorgerie E., Okpanachi M.A. Acute toxicity of water extracts of bark of the Neem ptarrt, Azadirachta indica (Lodd) to the Cichlid, Tilapia zilli (Gervais) Acta hydrobiologica (Cracow) 1997;39:47–51. [Google Scholar]
- Oniovosa U., Aina O., Alarape S., Babalola O., Adeyemo O. Effects of Neem leaves aqueous extract on organ histology, haematological parameters and biochemical indices in catfish. Alex. J. Vet. Sci. 2017;54(1):17. doi: 10.5455/ajvs.10.5455/ajvs.256015. [DOI] [Google Scholar]
- Pedge S.S., Ahirrao S.D. Antimicrobial activities of turmeric and ginger root used in the treatment of infectious fish disease. Int. J. Innovat. BioSci. 2012;2(2):81–84. [Google Scholar]
- Polakof S., Panserat S., Soengas J.L., Moon T.W. Glucose metabolism in fish: a review. J. Comp. Physiol. B: Biochem. 2012;182:1015–1045. doi: 10.1007/s00360-012-0658-7. [DOI] [PubMed] [Google Scholar]
- Şahin Ş.A., Başçinar N., Kocabaş M., Tufan B., Köse S., Okumuş I. Evaluation of meat yield, proximate composition and fatty acid profile of cultured brook trout (Salvelinus fontinalis Mitchill, 1814) and black sea trout (Salmo trutta labrax Pallas, 1811) in comparison with their hybrid. Turkish J. Fish. Aquat. Sci. 2011;11(2):261–271. [Google Scholar]
- Shah A.S., Gunjal M.A., Juvekar A.R. Immunostimulatory activity of aqueous extract of Azadirachta indica flowers on specific and non-specific immune response. J. Nat. Remedies. 2009;9(1):35–42. [Google Scholar]
- Siddique M.A.M., Mojumder P., Zamal H. Proximate composition of three commercially available marine dry fishes (Harpodon nehereus, Johnius dussumieri and Lepturacanthus savala) Am. J. Food Technol. 2012;7(7):429–436. [Google Scholar]
- Syed R., Masood Z., Hassan H.U., Khan W., Mushtaq S., Ali A., Gul Y., Jafari H., Shah M.I.A., Gabol K. Growth performance, hematological assessment, and chemical composition of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) fed different levels of Aloe vera extract as feed additives in a closed aquaculture system. Saudi J. Biol. Sci. 2021;S1319–562X(21) doi: 10.1016/j.sjbs.2021.08.098. 00786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpur A.D., Ikhwanuddin M. Azadirachta indica (Neem) leaf dietary effects on the immunity response and disease resistance of Asian seabass, Lates calcarifer challenged with Vibrio harveyi. Fish Shellfish Immunol. 2013;34(1):254–264. doi: 10.1016/j.fsi.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Zeng C., Naylor E. Endogenous tidal rhythms of vertical migration in field collected zoea-1 larvae of the shore crab Carcinus maenas: implications for ebb tide offshore Dispersal. Mar. Ecol. Prog. Ser. 1996;132:71–82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study are included in this published article.